2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity
Abstract
:1. Introduction
2. Results
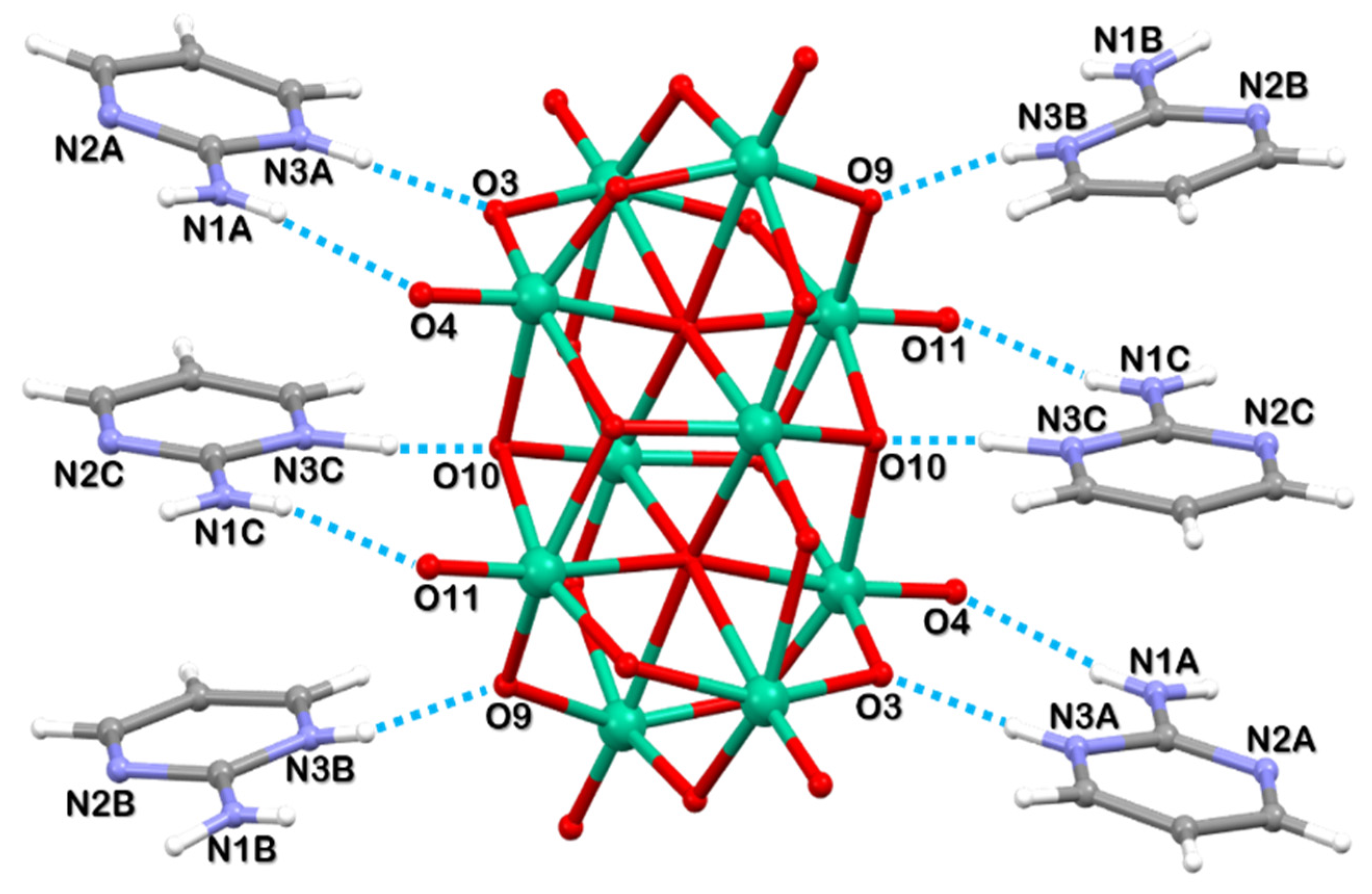
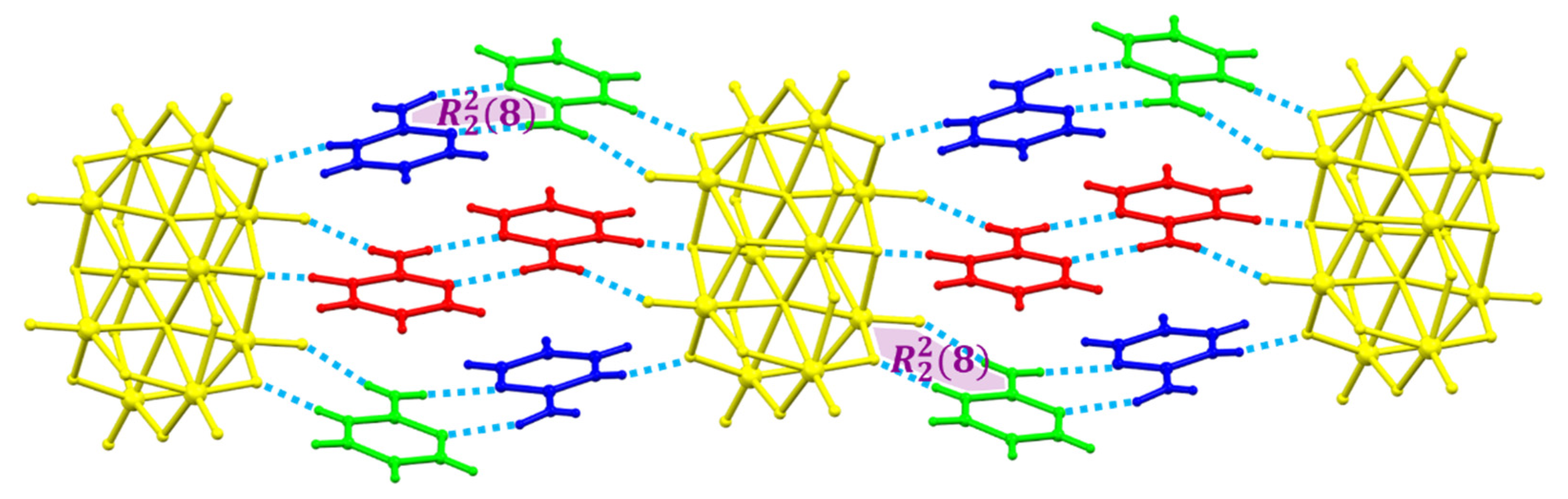
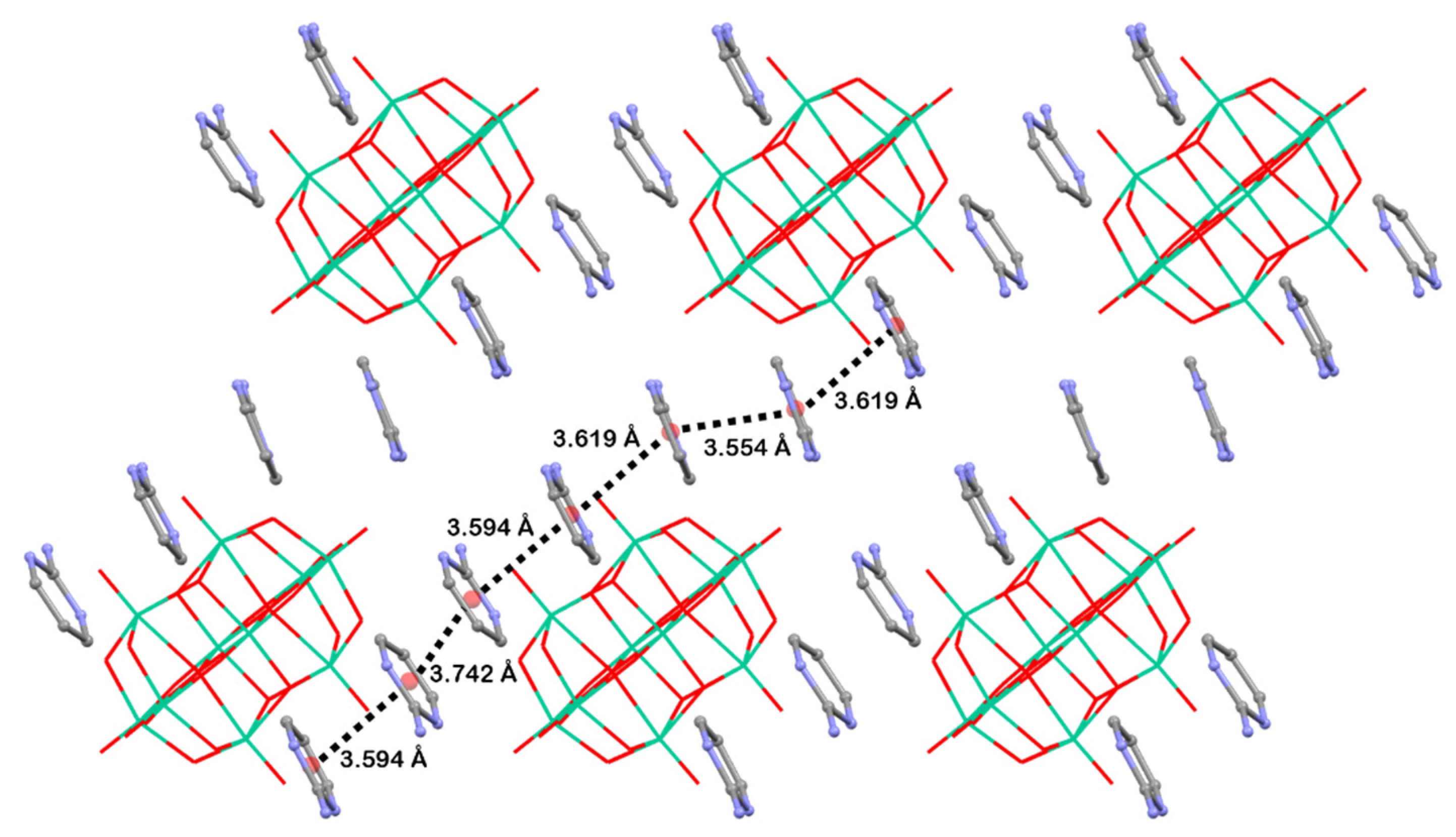
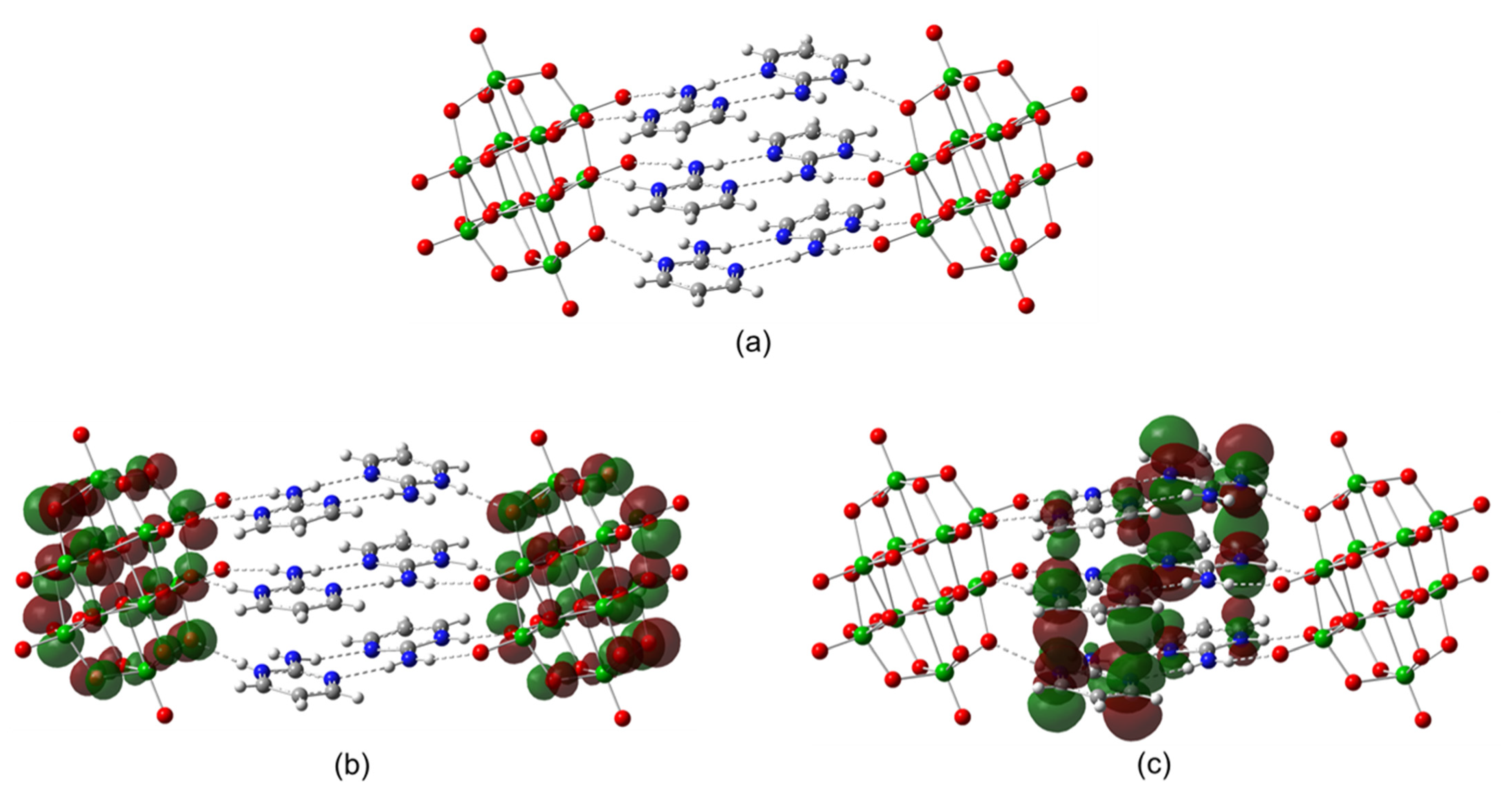
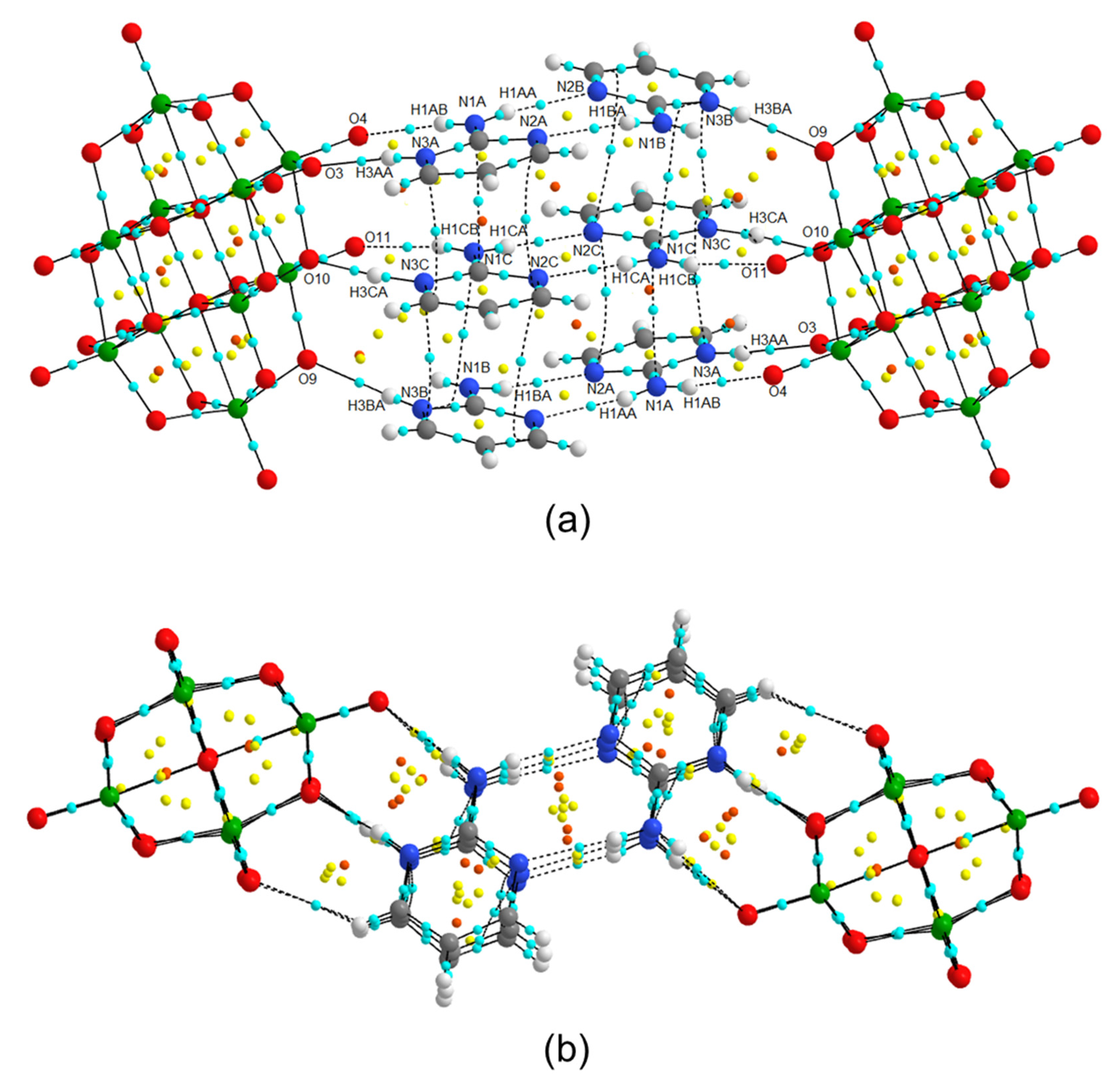
2.1. Structural Description of [2-ampymH]6[V10O28]·5H2O (1)
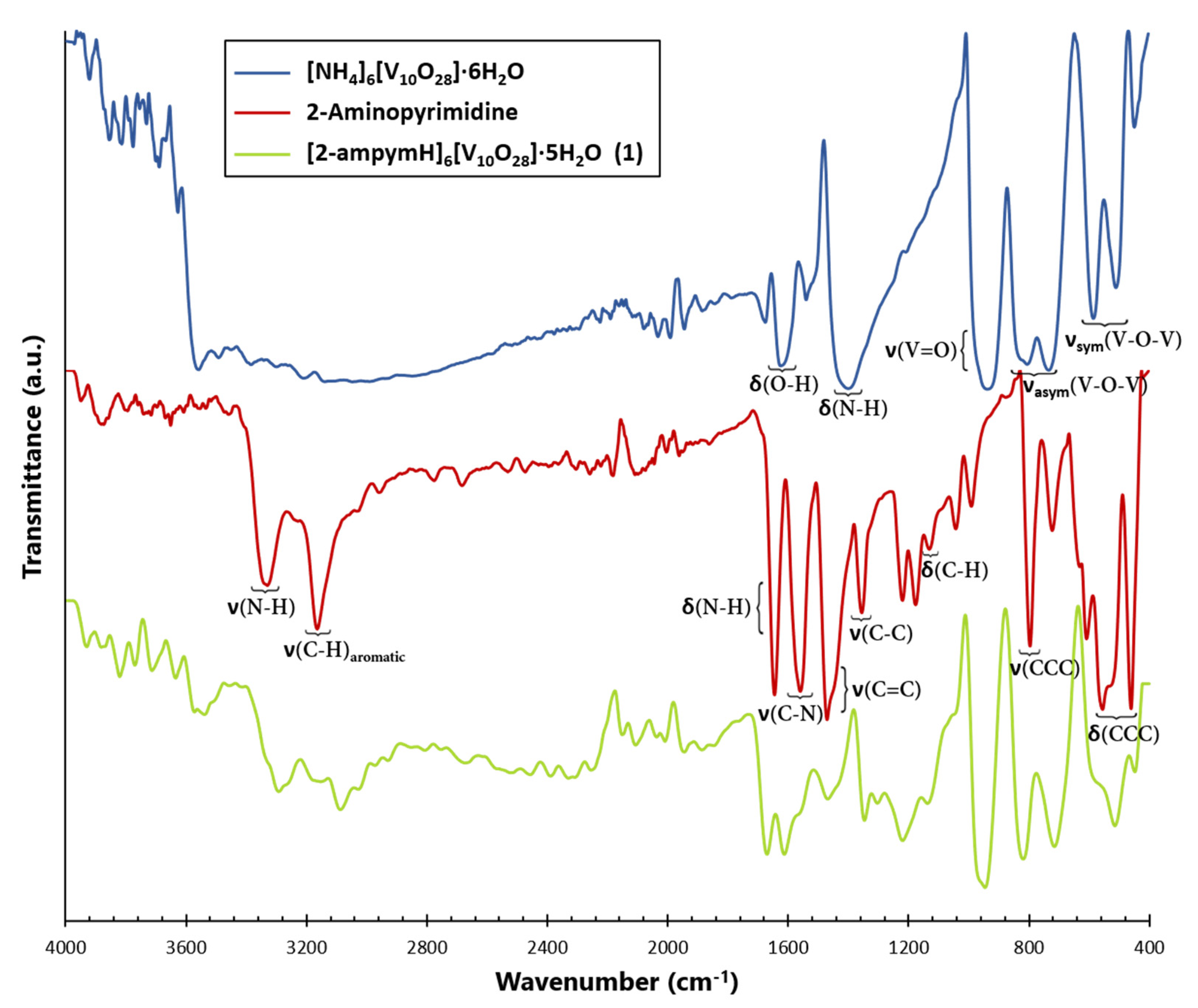
2.2. Infrared (IR) Spectroscopy
2.3. Thermal Study
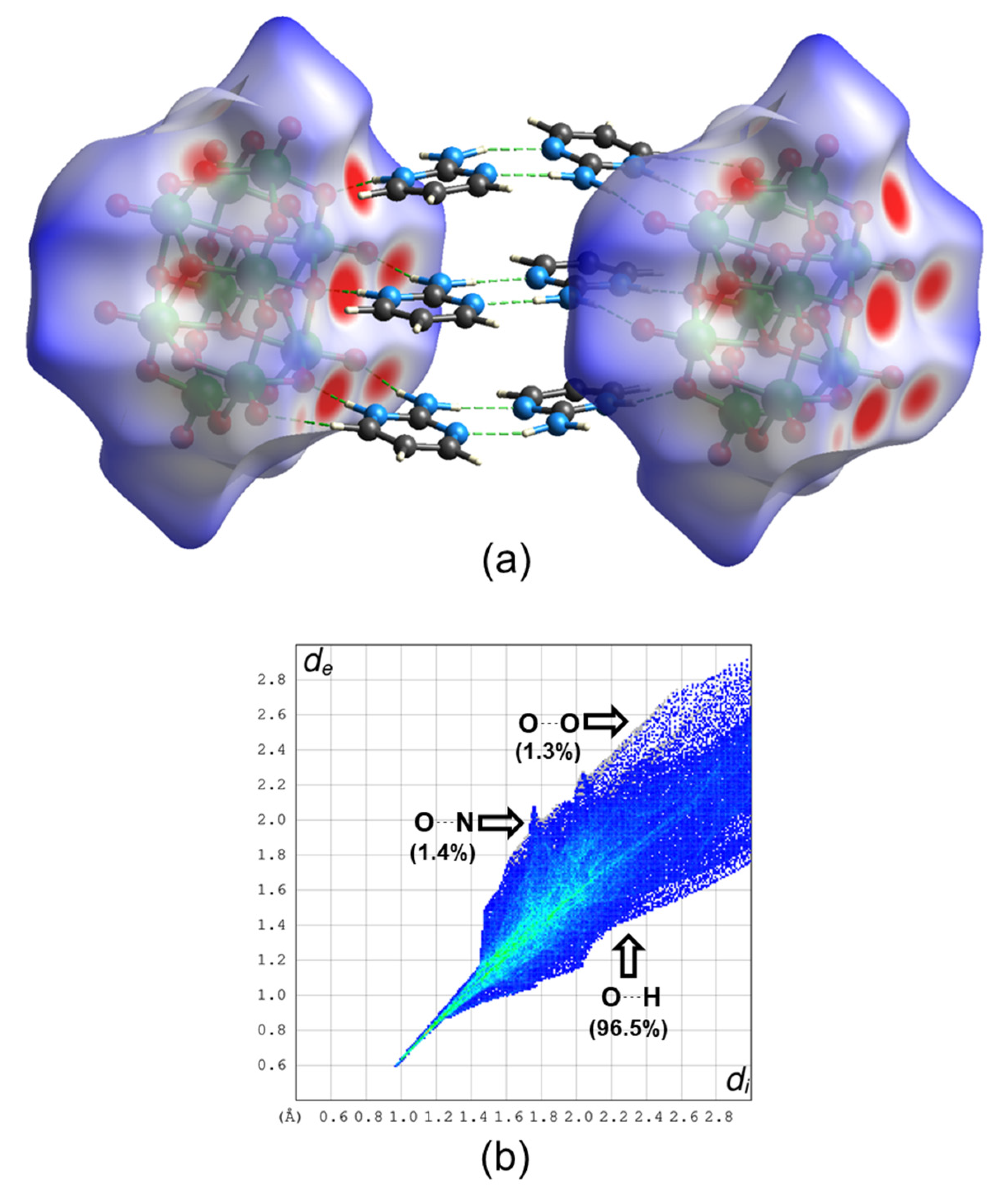
2.4. Theoretical Calculations
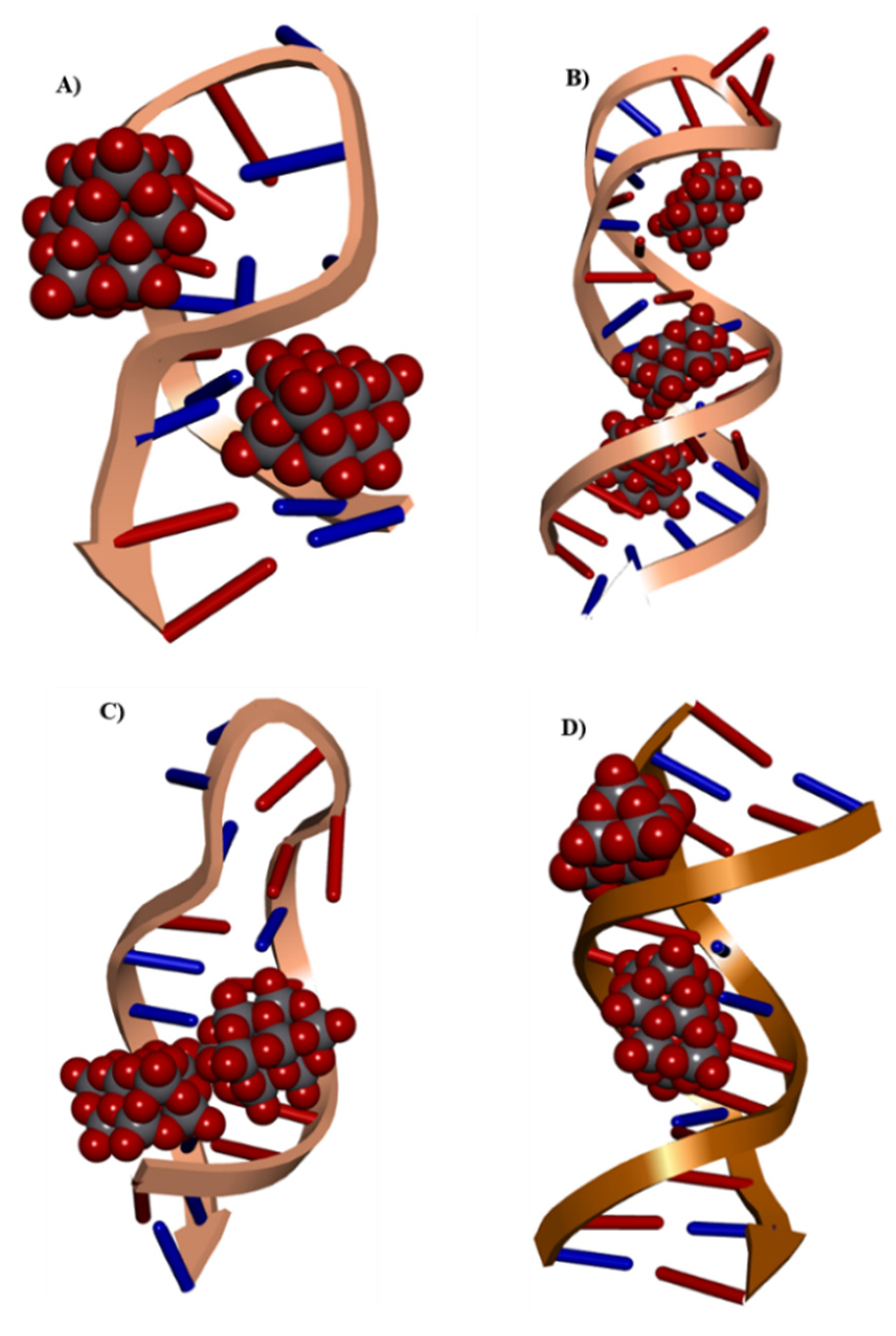
2.5. Docking Analysis
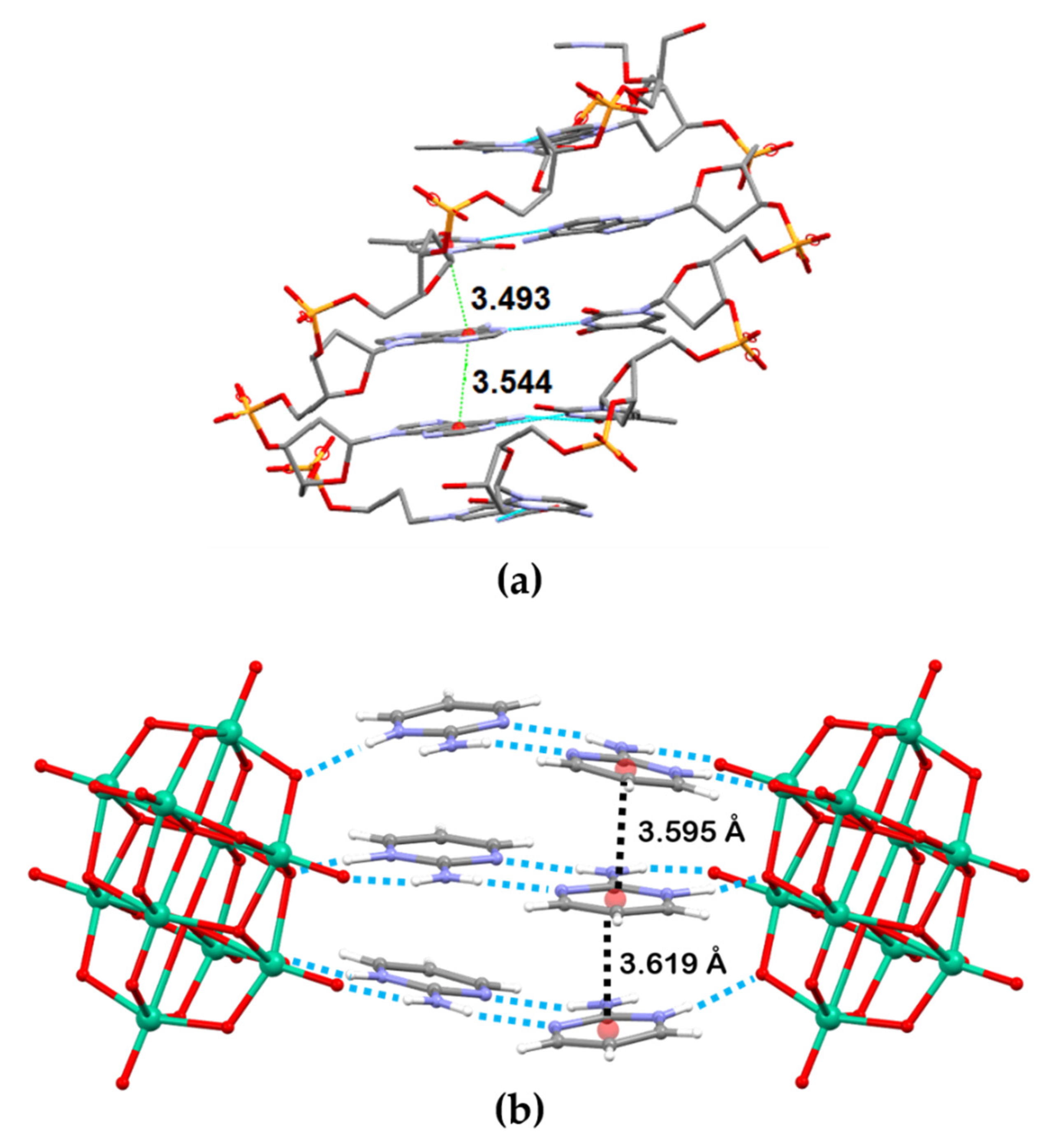
3. Discussion
4. Materials and Methods
4.1. Synthesis of [2-ampymH]6[V10O28]·5H2O (1)
4.2. Characterization Methods
4.2.1. Physicochemical Characterization
4.2.2. Single-Crystal X-ray Diffraction
4.2.3. Theoretical Methodology
4.2.4. Docking Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proust, A.; Thouvenot, R.; Gouzerh, P. Functionalization of polyoxidometalates: Towards advanced applications in catalysis and materials science. Chem. Commun. 2008, 16, 1837–1852. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxidometalates as Potential Next-Generation Metallodrugs in the Combat against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxidometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.T. The Molecular Structure of the Isopoly Complex Ion, Decavanadate (V10O286−). Inorg. Chem. 1966, 5, 967–977. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Sedghiniya, S.; Soleimannejad, J.; Jahani, Z.; Davoodi, J.; Janczak, J. Crystal engineering of an adenine-decavanadate molecular device towards label-free chemical sensing and biological screening. Acta Cryst. 2020, B76, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hartung, S.; Bucher, N.; Chen, H.-Y.; Al-Oweini, R.; Sreejith, S.; Borah, P.; Yanli, Z.; Kortz, U.; Stimming, U.; Hoster, H.E.; et al. Vanadium-based polyoxidometalate as new material for sodium-ion battery anodes. J. Power Sources 2015, 288, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.; Ma, C.-A.; Wang, L.; Chu, Y. Li6V10O28, a novel cathode material for Li-ion battery. Electrochim. Acta 2007, 52, 2945–2949. [Google Scholar] [CrossRef]
- Ji, Y.; Liu-Théato, X.; Xiu, Y.; Indris, S.; Njel, C.; Maibach, J.; Ehrenberg, H.; Fichtner, M.; Zhao-Karger, Z. Polyoxidometalate Modified Separator for Performance Enhancement of Magnesium-Sulfur Batteries. Adv. Funct. Mater. 2021, 2100868, 1–7. [Google Scholar]
- Steens, N.; Ramadan, A.M.; Absillis, G.; Parac-Vogt, T.N. Hydrolytic cleavage of DNA-model substrates promoted by polyoxidovanadates. Dalton Trans. 2010, 39, 585–592. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Yao, X.; Chao, Y.; Xun, S.; Xiong, J.; Fan, L.; Zhu, W.; Li, H. Decavanadates anchored into micropores of graphene-like boron nitride: Efficient heterogeneous catalysts for aerobic oxidative desulfurization. Fuel 2018, 230, 104–112. [Google Scholar] [CrossRef]
