Impact of Polymerization Technique and ZrO2 Nanoparticle Addition on the Fracture Load of Interim Implant-Supported Fixed Cantilevered Prostheses in Comparison to CAD/CAM Material
Abstract
1. Introduction
2. Materials and Methods
2.1. PMMA/ZrO2NP Mixture Preparation
2.2. Heat-Polymerized Acrylic Resin Specimens
2.3. CAD/CAM Specimen Preparation
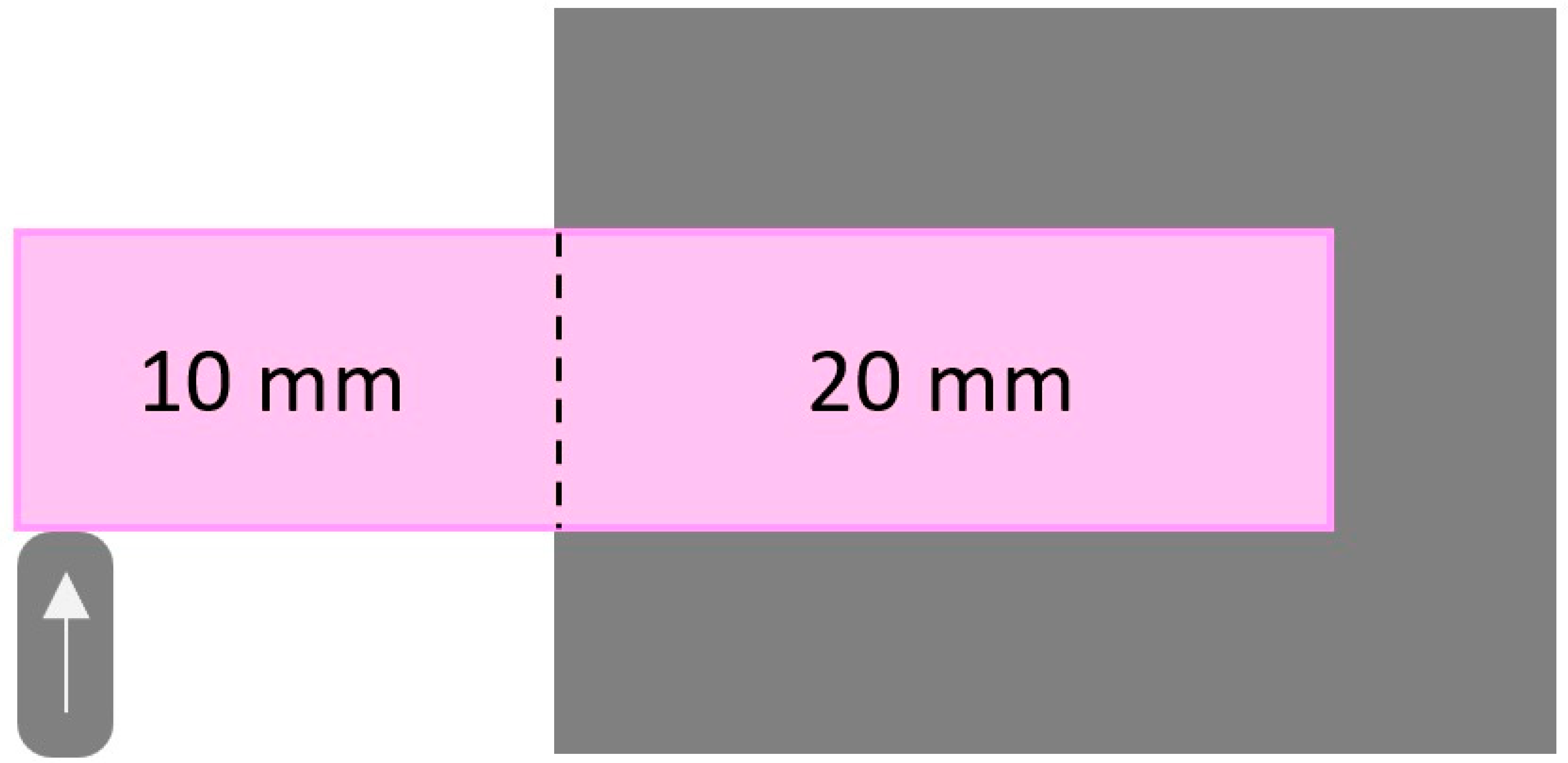
2.4. Specimen Testing
2.5. Statistical Analysis
3. Results
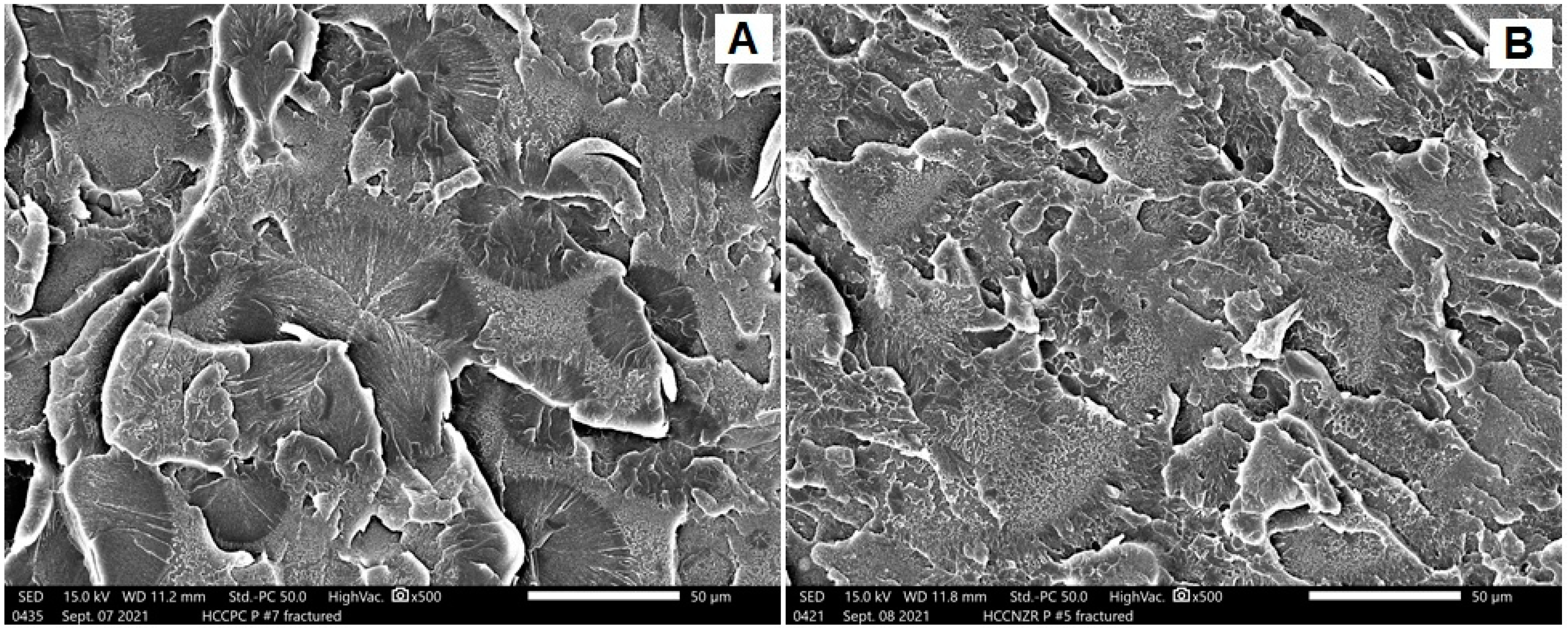
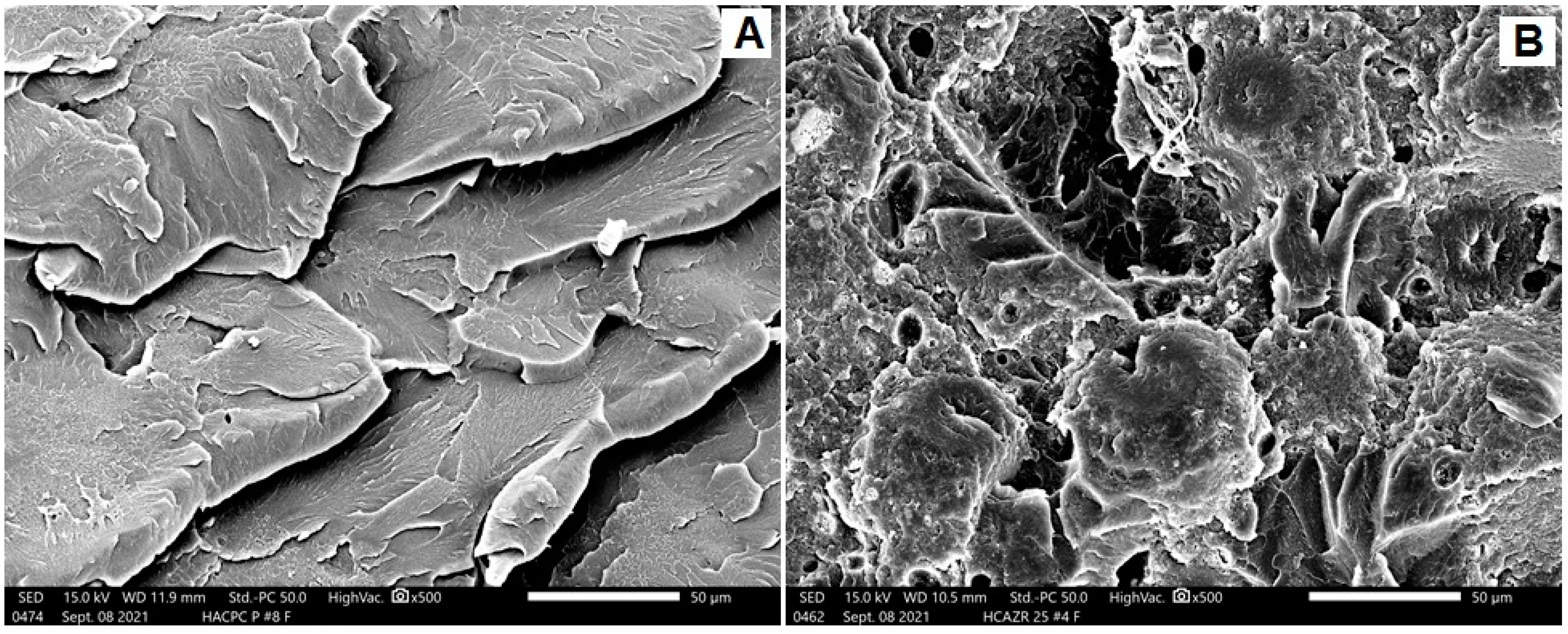
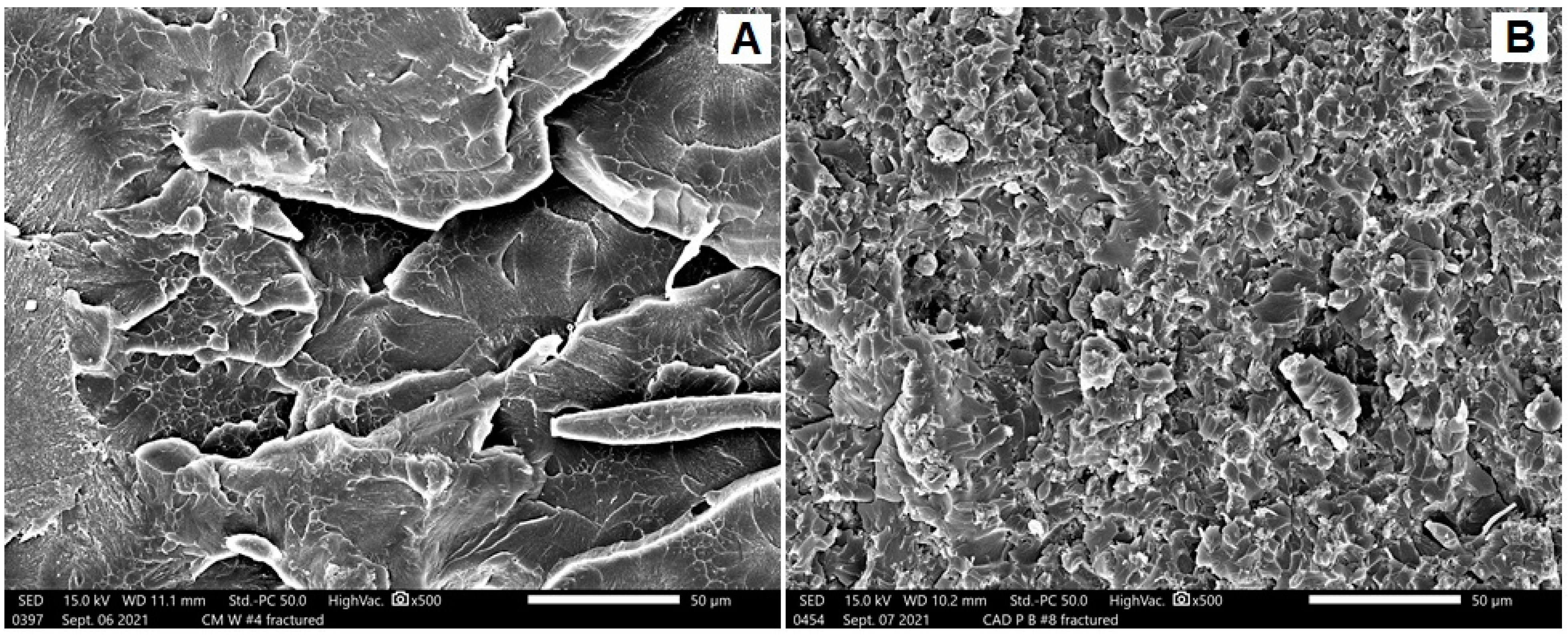
SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, S.; Aras, M.A.; Chitre, V. Guidelines for treatment planning of mandibular implant overdenture. J. Dent. Implant. 2014, 4, 86–90. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Lindquist, L.W. Ten-year longitudinal study of masticatory function in edentulous patients treated with fixed complete dentures on osseointegrated implants. Int. J. Prosthodont. 1994, 7, 448–453. [Google Scholar] [PubMed]
- Romanos, G.E.; Gupta, B.; Eckert, S.E. Distal cantilevers and implant dentistry. Int. J. Oral Maxillofac. Implants. 2012, 27, 1131–1136. [Google Scholar] [PubMed]
- Alshahrani, F.; Yilmaz, B.; Seidt, J.; McGlumphy, E.; Brantley, W. A load-to-fracture and strain analysis of monolithic zirconia cantilevered frameworks. J. Prosthet. Dent. 2017, 118, 752–758. [Google Scholar] [CrossRef]
