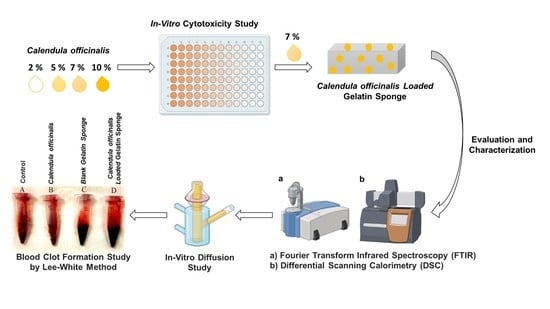
Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. In-Vitro Cytotoxicity Assay
2.2.2. Preparation and Evaluation of Calendula officinalis Oil-Loaded Gelatin Sponges
2.2.3. Drug Content
2.2.4. Solid-State Characterization of Calendula officinalis Oil-Loaded Sponge
Fourier-Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
In-Vitro Diffusion Study
2.2.5. Hemostasis In-Vitro (Clotting Time Determination)
3. Results
3.1. Cytotoxicity Assay
3.2. Preparation of Calendula Oil-Loaded Sponge
3.3. Drug Content
3.4. Evaluation and Characterization of Calendula Oil-Loaded Sponge
3.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4.2. Differential Scanning Calorimetry (DSC)
3.4.3. In-Vitro Diffusion study
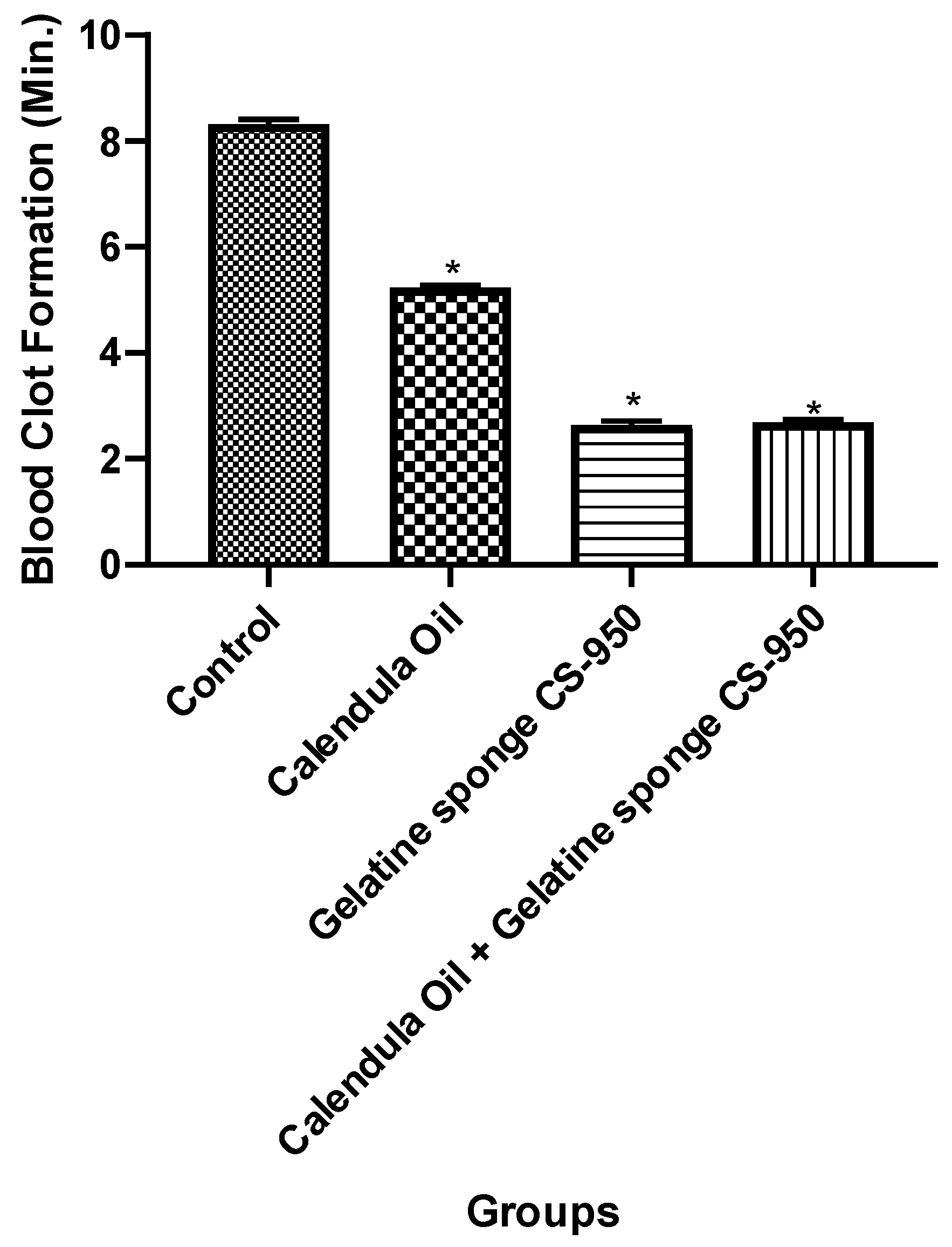
3.5. Hemostasis In-Vitro (Clotting Time Determination)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paepe, A.D.; Malfait, F. Bleeding and bruising in patients with Ehlers-Danlos syndrome and other collagen vascular disorders. Br. J. Haematol. 2004, 127, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Anarthe, R.; Kale, P.; Maniyar, S.; Anuraga, S. Hemostatic agents in dentistry. Galore Int. J. Health Sci. Res. 2018, 3, 40–46. [Google Scholar]
- Werner, E.J.; Broxson, E.H.; Tucker, E.L.; Giroux, D.S.; Shults, J.; Abshire, T.C. Prevalence of von Willebrand disease in children: A multiethnic study. J. Pediatr. 1993, 123, 893–898. [Google Scholar] [CrossRef]
- Kumar, M.; Pai, K.M.; Vineetha, R.; Kurien, A. Oral hygiene and dentition status in patients with congenital hemorrhagic disorders: A comparative study. Bras. Odontopediatria Clínica Integr. 2020, 20, 20. [Google Scholar] [CrossRef]