- Huang, X.; Gu, X.; Zhang, H.; Shen, G.; Gong, S.; Yang, B.; Wang, Y.; Chen, Y. Decavanadate-based clusters as bifunctional catalysts for efficient treatment of carbon dioxide and simulant sulfur mustard. J. CO2 Util. 2021, 45, 101419. [Google Scholar] [CrossRef]
- Diaz, A.; Muñoz-Arenas, G.; Venegas, B.; Vázquez-Roque, R.; Flores, G.; Guevara, J.; Gonzalez-Vergara, E.; Treviño, S. Metforminium decavanadate (MetfDeca) Treatment Ameliorates Hippocampal Neurodegeneration and Recognition Memory in a Metabolic Syndrome Model. Neurochem. Res. 2021, 46, 1151–1165. [Google Scholar] [CrossRef]
- Pereira, M.J.; Carvalho, E.; Eriksson, J.W.; Crans, D.C.; Aureliano, M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J. Inorg. Biochem. 2009, 103, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Wang, X.; Li, D.; Zhang, H.; Li, R.; Song, L. Synthesis and biological evaluation of decavanadate Na4Co(H2O)6V10O28·18H2O. Biomed. Pharmacother. 2009, 63, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Cantley, L.C.; Josephson, L.; Warner, R.; Yanagisawa, M.; Lechene, C.; Guidotti, G. Vanadate Is a Potent (Na, K)-ATPase Inhibitor Found in ATP Derived from Muscle. J. Biol. Chem. 1977, 252, 7421–7423. [Google Scholar] [CrossRef]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef]
- Aureliano, M. Decavanadate: A journey in a search of a role. Dalton Trans. 2009, 42, 9093–9100. [Google Scholar] [CrossRef] [Green Version]
- García-Vicente, S.; Yraola, F.; Marti, L.; González-Muñoz, E.; García-Barrado, M.J.; Cantó, C.; Abella, A.; Bour, S.; Artuch, R.; Sierra, C.; et al. Oral Insulin-Mimetic Compounds That Act Independently of Insulin. Diabetes 2007, 56, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Treviño, S.; Sánchez-Lara, E.; Sarmiento-Ortega, V.E.; Sánchez-Lombardo, I.; Flores-Hernández, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3[V10O28]·8H2O using hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J. Inorg. Biochem. 2015, 147, 85–92. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sarmiento-Ortega, V.E.; Flores-Hernández, J.Á.; Brambila, E.; Meléndez, F.J.; González-Vergara, E. Pharmacological and Toxicological Threshold of Bisammonium Tetrakis 4-(N,N-Dimethylamino)pyridinium Decavanadate in a Rat Model of Metabolic Syndrome and Insulin Resistance. Bioinorg. Chem. Appl. 2018, 2151079, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Turner, T.L.; Nguyen, V.H.; McLauchlan, C.C.; Dymon, Z.; Dorsey, B.M.; Hooker, J.D.; Jones, M.A. Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae In Vitro. J. Inorg. Biochem. 2012, 108, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Missina, J.M.; Gavinho, B.; Postal, K.; Santana, F.S.; Valdameri, G.; de Souza, E.M.; Hughes, D.L.; Ramirez, M.I.; Soares, J.F.; Nunes, G.G. Effects of Decavanadate Salts with Organic and Inorganic Cations on Escherichia coli, Giardia intestinalis, and Vero Cells. Inorg. Chem. 2018, 57, 11930–11941. [Google Scholar] [CrossRef]
- Samart, N.; Arhouma, Z.; Kumar, S.; Murakami, H.A.; Crick, D.C.; Crans, D.C. Decavanadate Inhibits Mycobacterial Growth More Potently Than Other Oxovanadates. Front. Chem. 2018, 6, 519. [Google Scholar] [CrossRef] [Green Version]
- Marques-da-Silva, D.; Fraqueza, G.; Lagoa, R.; Vannathan, A.A.; Mal, S.S.; Aureliano, M. Polyoxidovanadate inhibition of Escherichia coli growth shows a reverse correlation with Ca2+-ATPase inhibition. New. J. Chem. 2019, 43, 17577–17587. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhu, C.-Y.; Wu, Z.-Y.; Jiang, M.; Yan, C.-W. Synthesis, crystal structures and anticancer activities of two decavanadate compounds. Transit. Met. Chem. 2010, 35, 597–603. [Google Scholar] [CrossRef]
- Silva-Nolasco, A.M.; Camacho, L.; Saavedra-Díaz, R.O.; Hernández-Abreu, O.; León, I.E.; Sánchez-Lombardo, I. Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and In Vitro Cytotoxicity and Insulin-Like Activity. Inorganics 2020, 8, 67. [Google Scholar] [CrossRef]
- Louati, M.; Ksiksi, R.; Elbini-Dhouib, I.; Mlayah-Bellalouna, S.; Doghri, R.; Srairi-Abid, N.; Zid, M.-F. Synthesis, structure, and characterization of a novel decavanadate, Mg(H2O)6(C4N2H7)4V10O28·4H2O, with a potential antitumor activity. J. Mol. Struct. 2021, 1242, 130711. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sanchez-Gaytan, B.L.; Castro, M.E.; Bernès, S.; Méndez-Rojas, M.A.; Meléndez-Bustamante, F.J.; González-Vergara, E. A one-dimensional supramolecular chain based on [H2V10O28]4− units decorated with 4-dimethylaminopyridinium ions: An experimental and theoretical characterization. New J. Chem. 2019, 43, 17746–17755. [Google Scholar] [CrossRef]
- Bosnjakovic-Pavlovic, N.; Spasojevic-De-Biré, A. Cytosine-Cytosinium Dimer Behavior in a Cocrystal with a Decavanadate Anion as a Function of the Temperature. J. Phys. Chem. A. 2010, 114, 10664–10675. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Treviño, S.; Sánchez-Gaytán, B.L.; Sánchez-Mora, E.; Castro, M.E.; Meléndez-Bustamante, F.J.; Méndez-Rojas, M.A.; González-Vergara, E. Decavanadate Salts of Cytosine and Metformin: A Combined Experimental-Theoretical Study of Potential Metallodrugs Against Diabetes and Cancer. Front. Chem. 2018, 6, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Liu, S.; Zhao, W. The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10. Z. Kristallogr. NCS 2021, 236, 25–27. [Google Scholar] [CrossRef]
