Abstract
Our discussion focuses upon three possible features that a bonded halogen atom may exhibit on its outer side, on the extension of the bond. These are (1) a region of lower electronic density (a σ-hole) accompanied by a positive electrostatic potential with a local maximum, (2) a region of lower electronic density (a σ-hole) accompanied by a negative electrostatic potential that also has a local maximum, and (3) a buildup of electronic density accompanied by a negative electrostatic potential that has a local minimum. In the last case, there is no σ-hole. We show that for diatomic halides and halogen-substituted hydrides, the signs and magnitudes of these maxima and minima can be expressed quite well in terms of the differences in the electronegativities of the halogen atoms and their bonding partners, and the polarizabilities of both. We suggest that the buildup of electronic density and absence of a σ-hole on the extension of the bond to the halogen may be an operational indication of ionicity.
1. σ-Hole Interactions
The term “σ-hole”, introduced by Clark et al. [1], refers to a local region of lower electronic density on the outer side of a bonded atom, on the extension of the bond to that atom. If the electronic density of a σ-hole is sufficiently low, there will be a positive electrostatic potential associated with it, through which the atom can interact attractively with negative sites such as lone pairs, π-electrons, and anions.
Such positive regions were first observed on the halogen atoms of some organic halides, by Brinck et al. in 1992 [2,3]. They pointed out that these unexpected positive regions provide an explanation of halogen bonding, the long-known but puzzling noncovalent interactions between bonded halogen atoms (which are presumed to be negative in character) and negative sites.
It must be emphasized that the interactions are with the positive potentials, not with the σ-holes, and σ-holes do not always give rise to positive electrostatic potentials. For instance, fluorines often have σ-holes with negative potentials, although less negative than their surroundings on the molecular surfaces. Such fluorines do not form halogen bonds (unless the negative site has a strong enough electric field to induce a positive potential [4,5]).
During the years 2007–2009, it was shown by Murray et al. [6,7,8] that localized positive electrostatic potentials are also often present on the extensions of bonds to atoms in Groups IV–VI, again allowing interactions with negative sites. This has led to understanding numerous instances of noncovalent bonding involving these atoms [9,10,11]. There have been several reviews of halogen bonding and other noncovalent interactions arising from σ-holes [9,12,13,14,15].
What is the origin of a σ-hole on a bonded atom? When two atoms interact to form a bond, the charge distribution of each undergoes some degree of polarization due to the electric field of the other. Atoms in Groups V–VII commonly develop a lower electronic density and shorter radius on the extension of the bond (a σ-hole) and a higher electronic density and larger radius on one or more lateral sides [16,17,18,19]. There is often (not always) a positive electrostatic potential associated with the σ-hole and negative one(s) on the lateral side(s). In such cases, the atom can interact favorably with negative sites along the extension of the bond and with positive sites through its lateral side(s). This has indeed been observed, both crystallographically [20,21] and computationally [22].
In the present work, our objective is two-fold: (1) To address, more comprehensively than in the past, the long-standing issue of what factors govern the signs and strengths of the electrostatic potentials on the extensions of bonds, focusing upon bonds to halogen atoms, and (2) to extend the analysis to bonds between the halogens and Groups I–III of the periodic table. These have received much less attention than bonds to Groups IV–VII.
2. The Molecular Electrostatic Potential
Since much of our discussion will deal with electrostatic potentials, we will briefly review this property. Any system of nuclei and electrons, such as a molecule or molecular complex, has an electrostatic potential V(r) at every point r in the surrounding space, given by Equation (1):
ZA is the charge on nucleus A, located at RA and ρ(r) is the molecule’s electronic density. Those regions in which V(r) is positive, reflecting dominant nuclear contributions, will interact attractively with nucleophiles (negative sites); regions of negative V(r), in which the effect of the electrons dominates, are attractive to electrophiles (positive sites).
