Adsorption Properties of Ce5(BDC)7.5(DMF)4 MOF
Abstract
1. Introduction
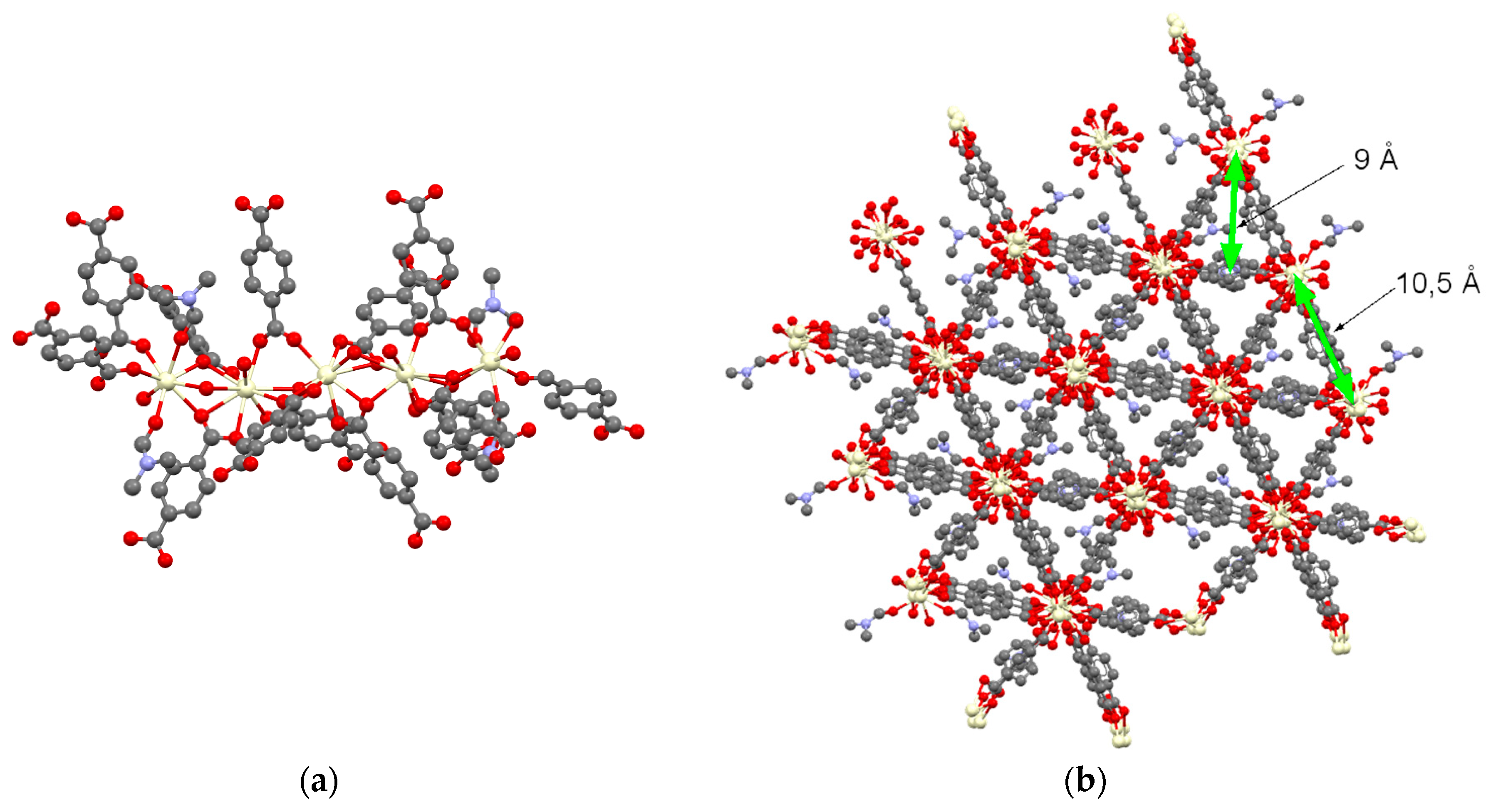