- Zarroug, R.; Abdallah, A.H.; Guionneau, P.; Masip-Sánchez, A.; López, X.; Ayed, B. Decavanadate salts of piperidine and triethanolamine: A combined experimental and theoretical study. J. Mol. Struct. 2021, 1241, 130677. [Google Scholar] [CrossRef]
- Sgambellone, M.A.; David, A.; Garner, R.N.; Dunbar, K.R.; Turro, C. Cellular Toxicity Induced by the Photorelease of a Caged Bioactive Molecule: Design of a Potential Dual-Action Ru(II) Complex. J. Am. Chem. Soc. 2013, 135, 11274–11282. [Google Scholar] [CrossRef]
- Amr, A.-G.E.; Mohamed, A.M.; Mohamed, S.F.; Abdel-Hafez, N.A.; Hammam, A.E.-F.G. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg. Med. Chem. 2006, 14, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 269–310, 398–408. [Google Scholar]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the Decavanadate(V) and Oxidovanadium(IV) Binding to G-Actin and the Competition with Decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.P.; Jiang, H.J.; Wang, Y.; Zhang, D.P.; Mei, J.; Cui, S.H. Synthesis and Crystal Structure of Decavanadate-Based Coordination Polymers. J. Cluster Sci. 2018, 29, 785–792. [Google Scholar] [CrossRef]
- Scheinbeim, J.; Schempp, E. 2-Aminopyrimidine. Acta Cryst. 1976, B32, 607–609. [Google Scholar] [CrossRef]
- Correia, I.; Avecilla, F.; Marcao, S.; Pessoa, J.C. Structural studies of decavanadate compounds with organic molecules and inorganic ions in their crystal packing. Inorg. Chim. Acta 2004, 357, 4476–4487. [Google Scholar] [CrossRef]
- Aissa, T.; Ksiksi, R.; Elbini-Dhouib, I.; Doghri, R.; Srairi-Abid, N.; Zid, M.F. Synthesis of a new vanadium complex (V), hexa[4-methylimidazolium]decavanadate trihydrate (C4H7N2)6V10O28·3H2O: Physico-chemical and biological characterizations. J. Mol. Struct. 2021, 1236, 130331. [Google Scholar] [CrossRef]
- Mahmoud, G.A.-E.; Ibrahim, A.B.M.; Mayer, P. (NH4)2[Ni(H2O)6]2V10O28·4H2O; Structural Analysis and Bactericidal Activity against Pathogenic Gram-Negative Bacteria. ChemistrySelect 2021, 6, 3782–3787. [Google Scholar] [CrossRef]
- Hou, W.; Guo, J.; Wang, Z.; Xu, Y. Synthesis, structural characterization, and properties of two new polyoxidovanadates based on decavanadate [V10O28]6−. J. Coord. Chem. 2013, 66, 2434–2443. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Cryst. 1990, B46, 256–262. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Li, J.; Mohammadnia, M. Pd based on 2-Aminopyrimidine and 1H-benzo[d]imidazol-2-amine functionalized Fe3O4 nanoparticles as novel recyclable magnetic nanocatalysts for Ullmann coupling reaction. Appl. Organomet. Chem. 2020, 34, e5708. [Google Scholar] [CrossRef]
