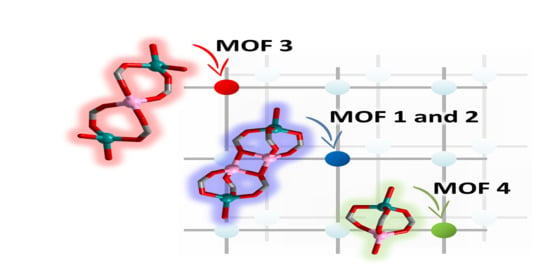
Influence of Substituents in Terephthalate Linker on the Structure of MOFs Obtained from Presynthesized Heterometallic Complex
Abstract
1. Introduction
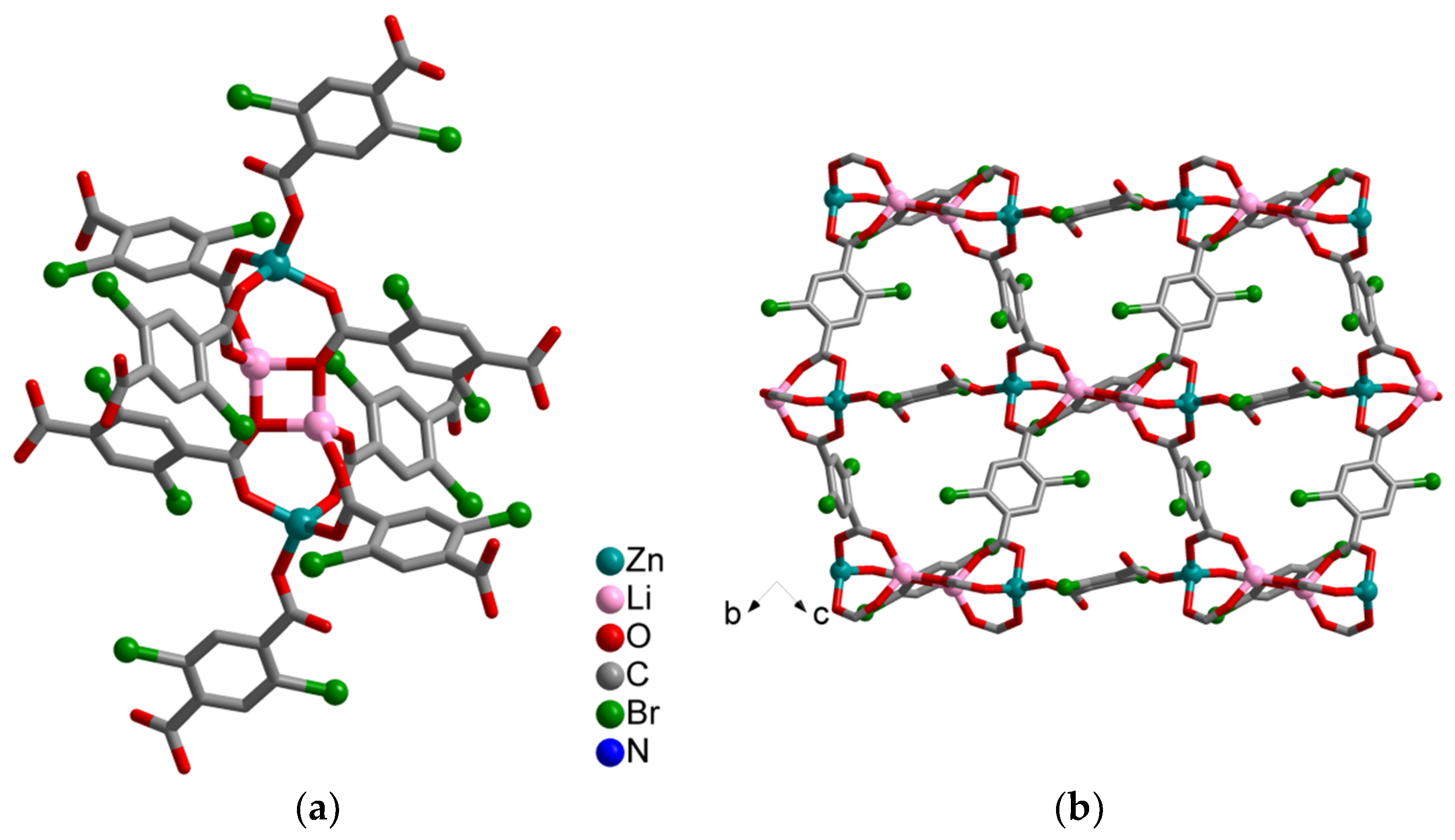
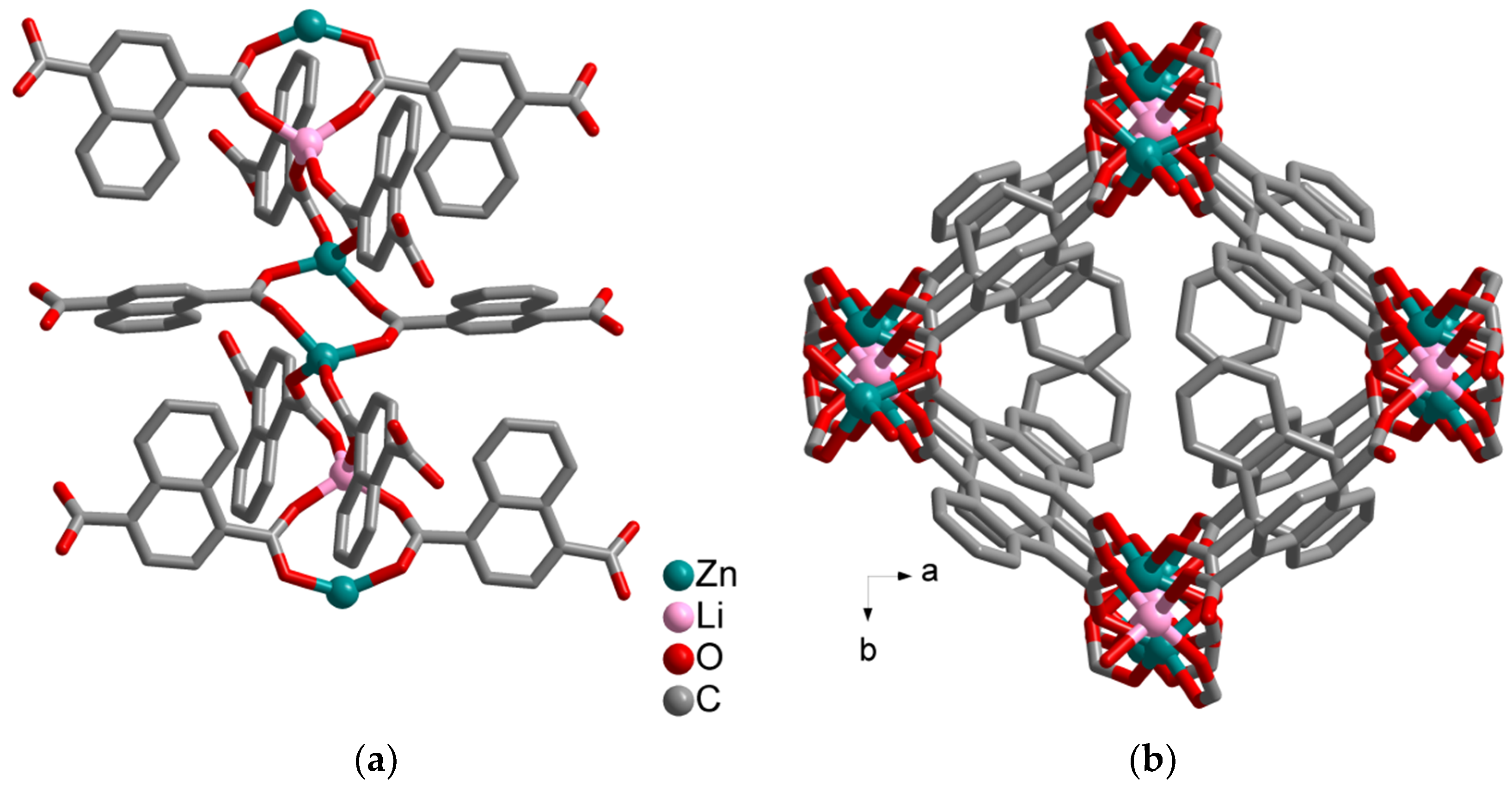
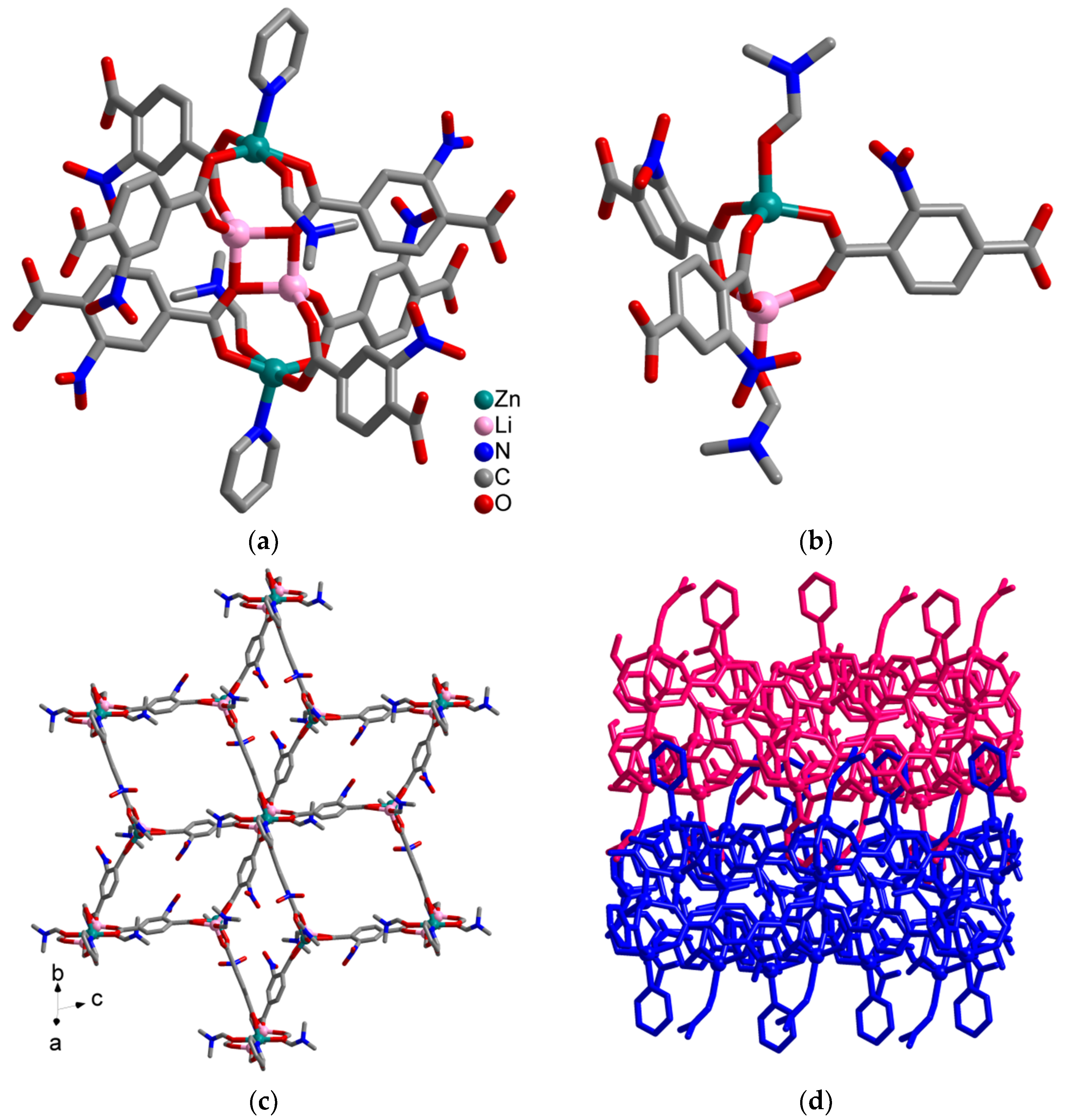
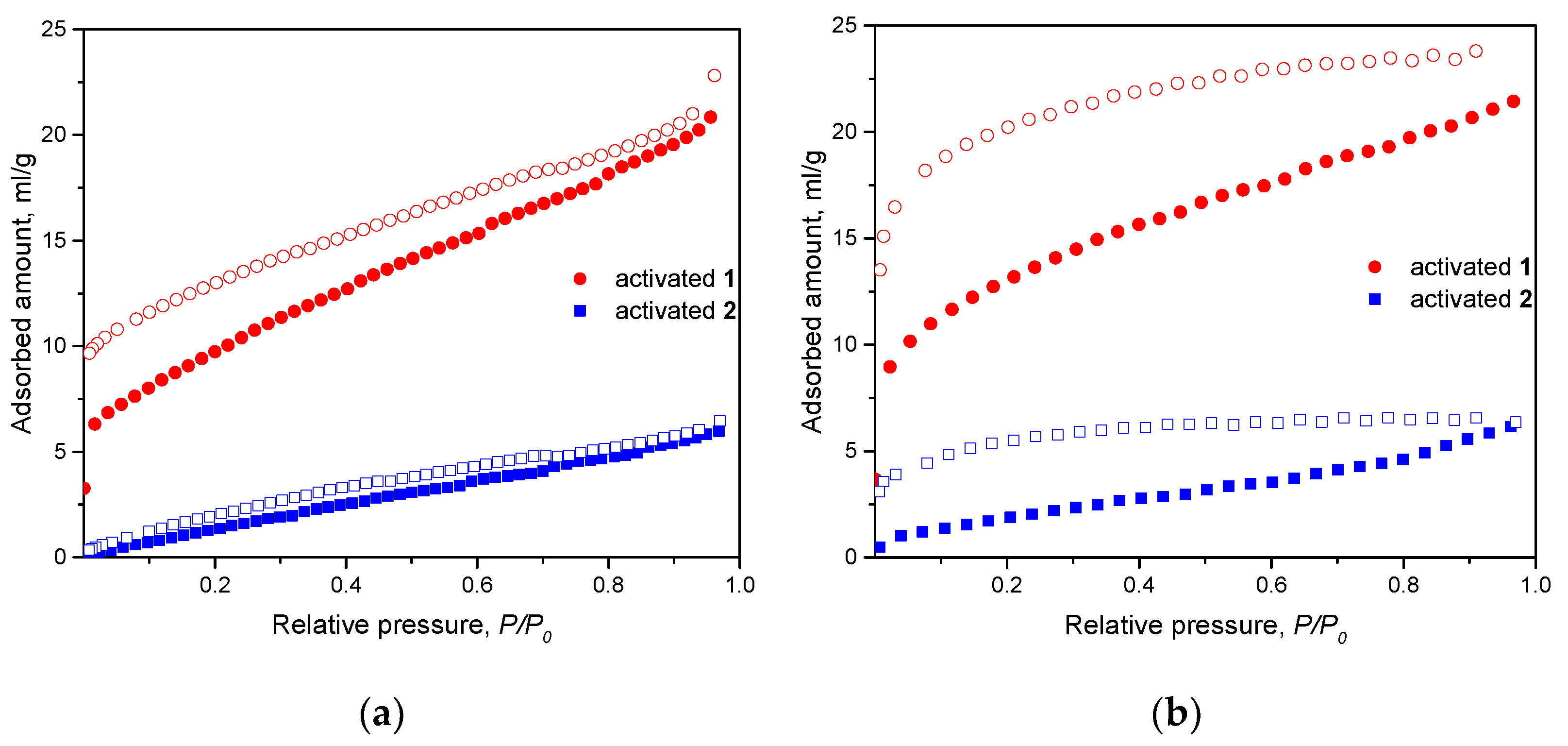
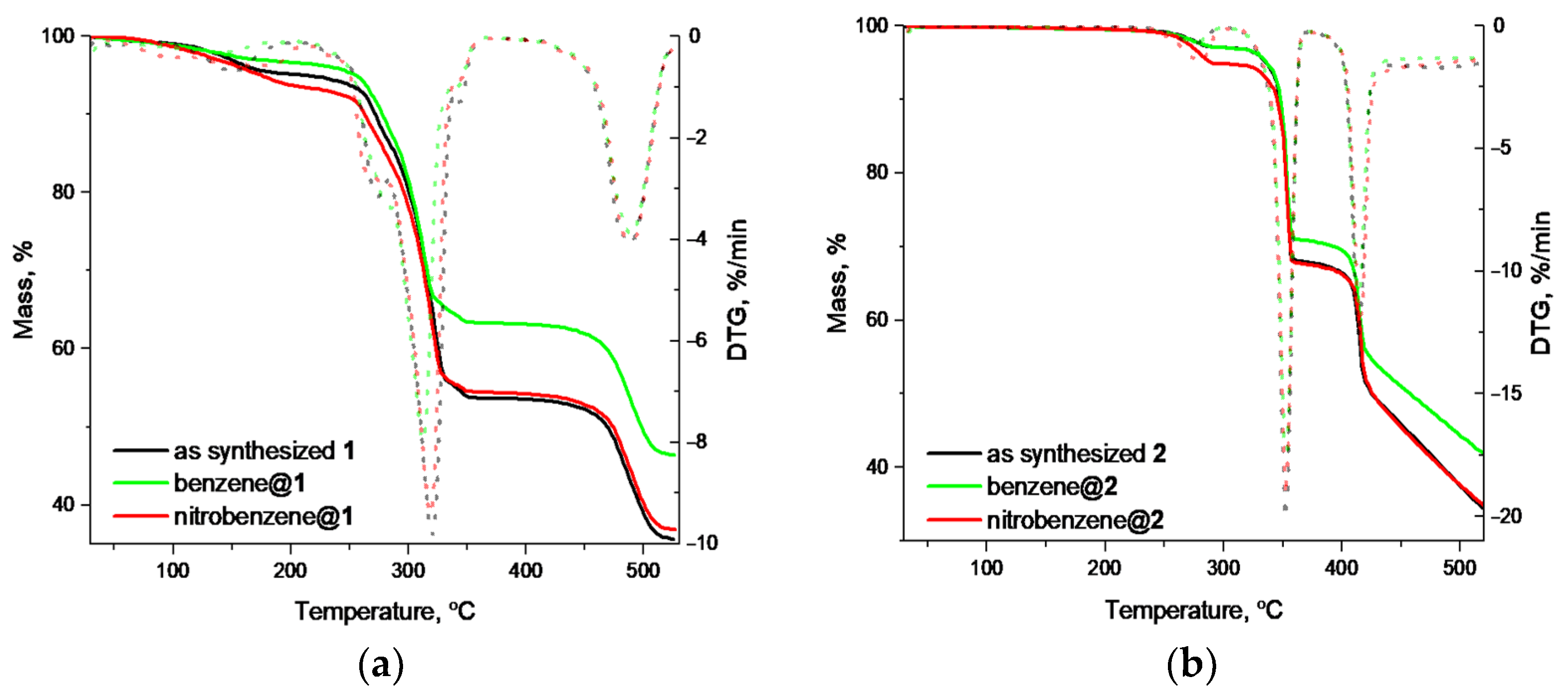
2. Results
Synthesis and Crystal Structures
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. Synthesis of [H2N(CH3)2]2[Li2Zn2(bdc)4]·CH3CN·DMF (1)
4.1.2. Synthesis of [Li2Zn2(H2Br2-bdc)(Br2-bdc)3]·2DMF (2)
4.1.3. Synthesis of [H2N(CH3)2][LiZn2(ndc)3]·CH3CN (3)
4.1.4. Synthesis of [{Li2Zn2(dmf)(py)2}{LiZn(dmf)2}2(NO2-bdc)6]·5DMF (4)
4.2. X-Ray Crystallography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal–organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wang, Z.; Qin, L.; Fu, Y.; Li, B.; Zhang, M.; Liu, S.; Li, L.; Yi, H.; Zhou, X.; et al. Metal–organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev. 2021, 427, 213565. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, J. Designer Metal–Organic Frameworks for Size-Exclusion-Based Hydrocarbon Separations: Progress and Challenges. Adv. Mater. 2020, 32, 2002603. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, E.; Peng, Y.-L.; Chen, Y.; Cheng, P.; Zhang, Z. Rational design and synthesis of ultramicroporous metal–organic frameworks for gas separation. Coord. Chem. Rev. 2020, 423, 213485. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kovalenko, K.A.; Samsonenko, D.G.; Barsukova, M.O.; Dybtsev, D.N.; Fedin, V.P. Exceptionally effective benzene/cyclohexane separation using a nitro-decorated metal–organic framework. Chem. Commun. 2020, 59, 8241–8244. [Google Scholar] [CrossRef] [PubMed]