- Yilmaz, B.; Alp, G.; Seidt, J.; Johnston, W.M.; Vitter, R.; McGlumphy, E.A. Fracture analysis of CAD-CAM high-density polymers used for interim implant-supported fixed, cantilevered prostheses. J. Prosthet. Dent. 2018, 120, 79–84. [Google Scholar] [CrossRef]
- Davidowitz, G.; Kotick, P. The use of CAD/CAM in dentistry. Dent. Clin. N. Am. 2011, 55, 559–570. [Google Scholar] [CrossRef]
- Al-Dharrab, A. The residual monomer content and mechanical properties of CAD\CAM resins used in the fabrication of complete dentures as compared to heat cured resins. Electron. Physician 2017, 9, 4766–4772. [Google Scholar]
- Ayaz, E.; Durkan, R.; Koroglu, A.; Bagis, B. Comparative effect of different polymerization techniques on residual monomer and hardness properties of PMMA-based denture resins. J. Appl. Biomater. Funct. Mater. 2014, 12, 228–233. [Google Scholar] [CrossRef]
- Ozkir, S.; Yilmaz, B.; Unal, S.; Culhaoglu, A.; Kurkcuoglu, I. Effect of heat polymerization conditions and microwave on the flexural strength of polymethyl methacrylate. Eur. J. Dent. 2018, 12, 116–119. [Google Scholar] [CrossRef]
- Durkan, R.; Özel, M.; Bagis, B.; Usanmaz, A. In vitro comparison of autoclave polymerization on the transverse strength of denture base resins. Dent. Mater. J. 2008, 27, 640–642. [Google Scholar] [CrossRef][Green Version]
