Abstract
Tetradentate N2S2 ligands (such as bismercaptoethanediazacycloheptane in this study) have seen extensive use in combination with transition metals. Well-oriented N2S2 binding sites are ideal for d8 transition metals with square planar preferences, especially NiII, but also as a square pyramidal base for those metals with pentacoordinate preferences, such as [V≡O]2+, [Fe(NO)]2+, and [Co(NO)]2+. Further reactivity at the thiolate sulfurs generates diverse bi, tri, and tetra/heterometallic compounds. Few N2S2 ligands have been explored to investigate the possibility of binding to main group metals, especially group III (MIII) metals, and their utility as synthons for main group/transition metal bimetallic complexes. To open up this area of chemistry, we synthesized three new five-coordinate main group XMN2S2 complexes with methyl as the fifth binding ligand for M = Al, and chloride for M = Ga and In. The seven-membered diazacycle, dach, was engaged as a rigid stabilized connector between the terminal thiolate sulfurs. The pentacoordinate XMN2S2 complexes were characterized by 1H-NMR, 13C-NMR, +ESI-Mass spectra, and X-ray diffraction. Their stabilities and reactivities were probed by adding NiII sources and W(CO)5(THF). The former replaces the main group metals in all cases in the N2S2 coordination environment, demonstrating the weak coordinate bonds of MIII–N/S. The reaction of XMN2S2 (XM = ClGaIII or ClInIII) with the labile ligand W(0) complex W(CO)5(THF) resulted in Ga/In–W bimetallic complexes with a thiolate S-bridge. The synthesis of XMN2S2 complexes provide examples of MIII–S coordination, especially Al–S, which is relatively rare. The bimetallic Ga/In–S–W complex formation indicates that the nucleophilic ability of sulfur is retained in MIII–S–R, resulting in the ability of main group MIII–N2S2 complexes to serve as metalloligands.
1. Introduction
In the early 1960s, Busch and co-workers reported the complexing ability of mercaptamines (NS ligands) for NiII [1]. Since then, various NS ligands have been reported, including those with a contiguous S–N–N–S, N2S2, tetradentate donor set, an arrangement that mimics the N2S2 coordination environment rendered by a cysteine-glycine/serine-cysteine tripeptide motif found at three enzyme active sites [2]. Further development has been robust. Of special note is the bimetallic Ni–Ni site in acetyl co-A synthase, ACS, in which N2S2 is viewed as a tight Ni-binding site, while the second nickel is labile and catalytically active in the C–C coupling reactions required of ACS, as shown in Figure 1 [3].
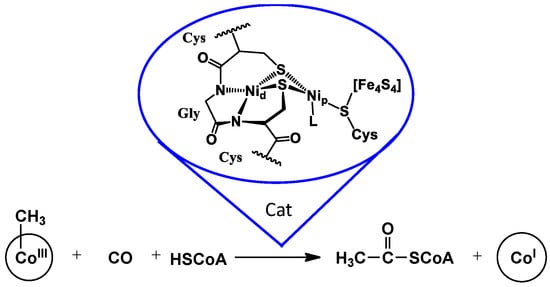
Figure 1.
Structure and function of the dinickel enzyme active site of acetyl co-A synthase [3].
We developed NiN2S2 complexes as metallodithiolate ligands, especially engaging diazamesocycles N2C6H8 (daco), N2C5H10 (dach), and N2C4H8 (dach*) as stabilizing units in N to N connections [4,5,6]. Those containing the more flexible “open-chain” ethylene or propylene N to N linkers bring more flexibility to the N2S2 binding unit, resulting in subtle differences in reactivity and stability properties. The ability to tune the MN2S2 ligands by variations of M has resulted in a range of complexes, such as V4+ in [V≡O]2+, Fe2+ in {Fe(NO)}7, Ni2+, Pd2+, Cu2+,and Zn2+ [7]. The ability of the MN2S2 metalloligands to serve as monodentate as well as bidentate ligands to a single metal or as bridging bidentate ligands to two metals has led to multiple new compositions and various diverse structural forms [7]. A few of these are illustrated in Figure 2 [7]. However, the chemistry of N2S2 derivatives of main group metals remains relatively unexplored, and their ability to serve as aggregation sites for exogeneous metals has until now, to our knowledge, been unreported.