- Gupta, P.K.; Arora, K. Studies on Simulation of Spectra of some Organic Compounds. Orient. J. Chem. 2019, 35, 1655–1668. [Google Scholar] [CrossRef]
- Thangarasu, S.; Athimoolam, S.; Bahadur, S.A.; Manikandan, A. Structural, Spectroscopic Investigation and Computational Study on Nitrate and Hydrogen Oxalate Salts of 2-Aminopyrimidine. J. Nanosci. Nanotechnol. 2018, 18, 2450–2462. [Google Scholar] [CrossRef]
- Ortaboy, S.; Acar, E.T.; Atun, G. The removal of radioactive strontium ions from aqueous solutions by isotopic exchange using strontium decavanadates and corresponding mixed oxides. Chem. Eng. J. 2018, 344, 194–205. [Google Scholar] [CrossRef]
- Omri, I.; Mhiri, T.; Graia, M. Novel decavanadate cluster complex (HImz)12(V10O28)2·3H2O: Synthesis, characterization, crystal structure, optical and thermal properties. J. Mol. Struct. 2015, 1098, 324–331. [Google Scholar] [CrossRef]
- Luo, S.-Y.; Wu, X.-L.; Hu, Q.-P.; Wang, J.-X.; Liu, C.-Z. Structural characterization of a new decavanadate compound with organic molecules and inorganic ions. J. Struct. Chem. 2012, 53, 915–920. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Gabriel, C.; Petanidis, S.; Psycharis, V.; Raptopoulou, C.P.; Terzis, A.; Salifoglou, A. Binary Decavanadate-Betaine Composite Materials of Potential Anticarcinogenic Activity. Z. Anorg. Allg. Chem. 2013, 639, 1407–1416. [Google Scholar] [CrossRef]
- Riou, D.; Roubeau, O.; Férey, G. Evidence for the Solid State Structural Transformation of the Network-Type Decavanadate (NC7H14)4[H2V10O28] into a Lamellar Topology (NC7H14)[V4O10]. Z. Anorg. Allg. Chem. 1998, 624, 1021–1025. [Google Scholar] [CrossRef]
- Filho, E.V.; Pinheiro, E.M.C.; Pinheiro, S.; Greco, S.J. Aminopyrimidines: Recent synthetic procedures and anticancer activities. Tetrahedron 2021, 92, 132256. [Google Scholar] [CrossRef]
- Aureliano, M. The Role of Decavanadate in Anti-Tumour Activity. Glob. J. Cancer Ther. 2017, 3, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Chitranshi, N.; Agarwal, A.K. Significance and Biological Importance of Pyrimidine in the Microbial World. Int. J. Med. Chem. 2014, 202784, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef]
- Cheng, M.; Li, N.; Wang, N.; Hu, K.; Xiao, Z.; Wu, P.; Wei, Y. Synthesis, structure and antitumor studies of a novel decavanadate complex with a wavelike two-dimensional network. Polyhedron 2018, 155, 313–319. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Q.; Huang, Y.; Zhu, Y.; Wei, Y.; Ruhlmann, L. Polyoxidovanadate-iodobodipy supramolecular assemblies: New agents for high efficiency cancer photochemotherapy. Chem. Commun. 2020, 56, 2869–2872. [Google Scholar] [CrossRef]