The electrostatic potential is a real physical property that is observable. It can be determined experimentally, by diffraction techniques [23,24,25], as well as computationally. It is important to recognize that the value of the potential at any point r reflects contributions from all of the nuclei and electrons of the molecule [26,27,28,29,30]. Among the consequences of this are that the electrostatic potential does not in general follow the electron density, i.e., electron-rich regions do not necessarily have negative potentials, and that the positive potentials due to σ-holes may not be directly at the σ-hole [19,30,31,32].
In analyzing the noncovalent interactions of a molecule, V(r) is typically computed on its surface, as defined by the 0.001 au contour of its electronic density [33], and is labeled VS(r). The locally most positive and most negative values, of which there may be several on a given molecular surface, are designated as VS,max and VS,min, respectively.
In the present study, all calculations are at the density functional M06-2X/6-311G* level, with the Gaussian 09 [34] and the WFA-SAS [35] codes. The M06-2X functional was especially designed for noncovalent interactions and has been proven to be very effective for this purpose [36,37,38]. Extensive comparisons have demonstrated that the 6-311G* basis set gives reliable electrostatic potentials [39].
3. Electrostatic Potentials Associated with σ-Holes
What determines the sign and magnitude of the electrostatic potential due to a σ-hole? Two common generalizations invoke (a) the polarizability of the atom that has the σ-hole, and (b) the electron-attracting power of that atom relative to that of its bonding partner [9,12]. The more polarizable is the atom, and the more electron-attracting is its partner, the more positive will be the potential, and specifically the VS,mas, corresponding to the atom’s σ-hole. First-row atoms, having low polarizabilities and high electronegativities, often have negative VS,mas associated with their σ-holes [9,12,40]. This is sometimes true of second-row atoms as well [40]. However, when such atoms are linked to strongly electron-attracting residues, their σ-holes do give rise to positive VS,max [6,40,41,42]. For instance, the fluorines in both H3C–F and NC–F have σ-holes. However, the VS,max are −21 and 16 kcal/mol, respectively [40].
The above generalizations are overall quite useful for atoms in Groups IV–VII. However, significant exceptions and complications do arise. In the molecule FPBr(CN), for instance, the three σ-holes on the phosphorus have VS,max of 39 kcal/mol (Br–P bond), 35 kcal/mol (F–P bond), and 34 kcal/mol (NC–P) [30]. This is exactly the opposite of what would be anticipated from the fact that the CN group is the most electron-attracting and Br is the least. The arsenic in (H3C)2AsCN is expected to have three VS,max, corresponding to its three bonds, but the three positive regions overlap and result in just a single VS,max [32]. In heterocyclic molecules, the VS,mas anticipated on the extensions of the ring bonds frequently overlap and produce single VS,max near the centers of the bonds [19,32]. The whole situation is further analyzed in two extensive studies [43,44].
A basic problem, as already emphasized, is that the electrostatic potential at any point r is composed of contributions from all of the nuclei and electrons of the molecule [26,27,28,29,30], more so as they are closer to r. Thus, the location and value of the VS,max on an atom depend not only upon the atom itself and its bonding partner, but also upon other nearby atoms. This is less of a problem for halogen atoms, because they tend to protrude from a molecular framework and accordingly do not usually have other atoms in their immediate proximities. Our present analysis will accordingly focus upon halides.
4. Electrostatic Potentials on Extensions of Bonds to Halogen Atoms
4.1. Diatomic Molecules
We begin by looking at the computed electrostatic potentials of the diatomic molecules AX (Table 1), where A ranges from H to F, plus Cl and Br, and X is F, Cl, and Br. These molecules include singlets, doublets, and triplets. Our focus is upon the electrostatic potentials on the atoms X, on the extensions of the A–X bonds. By considering only diatomics, we eliminate the effects of atoms other than the bonding partners of the X atoms.

Table 1.
Properties of AX diatomic molecules (X = F, Cl and Br). VS(X) is computed maximum or minimum of VS(r) on X, on the extension of the A–X bond. The minima are explicitly indicated. χA − χX is difference in electronegativities of A and X. R1 and R2 are distances from X nucleus to 0.001 au contour, along the extension of the A–X bond (R1) and to the lateral side of X (R2) a.