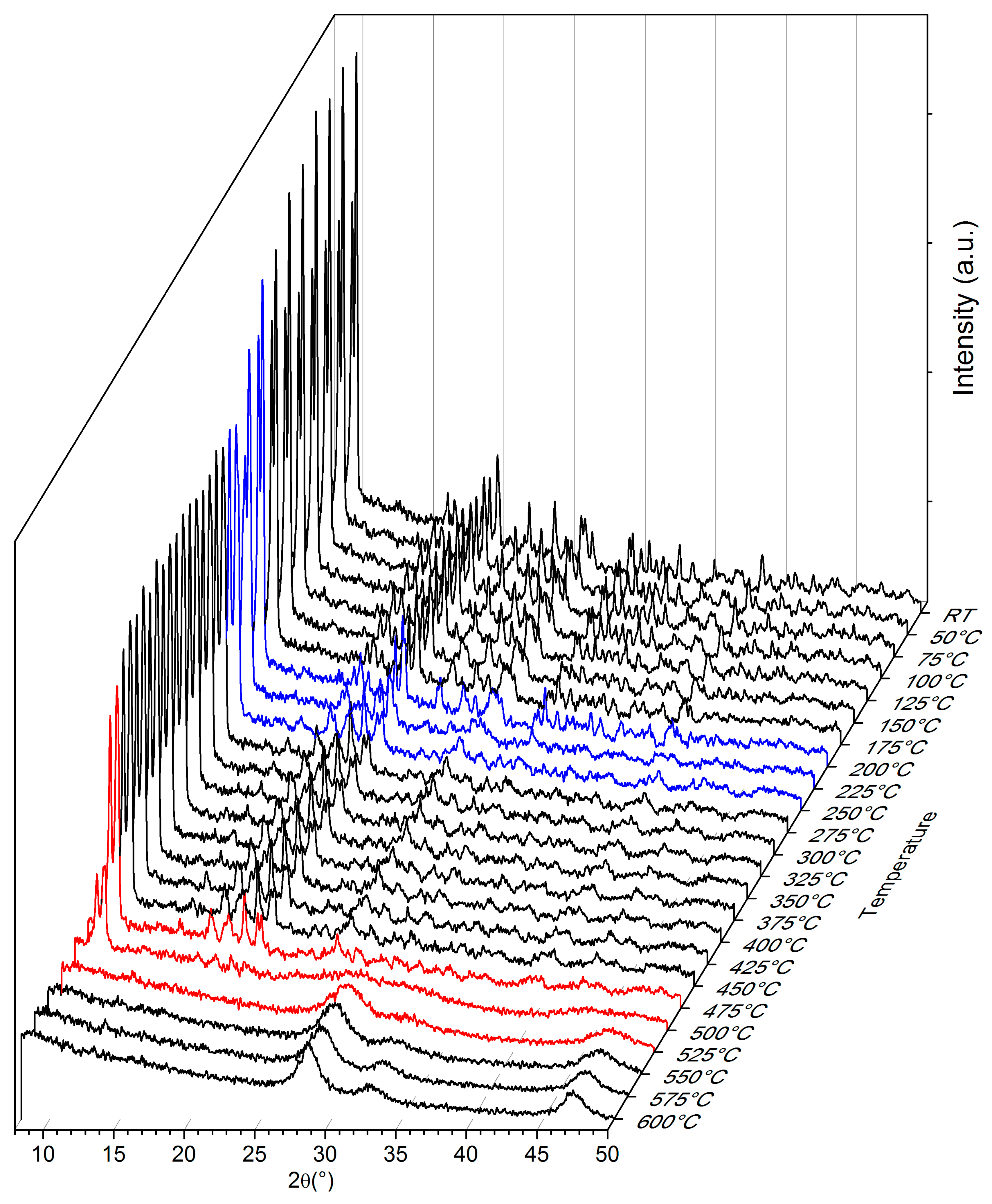
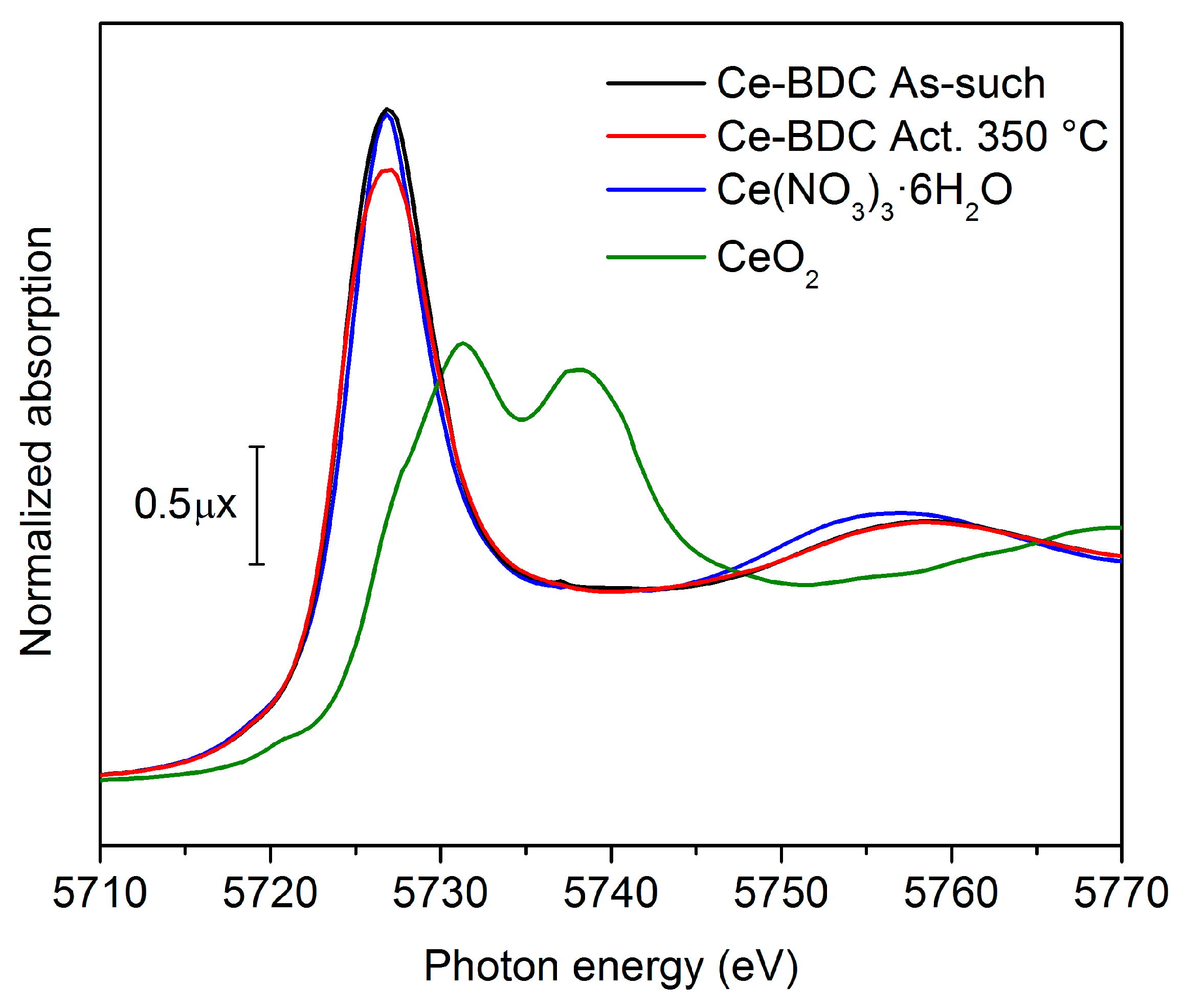