- Ksiksi, R.; Abdelkafi-Koubaa, Z.; Mlayah-Bellalouna, S.; Aissaoui, D.; Marrakchi, N.; Srairi-Abid, N.; Zid, M.F.; Graia, M. Synthesis, structural characterization and antitumoral activity of (NH4)4Li2V10O28·10H2O compound. J. Mol. Struct. 2021, 1229, 129492. [Google Scholar] [CrossRef]
- Francés-Monerris, A.; Segarra-Martí, J.; Merchán, M.; Roca-Sanjuán, D. Theoretical study on the excited-state π-stacking versus intermolecular hydrogen-transfer processes in the guanine-cytosine/cytosine trimer. Theor. Chem. Acc. 2016, 135, 31. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, S.; Peng, J.; Hu, N.; Jia, H. Synthesis, crystal structure, and thermal property of a novel supramolecular assembly: (NH4)2(C8H10N4O2)4[H4V10O28]·2H2O, constructed from decavanadate and caffeine. J. Chem. Crystallogr. 2004, 34, 541–548. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar] [CrossRef]
- Noda, M.F. MicroRNAs in cancer—From research to the clinical practice. Rev. Cubana Med. 2012, 51, 325–335. [Google Scholar]
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer (Review). Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Guo, Y.; Liu, Y.; Sun, L.; Chen, B.; Wang, C.; Chen, T.; Wang, Y.; Li, Y.; Dong, Q.; et al. Individualized lncRNA differential expression profile reveals heterogeneity of breast cancer. Oncogene 2021, 40, 4604–4614. [Google Scholar] [CrossRef]
- Gilles, M.-E.; Slack, F.J. Let-7 microRNA as a potential therapeutic target with implications for immunotherapy. Expert Opin. Ther. Targets 2018, 22, 929–939. [Google Scholar] [CrossRef]
- Chirshev, E.; Oberg, K.C.; Ioffe, Y.J.; Unternaehrer, J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Transl. Med. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, J.D. The Physical Basis of Life. Proc. Phys. Soc. A. 1949, 62, 537–558. [Google Scholar] [CrossRef]
- Brack, A. Clay Minerals and the Origin of Life. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 507–521. [Google Scholar]
- Wu, J.; Peng, D.; He, Y.; Du, X.; Zhang, Z.; Zhang, B.; Li, X.; Huang, Y. In Situ Formation of Decavanadate-Intercalated Layered Double Hydroxide Films on AA2024 and their Anti-Corrosive Properties when Combined with Hybrid Sol Gel Films. Materials 2017, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Amante, C.; Sousa-Coelho, D.; Luísa, A.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Scior, T.; Abdallah, H.H.; Mustafa, S.F.Z.; Guevara-García, J.A.; Rehder, D. Are vanadium complexes druggable against the main protease Mpro of SARS-CoV-2?—A computational approach. Inorg. Chim. Acta 2021, 519, 120287. [Google Scholar] [CrossRef]