Table 1 lists the extrema of VS(r) on the 0.001 au surfaces of the atoms X, on the extensions of the A–X bonds. These extrema include both maxima (VS,max) and minima (VS,min), and both the maxima and the minima can be either positive or negative. As far as we are aware, the possibility of electrostatic potential minima on the extensions of bonds to halogen atoms has received little or no attention in the past.
We will first discuss the maxima, both positive and negative. Most of the extrema in Table 1 are positive maxima. However, there are five maxima that are negative. These are the VS,max of the fluorines in HF, NF, ClF, and BrF, and that of the chlorine in BeCl. (The VS,max of the bromine in BeBr should be viewed as zero.) These negative VS,max are consistent with the generalizations mentioned in Section 3. Fluorine is the least polarizable and most electronegative of the elements, and chlorine also has a low polarizability and is much more electronegative than beryllium. All of these factors promote maxima being negative.
The relative electronegativities of A and X, χA and χB, are not, in themselves, sufficient to predict whether a VS,max on X will be positive or negative. The quantities (χA − χX) are listed in Table 1, using the electronegativity values in Table 2. (The electronegativity scale is a modified version [45,46] of the widely-used one proposed by Allen [47,48].) The data in Table 1 show that X being more electronegative than A does not mean that a VS,max on X will be negative. There are nine molecules in Table 1 for which the quantity (χA − χX) is negative but the VS,max of X is positive.

Table 2.
Electronegativities χ and polarizabilities α of ground-state atoms a.
All of the VS,max in Table 1, whether positive or negative, do correspond to σ-holes on the X atoms. This is shown by the dimensions of these atoms, also given in Table 1. For the molecules having a VS,max on X, the distance from the nucleus of X to the 0.001 au surface along the extension of the bond is in every case less than the distances to the lateral sides of X. This indicates a lower electronic density along the bond extension.
A particularly striking feature of Table 1 is that five molecules have minima (VS,min), not maxima, along the extensions of the bonds to X. The five molecules are LiF, BeF, BF, LiCl, and LiBr. For all of these except BF the VS,min are negative; for BF it is essentially neutral, 1.1 kcal/mol. In each of these five molecules, the distance from the nucleus of X to the 0.001 au contour along the bond extension is greater than the distance to the lateral sides of X. This means that the electronic density is increased along the bond extension (the opposite of a σ-hole). Thus, the five molecules with VS,min on the X atom do not have σ-holes.
The contrast between molecules that do and do not have σ-holes is illustrated by Figure 1, which displays the computed electrostatic potentials on the molecular surfaces of (a) FCl and (b) LiCl. In FCl, there is a maximum on the chlorine on the extension of the F–Cl bond, with VS,max = 45.0 kcal/mol. The distance from the chlorine nucleus to the 0.001 au surface along the bond extension is 1.81 Å, compared to 2.12 Å perpendicular to the bond. This confirms the presence of a σ-hole on the chlorine.
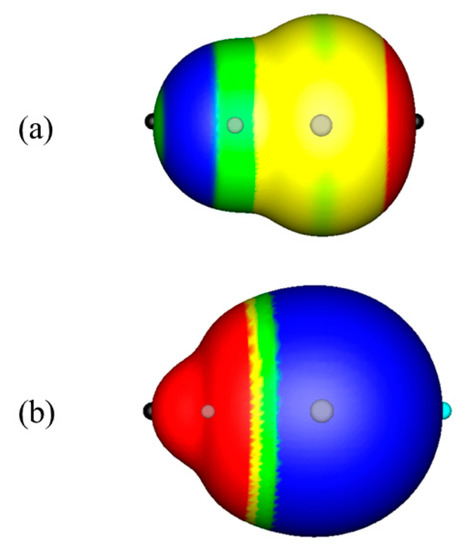
Figure 1.