- Brodbelt, A.R.; Miles, J.B.; Foy, P.M.; Broome, J.C. Intraspinal oxidised cellulose (Surgicel) causing delayed paraplegia after thoracotomy—A report of three cases. Ann. R. Coll. Surg. Engl. 2002, 84, 97–99. [Google Scholar]
- Hiwatashi, N.; Hirano, S.; Mizuta, M.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J.; Kawai, K.; Suzuki, S. Biocompatibility and efficacy of collagen/gelatin sponge scaffold with sustained release of basic fibroblast growth factor on vocal fold fibroblasts in 3-dimensional culture. Ann. Otol. Rhinol. Laryngol. 2015, 124, 116–125. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion 2008, 48, 1502–1516. [Google Scholar] [CrossRef]
- Rodrigues, R.N.; Lopes, A.A.; Torres, J.M.; Mundim, M.F.; Silva, L.L.; Silva, B.R. Compressive neuropathy of the first branch of the lateral plantar nerve: A study by magnetic resonance imaging. Radiol. Bras. 2015, 48, 368–372. [Google Scholar] [CrossRef][Green Version]
- Szpalski, M.; Gunzburg, R.; Sztern, B. An overview of blood-sparing techniques used in spine surgery during the perioperative period. Eur. Spine J. 2004, 13 (Suppl. 1), S18–S27. [Google Scholar] [CrossRef]
- Javan, R.; Yousefi, M.; Nazari, S.M.; Amiri, P.; Mosavi-Jarrahi, A.; Modiramani, P.; Naghedi-Baghdar, H. Herbal Medicines in Idiopathic Heavy Menstrual Bleeding: A Systematic Review. Phytother. Res. 2016, 30, 1584–1591. [Google Scholar] [CrossRef]
- Haznedaroglu, B.Z.; Haznedaroglu, I.C.; Walker, S.L.; Bilgili, H.; Goker, H.; Kosar, A.; Aktas, A.; Captug, O.; Kurt, M.; Özdemir, O.; et al. Ultrastructural and Morphological Analyses of the In Vitro and In Vivo Hemostatic Effects of Ankaferd Blood Stopper. Clin. Appl. Thromb. Hemostasis 2010, 16, 446–453. [Google Scholar] [CrossRef]
- Marigold (Calendula officinalis L.). Available online: https://www.tandfonline.com/doi/abs/10.1080/J157v06n03_08 (accessed on 10 March 2022).
