Absence of Oral Opportunistic Infections in Patients with Inflammatory Bowel Disease Receiving Anti-TNF-α and Anti-Integrin-α4β7 Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaire
2.3. Clinical Examination
2.4. Cultivation and Identification of Candida spp. and Oral Bacteria
2.5. Laboratory Tests and Activity of the Disease
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Demographic and Disease Data
3.2. Predisposing Factors for Candidiasis
3.3. Oral Opportunistic Infections
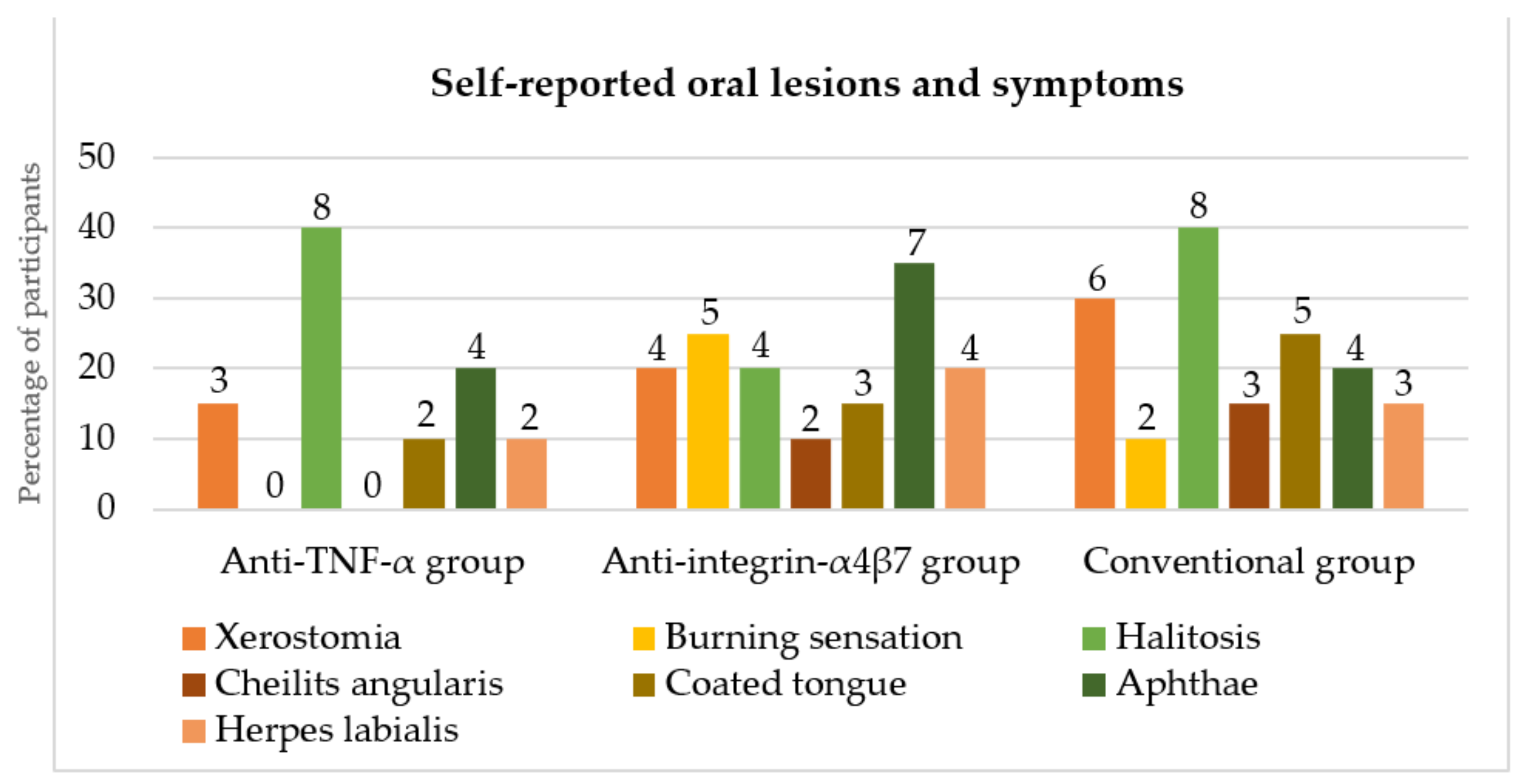
3.4. Self-Reported Oral Lesions and Symptoms
3.5. Oral Mucosal Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Group | Anti-TNF-α | Anti-TNF-α | Anti-integrin-α4β7 | Conventional |
| Sex | female | male | male | male |
| Age/years | 82 | 70 | 35 | 64 |
| Duration of biological therapy/years | 0.5 | 5 | 4 | |
| Hyperglycemia | + | − | − | − |
| Iron deficiency | − | − | + | − |
| Hyposalivation | + | + | + | + |
| Dentures | + | + | − | + |
| Smoking | − | − | + | − |
References
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns. Colitis. 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Lin, K.; Katz, S. Serious and opportunistic infections in elderly patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2019, 15, 593–605. [Google Scholar]
- Hindryckx, P.; Novak, G.; Bonovas, S.; Peyrin-Biroulet, L.; Danese, S. Infection risk with biologic therapy in patients with inflammatory bowel disease. Clin. Pharmacol. Ther. 2017, 102, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Purohit, T.; Razonable, R.; Loftus, E.V., Jr. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm. Bowel Dis. 2014, 20, 196–212. [Google Scholar] [CrossRef]
- Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association. Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication). Intest. Res. 2018, 16, 178–193. [Google Scholar] [CrossRef]
- Park, S.C.; Jeen, Y.T. Anti-integrin therapy for inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1868–1880. [Google Scholar] [CrossRef]
- Katsanos, K.H.; Papamichael, K.; Feuerstein, J.D.; Christodoulou, D.K.; Cheifetz, A.S. Biological therapies in inflammatory bowel disease: Beyond anti-TNF therapies. Clin. Immunol. 2019, 206, 9–14. [Google Scholar] [CrossRef]
- Hernandez-Rocha, C.; Vande Casteele, N. JAK inhibitors: Current position in treatment strategies for use in inflammatory bowel disease. Curr. Opin. Pharmacol. 2020, 55, 99–109. [Google Scholar] [CrossRef]
- Lamb, C.A.; O’Byrne, S.; Keir, M.E.; Butcher, E.C. Gut-selective integrin-targeted therapies for inflammatory bowel disease. J. Crohns. Colitis. 2018, 12 (Suppl. S2), S653–S668. [Google Scholar] [CrossRef]