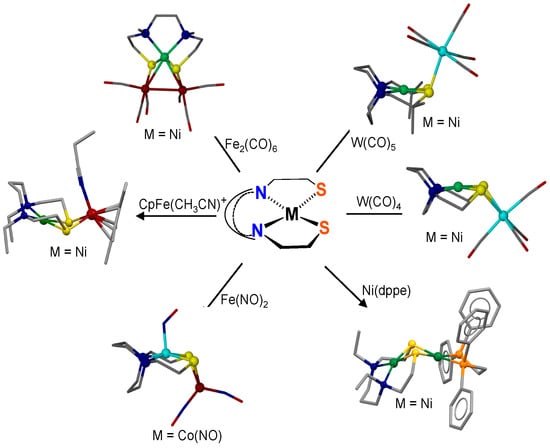
Figure 2.
Examples of bi- and trimetallic complexes accessed by connecting the MN2S2 metallodithiolate ligand to various transition metal receivers, adapted from [7].
While the expectation that the soft S will have a poor binding affinity for hard Al(III) is reasonable, a Cambridge database search located 36 results of N2S2 derivatives of aluminum. Most of them are derived from an aluminum (I) precursor [8,9]. The use of abundant aluminum in catalysis is well known, including as catalysts for CO2/epoxide cycloaddition [10,11], which comprises a tetradentate N2O2 ligand with an axial X ligand (X = alkyl, halogen completing the coordination sphere). In contrast, many more sulfur−coordinated gallium(III) and indium(III) complexes have been reported, owing to the softer core of Ga(III)/In(III) [12,13,14,15,16,17]. Explorations in those cases have been prompted by the wide use of 111In and 67Ga as radionuclides in PET (Positron Emission Tomography) and SPECT (Single Photon Emission Computed Tomography) [18].
In order to extend the coordination chemistry of main group III metals with this versatile ligand motif, we explored the synthesis and characterization of Group III elements, such as Ga/In–Cl or Al–R, bound within the N2S2 binding cavity. We probed the availability of the residual S lone pairs to serve as nucleophiles for the binding to exogeneous metals. In particular, heterobimetallics were formed with W(CO)4/5, a receiver unit with the CO reporter unit, which provides a reference point for establishing donor ability of such metalloligands [7].
2. Results
2.1. Synthesis and Characterizations of XMN2S2 Complexes
The H2bme-dach (N,N′-Bis(Mercaptoethyl)-1,4-Diazacycloheptane) was synthesized according to a reported procedure [4]. Under an N2 atmosphere, a solution of AlMe3, GaCl3, or InCl3 was transferred by a double-ended needle into a solution of H2bme-dach ligand in a Schlenk flask. White powdery products formed immediately, resulting in a white suspension. After stirring overnight, the solvent was removed under vacuum giving white solids, determined to be the compounds shown in Figure 3. They were further washed with Et2O and pentane, giving a yield of 75–80%.
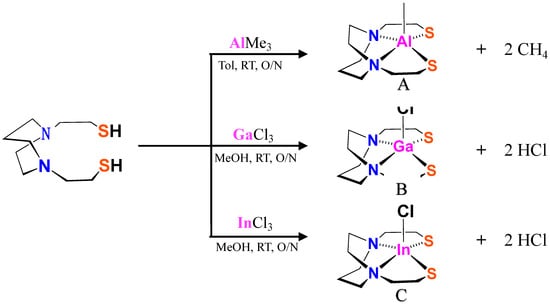
Figure 3.
Synthetic routes for XMN2S2 complexes; RT is ca. 22 °C and O/N is ca. 14 h.
The XMN2S2 complexes were characterized by XRD, ESI-mass spectra, 1H-NMR and 13C-NMR. Details are shown in the Supporting Information. The X-ray quality crystals of A were developed in pyridine solution at −28 °C. Crystals of B and C were obtained by slow evaporation of Et2O vapor into a pyridine solution. All crystals were colorless needles. Metric parameters for the molecular structures are given in Figure 4.
