Alkali-Activated Metakaolin as a Zeolite-Like Binder for the Production of Adsorbents
Abstract
1. Introduction
2. Results and Discussion
2.1. Density and Compressive Strength
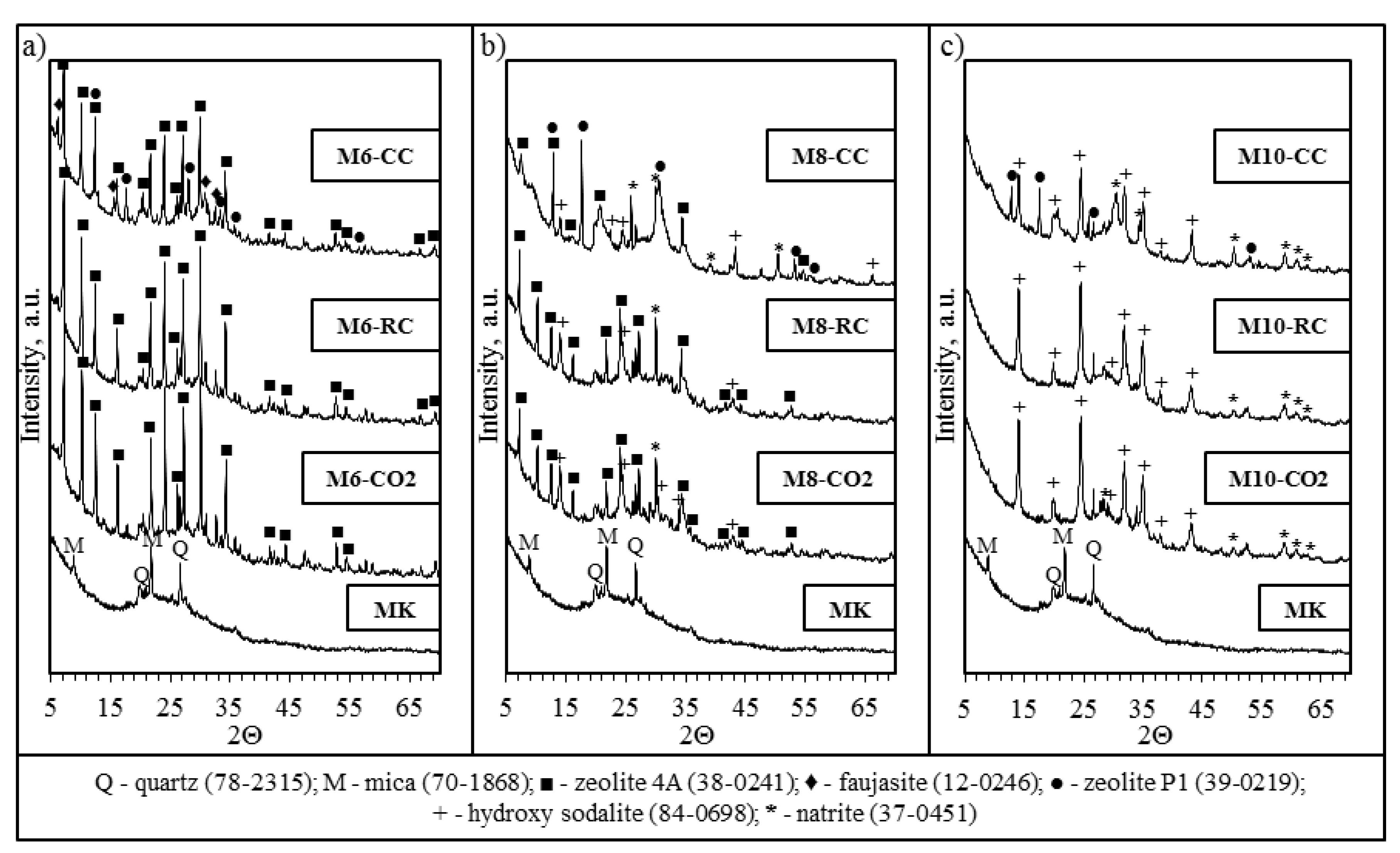
2.2. X-Ray Diffraction
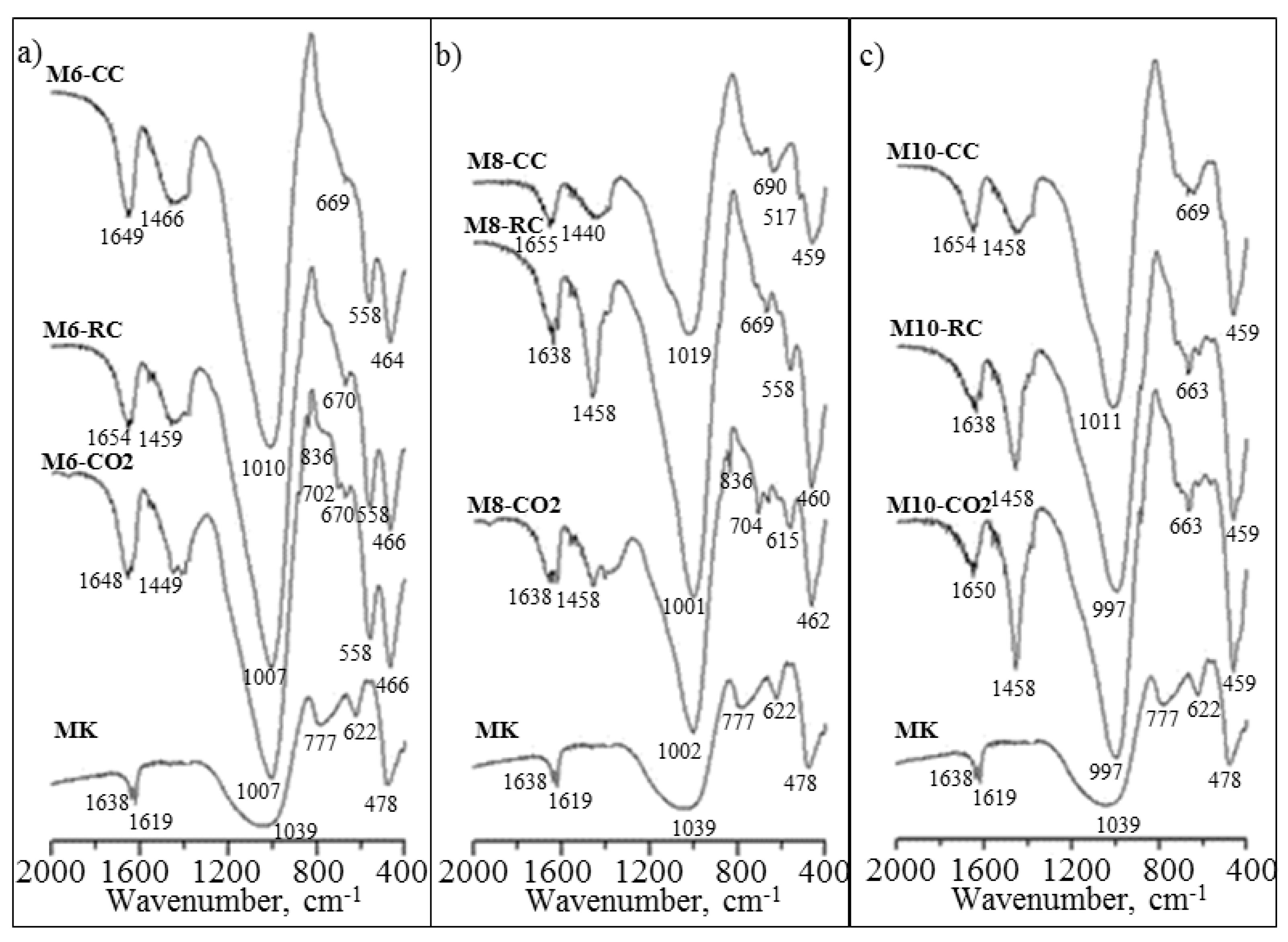
2.3. FTIR
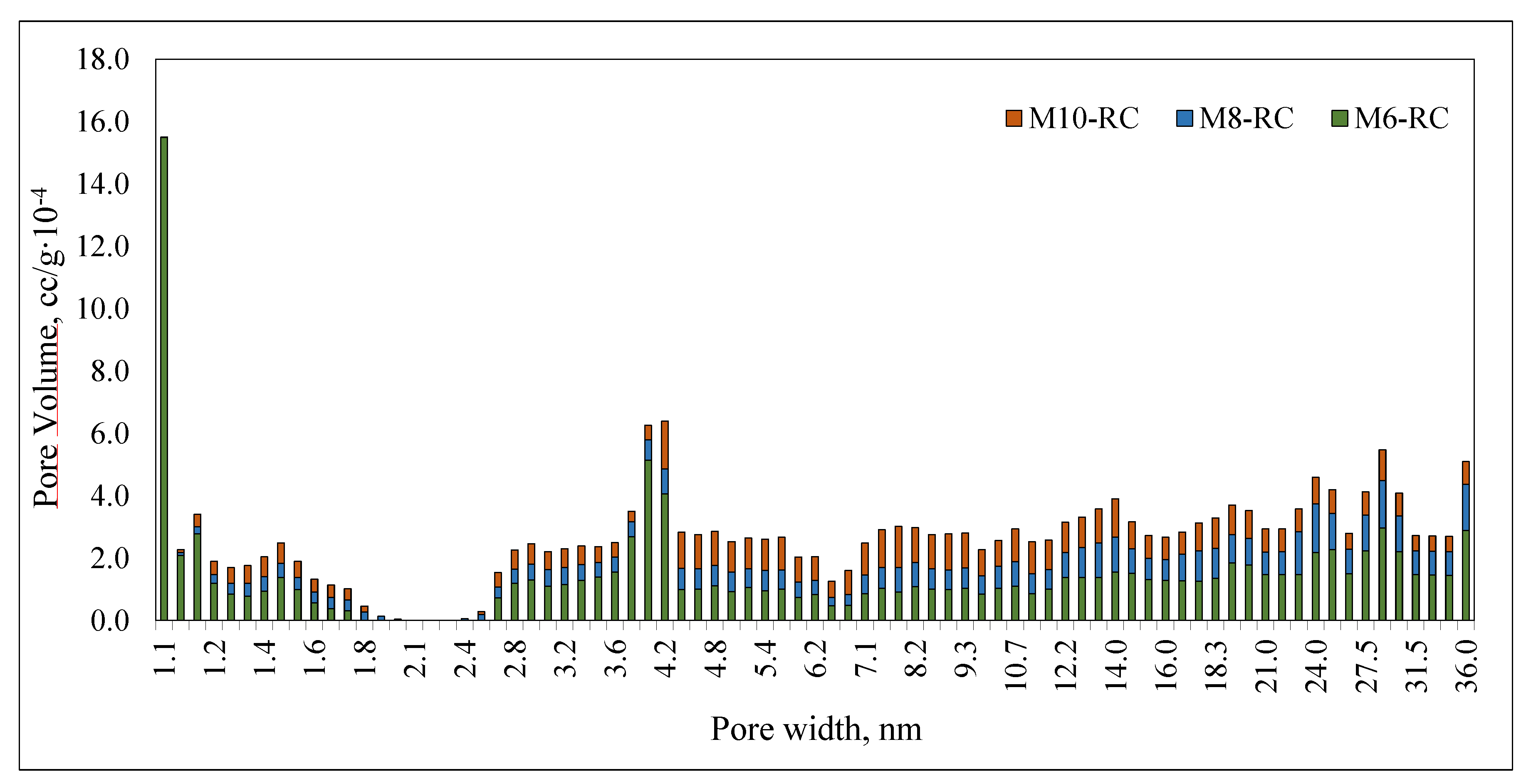
2.4. BET Analysis
2.5. SEM Analysis
2.6. Discussion on the Choice of Optimal Binder for Sorptive Applications
3. Materials and Methods
3.1. Raw Materials
3.2. Mixture Design and Sample Preparation
- Curing chamber (CC)-type: M6-CC, M8-CC, and M10-CC. After demoulding, the specimens were kept in a CC (at 85 °C with a humidity of >95%) until testing;
- Room condition (RC)-type: M6-RC, M8-RC, and M10-RC. After demoulding the specimens were kept at RCs (at 22 ± 2 °C with a humidity of 20%) until testing;
- CO2-type: M6-CO2, M8-CO2, and M10-CO2. After demoulding, the specimens were kept in the high CO2 environment (at 22 ± 2 °C with a humidity of 5%) until testing. A high CO2 environment was chosen, because of the required knowledge about material characteristics within CO2, to identify whether carbonization could occur. The high CO2 environment and the carbonization aspect could potentially influence mechanical properties of the final binder product.
3.3. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rugele, K.; Bumanis, G.; Bajare, D.; Lakevics, V.; Rubulis, J. Alkaline Activated Material for pH Control in Biotechnologies. Key Eng. Mater. 2014, 604, 223–226. [Google Scholar] [CrossRef]