Computed electrostatic potentials on the 0.001 au surfaces of (a) FCl and (b) LiCl. The gray circles indicate the positions of the atoms; the chlorines are on the right in both molecules. Color ranges, in kcal/mole: Red, more positive than 10; yellow, between 10 and zero; green, between zero and −10; blue, more negative than −10. The black hemispheres correspond to VS,max on both atoms in FCl and the lithium in LiCl; the blue hemisphere corresponds to a VS,min on the chlorine in LiCl. The VS,max on the fluorine in FCl is negative. Note that the outer surface of the chlorine in FCl is slightly flattened compared to that in LiCl; this is due to the σ-hole of the chlorine in FCl.
In LiCl, on the other hand, the chlorine has a minimum on the extension of the LiCl bond, with VS,min = −43.1 kcal/mol. The distance from the chlorine nucleus to the 0.001 au surface along the bond extension is 2.15 Å, and it is 2.05 Å perpendicular to the bond. This indicates an increase in electronic density on the outer side of the chlorine; there is no σ-hole on the chlorine. It can be seen in Figure 1 that the outer side of the chlorine is rounder in LiCl, Figure 1b than in FCl, Figure 1a; FCl displays the “polar flattening” that is associated with the presence of a σ-hole.
The five molecules in Table 1 that have a minimum (VS,min) on the extension of the bond to X (LiF, LiCl, LiBr, BeF, and BF) are the ones that have the most electronegative X relative to A, i.e., (χA − χX) has the most negative values. The electronic densities on their halogen atoms X are greater along the extensions of the bonds than on their lateral sides, and as we have already mentioned, the halogen atoms have no σ-holes.
Can the various VS,max and VS,min in Table 1, on the extensions of the bonds to the halogen atoms X, be represented analytically? We have already noted in Section 3 that the VS,max associated with σ-holes are linked to the relative electron attracting powers of X and its bonding partner A, and to the polarizability of X. Since the rearrangements of electronic density that lead to either a VS,max or a VS,min on X must involve A as well as X, it seems reasonable that the polarizability of A should also be taken explicitly into account [44].
Accordingly, we tested the three-parameter relationship Equation (2), using the electronegativity differences in Table 1 and the polarizabilities α in Table 2.
VS,max or VS,min = c1(χA − χX) + c2αA + c3αX + c4
The result obtained with the NCSS statistical analysis package [50], shown in Figure 2, is very satisfactory. The correlation between predicted and computed VS,max or VS,min is R2 = 0.943. The coefficients in Equation (2) are: c1 = 18.27, c2 = −1.233, c3 = 3.546, and c4 = 15.99.
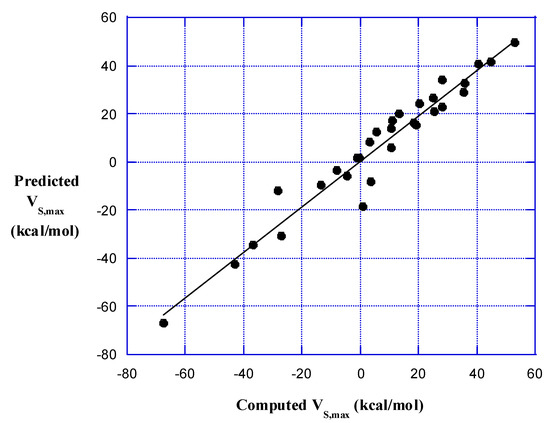
Figure 2.
Relationship between predicted and computed VS,max and VS,min of halogen atoms X in AX diatomic molecules. The R2 is 0.943.
Note that the coefficient of the polarizability of X in Equation (2) is positive, while the coefficient of the polarizability of A is negative. These signs are consistent with the polarization of X tending to make X more positive and the polarization of A tending to make X more negative.
4.2. Substituted Hydrides, HnA–X
Can Equation (2) be applied to polyatomic molecules? As an initial test, we considered the substituted hydrides HnA–X, where A ranges from H to Br (excluding the noble gases and transition elements) and X is again F, Cl, and Br. The extrema of V(r) on the 0.001 au surfaces of the atoms X, on the extensions of the A–X bonds, are in Table 3.

