Solvothermal Synthesis Routes to Substituted Cerium Dioxide Materials
Abstract
1. Introduction
2. Solvothermal Synthesis of Oxides
3. Survey of Substituted Cerium Oxides
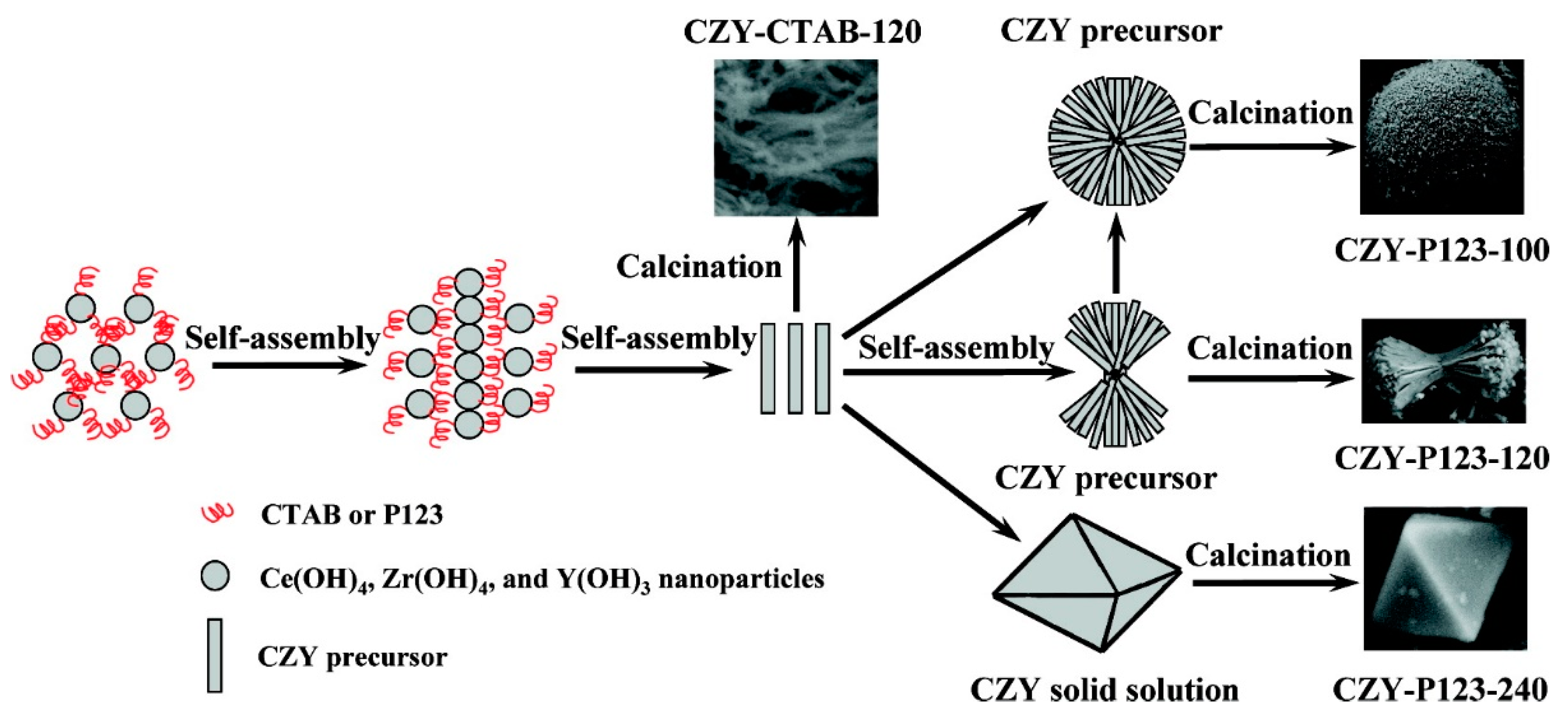
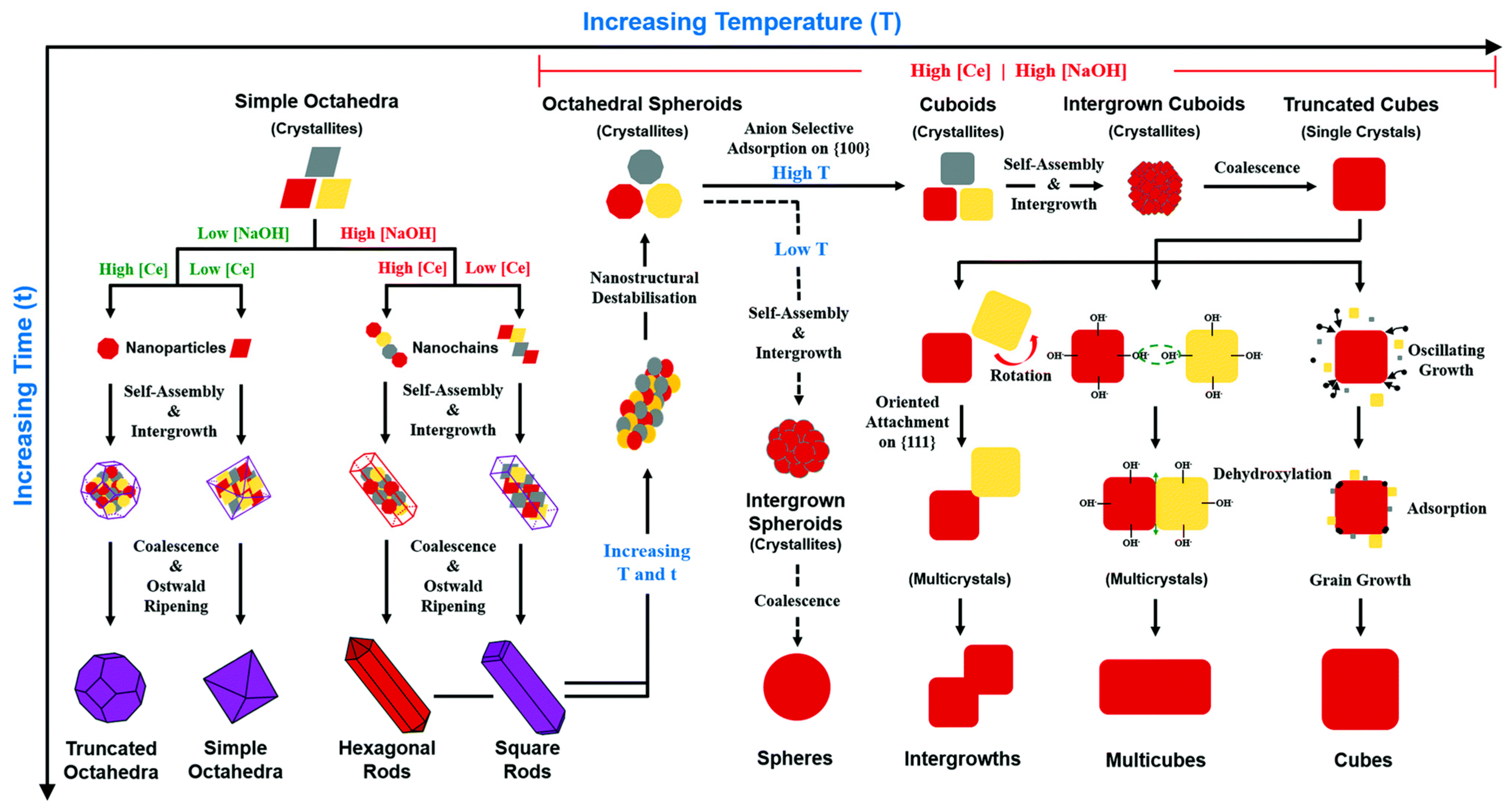
3.1. Ceria-Zirconia
| Crystal Morphology | Composition CexZr1−xO2 | Solvent Temperature Time a | Reagents | Reference |
|---|---|---|---|---|
| Facetted polyhedral 3–5 nm diameter | 0 ≤ x ≤ 1 | 6 M NaOH(aq) 150 °C 48 h | Ce(NO3)3·6H2O ZrO(NO3)2·xH2O | [49] |
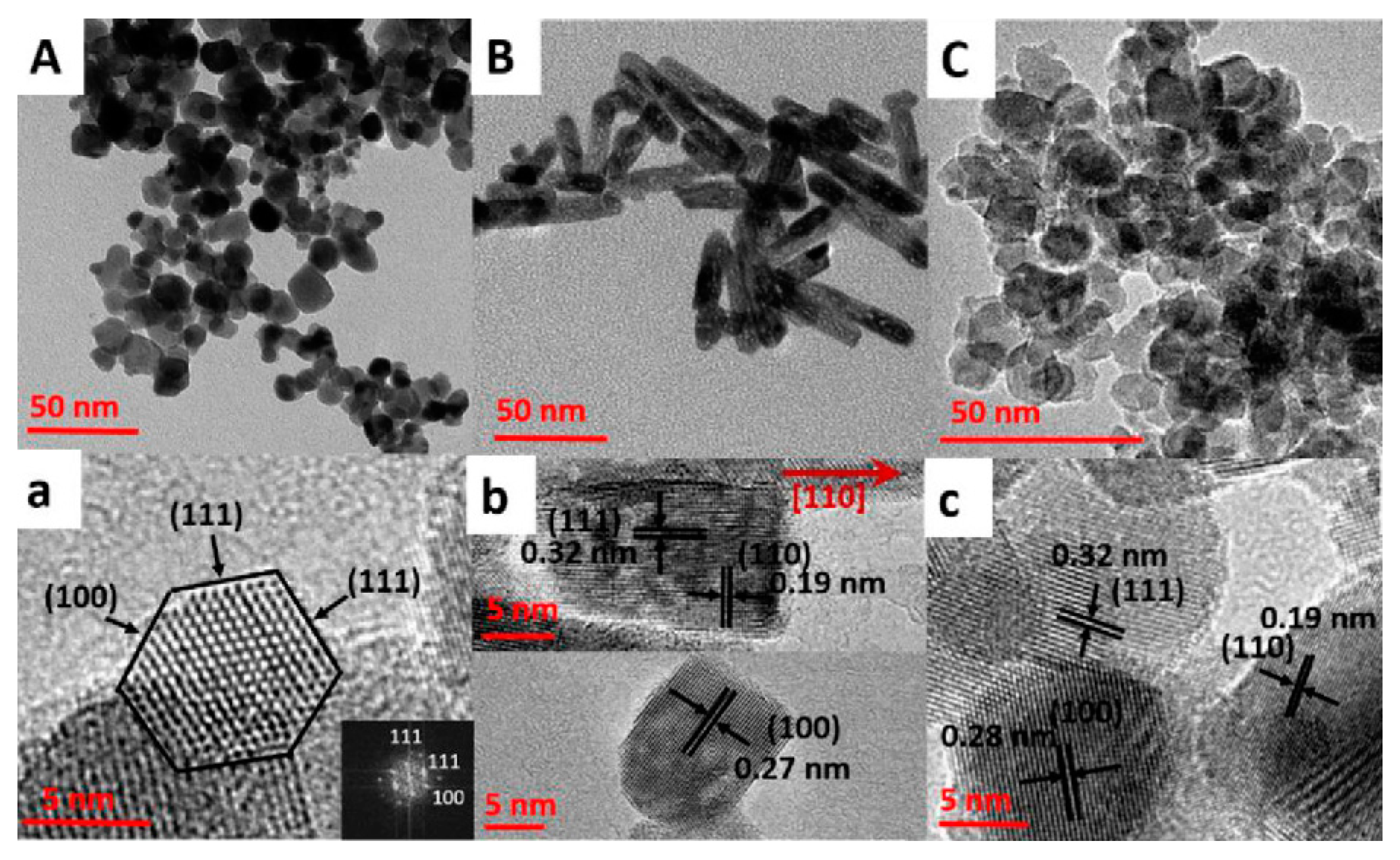
| Hollow, truncated octahedral ~80 nm edge | x = 0.01, 0.1, 0.2 | water 100 °C 18 h | CeCl3·7H2O ZrOCl2·8H2O Poly(vinylpyrrolidone) H2O2 formic acid tert-butylamine | [47] |
| Rods diameter ~8 nm length ~40 nm | 0 ≤ x ≤ 0.2 | 10 M NaOH(aq) 100 °C 20 h | Ce(NO3)3·6H2O Zr(NO3)4·5H2O | [48] |
| Facetted <10 nm diameter | 0 ≤ x ≤ 0.2 | water 95 °C 8 h | (NH4)2Ce(NO3)6 Zr(NO3)4·5H2O urea | [48] |
| Polyhedral 10–15 nm diameter | x = 0.2 | water 180 °C 12 h | Ce(NO3)3·6H2O ZrO(NO3)2·6H2O poly(vinylpyrrolidone) hydrazine | [46] |
| Rods diameter ~10 nm length ~20–70 nm | x = 0.2 | 6 M NaOH(aq) 100 °C 24 h | Ce(NO3)3·6H2O Zr(NO3)4·5H2O | [46] |
| Plates 6–12 nm diameter | x = 0.2 | NH3(aq) 100 °C 24 h | Ce(NO3)3·6H2O ZrO(NO3)2·6H2O CTAB b | [46] |
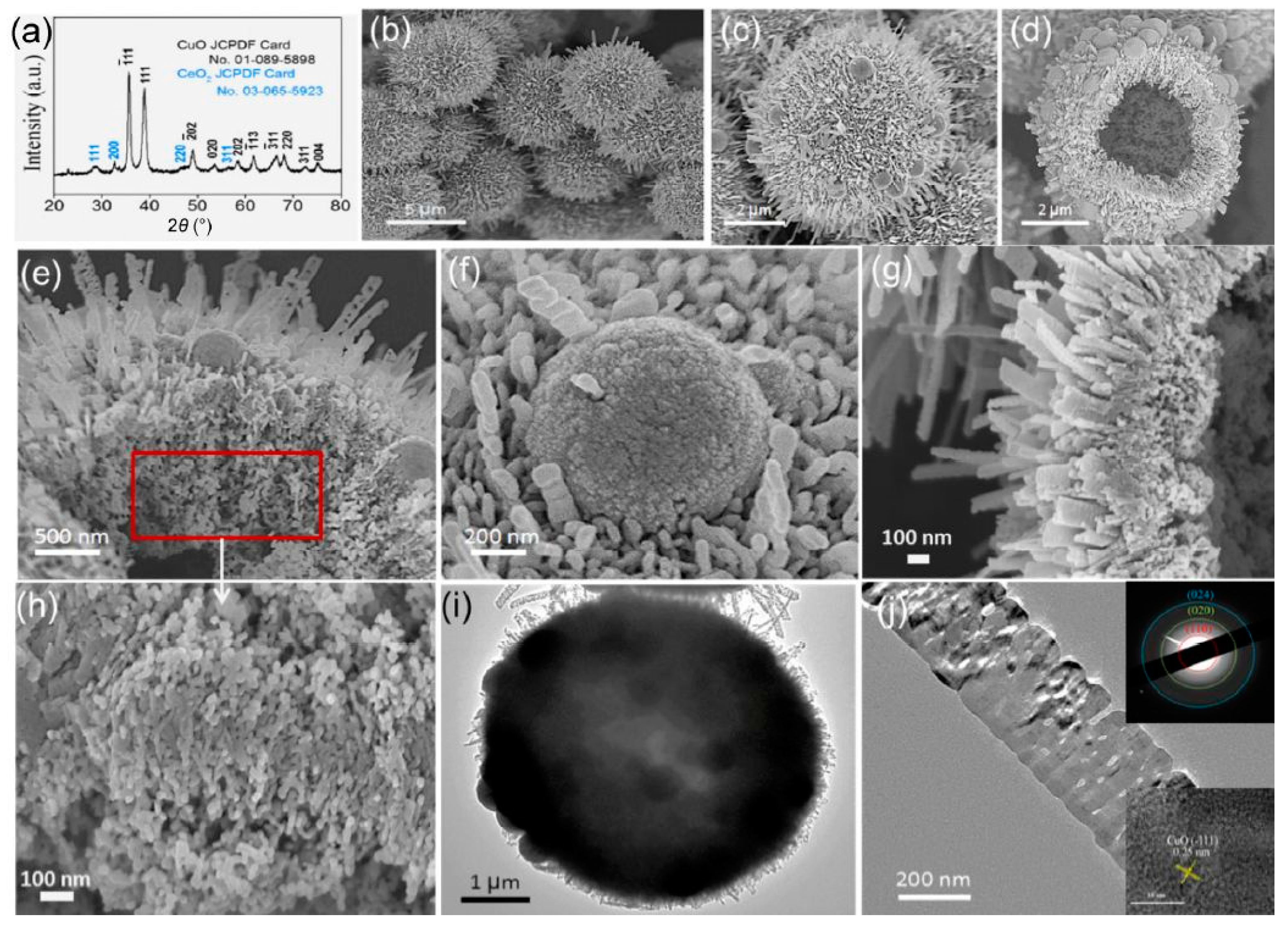
3.2. Transition Metal-Substituted Cerias
3.3. Main Group Substituents
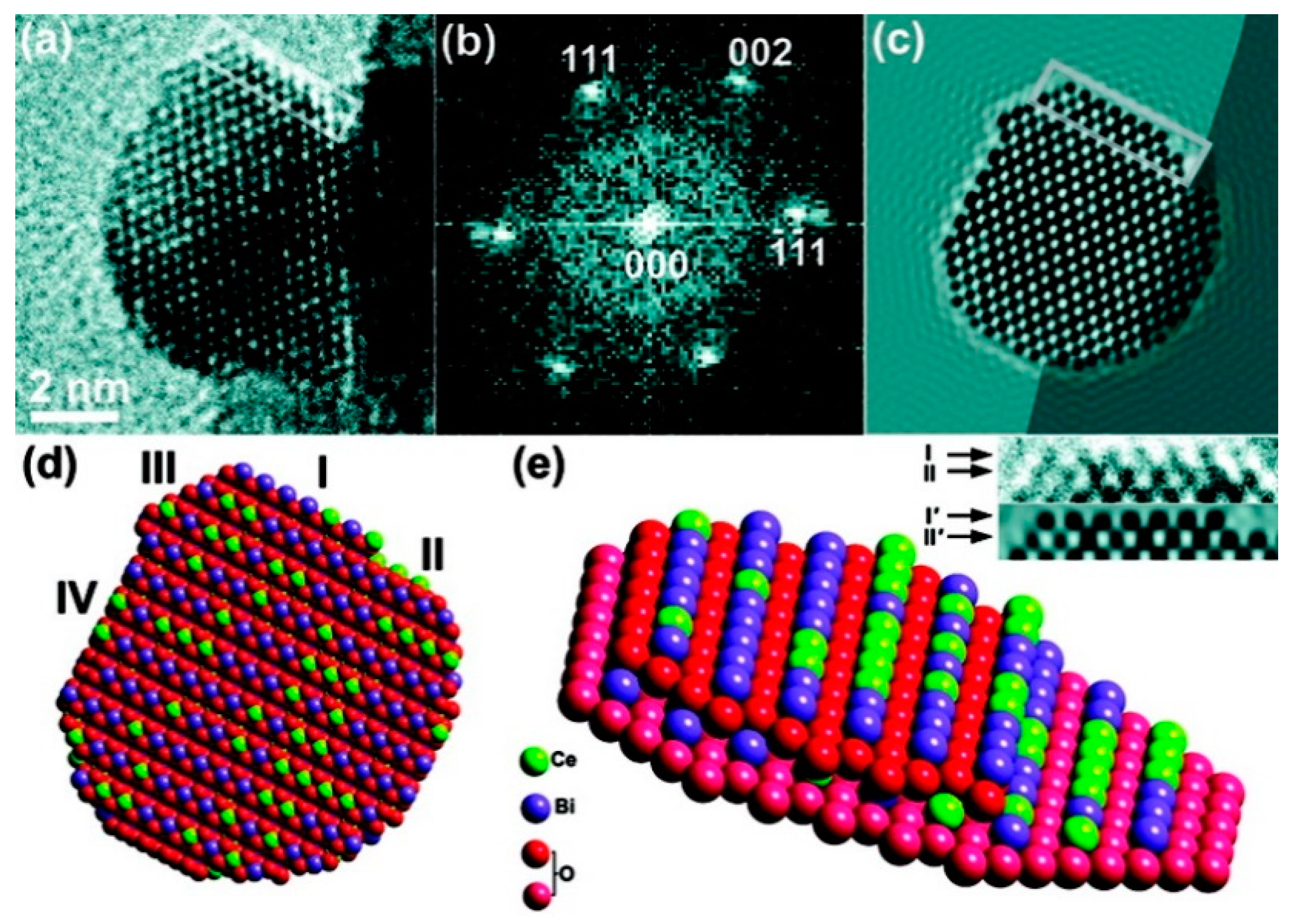
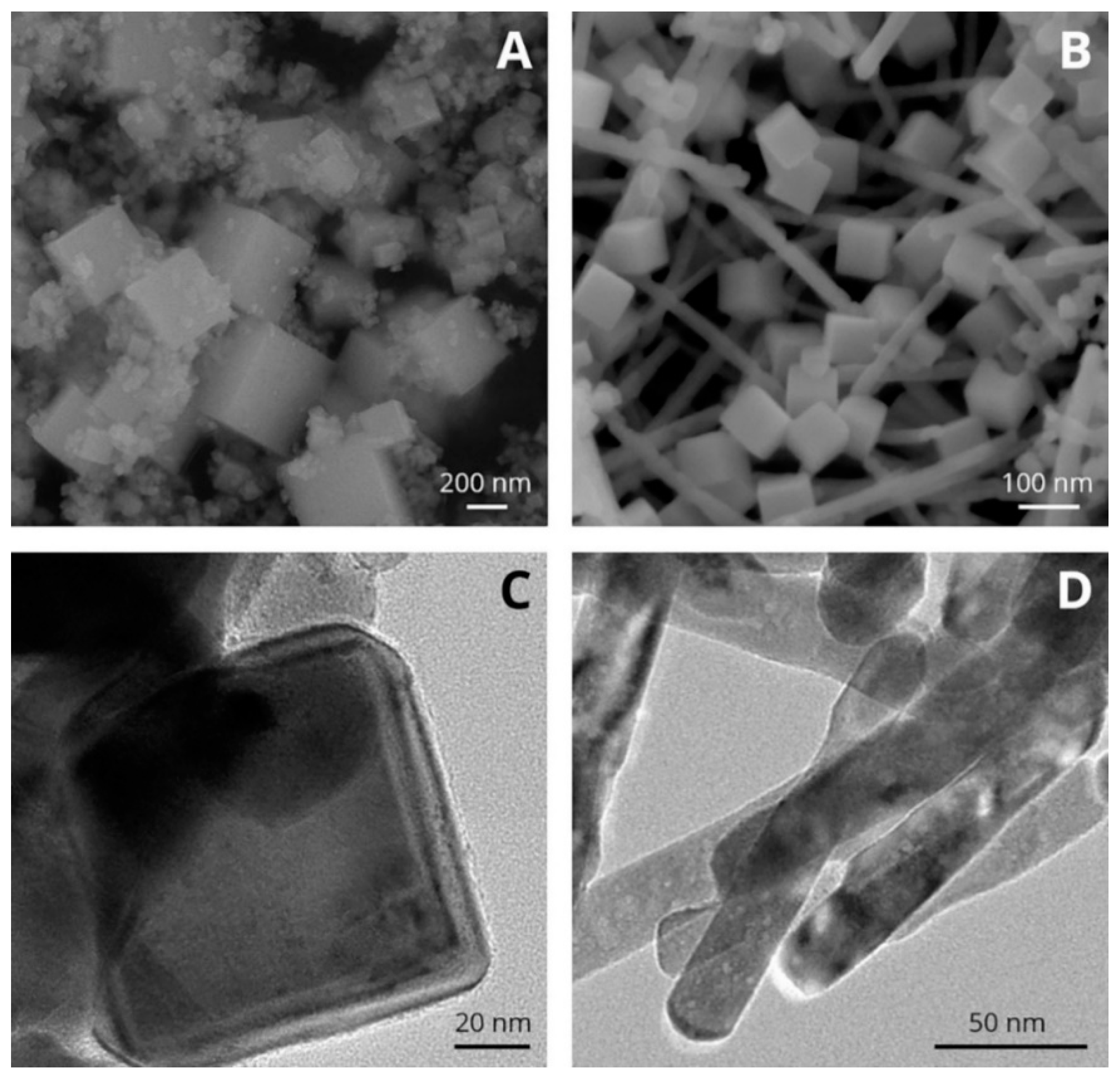
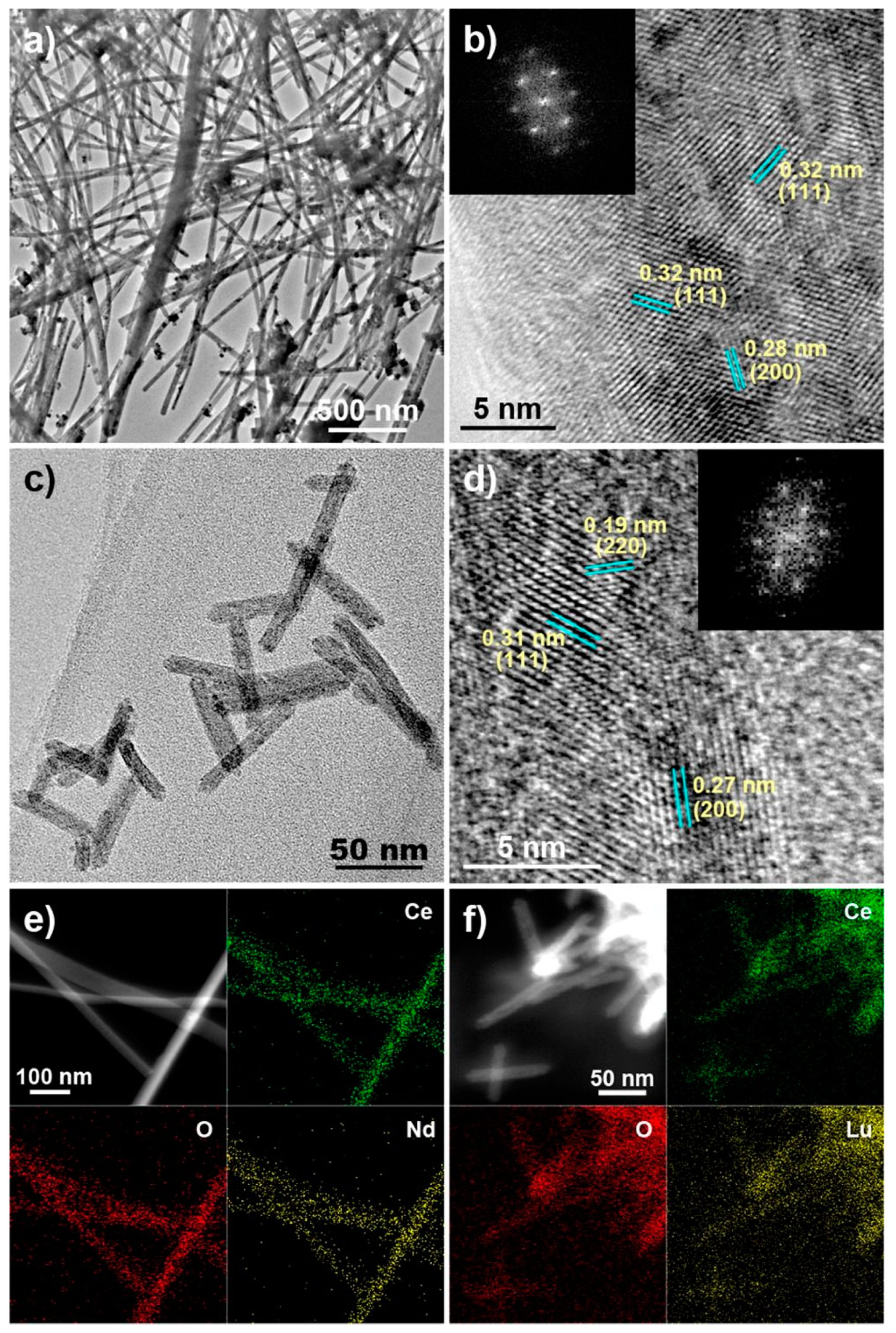
3.4. Rare Earth-Substituted Ceria
3.5. Multi-Element Substitutions
4. Summary, Challenges and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Kim, G. Ceria-promoted three-way catalysts for auto exhaust emission control. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 267–274. [Google Scholar] [CrossRef]
- Diwell, A.F.; Rajaram, R.R.; Shaw, H.A.; Truex, T.J. The Role of Ceria in Three-Way Catalysts. In Studies in Surface Science and Catalysis; Crucq, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 71, pp. 139–152. [Google Scholar]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Soykal, I.I.; Sohn, H.; Singh, D.; Miller, J.T.; Ozkan, U.S. Reduction Characteristics of Ceria under Ethanol Steam Reforming Conditions: Effect of the Particle Size. ACS Catal. 2014, 4, 585–592. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Gorte, R.J.; Zhao, S. Studies of the water-gas-shift reaction with ceria-supported precious metals. Catal. Today 2005, 104, 18–24. [Google Scholar] [CrossRef]
- Wang, Q.; Yeung, K.L.; Bañares, M.A. Ceria and its related materials for VOC catalytic combustion: A review. Catal. Today 2020, 356, 141–154. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S.; Bion, N.; Le Mercier, T.; Harlé, V. Reactivity of Doped Ceria-Based Mixed Oxides for Solar Thermochemical Hydrogen Generation via Two-Step Water-Splitting Cycles. Energy Fuels 2013, 27, 6068–6078. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Ivanov, V.K.; Zholobak, N.M.; Ivanova, O.S.; Krysanov, E.Y.; Baranchikov, A.E.; Spivak, N.Y.; Tretyakov, Y.D. Nanocrystalline ceria based materials—Perspectives for biomedical application. Biophysics 2011, 56, 987–1004. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X.G. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Casals, E.; Zeng, M.; Parra-Robert, M.; Fernández-Varo, G.; Morales-Ruiz, M.; Jiménez, W.; Puntes, V.; Casals, G. Cerium Oxide Nanoparticles: Advances in Biodistribution, Toxicity, and Preclinical Exploration. Small 2020, 16, 1907322. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.; Yamashita, M.; Momose, S.; Tahira, K.; Yoshida, S.; Li, R.; Yin, S.; Sato, T. Synthesis and UV-shielding properties of metal oxide doped ceria via soft solution chemical processes. Int. J. Inorg. Mater. 2001, 3, 1003–1008. [Google Scholar] [CrossRef]
- Yabe, S.; Sato, T. Cerium oxide for sunscreen cosmetics. J. Solid State Chem. 2003, 171, 7–11. [Google Scholar] [CrossRef]
- Caputo, F.; De Nicola, M.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef]
- Feng, X.; Sayle, D.C.; Wang, Z.L.; Paras, M.S.; Santora, B.; Sutorik, A.C.; Sayle, T.X.T.; Yang, Y.; Ding, Y.; Wang, X.; et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 2006, 312, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Q.; Wang, C.; Yu, H.C.; Wang, Y.G.; Wang, T.H. Fast humidity sensors based on CeO2 nanowires. Nanotechnology 2007, 18, 145503. [Google Scholar] [CrossRef]
- Schmitt, R.; Nenning, A.; Kraynis, O.; Korobko, R.; Frenkel, A.I.; Lubomirsky, I.; Haile, S.M.; Rupp, J.L.M. A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 2020, 49, 554–592. [Google Scholar] [CrossRef]
- Di Monte, R.; Kašpar, J. Heterogeneous environmental catalysis—A gentle art: CeO2–ZrO2 mixed oxides as a case history. Catal. Today 2005, 100, 27–35. [Google Scholar] [CrossRef]
- Gao, Y.X.; Wang, W.D.; Chang, S.J.; Huang, W.X. Morphology Effect of CeO2 Support in the Preparation, Metal-Support Interaction, and Catalytic Performance of Pt/CeO2 Catalysts. Chemcatchem 2013, 5, 3610–3620. [Google Scholar] [CrossRef]
- Qiao, Z.A.; Wu, Z.L.; Dai, S. Shape-Controlled Ceria-based Nanostructures for Catalysis Applications. ChemSusChem 2013, 6, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale 2013, 5, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent Activity of Ceria in Soot Combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Kašpar, J.; Di Monte, R.; Fornasiero, P.; Graziani, M.; Bradshaw, H.; Norman, C. Dependency of the Oxygen Storage Capacity in Zirconia–Ceria Solid Solutions upon Textural Properties. Top. Catal. 2001, 16, 83–87. [Google Scholar] [CrossRef]
- Monte, R.D.; Kašpar, J. Nanostructured CeO2–ZrO2 mixed oxides. J. Mater. Chem. 2005, 15, 633–648. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Walton, R.I. Solvothermal synthesis of cerium oxides. Prog. Cryst. Growth Charact. Mater. 2011, 57, 93–108. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–701. [Google Scholar] [CrossRef] [PubMed]