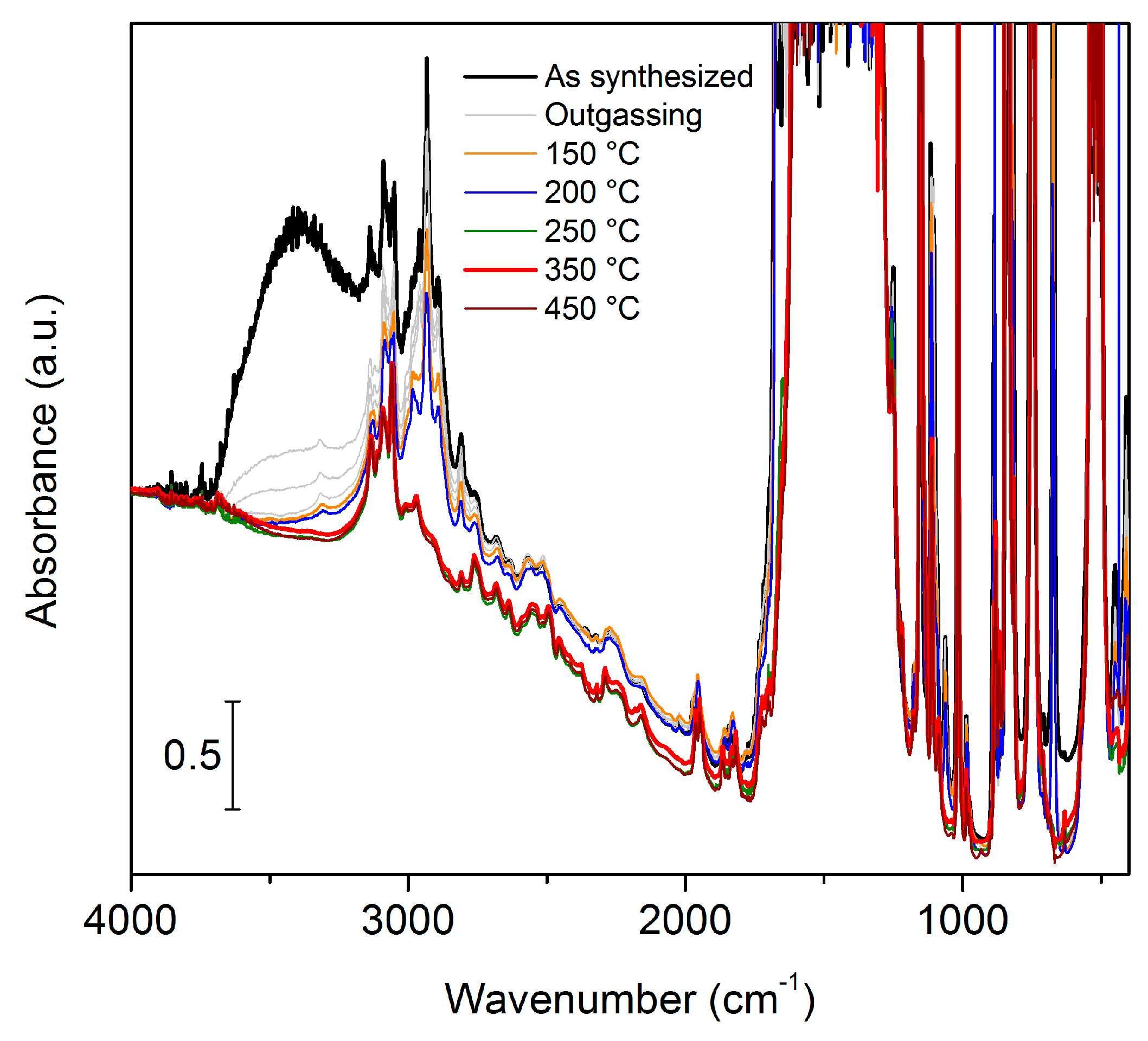
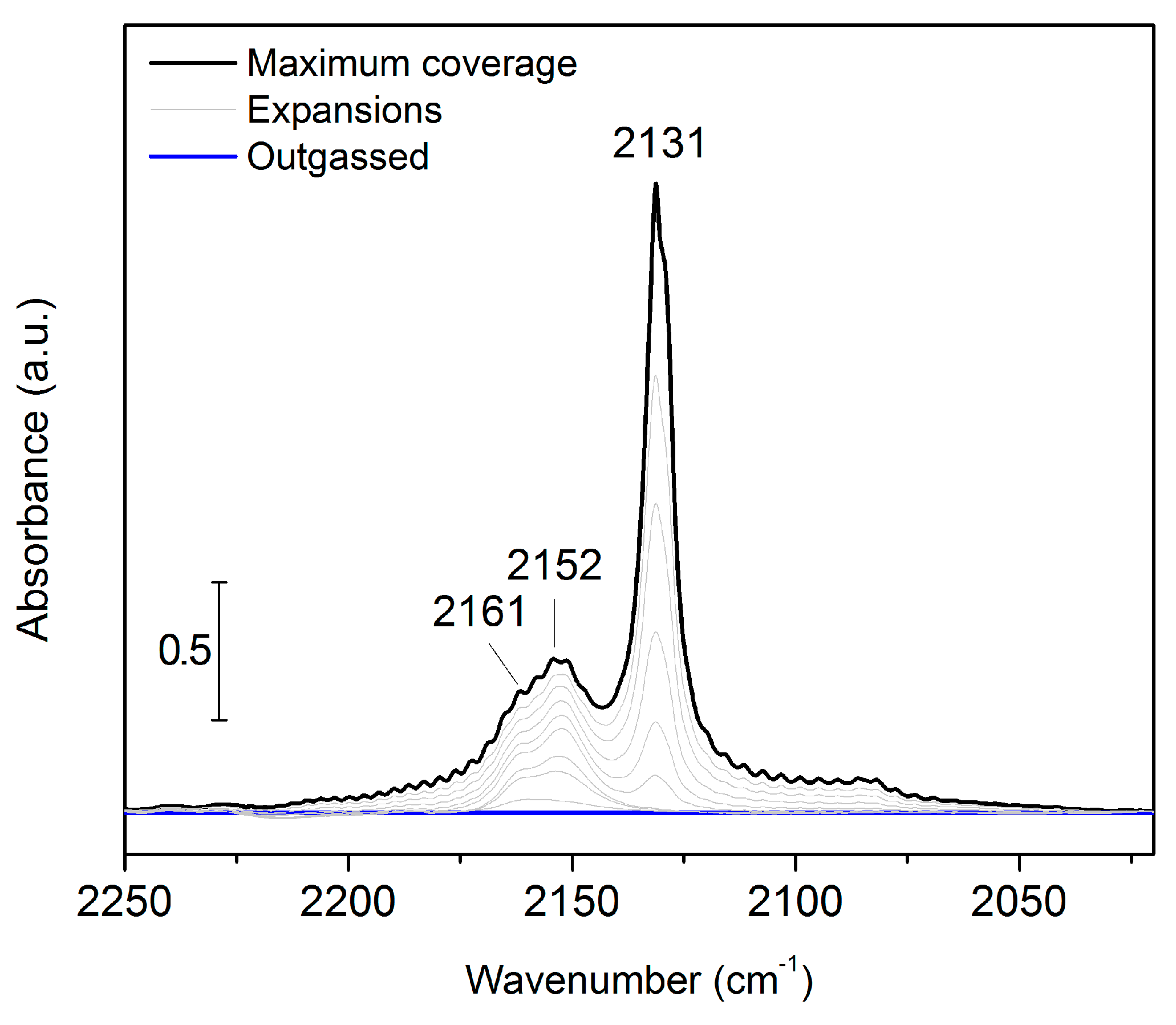
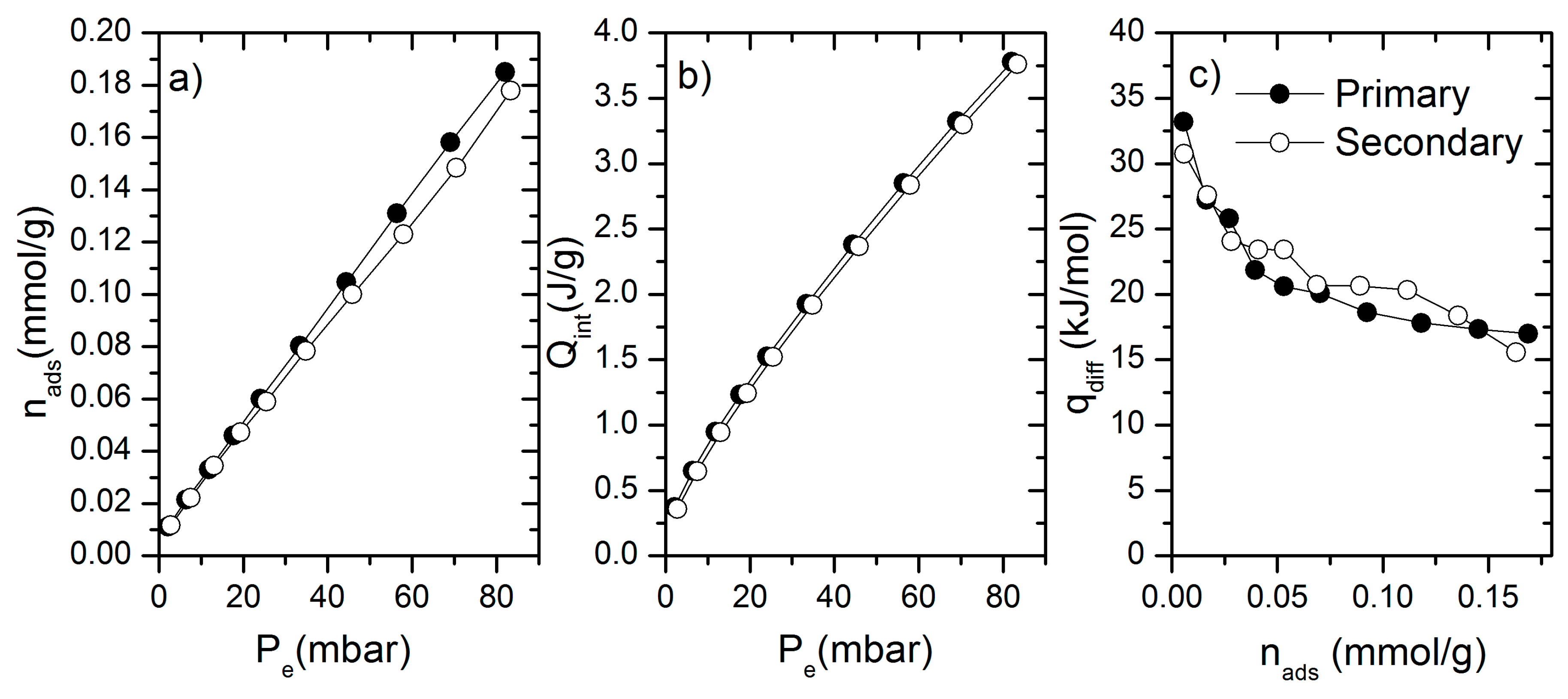
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare Earth Elements—Critical Resources for High Technology Supporting Sound Management of Our Mineral Resources; USGS: Reston, VA, USA, 2002.
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective; USGS: Reston, VA, USA, 2012.