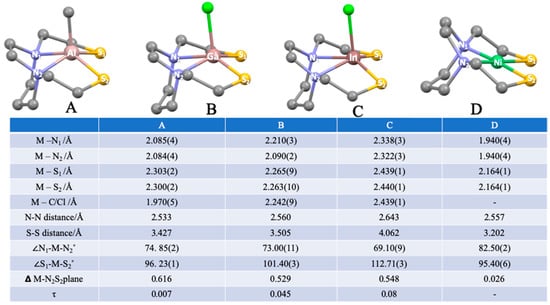
Figure 4.
Molecular structures of XMN2S2 complexes from XRD analysis with selected metric data.
The geometries of XMN2S2 (M = Al, Ga, and In) were similar, as the ideality of the square pyramidal structures is indicated by small τ values (near 0) [19]. The displacements of M from the best N2S2 planes showed aluminum to be the most out of plane, ∆ = 0.616 Å, while Ga and In showed displacements in the range of 0.53–0.55 Å. These displacements are likewise seen in some pentacoordinate transition metal complexes, such as MN2S2, where M = [Co(NO)]2+ (0.31 Å), [Fe(NO)]2+ (0.55 Å), and [V≡O]2+ (0.652 Å) [20,21]. In all cases of A, B, and C, the two-carbon linker within the diazacycloheptane ring is on the same side as the MIII–X bond vector. This means that the MN2C3 cyclohexane-type ring in the chair configuration is oriented “underneath” the N2S2 base of the square pyramidal X–MN2S2 structure. We saw no evidence of fluxionality in the solution.
Different from Ga and In, which are largely located on the midpoint line in the center of the N2S2 unit, Al showed a slight dissymmetry in its location, shifted towards the nitrogen atoms (Al–N is around 2.1 Å) and away from the sulfur atoms (Al–S is around 2.3 Å). This suggests a stronger electrostatic interaction to N from the AlIII. Table S5 lists the comparative ratios of the M–N/M–S distances that emphasize the similarity of Ga and In and their greater affinity for sulfur. From Al, Ga, to In, the angles of ∠N–M–N decreased with a concomitant increase in ∠S–M–S.
2.2. Reactions of XMN2S2 with Ni(II) Sources
The optimal sized N2S2 cavity of the bme-dach and analogous ligands, as well as the electronic structure preference of d8 NiII, leads to a well-known library of square planar NiN2S2 complexes [7]. Thus, we probed the possibility of NiII replacement of the Group III metal ions in the XMN2S2 complexes. Such metal exchange studies were not easily performed in the transition metal complexes, as aggregation at the sulfur elements was prominent.
The addition of NiCl2 or Ni(BF4)2 to XMIIIN2S2 complexes resulted in the formation of NiN2S2 with MIII replaced by NiII, concomitant with a color change from colorless to dark maroon, illustrated in Figure 5. The resulting maroon product gave a yield of 75–80% and was confirmed as the known NiN2S2 complex by mass spectroscopy (Figure S12) and X-ray structure analyses. To determine whether the XMIII unit might remain in the N2S2 cavity with the attachment of Ni(diphos) to the nucleophilic sulfurs of XMIIIN2S2, we added the (diphos)NiCl2 complex to a solution of XMIIIN2S2. The product of that reaction was determined to be the well-known dinickel complex derived from two (diphos)NiCl2 complexes with NiII moving into the tight binding site, replacing XMIII, and the second nickel holding on to the diphos ligand, Figure 5. The stability of the NiN2S2 complex relative to XMIIIN2S2 was confirmed.
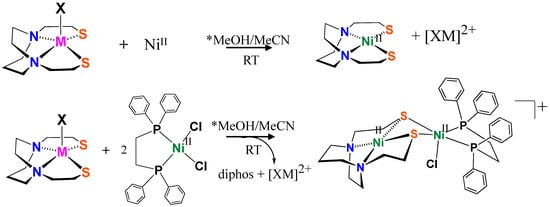
Figure 5.
Reactions of XMN2S2 with NiII salts and (diphos)NiCl2. * Pure MeOH, or MeCN, or a mixture of MeOH/MeCN (1:1) resulted in the same products.
