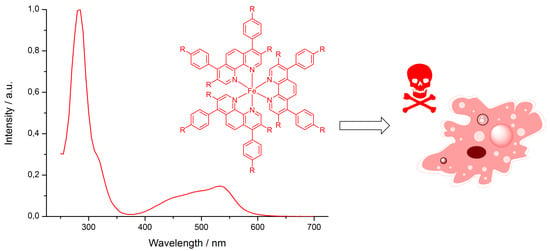
Synthesis, Characterization, and Biological Evaluation of Red-Absorbing Fe(II) Polypyridine Complexes
Abstract
1. Introduction
2. Results and Discussion
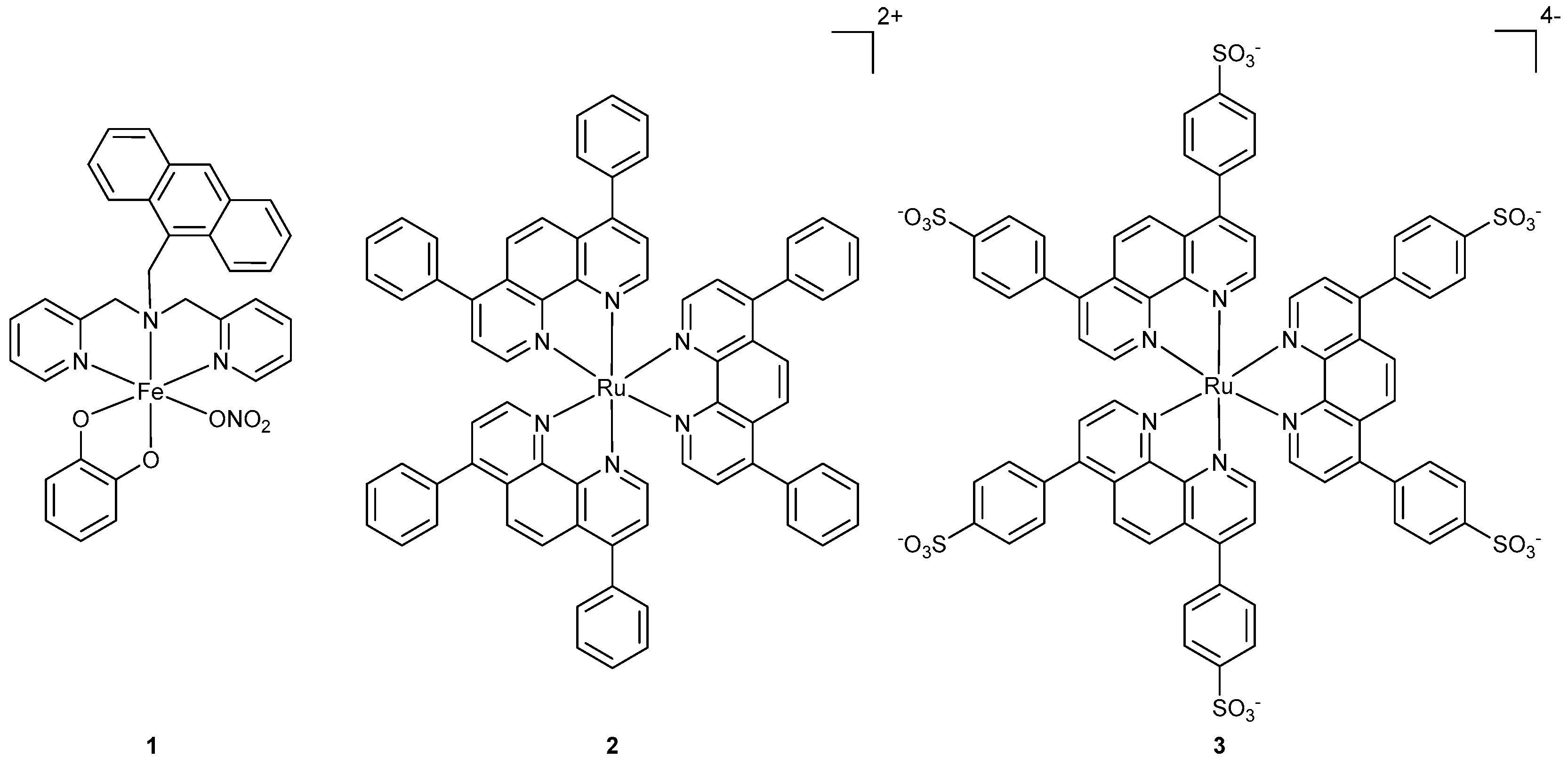
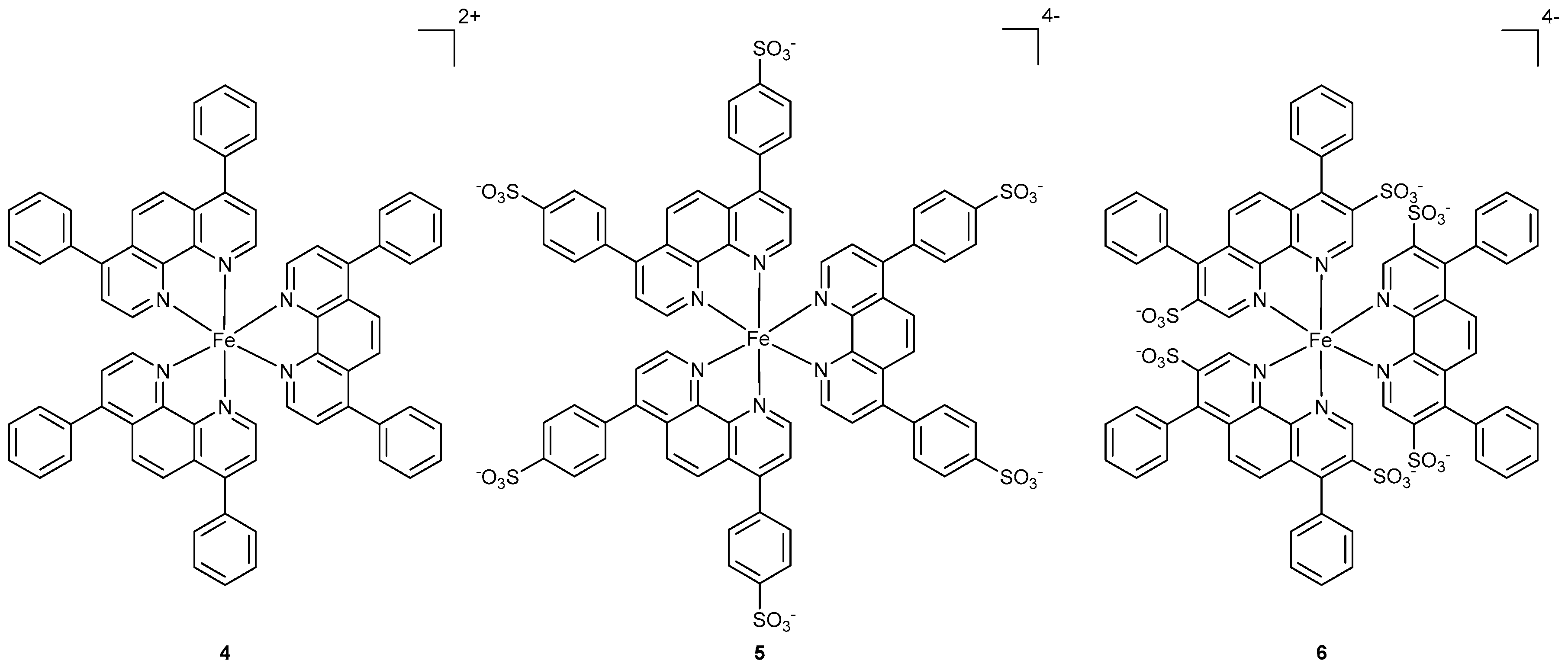
2.1. Synthesis and Characterization
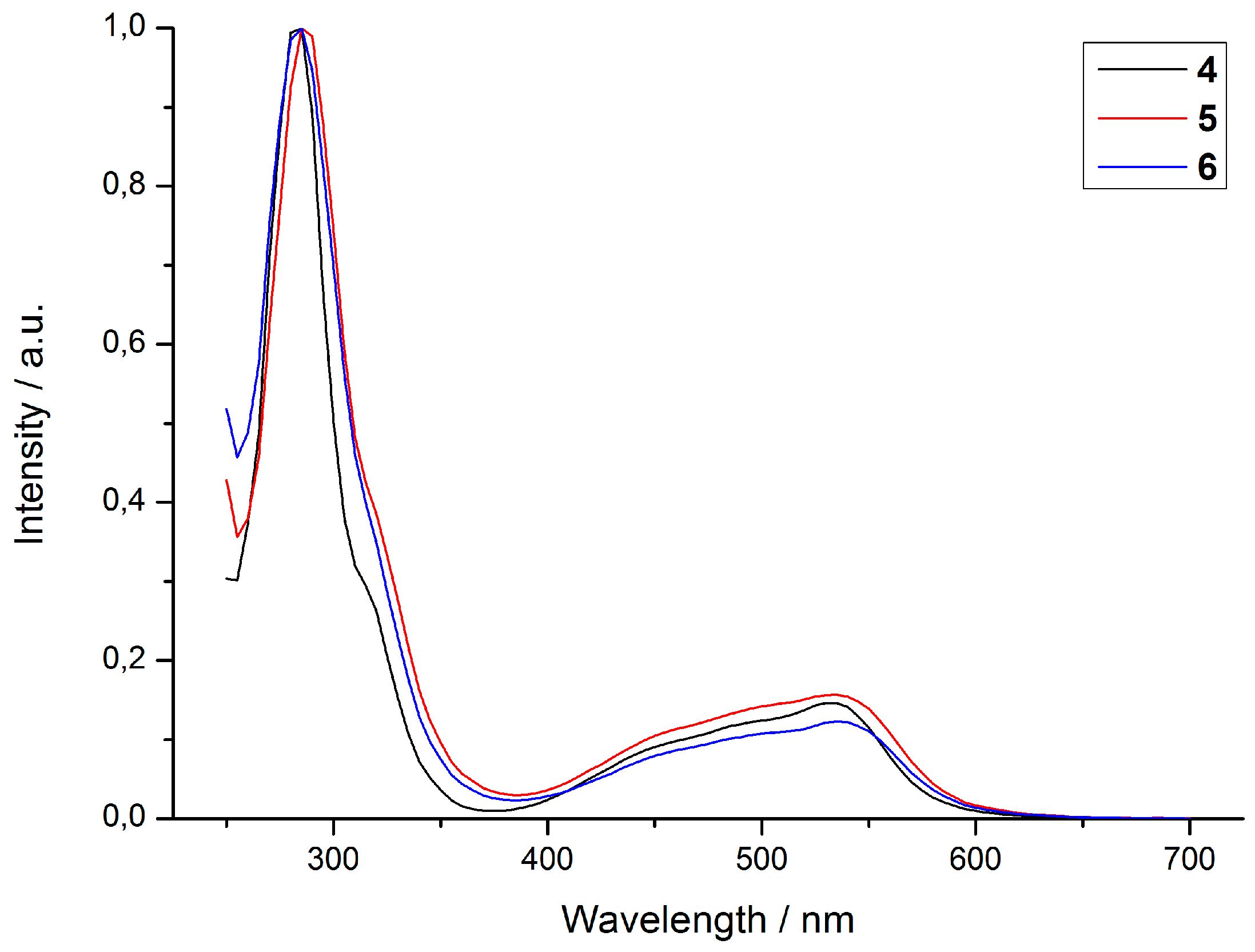
2.2. Photophysical Properties
2.3. Singlet Oxygen Generation
2.4. Stability in Human Plasma
2.5. Photostability
2.6. Distribution Coefficient
2.7. Cytotoxicity and Photocytotoxicity
3. Materials and Methods
3.1. Instrumentation and Methods
3.2. Materials
3.3. Synthesis
3.4. Spectroscopic Measurements
3.5. Luminescence Quantum Yield Measurements
F = 1 − 10−A
3.6. Lifetime Measurements
3.6.1. Singlet Oxygen Measurements - Direct evaluation
I = I0 * (1 − 10−A)
3.6.2. Singlet Oxygen Measurements - Indirect evaluation
3.7. Stability in Human Plasma
3.8. Photostability
3.9. Distribution Coefficient
3.10. Cell Culture
3.11. Cytotoxicity and Photocytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. Recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Metzler-Nolte, N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Pierroz, V.; Mari, C.; Gemperle, L.; Ferrari, S.; Gasser, G. A bis (dipyridophenazine)(2-(2-pyridyl) pyrimidine-4-carboxylic acid) ruthenium(II) complex with anticancer action upon photodeprotection. Angew. Chem. Int. Ed. 2014, 53, 2960–2963. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2010, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.; Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru(II) polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 2017, 46, 7317–7337. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Süss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010, 39, 1673–1688. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platinum Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Adhireksan, Z.; Davey, G.E.; Campomanes, P.; Groessl, M.; Clavel, C.M.; Yu, H.; Nazarov, A.A.; Yeo, C.H.F.; Ang, W.H.; Dröge, P.; et al. Ligand substitutions between ruthenium–cymene compounds can control protein versus DNA targeting and anticancer activity. Nat. Commun. 2014, 5, 3462. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Pracharova, J.; Stepankova, J.; Sadler, P.J.; Kasparkova, J. Photo-induced DNA cleavage and cytotoxicity of a ruthenium(II) arene anticancer complex. J. Inorg. Biochem. 