Bulky-Yet-Flexible Carbene Ligands and Their Use in Palladium Cross-Coupling
Abstract
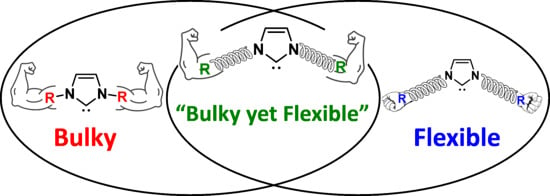
1. Introduction
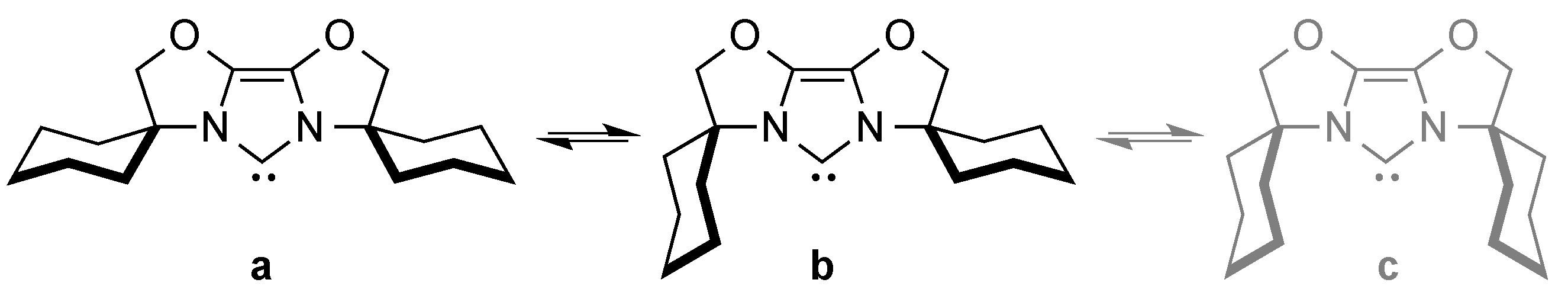
2. IBiox
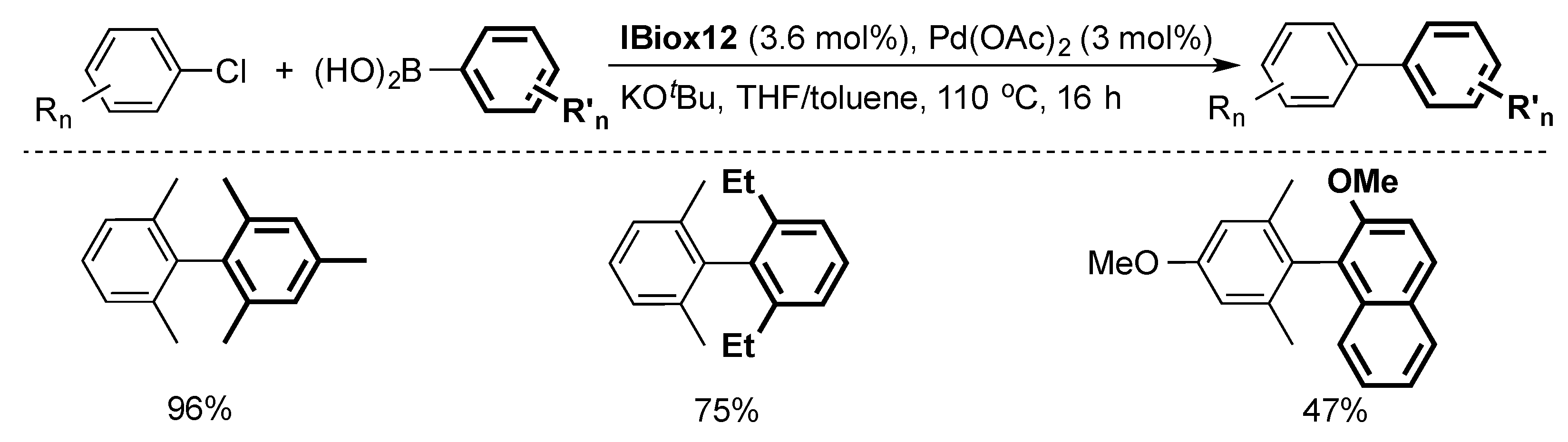
2.1. Suzuki–Miyaura Cross-Coupling
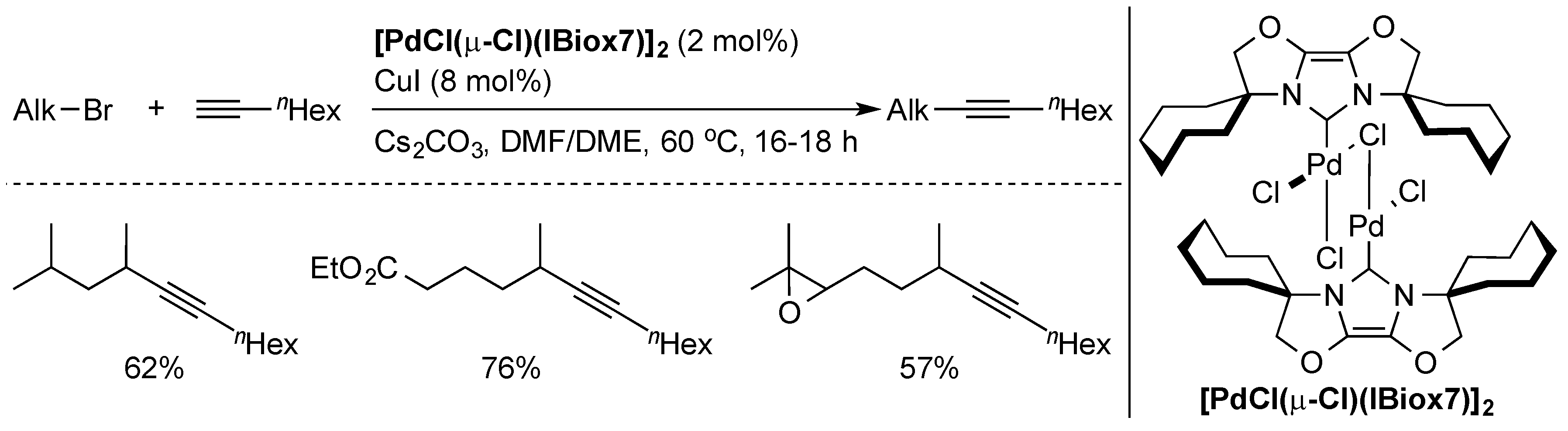
2.2. Alkyl Sonogashira Cross-Coupling
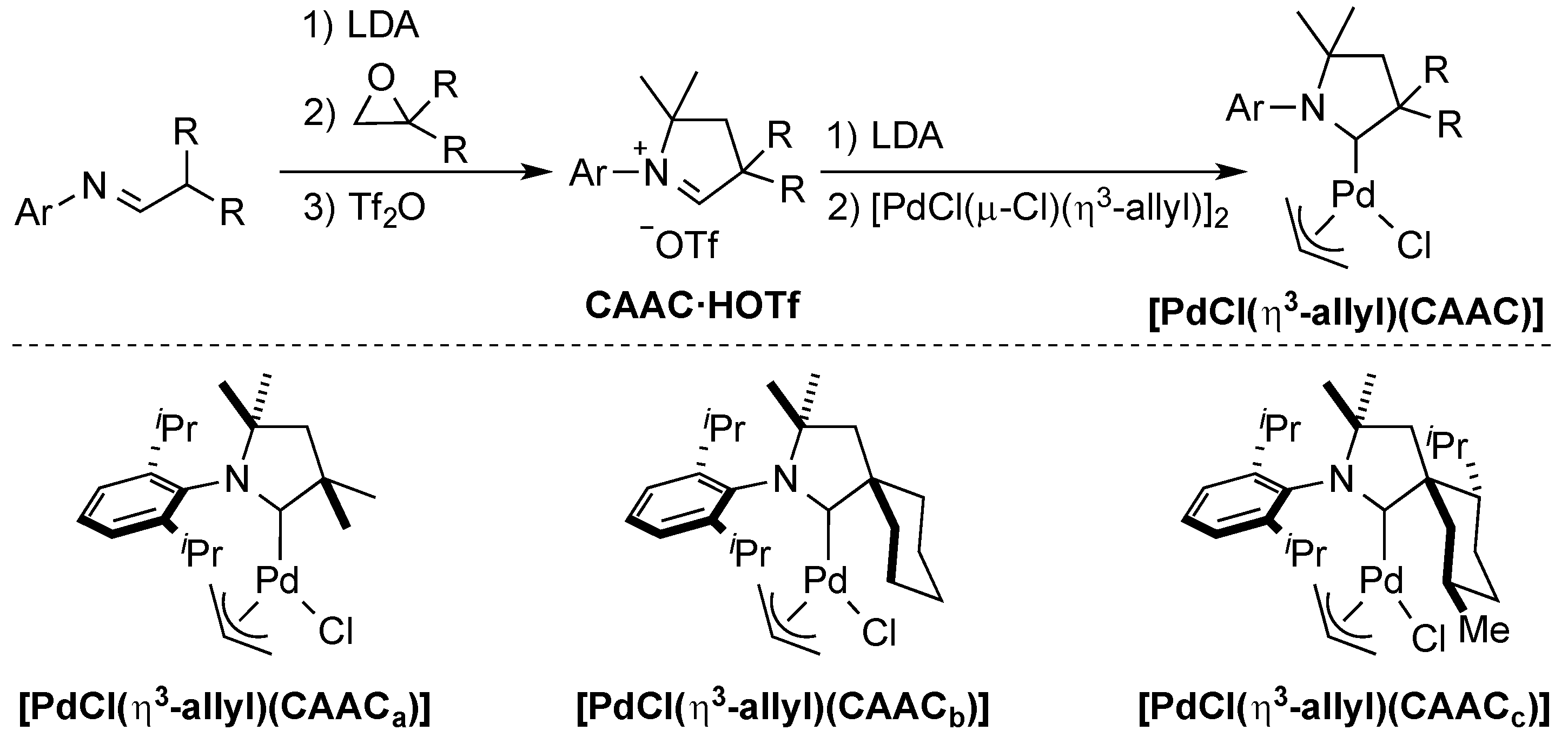
3. CAAC Series
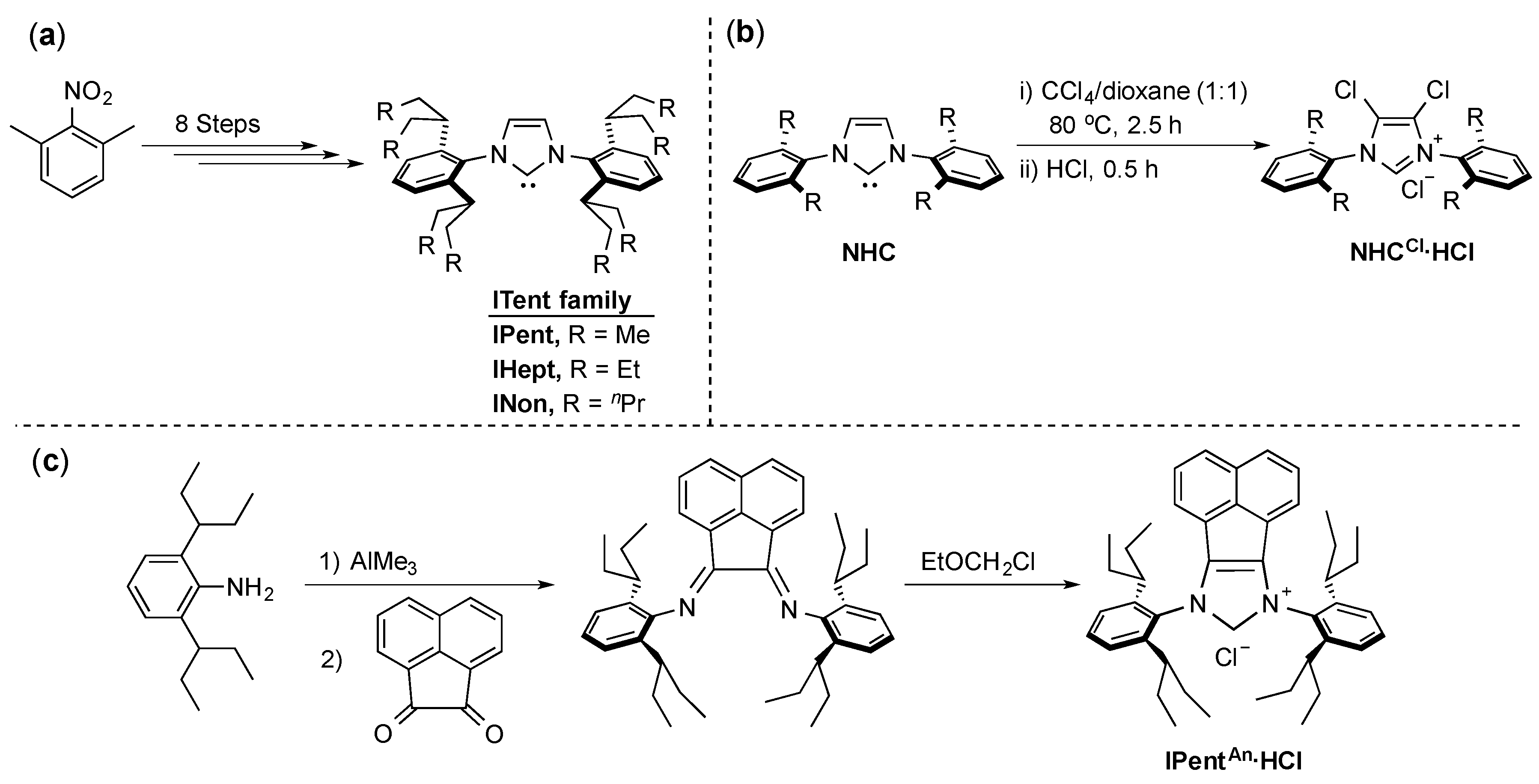
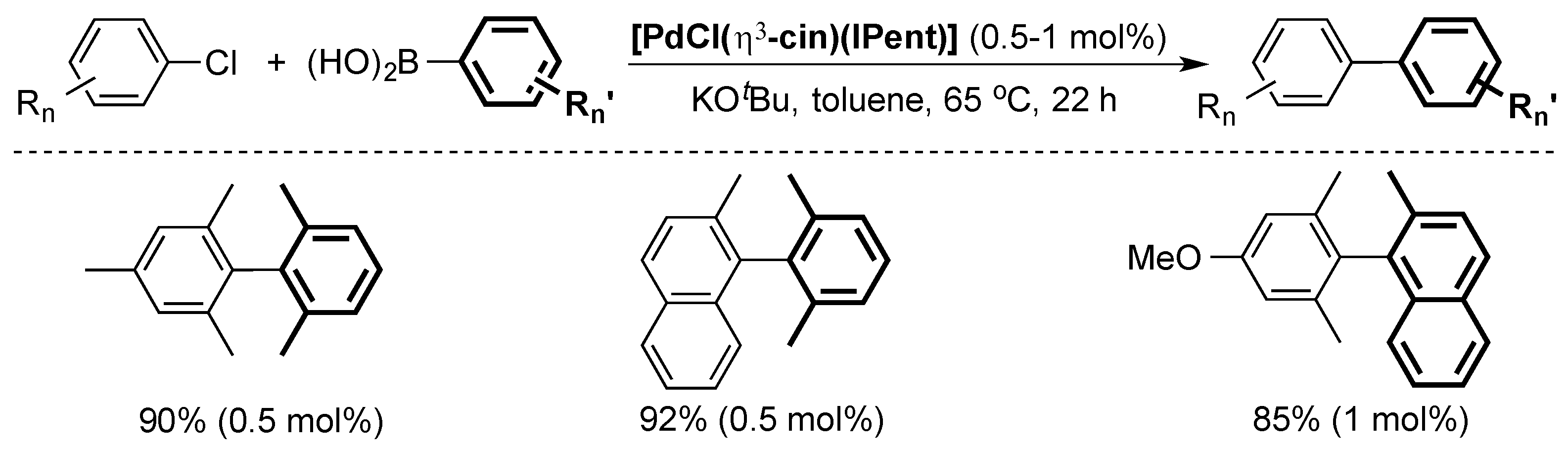
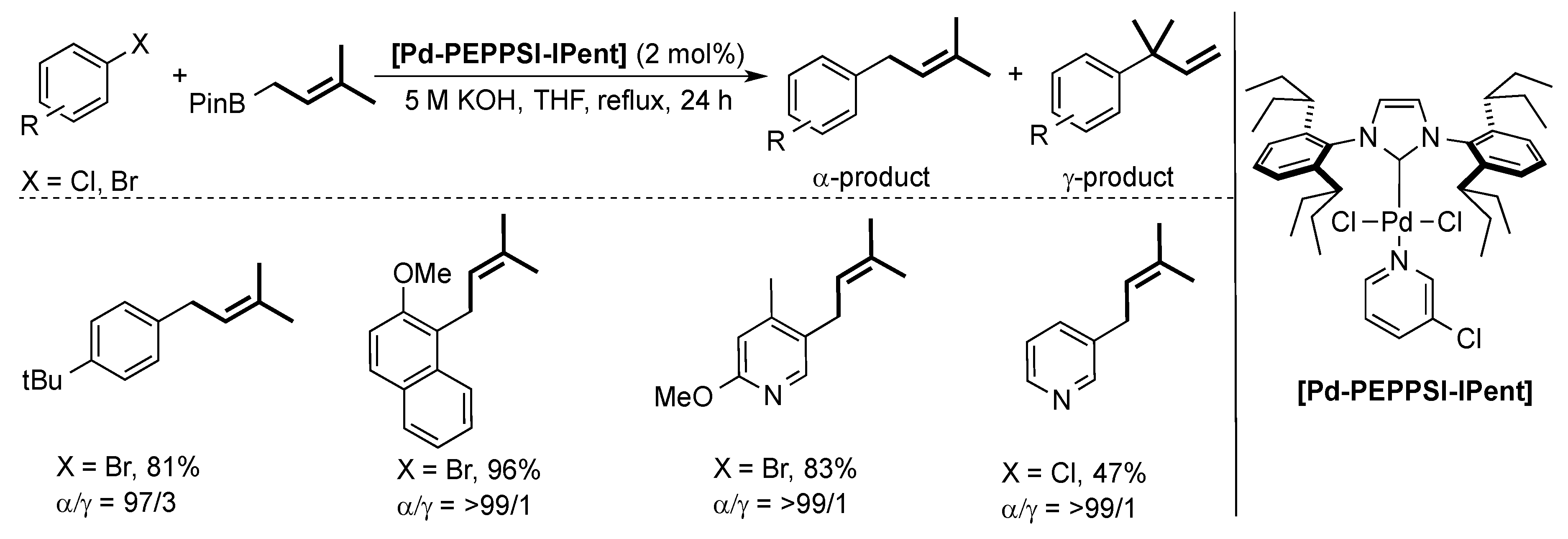
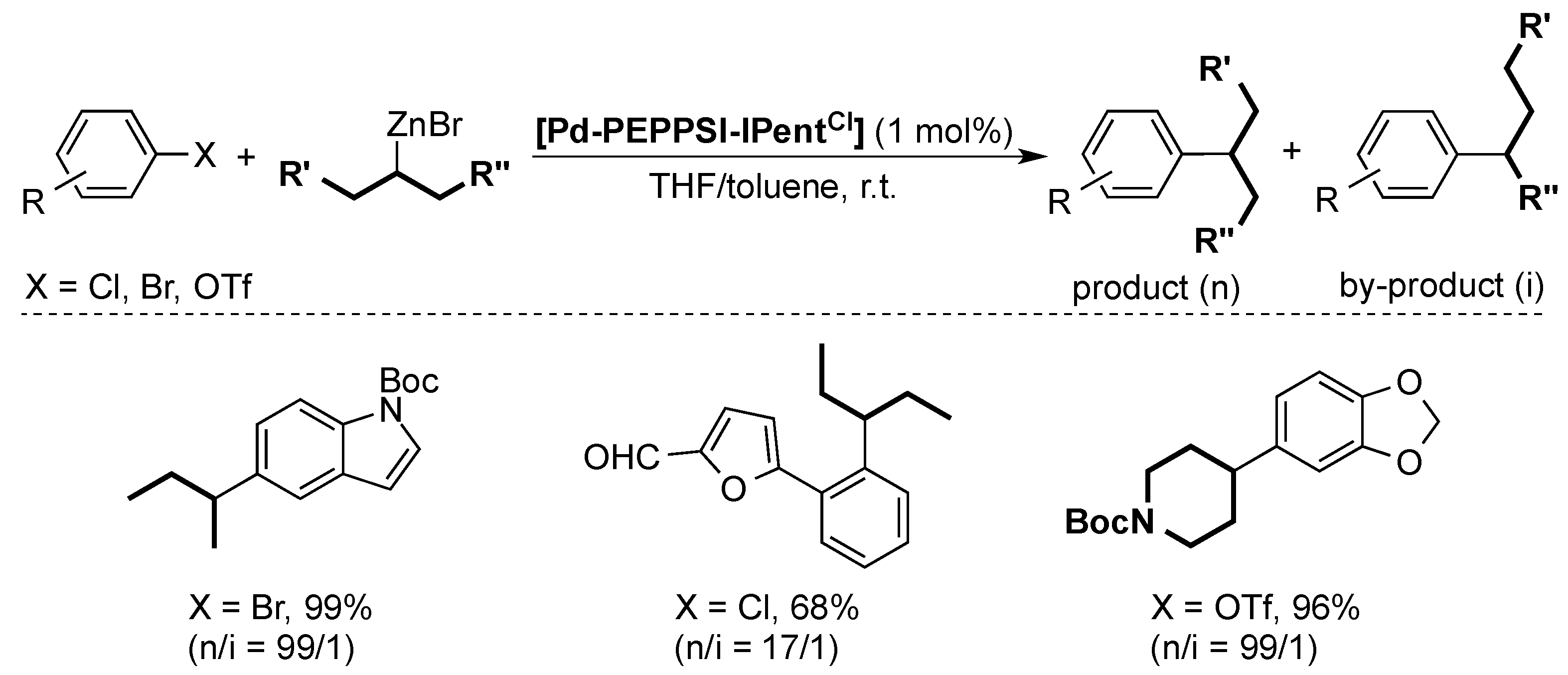
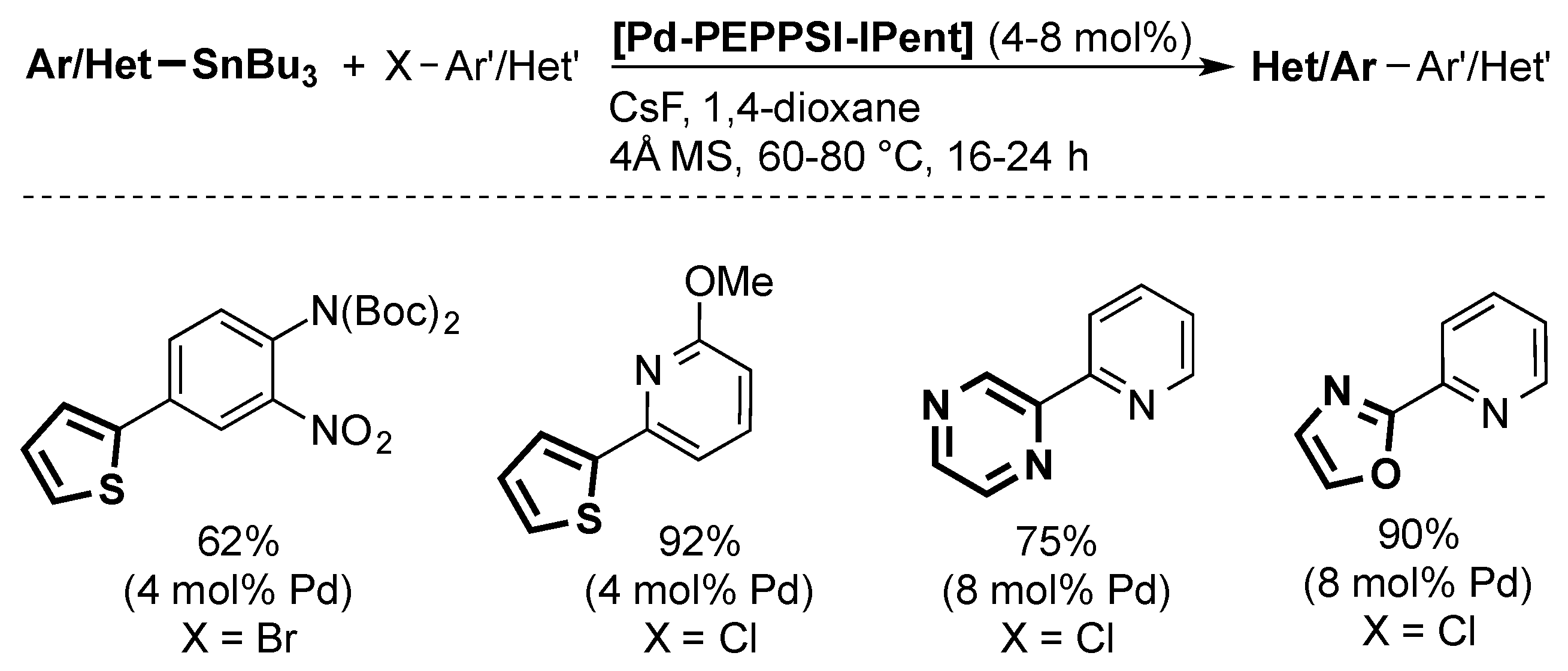
4. ITent
4.1. Suzuki–Miyaura Cross-Coupling
4.2. Negishi Cross-Coupling
4.3. Stille Coupling
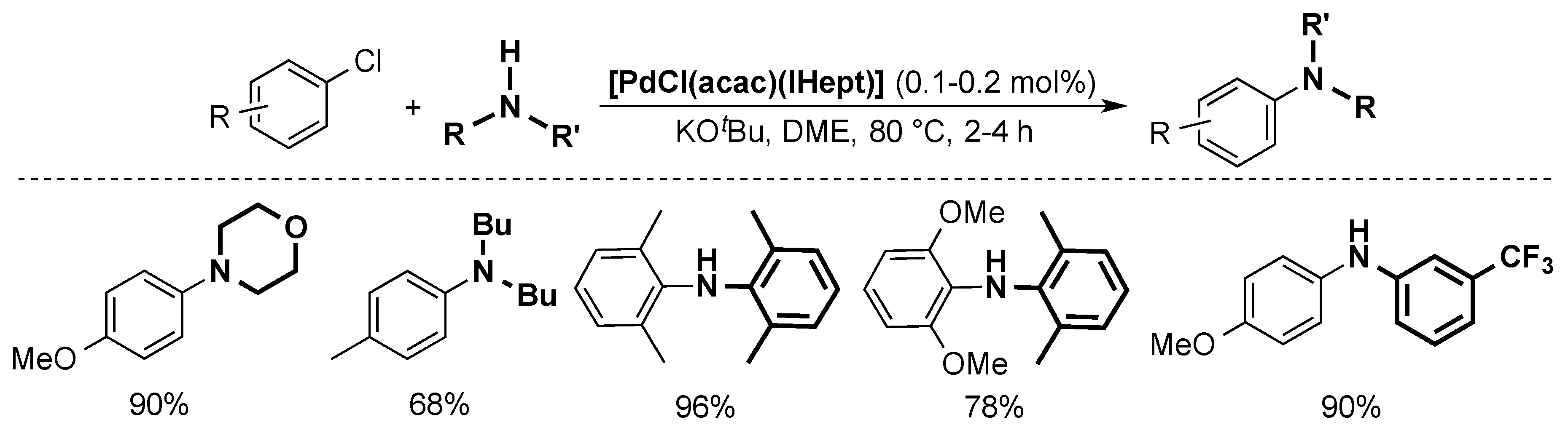
4.4. Buchwald-Hartwig Cross-Coupling
5. N-Naphthyl-Based NHCs
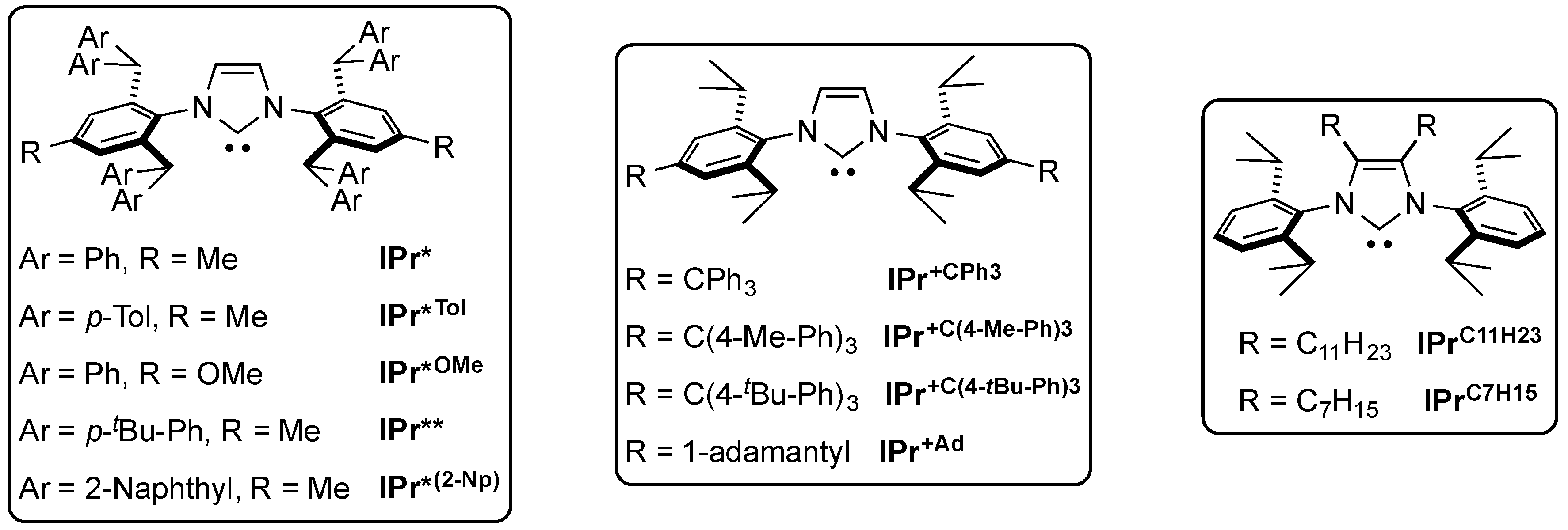
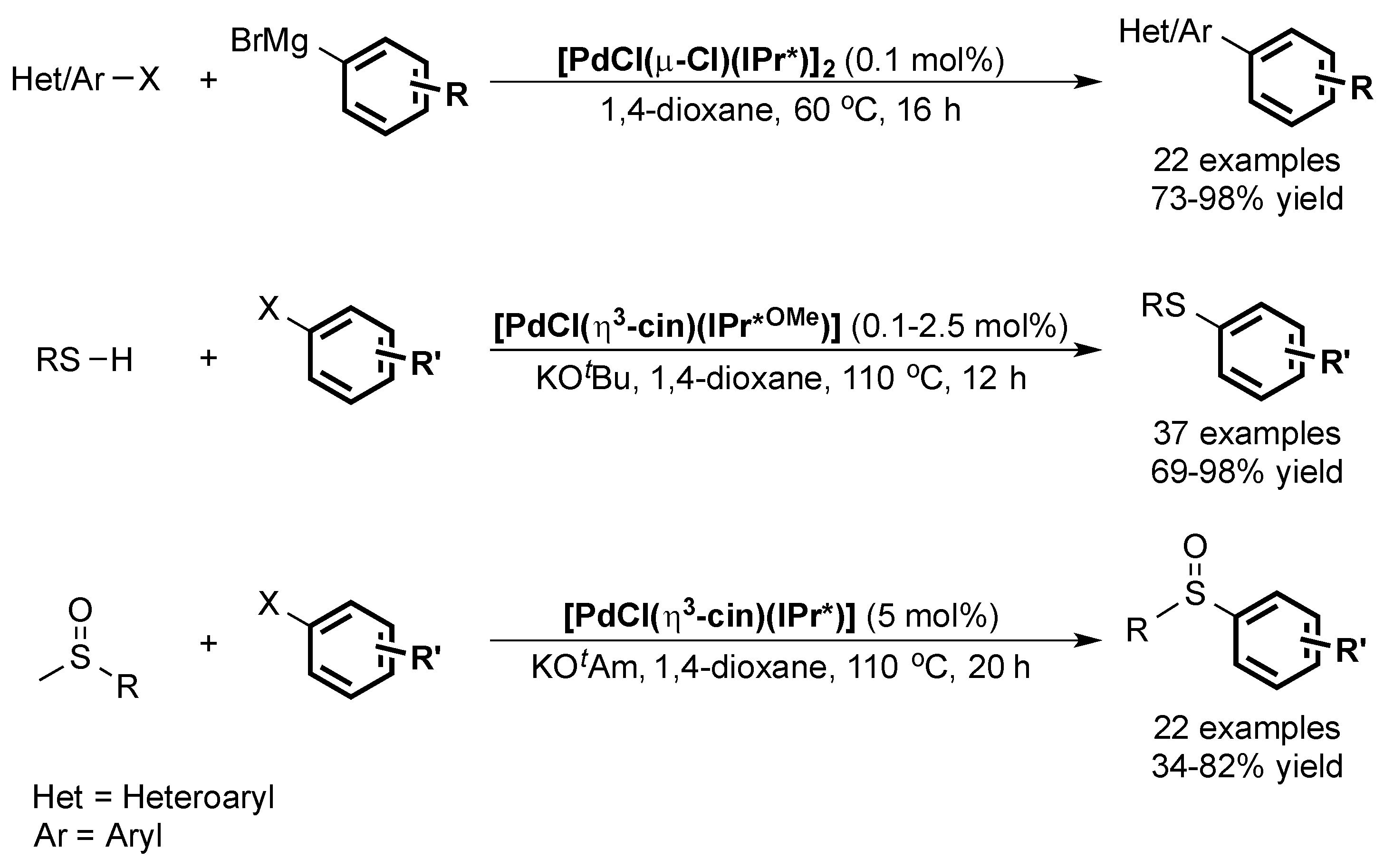
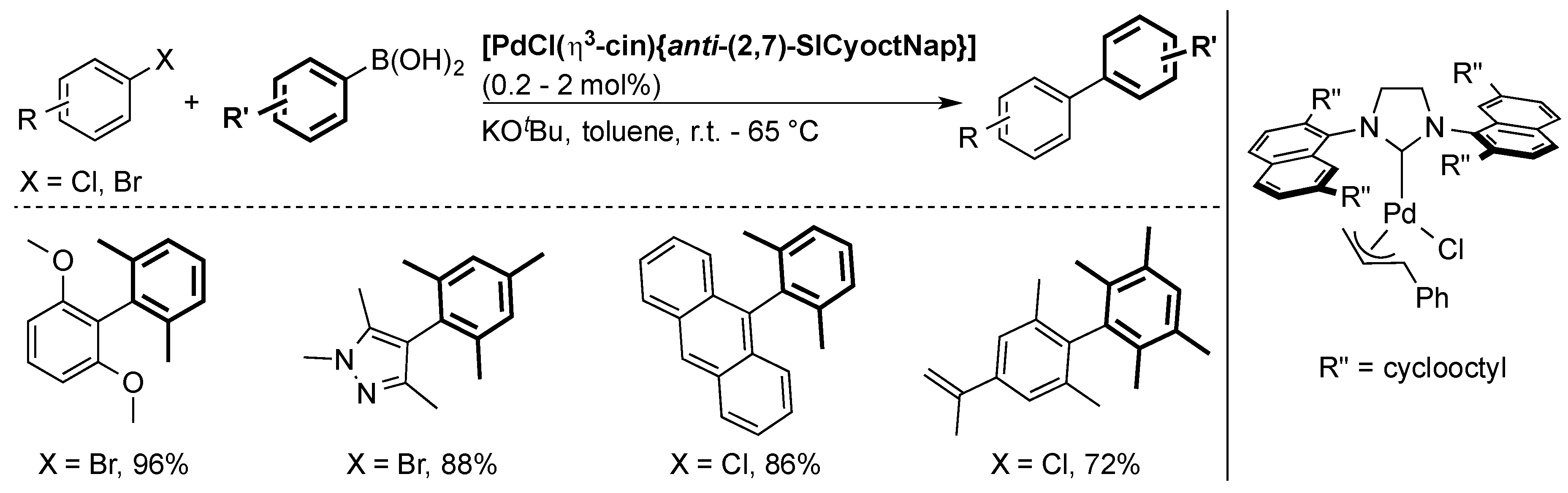
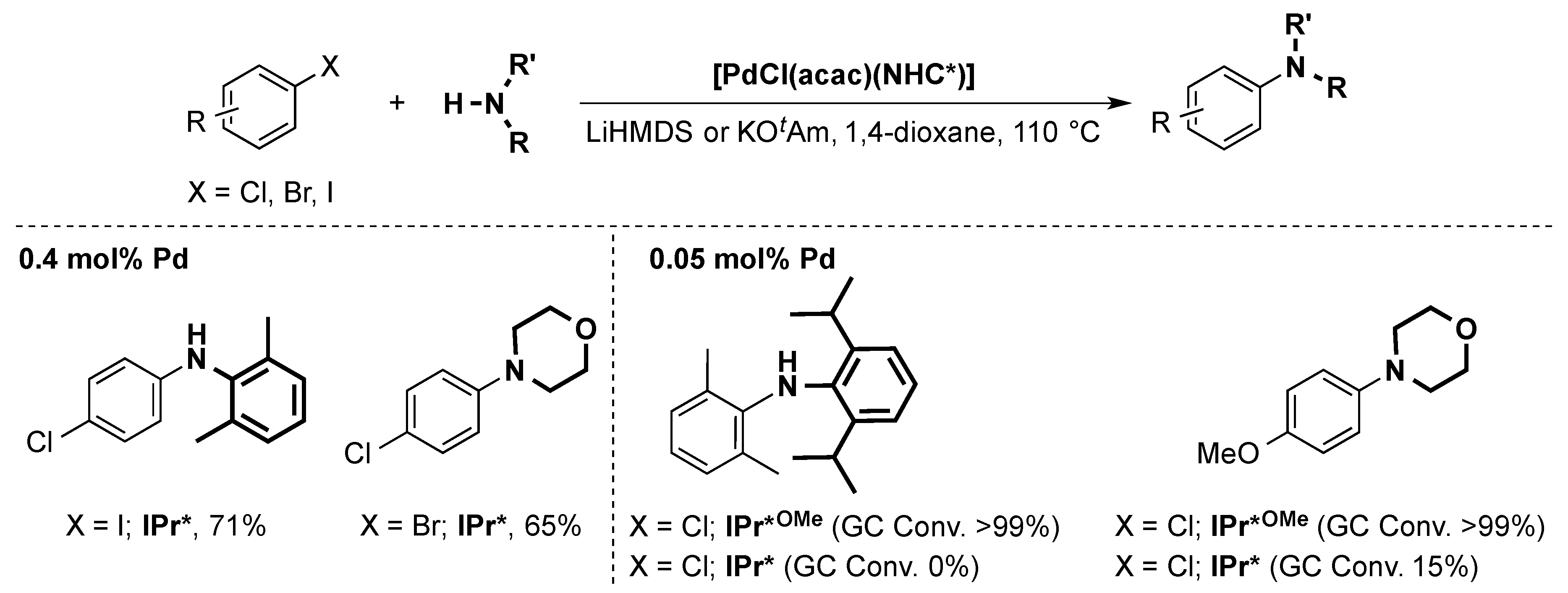
6. IPr* and Related NHCs
6.1. Suzuki Miyaura Cross-Coupling
6.2. Buchwald Hartwig Cross-Coupling
6.3. Other Palladium-Catalyzed Reactions
7. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Cazin, C.S.J. (Ed.) N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Springer: London, UK, 2011; ISBN 978-90-481-2866-2. [Google Scholar]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.D. Diverse Chemical Applications of N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2009, 131, 15075–15077. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.P.; Cazin, C.S.J. (Eds.) Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis; Thieme: Stuttgart, Germany, 2016; Volume 1, ISBN 978-31-320-1291-2. [Google Scholar]
- Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in gold catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Gade, L.H.; Bellemin-Laponnaz, S. Chiral N-Heterocyclic Carbenes as Stereodirecting Ligands in Asymmetric Catalysis. In N-Heterocyclic Carbenes in Transition Metal Catalysis; Glorius, F., Ed.; Springer: Berlin, Germany, 2007; pp. 117–157. ISBN 978-3-540-36930-1. [Google Scholar]
- Lin, I.J.B.; Vasam, C.S. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar] [CrossRef]
- Kuhl, O. The chemistry of functionalised N-heterocyclic carbenes. Chem. Soc. Rev. 2007, 36, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, M.; Guest, D.; Navarro, O. (N-Heterocyclic Carbene)–Palladium Complexes in Catalysis. In N-Heterocycle Carbenes: Effective Tools for Organometallic Synthesis; Nolan, S.P., Ed.; Wiley-VCH: Weinheim, Germany, 2014; pp. 85–110. ISBN 978-35-273-3490-2. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabba, F.P.; Bertrand, G. Stable carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef]