- Alzamly, A.; Bakiro, M.; Ahmed, S.H.; Alnaqbi, M.A.; Nguen, H.L. Rare-earth metal–organic frameworks as advanced catalytic platforms for organic synthesis. Coord. Chem. Rev. 2020, 425, 213543. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D. Lanthanide-functionalized metal–organic frameworks as ratiometric luminescent sensors. J. Mater. Chem. C 2020, 8, 12739–12754. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Liu, X.; Li, B.; Zhang, C.; Qin, L.; Huang, D.; Yi, H.; Zhang, M.; Li, L.; et al. Metal–organic frameworks and their derivatives as signal amplification elements for electrochemical sensing. Coord. Chem. Rev. 2020, 424, 213520. [Google Scholar] [CrossRef]
- Haldar, R.; Bhattacharyya, S.; Maji, T.K. Luminescent metal–organic frameworks and their potential applications. J. Chem. Sci. 2020, 132, 99. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Su, Z.; Zhang, X.; Zhang, W.; Srinivas, K. Organic carboxylate-based MOFs and derivatives for electrocatalytic water oxidation. Coord. Chem. Rev. 2021, 428, 213619. [Google Scholar] [CrossRef]
- Gong, W.; Liu, Y.; Li, H.; Cui, Y. Metal–organic frameworks as solid Brønsted acid catalysts for advanced organic transformations. Coord. Chem. Rev. 2020, 420, 213400. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-W.; Han, P.-C.; Chuang, C.-H.; Wu, K.C.-W. Electronically conductive metal–organic framework-based materials. APL Mater. 2019, 7, 110902. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Wang, Z.-L.; Kim, J.; Yamauchi, Y. Hollow Functional Materials Derived from Metal–Organic Frameworks: Synthetic Strategies, Conversion Mechanisms, and Electrochemical Applications. Adv. Mater. 2019, 31, 1804903. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Kung, C.-W. Metal–Organic Frameworks toward Electrochemical Sensors: Challenges and Opportunities. Electroanalysis 2020, 32, 1885. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Pombeiro, A.J.L. Recent advances on supramolecular isomerism in metal organic frameworks. CrystEngComm 2017, 19, 4666–4695. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Fedin, V.P. Main Approaches to the Synthesis of Heterometallic Metal–Organic Frameworks. Russ. J. Coord. Chem. 2020, 46, 443–457. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Sapianik, A.A.; Fedin, V.P. Pre-synthesized secondary building units in the rational synthesis of porous coordination polymers. Mendeleev Commun. 2017, 27, 321–331. [Google Scholar] [CrossRef]