- Pal, K.; Kundu, S.K.; Mandal, M.K.; Kundu, T.K. Prothrombin time test, Phytochemical screening, Thin layer chromatographic study and Quantitative study of Calendula officinalis leaves. Int. J. Pharmacol. Pharm. Sci. 2015, 2, 43–49. [Google Scholar]
- Scherer, W.; Gultz, J.; Lee, S.S.; Kaim, J. The ability of an herbal mouthrinse to reduce gingival bleeding. J. Clin. Dent. 1998, 9, 97–100. [Google Scholar]
- Chandran, P.K.; Kuttan, R. Effect of Calendula officinalis Flower Extract on Acute Phase Proteins, Antioxidant Defense Mechanism and Granuloma Formation During Thermal Burns. J. Clin. Biochem. Nutr. 2008, 43, 58–64. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Sosa, S.; Jurenitsch, J.; Schubert-Zsilavecz, M.; Della Loggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-oedematous activities of the main triterpendiol esters of marigold (calendula officinalis, L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef]
- Parente, L.M.; Andrade, M.A.; Brito, L.A.; Moura, V.M.; Miguel, M.P.; Lino-Júnior, R.D.S.; Tresvenzol, L.F.; Paula, J.R.; Paulo, N.M. Angiogenic activity of calendula officinalis flowers, L. in rats. Acta Cir. Bras. 2011, 26, 19–24. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory, anti-tumor-promoting, and cytotoxic activities of constituents of marigold (calendula officinalis) flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Patil, V.; Rathod, V.; Halkai, R. Cytotoxicity evaluation of fungal-derived silver nanoparticles on human gingival fibroblast cell line: An in vitro study. J. Conserv. Dent. JCD 2019, 22, 160. [Google Scholar] [CrossRef]
- Auddy, B.; Ferreira, M.; Blasina, F.; Lafon, L.; Arredondo, F.; Dajas, F.; Tripathi, P.C.; Seal, T.; Mukherjee, B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 2003, 84, 131–138. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Omer, A.M.; Abbas, E.; Eid, A.I.; Mohy-Eldin, M.S.; Hassan, M.A. Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep. 2021, 11, 3428. [Google Scholar] [CrossRef]
- Menda, J.P.; Reddy, T.; Deepika, R.; Sastry, T.P. Preparation and characterization of wound healing composites of chitosan, aloe vera and calendula officinalis—A comparative study. Am. J. Phytomedicine Clin. Ther. 2014, 2, 61–76. [Google Scholar]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Hernández, R.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.; de Campos, A.M.; Mijangos, C. Nanocomposite chitosan hydrogels based on PLGA nanoparticles as potential biomedical materials. Eur. Polym. J. 2018, 99, 456–463. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Sallam, A. The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Arana, L.; Salado, C.; Vega, S.; Aizpurua-Olaizola, O.; de la Arada, I.; Suarez, T.; Usobiaga, A.; Arrondo, J.L.; Alonso, A.; Goñi, F.M.; et al. Solid lipid nanoparticles for delivery of Calendula officinalis extract. Colloids Surf. B Biointerfaces 2015, 135, 18–26. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Wang, D.; Shen, M.; Ou, H.; Zhao, J.; Chen, M.; Yan, G.; Chen, J. Rapid Hemostatic Biomaterial from a Natural Bath Sponge Skeleton. Mar. Drugs 2021, 19, 220. [Google Scholar] [CrossRef]