- Chartoire, A.; Nolan, S.P. Advances in C–C and C–X Coupling Using Palladium–N-Heterocyclic Carbene (Pd–NHC) Complexes. In New Trends in Cross-Coupling: Theory and Applications; Colacot, T.J., Ed.; RSC: Cambridge, UK, 2014; pp. 139–227. ISBN 978-1-84973-896-5. [Google Scholar]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Würtz, S.; Glorius, F. Surveying Sterically Demanding N-Heterocyclic Carbene Ligands with Restricted Flexibility for Palladium-catalyzed Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1523–1533. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hou Hoi, K.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, F.; Manzini, S.; Nolan, S.P. The Use of the Sterically Demanding IPr* and Related Ligands in Catalysis. Chem. Commun. 2014, 50, 14926–14937. [Google Scholar] [CrossRef] [PubMed]
- Szilvási, T.; Veszprémi, T. Internal Catalytic Effect of Bulky NHC Ligands in Suzuki–Miyaura Cross-Coupling Reaction. ACS Catal. 2013, 3, 1984–1991. [Google Scholar] [CrossRef]
- Widenhoefer, R.A.; Buchwald, S.L. Halide and Amine Influence in the Equilibrium Formation of Palladium Tris(o-tolyl)phosphine Mono(amine) Complexes from Palladium Aryl Halide Dimers. Organometallics 1996, 15, 2755–2763. [Google Scholar] [CrossRef]
- Poater, A.; Cavallo, L. Deactivation of Ru-benzylidene Grubbs catalysts active in olefin metathesis. Theor. Chem. Acc. 2012, 131, 1155. [Google Scholar] [CrossRef]
- Poater, A.; Bahri-Laleh, N.; Cavallo, L. Rationalizing current strategies to protect N-heterocyclic carbene-based ruthenium catalysts active in olefin metathesis from C–H (de)activation. Chem. Commun. 2011, 47, 6674–6676. [Google Scholar] [CrossRef]
- Poater, A.; Falivene, L.; Urbina-Blanco, C.A.; Manzini, S.; Nolan, S.P.; Cavallo, L. How does the addition of steric hindrance to a typical N-heterocyclic carbene ligand affect catalytic activity in olefin metathesis? Dalton Trans. 2013, 42, 7433–7439. [Google Scholar] [CrossRef]
- Altenhoff, G.; Goddard, R.; Lehmann, C.W.; Glorius, F. Sterically Demanding, Bioxazoline-Derived N-Heterocyclic Carbene Ligands with Restricted Flexibility for Catalysis. J. Am. Chem. Soc. 2004, 126, 15195–15201. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem. Int. Ed. 2005, 44, 5705–5709. [Google Scholar] [CrossRef]
- Huang, J.; Nolan, S.P. Efficient Cross-Coupling of Aryl Chlorides with Aryl Grignard Reagents (Kumada Reaction) Mediated by a Palladium/Imidazolium Chloride System. J. Am. Chem. Soc. 1999, 121, 9889–9890. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Krafczyk, R.; Schmutzler, R.; Craig, H.A.; Goerlich, J.R.; Marshall, W.J.; Unverzagt, M. Imidazolylidenes, imidazolinylidenes and imidazolidines. Tetrahedron 1999, 55, 14523–14534. [Google Scholar] [CrossRef]
