Reaction of Dialumane Incorporating Bulky Eind Groups with Pyridines
Abstract
:1. Introduction
2. Results and Discussions
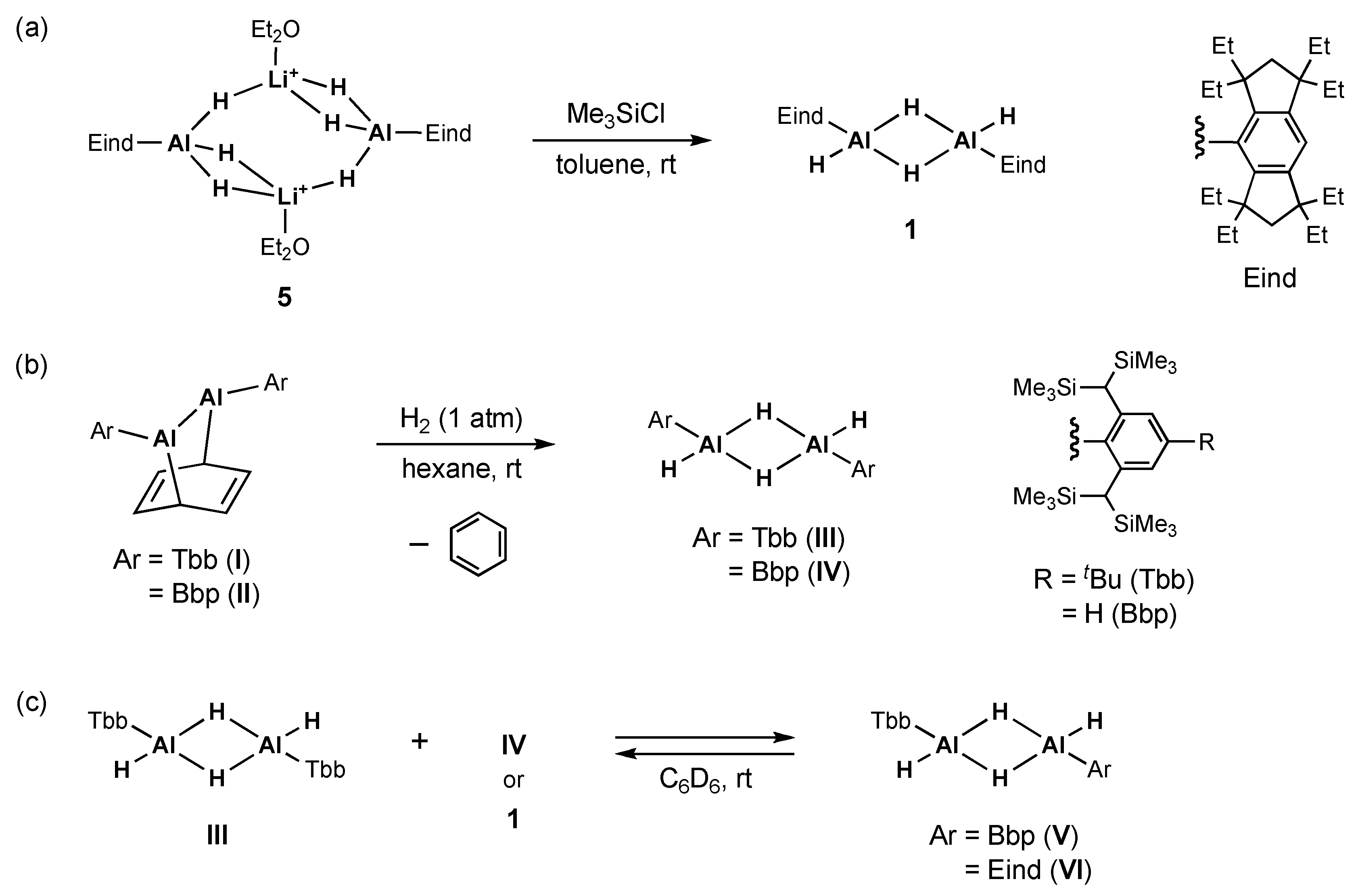
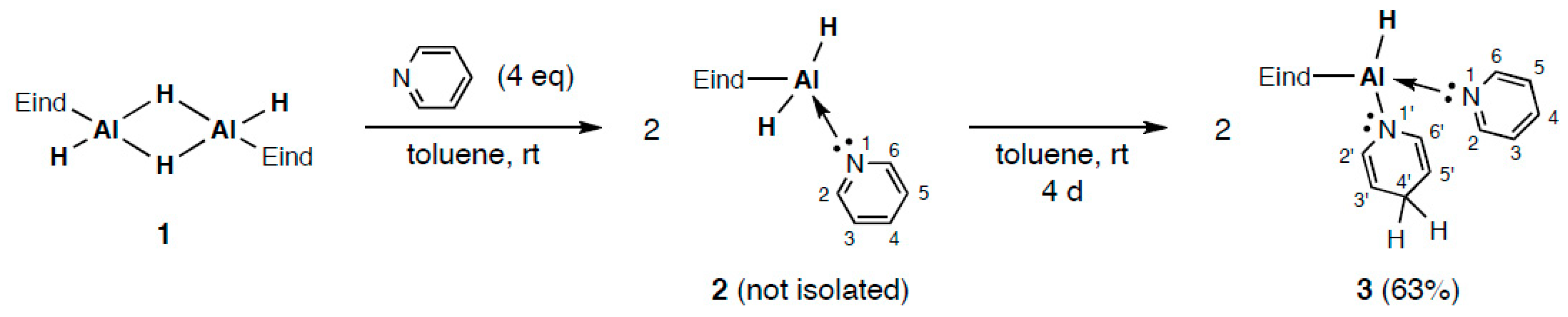
2.1. Reaction of (Eind)HAl(μ-H)2AlH(Eind) (1) with Pyridine (Py)
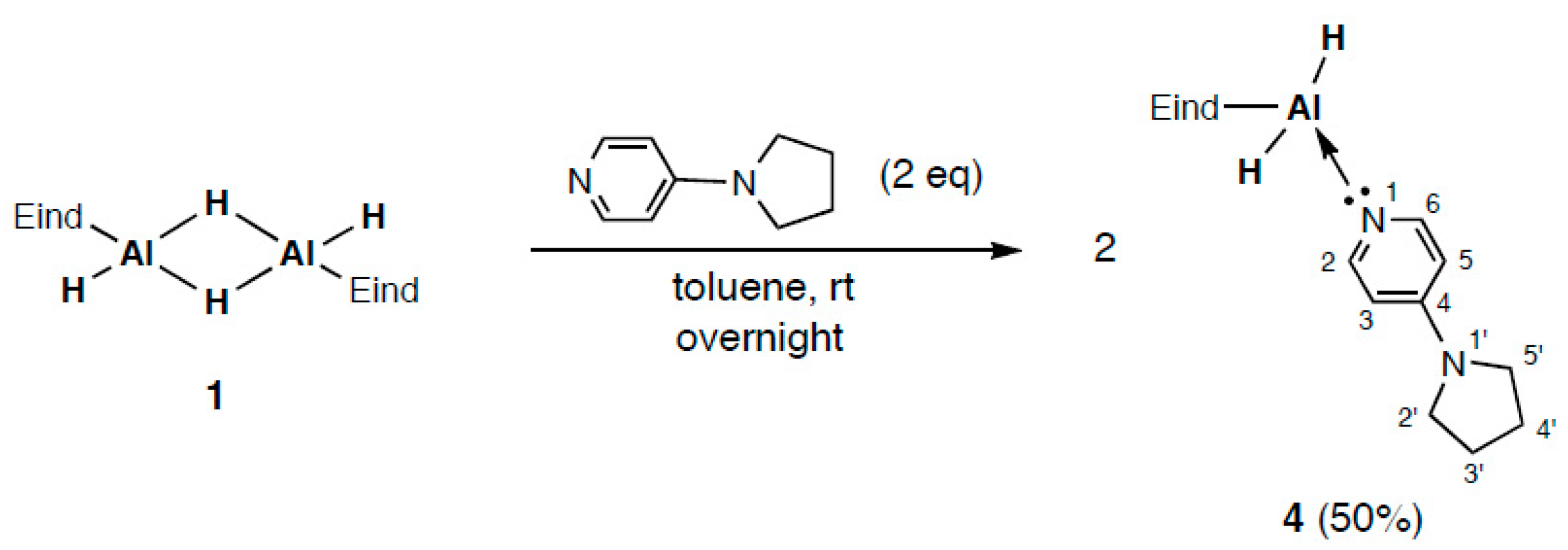
2.2. Reaction of (Eind)HAl(μ-H)2AlH(Eind) (1) with 4-Pyrrolidinopyridine (PPy)
2.3. Structures of Py→AlH(1,4-dihydropyrid-1-yl)(Eind) (3) and PPy→AlH2(Eind) (4)
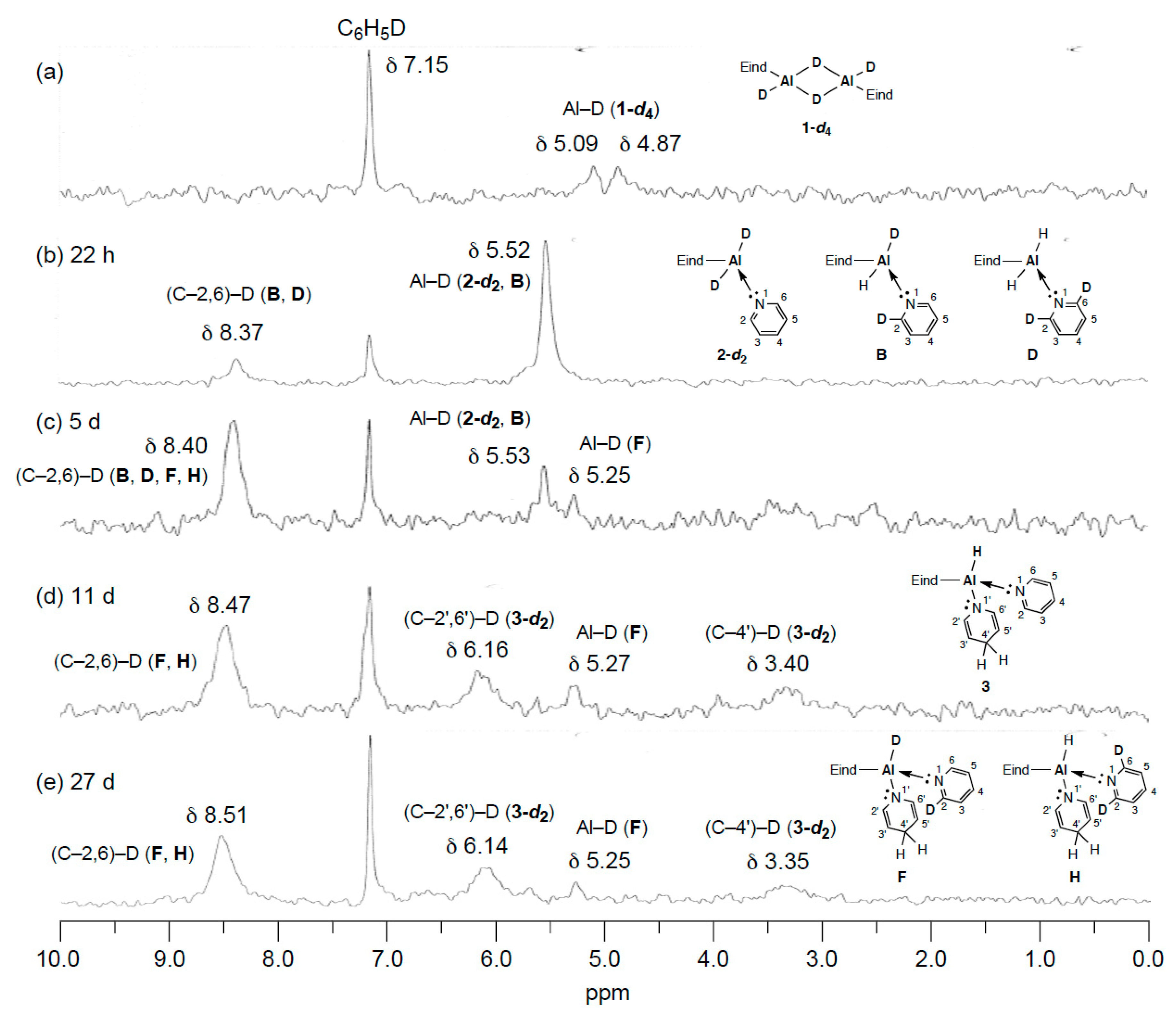
2.4. Mechanistic Studies on the Reaction from 1 to 3
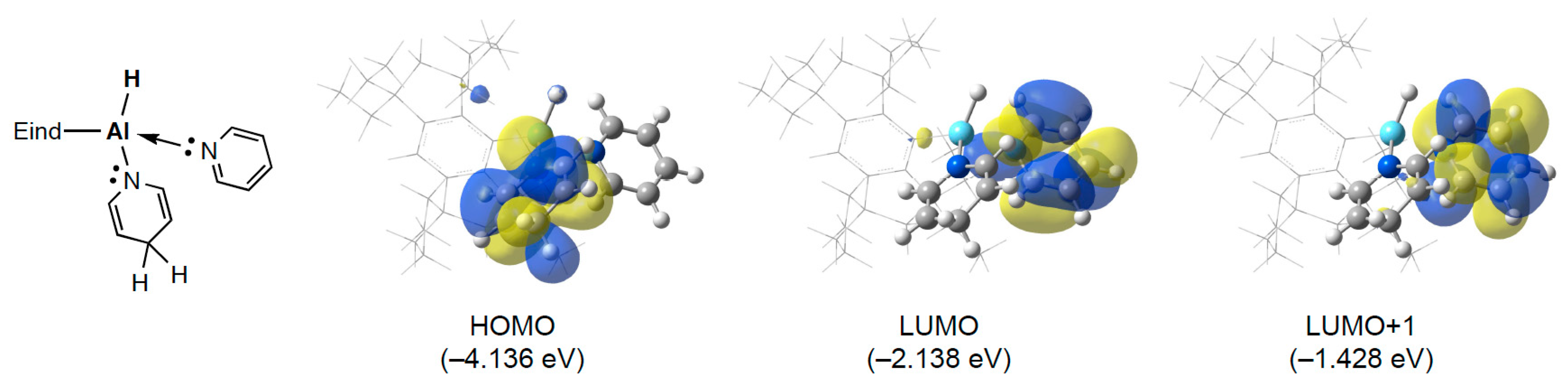
2.5. Theoretical Calculations of 3
3. Materials and Methods
3.1. General Procedures
3.1.1. Synthesis of Py→AlH(1,4-dihydropyrid-1-yl)(Eind) (3)
3.1.2. Synthesis of PPy→AlH2(Eind) (4)
3.1.3. Synthesis of [Li(OEt2)]2[(Eind)AlD3]2 (5-d6)
3.1.4. Synthesis of (Eind)DAl(μ-D)2AlD(Eind) (1-d4)
3.1.5. Reaction of (Eind)DAl(μ-D)2AlD(Eind) (1-d4) with Py
3.2. X-ray Crystallographic Studies of 3 and 4
3.2.1. Py→AlH(1,4-dihydropyrid-1-yl)(Eind) (3)
3.2.2. PPy→AlH2(Eind) (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowley, A.H.; Gabbaï, F.P.; Isom, H.S.; Dechen, A. New developments in the organoaluminum and organogallium hydrides. J. Organomet. Chem. 1995, 500, 81–88. [Google Scholar] [CrossRef]