2016, 160, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Heinemann, F.; Karges, J.; Gasser, G. Critical overview of the use of Ru(II) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Ferrari, S.; Gasser, G. Combination of Ru(II) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015, 6, 2660–2686. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J., III; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one-and two-photon photodynamic therapy. Coord. Chem. Rev. 2018, 379, 2–29. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Rubbiani, R.; Patra, M.; Hess, J.; Spingler, B.; Oehninger, L.; Schur, J.; Ott, I.; Salassa, L. DNA intercalating RuII polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem. Eur. J. 2014, 20, 14421–14436. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Leonidova, A.; Ferrari, S.; Gasser, G. Towards selective light-activated RuII-based prodrug candidates. Eur. J. Inorg. Chem. 2015, 2015, 3879–3891. [Google Scholar] [CrossRef]
- Huang, H.; Yu, B.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly charged ruthenium(II) polypyridyl complexes as lysosome-localized photosensitizers for two-photon photodynamic therapy. Angew. Chem. 2015, 127, 14255–14258. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalised with a photoactive ruthenium(II) complex: Exploring the formulation of a metal-based photodynamic therapy photosensitiser. Dalton Trans. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.; Kaspler, P.; Shi, G.; Monro, S.; Sainuddin, T.; Forward, S.; Kasimova, K.; Hennigar, R.; Mandel, A.; McFarland, S. Novel osmium-based coordination complexes as photosensitizers for panchromatic photodynamic therapy. Photochem. Photobiol. 2017, 93, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Joyce, L.E.; Dickson, N.M.; Turro, C. DNA photocleavage by an osmium(II) complex in the PDT window. Chem. Commun. 2010, 46, 6759–6761. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.A.; Zigler, D.F.; Tarrago-Trani, M.T.; Storrie, B.; Brewer, K.J. Photobiological impact of [{(bpy)2Ru(dpp)}2RhCl2]Cl5 and [{(bpy)2Os(dpp)}2RhCl2]Cl5 [bpy= 2,2′-bipyridine; dpp= 2,3-bis(2-pyridyl) pyrazine] on vero cells. Inorg. Chem. 2007, 46, 4760–4762. [Google Scholar] [CrossRef] [PubMed]