- Bruker. Bruker AXS Inc. V2019.1; Bruker: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; Institute for Inorganic Chemistry, University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement, and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules. International Series of Monograph on Chemistry-16; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, B.; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. Gauss View, Version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Keith, T.A. TK Gristmill Software, Version 19.02.13; AIMAll: Overland Park, KS, USA, 2019. [Google Scholar]
- Turner, M.J.; MacKiinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 24 July 2021).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Ortega, E.; Vigueras, G.; Ballester, F.J.; Ruiz, J. Targeting translation: A promising strategy for anticancer metallodrugs. Coord. Chem. Rev. 2021, 446, 214129. [Google Scholar] [CrossRef]


| Compound | 1 |
|---|---|
| Empirical formula | C24H46N28O33V10 |
| Formula mass (g·mol−1) | 1764.20 |
| CCDC | 2099300 |
| Crystal system | Triclinic |
| Space group | |
| a (Å) | 9.783(5) |
| b (Å) | 11.309(5) |
| c (Å) | 12.853(5) |
| α (°) | 110.166(5) |
| β (°) | 95.645(5) |
| λ (°) | 97.551(5) |
| Volume (Å3) | 1307.4(10) |
| Z | 2 |
| Density (calcd) (g·cm−3) | 2.063 |
| μ(Mo/CuKα) (mm−1) | 1.815 |
| Temperature (K) | 300(2) |
| GoF on F2 a | 1.065 |
| R1 [1 > 2σ(I)] b | 0.0386 |
| R1 [all data] b | 0.0621 |
| wR2 [1 > 2σ(I)] c | 0.0821 |
| wR2 [all data] c | 0.0958 |
| Angle | [2-AmpymH]+ (°) | 2-Ampym (°) | ∆° |
|---|---|---|---|
| N2A-C1A-N3A | 121.3 | 125.2 | 3.9 |
| N2B-C1B-N3B | 120.9 | 4.3 | |
| N2C-C1C-N3C | 123.2 | 2 | |
| C1A-N2A-C2A | 117.3 | 115.7 | 1.6 |
| C1B-N2B-C2B | 117.3 | 1.6 | |
| C1C-N2C-C2C | 116.6 | 0.9 | |
| C1A-N3A-C4A | 120.7 | 116.2 | 4.5 |
| C1B-N3B-C4B | 120.9 | 4.7 | |
| C1C-N3C-C4C | 118.4 | 2.2 |
| D-H···A b | D-H | H···A | D-H···A | Angle (°) |
|---|---|---|---|---|
| C2A-H2A···O12 | 0.93 | 2.57 | 3.176(4) | 122.9 |
| C3A-H3A···O5(i) | 0.93 | 2.28 | 3.133(4) | 152.6 |
| N1A-H1AA···N2B(ii) | 0.83 | 2.17 | 2.997(4) | 171.8 |
| N1A-H1AB···O4(iii) | 0.79 | 2.15 | 2.933(4) | 171.2 |
| N3A-H3AA···O3(iii) | 0.85 | 1.80 | 2.650(3) | 174.1 |
| N1B-H1BA···N2A(ii) | 0.93 | 2.14 | 3.043(4) | 165.6 |
| N1B-H1BB···O1W | 0.70 | 2.34 | 3.024(6) | 168.1 |
| N3B-H3BA···O9 | 0.78 | 1.87 | 2.625(3) | 163.2 |
| N1C-H1CA···N2C(iv) | 0.81 | 2.14 | 2.954(4) | 179.6 |
| N1C-H1CB···O11(v) | 0.81 | 2.10 | 2.896(3) | 168.8 |
| N3C-H3CA···O10 | 1.09 | 1.49 | 2.585(3) | 174.9 |
| O1W-H1WA···O3W(ii) | 0.85 | 2.36 | 3.162(13) | 157.2 |
| O1W-H1WB···O1 | 0.85 | 2.29 | 3.083(4) | 154.4 |
| O2W-H2WA···O11(vi) | 0.85 | 2.79 | 3.352(7) | 125.5 |
| O2W-H2WB···O11(ii) | 0.85 | 2.09 | 2.729(8) | 131.2 |
| O3W-H3WA···O6 | 0.85 | 2.30 | 3.049(9) | 147.7 |
| O3W-H3WA···O1W(ii) | 0.85 | 2.64 | 3.162(13) | 121.3 |
| O3W-H3WB···O4(i) | 0.85 | 2.30 | 3.143(11) | 174.7 |
| Vibrational Mode | (NH4)6V10O28·6H2O | 2-Ampym | Compound 1 |