- Seyden-Penna, J. Reductions by the Alumino and Borohydrides in Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Downs, A.J. Recent advances in the chemistry of Group 13 metals: Hydride derivatives and compounds involving multiply bonded Group 13 metal atom. Coord. Chem. Rev. 1999, 189, 59–100. [Google Scholar] [CrossRef]
- Wehmschulte, R.J.; Power, P.P. Primary alanes and alanates: Useful synthetic reagents in aluminum chemistry. Polyhedron 2000, 19, 1649–1661. [Google Scholar] [CrossRef]
- Aldridge, S.; Downs, A.J. Hydrides of Main-Group Metals: New Variations on an old theme. Chem. Rev. 2001, 104, 3305–3365. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensan, C.M. Complex Hydride for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Grabis, I.E. Aluminum hydride as a hydrogen and energy storage material: Past, present and future. J. Alloy. Compd. 2011, 509, S517–S528. [Google Scholar] [CrossRef]
- Li, W.; Ma, X.; Walawalkar, M.G.; Yang, Z.; Roesky, H.W. Soluble aluminum hydrides function as catalysts in deprotonation, insertion, and activation reactions. Coord. Chem. Rev. 2017, 350, 14–29. [Google Scholar] [CrossRef]
- Lansbury, P.T.; Peterson, J.O. Lithium N-Dihydroaluminum Hydride—A Selective Reducing Agent for Highly Electrophilic Carbonyl Compounds. J. Am. Chem. Soc. 1961, 83, 3537–3538. [Google Scholar] [CrossRef]
- Lansbury, P.T.; Peterson, J.O. Novel Selectivity in Reduction of Ketones by Lithium N-Dihydroaluminum Hydride. J. Am. Chem. Soc. 1962, 84, 1756–1757. [Google Scholar] [CrossRef]
- Lansbury, P.T.; Peterson, J.O. Lithium Tetrakis-(N-dihydropyridyl)-aluminate: Structure and Reducing Properties. J. Am. Chem. Soc. 1963, 85, 2236–2242. [Google Scholar] [CrossRef]
- Tanner, D.D.; Yang, C.-M. On the Structure and Mechanism of Formation of the Lansbury Reagent, Lithium Tetrakis(N-dihydropyridyl)aluminate. J. Org. Chem. 1993, 58, 1840–1846. [Google Scholar] [CrossRef]
- Hensen, K.; Lemke, A.; Stumpf, T.; Bolte, M.; Fleischer, H.; Pulham, C.R.; Gould, R.O.; Harris, S. Synthesis and Structural Characterization of (1,4-Dihydropyrid-1-yl)aluminum Complexes. Inorg. Chem. 1999, 38, 4700–4704. [Google Scholar] [CrossRef] [PubMed]