- Laudise, R.A. Hydrothermal Synthesis of Crystals. Chem. Eng. News 1987, 65, 30–43. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal reactions: An original route for the synthesis of novel materials. J. Mater. Sci. 2008, 43, 2104–2114. [Google Scholar] [CrossRef]
- Sōmiya, S.; Roy, R. Hydrothermal synthesis of fine oxide powders. Bull. Mater. Sci. 2000, 23, 453–460. [Google Scholar] [CrossRef]
- Riman, R.E.; Suchanek, W.L.; Lencka, M.M. Hydrothermal crystallization of ceramics. Ann. Chim. Sci. Mater. 2002, 27, 15–36. [Google Scholar] [CrossRef]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef]
- Komarneni, S. Nanophase materials by hydrothermal, microwave-hydrothermal and microwave-solvothermal methods. Curr. Sci. 2003, 85, 1730–1734. [Google Scholar]
- Kumada, N. Preparation and crystal structure of new inorganic compounds by hydrothermal reaction. J. Ceram. Soc. Jpn. 2013, 121, 135–141. [Google Scholar] [CrossRef][Green Version]
- Tani, E.; Yoshimura, M.; Sōmiya, S. Crystallization and crystal growth of CeO2 under hydrothermal conditions. J. Mater. Sci. Lett. 1982, 1, 461–462. [Google Scholar] [CrossRef]
- Montini, T.; Speghini, A.; Rogatis, L.D.; Lorenzut, B.; Bettinelli, M.; Graziani, M.; Fornasiero, P. Identification of the Structural Phases of CexZr1−xO2 by Eu(III) Luminescence Studies. J. Am. Chem. Soc. 2009, 131, 13155–13160. [Google Scholar] [CrossRef]
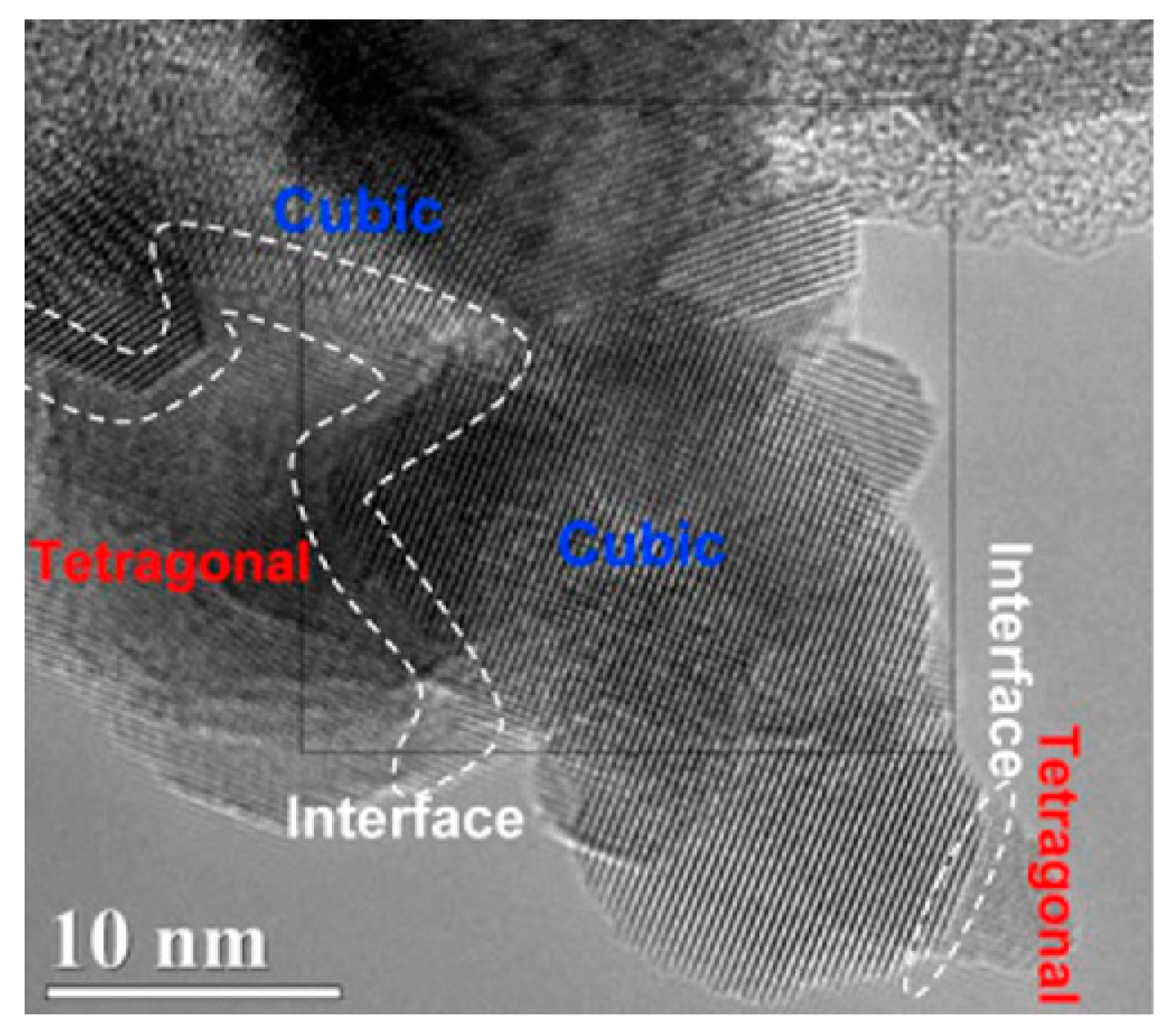
- Yang, Z.; Hu, W.; Zhang, N.; Li, Y.; Liao, Y. Facile synthesis of ceria–zirconia solid solutions with cubic–tetragonal interfaces and their enhanced catalytic performance in diesel soot oxidation. J. Catal. 2019, 377, 98–109. [Google Scholar] [CrossRef]
- Luciani, G.; Landi, G.; Imparato, C.; Vitiello, G.; Deorsola, F.A.; Di Benedetto, A.; Aronne, A. Improvement of splitting performance of Ce0.75Zr0.25O2 material: Tuning bulk and surface properties by hydrothermal synthesis. Int. J. Hydrogen Energy 2019, 44, 17565–17577. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.-Q.; Song, Y.-H.; Liu, Z.-T.; Liu, Z.-W. Defect-rich Ce1−xZrxO2 solid solutions for oxidative dehydrogenation of ethylbenzene with CO2. Catal. Today 2019, 324, 39–48. [Google Scholar] [CrossRef]
- Wan, J.; Lin, J.; Guo, X.; Wang, T.; Zhou, R. Morphology effect on the structure-activity relationship of Rh/CeO2-ZrO2 catalysts. Chem. Eng. J. 2019, 368, 719–729. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Zhang, X.; Han, L.; Zhang, C.; Yang, Y. Zr-doped CeO2 Hollow slightly-truncated nano-octahedrons: One-pot synthesis, characterization and their application in catalysis of CO oxidation. Cryst. Res. Technol. 2014, 49, 383–392. [Google Scholar] [CrossRef]
- Chen, A.; Zhou, Y.; Ta, N.; Li, Y.; Shen, W. Redox properties and catalytic performance of ceria–zirconia nanorods. Catal. Sci. Technol. 2015, 5, 4184–4192. [Google Scholar] [CrossRef]
- Wang, R.; Mutinda, S.I.; Fang, M. One-pot hydrothermal synthesis and high temperature thermal stability of CexZr1−xO2 nanocrystals. RSC Adv. 2013, 3, 19508–19514. [Google Scholar] [CrossRef]
- Das, S.; Gupta, R.; Kumar, A.; Shah, M.; Sengupta, M.; Bhandari, S.; Bordoloi, A. Facile Synthesis of Ruthenium Decorated Zr0.5Ce0.5O2 Nanorods for Catalytic Partial Oxidation of Methane. ACS Appl. Nano Mater. 2018, 1, 2953–2961. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics in oxygen storage capacity of ceria–zirconia mixed oxides prepared by continuous hydrothermal synthesis in supercritical water. Appl. Catal. B Environ. 2007, 71, 57–63. [Google Scholar] [CrossRef]
- Kim, J.-R.; Lee, K.-Y.; Suh, M.-J.; Ihm, S.-K. Ceria–zirconia mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as catalyst support. Catal. Today 2012, 185, 25–34. [Google Scholar] [CrossRef]
- Auxéméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous supercritical solvothermal preparation of nanostructured ceria-zirconia as supports for dry methane reforming catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. CuO/CeO2 catalysts: Redox features and catalytic behaviors. Appl. Catal. A Gen. 2005, 288, 116–125. [Google Scholar] [CrossRef]
- Bernardi, M.I.B.; Mesquita, A.; Béron, F.; Pirota, K.R.; De Zevallos, A.O.; Doriguetto, A.C.; De Carvalho, H.B. The role of oxygen vacancies and their location in the magnetic properties of Ce1−xCuxO2−δ nanorods. Phys. Chem. Chem. Phys. 2015, 17, 3072–3080. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Liu, Q. Ceria- and Cu-doped ceria nanocrystals synthesized by the hydrothermal methods. J. Am. Ceram. Soc. 2008, 91, 2706–2708. [Google Scholar] [CrossRef]
- Yang, F.; Wei, J.; Liu, W.; Guo, J.; Yang, Y. Copper doped ceria nanospheres: Surface defects promoted catalytic activity and a versatile approach. J. Mater. Chem. A 2014, 2, 5662–5667. [Google Scholar] [CrossRef]
- Yang, H.; Pan, Y.; Xu, Y.; Yang, Y.; Sun, G. Enhanced catalytic performance of (CuO)x/Ce0.9Cu0.1O2 nanospheres: Combined contribution of the synergistic effect and surface defects. ChemPlusChem 2015, 80, 886–894. [Google Scholar] [CrossRef]
- Rood, S.C.; Pastor-Algaba, O.; Tosca-Princep, A.; Pinho, B.; Isaacs, M.; Torrente-Murciano, L.; Eslava, S. Synergistic Effect of Simultaneous Doping of Ceria Nanorods with Cu and Cr on CO Oxidation and NO Reduction. Chem. Eur. J. 2021, 27, 2165–2174. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Liu, W.; Wang, S.; Xie, A.; Liu, X.; Wang, J.; Yang, Y. Facile preparation of Mnn+-Doped (M = Cu, Co, Ni, Mn) hierarchically mesoporous CeO2 nanoparticles with enhanced catalytic activity for CO oxidation. Eur. J. Inorg. Chem. 2015, 2015, 969–976. [Google Scholar] [CrossRef]
- Kurajica, S.; Mužina, K.; Dražić, G.; Matijašić, G.; Duplančić, M.; Mandić, V.; Župančić, M.; Munda, I.K. A comparative study of hydrothermally derived Mn, Fe, Co, Ni, Cu and Zn doped ceria nanocatalysts. Mater. Chem. Phys. 2020, 244, 122689. [Google Scholar] [CrossRef]
- Syed Khadar, Y.A.; Balamurugan, A.; Devarajan, V.P.; Subramanian, R.; Dinesh Kumar, S. Synthesis, characterization and antibacterial activity of cobalt doped cerium oxide (CeO2:Co) nanoparticles by using hydrothermal method. J. Mater. Res. 2019, 8, 267–274. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Yang, H.; Liu, X.; Liu, W.; Zhang, C.; Yang, Y. One-Pot Synthesis of Mn-Doped CeO2 Nanospheres for CO Oxidation. Eur. J. Inorg. Chem. 2013, 2013, 4443–4449. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Gao, R.; Dai, W.L. Manganese-doped CeO2 nanocubes as highly efficient catalysts for styrene epoxidation with TBHP. App.Surf. Sci. 2019, 471, 767–775. [Google Scholar]