Table 3.
Properties of HnA–X molecules (X = F, Cl, and Br). VS(X) is computed maximum or minimum of VS(r) on X, on the extension of the A–X bond. The minima are explicitly indicated. χA − χX is difference in electronegativities of A and X a.
A noteworthy feature of Table 3 is that there are nearly as many negative VS,max on the X atoms as there are positive ones. Furthermore there are many X with VS,min. In all of the molecules having a VS,min, the difference in electronegativities, χA − χX, is more negative than −1.50.
Nearly all of the fluorides have either a negative VS,max or a negative VS,min on the fluorine. The only exceptions are F2, which has a positive VS,max, and the molecules H2N–F, H2P–F, and HS–F, in which the negative potentials of the fluorines overlap with those of the lone pairs at the A atoms and no VS,max or VS,min can be attributed specifically to the fluorine.
When Equation (2) was applied to the HnA–X molecules in Table 3, using the data in Table 2, the result was very encouraging, as is shown in Figure 3. The R2 is 0.953 and the coefficients are: c1 = 18.89, c2 = −0.9769, c3 = 3.173, and c4 = 10.80. The signs of the coefficients of the polarizabilities are consistent with the polarization effects noted above for the AX molecules.
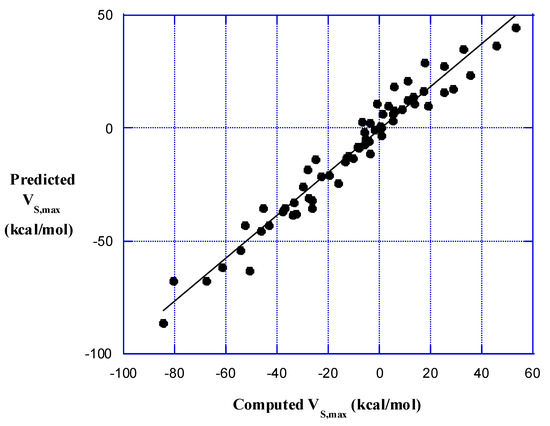
Figure 3.
Relationship between predicted and computed VS,max and VS,min of halogen atoms X in HnA–X molecules. The R2 is 0.953.
5. Discussion and Summary
We have discussed three possible features that a bonded halogen atom can have on its outer side, on the extension of the bond:
- (1)
- A lower electronic density (a σ-hole) accompanied by a positive electrostatic potential with a VS,max.
- (2)
- A lower electronic density (a σ-hole) accompanied by a negative electrostatic potential with a VS,max.
- (3)
- A buildup of electronic density (no σ-hole), usually accompanied by a negative electrostatic potential with a VS,min.
It is especially notable that for each set of molecules, the diatomic halides AX and the substituted hydrides HnA–X, the various VS,max and VS,min can all be expressed by a single three-parameter relationship of the form of Equation (2). The difference between the electronegativities of A and X, the polarizability of A, and the polarizability of X suffice to represent well all of the VS,max and VS,min of the diatomics AX (Figure 2) and the substituted hydrides HnA–X (Figure 3). It is actually somewhat surprising that Equation (2) is as effective for the hydrides as for the diatomics, given that the electronegativities and polarizabilities of free A atoms were used for the A in the HnA–X molecules.
The fact that a covalently-bonded halogen can have a buildup of electronic charge on the extension of the bond has not, to our knowledge, been emphasized in the past. In such instances, the halogen does not have a σ-hole. Instead of a VS,max, it has a VS,min on its outer side, on the extension of the bond. Such VS,min are found only when the halogen atom is considerably more electronegative than its bond partner; a small difference is not sufficient to produce a VS,min (Table 1 and Table 3).
A buildup of electronic density on the outer side of the halogen X indicates polarization of the electronic density of A toward X. This can be interpreted as resulting in some degree of ionic character, A+X−. While there is no unique or rigorous basis for distinguishing between covalency and ionicity, it may be that the formation of a VS,min can provide a useful operational distinction between them. On this basis, the ionic diatomics in Table 1 are LiF, BeF, BF, LiCl, and LiBr. In the HnA–X molecules, more of the A–X bonds have ionic character.
