Interfacial Biomaterial–Dentin Bacterial Biofilm Proliferation and Viability Is Affected by the Material, Aging Media and Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Specimen Aging
2.3. Biofilm Cultivation
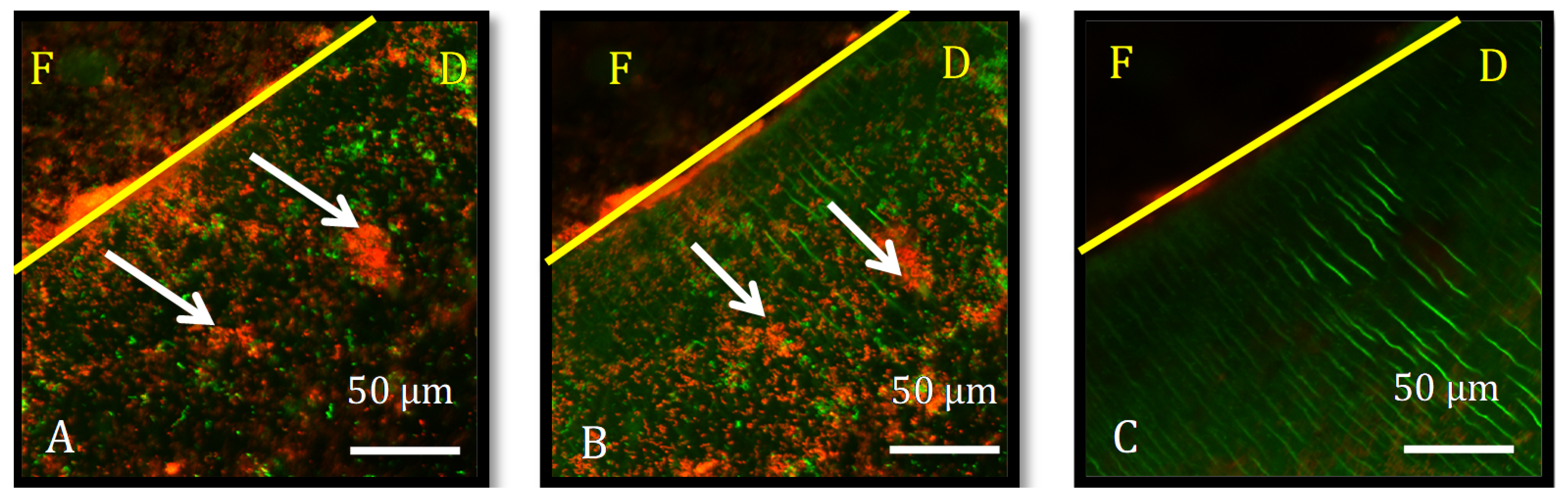
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
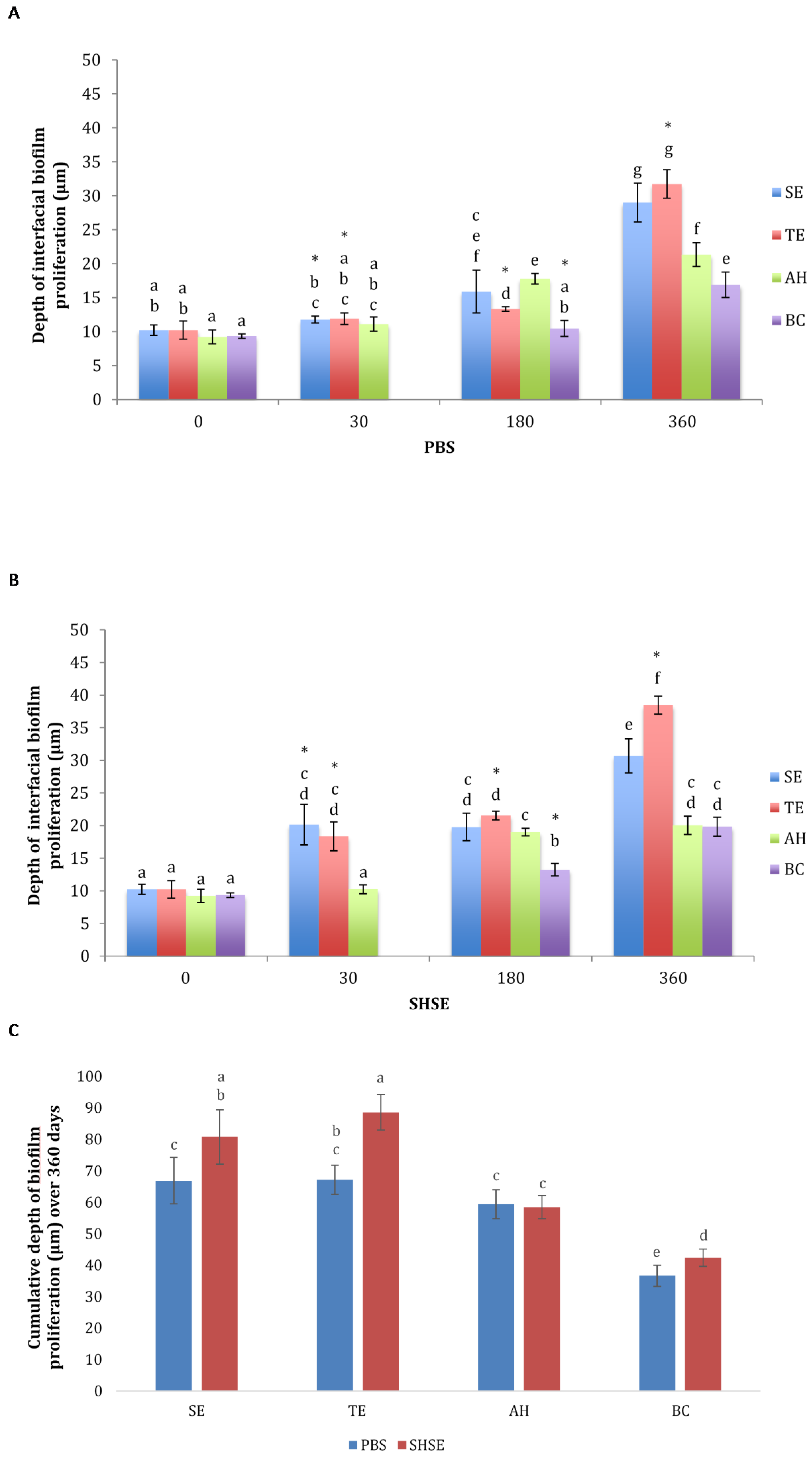
3.1. Bacterial Biofilm Proliferation Depth
3.2. Proportion of Live Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rôças, I.N.; Santos, S.R.L.D.; Lima, K.C.; Magalhães, F.A.C.; De Uzeda, M. Efficacy of Instrumentation Techniques and Irrigation Regimens in Reducing the Bacterial Population within Root Canals. J. Endod. 2002, 28, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Marashdeh, M.Q.; Gitalis, R.; Levesque, C.; Finer, Y. Enterococcus faecalis Hydrolyzes Dental Resin Composites and Adhesives. J. Endod. 2018, 44, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Marashdeh, M.Q.; Gitalis, R.; Lévesque, C.; Finer, Y. Endodontic pathogens possess collagenolytic properties that degrade human dentine collagen matrix. Int. Endod. J. 2019, 52, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Grossman, L.I. Endodontic Practice; Lea & Febiger: Philadelphia, PA, USA, 1988. [Google Scholar]
- Gomes, B.P.F.D.A.; Pedroso, J.L.; Jacinto, R.; Vianna, M.E.; Ferraz, C.; Zaia, A.A.; De Souza-Filho, F.J. In vitro evaluation of the antimicrobial activity of five root canal sealers. Braz. Dent. J. 2004, 15, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Hydration Characteristics of Calcium Silicate Cements with Alternative Radiopacifiers Used as Root-end Filling Materials. J. Endod. 2010, 36, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Grandini, S.; Ames, J.M.; Gu, L.-S.; Kim, S.K.; Pashley, D.H.; Gutmann, J.L.; Tay, F.R. Critical Review on Methacrylate Resin–based Root Canal Sealers. J. Endod. 2010, 36, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Sterzenbach, G.; Dietrich, T.; Bitter, K.; Frankenberger, R.; von Stein-Lausnitz, M. Dentin-like versus Rigid Endodontic Post: 11-year Randomized Controlled Pilot Trial on No-wall to 2-wall Defects. J. Endod. 2017, 43, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Slutzky-Goldberg, I.; Slutzky, H.; Gorfil, C.; Smidt, A. Restoration of Endodontically Treated Teeth Review and Treatment Recommendations. Int. J. Dent. 2009, 2009, 150251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi, S.; Santerre, J.; Cvitkovitch, D.; Finer, Y. Biodegradation of Resin-Dentin Interfaces Increases Bacterial Microleakage. J. Dent. Res. 2010, 89, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.A.; Friedman, S.; Lévesque, C.M.; Basrani, B.R.; Finer, Y. Microbial biofilm proliferation within sealer-root dentin interfaces is affected by sealer type and aging period. J. Endod. 