- Banerjee, K.; Prithviraj, M.; Augustine, N.; Pradeep, S.P.; Thiagarajan, P. Analytical characterization and antimicrobial activity of nano zirconia particles. J. Chem. Pharm. Sci. 2016, 9, 1186–1190. [Google Scholar]
- Gowri, S.; Rajiv, R.; Sundrarajan, M. Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J. Mater. Sci. Technol. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Arendorf, T.M.; Walker, D.M. Denture stomatitis: A review. J. Oral. Rehabil. 1987, 14, 2017–2277. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.; Alabidi, K.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 13, 283–292. [Google Scholar] [CrossRef]
- Chęcińska, K.; Chęciński, M.; Sikora, M.; Nowak, Z.; Karwan, S.; Chlubek, D. The Effect of Zirconium Dioxide (ZrO2) Nanoparticles Addition on the Mechanical Parameters of Polymethyl Methacrylate (PMMA): A Systematic Review and Meta-Analysis of Experimental Studies. Polymers 2022, 14, 1047. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural Strength and Hardness of Filler-Reinforced PMMA Targeted for Denture Base Application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef]
- Hamid, S.; Alghamdi, L.; Alshahrani, F.; Khan, S.; Matin, A.; Gad, M. In vitro assessment of artificial aging on the antifungal activity of PMMA denture base material modified with ZrO2 nanoparticles. Int. J. Dent. 2021, 2021, 5560443. [Google Scholar] [CrossRef]
