Study on Physicochemical and Thermal Properties of Tetrabutylammonium-Based Cation Ionic Salts Induced by Al2O3 Additive for Thermal Energy Storage Application
Abstract
1. Introduction
2. Results and Discussion
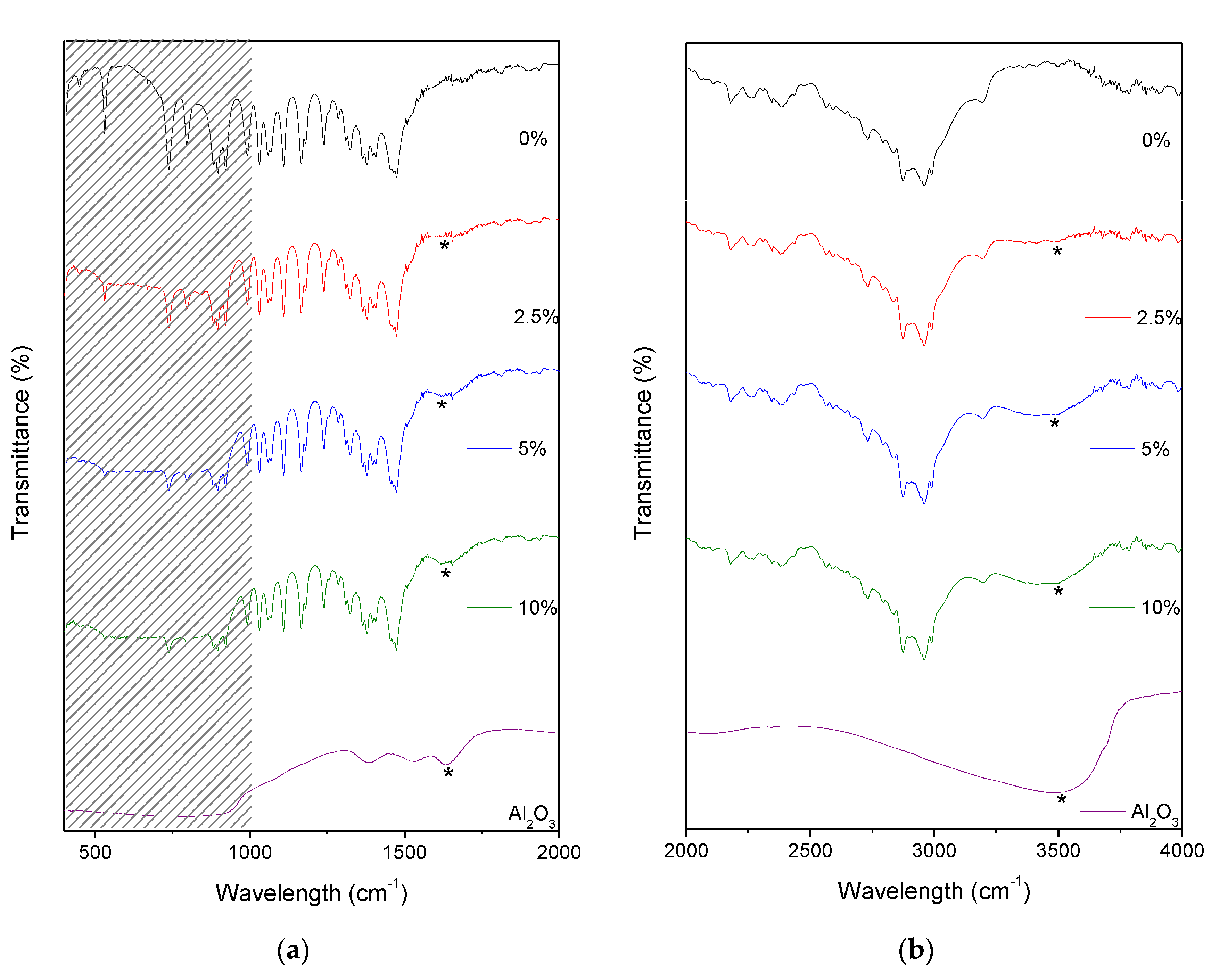
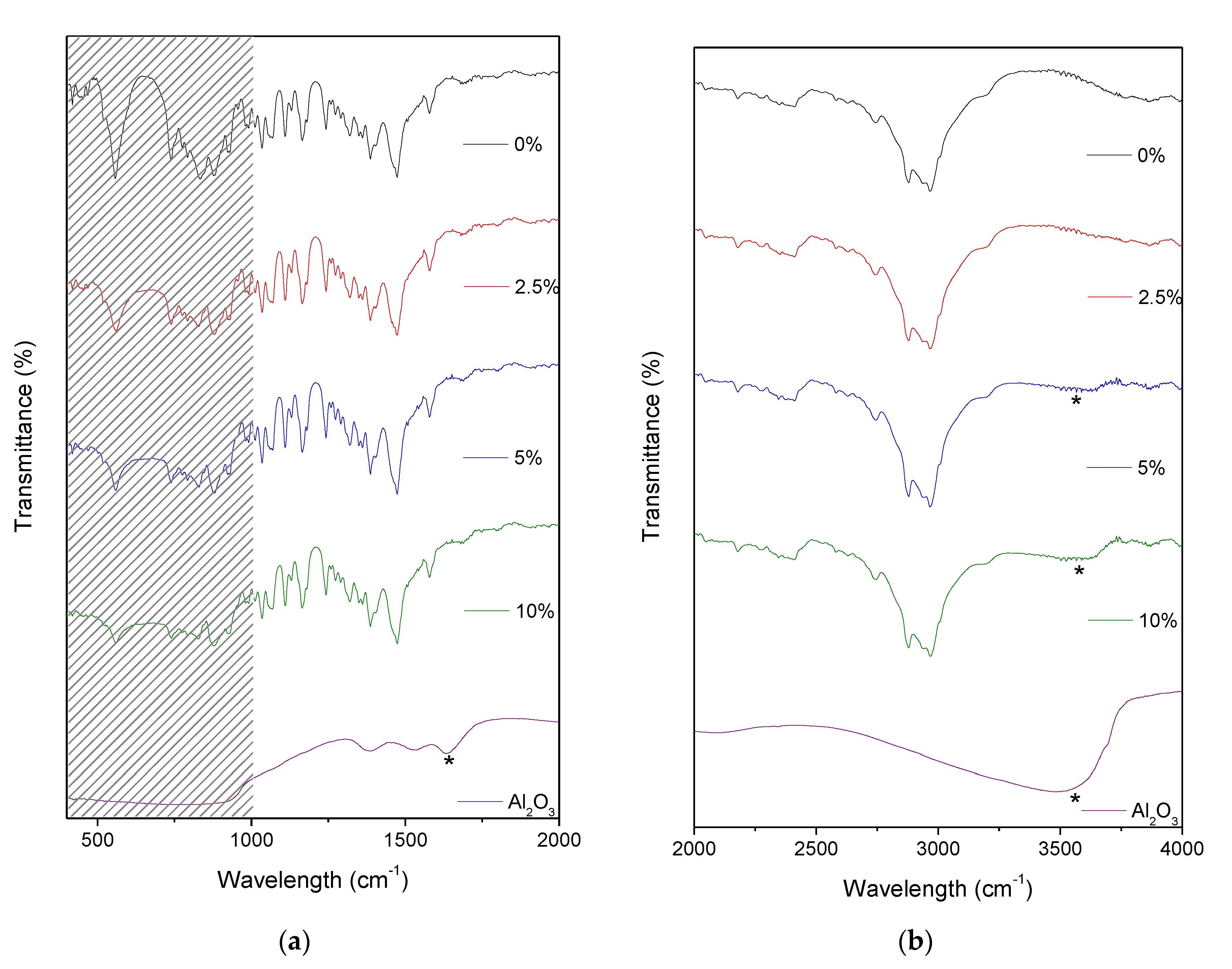
2.1. IR Spectra
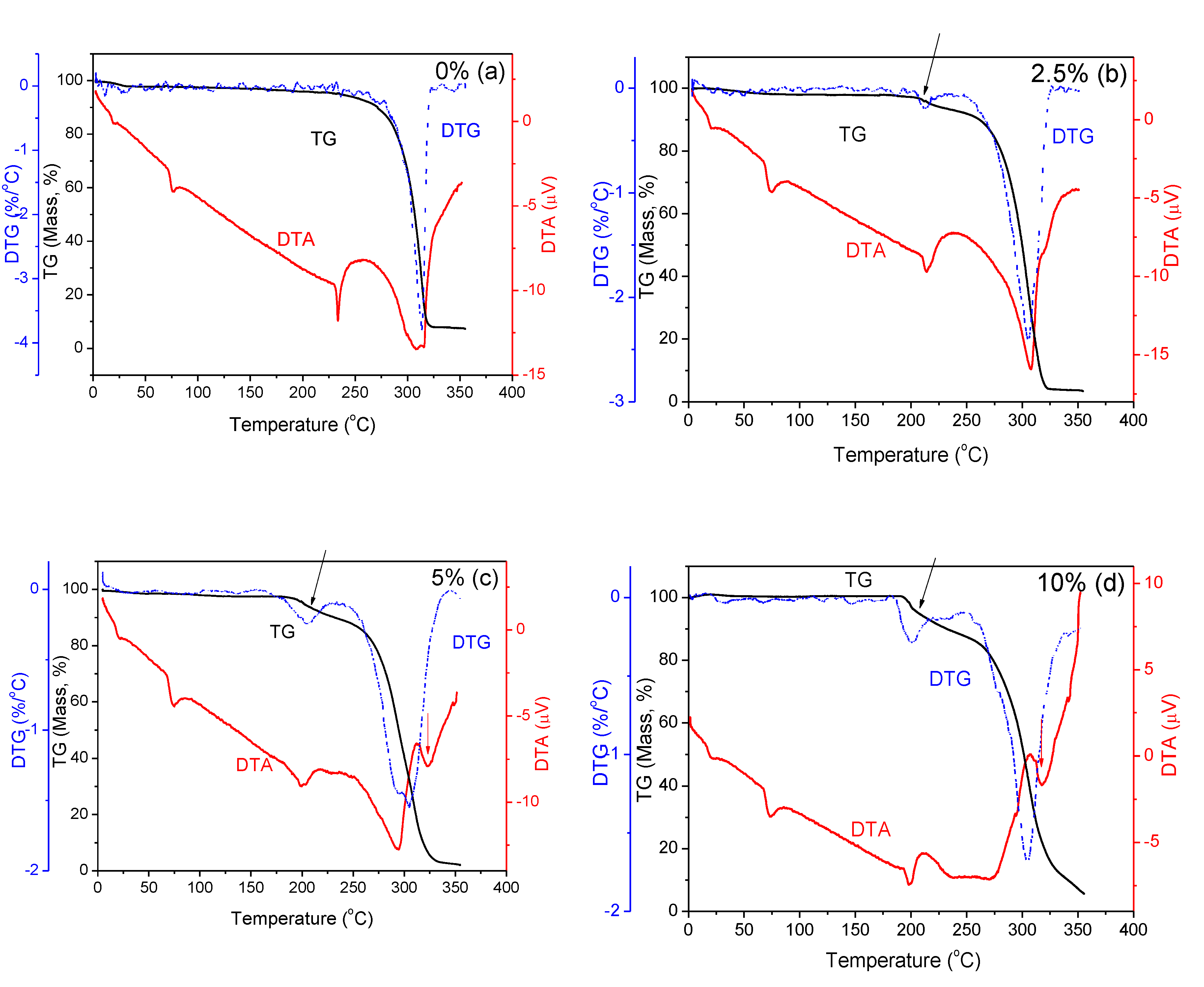
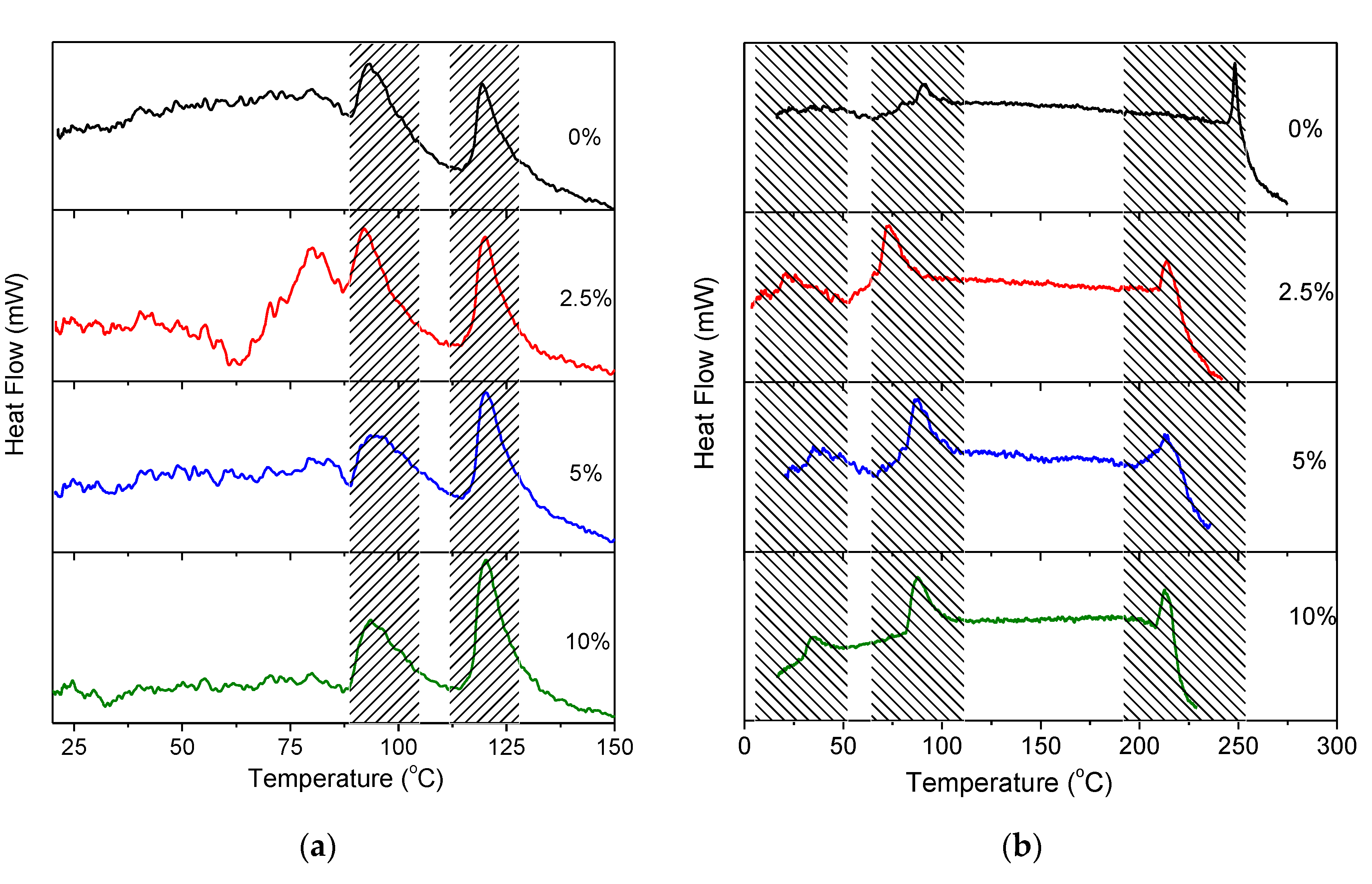
2.2. Thermal Properties
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rooney, D.; Jacquemin, J.; Gardas, R. Thermophysical Properties of Ionic Liquids. In Ionic Liquids; Kirchner, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 185–212. [Google Scholar]
- MacFarlane, D.R.; Kar, M.; Pringle, J.M. Fundamentals of Ionic Liquids, From Chemistry to Applications; Wiley: Weinheim, Germany, 2017. [Google Scholar]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Progress and Prospects. In Green Solvents II Properties and Application of Ionic Liquids; Mohammad, A., Inamuddin, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Wu, B.; Reddy, R.G.; Rogers, R.D. Novel ionic liquid thermal storage for solar thermal electric power systems In Proceedings, Solar Forum 2001 Solar Energy; The Power to Choose: Washington, DC, USA, 2001; pp. 445–451. [Google Scholar]
- Valkenburg, M.E.V.; Vaughn, R.L.; Williams, M.; Wilkes, J.S. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim. Acta 2005, 425, 181–188. [Google Scholar] [CrossRef]