|---|---|---|---|
| ν(N-H) | - | 3331 | 3290 |
| ν(C-H)aromatic | - | 3165 | 3082 |
| δ(O-H) | 1622 | - | - |
| δ(N-H) | 1394 | 1645 | 1670 |
| ν(C-N) | - | 1556 | 1610 |
| ν(C=C) | - | 1469 | 1462 |
| ν(C-C) | - | 1356 | 1346 |
| δ(C-H) | - | 1128 | 1132 |
| ν(V=O) | 927 | - | 941 |
| νasym(V-O-V) | 825 | - | 814 |
| 802 | 711 | ||
| 731 | |||
| ν(CCC) | - | 794 | |
| νsym(V-O-V) | 580 | - | 580 |
| 505 | 511 | ||
| δ(C-C-C) | - | 555 | |
| 459 |
| BCP | ρ(r) | ∇2ρ(r) | EH···Y | Dint |
|---|---|---|---|---|
| O3···H3AA | 0.0377 | 0.1440 | 11.26 | 1.8016 |
| O4···H1AB | 0.0166 | 0.0636 | 4.51 | 2.1456 |
| O9···H3BA | 0.0322 | 0.1345 | 9.88 | 1.8737 |
| O10···H3CA | 0.0788 | 0.1830 | 23.02 | 1.4946 |
| O11···H1CB | 0.0185 | 0.0705 | 5.11 | 2.0981 |
| N2A···H1BA | 0.0205 | 0.0684 | 4.80 | 2.1356 |
| N2B···H1AA | 0.0188 | 0.0691 | 4.54 | 2.1746 |
| N2C···H1CA | 0.0203 | 0.0758 | 5.05 | 2.9542 |
| Compound | Target | Binding Energies (Kcal/mol) | H Bonds | Interactions |
|---|---|---|---|---|
| Decavanadate | 2MNC (pre-miR-21) | −8.91 | 2 | C8, G10, C9, C21, A20 |
| −8.67 | 3 | G13, A14, C21, G13, A14, C17 | ||
| 2JXV (let-7 miRNA) | −8.66 | 1 | G8, U24, U25, U9, C26, A7I6 | |
| −8.58 | 5 | G11, G12, G19, A20, G15 | ||
| −8.51 | 3 | A3, G4, G5, U27, C29, C30 | ||
| 6PK9 (lncRNA) | −8.48 | 1 | A3, G1, G2, C17, U16 | |
| −8.39 | 3 | G4, G6, C15, G5 | ||
| 1BNA (DNA) | −10.79 | 4 | G4, A5, G22, A6, C23 | |
| −9.17 | 5 | A6, A17, A18, A5, G16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, A.; Noriega, L.; Meléndez-Bustamante, F.J.; Castro, M.E.; Sánchez-Gaytán, B.L.; Choquesillo-Lazarte, D.; González-Vergara, E.; Rodríguez-Diéguez, A. 2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity. Inorganics 2021, 9, 67. https://doi.org/10.3390/inorganics9090067
García-García A, Noriega L, Meléndez-Bustamante FJ, Castro ME, Sánchez-Gaytán BL, Choquesillo-Lazarte D, González-Vergara E, Rodríguez-Diéguez A. 2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity. Inorganics. 2021; 9(9):67. https://doi.org/10.3390/inorganics9090067
Chicago/Turabian StyleGarcía-García, Amalia, Lisset Noriega, Francisco J. Meléndez-Bustamante, María Eugenia Castro, Brenda L. Sánchez-Gaytán, Duane Choquesillo-Lazarte, Enrique González-Vergara, and Antonio Rodríguez-Diéguez. 2021. "2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity" Inorganics 9, no. 9: 67. https://doi.org/10.3390/inorganics9090067
APA StyleGarcía-García, A., Noriega, L., Meléndez-Bustamante, F. J., Castro, M. E., Sánchez-Gaytán, B. L., Choquesillo-Lazarte, D., González-Vergara, E., & Rodríguez-Diéguez, A. (2021). 2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity. Inorganics, 9(9), 67. https://doi.org/10.3390/inorganics9090067