- Gruskevica, K.; Bumanis, G.; Tihomirova, K.; Bajare, D.; Juhna, T. Alkaline Activated Material as the Adsorbent for Uptake of High Concentration of Zinc from Wastewater. Key Eng. Mater. 2016, 721, 123–127. [Google Scholar] [CrossRef]
- Liu, B.; Huang, T.; Zhang, Z.; Wang, Z.; Zhang, Y.; Li, J. The effect of the alkali additive on the highly active Ru/C catalyst for water gas shift reaction. Catal. Sci. Technol. 2014, 4, 1286. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Bajare, D.; Labrincha, J.A. Novel porous fly ash-containing geopolymers for pH buffering applications. J. Clean. Prod. 2016, 124, 395–404. [Google Scholar] [CrossRef]
- Minkiewicz, J.; Mozgawa, W.; Kr, M. IR spectroscopy studies of zeolites in geopolymeric materials derived from kaolinite. J. Mol. Struct. 2016, 1126, 200–206. [Google Scholar]
- Criado, M.; Fernandez-Jimenez, A.; Torre, G.; Aranda, M.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Grutzeck, M.; Kwan, S.; DiCola, M. Zeolite formation in alkali-activated cementitious systems. Cem. Concr. Res. 2004, 34, 949–955. [Google Scholar] [CrossRef]
- Pitcher, S.K.; Slade, R.C.T.; Ward, N.I. Heavy metal removal from motorway stormwater using zeolites. Sci. Total Environ. 2004, 161, 334–335. [Google Scholar] [CrossRef]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of Heavy Metals and Other Cations from Wastewater Using Zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Cooney, E.L.; Booker, N.A. Ammonia Removal from Wastewaters Using Natural Australian Zeolite. II. Pilot-Scale Study Using Continuous Packed Column Process. Sep. Sci. Technol. 1999, 34, 2741–2760. [Google Scholar] [CrossRef]