We recognize that the electronegativity differences χA − χX are only approximate measures of the relative effects of A and X upon each other. Perhaps a better means of doing this can be found. It is also clearly of interest to investigate the extension of the present analysis to atoms X other than halogens.
Author Contributions
The conceptualization and the formal analysis of this paper have been done by P.P. and J.S.M. The original draft preparation was done by P.P., while both P.P. and J.S.M. carried out review and editing. J.S.M. carried out the calculations and prepared the graphics for this paper.
Funding
This research received no external funding.
Acknowledgments
We greatly appreciate helpful discussions with Professor Tim Clark.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T.; Murray, J.S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. 1992, 44 (Suppl. 19), 57–64. [Google Scholar] [CrossRef]
- Brinck, T.; Murray, J.S.; Politzer, P. Molecular electrostatic potentials and local ionization energies of Group V-VII hydrides and their anions. Relationships for aqueous and gas-phase acidities. Int. J. Quantum Chem. 1993, 48 (Suppl. 20), 73–88. [Google Scholar] [CrossRef]
- Hennemann, M.; Murray, J.S.; Riley, K.E.; Politzer, P.; Clark, T. Polarization-induced σ-holes and hydrogen bonding. J. Mol. Model. 2012, 18, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Mathematical modeling and physical reality in noncovalent interactions. J. Mol. Model. 2015, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. σ-Hole bonding: Molecules containing Group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. A predicted new type of directional noncovalent interaction. Int. J. Quantum Chem. 2007, 107, 2286–2292. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Janjić, G.V.; Zarić, S.D. σ-Hole interactions of covalently-bonded nitrogen, phosphorus and arsenic: A survey of crystal structures. Crystals 2014, 4, 12–31. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Resnati, G. The chalcogen bond in crystalline solids: A world parallel to hydrogen bond. Acc. Chem. Res. 2019, 52, 1314–1324. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen bonding: An interim discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The bright future of unconventional σ/π-hole interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.H.; Hobza, P. Computer modeling of halogen bonds and other σ-hole interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole bond vs. π-hole bond: A comparison based on halogen bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef] [PubMed]
- Nyburg, S.C.; Faerman, C.H. A revision of van der Waals atomic radii for molecular crystals: N, O, F, S, Cl, Se, Br and I bonded to carbon. Acta Cryst. B 1985, 41, 274–279. [Google Scholar] [CrossRef]
- Ikuta, S. Anisotropy of electron density distribution around atoms in molecules: N, P, O and S atoms. J. Mol. Struct. 1990, 205, 191–201. [Google Scholar] [CrossRef]
- Eramian, H.; Tian, Y.-H.; Fox, Z.; Beneberu, H.Z.; Kertesz, M. On the anisotropy of van der Waals atomic radii of O, S, Se, F, Cl, Br and I. J. Phys. Chem. A 2013, 117, 14184–14190. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef]
- Rosenfeld, R.E., Jr.; Parthasarathy, R.; Dunitz, J.D. Directional preferences of nonbonded atomic contacts with divalent sulfur. 1. Electrophiles and nucleophiles. J. Am. Chem. Soc. 1977, 99, 4860–4862. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Parthasarathy, R.; Murray-Rust, P. Angular preferences of intermolecular forces around halogen centers: Preferred directions of approach of electrophiles and nucleophiles around carbon-halogen bond. J. Am. Chem. Soc. 1986, 108, 4308–4314. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Concha, M.C. σ-Hole bonding between like atoms: A fallacy of atomic charges. J. Mol. Model. 2008, 14, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.F. On the mapping of electrostatic properties from Bragg diffraction data. Chem. Phys. Lett. 1979, 65, 335–342. [Google Scholar] [CrossRef]
