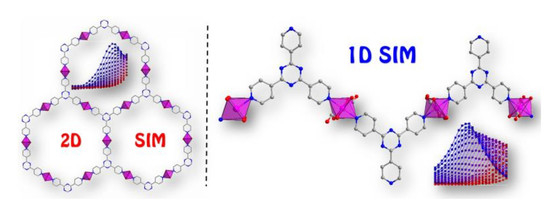
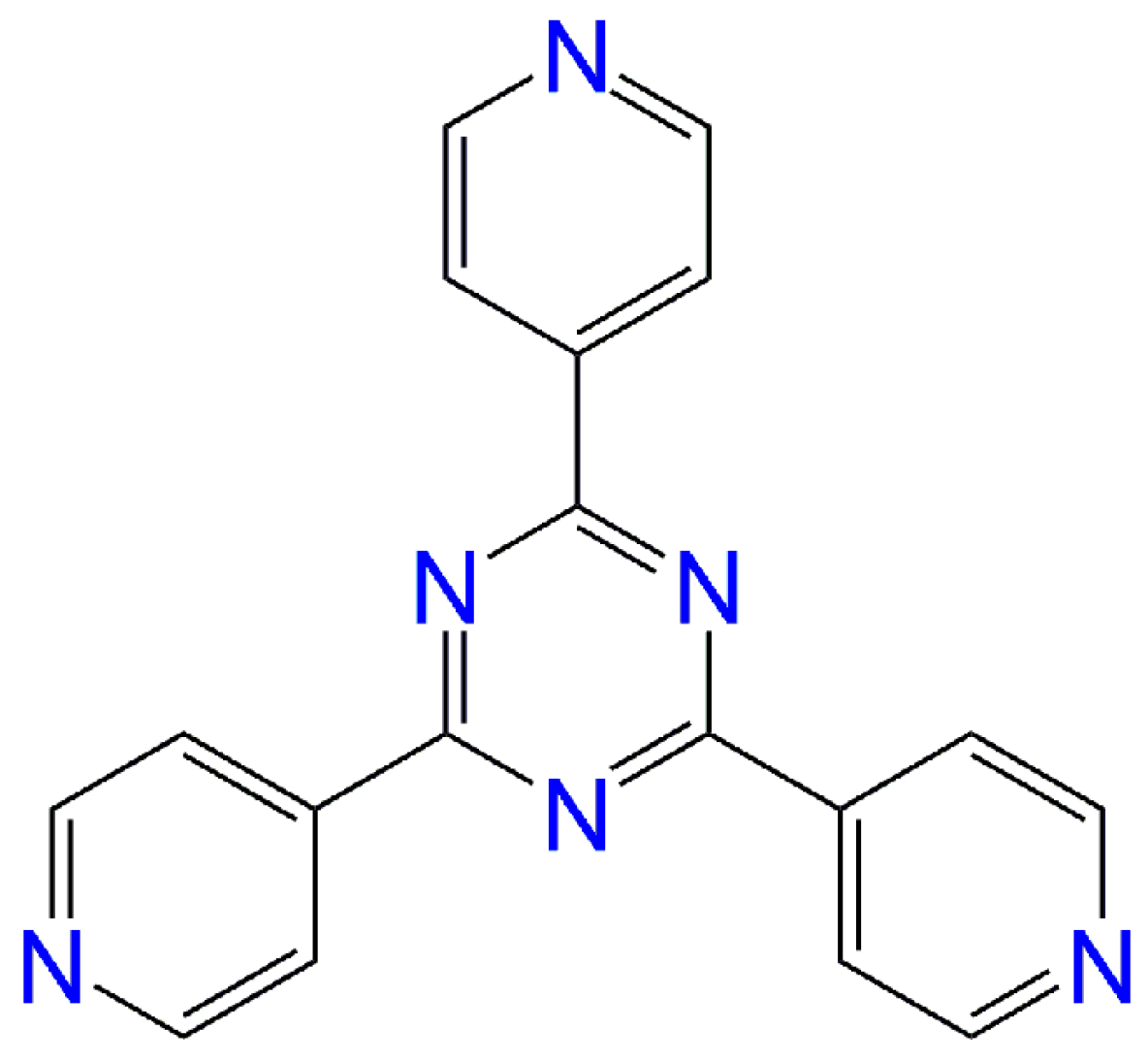
Field-Induced Single-Ion Magnet Behaviour in Two New Cobalt(II) Coordination Polymers with 2,4,6-Tris(4-pyridyl)-1,3,5-triazine
Abstract
:1. Introduction
2. Results and Discussion
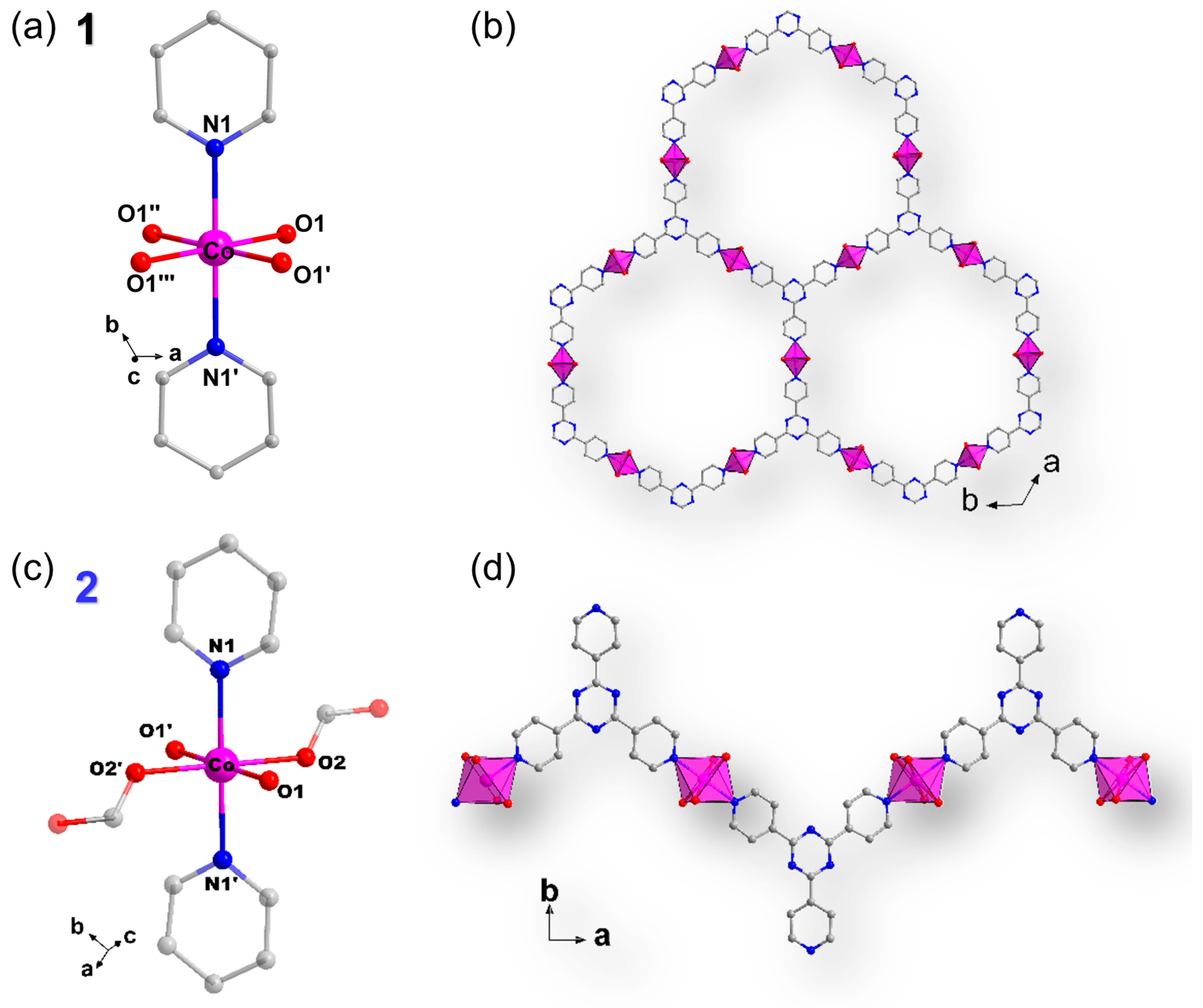
2.1. Crystal Structure Description
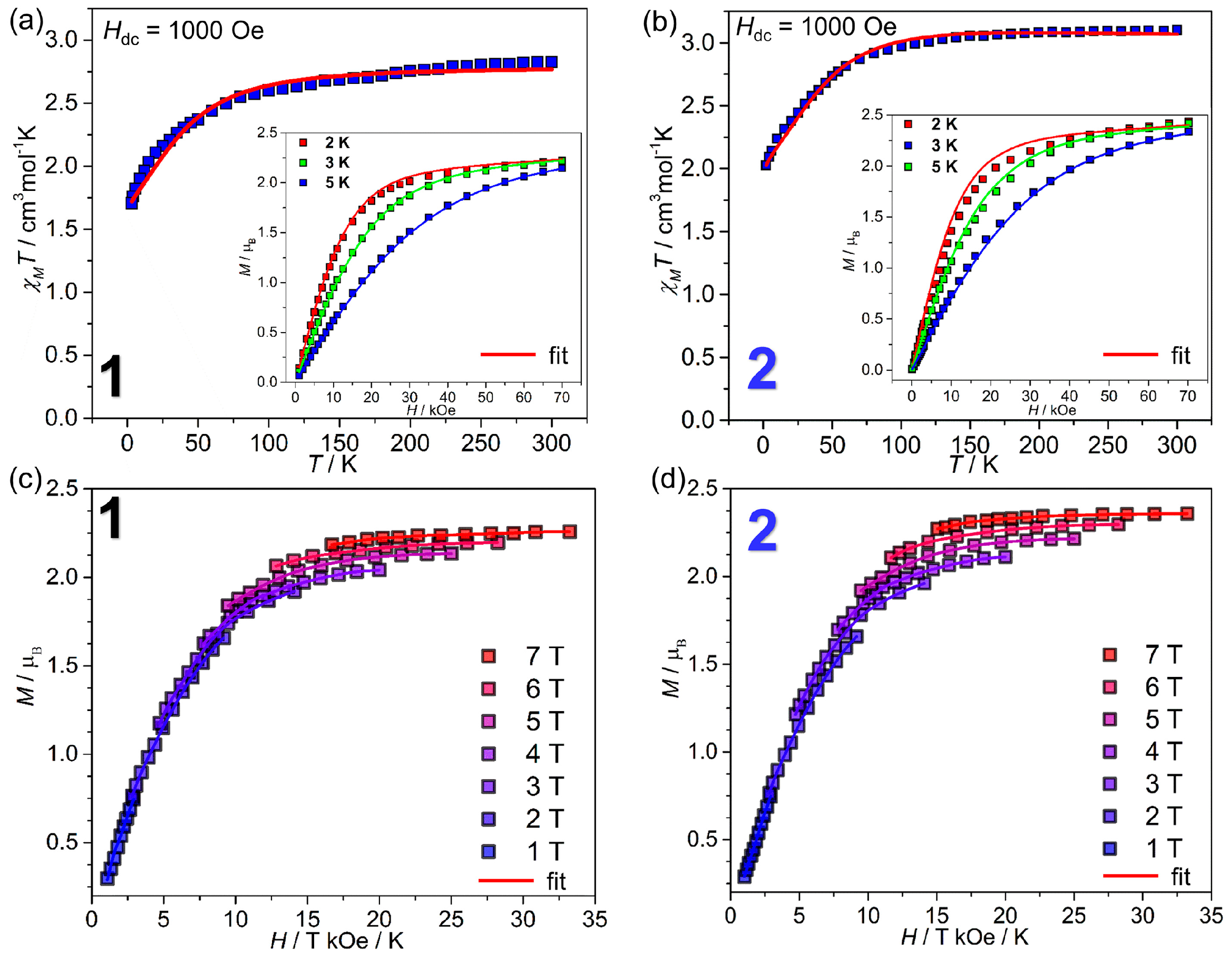
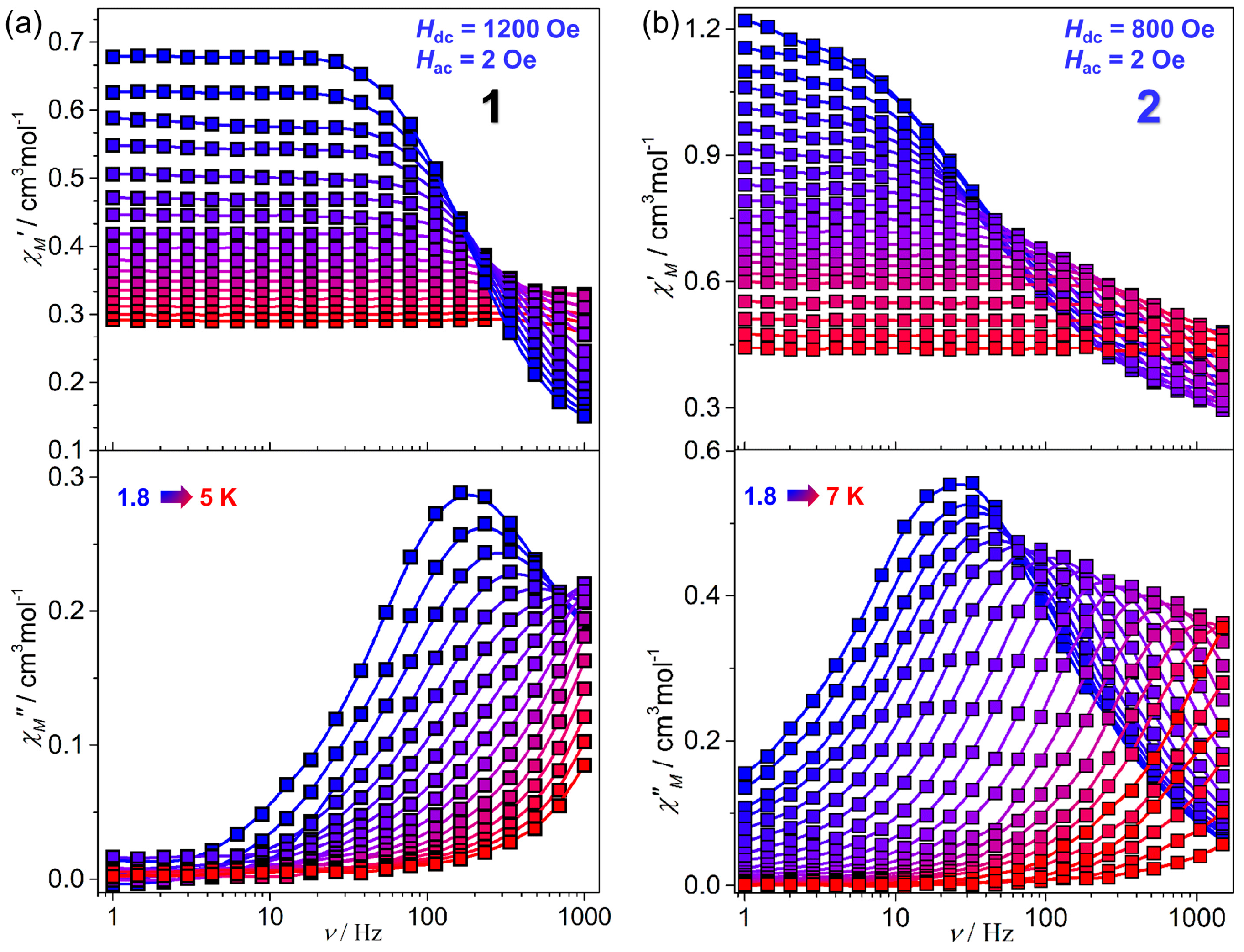
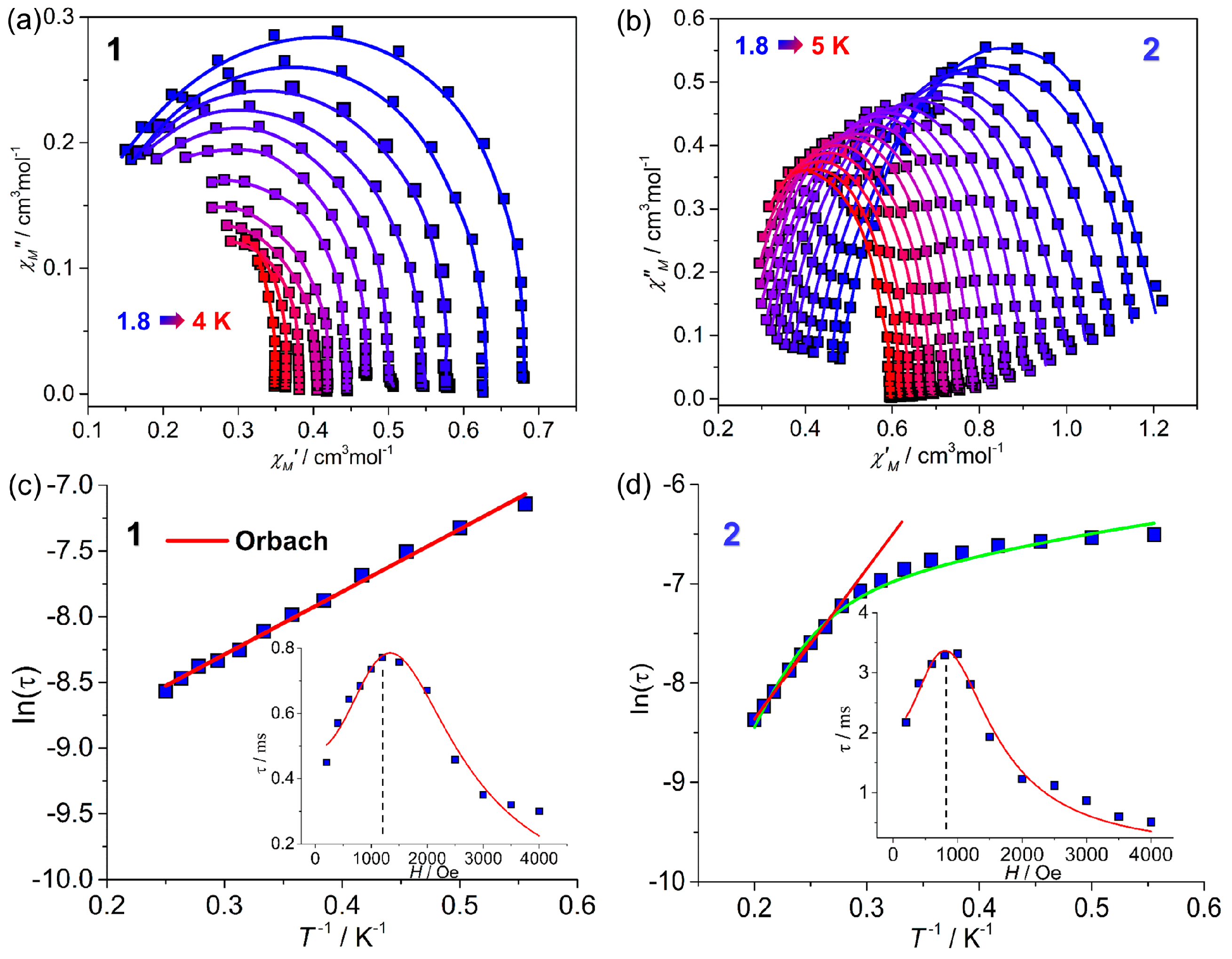
2.2. Magnetic Properties
3. Materials and Methods