- Organ, M.G.; Çalimsiz, S.; Sayah, M.; Hoi, K.H.; Lough, A.J. Pd-PEPPSI-IPent: An Active, Sterically Demanding Cross-Coupling Catalyst and Its Application in the Synthesis of Tetra-Ortho-Substituted Biaryls. Angew. Chem. Int. Ed. 2009, 48, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Berthon-Gelloz, G.; Siegler, M.A.; Spek, A.L.; Tinant, B.; Reek, J.N.H.; Markó, I.E. IPr* an easily accessible highly hindered N-heterocyclic carbene. Dalton Trans. 2010, 39, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Drinkel, E.; Gaggia, F.; Capolicchio, S.; Linden, A.; Falivene, L.; Cavallo, L.; Dorta, R. Room-Temperature Synthesis of Tetra-ortho-Substituted Biaryls by NHC-Catalyzed Suzuki–Miyaura Couplings. Chem. Eur. J. 2011, 17, 12886–12890. [Google Scholar] [CrossRef] [PubMed]
- Altenhoff, G.; Goddard, R.; Lehmann, C.W.; Glorius, F. An N-Heterocyclic Carbene Ligand with Flexible Steric Bulk Allows Suzuki Cross-Coupling of Sterically Hindered Aryl Chlorides at Room Temperature. Angew. Chem. Int. Ed. 2003, 42, 3690–3693. [Google Scholar] [CrossRef] [PubMed]
- Bexrud, J.; Lautens, M. A Rhodium IBiox[(−)-menthyl] Complex as a Highly Selective Catalyst for the Asymmetric Hydroarylation of Azabicyles: An Alternative Route to Epibatidine. Org. Lett. 2010, 12, 3160–3163. [Google Scholar] [CrossRef] [PubMed]
- Würtz, S.; Lohre, C.; Fröhlich, R.; Bergander, K.; Glorius, F. IBiox[(−)-menthyl]: A Sterically Demanding Chiral NHC Ligand. J. Am. Chem. Soc. 2009, 131, 8344–8345. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Oxazolines as chiral building blocks for imidazolium salts and N-heterocyclic carbene ligands. Chem. Commun. 2002, 2704–2705. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Anastasia, L. Palladium-Catalyzed Alkynylation. Chem. Rev. 2003, 103, 1979–2018. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Fu, G.C. The First Applications of Carbene Ligands in Cross-Couplings of Alkyl Electrophiles: Sonogashira Reactions of Unactivated Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003, 125, 13642–13643. [Google Scholar] [CrossRef] [PubMed]
- Altenhoff, G.; Würtz, S.; Glorius, F. The first palladium-catalyzed Sonogashira coupling of unactivated secondary alkyl bromides. Tetrahedron Lett. 2006, 47, 2925–2928. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; DeHope, A.; Donnadieu, B.; Bertrand, G. A Rigid Cyclic (Alkyl)(amino)carbene Ligand Leads to Isolation of Low-Coordinate Transition-Metal Complexes. Angew. Chem. Int. Ed. 2005, 44, 7236–7239. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Tang, H.; Zeng, X.; Liu, L.; Melaimi, M.; Bertrand, G. Cyclic (Amino)(aryl)carbenes (CAArCs) as Strong σ-Donating and π-Accepting Ligands for Transition Metals. Angew. Chem. Int. Ed. 2015, 54, 14915–14919. [Google Scholar] [CrossRef] [PubMed]