- Masayoshi, W.; Morgan, L.T.; Shiguo, Z.; Kazuhide, U.; Tomohiro, Y.; Kaoru, D. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Fan, L.; Kang, S.; Wu, J.; Hao, S.; Lan, Z.; Lin, J. Quasi-Solid State Dye-sensitized Solar Cells Based on Polyvinylpyrrolidone With Ionic Liquid. Energy Sources A 2010, 32, 1559–1568. [Google Scholar] [CrossRef]
- Devaki, S.J.; Sasi, R. Ionic Liquids/Ionic Liquid Crystals for Safe and Sustainable Energy Storage Systems. In Progress and Developments in Ionic Liquids; Handy, S., Ed.; Intech Europe: Rijeka, Croatia, 2017; pp. 313–336. [Google Scholar] [CrossRef]
- Burns, J.A.; Verrall, R.E. Thermodynamics of tetraalkyl- and bis-tetraalkylammonium bromides: II. Heat capacities of solid state from 273 to 373 K. Thermochim. Acta 1974, 9, 277–288. [Google Scholar] [CrossRef]
- Zhu, J.; Bai, L.; Chen, B.; Fei, W. Thermodynamical properties of phase change materials based on ionic liquids. Chem. Eng. J. 2009, 147, 58–62. [Google Scholar] [CrossRef]
- Zhang, Z.; Salih, A.A.M.; Li, M.; Yang, B. Synthesis and Characterization of Functionalized Ionic Liquids for Thermal Storage. Energy Fuels 2014, 28, 2802–2810. [Google Scholar] [CrossRef]
- Fukushima, T.; Aida, T. Ionic liquids for soft functional materials with carbon nanotubes. Chem. Eur. J. 2007, 13, 5048–5058. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Lourenço, M.J.V.; Ribeiro, A.P.C.; Langa, E.; Vieira, S.I.C.; Goodrich, P.; Hardacre, C. Thermal properties of ionic liquids and ionanofluids of imidazolium and pyrrolidinium liquids. J. Chem. Eng. Data 2010, 55, 653–661. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Vieira, S.I.C.; França, J.M.; Queirós, C.S.; Langa, E.; Lourenço, M.J.V.; Murshed, S.M.S.; Nieto de Castro, C.A. Thermal Properties of Ionic Liquids and Ionanofluids. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; Intech Europe: Rijeka, Croatia, 2011; pp. 37–60. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Nieto de Castro, C.A.; Lourenço, M.J.V.; França, J.; Ribeiro, A.P.C.; Vieira, S.I.C.; Queirós, C.S. Ionanofluids as Novel Fluids for Advanced Heat Transfer Applications, World Academy of Science. Int. J. Math. Comput. Phys. Electr. Comput. Eng. 2011, 5, 795–798. [Google Scholar]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Rheological and tribological properties of ionic liquid-based nanofluids containing functionalized multi-walled carbon nanotubes. J. Phys. Chem. C 2010, 114, 8749–8754. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lou, W.; Hao, J. Ionic liquid-based stable nanofluids containing gold nanoparticles. J. Colloid Interface Sci. 2011, 362, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Jwo, C.S.; Change, H.; Teng, T.P.; Kao, M.J.; Guo, Y.T. A study on the effects of temperature and volume fraction on thermal conductivity of copper oxide nanofluid. J. Nanosci. Nanotechnol. 