- Fei, H.; Cahill, J.F.; Prather, K.A.; Cohen, S.M. Tandem Postsynthetic Metal Ion and Ligand Exchange in Zeolitic Imidazolate Frameworks. Inorg. Chem. 2013, 52, 4011–4016. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 30, 2000238. [Google Scholar] [CrossRef]
- Hao, J.-N.; Xu, X.-Y.; Lian, X.; Zhang, C.; Yan, B. A Luminescent 3d-4f-4d MOF Nanoprobe as a Diagnosis Platform for Human Occupational Exposure to Vinyl Chloride Carcinogen. Inorg. Chem. 2017, 56, 11176–11183. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Yan, B. Ag+-induced photoluminescence enhancement in lanthanide post-functionalized MOFs and Ag+ sensing. Phys. Chem. Chem. Phys. 2017, 19, 9174–9180. [Google Scholar] [CrossRef] [PubMed]
- Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabien, A.; Pérez-Yáñez, S. Synthesis of heterometallic metal–organic frameworks and their performance as electrocatalyst for CO2 reduction. RSC Adv. 2018, 8, 21092–21099. [Google Scholar] [CrossRef]
- Kim, M.; Cahill, J.F.; Fei, H.; Prather, K.A.; Cohen, S.M. Postsynthetic Ligand and Cation Exchange in Robust Metal–Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 18082–18088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, N.; Gao, M.; Deng, C.-H. Magnetic Binary Metal–Organic Framework as a Novel Affinity Probe for Highly Selective Capture of Endogenous Phosphopeptides. ACS Sustain. Chem. Eng. 2018, 6, 4382–4389. [Google Scholar] [CrossRef]
- Cui, P.; Wang, P.; Zhao, Y.; Sun, W.-Y. Fabrication of Desired Metal–Organic Frameworks via Postsynthetic Exchange and Sequential Linker Installation. Cryst. Growth Des. 2019, 19, 1454–1470. [Google Scholar] [CrossRef]