- Jampaiah, D.; Venkataswamy, P.; Coyle, V.E.; Reddy, B.M.; Bhargava, S.K. Low-temperature CO oxidation over manganese, cobalt, and nickel doped CeO2 nanorods. RSC Adv. 2016, 6, 80541–80548. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Ding, J.; Wu, X.; Si, Z.; Weng, D. The controlled preparation and performance of Fe, Co-modified porous ceria nanorods for the total oxidation of propane. Mol. Catal. 2020, 480, 110663. [Google Scholar] [CrossRef]
- Xing, X.; Cai, Y.; Chen, N.; Li, Y.; Deng, D.; Wang, Y. Synthesis of mixed Mn–Ce–Ox one dimensional nanostructures and their catalytic activity for CO oxidation. Ceram. Int. 2015, 41, 4675–4682. [Google Scholar] [CrossRef]
- Liu, X.; Han, L.; Liu, W.; Yang, Y. Synthesis of Co/Ni unitary- or binary-doped CeO2 mesoporous nanospheres and their catalytic performance for CO oxidation. Eur. J. Inorg. Chem. 2014, 2014, 5370–5377. [Google Scholar]
- Du, X.; Dai, Q.; Wei, Q.; Huang, Y. Nanosheets-assembled Ni (Co) doped CeO2 microspheres toward NO + CO reaction. Appl. Catal. A Gen. 2020, 602, 117728. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Damma, D.; Jampaiah, D.; Mukherjee, D.; Vithal, M.; Reddy, B.M. Cr-Doped CeO2 Nanorods for CO Oxidation: Insights into Promotional Effect of Cr on Structure and Catalytic Performance. Catal. Lett. 2020, 150, 948–962. [Google Scholar]
- Phokha, S.; Pinitsoontorn, S.; Maensiri, S. Structure and Magnetic Properties of Monodisperse Fe3+-doped CeO2 Nanospheres. Nano-Micro Lett. 2013, 3, 223–233. [Google Scholar]
- Wang, W.; Zhu, Q.; Qin, F.; Dai, Q.; Wang, X. Fe doped CeO2 nanosheets as Fenton-like heterogeneous catalysts for degradation of salicylic acid. Chem. Eng. J. 2018, 333, 226–239. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zheng, Y.; Shen, L.; Xiao, Y.; Cao, Y.; Zhang, Y.; Au, C.; Jiang, L. Highly Efficient Porous FexCe1−xO2−δ with Three-Dimensional Hierarchical Nanoflower Morphology for H2S-Selective Oxidation. ACS Catal. 2020, 10, 3968–3983. [Google Scholar] [CrossRef]
- Liu, W.; Wang, W.; Tang, K.; Guo, J.; Ren, Y.; Wang, S.; Feng, L.; Yang, Y. The promoting influence of nickel species in the controllable synthesis and catalytic properties of nickel–ceria catalysts. Catal. Sci. Tech. 2016, 6, 2427–2434. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Rosmini, C.; Dimitrov, M.; Issa, G.; Henych, J.; Němečková, Z.; Kovacheva, D.; Velinov, N.; Atanasova, G.; Spassova, I. Formation of Catalytic Active Sites in Hydrothermally Obtained Binary Ceria–Iron Oxides: Composition and Preparation Effects. ACS Appl. Mater. Inter. 2021, 13, 1838–1852. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.C.S.; Peixoto, E.B.; Jesus, A.C.B.; Jesus, J.R.; Fabian, F.A.; Costa, I.M.; Almeida, J.M.A.; Duque, J.G.S.; Meneses, C.T. Effect of doping in Ce1−xTMxO2 (TM = Mn, Cr, Co and Fe) nanoparticles obtained by hydrothermal method. Mater. Chem. Phys. 2019, 225, 187–191. [Google Scholar] [CrossRef]
- Radović, M.; Dohčević-Mitrović, Z.; Golubović, A.; Fruth, V.; Preda, S.; Šćepanović, M.; Popović, Z.V. Influence of Fe3+-doping on optical properties of CeO2−y nanopowders. Ceram. Int. 2013, 39, 4929–4936. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, S.; Chen, D.; He, C.; Tian, S.; Xiong, Y. Grey Fe-CeO2−σ for boosting photocatalytic ozonation of refractory pollutants: Roles of surface and bulk oxygen vacancies. Appl. Catal. B Environ. 2021, 286, 119928. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Giuli, G.; Euchner, H.; Groß, A.; Lepore, G.O.; d’Acapito, F.; Geiger, D.; Biskupek, J.; Kaiser, U.; et al. Introducing Highly Redox-Active Atomic Centers into Insertion-Type Electrodes for Lithium-Ion Batteries. Adv. Ener. Mater. 2020, 10, 2000783. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Zhao, X.; Xu, J.; Zhang, Y. The variation of microstructures, spectral characteristics and catalysis effects of Fe3+ and Zn2+ co-doped CeO2 solid solutions. J. Rare Earths 2020, 38, 241–249. [Google Scholar] [CrossRef]
- Dosa, M.; Piumetti, M.; Bensaid, S.; Andana, T.; Novara, C.; Giorgis, F.; Fino, D.; Russo, N. Novel Mn–Cu-Containing CeO2 Nanopolyhedra for the Oxidation of CO and Diesel Soot: Effect of Dopants on the Nanostructure and Catalytic Activity. Catal. Lett. 2018, 148, 298–311. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Gao, Y.; Liu, W.; Wang, Z.; Cui, C.; Liu, W.; Wang, L. Catalytic oxidation of CO on mesoporous codoped ceria catalysts: Insights into correlation of physicochemical property and catalytic activity. J. Rare Earths 2019, 37, 961–969. [Google Scholar] [CrossRef]
- Ji, Y.; Jin, Z.; Li, J.; Zhang, Y.; Liu, H.; Shi, L.; Zhong, Z.; Su, F. Rambutan-like hierarchically heterostructured CeO2-CuO hollow microspheres: Facile hydrothermal synthesis and applications. Nano Res. 2017, 10, 381–396. [Google Scholar] [CrossRef]
- Hiley, C.I.; Fisher, J.M.; Thompsett, D.; Kashtiban, R.J.; Sloan, J.; Walton, R.I. Incorporation of square-planar Pd2+ in fluorite CeO2: Hydrothermal preparation, local structure, redox properties and stability. J. Mater. Chem. A 2015, 3, 13072–13079. [Google Scholar] [CrossRef]
- Hiley, C.I.; Playford, H.Y.; Fisher, J.M.; Felix, N.C.; Thompsett, D.; Kashtiban, R.J.; Walton, R.I. Pair Distribution Function Analysis of Structural Disorder by Nb5+ Inclusion in Ceria: Evidence for Enhanced Oxygen Storage Capacity from Under-Coordinated Oxide. J. Am. Chem. Soc. 2018, 140, 1588–1591. [Google Scholar] [CrossRef]
- Ma, X.; Lu, P.; Wu, P. Structural, optical and magnetic properties of CeO2 nanowires with nonmagnetic Mg2+ doping. J. Alloys Compd. 2018, 734, 22–28. [Google Scholar] [CrossRef]
- Siqueira Júnior, J.M.; Brum Malta, L.F.; Garrido, F.M.S.; Ogasawara, T.; Medeiros, M.E. Raman and Rietveld structural characterization of sintered alkaline earth doped ceria. Mater. Chem. Phys. 2012, 135, 957–964. [Google Scholar] [CrossRef]