- Swavey, S.; Brewer, K.J. Visible light induced photocleavage of DNA by a mixed-metal supramolecular complex:[{(bpy)2Ru(dpp)}2RhCl2]5+. Inorg. Chem. 2002, 41, 6196–6198. [Google Scholar] [CrossRef]
- Zamora, A.; Vigueras, G.; Rodríguez, V.; Santana, M.D.; Ruiz, J. Cyclometalated iridium(III) luminescent complexes in therapy and phototherapy. Coord. Chem. Rev. 2018, 360, 34–76. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Sazanovich, I.V.; Baggaley, E.; Bonneau, M.; Guerchais, V.; Williams, J.A.; Weinstein, J.A.; Bryant, H.E. Metal complexes for two-photon photodynamic therapy: A cyclometallated iridium complex induces two-photon photosensitization of cancer cells under near-IR light. Chem. Eur. J. 2017, 23, 234–238. [Google Scholar] [CrossRef]
- Huang, H.; Banerjee, S.; Sadler, P.J. Recent advances in the design of targeted iridium(III) photosensitizers for photodynamic therapy. ChemBioChem 2018, 19, 1574–1589. [Google Scholar] [CrossRef]
- Basu, U.; Otto, S.; Heinze, K.; Gasser, G. Biological evaluation of the NIR-emissive ruby analogue [Cr(ddpd)2][BF4]3 as a photodynamic therapy photosensitizer. Eur. J. Inorg. Chem. 2018. [Google Scholar] [CrossRef]
- Mengel, A.K.; Bissinger, C.; Dorn, M.; Back, O.; Förster, C.; Heinze, K. Boosting Vis/NIR charge-transfer absorptions of iron(II) complexes by N-alkylation and N-deprotonation in the ligand backbone. Chem. Eur. J. 2017, 23, 7920–7931. [Google Scholar] [CrossRef]
- Zhang, W.; Alonso-Mori, R.; Bergmann, U.; Bressler, C.; Chollet, M.; Galler, A.; Gawelda, W.; Hadt, R.G.; Hartsock, R.W.; Kroll, T.; et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 2014, 509, 345. [Google Scholar] [CrossRef]
- Basu, U.; Khan, I.; Hussain, A.; Kondaiah, P.; Chakravarty, A.R. Photodynamic effect in near-IR light by a photocytotoxic iron(III) cellular imaging agent. Angew. Chem. Int. Ed. 2012, 51, 2658–2661. [Google Scholar] [CrossRef]
- Roy, M.; Saha, S.; Patra, A.K.; Nethaji, M.; Chakravarty, A.R. Ternary iron(III) complex showing photocleavage of DNA in the photodynamic therapy window. Inorg. Chem. 2007, 46, 4368–4370. [Google Scholar] [CrossRef]
- Saha, S.; Majumdar, R.; Roy, M.; Dighe, R.R.; Chakravarty, A.R. An iron complex of dipyridophenazine as a potent photocytotoxic agent in visible light. Inorg. Chem. 2009, 48, 2652–2663. [Google Scholar] [CrossRef]