- Organ, M.G.; Chass, G.A.; Fang, D.-C.; Hopkinson, A.C.; Valente, C. Pd-NHC (PEPPSI) Complexes: Synthetic Utility and Computational Studies into Their Reactivity. Synthesis 2008, 2776–2797. [Google Scholar] [CrossRef]
- Meiries, S.; Le Duc, G.; Chartoire, A.; Collado, A.; Speck, K.; Arachchige, K.S.A.; Slawin, A.M.Z.; Nolan, S.P. Large yet Flexible N-Heterocyclic Carbene Ligands for Palladium Catalysis. Chem. Eur. J. 2013, 19, 17358–17368. [Google Scholar] [CrossRef]
- Pompeo, M.; Froese, R.D.J.; Hadei, N.; Organ, M.G. Pd-PEPPSI-IPent(Cl): A highly effective catalyst for the selective cross-coupling of secondary organozinc reagents. Angew. Chem. Int. Ed. 2012, 51, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.D.; He, X.X.; Liu, F.S. Bulky yet flexible Pd-PEPPSI-IPentAn for the synthesis of sterically hindered biaryls in air. J. Org. Chem. 2017, 82, 10898–10911. [Google Scholar] [CrossRef]
- Farmer, J.L.; Hunter, H.N.; Organ, M.G. Regioselective Cross-Coupling of Allylboronic Acid Pinacol Ester Derivatives with Aryl Halides via Pd-PEPPSI-IPent. J. Am. Chem. Soc. 2012, 134, 17470–17473. [Google Scholar] [CrossRef]
- Organ, M.G.; Avola, S.; Dubovyk, I.; Hadei, N.; Kantchev, E.A.B.; O’Brien, C.J.; Valente, C. A User-Friendly, All-Purpose Pd–NHC (NHC = N-Heterocyclic Carbene) Precatalyst for the Negishi Reaction: A Step Towards a Universal Cross-Coupling Catalyst. Chem. Eur. J. 2006, 12, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Calimsiz, S.; Sayah, M.; Mallik, D.; Organ, M.G. Pd-PEPPSI-IPent: Low-Temperature Negishi Cross-Coupling for the Preparation of Highly Functionalized, Tetra-ortho-Substituted Biaryls. Angew. Chem. Int. Ed. 2010, 49, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Belowich, M.E.; Hadei, N.; Organ, M.G. Pd-PEPPSI Complexes and the Negishi Reaction. Eur. J. Org. Chem. 2010, 2010, 4343–4354. [Google Scholar] [CrossRef]
- Calimsiz, S.; Organ, M.G. Negishi cross-coupling of secondary alkylzinc halides with aryl/heteroaryl halides using Pd–PEPPSI–IPent. Chem. Commun. 2011, 47, 5181–5183. [Google Scholar] [CrossRef] [PubMed]
- Price, G.A.; Hassan, A.; Chandrasoma, N.; Bogdan, A.R.; Djuric, S.W.; Organ, M.G. Pd-PEPPSI-IPent-SiO2: A supported catalyst for challenging Negishi coupling reactions in flow. Angew. Chem. Int. Ed. 2017, 56, 13347–13350. [Google Scholar] [CrossRef]
- Dowlut, M.; Mallik, D.; Organ, M.G. An Efficient Low-Temperature Stille–Migita Cross-Coupling Reaction for Heteroaromatic Compounds by Pd–PEPPSI–IPent. Chem. Eur. J. 2010, 16, 4279–4283. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Grasa, G.; Nolan, S.P. General and Efficient Catalytic Amination of Aryl Chlorides Using a Palladium/Bulky Nucleophilic Carbene System. Org. Lett. 1999, 1, 1307–1309. [Google Scholar] [CrossRef]