2007, 7, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.J.; Guo, L.J. Ionic current rectification, breakdown, and switching in heterogeneous oxide nanofluidic devices. ACS Nano 2009, 3, 575–584. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.J.; Chang, Y.C.; Huang, D.Y. A new approach of synthesis of Al2O3 nanofluid. In Proceedings of the 3rd Workshop on Metastable and Nanostructured Materials, NANOMAT, Rio de Janeiro, Brazil, 5–8 June 2006; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2008; pp. 155–161. [Google Scholar]
- Titan, C.P.; Morshed, A.K.M.M.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Enhanced Thermal Performance of Ionic Liquid-Al2O3 Nanofluid as Heat Transfer Fluid for Solar Collector. In Proceedings of the ASME 7th International Conference on Energy Sustainability, ES-FuelCell2013, Minneapolis, MN, USA, 14–19 July 2013. [Google Scholar]
- Zhang, L.; Liu, J.; He, G.; Ye, Z.; Fang, X.; Zhang, Z. Radiative properties of ionic liquid-based nanofluids for medium-to-high-temperature direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2014, 130, 521–528. [Google Scholar] [CrossRef]
- Bridges, N.J.; Visser, A.E.; Fox, E.B. Potential of Nanoparticle-Enhanced Ionic Liquids (NEILs) as Advanced Heat-Transfer Fluids. Energy Fuels 2011, 25, 4862–4864. [Google Scholar] [CrossRef]
- Bai, L.; Li, X.; Zhu, J.; Chen, B. Effects of Nucleators on the Thermodynamic Properties of Seasonal Energy Storage Materials Based on Ionic Liquids. Energy Fuels 2011, 25, 1811–1816. [Google Scholar] [CrossRef]