- Saha, S.; Mallick, D.; Majumdar, R.; Roy, M.; Dighe, R.R.; Jemmis, E.D.; Chakravarty, A.R. Structure− activity relationship of photocytotoxic iron(III) complexes of modified dipyridophenazine ligands. Inorg. Chem. 2011, 50, 2975–2987. [Google Scholar] [CrossRef]
- Basu, U.; Pant, I.; Kondaiah, P.; Chakravarty, A.R. Mitochondria-targeting iron(III) catecholates for photoactivated anticancer activity under red light. Eur. J. Inorg. Chem. 2016, 2016, 1002–1012. [Google Scholar] [CrossRef]
- Basu, U.; Pant, I.; Khan, I.; Hussain, A.; Kondaiah, P.; Chakravarty, A.R. Iron(III) catecholates for cellular imaging and photocytotoxicity in red light. Chem. Asian J. 2014, 9, 2494–2504. [Google Scholar] [CrossRef]
- Sahoo, S.; Podder, S.; Garai, A.; Majumdar, S.; Mukherjee, N.; Basu, U.; Nandi, D.; Chakravarty, A.R. Iron(III) complexes of vitamin b6 schiff base with boron-dipyrromethene pendants for lysosome-selective photocytotoxicity. Eur. J. Inorg. Chem. 2018, 2018, 1522–1532. [Google Scholar] [CrossRef]
- Garai, A.; Pant, I.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A.R. Mitochondria-targeted anticancer activity of bodipy-appended iron(III) catecholates in red light. ChemistrySelect 2017, 2, 11686–11692. [Google Scholar] [CrossRef]
- Basu, U.; Khan, I.; Hussain, A.; Gole, B.; Kondaiah, P.; Chakravarty, A.R. Carbohydrate-appended tumor targeting iron(III) complexes showing photocytotoxicity in red light. Inorg. Chem. 2014, 53, 2152–2162. [Google Scholar] [CrossRef]
- Basu, U.; Pant, I.; Hussain, A.; Kondaiah, P.; Chakravarty, A.R. Iron(III) complexes of a pyridoxal schiff base for enhanced cellular uptake with selectivity and remarkable photocytotoxicity. Inorg. Chem. 2015, 54, 3748–3758. [Google Scholar] [CrossRef]
- Garai, A.; Basu, U.; Khan, I.; Pant, I.; Hussain, A.; Kondaiah, P.; Chakravarty, A.R. Iron(III) benzhydroxamates of dipicolylamines for photocytotoxicity in red light and cellular imaging. Polyhedron 2014, 73, 124–132. [Google Scholar] [CrossRef]
- Garai, A.; Pant, I.; Kondaiah, P.; Chakravarty, A.R. Iron(III) salicylates of dipicolylamine bases showing photo-induced anticancer activity and cytosolic localization. Polyhedron 2015, 102, 668–676. [Google Scholar] [CrossRef]