- Less, R.J.; Simmonds, H.R.; Wright, D.S. Reactivity and catalytic activity of tert-butoxy-aluminium hydeide reagents. Dalton Trans. 2014, 43, 5785–5792. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.M.; Meyers, A.I. Recent Advances in the Chemistry of Dihydropyridines. Chem. Rev. 1982, 82, 223–243. [Google Scholar] [CrossRef]
- Murosaki, T.; Kaneda, S.; Maruhashi, R.; Sadamori, K.; Shoji, Y.; Tamao, K.; Hashizume, D.; Hayakawa, N.; Matsuo, T. Synthesis and Structural Characteristics of Discrete Organoboron and Organoaluminum Hydirdes Incorporating Bulky Eind Groups. Organometallics 2016, 35, 3397. [Google Scholar] [CrossRef]
- Nagata, K.; Murosaki, T.; Agou, T.; Sasamori, T.; Matsuo, T.; Tokitoh, N. Activation of Dihydrogen by Masked Doubly Bonded Aluminum Species. Angew. Chem. Int. Ed. 2016, 55, 12877–12880. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J. The Elements, 3rd ed.; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Matsuo, T.; Suzuki, K.; Fukawa, T.; Li, B.L.; Ito, M.; Shoji, Y.; Otani, T.; Li, L.C.; Kobayashi, M.; Hachiya, M.; et al. Synthesis and structures of a series of bulky “Rind-Br” based on a rigid fused-ring s-hydrindacene skeleton. Bull. Chem. Soc. Jpn. 2011, 84, 1178–1191. [Google Scholar] [CrossRef]
- CrysAlisPro; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2014.