- Du, J.-J.; Zhang, X.; Zhou, X.-P.; Li, D. Robust heterometallic MOF catalysts for the cyanosilylation of aldehydes. Inorg. Chem. Front. 2018, 5, 2772–2776. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Cheng, X.; Guo, X.; Tang, L.; Yang, H.; Wu, H.; Pan, F.; Zhang, P.; Cao, X.; et al. Bimetallic metal–organic frameworks nanocages as multi-functional fillers for water-selective membranes. J. Mem. Sci. 2018, 545, 19–28. [Google Scholar] [CrossRef]
- Rubio-Giménez, V.; Waerenborgh, J.C.; Clemente-Juan, J.M.; Martí-Gastaldo, C. Spontaneous Magnetization in Heterometallic NiFe-MOF-74 Microporous Magnets by Controlled Iron Doping. Chem. Mater. 2017, 29, 6181–6185. [Google Scholar] [CrossRef]
- Han, Y.; Zheng, H.; Liu, K.; Wang, H.; Huang, H.; Xie, L.-H.; Wang, L.; Li, J.-R. In-Situ Ligand Formation-Driven Preparation of a Heterometallic Metal–Organic Framework for Highly Selective Separation of Light Hydrocarbons and Efficient Mercury Adsorption. ACS Appl. Mater. Interfaces 2016, 8, 23331–23337. [Google Scholar] [CrossRef]
- Hu, H.-C.; Kang, X.-M.; Cao, C.-S.; Cheng, P.; Zhao, B. First tetrazole-bridged d–f heterometallic MOFs with a large magnetic entropy change. Chem. Commun. 2015, 51, 10850–10853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, D.-D.; Wu, Y.-P.; Zhao, J.; Wu, T.; Zhang, J.; Li, D.-S.; Sun, C.; Feng, P.; Bu, X. Stable Hierarchical Bimetal–Organic Nanostructures as HighPerformance Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 4227–4237. [Google Scholar] [CrossRef] [PubMed]
- Bratsos, I.; Tampaxis, C.; Spanopoulos, I.; Demitri, N.; Charalambopoulou, G.; Vourloumis, D.; Steriotis, T.A.; Trikalitis, P.N. Heterometallic In(III)–Pd(II) Porous Metal–Organic Framework with Square-Octahedron Topology Displaying High CO2 Uptake and Selectivity toward CH4 and N2. Inorg. Chem. 2018, 57, 7244–7251. [Google Scholar] [CrossRef]