- Roy, M.; Santhanagopal, R.; Chakravarty, A.R. DNA binding and oxidative DNA cleavage activity of (μ-oxo) diiron(III) complexes in visible light. Dalton Trans. 2009, 6, 1024–1033. [Google Scholar] [CrossRef]
- Roy, M.; Bhowmick, T.; Ramakumar, S.; Nethaji, M.; Chakravarty, A.R. Double-strand DNA cleavage from photodecarboxylation of (μ-oxo) diiron(III) l-histidine complex in visible light. Dalton Trans. 2008, 27, 3542–3545. [Google Scholar] [CrossRef]
- Roy, M.; Bhowmick, T.; Santhanagopal, R.; Ramakumar, S.; Chakravarty, A.R. Photo-induced double-strand DNA and site-specific protein cleavage activity of l-histidine (μ-oxo) diiron(III) complexes of heterocyclic bases. Dalton Trans. 2009, 24, 4671–4682. [Google Scholar] [CrossRef]
- Saha, S.; Majumdar, R.; Hussain, A.; Dighe, R.R.; Chakravarty, A.R. Biotin-conjugated tumour-targeting photocytotoxic iron(III) complexes. Phil. Trans. R. Soc. A 2013, 371, 20120190. [Google Scholar] [CrossRef]
- Li, Q.; van den Berg, T.A.; Feringa, B.L.; Roelfes, G. Mononuclear Fe(II)-N4Py complexes in oxidative DNA cleavage: Structure, activity and mechanism. Dalton Trans. 2010, 39, 8012–8021. [Google Scholar] [CrossRef]
- Li, Q.; Browne, W.R.; Roelfes, G. DNA cleavage activity of Fe(II) N4Py under photo irradiation in the presence of 1,8-naphthalimide and 9-aminoacridine: Unexpected effects of reactive oxygen species scavengers. Inorg. Chem. 2011, 50, 8318–8325. [Google Scholar] [CrossRef]
- Basu, U.; Khan, I.; Koley, D.; Saha, S.; Kondaiah, P.; Chakravarty, A.R. Nuclear targeting terpyridine iron(II) complexes for cellular imaging and remarkable photocytotoxicity. J. Inorg. Biochem. 2012, 116, 77–87. [Google Scholar] [CrossRef]
- Garai, A.; Basu, U.; Pant, I.; Kondaiah, P.; Chakravarty, A.R. Polypyridyl iron(II) complexes showing remarkable photocytotoxicity in visible light. J. Chem. Sci. 2015, 127, 609–618. [Google Scholar] [CrossRef]
- Dickerson, M.; Sun, Y.; Howerton, B.; Glazer, E.C. Modifying charge and hydrophilicity of simple Ru(II) polypyridyl complexes radically alters biological activities: Old complexes, surprising new tricks. Inorg. Chem. 2014, 53, 10370–10377. [Google Scholar] [CrossRef]
- Castellano, F.N.; Lakowicz, J.R. A water-soluble luminescence oxygen sensor. Photochem. Photobiol. 1998, 67, 179–183. [Google Scholar] [CrossRef]