- Tighe, C.J.; Cabrera, R.Q.; Gruar, R.I.; Darr, J.A. Scale Up Production of Nanoparticles: Continuous Supercritical Water Synthesis of Ce–Zn Oxides. Ind. Eng. Chem. Res. 2013, 52, 5522–5528. [Google Scholar] [CrossRef]
- Rajkumar, T.; Sápi, A.; Ábel, M.; Kiss, J.; Szenti, I.; Baán, K.; Gómez-Pérez, J.F.; Kukovecz, Á.; Kónya, Z. Surface Engineering of CeO2 Catalysts: Differences Between Solid Solution Based and Interfacially Designed Ce1−xMxO2 and MO/CeO2 (M = Zn, Mn) in CO2 Hydrogenation Reaction. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Das, A.; Patra, M.; Bhagavathiachari, M.; Nair, R.G. Defect-induced visible-light-driven photocatalytic and photoelectrochemical performance of ZnO–CeO2 nanoheterojunctions. J. Alloys Compd. 2021, 858, 157730. [Google Scholar] [CrossRef]
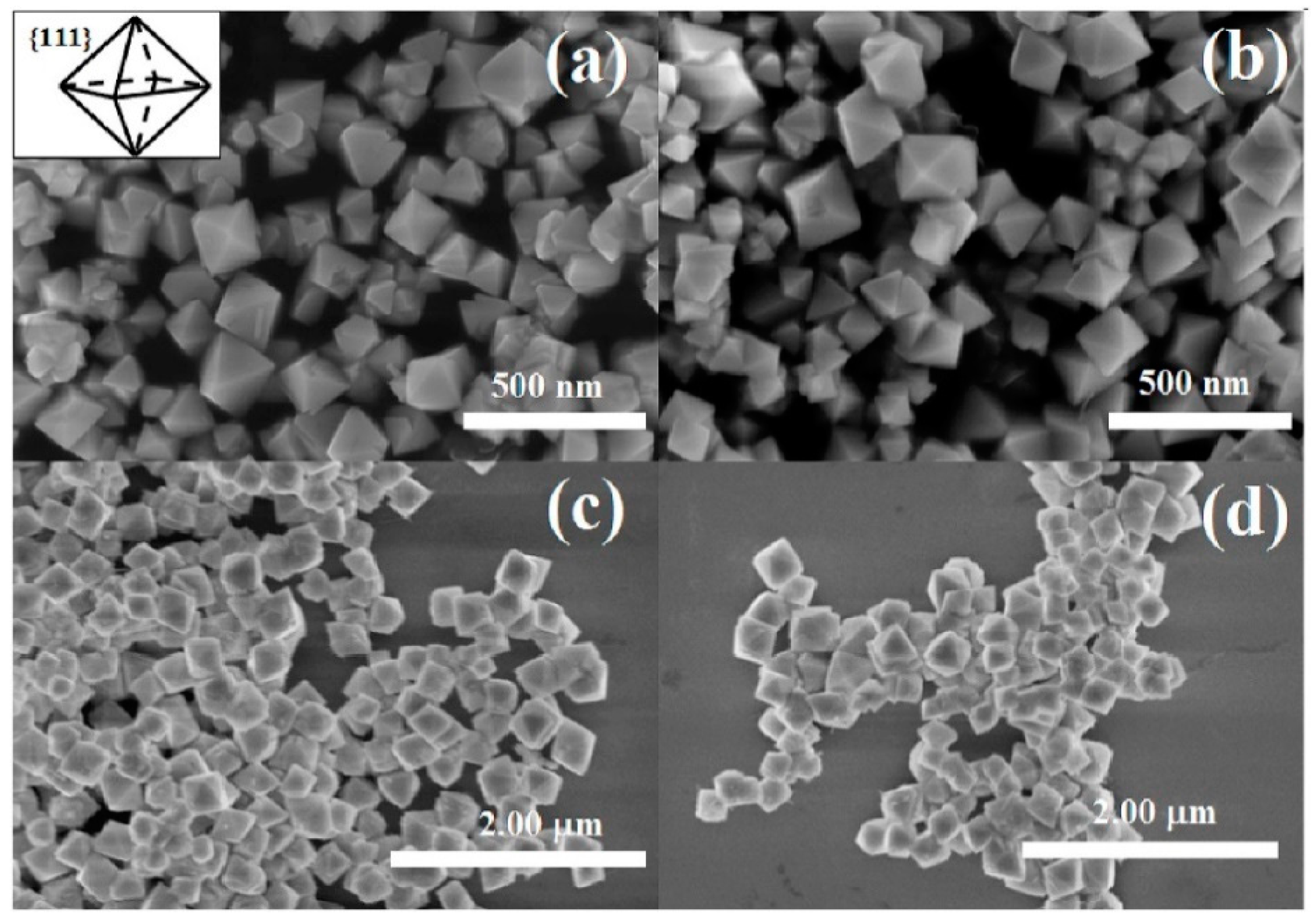
- Younis, A.; Chu, D.; Kaneti, Y.V.; Li, S. Tuning the surface oxygen concentration of {111} surrounded ceria nanocrystals for enhanced photocatalytic activities. Nanoscale 2016, 8, 378–387. [Google Scholar] [CrossRef]
- Abbas, F.; Iqbal, J.; Maqbool, Q.; Jan, T.; Ullah, M.O.; Nawaz, B. ROS mediated malignancy cure performance of morphological, optical, and electrically tuned Sn doped CeO2 nanostructures. AIP Adv. 2017, 7, 095205. [Google Scholar] [CrossRef]
- Shajahan, S.; Arumugam, P.; Rajendran, R.; Munusamy, A.P. Optimization and detailed stability study on Pb doped ceria nanocubes for enhanced photodegradation of several anionic and cationic organic pollutants. Arab. J. Chem. 2020, 13, 1309–1322. [Google Scholar] [CrossRef]
- Sardar, K.; Playford, H.Y.; Darton, R.J.; Barney, E.R.; Hannon, A.C.; Tompsett, D.; Fisher, J.; Kashtiban, R.J.; Sloan, J.; Ramos, S.; et al. Nanocrystalline Cerium−Bismuth Oxides: Synthesis, Structural Characterization, and Redox Properties. Chem. Mater. 2010, 22, 6191–6201. [Google Scholar]
- Frerichs, H.; Pütz, E.; Reich, T.; Gazanis, A.; Panthöfer, M.; Hartmann, J.; Jegel, O.; Tremel, W. Nanocomposite antimicrobials prevent bacterial growth through the enzyme-like activity of Bi-doped cerium dioxide (Ce1−xBixO2−δ). Nanoscale 2020, 12, 21344–21358. [Google Scholar] [CrossRef]
- Houlberg, K.; Bøjesen, E.D.; Mamakhel, A.; Wang, X.; Su, R.; Besenbacher, F.; Iversen, B.B. Hydrothermal Synthesis and in Situ Powder X-ray Diffraction Study of Bismuth-Substituted Ceria Nanoparticles. Cryst. Growth Des. 2015, 15, 3628–3636. [Google Scholar]
- Shanavas, S.; Priyadharsan, A.; Dharmaboopathi, K. Ultrasonically and Photonically Simulatable Bi-Ceria Nanocubes for Enhanced Catalytic Degradation of Aqueous Dyes: A Detailed Study on Optimization, Mechanism and Stability. ChemistrySelect 2018, 3, 12841–12853. [Google Scholar] [CrossRef]
- Veedu, S.N.; Jose, S.; Narendranath, S.B.; Prathapachandra Kurup, M.R.; Periyat, P. Visible light-driven photocatalytic degradation of methylene blue dye over bismuth-doped cerium oxide mesoporous nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 4147–4155. [Google Scholar] [CrossRef]
- Hiley, C.I.; Fisher, J.M.; Kashtiban, R.J.; Cibin, G.; Thompsett, D.; Walton, R.I. Incorporation of Sb5+ into CeO2: Local structural distortion of the fluorite structure from a pentavalent substituent. Dalton Trans. 2018, 47, 9693–9700. [Google Scholar]
- Xu, Y.; Li, R. Wet-chemical synthesis and characterization of nitrogen-doped CeO2 powders for oxygen storage capacity. Appl. Surf. Sci. 2018, 455, 997–1004. [Google Scholar] [CrossRef]
- Liyanage, A.D.; Perera, S.D.; Tan, K.; Chabal, Y.; Balkus, K.J. Synthesis, Characterization, and Photocatalytic Activity of Y-Doped CeO2 Nanorods. ACS Catal. 2014, 4, 577–584. [Google Scholar]
- Mendiuk, O.; Kepinski, L. Synthesis of Ce1−xErxO2−y nanoparticles by the hydrothermal method: Effect of microwave radiation on morphology and phase composition. Ceram. Int. 2014, 40, 14833–14843. [Google Scholar] [CrossRef]
- Małecka, M.A. Characterization and thermal stability of Yb-doped ceria prepared by methods enabling control of the crystal morphology. CrystEngComm 2017, 19, 6199–6207. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Jiang, L.; Tinoco, M.; Hungria, A.B.; Han, J.; Blanco, G.; Calvino, J.J.; Chen, X. Enhanced Hydroxyl Radical Scavenging Activity by Doping Lanthanum in Ceria Nanocubes. J. Phys. Chem. C 2016, 120, 1891–1901. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Nanostructured ceria-praseodymia catalysts for diesel soot combustion. Appl. Catal. B Environ. 2016, 197, 125–137. [Google Scholar] [CrossRef]
- Jiang, L.; Fernandez-Garcia, S.; Tinoco, M.; Yan, Z.; Xue, Q.; Blanco, G.; Calvino, J.J.; Hungria, A.B.; Chen, X. Improved Oxidase Mimetic Activity by Praseodymium Incorporation into Ceria Nanocubes. ACS Appl. Mater. Inter. 2017, 9, 18595–18608. [Google Scholar] [CrossRef] [PubMed]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. Nanostructured equimolar ceria-praseodymia for NOx-assisted soot oxidation: Insight into Pr dominance over Pt nanoparticles and metal–support interaction. Appl. Catal. B Environ. 2018, 226, 147–161. [Google Scholar] [CrossRef]
- Sartoretti, E.; Martini, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured Equimolar Ceria-Praseodymia for Total Oxidations in Low-O2 Conditions. Catalysts 2020, 10, 165. [Google Scholar] [CrossRef]
- Mendiuk, O.; Nawrocki, M.; Kepinski, L. The synthesis of Ce1−xLnxO2−y (Ln = Pr, Sm, Gd, Tb) nanocubes by hydrothermal methods. Ceram. Int. 2016, 42, 1998–2012. [Google Scholar] [CrossRef]
- Hong, S.; Lee, D.; Yang, H.; Kim, Y.-B. Direct hydrothermal growth of GDC nanorods for low temperature solid oxide fuel cells. Appl. Surf. Sci. 2018, 444, 430–435. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujihara, S. Shape-Controlled Synthesis and Luminescent Properties of CeO2:Sm3+ Nanophosphors. Eur. J. Inorg. Chem. 2011, 2011, 1577–1583. [Google Scholar] [CrossRef]
- Bhatta, U.M.; Reid, D.; Sakthivel, T.; Sayle, T.X.T.; Sayle, D.; Molinari, M.; Parker, S.C.; Ross, I.M.; Seal, S.; Möbus, G. Morphology and Surface Analysis of Pure and Doped Cuboidal Ceria Nanoparticles. J. Phys. Chem. C 2013, 117, 24561–24569. [Google Scholar] [CrossRef]