- Li, C.; Tang, H.; Fang, Y.; Xiao, Z.; Wang, K.; Wu, X.; Niu, H.; Zhu, C.; Zhou, H. Bottom-Up Assembly of a Highly Efficient Metal–Organic Framework for Cooperative Catalysis. Inorg. Chem. 2018, 57, 13912–13919. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, P.F.; Liu, C.; Miller, C.C.; Koby, S.B.; Jarvi, A.G.; Luo, T.-Y.; Saxena, S.; O’Keeffe, M.O.; Rossi, N.L. Programmable Topology in New Families of Heterobimetallic Metal–Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 6194–6198. [Google Scholar] [CrossRef] [PubMed]
- Sapianik, A.A.; Lutsenko, I.A.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Heterometallic molecular complex [Co2Gd(NO3)(piv)6(py)2] and coordination polymer [{CoGd(dma)2}2(bdc)5]·4DMA: The synthesis, structure, and properties. Russ. Chem. Bull. 2016, 65, 2601–2606. [Google Scholar] [CrossRef]
- Lytvynenko, A.S.; Kolotilov, S.V.; Kiskin, M.A.; Cador, O.; Golhen, S.; Aleksandrov, G.G.; Mishura, A.M.; Titov, V.E.; Ouahab, L.; Eremenko, I.L.; et al. Redox-Active Porous Coordination Polymers Prepared by Trinuclear Heterometallic Pivalate Linking with the Redox-Active Nickel(II) Complex: Synthesis, Structure, Magnetic and Redox Properties, and Electrocatalytic Activity in Organic Compound Dehalogenation in Heterogeneous Medium. Inorg. Chem. 2018, 57, 4970–4979. [Google Scholar] [CrossRef]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic Coordination Polymers Assembled from Trigonal Trinuclear Fe2Ni-Pivalate Blocks and Polypyridine Spacers: Topological Diversity, Sorption, and Catalytic Properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef]
- Dorofeeva, V.N.; Kolotilov, S.V.; Kiskin, M.A.; Polunin, R.A.; Dobrokhotova, Z.V.; Cador, O.; Golhen, S.; Eremenko, I.L.; Novotortsev, V.M. 2D Porous Honeycomb Polymers versus Discrete Nanocubes from Trigonal Trinuclear Complexes and Ligands with Variable Topology. Chem. Eur. J. 2012, 18, 5006–5012. [Google Scholar] [CrossRef]