- Teresa, M.; Deng, S. Ce–Fe-modified zeolite-rich tuff to remove Ba2+-like 226Ra2+ in presence of As(V) and F− From aqueous media as pollutants of drinking water. J. Hazard. Mater. 2016, 302, 341–350. [Google Scholar]
- Bumanis, G.; Vitola, L.; Fernandez-Jimenez, A.; Palomo, A.; Bajare, D. The Effect of Heat Treatment on Alkali Activated Materials. Mater. Sci. 2017, 23, 266. [Google Scholar] [CrossRef]
- Kim, J.; Baek, G.; Kim, J.; Lee, C. Energy production from different organic wastes by anaerobic co-digestion: Maximizing methane yield versus maximizing synergistic effect. Renew. Energy 2019, 136, 683–690. [Google Scholar] [CrossRef]
- Rikkonen, P.; Tapio, P.; Rintamäki, H. Visions for small-scale renewable energy production on Finnish farms—A Delphi study on the opportunities for new business. Energy Policy 2019, 129, 939–948. [Google Scholar] [CrossRef]
- Xie, F.; Lin, Z.; Podkaminer, K. Could a bioenergy program stimulate electric vehicle market penetration? Potential impacts of biogas to electricity annual rebate program. GCB Bioenergy 2019, 11, 623. [Google Scholar] [CrossRef]
- Abdul Aziz, N.I.H.; Hanafiah, M.M.; Mohamed Ali, M.Y. Sustainable biogas production from agrowaste and effluents—A promising step for small-scale industry income. Renew. Energy 2019, 132, 363–369. [Google Scholar] [CrossRef]
- Vo, T.T.Q.; Rajendran, K.; Murphy, J.D. Can power to methane systems be sustainable and can they improve the carbon intensity of renewable methane when used to upgrade biogas produced from grass and slurry? Appl. Energy 2018, 228, 1046–1056. [Google Scholar] [CrossRef]