- Ke, J.; Xiao, J.-W.; Zhu, W.; Liu, H.; Si, R.; Zhang, Y.-W.; Yan, C.-H. Dopant-Induced Modification of Active Site Structure and Surface Bonding Mode for High-Performance Nanocatalysts: CO Oxidation on Capping-free (110)-oriented CeO2:Ln (Ln = La–Lu) Nanowires. J. Am. Chem. Soc. 2013, 135, 15191–15200. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, Q.; Yuan, S.; Zhang, M.; Ohno, T. Morphology control and photocatalytic characterization of yttrium-doped hedgehog-like CeO2. Appl. Catal. B Environ. 2015, 164, 120–127. [Google Scholar] [CrossRef]
- Małecka, M.A.; Kraszkiewicz, P.; Bezkrovnyi, O. Catalysis by shapely nanocrystals of the Ce1−xYbxO2−x/2 mixed oxides—Synthesis and phase stability. Mater. Char. 2019, 155, 109796. [Google Scholar] [CrossRef]
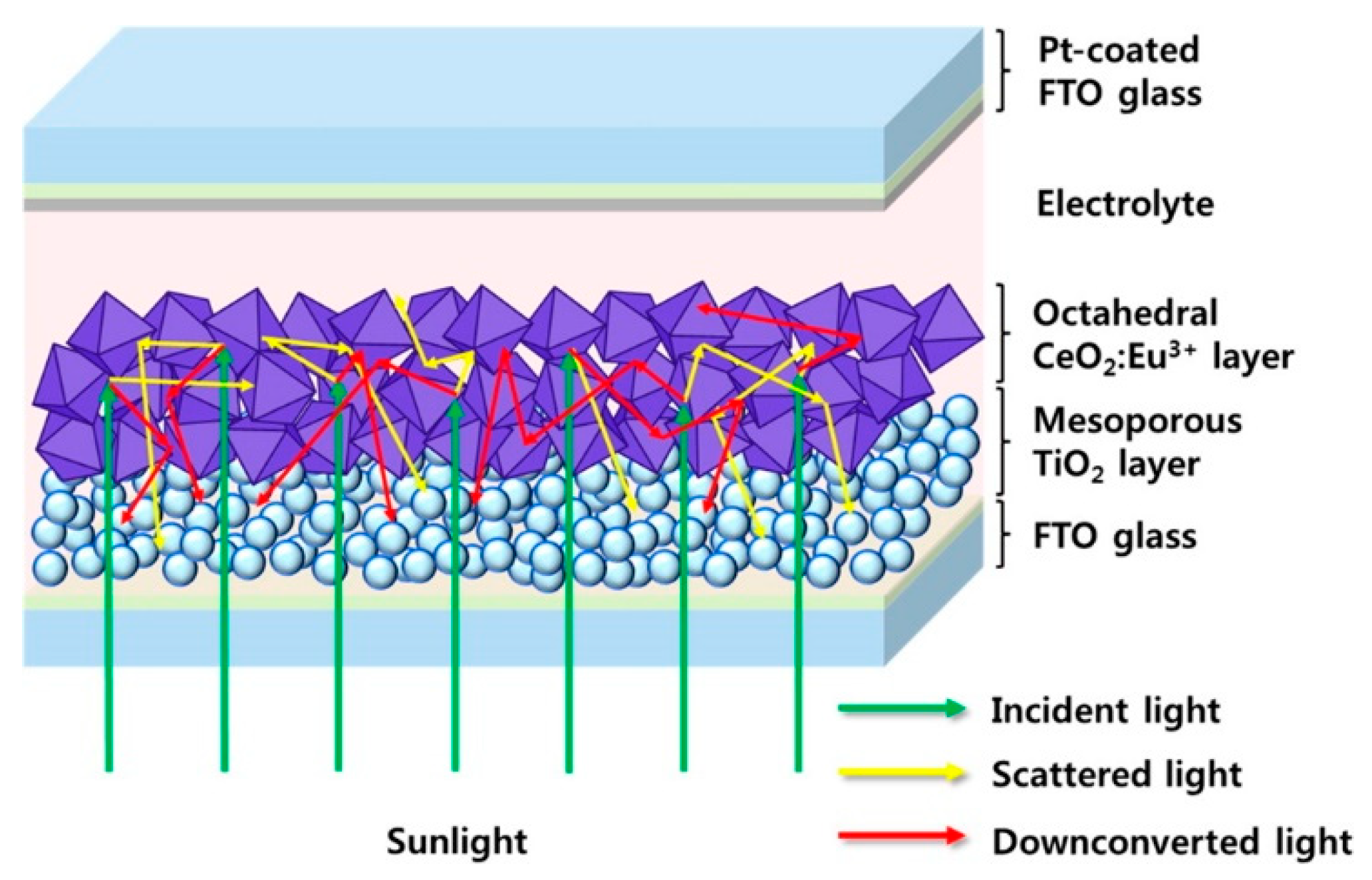
- Roh, J.; Hwang, S.H.; Jang, J. Dual-Functional CeO2:Eu3+ Nanocrystals for Performance-Enhanced Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 19825–19832. [Google Scholar] [CrossRef]
- Bo, Q.; Wang, J. Structural and optical properties of erbium doped ceria nanoparticles synthesized by hydrothermal method. Spectrosc. Lett. 2021, 54, 165–170. [Google Scholar] [CrossRef]
- Yang, Y.; Cong, Y.; Dong, D.P.; Xiao, Y.; Shang, J.Y.; Tong, Y.; Zhang, H.M.; He, M.; Zhang, J.H. Structural and excitation dependent emission properties of octahedral CeO2:Er3+ nanocrystal. J. Lumin. 2019, 213, 427–432. [Google Scholar] [CrossRef]
- Wang, Z.L.; Feng, X. Polyhedral Shapes of CeO2 Nanoparticles. J. Phys. Chem. B 2003, 107, 13563–13566. [Google Scholar] [CrossRef]
- Yan, L.; Yu, R.; Chen, J.; Xing, X. Template-Free Hydrothermal Synthesis of CeO2 Nano-octahedrons and Nanorods: Investigation of the Morphology Evolution. Cryst. Growth Des. 2008, 8, 1474–1477. [Google Scholar] [CrossRef]
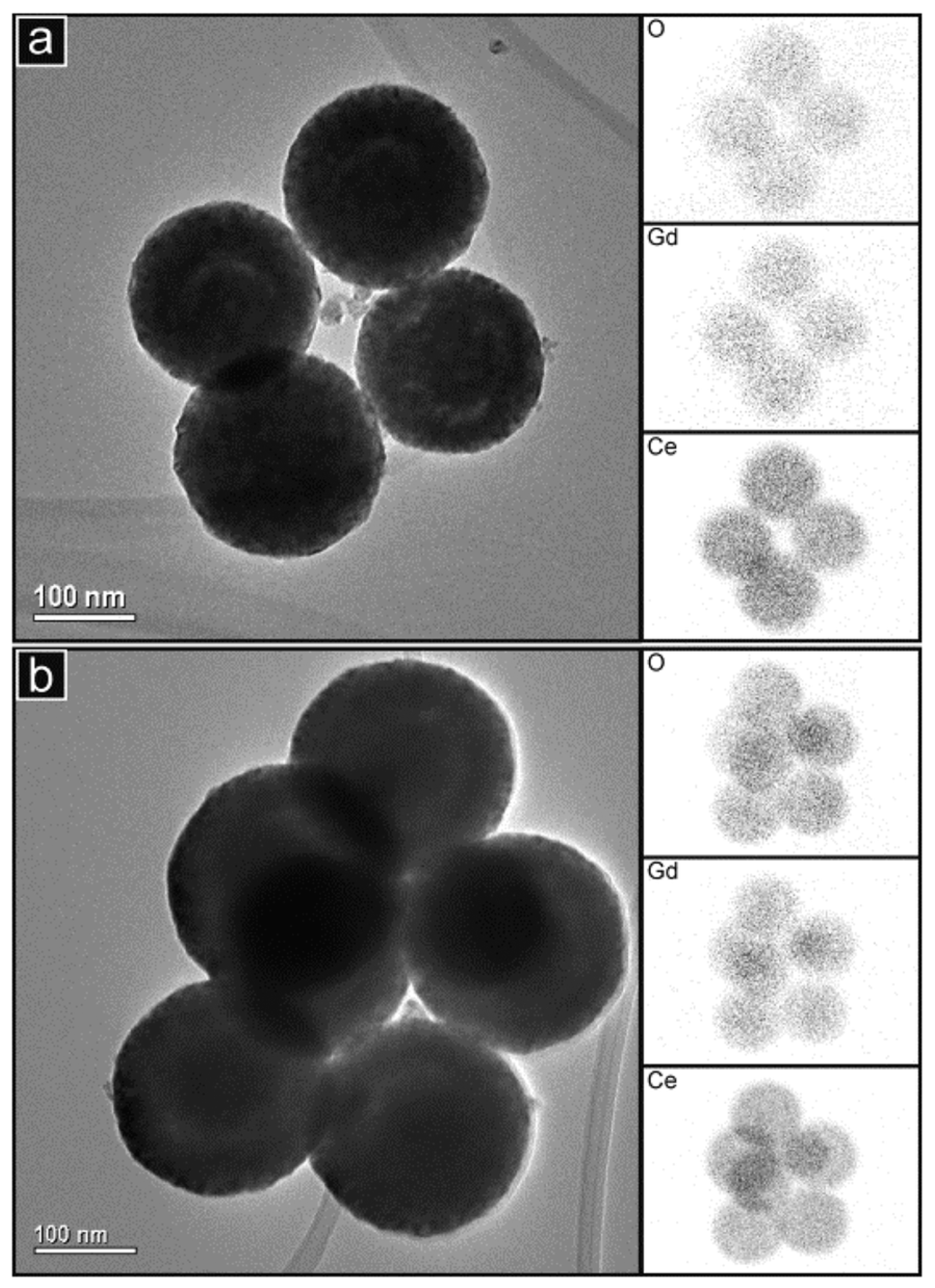
- Muñoz, F.F.; Acuña, L.M.; Albornoz, C.A.; Leyva, A.G.; Baker, R.T.; Fuentes, R.O. Redox properties of nanostructured lanthanide-doped ceria spheres prepared by microwave assisted hydrothermal homogeneous co-precipitation. Nanoscale 2015, 7, 271–281. [Google Scholar] [CrossRef]
- Llusar, M.; Vitásková, L.; Šulcová, P.; Tena, M.A.; Badenes, J.A.; Monrós, G. Red ceramic pigments of terbium-doped ceria prepared through classical and non-conventional coprecipitation routes. J. Eur. Ceram. Soc. 2010, 30, 37–52. [Google Scholar] [CrossRef]
- Acuña, L.M.; Muñoz, F.F.; Albornoz, C.A.; Leyva, A.G.; Baker, R.T.; Fuentes, R.O. Nanostructured terbium-doped ceria spheres: Effect of dopants on their physical and chemical properties under reducing and oxidizing conditions. J. Mater. Chem. A 2015, 3, 16120–16131. [Google Scholar] [CrossRef]
- Xu, B.; Yang, H.; Zhang, Q.; Yuan, S.; Xie, A.; Zhang, M.; Ohno, T. Design and Synthesis of Sm, Y, La and Nd-doped CeO2 with a broom-like hierarchical structure: A photocatalyst with enhanced oxidation performance. ChemCatChem 2020, 12, 2638–2646. [Google Scholar] [CrossRef]
- Hirano, M.; Kato, E. Hydrothermal Synthesis of Nanocrystalline Cerium(IV) Oxide Powders. J. Am. Ceram. Soc. 1999, 82, 786–788. [Google Scholar] [CrossRef]
- Basu, J.; Divakar, R.; Winterstein, J.P.; Carter, C.B. Low-temperature and ambient-pressure synthesis and shape evolution of nanocrystalline pure, La-doped and Gd-doped CeO2. Appl. Surf. Sci. 2010, 256, 3772–3777. [Google Scholar] [CrossRef]
- Polezhaeva, O.S.; Ivanov, V.K.; Dolgopolova, E.A.; Baranchikov, A.E.; Shcherbakov, A.B.; Tret’yakov, Y.D. Synthesis of Nanocrystalline Solid Solutions Ce1-xRxO2-( R = Nd, Eu) by the Homogeneous Hydrolysis Method. Dokl. Chem. 2010, 433, 183–185. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Ivanova, O.S.; Ivanov, V.K.; Sharikov, F.Y.; Baranchikov, A.E.; Shcherbakov, A.B.; Trietyakov, Y.D. Microwave-hydrothermal synthesis of gadolinium-doped nanocrystalline ceria in the presence of hexamethylenetetramine. Russ. J. Inorg. Chem. 2012, 57, 1303–1307. [Google Scholar] [CrossRef]
- Dunnick, K.M.; Pillai, R.; Pisane, K.L.; Stefaniak, A.B.; Sabolsky, E.M.; Leonard, S.S. The Effect of Cerium Oxide Nanoparticle Valence State on Reactive Oxygen Species and Toxicity. Biol. Trace Elem. Res. 2015, 166, 96–107. [Google Scholar] [CrossRef]
- Dunnick, K.M.; Morris, A.M.; Badding, M.A.; Barger, M.; Stefaniak, A.B.; Sabolsky, E.M.; Leonard, S.S. Evaluation of the effect of valence state on cerium oxide nanoparticle toxicity following intratracheal instillation in rats. Nanotoxicology 2016, 10, 992–1000. [Google Scholar] [CrossRef]
- Sato, K.; Arai, M.; Valmalette, J.-C.; Abe, H. Surface Capping-Assisted Hydrothermal Growth of Gadolinium-Doped CeO2 Nanocrystals Dispersible in Aqueous Solutions. Langmuir 2014, 30, 12049–12056. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Zhang, C.; Xiao, Y.; Xie, A.; Pang, Y.U.E.; Yang, Y. One-step synthesis of samarium-doped ceria and its CO catalysis. Bull. Mater. Sci. 2015, 38, 1149–1154. [Google Scholar] [CrossRef][Green Version]
- Anantharaman, A.P.; Gadiyar, H.J.; Surendran, M.; Rao, A.S.; Dasari, H.P.; Dasari, H.; Babu, G.U.B. Effect of synthesis method on structural properties and soot oxidation activity of gadolinium-doped ceria. Chem. Pap. 2018, 72, 3179–3188. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hashishin, T.; Matsuda, M.; Qiu, N.; Tan, Z.; Ohara, S. High-performance Ni nanocomposite anode fabricated from Gd-doped ceria nanocubes for low-temperature solid-oxide fuel cells. Nano Energy 2014, 6, 103–108. [Google Scholar] [CrossRef]
- Kempaiah, D.M.; Yin, S.; Sato, T. A facile and quick solvothermal synthesis of 3D microflower CeO2 and Gd:CeO2 under subcritical and supercritical conditions for catalytic applications. CrystEngComm 2011, 13, 741–746. [Google Scholar] [CrossRef]
- Öksüzömer, M.A.F.; Dönmez, G.; Sariboğa, V.; Altinçekiç, T.G. Microstructure and ionic conductivity properties of gadolinia doped ceria (GdxCe1−xO2−x/2) electrolytes for intermediate temperature SOFCs prepared by the polyol method. Ceram. Int. 2013, 39, 7305–7315. [Google Scholar] [CrossRef]