- Hossein, N.; Abolfazl, M.; Mahdi, S.; Ali, K. Effect of Cinnamon zeylanicum essence and distillate on the clotting time. J. Med. Plants Res. 2013, 7, 1339–1343. [Google Scholar]
- Kee, N.L.; Mnonopi, N.; Davids, H.; Naude, R.J.; Frost, C.L. Antithrombotic/anticoagulant and anticancer activities of selected medicinal plants. Afr. J. Biotechnol. 2008, 7, 217–223. [Google Scholar]
- Hess, J.R.; Brohi, K.; Dutton, R.P.; Hauser, C.J.; Holcomb, J.B.; Kluger, Y.; Mackway-Jones, K.; Parr, M.; Rizoli, S.B.; Yukioka, T.; et al. The coagulopathy of trauma: A review of mechanisms. J. Trauma Acute Care Surg. 2008, 65, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.; Strayer, D.S.; Rubin, E. (Eds.) Rubin’s Pathology: Clinicopathologic Foundations of Medicine, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Anti-inflammatory activity of flower extract of Calendula officinalis Linn. and its possible mechanism of action. Indian J. Exp. Biol. 2009, 47, 113–120. [Google Scholar] [PubMed]
- Lagarto, A.; Bueno, V.; Guerra, I.; Valdés, O.; Vega, Y.; Torres, L. Acute and subchronic oral toxicities of Calendula officinalis extract in Wistar rats. Exp. Toxicol. Pathol. 2011, 63, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Shafeie, N.; Naini, A.T.; Jahromi, H.K. Comparison of Different Concentrations of Calendula Officinalis Gel on Cutaneous Wound Healing. Biomed. Pharmacol. J. 2015, 8, 979–992. [Google Scholar] [CrossRef]
- Saini, P.; Al-Shibani, N.; Sun, J.; Zhang, W.; Song, F.; Gregson, K.S.; Windsor, L.J. Effects of Calendula officinalis on human gingival fibroblasts. Homeopathy 2012, 101, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Matysik, M.G.; Wójciak-Kosior, M.; Paduch, R. The influence of Calendulae officinalis flos extracts on cell cultures, and the chromatographic analysis of extracts. J. Pharm. Biomed. Anal. 2005, 38, 285–292. [Google Scholar] [CrossRef]
- Rodríguez-Acosta, H.; Tapia-Rivera, J.M.; Guerrero-Guzmán, A.; Hernández-Elizarraráz, E.; Hernández-Díaz, J.A.; Garza-García, J.J.O.; Pérez-Ramírez, P.E.; Velasco-Ramírez, S.F.; Ramírez-Anguiano, A.C.; Velázquez-Juárez, G.; et al. Chronic wound healing by controlled release of chitosan hydrogels loaded with silver nanoparticles and calendula extract. J. Tissue Viability 2022, 31, 173–179. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.K.; Hommos, A.M.; Kotry, G.S.; Labib, G.S. The effect of a calendula based topical formula versus oxidized regenerated cellulose on palatal wound healing: A randomized controlled clinical trial. Alex. Dent. J. 2021, 46, 45–53. [Google Scholar] [CrossRef]
- de Brito Sousa, J.D.; Sachett, J.A.G.; de Oliveira, S.S.; Mendonça-da-Silva, I.; Marques, H.O.; de Lacerda, M.V.G.; Fan, H.W.; Monteiro, W.M. Accuracy of the Lee-White Clotting Time Performed in the Hospital Routine to Detect Coagulopathy in Bothrops atrox Envenomation. Am. J. Trop. Med. Hyg. 2018, 98, 1547–1551. [Google Scholar] [CrossRef]