- Polunin, R.A.; Kolotilov, S.V.; Kiskin, M.A.; Cador, O.; Golhen, S.; Shvets, O.V.; Ouahab, L.; Dobrokhotova, Z.V.; Ovcharenko, V.I.; Eremenko, I.L.; et al. Structural Flexibility and Sorption Properties of 2D Porous Coordination Polymers Constructed from Trinuclear Heterometallic Pivalates and 4,4′-Bipyridine. Eur. J. Inorg. Chem. 2011, 2011, 4985–4992. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Zorina-Tikhonova, E.N.; Kiskin, M.A.; Samsonenko, D.G.; Kovalenko, K.A.; Sidorov, A.A.; Eremenko, I.L.; Dybtsev, D.N.; Blake, A.J.; Argent, S.P.; et al. Rational Synthesis and Investigation of Porous Metal–Organic Framework Materials from a Preorganized Heterometallic Carboxylate Building Block. Inorg. Chem. 2017, 56, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, G.P.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Muller-Buschbaum, K. Engineering Metal-based Luminescence in Coordination Polymers and Metal–Organic Frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- Han, L.-L.; Hu, T.-P.; Mei, K.; Guo, Z.-M.; Yin, C.; Wang, Y.X.; Zheng, J.; Wang, X.-P.; Sun, D. Solvent-controlled three families of Zn(II) coordination compounds: Synthesis, crystal structure, solvent-induced structural transformation, supramolecular isomerism and photoluminescence. Dalton Trans. 2015, 44, 6052–6061. [Google Scholar] [CrossRef] [PubMed]
- Goldsworthy, J.S.; Staples, R.J.; LaDuca, R.L. Luminescent zinc terephthalate coordination polymers with pyridylnicotinamide ligands: Effect of added base and nitrogen donor disposition on topology. J. Molec. Struct. 2014, 1062, 116–124. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kiskin, M.A.; Samsonenko, D.G.; Ryadun, A.A.; Dybtsev, D.N.; Fedin, V.P. Luminescent detection by coordination polymers derived from a pre-organized heterometallic carboxylic building unit. Polyhedron 2018, 145, 145–153. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kiskin, M.A.; Kovalenko, K.A.; Samsonenko, D.G.; Dybtsev, D.N.; Audebrand, N.; Sun, Y.; Fedin, V.P. Rational synthesis and dimensionality tuning of MOFs from preorganized heterometallic molecular complexes. Dalton Trans. 2019, 48, 3676–3686. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Semenenko, E.E.