- Mineaa, A.A.; Murshed, S.S. A review on development of ionic liquid based nanofluids and their heat transfer behaviour. Renew. Sustain. Energy Rev. 2018, 91, 584–599. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Gohil, K. Performance evaluation of solar cooker using some [N+4444] based ionic liquids as thermal energy storage materials. Adv. Mater. Lett. 2013, 4, 277–282. [Google Scholar] [CrossRef]
- Fifield, F.W.; Kealey, D. Principles and Practice of Analytical Chemistry, 5th ed.; Blackwell Science Ltd.: Cambridge, UK, 2000; ISBN 0-632-05384-4. [Google Scholar]
- Wang, Q.; Habenschuss, A.; Xenopoulos, A.; Wunderlich, B. Mesophases of Alkylammonium Salts. VI. The Crystal Structures of Tetra-nbutylammonium Bromide and Iodide. Mol. Cryst. Liq. Cryst. 1995, 264, 115–129. [Google Scholar] [CrossRef]
- Xenopoulos, A.; Cheng, J.; Yasuniwat, M.; Wunderlich, B. Mesophases of Alkylammonium Salts. I. First-Order Transitions. Mol. Cryst. Liq. Cryst. 1992, 214, 63–79. [Google Scholar] [CrossRef]
- Zhuravlev, O.E.; Nikol’skii, V.M.; Voronchikhina, L.I. Thermal Stability of Quaternary Ammonium Hexafluorophosphates and Halides. Russ. J. Appl. Chem. 2013, 86, 824–830. [Google Scholar] [CrossRef]
- Blundell, R.K.; Licence, P. Quaternary ammonium and phosphonium based ionic liquids: A comparison of common anions. Phys. Chem. Chem. Phys. 2014, 16, 15278–15288. [Google Scholar] [CrossRef]