- Ogle, C.W.; Dai, S.; Ma, J.C. The haemostatic effects of the Chinese herbal drug Yunnan Bai Yao: A pilot study. Am. J. Chin. Med. 1976, 4, 147–152. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Torbati, M.; Mahmoudi, J.; Valizadeh, H. Medicinal Plants as Potential Hemostatic Agents. J. Pharm. Pharm. Sci. 2020, 23, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Kooshki, F.; Tabatabaei, F.S.; Tajik, S.; Aayan, A. The comparison of antimicrobial effects of herbal and chemical agents on toothpaste: An experimental study. Dent. Res. J. 2018, 15, 289–294. [Google Scholar]
- Scarborough, J.E.; Schumacher, J.; Pappas, T.N.; McCoy, C.C.; Englum, B.R.; Agarwal, S.K.J.; Greenberg, C.C. Which Complications Matter Most? Prioritizing Quality Improvement in Emergency General Surgery. J. Am. Coll. Surg. 2016, 222, 515–524. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs. 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Mp, S.K. Local hemostatic agents in the management of bleeding in oral surgery. Asian J. Pharm. Clin. Res. 2016, 9, 35–41. [Google Scholar]
- AshwlayanVD, K.A.; Verma, M. Therapeutic potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar]
- Della Loggia, R.; Tubaro, A.; Sosa, S.; Becker, H.; Saar, S.; Isaac, O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Med. 1994, 60, 516–520. [Google Scholar] [CrossRef]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma Mon. 2016, 21, e26023. [Google Scholar] [CrossRef]
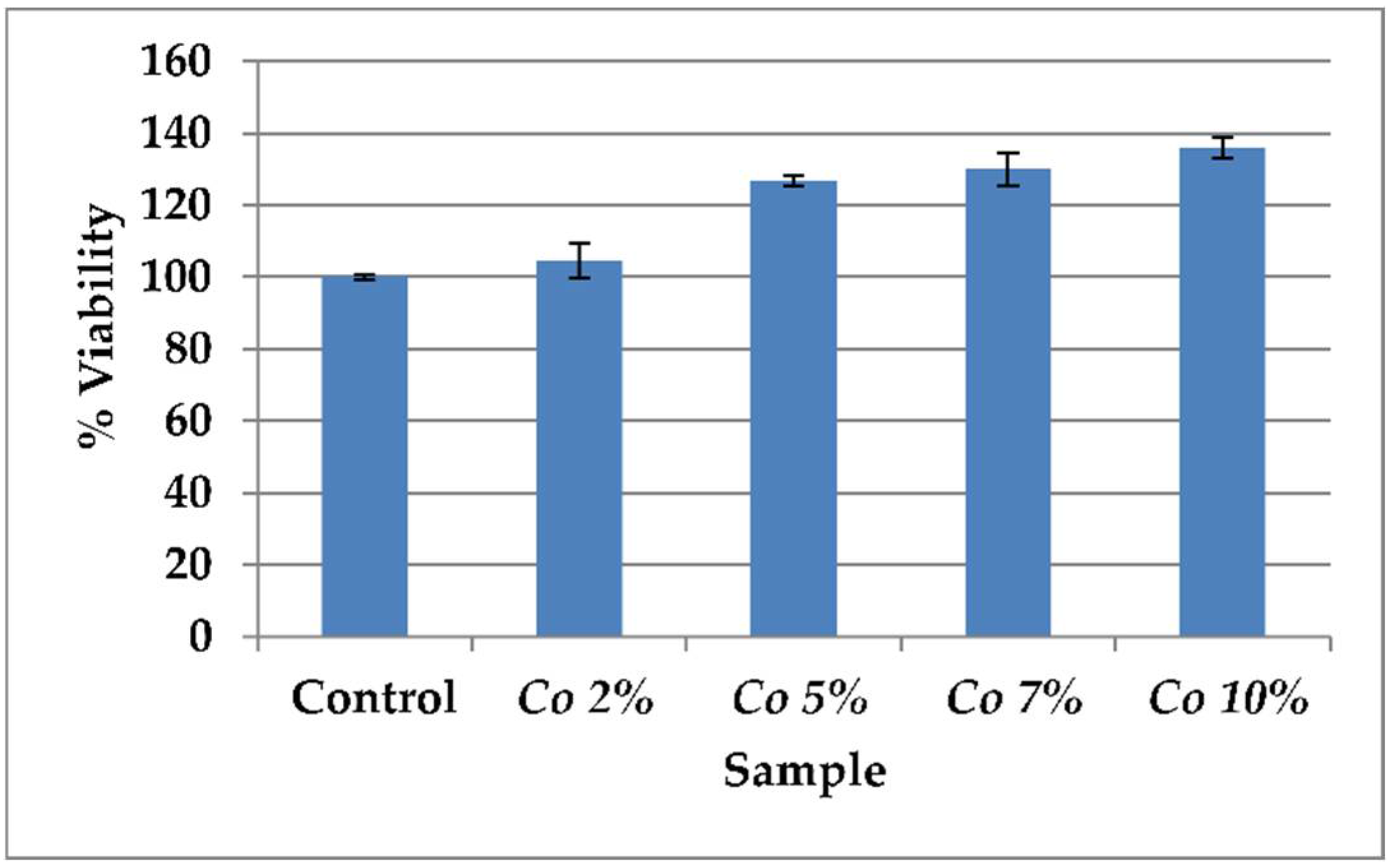









| Sample | % Viability |
|---|---|
| Control | 100 ± 0.5 |
| 2% Calendula officinalis | 104.7 ± 4.9 |
| 5% Calendula officinalis | 126.7 ± 1.5 |
| 7% Calendula officinalis | 130.1 ± 4.5 |
| 10% Calendula officinalis | 136.0 ± 3.0 |
| Groups | Clotting Time (Seconds) | Clotting Time (Minutes) |
|---|---|---|
| Control | 499.7 ± 5.37 | 8.32 ± 0.089 |
| Calendula officinalis oil + gelatin sponge | 161.70 ± 3.11 | 2.69 ± 0.051 |
| Gelatin sponge | 158.75 ± 4.60 | 2.64 ± 0.076 |
| Calendula officinalis oil | 314.30 ± 2.40 | 5.23 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyanahalli Matta, B.K.; Kumar, S.; Mehta, C.H.; Nayak, U.Y.; Rodriguez, P.G. Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study. Dent. J. 2022, 10, 76. https://doi.org/10.3390/dj10050076
Ayyanahalli Matta BK, Kumar S, Mehta CH, Nayak UY, Rodriguez PG. Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study. Dentistry Journal. 2022; 10(5):76. https://doi.org/10.3390/dj10050076
Chicago/Turabian StyleAyyanahalli Matta, Bharath Kumar, Santhosh Kumar, Chetan Hasmukh Mehta, Usha Yogendra Nayak, and Patricia Garcia Rodriguez. 2022. "Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study" Dentistry Journal 10, no. 5: 76. https://doi.org/10.3390/dj10050076
APA StyleAyyanahalli Matta, B. K., Kumar, S., Mehta, C. H., Nayak, U. Y., & Rodriguez, P. G. (2022). Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study. Dentistry Journal, 10(5), 76. https://doi.org/10.3390/dj10050076