- Friedman, A.E.; Kumar, C.V.; Turro, N.J.; Barton, J.K. Luminescence of ruthenium(II) polypyridyls: Evidence for intercalative binding to z-DNA. Nucleic Acids Res. 1991, 19, 2595. [Google Scholar]
- Tan, C.; Lai, S.; Wu, S.; Hu, S.; Zhou, L.; Chen, Y.; Wang, M.; Zhu, Y.; Lian, W.; Peng, W. Nuclear permeable ruthenium(II) β-carboline complexes induce autophagy to antagonize mitochondrial-mediated apoptosis. J. Med. Chem. 2010, 53, 7613–7624. [Google Scholar] [CrossRef]
- Tan, C.; Wu, S.; Lai, S.; Wang, M.; Chen, Y.; Zhou, L.; Zhu, Y.; Lian, W.; Peng, W.; Ji, L. Synthesis, structures, cellular uptake and apoptosis-inducing properties of highly cytotoxic ruthenium–norharman complexes. Dalton Trans. 2011, 40, 8611–8621. [Google Scholar] [CrossRef]
- Qian, C.; Wang, J.-Q.; Song, C.-L.; Wang, L.-L.; Ji, L.-N.; Chao, H. The induction of mitochondria-mediated apoptosis in cancer cells by ruthenium(II) asymmetric complexes. Metallomics 2013, 5, 844–854. [Google Scholar] [CrossRef]
- Jiang, G.-B.; Xie, Y.-Y.; Lin, G.-J.; Huang, H.-L.; Liang, Z.-H.; Liu, Y.-J. Synthesis, characterization, DNA interaction, antioxidant and anticancer activity studies of ruthenium(II) polypyridyl complexes. J. Photochem. Photobiol. B Biol. 2013, 129, 48–56. [Google Scholar] [CrossRef]
- Mazuryk, O.; Maciuszek, M.; Stochel, G.; Suzenet, F.; Brindell, M. 2-Nitroimidazole-ruthenium polypyridyl complex as a new conjugate for cancer treatment and visualization. J. Inorg. Biochem. 2014, 134, 83–91. [Google Scholar] [CrossRef]
- Griffith, C.; Dayoub, A.S.; Jaranatne, T.; Alatrash, N.; Mohamedi, A.; Abayan, K.; Breitbach, Z.S.; Armstrong, D.W.; MacDonnell, F.M. Cellular and cell-free studies of catalytic DNA cleavage by ruthenium polypyridyl complexes containing redox-active intercalating ligands. Chem. Sci. 2017, 8, 3726–3740. [Google Scholar] [CrossRef]
- Gill, M.R.; Cecchin, D.; Walker, M.G.; Mulla, R.S.; Battaglia, G.; Smythe, C.; Thomas, J.A. Targeting the endoplasmic reticulum with a membrane-interactive luminescent ruthenium(II) polypyridyl complex. Chem. Sci. 2013, 4, 4512–4519. [Google Scholar] [CrossRef]
- Audi, H.; Azar, D.; Mahjoub, F.; Farhat, S.; El-Masri, Z.; El-Sibai, M.; Abi-Habib, R.J.; Khnayzer, R.S. Cytotoxicity modulation of ruthenium(II) tris-bathophenantholine complexes with systematically varied charge. J. Photochem. Photobiol. A 2018, 351, 59–68. [Google Scholar] [CrossRef]