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Ethiraj, J.; Bonino, F.; Vitillo, J.G.; Lomachenko, K.A.; Lamberti, C.; Reinsch, H.; Lillerud, K.P.; Bordiga, S. Solvent-Driven Gate Opening in MOF-76-Ce: Effect on CO2 Adsorption. ChemSusChem 2016, 9, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Atzori, C.; Lomachenko, K.A.; Øien-ØDegaard, S.; Lamberti, C.; Stock, N.; Barolo, C.; Bonino, F. Disclosing the Properties of a New Ce(III)-Based MOF: Ce2(NDC)3(DMF)2. Cryst. Growth Des. 2019, 19, 787–796. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; De Vos, D.; Stock, N. Cerium-based metal organic frameworks with UiO-66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef]
- Smolders, S.; Lomachenko, K.A.; Bueken, B.; Struyf, A.; Bugaev, A.L.; Atzori, C.; Stock, N.; Lamberti, C.; Roeffaers, M.B.J.; De Vos, D.E. Unravelling the Redox-catalytic Behavior of Ce4+ Metal–Organic Frameworks by X-ray Absorption Spectroscopy. ChemPhysChem 2018, 19, 373–378. [Google Scholar] [CrossRef]
- Lammert, M.; Glißmann, C.; Reinsch, H.; Stock, N. Synthesis and Characterization of New Ce(IV)-MOFs Exhibiting Various Framework Topologies. Cryst. Growth Des. 2017, 17, 1125–1131. [Google Scholar] [CrossRef]
- Griffin, S.L.; Wilson, C.; Forgan, R.S. Uncovering the structural diversity of Y(III) naphthalene-2,6-dicarboxylate MOFs through coordination modulation. Front. Chem. 2019, 7, 36. [Google Scholar] [CrossRef]
- D’Arras, L.; Sassoye, C.; Rozes, L.; Sanchez, C.; Marrot, J.; Marre, S.; Aymonier, C. Fast and continuous processing of a new sub-micronic lanthanide-based metal–organic framework. New J. Chem. 2014, 38, 1477. [Google Scholar] [CrossRef]
- Rhauderwiek, T.; Heidenreich, N.; Reinsch, H.; Øien-ØDegaard, S.; Lomachenko, K.A.; Rütt, U.; Soldatov, A.V.; Lillerud, K.P.; Stock, N. Co-Ligand Dependent Formation and Phase Transformation of Four Porphyrin-Based Cerium Metal–Organic Frameworks. Cryst. Growth Des. 2017, 17, 3462–3474. [Google Scholar] [CrossRef]
- Lammert, M.; Reinsch, H.; Murray, C.A.; Wharmby, M.T.; Terraschke, H.; Stock, N. Synthesis and structure of Zr(IV)- and Ce(IV)-based CAU-24 with 1,2,4,5-tetrakis(4-carboxyphenyl)benzene. Dalton Trans. 2016, 45, 18822–18826. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Glißmann, C.; Stock, N. Tuning the stability of bimetallic Ce(IV)/Zr(IV)-based MOFs with UiO-66 and MOF-808 structures. Dalton Trans. 2017, 46, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Lomachenko, K.A.; Jacobsen, J.; Bugaev, A.L.; Atzori, C.; Bonino, F.; Bordiga, S.; Stock, N.; Lamberti, C. Exact Stoichiometry of CexZr6−x Cornerstones in Mixed-Metal UiO-66 Metal–Organic Frameworks Revealed by Extended X-ray Absorption Fine Structure Spectroscopy. J. Am. Chem. Soc. 2018, 140, 17379–17383. [Google Scholar] [CrossRef] [PubMed]
- Atzori, C. Synthesis and Characterization of a Ce-Based Metal–Organic Framework (MOF). Master’s Thesis, Università di Torino, Turin, Italy, 2014. [Google Scholar]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Chavan, S.; Bonino, F.; Vitillo, J.G.; Groppo, E.; Lamberti, C.; Dietzel, P.D.C.; Zecchina, A.; Bordiga, S. Response of CPO-27-Ni towards CO, N2 and C2H4. Phys. Chem. Chem. Phys. 2009, 11, 9811–9822. [Google Scholar] [CrossRef]