- Dönmez, G.; Sarıboğa, V.; Gürkaynak Altınçekiç, T.; Öksüzömer, M.A.F. Polyol Synthesis and Investigation of Ce1−xRExO2−x/2 (RE = Sm, Gd, Nd, La, 0 ≤ x ≤ 0.25) Electrolytes for IT-SOFCs. J. Am. Ceram. Soc. 2015, 98, 501–509. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, Z.; Zhao, Z.; Yu, J.; Li, J.; Ren, Z.; Yu, G. Morphologies and magnetic properties of La-doped CeO2 nanoparticles by the solvothermal method in a low magnetic field. Mater. Chem. Phys. 2020, 240, 122148. [Google Scholar] [CrossRef]
- Thorat, A.V.; Ghoshal, T.; Carolan, P.; Holmes, J.D.; Morris, M.A. Defect Chemistry and Vacancy Concentration of Luminescent Europium Doped Ceria Nanoparticles by the Solvothermal Method. J. Phys. Chem. C 2014, 118, 10700–10710. [Google Scholar] [CrossRef]
- Karaca, T.; Altınçekiç, T.G.; Faruk Öksüzömer, M. Synthesis of nanocrystalline samarium-doped CeO2 (SDC) powders as a solid electrolyte by using a simple solvothermal route. Ceram. Int. 2010, 36, 1101–1107. [Google Scholar] [CrossRef]
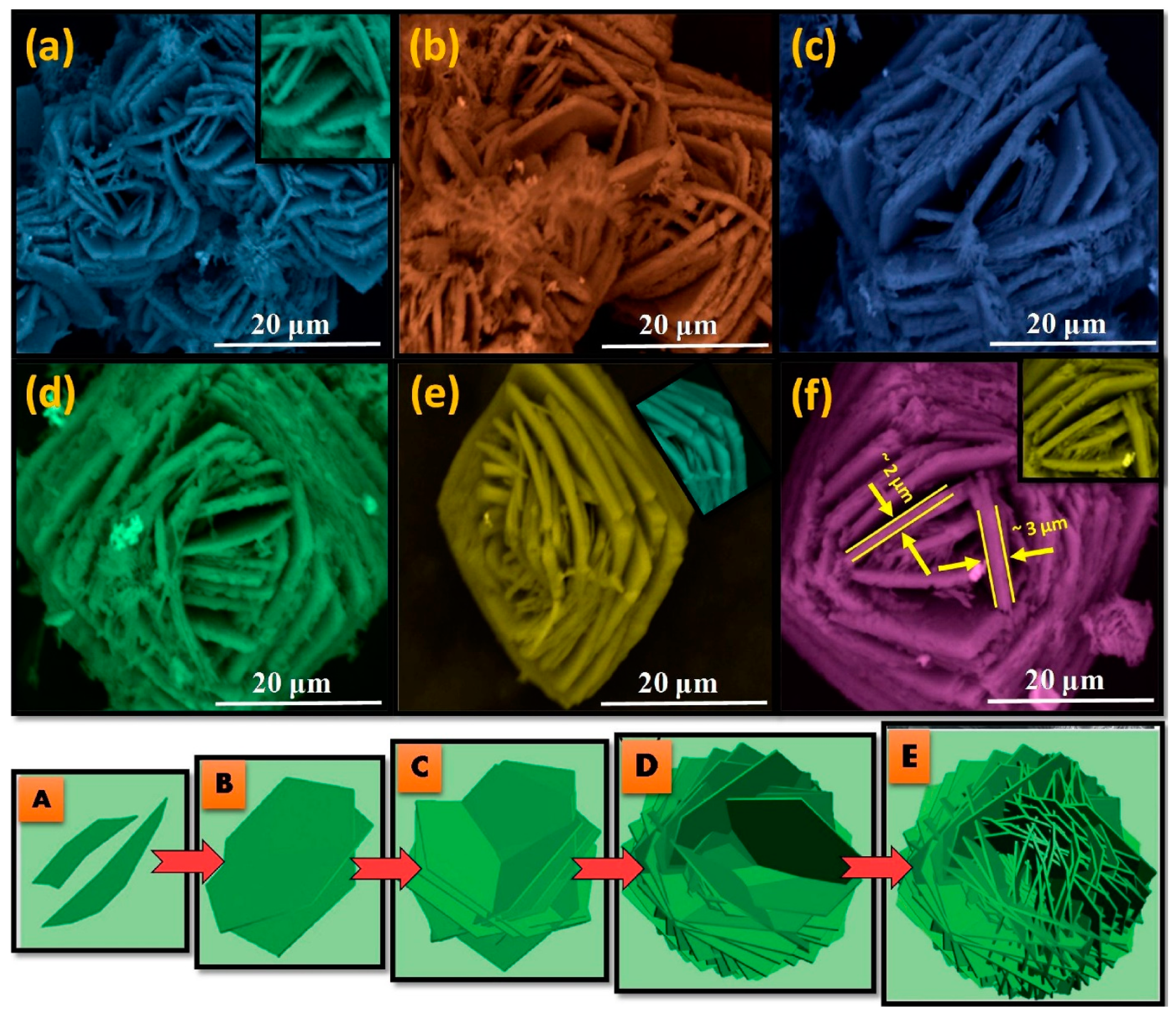
- Deepthi, N.H.; Darshan, G.P.; Basavaraj, R.B.; Prasad, B.D.; Nagabhushana, H. Large-scale controlled bio-inspired fabrication of 3D CeO2:Eu3+ hierarchical structures for evaluation of highly sensitive visualization of latent fingerprints. Sens. Actuators B Chem. 2018, 255, 3127–3147. [Google Scholar] [CrossRef]
- Godinho, M.; Gonçalves, R.d.F.; Leite, E.R.; Raubach, C.W.; Carreño, N.L.V.; Probst, L.F.D.; Longo, E.; Fajardo, H.V. Gadolinium-doped cerium oxide nanorods: Novel active catalysts for ethanol reforming. J. Mater. Sci. 2010, 45, 593–598. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Schmidt, R.; Espíndola-Canuto, J.; Ramos-Alvarez, P.; Morán, E. Increased ionic conductivity in microwave hydrothermally synthesized rare-earth doped ceria Ce1−xRExO2−(x/2). J. Power Source 2012, 209, 163–171. [Google Scholar] [CrossRef]
- Deus, R.C.; Cortés, J.A.; Ramirez, M.A.; Ponce, M.A.; Andres, J.; Rocha, L.S.R.; Longo, E.; Simões, A.Z. Photoluminescence properties of cerium oxide nanoparticles as a function of lanthanum content. Mater. Res. Bull. 2015, 70, 416–423. [Google Scholar] [CrossRef]
- Silva, A.G.M.; Rodrigues, T.S.; Dias, A.; Fajardo, H.V.; Gonçalves, R.F.; Godinho, M.; Robles-Dutenhefner, P.A. Ce1−xSmxO1.9−δ nanoparticles obtained by microwave-assisted hydrothermal processing: An efficient application for catalytic oxidation of α-bisabolol. Catal. Sci. Tech. 2014, 4, 814–821. [Google Scholar] [CrossRef]
- Bezkrovnyi, O.; Małecka, M.A.; Lisiecki, R.; Ostroushko, V.; Thomas, A.G.; Gorantla, S.; Kepinski, L. The effect of Eu doping on the growth, structure and red-ox activity of ceria nanocubes. CrystEngComm 2018, 20, 1698–1704. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Bassat, J.-M.; Aymonier, C. Synthesis of cerium oxide-based nanostructures in near- and supercritical fluids. J. Supercrit. Fluids 2013, 84, 89–97. [Google Scholar] [CrossRef]
- Xu, Y.; Farandos, N.; Rosa, M.; Zielke, P.; Esposito, V.; Vang Hendriksen, P.; Jensen, S.H.; Li, T.; Kelsall, G.; Kiebach, R. Continuous hydrothermal flow synthesis of Gd-doped CeO2 (GDC) nanoparticles for inkjet printing of SOFC electrolytes. Int. J. Appl. Ceram. Technol. 2018, 15, 315–327. [Google Scholar] [CrossRef]
- Karl Chinnu, M.; Vijai Anand, K.; Mohan Kumar, R.; Alagesan, T.; Jayavel, R. Synthesis and enhanced electrochemical properties of Sm:CeO2 nanostructure by hydrothermal route. Mater. Lett. 2013, 113, 170–173. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Spiridigliozzi, L.; Marocco, A.; Accardo, G.; Ferone, C.; Cioffi, R. Effect of the Mineralizer Solution in the Hydrothermal Synthesis of Gadolinium-Doped (10% mol Gd) Ceria Nanopowders. J. Appl. Biomater. Funct. Mater. 2016, 14, 189–196. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Liao, Y.; Shen, G.; Gong, X.; Han, N.; Liu, H.; Chen, Y. Comparative Study of CeO2 and Doped CeO2 with Tailored Oxygen Vacancies for CO Oxidation. ChemPhysChem 2011, 12, 2763–2770. [Google Scholar] [CrossRef]
- Kim, G.; Lee, N.; Kim, K.-B.; Kim, B.-K.; Chang, H.; Song, S.-J.; Park, J.-Y. Various synthesis methods of aliovalent-doped ceria and their electrical properties for intermediate temperature solid oxide electrolytes. Int. J. Hydrogen Energy 2013, 38, 1571–1587. [Google Scholar] [CrossRef]
- Sohn, Y. Lanthanide (III) (La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Yb) Ions Loaded in CeO2 Support; Fundamental Natures, Hydrogen Reduction, and CO Oxidation Activities. Appl. Sci. Converg. Technol. 2019, 28, 35–40. [Google Scholar] [CrossRef]
- Gnanam, S.; Gajendiran, J.; Ramana Ramya, J.; Ramachandran, K.; Gokul Raj, S. Glycine-assisted hydrothermal synthesis of pure and europium doped CeO2 nanoparticles and their structural, optical, photoluminescence, photocatalytic and antibacterial properties. Chem. Phys. Lett. 2021, 763, 138217. [Google Scholar] [CrossRef]
- Palard, M.; Balencie, J.; Maguer, A.; Hochepied, J.-F. Effect of hydrothermal ripening on the photoluminescence properties of pure and doped cerium oxide nanoparticles. Mater. Chem. Phys. 2010, 120, 79–88. [Google Scholar] [CrossRef]
- Cabral, A.C.; Cavalcante, L.S.; Deus, R.C.; Longo, E.; Simões, A.Z.; Moura, F. Photoluminescence properties of praseodymium doped cerium oxide nanocrystals. Ceram. Int. 2014, 40, 4445–4453. [Google Scholar] [CrossRef]
- Wang, P.; Kobiro, K. Synthetic versatility of nanoparticles: A new, rapid, one-pot, single-step synthetic approach to spherical mesoporous (metal) oxide nanoparticles using supercritical alcohols. Pure Appl. Chem. 2014, 86, 785–800. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Liu, S.; Zhang, M.; Ohno, T. Synthesis and photocatalytic performance of yttrium-doped CeO2 with a hollow sphere structure. Catal. Today 2017, 281, 135–143. [Google Scholar] [CrossRef]