2.3. Nucleophilicity of S Lone Pairs on XMN2S2 Towards the Soft Receiver W(CO)5
The stability and electronic reporting capability of tungsten carbonyls have proven useful to rank the donor ability of various MN2S2 metalloligands. The labile ligand synthons W(CO)5(solv) and W(CO)4(solv)2 were generated by appropriate methods [22,23]. Carbon monoxide was lost in solutions of W(CO)6 in THF under UV light, after which the golden yellow solution showed the ν(CO) C4v pattern typical of W(CO)5(THF) and was transferred via cannula to a solution of XMN2S2 (XM = ClGa/ClIn). Over the course of several hours at room temperature, with no further photolysis, the color changed to brown-orange. As shown in the overlaid spectra in Figure 6, the shift in ν(CO) was not large, indicating the donor ability of THF and monodentate XMN2S2 toward W(CO)5 is similar and weak. In contrast, NiN2S2 reacted with W(CO)5(THF), producing a much greater change in ν(CO) of 2062 (w), 1922 (s), and 1884 (m) [23]. The MeAlN2S2 metalloligand itself is not stable under UV light and gave products of degradation in the presence of W(CO)5(THF).
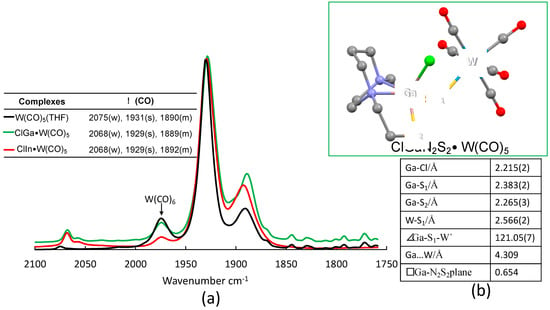
Figure 6.
(a) IR spectra in ν(CO) region of products from reactions of W(CO)5(THF) with XMN2S2; (b) XRD determined molecular structure of ClGaN2S2·W(CO)5 with selected metric data.
Orange sheet crystals of ClGaN2S2·W(CO)5 were obtained from THF solution by hexane layering. The X-ray diffraction study showed that the geometry and metric parameters were largely the same in the free and S-bound monodentate complexes. The Ga–S1 bridge bond was elongated compared with the ClGaN2S2 structure. The N2S2 “plane” was quite distorted, giving the ClGaN2S2 a geometry between square pyramidal and trigonal bipyramidal with the τ value 0.50, as shown in Figure S18 [19]. Gallium(III) was significantly more displaced from the N2S2 “plane”, giving a deviation of over 0.1 Å (0.529 Å in ClGaN2S2 to 0.654 Å in ClGaN2S2·W(CO)5). The distance between Ga and W was 4.309 Å.
On further exposing ClGaN2S2·W(CO)5 to UV light, a second CO was lost, yielding ClGaN2S2·W(CO)4. The ν(CO) IR spectrum displays a four-band pattern typical of C2v metal carbonyl derivatives; the values are 2004 (w), 1929 (m), 1890 (m), and 1846 (m) cm−1 with the color changing to light orange, Figure 7. Similar to the ClGaN2S2·W(CO)5 complex, the ClInN2S2·W(CO)5 converted to ClInN2S2·W(CO)4 under light, giving ν (CO) values of 2007 (w), 1929 (m), 1894 (m), and 1850 (m) cm−1, as shown in Figure S17. The final product was confirmed by −ESI-Mass, and details are given in the Supplementary Materials. The experimental results indicate that the thiolate sulfurs in the XMN2S2 complexes are still sufficiently active to bind another metal. However, the binding ability is weak. The latter statement was confirmed by the reversibility of the CO loss. On bubbling CO through the solution of ClGaN2S2·W(CO)4, the pentacarbonyl was readily reclaimed.
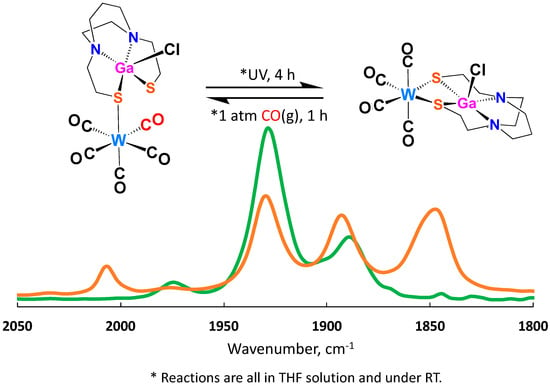
Figure 7.