- Ison, A.; Xu, C.; Weakley, G.K.; Richardson, D.E. Catalytic autoxidations using tris-diimine iron(II) coordination complexes. J. Mol. Catal. A Chem. 2008, 293, 1–7. [Google Scholar] [CrossRef]
- Ogawa, K.; Kobuke, Y. Recent advances in two-photon photodynamic therapy. Anti-Cancer Agents Med. Chem. 2008, 8, 269–279. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jeeves, W.P.; Lowe, D.M. In vivo and post mortem measurements of the attenuation spectra of light in mammalian tissues. Photochem. Photobiol. 1985, 42, 153–162. [Google Scholar] [CrossRef]
- Castellano, F.N. Inorganic chemistry: Making iron glow. Nature 2017, 543, 627. [Google Scholar] [CrossRef]
- Chábera, P.; Liu, Y.; Prakash, O.; Thyrhaug, E.; El Nahhas, A.; Honarfar, A.; Essén, S.; Fredin, L.A.; Harlang, T.C.; Kjær, K.S. A low-spin Fe(III) complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 2017, 543, 695. [Google Scholar] [CrossRef]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Heier, J.; Ferrari, S.; Gasser, G. Towards cancer cell-specific phototoxic organometallic rhenium(I) complexes. Dalton Trans. 2014, 43, 4287–4294. [Google Scholar] [CrossRef]
- Bruce, S.J.; Tavazzi, I.; Parisod, V.R.; Rezzi, S.; Kochhar, S.; Guy, P.A. Investigation of human blood plasma sample preparation for performing metabolomics using ultrahigh performance liquid chromatography/mass spectrometry. Anal. Chem. 2009, 81, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Martınez, G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Mang, T.S.; Dougherty, T.J.; Potter, W.R.; Boyle, D.G.; Somer, S.; Moan, J. Photobleaching of porphyrins used in photodynamic therapy and implications for therapy. Photochem. Photobiol. 1987, 45, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Puckett, C.A.; Ernst, R.J.; Barton, J.K. Exploring the cellular accumulation of metal complexes. Dalton Trans. 2010, 39, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Puckett, C.A.; Barton, J.K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 2007, 129, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, K. Solvent effect on the nonradiative deactivation of the excited state of tris(2,2′-bipyridyl)ruthenium(II) ion. Bull. Chem. Soc. Jpn. 1982, 55, 1639–1640. [Google Scholar] [CrossRef]