- Lassègues, J.C.; Grondin, J.; Cavagnat, D.; Johansson, P. New Interpretation of the CH Stretching Vibrations in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2009, 113, 6419–6421. [Google Scholar] [CrossRef] [PubMed]
- Wulf, A.; Fumino, K.; Ludwig, R. Comment on “New Interpretation of the CH Stretching Vibrations in Imidazolium-Based Ionic Liquids”. J. Phys. Chem. A 2010, 114, 685–686. [Google Scholar] [CrossRef]
- Lassegues, J.C.; Grondin, J.; Cavagnat, D.; Johansson, P. Reply to the “Comment on ‘New Interpretation of the CH stretching Vibrations in Imidazolium-Based Ionic Liquids’”. J. Phys. Chem. A 2010, 114, 687–688. [Google Scholar] [CrossRef]
- Harmon, K.M.; De Santis, N.J.; Brandt, D.O. Hydrogen bonding Part 39. Hydrogen bonding by α-CH in quaternary ammonium salts and the possible role of CHB hydrogen bonds in acetylcholine—Receptor interactions. J. Mol. Struct. 1992, 265, 47–57. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley and Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Paschoal, V.H.; Faria, L.F.O.; Ribeiro, M.C.C. Vibrational Spectroscopy of Ionic Liquids. Chem. Rev. 2017, 117, 7053–7112. [Google Scholar] [CrossRef]
- Falk, M.; Ford, T.A. Infrared spectrum and structure of liquid water. Can. J. Chem. 1966, 44, 1699–1707. [Google Scholar] [CrossRef]
- Ates, M.; Demir, V.; Arslan, Z.; Daniels, J.; Farah, I.O.; Bogatu, C. Evaluation of Alpha and Gamma Aluminum Oxide Nanoparticle Accumulation, Toxicity and Depuration in Artemia Salina Larvae. Environ. Toxicol. 