Reactions of ClGaN2S2·W(CO)5 and ClGaN2S2·W(CO)4 under UV light irradiation. ClGaN2S2·W(CO)5 converted to ClGaN2S2·W(CO)4 and, reversibility established by bubbling CO(g) to ClGaN2S2·W(CO)4 resulting in the reformation of ClGaN2S2·W(CO)5. The green line is for ClGaN2S2·W(CO)5 and the orange line is for ClGaN2S2·W(CO)4.
3. Materials and Methods
All reagents and solvents were obtained from commercial sources. All solvents were purified and dried by an MBRAUN Manual Solvent Purification System (MBRAUN, NH, USA) packed with Alcoa F200 activated alumina desiccant. All reactions and operations were carried out on a double manifold Schlenk vacuum line or in a glovebox under a N2 or Ar atmosphere.
Solution infrared spectra were recorded on a Bruker Tensor 37 Fourier transform IR (FTIR) spectrometer (Billerica, MA, USA) using a CaF2 cell with a 0.2 mm path length. Both High Resolution and Low Resolution Mass spectrometry (Thermo Fisher Q Exactive Mass Spectrometer, ESI-MS, (IET, IL, USA) were performed in the Laboratory for Biological Mass Spectrometry at Texas A&M University. Data collections for X-ray structure-determination were carried out using Bruker APEX2 (Billerica, MA, USA) or Venture with a graphite monochromated radiation source (λ = 0.71073 Å). All crystals were coated in paraffin oil and mounted on a nylon loop and placed under streaming N2 (110/150K). The structures were solved by direct methods (SHELXS-97) and refined by standard Fourier techniques against F square with a full-matrix least-squares algorithm using SHELXL-97 and the WinGX (1.80.05) software package (University Of Glasgow, Scotland, UK). Hydrogen atoms were placed in calculated positions and refined with a riding model. Graphical representations were prepared with ORTEP-III. Crystallographic data (including structure factors) have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. 1943010–1943013.
3.1. Synthesis of XMN2S2
MeAlN2S2: The H2bme-dach ligand (0.22 g, 1.0 mmol) was added to a 125 mL Schlenk flask by pipet in an inert atmosphere glove box and dissolved in ca. 10 mL toluene. A pentane solution of AlMe3 (1 M in pentane, 1 mL, 1 mmol) was added dropwise to the ligand solution. After reacting overnight at room temperature, the resulting powdery white solution was filtered, and solvent was removed under vacuum. The white solid was washed with Et2O and pentane, giving a ~75% yield. Proton NMR spectral values (300 MHz, 293 K, pyridine-d5) were as follows: δ (ppm) = 4.98 (s, 8H, N2S2) 2.63(q, 2H, N2S2), 2.61(q, 8H, N2S2), 1.70(q, 2H, N2S2), 0.10(s, 1H, Me). 13C NMR (300 MHz, 293 K, pyridine-d5): δ (ppm) = 61.40, 55.80, 54.39, 28.81, 23.69, −4.01. High Resolution +ESI-Mass: [M + H]+ (C10H22AlN2S2) Calculated 261.1040 (Most Abundant Isotopic Mass); Found: 261.1033. The detailed isotope abundance is shown in Figure S4.
ClGaN2S2: Similar to MeAlN2S2, ClGaN2S2 was derived from a MeOH solution of GaCl3 (0.176 g, 1.0 mmol) and H2bme-dach (0.22 g, 1.0 mmol) at room temperature. After overnight reaction, the solvent was removed from the resulting powdery white suspension under vacuum, and the white solid was washed with Et2O and pentane, giving an ~80% yield. 1H NMR (300 MHz, 293 K, pyridine-d5): δ (ppm) = 5.75(s, 8H, N2S2), 3.62(s, 4H, N2S2), 2.84(s, 2H, N2S2), 2.17(q, 2H, N2S2), 1.27(q, 2H, N2S2). 13C NMR (300 MHz, 293 K, pyridine-d5): δ (ppm) = 55.96, 51.54, 48.71, 24.62, 22.18. High Resolution +ESI-Mass: [M − Cl]+ (C9H18GaN2S2) Calculated 287.0162; Found: 287.0157 (Most Abundant Isotopic Mass). The detailed isotope abundance is shown in Figure S5.