- Helal, M.A.; Fadl-Alah, A.; Baraka, Y.M.; Gad, M.M.; Emam, A.N. In-vitro comparative evaluation for the surface properties and impact strength of CAD/CAM milled, 3D printed, and polyamide denture base resins. J. Int. Soc. Prevent. Communit. Dent. 2022, 12, 126. [Google Scholar]
- Gad, M.M.; Fouda, S.M.; Abualsaud, R.; Alshahrani, F.A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Helal, M.A.; Al-Harbi, F.A. Strength and surface properties of a 3D-printed denture base polymer. J. Prosthodont. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Abualsaud, R.; Al-Thobity, A.M.; Akhtar, S.; Siddiqui, I.A.; Al-Harbi, F.A. Influence of artificial aging and ZrO2 nanoparticle-reinforced repair resin on the denture repair strength. J. Clin. Exper. Dent. 2020, 12, e354–e362. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Chuang, S.K.; Weber, H.P.; Gallucci, G.O. A systematic review of biologic and technical complications with fixed implant rehabilitations for edentulous patients. Int. J. Oral Maxillofac. Implant. 2012, 27, 102–110. [Google Scholar]
- Deste, G.; Durkan, R.; Oyar, P.; Gurbuz, A. The effect of autoclave and heat polymerization techniques of internal adaptation of acrylic resins. J. Dent. Fac. Ataturk Uni. 2020, 30, 614–619. [Google Scholar] [CrossRef]