2015, 30, 109–118. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Synthesis and Dissolution of Metal Oxides in Ionic Liquids and Deep Eutectic Solvents. Molecules 2020, 25, 78. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Chen, L. Understanding of Effects of Nano-Al2O3 Particles on Ionic Conductivity of Composite Polymer Electrolytes. Electrochem. Solid St (ESL) 2003, 6, E40–E44. [Google Scholar] [CrossRef]
- Lü, R.; Lin, J.; Lu, Y.; Liu, D. The comparison of cation–anion interactions of phosphonium- and ammonium-based ionic liquids—A theoretical investigation. Chem. Phys. Lett. 2014, 597, 114–120. [Google Scholar] [CrossRef]
- Erdmenger, T.; Vitz, J.; Wiesbrock, F.; Schubert, U.S. Influence of different branched alkyl side chains on the properties of imidazolium-based ionic liquids. J. Mater. Chem. 2008, 18, 5267–5273. [Google Scholar] [CrossRef]
- Golding, J.; Forsyth, S.; MacFarlane, D.R.; Forsyth, M.; Deacon, G.B. Methanesulfonate and p-toluenesulfonate salts of the N-methyl-N-alkylpyrrolidinium and quaternary ammonium cations: Novel low cost ionic liquids. Green Chem. 2002, 4, 223–229. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
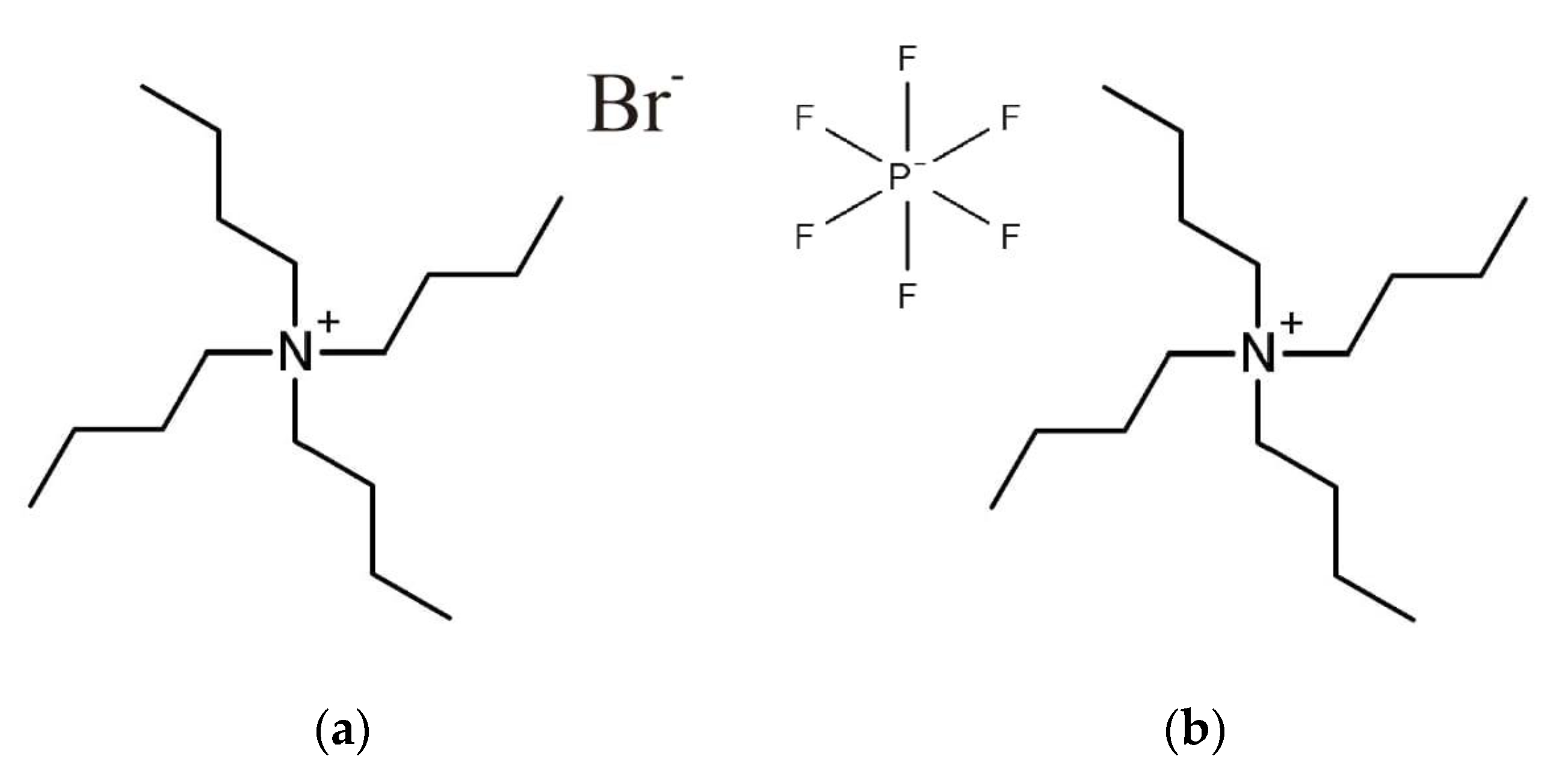

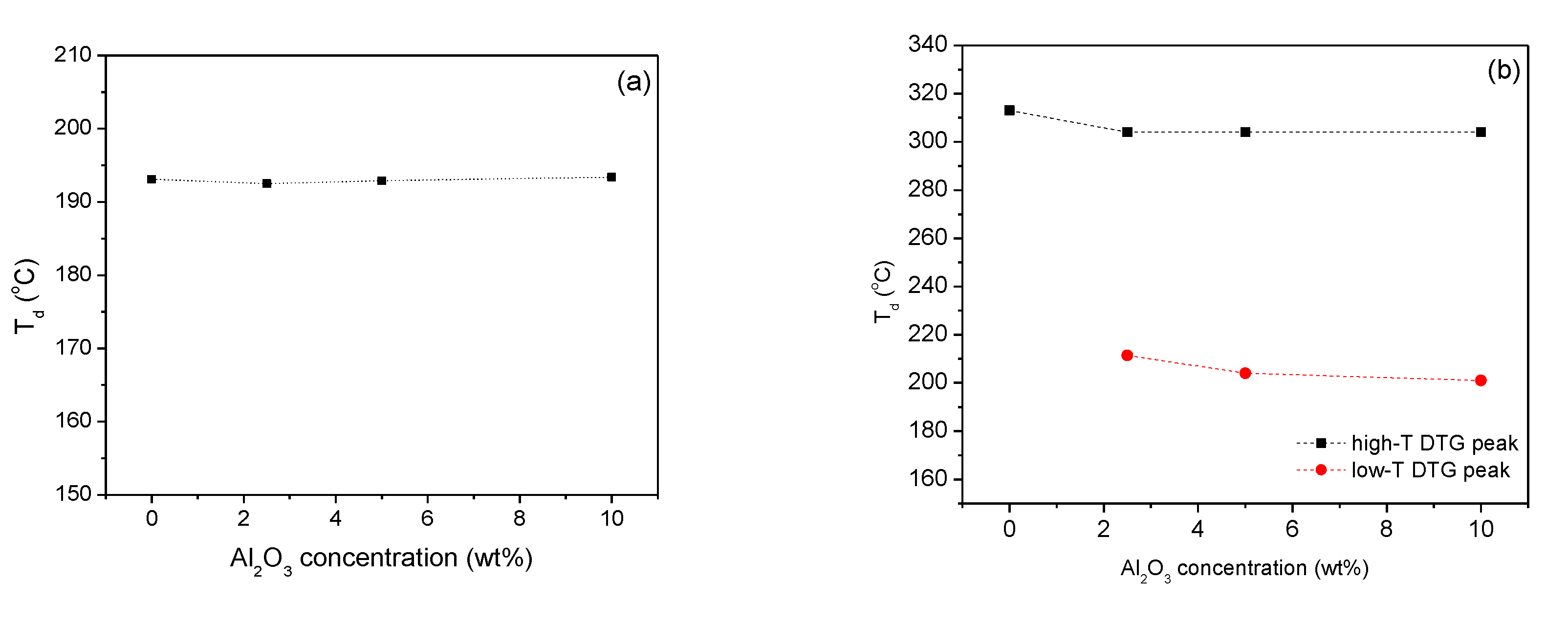
| Al2O3/(wt %) c | TBA-Br a + Al2O3 | TBA-PF6 b + Al2O3 | ||||||
| Phase Transition Temps. (°C) d | ∆Hm/ (kJ kg−1) f | Phase Transition Temps. (°C) d | ∆Hm/ (kJ kg−1) f | |||||
| 1st Peak e | 2nd Peak | 3rd Peak | 1st Peak | 2nd Peak | 3rd Peak | |||