ClInN2S2: ClInN2S2 was synthesized in a similar method to ClGaN2S2. A MeOH solution of InCl3 (0.221 g, 1.0 mmol) was added dropwise to a MeOH solution of H2bme-dach. The powdery suspension was dried, and the white solid was washed with Et2O and pentane. The yield was 85%. 1H NMR (300 MHz, 293 K, pyridine-d5): δ (ppm) = 5.75(s, 8H, N2S2), 3.62(s, 4H, N2S2), 2.84(s, 2H, N2S2), 2.17(q, 2H, N2S2), 1.27(q, 2H, N2S2). 13C NMR (300 MHz, 293 K, pyridine-d5): δ (ppm) = 58.28, 53.01, 48.11, 24.23, 22.83. High Resolution +ESI-Mass: [M − Cl]+ (C9H18InN2S2) Calculated 332.9945; Found: 332.9939 (Most Abundant Isotopic Mass). The detailed isotope abundance is shown in Figure S6.
3.2. Reactions of XMN2S2 with Ni(II)
The white solid of XMN2S2 (0.1 mmol) was placed in a 50 mL flask, and 10 mL of a MeOH/MeCN (1:1) mixture was added. The green solution of NiCl2·6H2O (0.1 mmol, 24 mg) in MeOH was then transferred to the 50 mL flask. The color immediately changed to maroon, which is typical of NiN2S2, and this product was confirmed by its X-ray structure. The same product, NiN2S2, was also formed by adding Ni(BF4)2 using the same method.
Similar to the reaction with the chloride salt of NiII, the white solid of XMN2S2 (0.1 mmol) was stirred with 10 mL MeCN in the 50 mL round flask. The NiP2Cl2 (P2 = diphos or 1,1′-diphenylphosphinoethane, 0.105 g, 0.2 mmol) was dissolved in 10 mL MeCN and transferred to the flask. The resulting dark brown solution was dried in vacuo, giving a dark brown solid. Dark brown needle crystals were formed under Et2O diffusion into a concentrated CH3CN solution, proven to be the dinickel complex by X-ray structure and +ESI-MS analyses.
3.3. Synthesis of ClMW(CO)5
The W(CO)5(solv) was generated in situ by W(CO)6 in THF under UV light, after which the golden yellow solution was directly transferred to a white suspension of XMN2S2 (M = GaCl/InCl) in THF. Then, the mixture was stirred at room temperature and monitored by FTIR. After several hours, the color changed to a clear brown-orange and without further ν(CO) change. IR (cm−1): ClGaN2S2W(CO)5, ν(CO) 2068(w), 1929(s), 1889(m); ClInN2S2W(CO)5, ν(CO) 2068(w), 1929(s), 1892(m). −ESI-Mass: [ClInN2S2W(CO)5 + Cl]+ (C14H18ClGaN2O5S2W) Calculated 680.88; Found: 680.92; [ClInN2S2W(CO)5 + Cl]+ (C14H18ClInN2O5S2W) Calculated 726.86; Found: 726.94.
4. Conclusions
Through this study, we have demonstrated that the main group metals Al, Ga, and In can bind within the tetradentate chelating pocket of an N2S2 ligand, yielding square pyramidal complexes. The MIII sits above the N2S2 plane and each is capped with an additional ligand, CH3− in the case of AlIII and Cl− for GaIII and InIII. Known to be tunable via the N to N and N to S linkers of the MN2S2 metallodithiolates, our account extends the range of metals that define the nucleophilicity of the sulfurs in metallodithiolates as aggregating sites for exogeneous metals. The system demonstrates that the XMN2S2 complexes bind to W(CO)5 and W(CO)4, forming isolable complexes, one of which was subjected to XRD analysis. Solution ν(CO) IR spectral values permit comparisons of donor ability with transition metals such as NiN2S2 where the Nidπ–Spπ antibonding interaction leads to greater donor ability. In addition, compared with NiN2S2, the XMIIIN2S2 complexes are less stable and can readily convert to NiN2S2 on exposure to NiII when made available as a salt or when bound by diphos, bis-diphenylphosphino ethane. Such fundamental studies are useful for extending main group coordination chemistry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-6740/7/9/115/s1: 1H-NMR and 13C-NMR Spectra (Figures S1–S3); Mass Spectra (Figures S4–S12); X-ray Crystal Structures (Figures S13–S16, Table S1–S4); FTIR Spectra of ClInN2S2·W(CO)4 (Figure S17); The deviations of Nitrogen and Sulfur atoms from N2S2 plane (Figure S18); The ratio of M–N/M–S in XMN2S2 (Table S5); Different ν(CO) values in MW(CO)4 comparison (Table S6).