2012, 38, 1253–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Sadeghinejad, L.; Adebayo, O.I.; Ma, D.; Xiao, Y.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Gene expression and protein synthesis of esterase from Streptococcus mutans are affected by biodegradation by-product from methacrylate resin composites and adhesives. Acta Biomater. 2018, 81, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Gitalis, R.; Zhou, L.; Marashdeh, M.Q.; Sun, C.; Glogauer, M.; Finer, Y. Human neutrophils degrade methacrylate resin composites and tooth dentin. Acta Biomater. 2019, 88, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Marashdeh, M.; Friedman, S.; Lévesque, C.; Finer, Y. Esterases affect the physical properties of materials used to seal the endodontic space. Dent. Mater. 2019, 35, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Sadeghinejad, L.; Cvitkovitch, D.G.; Siqueira, W.L.; Santerre, J.P.; Finer, Y. Triethylene Glycol Up-Regulates Virulence-Associated Genes and Proteins in Streptococcus mutans. PLoS ONE 2016, 11, e0165760. [Google Scholar] [CrossRef] [PubMed]
- Serkies, K.B.; Garcha, R.; Tam, L.E.; De Souza, G.M.; Finer, Y. Matrix metalloproteinase inhibitor modulates esterase-catalyzed degradation of resin–dentin interfaces. Dent. Mater. 2016, 32, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Sato, E.; Ferraz, C.; Teixeira, F.B.; Zaia, A.A.; Souza-Filho, F.J. Evaluation of time required for recontamination of coronally sealed canals medicated with calcium hydroxide and chlorhexidine. Int. Endod. J. 2003, 36, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Biodegradation of resin–dentin interfaces is dependent on the restorative material, mode of adhesion, esterase or MMP inhibition. Dent. Mater. 2018, 34, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Attam, K.; Talwar, S.; Yadav, S.; Miglani, S. Comparative analysis of the effect of autoclaving and 10% formalin storage on extracted teeth: A microleakage evaluation. J. Conserv. Dent. 2009, 12, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Grossi, M.; Morgunova, M.; Cheung, S.; Scholz, D.; Conroy, E.; Terrile, M.; Panarella, A.; Simpson, J.C.; Gallagher, W.; O’Shea, D.F. Lysosome triggered near-infrared fluorescence imaging of cellular trafficking processes in real time. Nat. Commun. 2016, 7, 10855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourbia, M.; Ma, D.; Cvitkovitch, D.; Santerre, J.; Finer, Y. Cariogenic Bacteria Degrade Dental Resin Composites and Adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, P.D. Dental Plaque as a Microbial Biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pitts, B.; Stewart, P.S.; Camper, A.; Yoon, J. Comparison of the Antimicrobial Effects of Chlorine, Silver Ion, and Tobramycin on Biofilm. Antimicrob. Agents Chemother. 