- Leão, R.D.S.; de Moraes, S.L.D.; Gomes, J.M.D.L.; Lemos, C.A.A.; Casado, B.G.D.S.; Vasconcelos, B.C.D.E.; Pellizzer, E.P. Influence of addition of zirconia on PMMA: A systematic review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110292. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Assessing fracture toughness and impact strength of PMMA reinforced with nano-particles and fibre as advanced denture base materials. Materials 2021, 14, 4127. [Google Scholar] [CrossRef] [PubMed]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the mechanical properties of ZrO2-impregnated PMMA nanocomposite for denture-based applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef]
- Edelhoff, D.; Beuer, F.; Schweiger, J.; Brix, O.; Stimmelmayr, M.; Guth, J.F. CAD/CAM-generated high density polymer restorations for the pretreatment of complex cases: A case report. Quintessence Int. 2012, 43, 457–467. [Google Scholar]
- Saad, Y.; Abdelhamid, A.; ElShabrawy, S. Laboratory evaluation of pre-polymerized denture base material used for CAD/CAM complete denture manufacturing. Alex. Dent. J. 2018, 43, 94–101. [Google Scholar] [CrossRef]
- Nguyen, J.; Migonney, V.; Ruse, N.; Sadoun, M. Resin composite blocks via high-pressure high-temperature polymerization. Dent. Mater. 2012, 28, 529–534. [Google Scholar] [CrossRef]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.; Lassila, L. 3D-Printed vs. heat-polymerizing and autopolymerizing denture base acrylic resins. Materials 2021, 14, 5781. [Google Scholar]
- Prpić, V.; Schauperl, Z.; Ćatić, A.; Dulčić, N.; Čimić, S. Comparison of mechanical properties of 3D-printed, CAD/CAM, and conventional denture base materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Es-Said, O.; Foyos, J.; Noorani, R.; Mendelson, M.; Marloth, R.; Pregger, B. Effect of layer orientation on mechanical properties of rapid prototyped samples. Mater. Manuf. Process. 2000, 15, 107–122. [Google Scholar] [CrossRef]
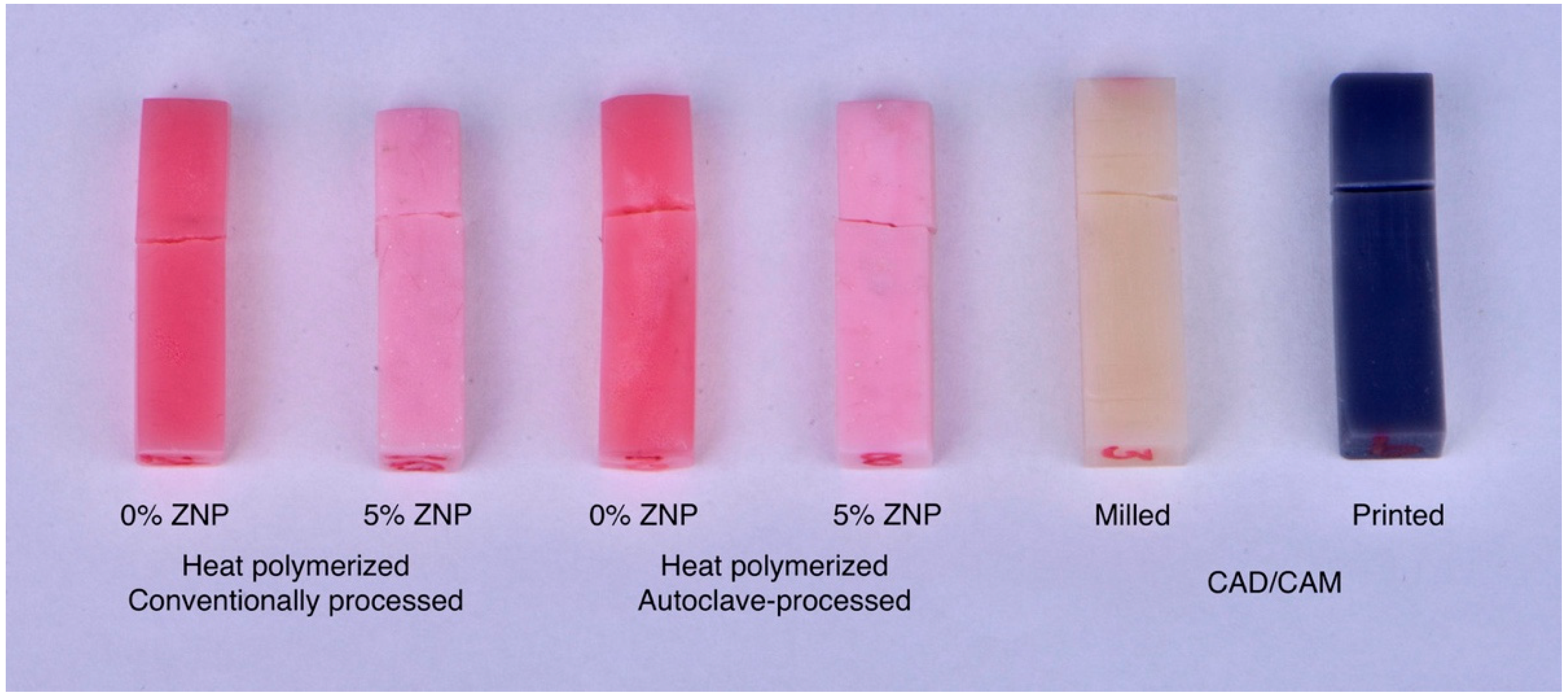
| Heat Polymerized | CAD/CAM | |||||
|---|---|---|---|---|---|---|
| Conventionally Processed | Autoclave-Processed | |||||
| 0% ZNP | 5% ZNP | 0% ZNP | 5% ZNP | Milled | 3D-Printed | |
| Mean (SD) | 635.5 (43.4) a | 583.4 (58.1) a | 744.0 (76.1) b | 380.7 (52.8) | 926.6 (82.8) | 739.4 (58.8) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshahrani, F.A.; Hamid, S.K.; Alghamdi, L.A.; Alqarawi, F.K.; Al-Dulaijan, Y.A.; AlRumaih, H.S.; Alalawi, H.; Al Ghamdi, M.A.; Alzoubi, F.; Gad, M.M. Impact of Polymerization Technique and ZrO2 Nanoparticle Addition on the Fracture Load of Interim Implant-Supported Fixed Cantilevered Prostheses in Comparison to CAD/CAM Material. Dent. J. 2022, 10, 102. https://doi.org/10.3390/dj10060102