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Crystal Structure of Coordination Polymers Based on A Heterometallic Carboxylate Complex. J. Struct. Chem. 2018, 59, 487–493. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Smirnov, K.D.; Barsukova, M.O.; Samsonenko, D.G.; Fedin, V.P. Crystal Structures of Compounds Obtained in Reactions of Heterometallic Pivalate Complexes with Dicarboxylic Acids. J. Struct. Chem. 2019, 60, 609–616. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Dudko, E.R.; Samsonenko, D.G.; Fedin, V.P. Crystal Structures of Metal–Organic frameworks obtained from a heterometallic pivalate complex. J. Struct. Chem. 2020, 61, 2064–2071. [Google Scholar]
- CrysAlisPro 1.171.40.84a. Rigaku Oxford Diffraction; Rigaku Corporation: The Woodlands, TX, USA, 2020. [Google Scholar]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]





| Identification Code | 1 | 2DMA | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C41H42Li2N4O17Zn2 | C40H28Br8Li2N2O18Zn2 | C40H29LiN2O12Zn2 | C88H98Li4N18O46Zn4 |
| M, g·mol−1 | 1007.40 | 1608.54 | 867.33 | 2433.08 |
| T, K | 150(2) | 100(2) | 150(2) | 150(2) |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Triclinic |
| Space group | P21/n | P–1 | C2/c | P–1 |
| a, Å | 9.9681(3) | 9.675(5) | 15.6794(4) | 13.4894(6) |
| b, Å | 15.9931(6) | 12.346(3) | 13.2596(4) | 14.8036(6) |
| c, Å | 16.2939(6) | 12.523(2) | 19.9294(6) | 17.0021(6) |
| α, deg | 90 | 80.897(16) | 90 | 64.769(4) |
| β, deg | 92.160(3) | 71.847(16) | 104.551(3) | 73.282(4) |
| γ, deg | 90 | 70.278(16) | 90 | 86.538(4) |
| V, Å3 | 2595.74(16) | 1335.7(8) | 4010.5(2) | 2933.9(2) |
| Z | 2 | 1 | 4 | 1 |
| D(calcd), g·cm−3 | 1.289 | 2.000 | 1.436 | 1.377 |
| μ, mm−1 | 0.990 | 9.131 | 1.260 | 0.899 |
| F(000) | 1036 | 772 | 1768 | 1252 |
| Crystal size, mm | 0.29 × 0.21 × 0.18 | 0.10 × 0.10 × 0.05 | 0.32 × 0.20 × 0.09 | 0.34 × 0.30 × 0.05 |
| θ range for data collection, deg | 2.43–28.51 | 1.9–31.0 | 2.68–28.74 | 2.37–28.35 |