- CrystalClear; Rigaku/MSC. Inc.: The Woodlands, TX, USA, 2005.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.; Spagna, R. SIR2011. J. Appl. Cryst. 2012, 45, 357–361. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2005. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2018/3. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murosaki, T.; Ohno, R.; Agou, T.; Hashizume, D.; Matsuo, T. Reaction of Dialumane Incorporating Bulky Eind Groups with Pyridines. Inorganics 2019, 7, 129. https://doi.org/10.3390/inorganics7110129
Murosaki T, Ohno R, Agou T, Hashizume D, Matsuo T. Reaction of Dialumane Incorporating Bulky Eind Groups with Pyridines. Inorganics. 2019; 7(11):129. https://doi.org/10.3390/inorganics7110129
Chicago/Turabian StyleMurosaki, Takahiro, Ryoma Ohno, Tomohiro Agou, Daisuke Hashizume, and Tsukasa Matsuo. 2019. "Reaction of Dialumane Incorporating Bulky Eind Groups with Pyridines" Inorganics 7, no. 11: 129. https://doi.org/10.3390/inorganics7110129
APA StyleMurosaki, T., Ohno, R., Agou, T., Hashizume, D., & Matsuo, T. (2019). Reaction of Dialumane Incorporating Bulky Eind Groups with Pyridines. Inorganics, 7(11), 129. https://doi.org/10.3390/inorganics7110129