Alshahrani FA, Hamid SK, Alghamdi LA, Alqarawi FK, Al-Dulaijan YA, AlRumaih HS, Alalawi H, Al Ghamdi MA, Alzoubi F, Gad MM. Impact of Polymerization Technique and ZrO2 Nanoparticle Addition on the Fracture Load of Interim Implant-Supported Fixed Cantilevered Prostheses in Comparison to CAD/CAM Material. Dentistry Journal. 2022; 10(6):102. https://doi.org/10.3390/dj10060102
Chicago/Turabian StyleAlshahrani, Faris A., Shorouq Khalid Hamid, Lujain Ali Alghamdi, Firas K. Alqarawi, Yousif A. Al-Dulaijan, Hamad S. AlRumaih, Haidar Alalawi, Maram A. Al Ghamdi, Fawaz Alzoubi, and Mohammed M. Gad. 2022. "Impact of Polymerization Technique and ZrO2 Nanoparticle Addition on the Fracture Load of Interim Implant-Supported Fixed Cantilevered Prostheses in Comparison to CAD/CAM Material" Dentistry Journal 10, no. 6: 102. https://doi.org/10.3390/dj10060102
APA StyleAlshahrani, F. A., Hamid, S. K., Alghamdi, L. A., Alqarawi, F. K., Al-Dulaijan, Y. A., AlRumaih, H. S., Alalawi, H., Al Ghamdi, M. A., Alzoubi, F., & Gad, M. M. (2022). Impact of Polymerization Technique and ZrO2 Nanoparticle Addition on the Fracture Load of Interim Implant-Supported Fixed Cantilevered Prostheses in Comparison to CAD/CAM Material. Dentistry Journal, 10(6), 102. https://doi.org/10.3390/dj10060102