- Rajendran, K.; O’Gallachoir, B.; Murphy, J.D. The combined role of policy and incentives in promoting cost efficient decarbonisation of energy: A case study for biomethane. J. Clean. Prod. 2019, 219, 278–290. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Cucchiella, F.; Dadamo, I.; Gastaldi, M. Biomethane: A Renewable Resource as Vehicle Fuel. Resources 2017, 6, 58. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, X.; Ma, J.; Li, R. Adsorption of carbon dioxide on alkali-modified zeolite 13X adsorbents. Int. J. Greenh. Gas. Control 2007, 1, 355–359. [Google Scholar] [CrossRef]
- Shams, K.; Mirmohammadi, S.J. Preparation of 5A zeolite monolith granular extrudates using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Microporous Mesoporous Mater. 2007, 106, 268–277. [Google Scholar] [CrossRef]
- Peglow, M.; Antonyuk, S.; Jacob, M.; Palzer, S.; Heinrich, S.; Tsotsas, E. Particle Formulation in Spray Fluidized Beds. In Modern Drying Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; p. 295. [Google Scholar]
- Mörl, L.; Heinrich, S.; Peglow, M. Chapter 2 Fluidized bed spray granulation. In Handbook of Powder Technology; Elsevier Science: Burlington, MA, USA, 2007; Volume 11, pp. 21–188. [Google Scholar]
- Müller, P.; Russell, A.; Tomas, J. Influence of binder and moisture content on the strength of zeolite 4A granules. Chem. Eng. Sci. 2015, 126, 204–215. [Google Scholar] [CrossRef]
- He, Y.; Cui, X.; Liu, X.; Wang, Y.; Zhang, J.; Liu, K. Preparation of self-supporting NaA zeolite membranes using geopolymers. J. Memb. Sci. 2013, 447, 66–72. [Google Scholar] [CrossRef]