| 0 | 58 | 93 | 119 | 45 | ~20 e | 91 | 248 | 35 |
| 2.5 | 66 | 92 | 120 | 67 | 21 | 73 | 214 | 22 |
| 5 | 67 | 94 | 120 | 43 | 35 | 87 | 213 | 68 |
| 10 | 58 | 93 | 120 | 44 | 34 | 88 | 213 | 27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutjahja, I.M.; Wonorahardjo, S.; Wonorahardjo, S. Study on Physicochemical and Thermal Properties of Tetrabutylammonium-Based Cation Ionic Salts Induced by Al2O3 Additive for Thermal Energy Storage Application. Inorganics 2020, 8, 51. https://doi.org/10.3390/inorganics8090051
Sutjahja IM, Wonorahardjo S, Wonorahardjo S. Study on Physicochemical and Thermal Properties of Tetrabutylammonium-Based Cation Ionic Salts Induced by Al2O3 Additive for Thermal Energy Storage Application. Inorganics. 2020; 8(9):51. https://doi.org/10.3390/inorganics8090051
Chicago/Turabian StyleSutjahja, Inge M., Surjani Wonorahardjo, and Surjamanto Wonorahardjo. 2020. "Study on Physicochemical and Thermal Properties of Tetrabutylammonium-Based Cation Ionic Salts Induced by Al2O3 Additive for Thermal Energy Storage Application" Inorganics 8, no. 9: 51. https://doi.org/10.3390/inorganics8090051
APA StyleSutjahja, I. M., Wonorahardjo, S., & Wonorahardjo, S. (2020). Study on Physicochemical and Thermal Properties of Tetrabutylammonium-Based Cation Ionic Salts Induced by Al2O3 Additive for Thermal Energy Storage Application. Inorganics, 8(9), 51. https://doi.org/10.3390/inorganics8090051