- Hoi, K.H.; Calimsiz, S.; Froese, R.D.J.; Hopkinson, A.C.; Organ, M.G. Amination with Pd–NHC Complexes: Rate and Computational Studies on the Effects of the Oxidative Addition Partner. Chem. Eur. J. 2011, 17, 3086–3090. [Google Scholar] [CrossRef]
- Hoi, K.H.; Calimsiz, S.; Froese, R.D.J.; Hopkinson, A.C.; Organ, M.G. Amination with Pd–NHC Complexes: Rate and Computational Studies Involving Substituted Aniline Substrates. Chem. Eur. J. 2012, 18, 145–151. [Google Scholar] [CrossRef]
- Sharif, S.; Day, J.; Hunter, H.N.; Lu, Y.; Mitchell, D.; Rodriguez, M.J.; Organ, M.G. Cross-coupling of primary amides to aryl and heteroaryl partners using (DiMeIHeptCl)Pd promoted by trialkylboranes or B(C6F5)3. J. Am. Chem. Soc. 2017, 139, 18436–18439. [Google Scholar] [CrossRef]
- Khadra, A.; Mayer, S.; Organ, M.G. Pd-PEPPSI-IPentCl: A useful catalyst for the coupling of 2-aminopyridine derivatives. Chem. Eur. J. 2017, 23, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-D.; Xu, C.; Lu, D.-D.; Shen, D.-S.; Li, T.; Liu, F.-S. Pd-PEPPSI-IPentAn promoted deactivated amination of aryl chlorides with amines under aerobic conditions. J. Org. Chem. 2018, 83, 9144–9155. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Mariz, R.; Gatti, M.; Costabile, C.; Poater, A.; Cavallo, L.; Linden, A.; Dorta, R. Identification and Characterization of a New Family of Catalytically Highly Active Imidazolin-2-ylidenes. J. Am. Chem. Soc. 2008, 130, 6848–6858. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Luan, X.; Mariz, R.; Blumentritt, S.; Linden, A.; Dorta, R. A New Class of Stable, Saturated N-Heterocyclic Carbenes with N-Naphthyl Substituents: Synthesis, Dynamic Behavior, and Catalytic Potential. Eur. J. Inorg. Chem. 2009, 2009, 1861–1870. [Google Scholar] [CrossRef]
- Gatti, M.; Wu, L.; Drinkel, E.; Gaggia, F.; Blumentritt, S.; Linden, A.; Dorta, R. The effect of substituents on the syn-anti conformer ratio in naphthyl-based imidazolinium salts and their corresponding N-heterocyclic carbenes. ARKIVOC 2011, 6, 176–198. [Google Scholar] [CrossRef]
- Martin, A.R.; Chartoire, A.; Slawin, A.M.Z.; Nolan, S.P. Extending the utility of [Pd(NHC)(cinnamyl)Cl] precatalysts: Direct arylation of heterocycles. Beilstein J. Org. Chem. 2012, 8, 1637–1643. [Google Scholar] [CrossRef]
- Meiries, S.; Speck, K.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*OMe)(acac)Cl]: Tuning the N-Heterocyclic Carbene in Catalytic C–N Bond Formation. Organometallics 2013, 32, 330–339. [Google Scholar] [CrossRef]
- Weber, S.G.; Loos, C.; Rominger, F.; Straub, B.F. Synthesis of an extremely sterically shielding N-heterocyclic carbene ligand. ARKIVOC 2012, 3, 226–242. [Google Scholar] [CrossRef]
- Dierick, S.; Dewez, D.F.; Markó, I.E. IPr*(2-Np)—An Exceedingly Bulky N-Heterocyclic Carbene. Organometallics 2014, 33, 677–683. [Google Scholar] [CrossRef]