| Index ranges | −10 ≤ h ≤ 12, −20 ≤ k ≤ 16, −20 ≤ l ≤ 20 | −12 ≤ h ≤ 12, −15 ≤ k ≤ 15, −16 ≤ l ≤ 16 | −14 ≤ h ≤ 19, −16 ≤ k ≤ 13, −20 ≤ l ≤ 26 | −17 ≤ h ≤ 18, −13 ≤ k ≤ 18, −19 ≤ l ≤ 22 |
| Reflections collected/indep. | 12,793/5802 | 15,935/5956 | 10,384/4510 | 26,115/12,851 |
| Rint | 0.0303 | 0.0585 | 0.0120 | 0.0330 |
| Reflections with I > 2σ(I) | 4603 | 5265 | 4127 | 9028 |
| GOF on F2 | 1.093 | 1.063 | 1.087 | 1.067 |
| Final R indices [I > 2σ(I)] | R1 = 0.0536, wR2 = 0.0713 | R1 = 0.0624, wR2 = 0.0683 | R1 = 0.0493, wR2 = 0.0530 | R1 = 0.0633, wR2 = 0.0902 |
| R indices (all data) | R1 = 0.1592, wR2 = 0.1717 | R1 = 0.1663, wR2 = 0.1691 | R1 = 0.1358, wR2 = 0.1383 | R1 = 0.1922, wR2 = 0.2064 |
| Largest diff. peak/hole, e·Å−3 | 0.916/−0.697 | 1.288/−1.267 | 1.441/−0.902 | 0.900/−0.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsukova, M.; Dudko, E.; Samsonenko, D.; Kovalenko, K.; Ryadun, A.; Sapianik, A.; Fedin, V. Influence of Substituents in Terephthalate Linker on the Structure of MOFs Obtained from Presynthesized Heterometallic Complex. Inorganics 2021, 9, 4. https://doi.org/10.3390/inorganics9010004
Barsukova M, Dudko E, Samsonenko D, Kovalenko K, Ryadun A, Sapianik A, Fedin V. Influence of Substituents in Terephthalate Linker on the Structure of MOFs Obtained from Presynthesized Heterometallic Complex. Inorganics. 2021; 9(1):4. https://doi.org/10.3390/inorganics9010004
Chicago/Turabian StyleBarsukova, Marina, Evgeny Dudko, Denis Samsonenko, Konstantin Kovalenko, Alexey Ryadun, Aleksandr Sapianik, and Vladimir Fedin. 2021. "Influence of Substituents in Terephthalate Linker on the Structure of MOFs Obtained from Presynthesized Heterometallic Complex" Inorganics 9, no. 1: 4. https://doi.org/10.3390/inorganics9010004
APA StyleBarsukova, M., Dudko, E., Samsonenko, D., Kovalenko, K., Ryadun, A., Sapianik, A., & Fedin, V. (2021). Influence of Substituents in Terephthalate Linker on the Structure of MOFs Obtained from Presynthesized Heterometallic Complex. Inorganics, 9(1), 4. https://doi.org/10.3390/inorganics9010004