- Jin, M.; Liu, L.; Xue-min, C.; Yan, H.; Chen, J.; Liu, X. The hydrothermal transformation of solid geopolymers into zeolites. Microporous Mesoporous Mater. 2012, 161, 187–192. [Google Scholar]
- Lee, N.K.; Choudhry, I.; Lee, H.K.; Wang, Z.; Khalid, H.R. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2018, 239, 33–36. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014; Volume 13, p. 388. [Google Scholar]
- Wang, J.; Ge, Y.; He, Y.; Xu, M.; Cui, X. A porous gradient geopolymer-based tube membrane with high PM removal rate for air pollution. J. Clean. Prod. 2019, 217, 335–343. [Google Scholar] [CrossRef]
- Xu, M.; He, Y.; Liu, Z.; Tong, Z.; Cui, X. Preparation of geopolymer inorganic membrane and purification of pulp-papermaking green liquor. Appl. Clay Sci. 2019, 168, 269–275. [Google Scholar] [CrossRef]
- Innocentini, M.D.M.; Botti, R.F.; Bassi, P.M.; Paschoalato, C.F.P.R.; Flumignan, D.L.; Franchin, G.; Colombo, P. Lattice-shaped geopolymer catalyst for biodiesel synthesis fabricated by additive manufacturing. Ceram. Int. 2019, 45, 1443–1446. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Zhang, Z.; Li, Y. In-situ transition of amorphous gels to Na-P1 zeolite in geopolymer: Mechanical and adsorption properties. Constr. Build. Mater. 2019, 202, 851–860. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Minelli, M.; Medri, V.; Papa, E.; Miccio, F.; Landi, E.; Doghieri, F. Geopolymers as solid adsorbent for CO2 capture. Chem. Eng. Sci. 2016, 148, 267–274. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Amari, S.; Manaud, J.; Benito, P.; Vaccari, A.; Landi, E. Zeolite-geopolymer composite materials: Production and characterization. J. Clean. Prod. 2018, 171, 76–84. [Google Scholar] [CrossRef]