- Dible, B.R.; Cowley, R.E.; Holland, P.L. Remote Substitution on N-Heterocyclic Carbenes Heightens the Catalytic Reactivity of Their Palladium Complexes. Organometallics 2011, 30, 5123–5132. [Google Scholar] [CrossRef]
- Rühling, A.; Rakers, L.; Glorius, F. Long Alkyl Chain NHC Palladium Complexes for the Amination and Hydrodehalogenation of Aryl Chlorides in Lipophilic Media. ChemCatChem 2017, 9, 547–550. [Google Scholar] [CrossRef]
- Chartoire, A.; Lesieur, M.; Falivene, L.; Slawin, A.M.Z.; Cavallo, L.; Cazin, C.S.J.; Nolan, S.P. [Pd(IPr*)(cinnamyl)Cl]: An Efficient Pre-catalyst for the Preparation of Tetra-ortho-substituted Biaryls by Suzuki–Miyaura Cross-Coupling. Chem. Eur. J. 2012, 18, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, W.; Deng, C.; Wang, Z.; Zhang, Y.; Qian, H. Preparation of poly(bulky N-heterocyclic carbene) as the self-supporting catalyst toward Suzuki–Miyaura Reaction. Macromol. Chem. Phys. 2018, 219, 1800156. [Google Scholar] [CrossRef]
- Ormerod, D.; Lefevre, N.; Dorbec, M.; Eyskens, I.; Vloemans, P.; Duyssens, K.; Diez de la Torre, V.; Kaval, N.; Merkul, E.; Sergeyev, S.; et al. Potential of homogeneous Pd catalyst separation by ceramic membranes. Application to downstream and continuous flow processes. Org. Process Res. Dev. 2016, 20, 911–920. [Google Scholar] [CrossRef]
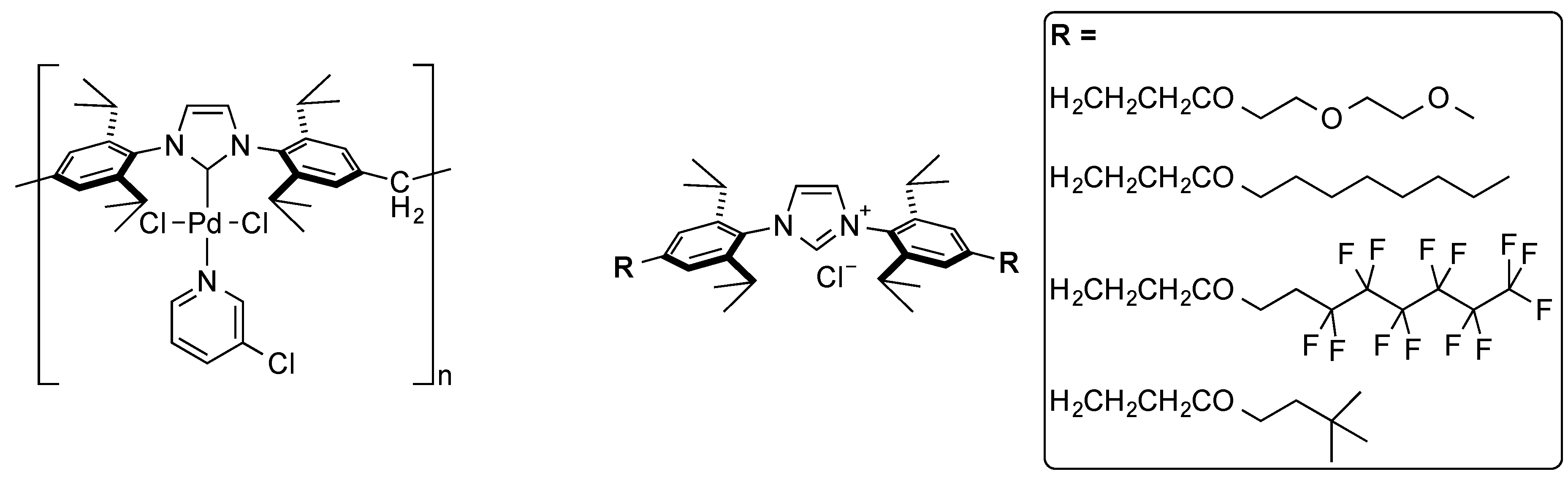
- Ormerod, D.; Dorbec, M.; Merkul, E.; Kaval, N.; Lefèvre, N.; Hostyn, S.; Eykens, L.; Lievens, J.; Sergeyev, S.; Maes, B.U.W. Synthesis of Pd complexes containing tailed NHC ligands and their use in a semicontinuous membrane-assisted Suzuki cross-coupling process. Org. Process Res. Dev. 2018, 22, 1509–1517. [Google Scholar] [CrossRef]
- Meiries, S.; Chartoire, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(acac)Cl]: An Easily Synthesized, Bulky Precatalyst for C–N Bond Formation. Organometallics 2012, 31, 3402–3409. [Google Scholar] [CrossRef]
- Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*)(3-Cl-pyridinyl)Cl2]: A Novel and Efficient PEPPSI Precatalyst. Organometallics 2012, 31, 6947–6951. [Google Scholar] [CrossRef]
- Chartoire, A.; Frogneux, X.; Nolan, S.P. An Efficient Palladium-NHC (NHC = N-Heterocyclic Carbene) and Aryl Amination Pre-Catalyst: [Pd(IPr*)(cinnamyl)Cl]. Adv. Synth. Catal. 2012, 354, 1897–1901. [Google Scholar] [CrossRef]
- Chartoire, A.; Boreux, A.; Martin, A.R.; Nolan, S.P. Solvent-free aryl amination catalysed by [Pd(NHC)] complexes. RSC Adv. 2013, 3, 3840–3843. [Google Scholar] [CrossRef]
- Lesieur, M.; Slawin, A.M.Z.; Cazin, C.S.J. [Pd(μ-Cl)Cl(IPr*)]2: A highly hindered pre-catalyst for the synthesis of tetra-ortho-substituted biaryls via Grignard reagent cross-coupling. Org. Biomol. Chem. 2014, 12, 5586–5589. [Google Scholar] [CrossRef]