- Maria Magdalane, C.; Kaviyarasu, K.; Raja, A.; Arularasu, M.V.; Mola, G.T.; Isaev, A.B.; Al-Dhabi, N.A.; Arasu, M.V.; Jeyaraj, B.; Kennedy, J.; et al. Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: Investigation of cytotoxicity, antibacterial growth inhibition using catalyst. J. Photochem. Photobiol. B 2018, 185, 275–282. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; Cabral, L.; Cabral, A.C.; Almeida, P.B.; Tibaldi, N.; Sambrano, J.R.; Simões, A.Z.; Macchi, C.E.; Moura, F.; Marques, G.E.; et al. Charge transfer in Pr-Doped cerium oxide: Experimental and theoretical investigations. Mater. Chem. Phys. 2020, 249, 122967. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, J.; Ai, D.; Zan, Q.; Lin, X.; Deng, C. Fabrication and performance of Pr-doped CeO2 nanorods-impregnated Sr-doped LaMnO3–Y2O3-stabilized ZrO2 composite cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2012, 22, 25042–25049. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, L.; Cheng, J.; Huang, Y.; Wang, Z.; Chi, B.; Pu, J.; Li, J. Gadolinium doped ceria on graphene cathode with enhanced cycle stability for non-aqueous lithium-oxygen batteries. J. Power Source 2018, 400, 1–8. [Google Scholar] [CrossRef]
- Shi, S.-J.; Zhou, S.-S.; Liu, S.-Q.; Chen, Z.-G. Photocatalytic activity of erbium-doped CeO2 enhanced by reduced graphene Oxide/CuO cocatalyst for the reduction of CO2 to methanol. Environ. Prog. Sustain. Energy 2018, 37, 655–662. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, F.; Wang, Z.; Han, D. Solvothermal synthesis of CeO2:Er/Yb nanorods and upconversion luminescence characterization. Mater. Res. Bull. 2014, 53, 141–144. [Google Scholar] [CrossRef]
- Aouissi, M.L.; Ghrib, A. Effect of Synthesis Route on Structural, Morphological Properties of Ce0.8Y0.2−xLaxO2 (x = 0, 0.1, 0.2). Bull. Chem. Soc. Jpn. 2015, 88, 1159–1163. [Google Scholar] [CrossRef]
- Zhao, R.; Huan, L.; Gu, P.; Guo, R.; Chen, M.; Diao, G. Yb,Er-doped CeO2 nanotubes as an assistant layer for photoconversion-enhanced dye-sensitized solar cells. J. Power Source 2016, 331, 527–534. [Google Scholar] [CrossRef]
- Han, D.; Yang, Y.; Gu, F.; Wang, Z. Tuning the morphology and upconversion fluorescence of CeO2: Er/Yb nano-octahedra. J. Alloys Compd. 2016, 656, 524–529. [Google Scholar] [CrossRef]
- Shirbhate, S.C.; Singh, K.; Acharya, S.A.; Yadav, A.K. Review on local structural properties of ceria-based electrolytes for IT-SOFC. Ionics 2017, 23, 1049–1057. [Google Scholar] [CrossRef]
- Piumetti, M.; Andana, T.; Bensaid, S.; Fino, D.; Russo, N.; Pirone, R. Ceria-based nanomaterials as catalysts for CO oxidation and soot combustion: Effect of Zr-Pr doping and structural properties on the catalytic activity. AIChE J. 2017, 63, 216–225. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, J.; Lan, L.; Wang, J.; Yuan, S.; Gong, M.; Chen, Y. Remarkably promoted low-temperature reducibility and thermal stability of CeO2–ZrO2–La2O3–Nd2O3 by a urea-assisted low-temperature (90 °C) hydrothermal procedure. J. Mater. Sci. 2017, 52, 5894–5907. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Huang, W.; Zou, B.; Khan, Z.S.; Zhao, Y.; Yang, J.; Cao, X. Influence of CeO2 addition on crystal growth behavior of CeO2–Y2O3–ZrO2 solid solution. Ceram. Int. 2012, 38, 2087–2094. [Google Scholar] [CrossRef]
- Khajavi, P.; Xu, Y.; Frandsen, H.L.; Chevalier, J.; Gremillard, L.; Kiebach, R.; Hendriksen, P.V. Tetragonal phase stability maps of ceria-yttria co-doped zirconia: From powders to sintered ceramics. Ceram. Int. 2020, 46, 9396–9405. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Deng, J.; Dai, H.; He, H. Controlled Synthesis, Characterization, and Morphology-Dependent Reducibility of Ceria−Zirconia−Yttria Solid Solutions with Nanorod-like, Microspherical, Microbowknot-like, and Micro-octahedral Shapes. Inorg. Chem. 2009, 48, 2181–2192. [Google Scholar] [PubMed]
- Zhou, Y.; Xiong, L.; Deng, J.; Wang, J.; Yuan, S.; Lan, L.; Chen, Y. Facile synthesis of high surface area nanostructured ceria-zirconia-yttria-lanthana solid solutions with the assistance of lauric acid and dodecylamine. Mater. Res. Bull. 2018, 99, 281–291. [Google Scholar] [CrossRef]
- Ozawa, M.; Yoshimura, Y.; Kobayashi, K. Structure and photoluminescence properties of Ce0.5Zr0.5O2:Eu3+ nanoparticles synthesized by hydrothermal method. Jpn. J. Appl. Phys. 2017, 56, 01ae07. [Google Scholar] [CrossRef]
- Ozawa, M.; Matsumoto, M.; Hattori, M. Photoemission properties of Eu-doped Zr1-xCexO2 (x=0-0.2) nanoparticles prepared by hydrothermal method. Jpn. J. Appl. Phys. 2018, 57, 01ae04. [Google Scholar] [CrossRef]
- Chen, H.; Shi, J.; Hua, Z.; Zhao, J.; Gao, J.; Lei, L.; Yan, D. Synthesis and Characteristics of La Doped Ceria–Zirconia Composite with Uniform Nano-Crystallite Dispersion. Sci. Adv. Mater. 2010, 2, 43–50. [Google Scholar]
- Dudek, M. Ceramic Electrolytes in the CeO2-Gd2O3-SrO System—Preparation, Properties and Application for Solid Oxide Fuel Cells. Int. J. Electrochem. Sci. 2012, 7, 2874–2889. [Google Scholar]
- Dikmen, S. Effect of co-doping with Sm3+, Bi3+, La3+, and Nd3+ on the electrochemical properties of hydrothermally prepared gadolinium-doped ceria ceramics. J. Alloys Compd. 2010, 491, 106–112. [Google Scholar] [CrossRef]
- Dikmen, S.; Aslanbay, H.; Dikmen, E.; Şahin, O. Hydrothermal preparation and electrochemical properties of Gd3+ and Bi3+, Sm3+, La3+, and Nd3+ codoped ceria-based electrolytes for intermediate temperature-solid oxide fuel cell. J. Power Source 2010, 195, 2488–2495. [Google Scholar] [CrossRef]
- Hiley, C.I.; Walton, R.I. Controlling the crystallisation of oxide materials by solvothermal chemistry: Tuning composition, substitution and morphology of functional solids. CrystEngComm 2016, 18, 7656–7670. [Google Scholar] [CrossRef]
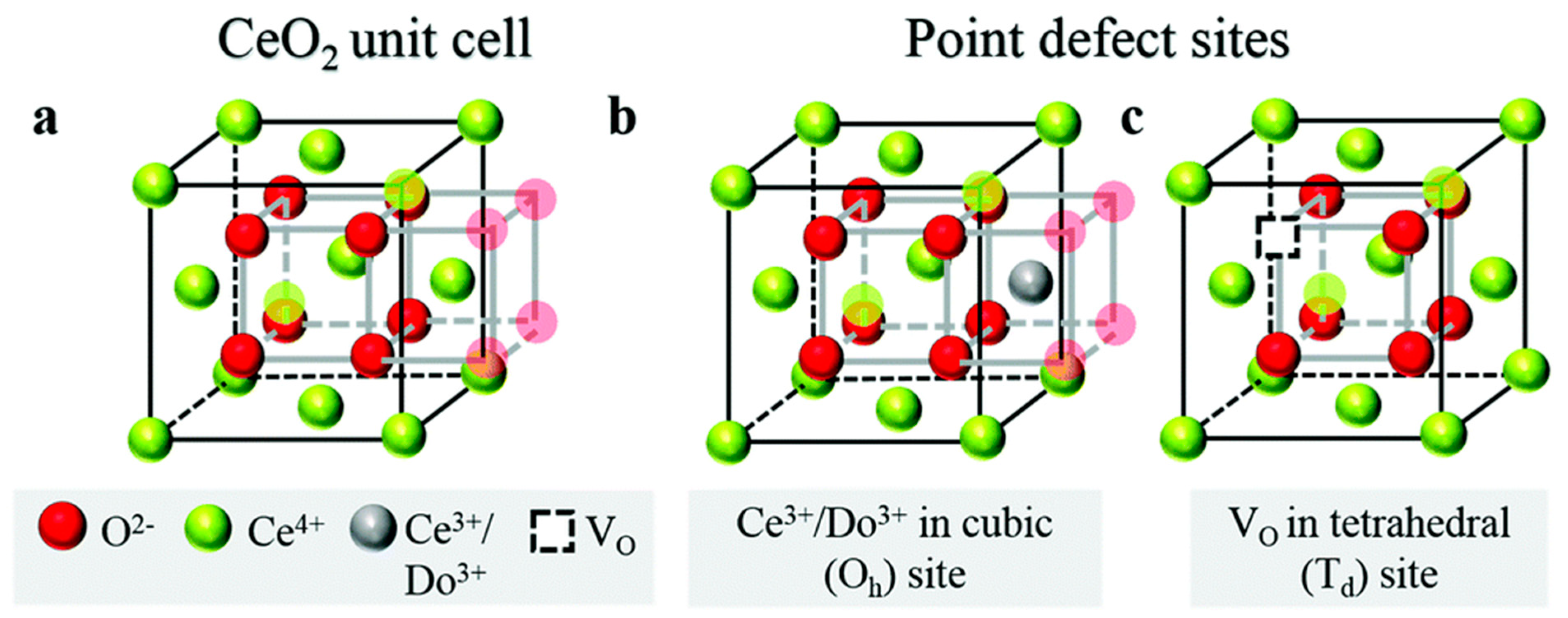
- Minervini, L.; Zacate, M.O.; Grimes, R.W. Defect cluster formation in M2O3-doped CeO2. Solid State Ion. 1999, 116, 339–349. [Google Scholar] [CrossRef]
- Wang, J.D.; Xiao, X.; Liu, Y.; Pan, K.M.; Pang, H.; Wei, S.Z. The application of CeO2-based materials in electrocatalysis. J. Mater. Chem. A 2019, 7, 17675–17702. [Google Scholar] [CrossRef]
- Tyrsted, C.; Jensen, K.M.Ø.; Bøjesen, E.D.; Lock, N.; Christensen, M.; Billinge, S.J.L.; Iversen, B.B. Understanding the Formation and Evolution of Ceria Nanoparticles Under Hydrothermal Conditions. Angew. Chem. Int. Ed. 2012, 51, 9030–9033. [Google Scholar] [CrossRef]
- Promethean Particles Products: Metal Oxides. Available online: https://prometheanparticles.co.uk/metal-oxides/ (accessed on 29 March 2021).