- Kochevar, I.E.; Redmond, R.W. [2] Photosensitized production of singlet oxygen. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2000; Volume 319, pp. 20–28. [Google Scholar]
- Garcìa-Fresnadillo, D.; Georgiadou, Y.; Orellana, G.; Braun, A.M.; Oliveros, E. Singlet-oxygen (1∆g) production by ruthenium(II) complexes containing polyazaheterocyclic ligands in methanol and in water. Helv. Chim. Acta 1996, 79, 1222–1238. [Google Scholar] [CrossRef]
| Compounds | CH3CN | CH3CN | D2O | PBS |
|---|---|---|---|---|
| Direct Method | Indirect Method | Direct Method | Indirect Method | |
| 4 | n.d. | 4% | n.d. | <1% |
| 5 | n.d. | 2% | n.d. | <1% |
| 6 | n.d. | 2% | n.d. | <1% |
| Compound | log P |
|---|---|
| 4 | +1.7 ± 0.1 |
| 5 | −2.2 ± 0.1 |
| 6 | −1.9 ± 0.1 |
| HeLa | RPE-1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50/μM Dark | IC50/μM 480 nm | PI | IC50/μM 540 nm | PI | IC50/μM Dark | IC50/μM 480 nm | PI | IC50/μM 540 nm | PI | |
| 4 | 9.3 ± 0.8 | 7.2 ± 0.6 | 1.3 | 6.6 ± 0.1 | 1.4 | 10.8 ± 1.0 | 9.3 ± 0.5 | 1.2 | 9.8 ± 0.5 | 1.1 |
| 5 | >100 | >100 | n.d. | >100 | n.d. | >100 | >100 | n.d. | >100 | n.d. |
| 6 | >100 | >100 | n.d. | >100 | n.d. | >100 | >100 | n.d. | >100 | n.d. |
| PpIX | >100 | 2.5 ± 0.1 | >40 | 2.1 ± 0.3 | >48 | >100 | 3.8 ± 0.1 | >26 | 3.1 ± 0.1 | >32 |
| Cisplatin | 10.5 ± 0.8 | - | - | - | - | 29.3 ± 1.4 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karges, J.; Goldner, P.; Gasser, G. Synthesis, Characterization, and Biological Evaluation of Red-Absorbing Fe(II) Polypyridine Complexes. Inorganics 2019, 7, 4. https://doi.org/10.3390/inorganics7010004
Karges J, Goldner P, Gasser G. Synthesis, Characterization, and Biological Evaluation of Red-Absorbing Fe(II) Polypyridine Complexes. Inorganics. 2019; 7(1):4. https://doi.org/10.3390/inorganics7010004
Chicago/Turabian StyleKarges, Johannes, Philippe Goldner, and Gilles Gasser. 2019. "Synthesis, Characterization, and Biological Evaluation of Red-Absorbing Fe(II) Polypyridine Complexes" Inorganics 7, no. 1: 4. https://doi.org/10.3390/inorganics7010004
APA StyleKarges, J., Goldner, P., & Gasser, G. (2019). Synthesis, Characterization, and Biological Evaluation of Red-Absorbing Fe(II) Polypyridine Complexes. Inorganics, 7(1), 4. https://doi.org/10.3390/inorganics7010004