- Politzer, P.; Truhlar, D.G. (Eds.) Chemical Applications of Atomic and Molecular Electrostatic Potentials; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Klein, C.L.; Stevens, E.D. Charge density studies of drug molecules. In Structure and Reactivity; Liebman, J.F., Goldberg, A., Eds.; VCH Publishers: New York, NY, USA, 1988; pp. 26–64. [Google Scholar]
- Politzer, P.; Murray, J.S. Molecular electrostatic potentials and chemical reactivity. In Reviews in Computational Chemistry; Lipkowitz, K.B., Boyd, D.B., Eds.; VCH Publishers: New York, NY, USA, 1991; Volume 2, pp. 273–312. [Google Scholar]
- Wheeler, S.E.; Houk, K.N. Through-space effects of substituents dominate molecular electrostatic potentials of substituted arenes. J. Chem. Theory Comput. 2009, 5, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Shields, Z.P.-I.; Seybold, P.G.; Politzer, P. Intuitive and counterintuitive noncovalent interactions of aromatic π-regions with the hydrogen and the nitrogen of HCN. J. Comput. Sci. 2015, 10, 209–216. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. WIREs Comput. Mol. Sci. 2017, 7, e1236. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Holes and π-holes: Similarities and differences. J. Comput. Chem. 2018, 39, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. σ-Hole interactions: Perspectives and misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef]
- Murray, J.S.; Resnati, G.; Politzer, P. Close contacts and noncovalent interactions in crystals. Faraday Discuss. 2017, 203, 113–130. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bulat, F.A.; Toro-Labbé, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative analysis of molecular surface properties. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Paykatov, G.; Dinadayalane, T.; Leszczynski, J. Towards selection of efficient density functionals for van der Waals molecular complexes: Comparative study of C–H···π and N–H···π interactions. J Phys. Chem. A 2015, 119, 1190–1200. [Google Scholar]
- Riley, K.E.; Tran, K.; Lane, P.; Murray, J.S.; Politzer, P. Comparative analysis of electrostatic potential maxima and minima on molecular surfaces, as determined by three methods and a variety of basis sets. J. Comput. Sci. 2016, 17, 273–284. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Concha, M.C. Halogen bonding and the design of new materials: Organic bromides, chlorides and perhaps even fluorides as donors. J. Mol. Model. 2007, 13, 643–650. [Google Scholar] [CrossRef]
- Chopra, D.; Row, T.N.G. Role of organic fluorine in crystal engineering. CrystEngComm 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. Fluorine-centered halogen bonding: A factor in recognition phenomena and reactivity. Cryst. Growth Des. 2011, 11, 4238–4246. [Google Scholar] [CrossRef]
- Bundhun, A.; Ramasami, P.; Murray, J.S.; Politzer, P. Trends in σ-hole strengths and interactions of F3MX molecules (M = C, Si, Ge and X = F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef]
- Murray, J.S.; Macaveiu, L.; Politzer, P. Factors affecting the strengths of σ-hole electrostatic potentials. J. Comput. Sci. 2014, 5, 590–596. [Google Scholar] [CrossRef]
- Politzer, P.; Shields, Z.P.-I.; Bulat, F.A.; Murray, J.S. Average Local Ionization Energies as a Route to Intrinsic Atomic Electronegativities. J. Chem. Theory Comput. 2011, 7, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Electronegativity—A perspective. J. Mol. Model. 2018, 24, 214. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.C. Electronegativity Is the Average One-Electron Energy of the Valence-Shell Electrons in Ground-State Free Atoms. J. Am. Chem. Soc. 1989, 111, 9003–9014. [Google Scholar] [CrossRef]
- Allen, L.C. Encyclopedia of Computational Chemistry; Schleyer, P.V.R., Ed.; Wiley: New York, NY, USA, 1998; Volume 2, pp. 835–852. [Google Scholar]
- Lide, D.R. Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- NCSS 11 Statistical Software (2016). NCSS, LLC.: Kaysville, UT, USA. Available online: Ncss.com/software/ncss (accessed on 12 May 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).