- Borman, Z.A.; Côté-Daigneault, J.; Colombel, J.F. The risk for opportunistic infections in inflammatory bowel disease with biologics: An update. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 1101–1108. [Google Scholar] [CrossRef]
- Rahier, J.F. Prevention and management of infectious complications in IBD. Dig. Dis. 2012, 30, 408–414. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohns. Colitis. 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns. Colitis. 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Kramer, I.R.; Pindborg, J.J.; Bezroukov, V.; Infirri, J.S. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Community. Dent. Oral Epidemiol. 1980, 8, 1–24. [Google Scholar] [CrossRef]
- Jontell, M.; Holmstrup, P. Red and white lesion of oral mucosa. In Burket’s Oral Medicine, 11th ed.; Greenberg, M.S., Glick, M., Ship, J.A., Eds.; BC Decker, Inc.: Hamilton, ON, Canada, 2008; pp. 77–106. [Google Scholar]
- Pravin Charles, M.V.; Kali, A.; Joseph, N.M. Performance of chromogenic media for Candida in rapid presumptive identification of Candida species from clinical materials. Pharmacogn. Res. 2015, 7 (Suppl. S1), S69–S73. [Google Scholar] [CrossRef]
- Bryant, P.A.; Baddley, J.W. Opportunistic infections in biological therapy, risk and prevention. Rheum. Dis. Clin. 2017, 43, 27–41. [Google Scholar] [CrossRef]
- Kourbeti, I.S.; Ziakas, P.D.; Mylonakis, E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: A meta-analysis. Clin. Infect. Dis. 2014, 58, 1649–1657. [Google Scholar] [CrossRef]
- Toruner, M.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Orenstein, R.; Sandborn, W.J.; Colombel, J.F.; Egan, L.J. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008, 134, 929–936. [Google Scholar] [CrossRef]
- Nanau, R.M.; Cohen, L.E.; Neuman, M.G. Risk of infections of biological therapies with accent on inflammatory bowel disease. J. Pharm. Pharm. Sci. 2014, 17, 485–531. [Google Scholar] [CrossRef][Green Version]
- Osterman, M.T.; Sandborn, W.J.; Colombel, J.F.; Peyrin-Biroulet, L.; Robinson, A.M.; Zhou, Q.; Lewis, J.D. Crohn’s disease activity and concomitant immunosuppressants affect the risk of serious and opportunistic infections in patients treated with adalimumab. Am. J. Gastroenterol. 2016, 111, 1806–1815. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Rodríguez-Fernández, S.; Teijón, S.; Esteve, M.; Rodríguez-Carballeira, M.; Lacasa, J.M.; Salvador, G.; Garau, J. Risk factors for opportunistic infections in infliximab-treated patients: The importance of screening in prevention. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Winthrop, K.L.; Chen, L.; Liu, L.; Grijalva, C.G.; Delzell, E.; Beukelman, T.; Patkar, N.M.; Xie, F.; Saag, K.G.; et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: Results of the safety assessment of biologic therapy (SABER) study. Ann. Rheum. Dis. 2014, 73, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Adar, T.; Faleck, D.; Sasidharan, S.; Cushing, K.; Borren, N.Z.; Nalagatla, N.; Ungaro, R.; Sy, W.; Owen, S.C.; Patel, A.; et al. Comparative safety and effectiveness of tumor necrosis factor α antagonists and vedolizumab in elderly IBD patients: A multicentre study. Aliment. Pharmacol. Ther. 2019, 49, 873–879. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: A systematic review and network meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: Meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 2013, 108, 1268–1276. [Google Scholar] [CrossRef]
- Salmon-Ceron, D.; Tubach, F.; Lortholary, O.; Chosidow, O.; Bretagne, S.; Nicolas, N.; Cuillerier, E.; Fautrel, B.; Michelet, C.; Morel, J.; et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann. Rheum. Dis. 2011, 70, 616–623. [Google Scholar] [CrossRef]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Van Assche, G.; Vermeire, S.; Rutgeerts, P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: A single-centre cohort study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef]
- Shah, E.D.; Farida, J.P.; Siegel, C.A.; Chong, K.; Melmed, G.Y. Risk for overall infection with anti-TNF and anti-integrin agents used in IBD: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2017, 23, 570–577. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V., Jr.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Luthra, P.; Peyrin-Biroulet, L.; Ford, A.C. Systematic review and meta-analysis: Opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 1227–1236. [Google Scholar] [CrossRef]
- Ali, T.; Kaitha, S.; Mahmood, S.; Ftesi, A.; Stone, J.; Bronze, M.S. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc. Patient Saf. 2013, 5, 79–99. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, Z. Invasive candidiasis: A review of nonculture-based laboratory diagnostic methods. Indian J. Med. Microbiol. 2012, 30, 264–269. [Google Scholar] [CrossRef]
- Corazza, M.; Zauli, S.; Ricci, M.; Borghi, A.; Rossi, R.; Virgili, A. Does anti-tumour necrosis factor-alpha increase oral candida colonization? A case-control study in psoriatic patients. Acta Derm. Venereol. 2013, 93, 352–353. [Google Scholar] [CrossRef][Green Version]


| Anti-TNF-α | Groups | Conventional | p-Value | |
|---|---|---|---|---|
| Anti-Integrin-α4β7 | ||||
| Sex | ||||
| Female/n (%) | 9 (45) | 9 (45) | 10 (50) | 0.935 a |
| Male/n (%) | 11 (55) | 11 (55) | 10 (50) | |
| Age (years) | ||||
| Average | 47 | 46.25 | 46 | 0.980 b |
| Standard deviation | 18.01 | 15.28 | 15.68 | |
| Minimum | 21 | 20 | 19 | |
| Maximum | 82 | 68 | 76 | |
| Type of disease | ||||