- Minelli, M.; Papa, E.; Medri, V.; Miccio, F.; Benito, P.; Doghieri, F.; Landi, E. Characterization of novel geopolymer—Zeolite composites as solid adsorbents for CO2 capture. Chem. Eng. J. 2018, 341, 505–515. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Paillard, C.; Contri, B.; Natali Murri, A.; Vaccari, A.; Landi, E. Geopolymer-hydrotalcite composites for CO2 capture. J. Clean. Prod. 2019, 237, 117738. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Lizion, J.; Landi, E. Metakaolin-based geopolymer beads: Production methods and characterization. J. Clean. Prod. 2019. [Google Scholar] [CrossRef]
- Özen, S.; Alam, B. Compressive Strength and Microstructural Characteristics of Natural Zeolite-based Geopolymer. Period. Polytech. Civ. Eng. 2017, 62, 64–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol-gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol.-Gel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A. Fernandez-Jimenez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Palomo, A.; Blanco-varela, M.T.; Granizo, M.L.; Puertas, F.; Vazquez, T.; Grutzeck, M.W. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Ugal, J.R.; Mustafa, M.; Abdulhadi, A.A. Preparation of Zeolite Type 13X from Locally Available Raw Materials. 51 Iraqi J. Chem. Pet. Eng. 2008, 9, 51–56. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
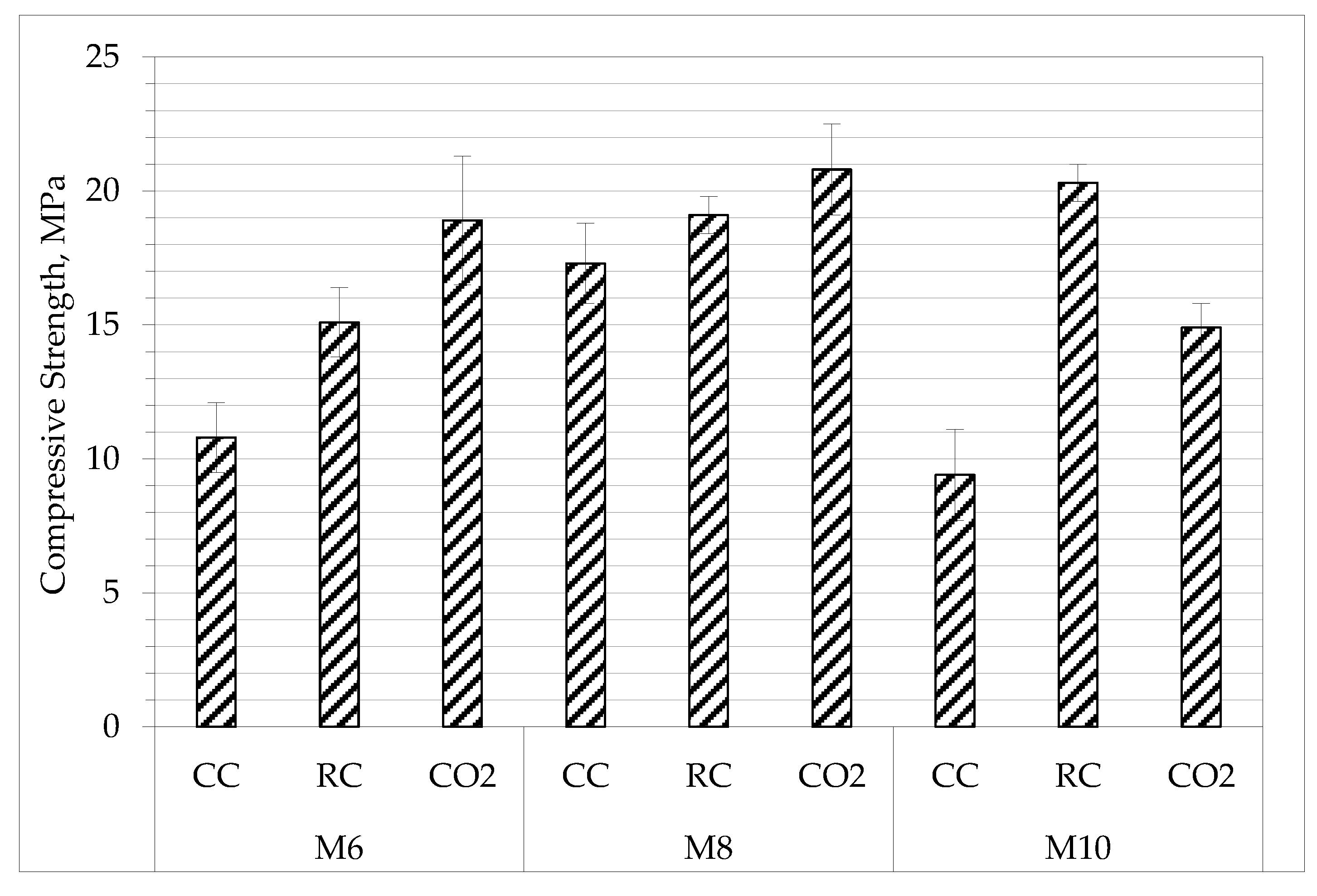
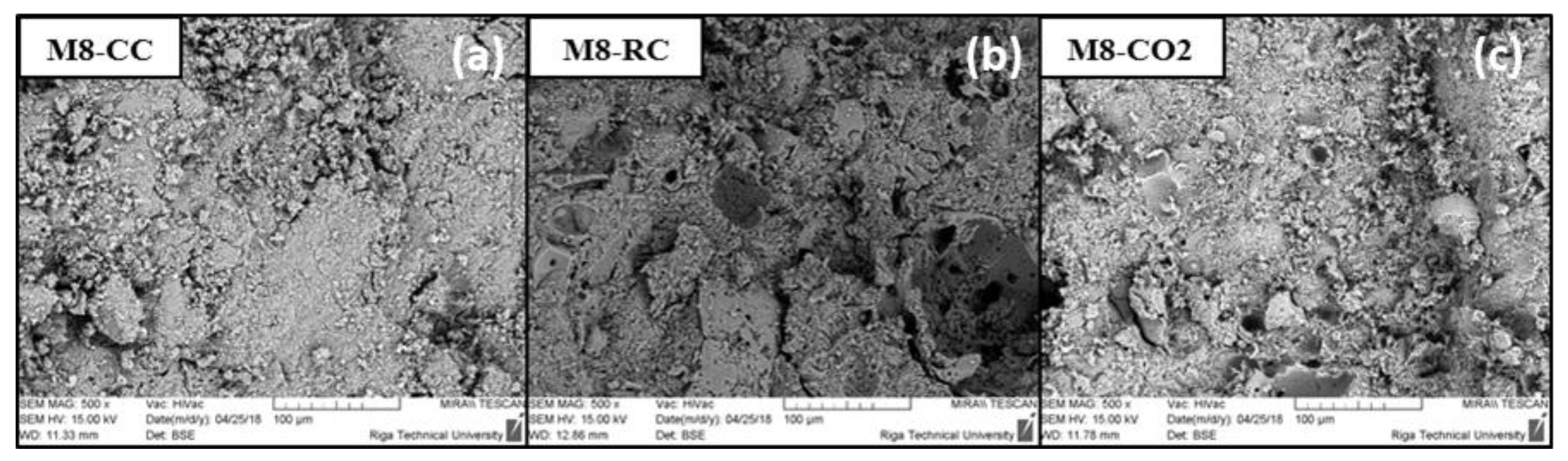