- Bastug, G.; Nolan, S.P. Carbon–Sulfur Bond Formation Catalyzed by [Pd(IPr*OMe)(cin)Cl] (cin = cinnamyl). J. Org. Chem. 2013, 78, 9303–9308. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, F.; Chartoire, A.; Nolan, S.P. Direct S-Arylation of Unactivated Arylsulfoxides Using [Pd(IPr*)(cin)Cl]. ACS Catal. 2013, 3, 2190–2193. [Google Scholar] [CrossRef]
- Martin, A.R.; Makida, Y.; Meiries, S.; Slawin, A.M.Z.; Nolan, S.P. Enhanced Activity of [Ni(NHC)CpCl] Complexes in Arylamination Catalysis. Organometallics 2013, 32, 6265–6270. [Google Scholar] [CrossRef]
- Martin, A.R.; Nelson, D.J.; Meiries, S.; Slawin, A.M.Z.; Nolan, S.P. Efficient C-N and C-S Bond Formation Using the Highly Active [Ni(allyl)Cl(IPr*OMe)] Precatalyst. Eur. J. Org. Chem. 2014, 15, 3127–3131. [Google Scholar] [CrossRef]
- Fernández-Salas, J.A.; Marelli, E.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. General and Mild Ni0-Catalyzed α-arylation of ketones Using Aryl Chlorides. Chem. Eur. J. 2015, 21, 3906–3909. [Google Scholar] [CrossRef]
- Fernández-Salas, J.A.; Marelli, E.; Nolan, S.P. Synthesis of (diarylmethyl)amines using Ni-catalyzed arylation of C(sp3)-H bonds. Chem. Sci. 2015, 6, 4973–4977. [Google Scholar] [CrossRef]
- Matsubara, K.; Fukahori, Y.; Inatomi, T.; Tazaki, S.; Yamada, Y.; Koga, Y.; Kanegawa, S.; Nakamura, T. Monomeric Three-Coordinate N-Heterocyclic Carbene Nickel(I) Complexes: Synthesis, Structures, and catalytic Applications in Cross-Coupling Reactions. Organometallics 2016, 35, 3281–3287. [Google Scholar] [CrossRef]
- Nakanowatari, S.; Müller, T.; Oliveira, J.C.A.; Ackermann, L. Bifurcated Nickel-Catalyzed Functionalizations: Heteroarene C–H Activation with Allenes. Angew. Chem. Int. Ed. 2017, 56, 15891–15895. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, G.; Xu, J.; Sun, H.; Shen, Q. Nickel-Catalyzed Reductive Cross-Coupling of Benzyl Chlorides with Aryl Chlorides/Fluorides: A One-Pot Synthesis of Diarylmethanes. Org. Lett. 2016, 18, 2860–2863. [Google Scholar] [CrossRef]
- Diesel, J.; Finogenova, A.M.; Cramer, N. Nickel-Catalyzed Enantioselective Pyridone C–H Functionalizations Enabled by a Bulky N-Heterocyclic Carbene Ligand. J. Am. Chem. Soc. 2018, 140, 4489–4493. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Newman, S.G. Methyl Esters as Cross-Coupling Electrophiles: Direct Synthesis of Amide bonds. ACS Catal. 2019, 9, 4426–4433. [Google Scholar] [CrossRef]
- Inatomi, T.; Fukahori, Y.; Yamada, Y.; Ishikawa, R.; Kanegawa, S.; Koga, Y.; Matsubara, K. Ni(I)-Ni(III) cycle in Buchwald-Hartwig amination of aryl bomide mediated by NHC-ligated Ni(I) complexes. Catal. Sci. Technol. 2019, 9, 1784–1793. [Google Scholar] [CrossRef]
- Pelties, S.; Wolf, R. Iron(II), Cobalt(II), and Nickel(II) Complexes of a Cyclic (Alkyl)(amino)carbene. Z. Anorg. Allg. Chem. 2013, 639, 2581–2585. [Google Scholar] [CrossRef]