- Bordiga, S.; Regli, L.; Bonino, F.; Groppo, E.; Lamberti, C.; Xiao, B.; Wheatley, P.S.; Morris, R.E.; Zecchina, A. Adsorption properties of HKUST-1 toward hydrogen and other small molecules monitored by IR. Phys. Chem. Chem. Phys. 2007, 9, 2676–2685. [Google Scholar] [CrossRef]
- Vindigni, F.; Manzoli, M.; Tabakova, T.; Idakiev, V.; Boccuzzi, F.; Chiorino, A. Effect of ceria structural properties on the catalytic activity of Au–CeO2 catalysts for WGS reaction. Phys. Chem. Chem. Phys. 2013, 15, 13400. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Chavan, S.M.; Shearer, G.C.; Svelle, S.; Olsbye, U.; Bonino, F.; Ethiraj, J.; Lillerud, K.P.; Bordiga, S. Synthesis and Characterization of Amine-Functionalized Mixed-Ligand Metal–Organic Frameworks of UiO-66 Topology. Inorg. Chem. 2014, 53, 9509–9515. [Google Scholar] [CrossRef]
- Grajciar, L.; Bludský, O.; Nachtigall, P. Water adsorption on coordinatively unsaturated sites in CuBTC MOF. J. Phys. Chem. Lett. 2010, 1, 3354–3359. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Auroux, A. Calorimetry and Thermal Methods in Catalysis; Auroux, A., Ed.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 154, ISBN 978-3-642-11953-8. [Google Scholar]
- Bolis, V.; Maggiorini, S.; Meda, L.; D’Acapito, F.; Turnes Palomino, G.; Bordiga, S.; Lamberti, C. X-ray photoelectron spectroscopy and X-ray absorption near edge structure study of copper sites hosted at the internal surface of ZSM-5 zeolite: A comparison with quantitative and energetic data on the CO and NH3 adsorption. J. Chem. Phys. 2000, 113, 9248–9261. [Google Scholar] [CrossRef]


| Treatment | BET Surface Area (m2/g) | C Value | Langmuir Surface Area (m2/g) | t-Plot Micropore Volume (cm3/g) |
|---|---|---|---|---|
| 150 °C-3 h | 3.63 ± 0.09 | 13.6 | 6.0 ± 0.2 | - |
| 200 °C-3 h | 11.57 ± 0.06 | 115.9 | 16.1 ± 0.2 | - |
| 250 °C-3 h | 223 ± 2 | 7110 | 217.3 ± 0.2 | 0.072 |
| 350 °C-3 h | 212 ± 2 | 6893 | 232.9 ± 0.5 | 0.073 |
| 450 °C-3 h | 216 ± 1 | 26,545 | 232 ± 0.4 | 0.075 |
| Treatment | CO2 Uptake at 1 Bar and 25 °C | nads/SLangmuir(1 bar) | ||
|---|---|---|---|---|
| (mol/kg) | (cm3/g) STP | Weight Percentage | (μmol/m2) | |
| 150 °C-3 h | 0.1281 | 2.87 | 0.56% | 21.4 ± 0.7 |
| 200 °C-3 h | 0.2728 | 6.12 | 1.19% | 16.93 ± 0.3 |
| 250 °C-3 h | 0.7954 | 17.83 | 3.38% | 3.660 ± 0.004 |
| 350 °C-3 h | 0.8352 | 18.72 | 3.55% | 3.586 ± 0.008 |
| 450 °C-3 h | 0.8301 | 18.61 | 3.52% | 3.586 ± 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzori, C.; Ethiraj, J.; Colombo, V.; Bonino, F.; Bordiga, S. Adsorption Properties of Ce5(BDC)7.5(DMF)4 MOF. Inorganics 2020, 8, 9. https://doi.org/10.3390/inorganics8020009
Atzori C, Ethiraj J, Colombo V, Bonino F, Bordiga S. Adsorption Properties of Ce5(BDC)7.5(DMF)4 MOF. Inorganics. 2020; 8(2):9. https://doi.org/10.3390/inorganics8020009
Chicago/Turabian StyleAtzori, Cesare, Jayashree Ethiraj, Valentina Colombo, Francesca Bonino, and Silvia Bordiga. 2020. "Adsorption Properties of Ce5(BDC)7.5(DMF)4 MOF" Inorganics 8, no. 2: 9. https://doi.org/10.3390/inorganics8020009
APA StyleAtzori, C., Ethiraj, J., Colombo, V., Bonino, F., & Bordiga, S. (2020). Adsorption Properties of Ce5(BDC)7.5(DMF)4 MOF. Inorganics, 8(2), 9. https://doi.org/10.3390/inorganics8020009