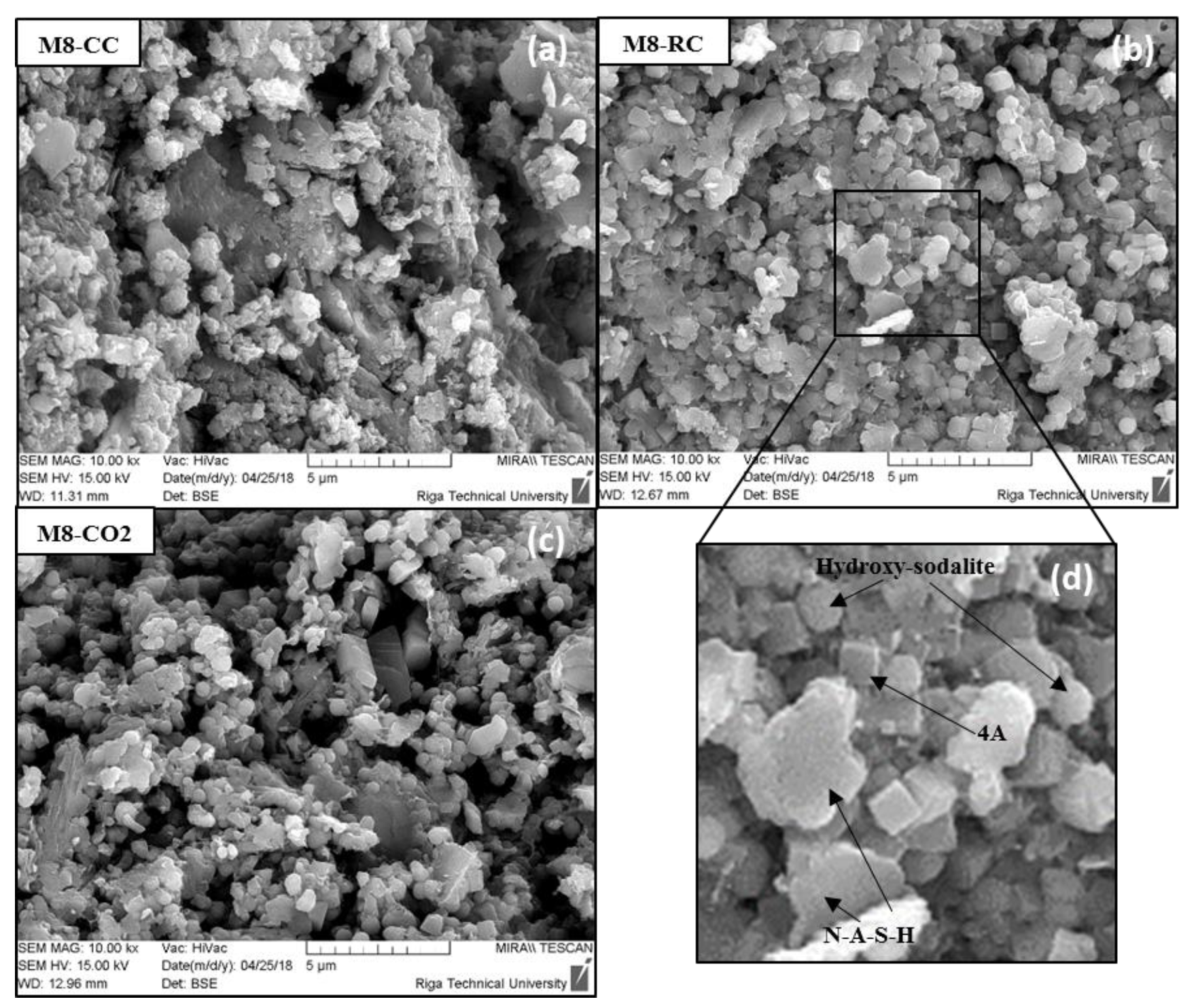
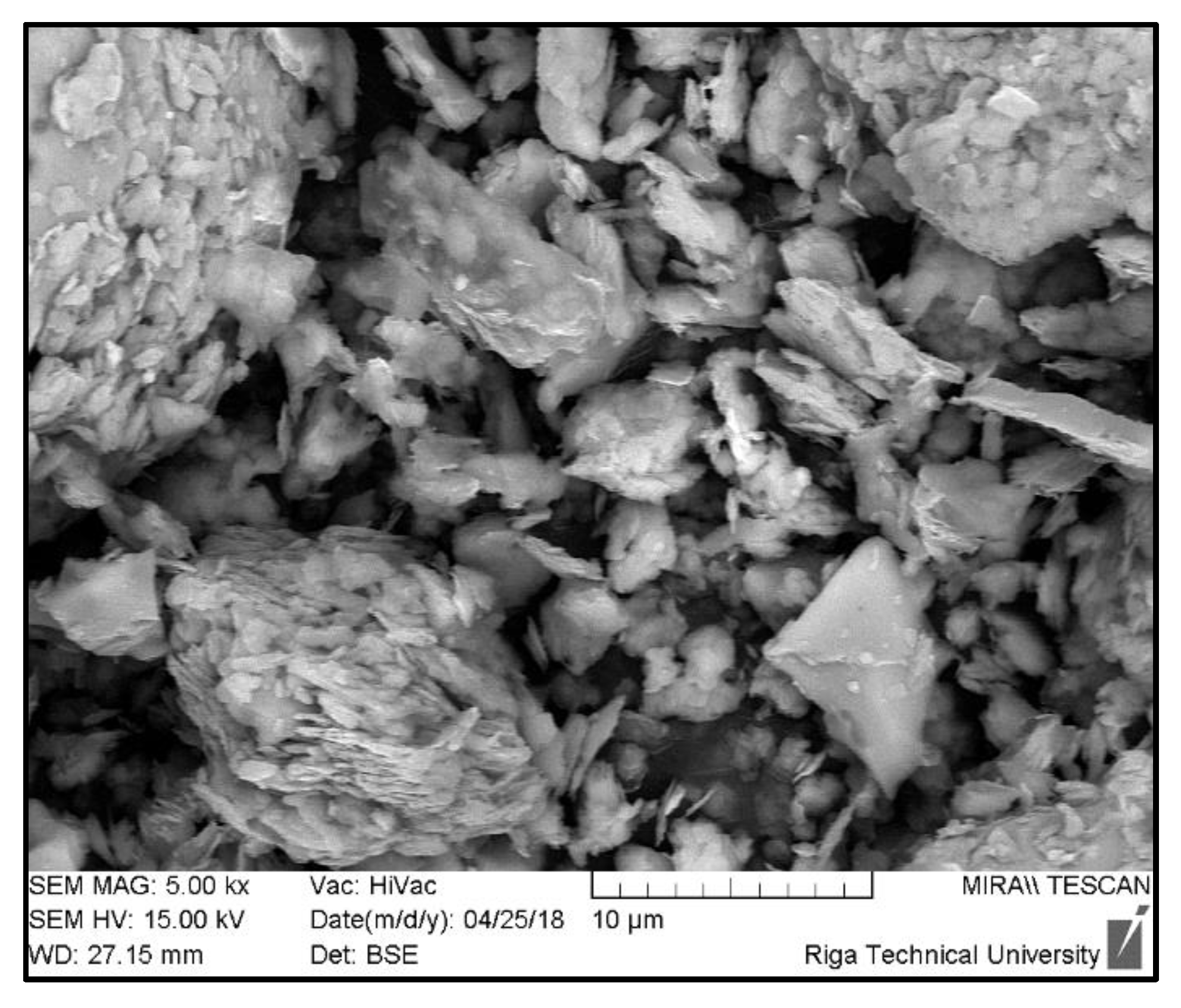
| Composition | Material Density, kg/m3 | Total Porosity, % | Compressive Strength, MPa | |
|---|---|---|---|---|
| M6 | CC | 1315 ± 22 | 37.9 ± 1.1 | 10.8 ± 1.3 |
| RC | 1285 ± 29 | 36.4 ± 1.4 | 15.1 ± 1.3 | |
| CO2 | 1285 ± 16 | 37.9 ± 0.8 | 18.9 ± 2.4 | |
| M8 | CC | 1255 ± 47 | 40.9 ± 2.2 | 17.3 ± 0.7 |
| RC | 1245 ± 16 | 40.6 ± 0.8 | 19.1 ± 1.5 | |
| CO2 | 1230 ± 34 | 41.7 ± 1.6 | 20.8 ± 1.7 | |
| M10 | CC | 1535 ± 11 | 28.9 ± 0.5 | 9.4 ± 0.7 |
| RC | 1530 ± 9 | 28.7 ± 0.4 | 20.3 ± 1.7 | |
| CO2 | 1535 ± 7 | 28.7 ± 0.3 | 14.9 ± 0.9 | |
| Wavenumbers, cm−1 | Assignments | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MK | MK6 | MK8 | MK10 | ||||||||
| CC | RC | CO2 | CC | RC | CO2 | CC | RC | CO2 | |||
| 1638 | 1648 | 1654 | 1648 | 1655 | 1638 | 1638 | 1654 | 1638 | 1650 | O–H bending vibrations | [5,43] |
| - | 1466 | 1459 | 1449 | 1440 | 1458 | 1458 | 1458 | 1458 | 1458 | ν3 C–O (CO32−) | [41] |
| 1039 | 1010 | 1007 | 1007 | 1019 | 1001 | 1002 | 1011 | 997 | 997 | ν3 T–O (T = Al, Si) | [20,42] |
| - | - | - | 836 | - | - | 836 | - | - | 836 | ν2 C–O (CO32−) | [41] |
| 777 | - | - | - | - | - | - | - | - | - | ν1 Si–O | [42] |
| - | 669 | 670 | 702 | 704 | 669 | 704 | 669 | 663 | 622 | ν4 Si–O–Si | [41,42] |
| 622 | 558 | 558 | 558 | 517 | 558 | 615 | - | - | - | ν4 Al–O–Al | [5,20,44] |
| 478 | 464 | 466 | 466 | 459 | 460 | 462 | 459 | 459 | 459 | ν4 Si–O | [41,42] |
| Compound | Al2O3 | SiO2 | CaO | TiO2 | MgO | Fe2O3 | Na2O | Others | LOI, 1000 °C |
|---|---|---|---|---|---|---|---|---|---|