- Henrion, M.; Cardoso, B.P.; César, V.; Chetcutti, M.J.; Ritleng, V. Nickel(II) Complexes of Highly σ-Donating Cyclic (Alkyl)(Amino)- and Malonate-Carbenes: Syntheses and Catalytic Studies. Organometallics 2017, 36, 1113–1121. [Google Scholar] [CrossRef]
- Vijaykumar, G.; Jose, A.; Vardhanapu, P.K.; Mandal, S.K. Abnormal-NHC-Supported Nickel Catalysts for Hydroheteroarylation of Vinylarenes. Organometallics 2017, 36, 4753–4758. [Google Scholar] [CrossRef]
- Nett, A.J.; Cañellas, S.; Higuchi, Y.; Robo, M.T.; Kochkodan, J.M.; Haynes, M.T., II; Kampf, J.W.; Montgomery, J. Stable, Well-Defined Nickel(0) Catalysts for Catalytic C–C and C–N Bond Formation. ACS Catal. 2018, 8, 6606–6611. [Google Scholar] [CrossRef]
- Felten, S.; Marshall, S.F.; Groom, A.J.; Vanderlinden, R.T.; Stolley, R.M.; Louie, J. Synthesis and Characterization of [(NHC)Ni(styrene)2] Complexes: Isolation of Monocarbene Nickel Complexes and Benchmarking of %Vbur in (NHC)Ni-π Systems. Organometallics 2018, 37, 3687–3697. [Google Scholar] [CrossRef]
- Elsby, M.R.; Liu, J.; Zhu, S.; Hu, L.; Huang, G.; Johnson, S.A. Influence of N-Heterocyclic Carbene dteric Bulk on Selectivity in Nickel Catalyzed C–H Bond Silylation, Germylation, and Stannylation. Organometallics 2019, 38, 436–450. [Google Scholar] [CrossRef]
- Iglesia, M.J.; Blandez, J.F.; Fructos, M.R.; Prieto, A.; Álvarez, E.; Belderrain, T.R.; Nicasio, M.C. Synthesis, Structural Characterization, and Catalytic Activity of IPrNi(styrene)2 in the Amination of Aryl Tosylates. Organometallics 2012, 31, 6312–6316. [Google Scholar] [CrossRef]
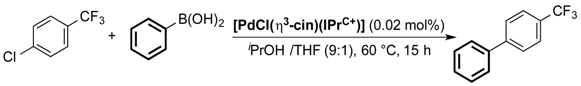
| Entry | [PdCl(η3-cin)(IPr+C)] | GC Conversion (%) |
|---|---|---|
| 1 | [PdCl(η3-cin)(IPr)] | 55% |
| 2 | [PdCl(η3-cin)(IPr+CPh3)] | 87% |
| 3 | [PdCl(η3-cin)(IPr+C(4-Me-Ph)3)] | 86% |
| 4 | [PdCl(η3-cin){IPr+C(4-tBu-Ph)3)] | 85% |
| 5 | [PdCl(η3-cin)(IPr+Ad)] | 69% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanden Broeck, S.M.P.; Nahra, F.; Cazin, C.S.J. Bulky-Yet-Flexible Carbene Ligands and Their Use in Palladium Cross-Coupling. Inorganics 2019, 7, 78. https://doi.org/10.3390/inorganics7060078
Vanden Broeck SMP, Nahra F, Cazin CSJ. Bulky-Yet-Flexible Carbene Ligands and Their Use in Palladium Cross-Coupling. Inorganics. 2019; 7(6):78. https://doi.org/10.3390/inorganics7060078
Chicago/Turabian StyleVanden Broeck, Sofie M. P., Fady Nahra, and Catherine S. J. Cazin. 2019. "Bulky-Yet-Flexible Carbene Ligands and Their Use in Palladium Cross-Coupling" Inorganics 7, no. 6: 78. https://doi.org/10.3390/inorganics7060078
APA StyleVanden Broeck, S. M. P., Nahra, F., & Cazin, C. S. J. (2019). Bulky-Yet-Flexible Carbene Ligands and Their Use in Palladium Cross-Coupling. Inorganics, 7(6), 78. https://doi.org/10.3390/inorganics7060078