2008, 52, 1446–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, K.; Neuhaus, J.; Donnermeyer, D.; Schäfer, E.; Dammaschke, T. Solubility and pH Value of 3 Different Root Canal Sealers: A Long-term Investigation. J. Endod. 2018, 44, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- McMichen, F.R.S.; Pearson, G.; Rahbaran, S.; Gulabivala, K. A comparative study of selected physical properties of five root-canal sealers. Int. Endod. J. 2003, 36, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Radovic, I.; Monticelli, F.; Goracci, C.; Vulicevic, Z.R.; Ferrari, M. Self-adhesive resin cements: A literature review. J. Adhes. Dent. 2008, 10, 251–258. [Google Scholar] [PubMed]
- Kindblom, C.; Davies, J.; Herzberg, M.; Svensäter, G.; Wickström, C. Salivary proteins promote proteolytic activity in Streptococcus mitis biovar 2 and Streptococcus mutans. Mol. Oral Microbiol. 2012, 27, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Bodrumlu, E.; Semiz, M. Antibacterial activity of a new endodontic sealer against Enterococcus faecalis. J. Can. Dent. Assoc. 2006, 72, 637. [Google Scholar] [PubMed]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial Activity of Endodontic Sealers by Modified Direct Contact Test Against Enterococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed]

| Group | Description |
|---|---|
| SE | Resin composite (BisfilTM 2B, Bisco, Schaumburg, IL, USA) bonded to root dentin using self-etch adhesive (AdperTM Easy Bond, 3M, Saint Paul, MN, USA). |
| TE | BisfilTM 2B bonded to root dentin using total-etch adhesive (ScotchbondTM, 3M, Saint Paul, MN, USA) |
| AH | epoxy-resin-based sealer (AH Plus®, Dentsply Sirona, York, PA, USA). |
| BC | Bioceramic sealer (EndoSequence BC Sealer, Brasseler USA, Savannah, GA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marashdeh, M.Q.; Lévesque, C.; Friedman, S.; Stewart, C.A.; Finer, Y. Interfacial Biomaterial–Dentin Bacterial Biofilm Proliferation and Viability Is Affected by the Material, Aging Media and Period. Dent. J. 2022, 10, 33. https://doi.org/10.3390/dj10030033
Marashdeh MQ, Lévesque C, Friedman S, Stewart CA, Finer Y. Interfacial Biomaterial–Dentin Bacterial Biofilm Proliferation and Viability Is Affected by the Material, Aging Media and Period. Dentistry Journal. 2022; 10(3):33. https://doi.org/10.3390/dj10030033
Chicago/Turabian StyleMarashdeh, Muna Q., Celine Lévesque, Shimon Friedman, Cameron A. Stewart, and Yoav Finer. 2022. "Interfacial Biomaterial–Dentin Bacterial Biofilm Proliferation and Viability Is Affected by the Material, Aging Media and Period" Dentistry Journal 10, no. 3: 33. https://doi.org/10.3390/dj10030033
APA StyleMarashdeh, M. Q., Lévesque, C., Friedman, S., Stewart, C. A., & Finer, Y. (2022). Interfacial Biomaterial–Dentin Bacterial Biofilm Proliferation and Viability Is Affected by the Material, Aging Media and Period. Dentistry Journal, 10(3), 33. https://doi.org/10.3390/dj10030033