| Crohn’s disease/n (%) | 13 (65) | 11 (55) | 7 (35) | 0.154 a |
| Ulcerative colitis/n (%) | 7 (35) | 9 (45) | 13 (65) | |
| Disease duration (years) | ||||
| Median | 7.5 | 11.5 | 7 | 0.005 c |
| Range | 33 | 36 | 14 | |
| Minimum | 2 | 4 | 1 | |
| Maximum | 35 | 40 | 15 | |
| C-reactive protein levels (mg/L) | ||||
| Median | 4.3 | 3.7 | 7.68 | 0.998 c |
| Range | 97.8 | 110.7 | 39.4 | |
| Minimum | 0.5 | 0.6 | 0.6 | |
| Maximum | 98.3 | 111.3 | 40 | |
| Fecal calprotectin levels (μg/g) | ||||
| Median | 319.5 | 136 | 275.3 | 0.975 c |
| Range | 1907 | 2717.09 | 872 | |
| Minimum | 20 | 21.91 | 20 | |
| Maximum | 1927 | 2739 | 892 | |
| Type of therapy/n (%) | ||||
| infliximab | vedolizumab 20 (100) | mesalazine | ||
| 17 (85) | 18 (90) | |||
| adalimumab | sulfasalazine | |||
| 3 (15) | 1 (5) | |||
| prednisone < 20 mg | ||||
| 1 (5) | ||||
| Duration of biological therapy (years) | ||||
| Average | 2.86 | 2.23 | 0.297 b | |
| Standard deviation | 2.36 | 1.31 | ||
| Minimum | 0.5 | 0.5 | ||
| Maximum | 7 | 4 | ||
| Anti-TNF-α Group | Anti-Integrin-α4β7 Group | Conventional Group | p-Value | |
|---|---|---|---|---|
| Candidiasis/n (%) | 2 (10) | 1 (5) | 1 (5) | 0.765 a |
| Types of | ||||
| Candida spp. | ||||
| C. albicans | + | + | + | |
| C. glabrata | − | − | + | |
| C. krusei | + | − | − | |
| C. dubliniensis | + | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltović, E.; Mijandrušić-Sinčić, B.; Braut, A.; Škrobonja, I.; Sever, E.; Glažar, I.; Pezelj-Ribarić, S.; Muhvić-Urek, M. Absence of Oral Opportunistic Infections in Patients with Inflammatory Bowel Disease Receiving Anti-TNF-α and Anti-Integrin-α4β7 Therapy. Dent. J. 2022, 10, 32. https://doi.org/10.3390/dj10030032
Saltović E, Mijandrušić-Sinčić B, Braut A, Škrobonja I, Sever E, Glažar I, Pezelj-Ribarić S, Muhvić-Urek M. Absence of Oral Opportunistic Infections in Patients with Inflammatory Bowel Disease Receiving Anti-TNF-α and Anti-Integrin-α4β7 Therapy. Dentistry Journal. 2022; 10(3):32. https://doi.org/10.3390/dj10030032
Chicago/Turabian StyleSaltović, Ema, Brankica Mijandrušić-Sinčić, Alen Braut, Ivana Škrobonja, Ella Sever, Irena Glažar, Sonja Pezelj-Ribarić, and Miranda Muhvić-Urek. 2022. "Absence of Oral Opportunistic Infections in Patients with Inflammatory Bowel Disease Receiving Anti-TNF-α and Anti-Integrin-α4β7 Therapy" Dentistry Journal 10, no. 3: 32. https://doi.org/10.3390/dj10030032
APA StyleSaltović, E., Mijandrušić-Sinčić, B., Braut, A., Škrobonja, I., Sever, E., Glažar, I., Pezelj-Ribarić, S., & Muhvić-Urek, M. (2022). Absence of Oral Opportunistic Infections in Patients with Inflammatory Bowel Disease Receiving Anti-TNF-α and Anti-Integrin-α4β7 Therapy. Dentistry Journal, 10(3), 32. https://doi.org/10.3390/dj10030032