Author Contributions
M.Y.D. and X.Y. designed the project. X.Y. synthesized and characterized the complexes. All authors contributed to the writing and editing the manuscript.
Funding
This work was financially supported by the National Science Foundation (CHE-1266097, CHE-1665258) and the Robert A. Welch Foundation (A-0924).
Acknowledgments
We thank Trung Le and Xiaogao Meng for their help in solving the crystal structure. We acknowledge the work of Michelle L. Hatley, M.S., TAMU, who initiated the gallium synthesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Busch, D.H.; Jicha, D.C.; Thompson, M.C.; Wrathall, J.W.; Blinn, E. Reactions of Coordinated Ligands. VIII. The Reactions of Alkyl Halides with Mercapto Groups in Transition Metal Complexes of Mercaptoamines. J. Am. Chem. Soc. 1964, 86, 3642–3650. [Google Scholar] [CrossRef]
- Can, M.; Armstrong, F.A.; Ragsdale, S.W. Structure, Function, and Mechanism of the Nickel Metalloenzymes, CO Dehydrogenase, and Acetyl-CoA Synthase. Chem. Rev. 2014, 114, 4149–4174. [Google Scholar] [CrossRef] [PubMed]
- Doukov, T.I.; Blasiak, L.C.; Seravalli, J.; Ragsdale, S.W.; Drennan, C.L. Xenon in and at the End of the Tunnel of Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase. Biochemistry 2008, 47, 3474–3483. [Google Scholar] [CrossRef] [PubMed]
- Smee, J.J.; Miller, M.L.; Grapperhaus, C.A.; Reibenspies, J.H.; Darensbourg, M.Y. Subtle Bite-Angle Influences on N2S2Ni Complexes. Inorg. Chem. 2001, 40, 3601–3605. [Google Scholar] [CrossRef]
- Mills, D.K.; Reibenspies, J.H.; Darensbourg, M.Y. Sterically protected nickel(II) in a N2S2 donor environment: 1,5-bis(mercaptoethyl)-1,5-diazacyclooctane and its methylated derivative. Inorg. Chem. 1990, 29, 4364–4366. [Google Scholar] [CrossRef]
- Zhao, T.; Ghosh, P.; Martinez, Z.; Liu, X.; Meng, X.; Darensbourg, M.Y. Discrete Air-Stable Nickel(II)–Palladium(II) Complexes as Catalysts for Suzuki-Miyaura Reactions. Organometallics 2017, 36, 1822–1827. [Google Scholar] [CrossRef]
- Denny, J.A.; Darensbourg, M.Y. Metallodithiolates as Ligands in Coordination, Bioinorganic, and Organometallic Chemistry. Chem. Rev. 2015, 115, 5248–5273. [Google Scholar] [CrossRef]
- Yang, Z.; Yi, Y.; Zhong, M.; De, S.; Mondal, T.; Koley, D.; Ma, X.; Zhang, D.; Roesky, H.W. Addition Reactions of Me3SiCN with Aldehydes Catalyzed by Aluminum Complexes Containing in Their Coordination Sphere O, S, and N Ligands. Chem. Eur. J. 2016, 22, 6932–6938. [Google Scholar] [CrossRef]
- Chu, T.; Vyboishchikov, S.F.; Gabidullin, B.; Nikonov, G.I. Oxidative Cleavage of C=S and P=S Bonds at an AlI Center: Preparation of Terminally Bound Aluminium Sulfides. Angew. Chem. Int. Ed. 2016, 55, 13306–13311. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.Y.; Kim, J.H.; Kang, Y.Y.; Lee, J.; Kim, Y. Monomeric or Dimeric Aluminium Complexes as Catalysts for Cycloaddition between CO2 and Epoxides. Eur. J. Inorg. Chem. 2015, 13, 2323–2329. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Billodeaux, D.R. Aluminum Salen Complexes and Tetrabutylammonium Salts: A Binary Catalytic System for Production of Polycarbonates from CO2 and Cyclohexene Oxide. Inorg. Chem. 2005, 44, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.S.; Arrowsmith, R.L.; Cortezon-Tamarit, F.; Twyman, F.; Kociok-Köhn, G.; Botchway, S.W.; Dilworth, J.R.; Carroll, L.; Aboagye, E.O.; Pascu, S.I. Microwave Gallium-68 Radiochemistry for Kinetically Stable bis(thiosemicarbazone) Complexes: Structural Investigations and Cellular Uptake under Hypoxia. Dalton Trans. 2015, 45, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tai, Y.; Li, M.; Ma, P.; Zhao, J.; Niu, J. Main Group Bismuth(III), Gallium(III) and Diorganotin(IV) Complexes derived from bis(2-acetylpyrazine) thiocarbonohydrazone: Synthesis, Crystal Structures and Biological Evaluation. Dalton Trans. 2014, 43, 5182–5189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dodonov, V.A.; Chen, W.; Zhao, Y.; Skatova, A.A.; Fedushkin, I.L.; Roesky, P.W.; Wu, B.; Yang, X.J. Cycloaddition versus Cleavage of the C=S Bond of Isothiocyanates Promoted by Digallane Compounds with Noninnocent α-Diimine Ligands. Chem. Eur. J. 2018, 24, 14994–15002. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Ji, Y.; Lu, Y.; Li, M.; Wu, Y.; Han, Q. Cadmium(II) and Indium(III) Complexes Derived from 2-benzoylpyridine N(4)-cyclohexylthiosemicarbazone: Synthesis, Crystal Structures, Spectroscopic Characterization and Cytotoxicity. Synth. Met. 2016, 219, 109–114. [Google Scholar] [CrossRef]
- Arrowsmith, R.L.; Waghorn, P.A.; Jones, M.W.; Bauman, A.; Brayshaw, S.K.; Hu, Z.; Kociok-Köhn, G.; Mindt, T.L.; Tyrrell, R.M.; Botchway, S.W.; et al. Fluorescent Gallium and Indium bis(thiosemicarbazonates) and Their Radiolabelled Analogues: Synthesis, Structures and Cellular Confocal Fluorescence Imaging Investigations. Dalton Trans. 2011, 2011. 40, 6238–6252. [Google Scholar] [CrossRef]
- Anderson, T.S.; Briand, G.G.; Brüning, R.; Decken, A.; Margeson, M.J.; Pickard, H.M.; Trevors, E.E. Synthesis, Characterization and Reactivity of (dithiolato)Indium Complexes. Polyhedron 2017, 135, 101–108. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Jacobus, V.R.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Hess, J.; Conder, H.L.; Green, K.N.; Darensbourg, M.Y. Electronic Effects of (N2S2)M(NO) Complexes (M = Fe, Co) as Metallodithiolate Ligands. Inorg. Chem. 2008, 47, 2056–2063. [Google Scholar] [CrossRef]
- Jenkins, R.; Pinder, T.A.; Hatley, M.L.; Reibenspies, J.H.; Darensbourg, M.Y. Tetradentate N2S2 Vanadyl(IV) Coordination Complexes: Synthesis, Characterization, and Reactivity Studies. Inorg. Chem. 2011, 50, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J.; Kump, R.L. A Convenient Synthesis of cis-Mo(CO)4L2 Derivatives (L = Group 5A Ligand) and a Qualitative Study of Their Thermal Reactivity toward Ligand Dissociation. Inorg. Chem. 1978, 17, 2680–2682. [Google Scholar] [CrossRef]
- Rampersad, M.V.; Jeffery, S.P.; Golden, M.L.; Lee, J.; Reibenspies, J.H.; Darensbourg, D.J.; Darensbourg, M.Y. Characterization of Steric and Electronic Properties of NiN2S2 Complexes as S-donor Metallodithiolate Ligands. J. Am. Chem. Soc. 2005, 127, 17323–17334. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).