- Wang, H.; Chen, X.; Gao, S.; Wu, Z.; Liu, Y.; Weng, X. Deactivation mechanism of Ce/TiO2 selective catalytic reduction catalysts by the loading of sodium and calcium salts. Catal. Sci. Tech. 2013, 3, 715–722. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Chang, H.; Li, X.; Crittenden, J.C.; Hao, J. Chemical poison and regeneration of SCR catalysts for NOx removal from stationary sources. Front. Environ. Sci. Eng. 2016, 10, 413–427. [Google Scholar] [CrossRef]
- Kennedy, E.M.; Cant, N.W. Oxidative dehydrogenation of ethane and the coupling of methane over sodium containing cerium oxides. Appl. Catal. A Gen. 1992, 87, 171–183. [Google Scholar] [CrossRef]
- Wright, C.S.; Walton, R.I.; Thompsett, D.; Fisher, J.; Ashbrook, S.E. One-step hydrothermal synthesis of nanocrystalline ceria-zirconia mixed oxides: The beneficial effect of sodium inclusion on redox properties. Adv. Mater. 2007, 19, 4500–4504. [Google Scholar] [CrossRef]
- Soria, J.; Martinez-Arias, A.; Coronado, J.M.; Conesa, J.C. Chloride-induced modifications of the properties of rhodia/ceria catalysts. Top. Catal. 2000, 11, 205–212. [Google Scholar] [CrossRef]
- Kȩpiński, L.; Okal, J. Occurrence and Mechanism of Formation of CeOCl in Pd/CeO2 Catalysts. J. Catal. 2000, 192, 48–53. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Dell’Agli, G.; Biesuz, M.; Sglavo, V.M.; Pansini, M. Effect of the Precipitating Agent on the Synthesis and Sintering Behavior of 20 mol% Sm-Doped Ceria. Adv. Mater. Sci. Eng. 2016, 2016, 6096123. [Google Scholar] [CrossRef]
- Mauro, M.; Crosera, M.; Monai, M.; Montini, T.; Fornasiero, P.; Bovenzi, M.; Adami, G.; Turco, G.; Filon, F.L. Cerium Oxide Nanoparticles Absorption through Intact and Damaged Human Skin. Molecules 2019, 24, 3759. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mofarah, S.S.; Mehmood, R.; Cazorla, C.; Koshy, P.; Sorrell, C.C. Design strategies for ceria nanomaterials: Untangling key mechanistic concepts. Mater. Horiz. 2021, 8, 102–123. [Google Scholar] [CrossRef]
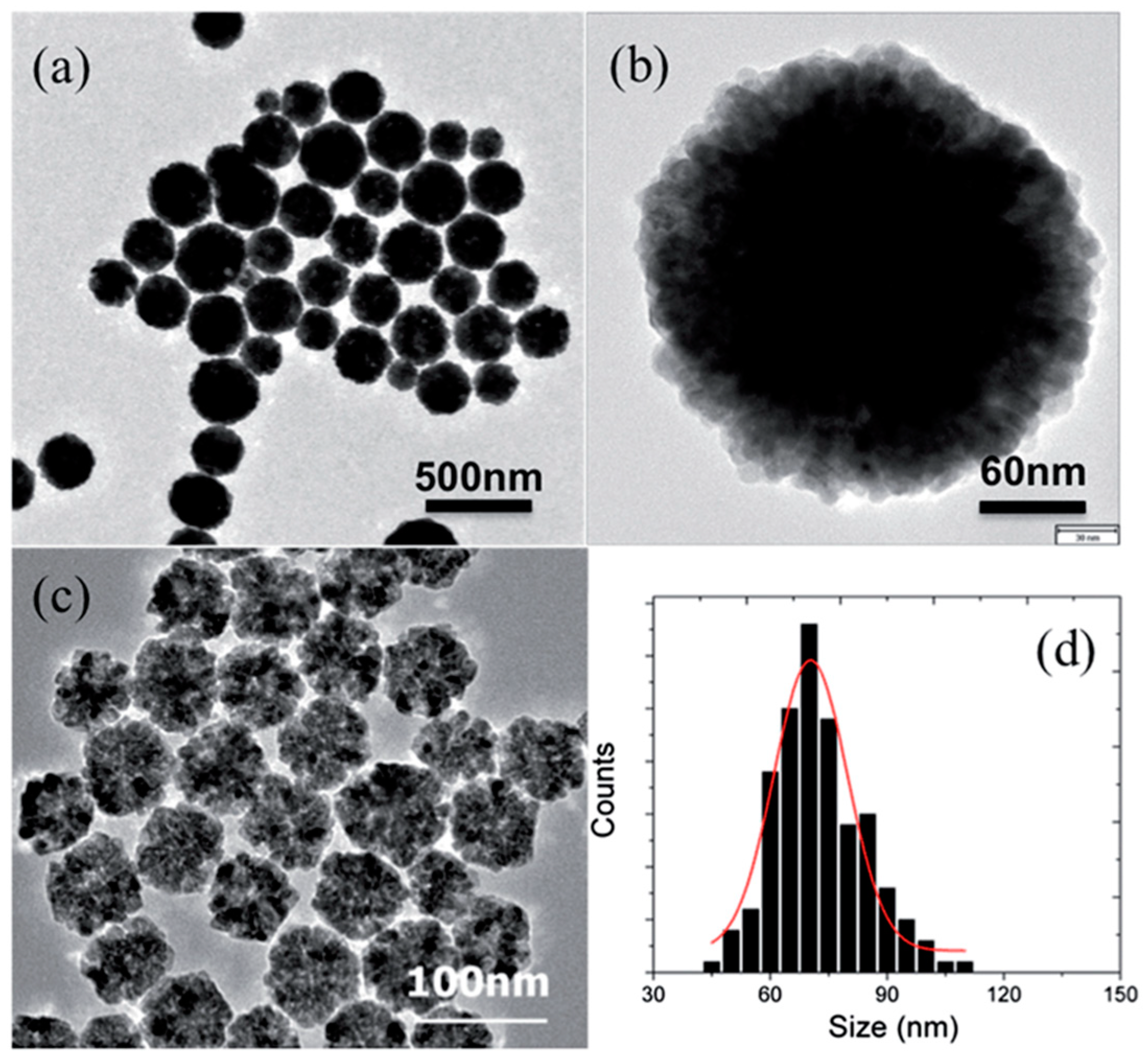
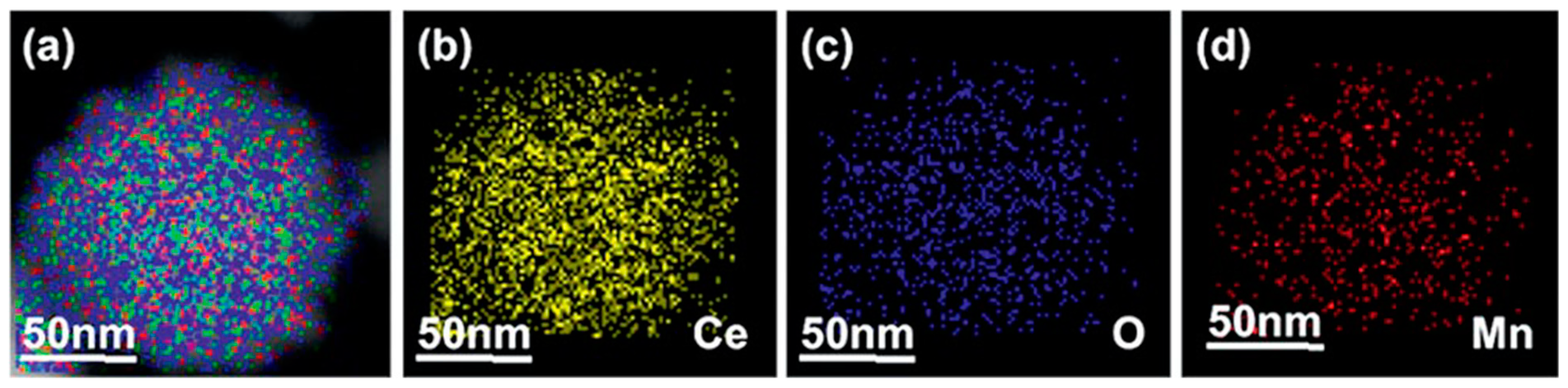
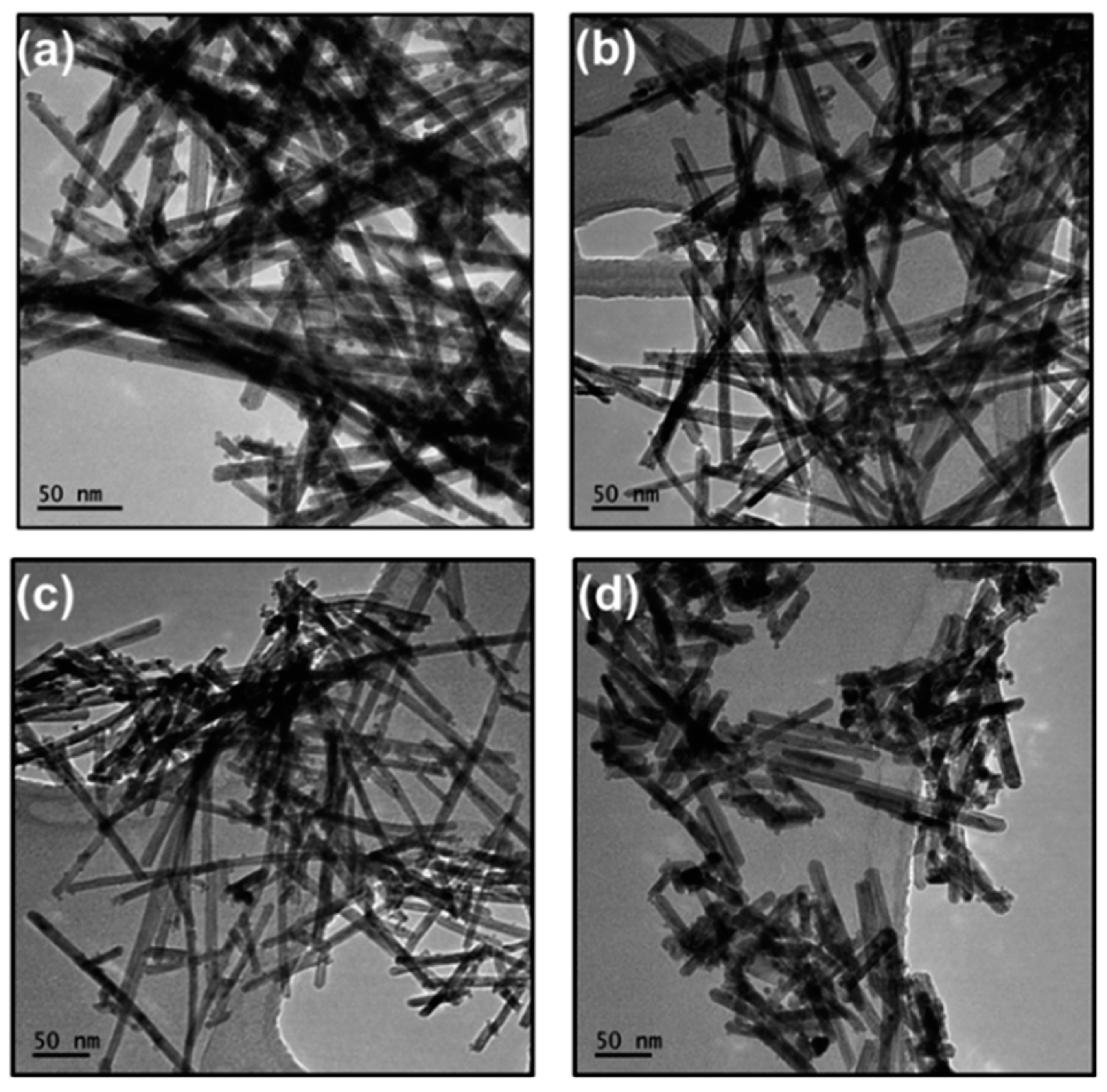

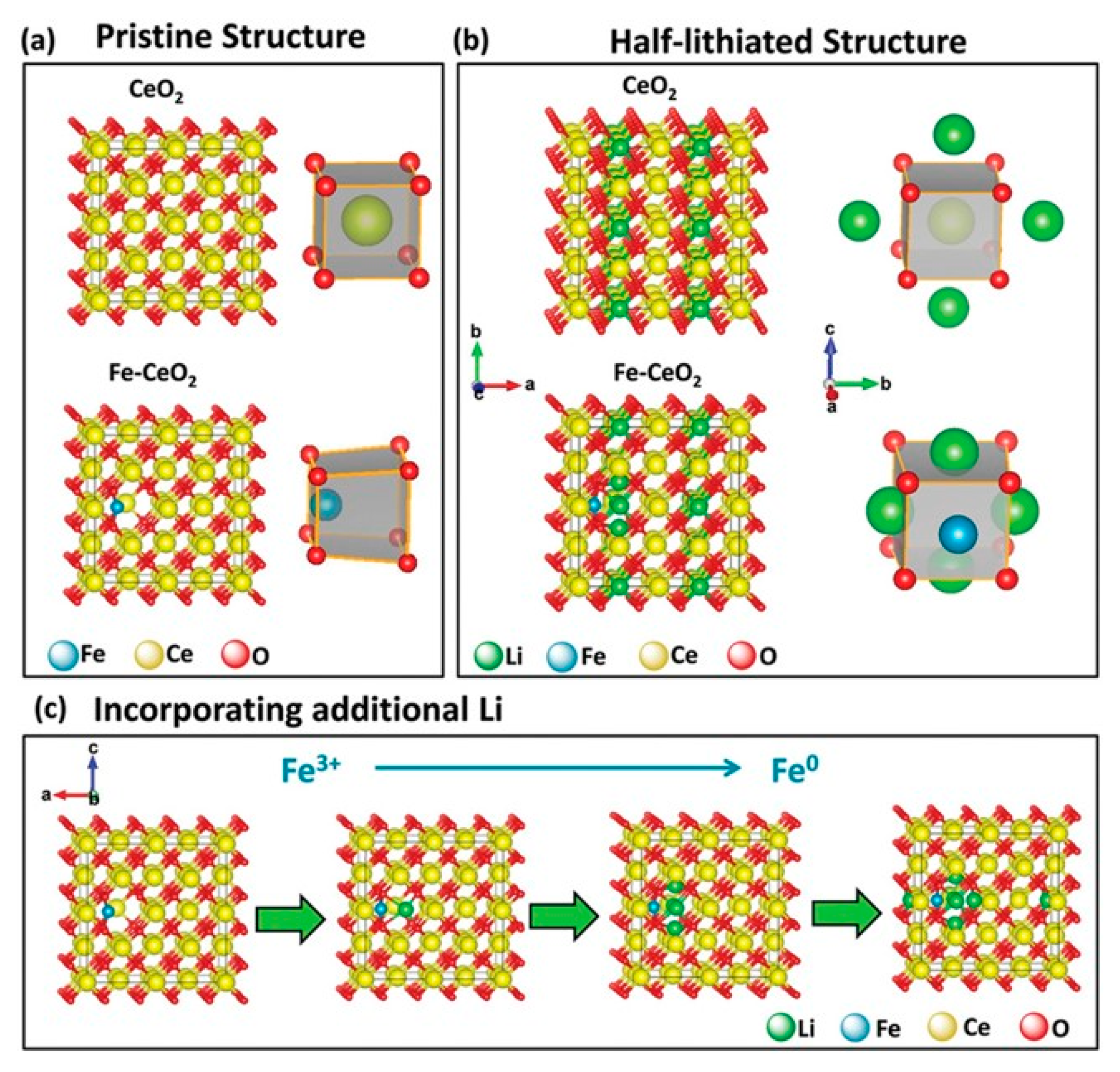



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annis, J.W.; Fisher, J.M.; Thompsett, D.; Walton, R.I. Solvothermal Synthesis Routes to Substituted Cerium Dioxide Materials. Inorganics 2021, 9, 40. https://doi.org/10.3390/inorganics9060040
Annis JW, Fisher JM, Thompsett D, Walton RI. Solvothermal Synthesis Routes to Substituted Cerium Dioxide Materials. Inorganics. 2021; 9(6):40. https://doi.org/10.3390/inorganics9060040
Chicago/Turabian StyleAnnis, James W., Janet M. Fisher, David Thompsett, and Richard I. Walton. 2021. "Solvothermal Synthesis Routes to Substituted Cerium Dioxide Materials" Inorganics 9, no. 6: 40. https://doi.org/10.3390/inorganics9060040
APA StyleAnnis, J. W., Fisher, J. M., Thompsett, D., & Walton, R. I. (2021). Solvothermal Synthesis Routes to Substituted Cerium Dioxide Materials. Inorganics, 9(6), 40. https://doi.org/10.3390/inorganics9060040