| MK | 34.2 | 51.8 | 0.1 | 0.6 | 0.1 | 0.5 | 0.6 | 12.1 | 11.9 |
| Composition | Raw Materials, Mass Parts | Curing Condition 2nd–7th Day After Making | ||||||
|---|---|---|---|---|---|---|---|---|
| MK | NaOH Solution | Climate Chamber | Room Conditions | High CO2 Environment | ||||
| 6 M | 8 M | 10 M | ||||||
| Series | Type | |||||||
| M6 | CC | 1 | 0.6 | + | ||||
| RC | 1 | 0.6 | + | |||||
| CO2 | 1 | 0.6 | + | |||||
| M8 | CC | 1 | 0.6 | + | ||||
| RC | 1 | 0.6 | + | |||||
| CO2 | 1 | 0.6 | + | |||||
| M10 | CC | 1 | 0.6 | + | ||||
| RC | 1 | 0.6 | + | |||||
| CO2 | 1 | 0.6 | + | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegere, K.; Vitola, L.; Argalis, P.P.; Bajare, D.; Krauklis, A.E. Alkali-Activated Metakaolin as a Zeolite-Like Binder for the Production of Adsorbents. Inorganics 2019, 7, 141. https://doi.org/10.3390/inorganics7120141
Vegere K, Vitola L, Argalis PP, Bajare D, Krauklis AE. Alkali-Activated Metakaolin as a Zeolite-Like Binder for the Production of Adsorbents. Inorganics. 2019; 7(12):141. https://doi.org/10.3390/inorganics7120141
Chicago/Turabian StyleVegere, Kristine, Laura Vitola, Pauls P. Argalis, Diana Bajare, and Andrey E. Krauklis. 2019. "Alkali-Activated Metakaolin as a Zeolite-Like Binder for the Production of Adsorbents" Inorganics 7, no. 12: 141. https://doi.org/10.3390/inorganics7120141
APA StyleVegere, K., Vitola, L., Argalis, P. P., Bajare, D., & Krauklis, A. E. (2019). Alkali-Activated Metakaolin as a Zeolite-Like Binder for the Production of Adsorbents. Inorganics, 7(12), 141. https://doi.org/10.3390/inorganics7120141