3.1. Syntheses of Compounds 1 and 2
3.2. Physical Measurements
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, L.; Lionti, F.; Ballou, R.; Gatteschi, D.; Sessoli, R.; Barbara, B. Macroscopic quantum tunneling of magnetization in a single crystal of nanomagnets. Nature 1996, 383, 145–147. [Google Scholar] [CrossRef]
- Wernsdorfer, W.; Aliaga-Alcalde, N.; Hendrickson, D.N.; Christou, G. Exchange-biased quantum tunnelling in a supramolecular dimer of single-molecule magnets. Nature 2002, 416, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-S.; Jiang, S.-D.; Wang, B.-W.; Gao, S. Understanding the magnetic anisotropy toward single-ion magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Murrie, M. 3D single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.E.; Harman, W.H.; Harris, T.D.; Long, G.J.; Chang, C.J.; Long, J.R. Slow magnetic relaxation in a high-spin iron(II) complex. J. Am. Chem. Soc. 2010, 132, 1224–1225. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.-K.; Pichon, C.; Sutter, J.-P. Magnetic anisotropy in two- to eight-coordinated transition–metal complexes: Recent developments in molecular magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Zhu, M.-S.; Yin, T.-T.; Meng, Y.-S.; Wu, Z.-Q.; Zhang, Y.-Q.; Gao, S. Cobalt(II) coordination polymer exhibiting single-ion-magnet-type field-induced slow relaxation behavior. Inorg. Chem. 2015, 54, 3716–3718. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Shi, L.; Shen, F.-X.; Wang, X.-Y. A cyano-bridged coordination polymer nanotube showing field-induced slow magnetic relaxation. CrystEngComm 2017, 19, 5707–5711. [Google Scholar] [CrossRef]
- Shao, D.; Shi, L.; Zhang, S.-L.; Zhao, X.-H.; Wu, D.-Q.; Wei, X.-Q.; Wang, X.-Y. Syntheses, structures, and magnetic properties of three new chain compounds based on the pentagonal bipyramidal Co(II) building block. CrystEngComm 2016, 18, 4150–4157. [Google Scholar] [CrossRef]
- Shao, D.; Zhou, Y.; Pi, Q.; Shen, F.-X.; Yang, S.-R.; Zhang, S.-L.; Wang, X.-Y. Two-dimensional frameworks formed by pentagonal bipyramidal cobalt(II) ions and hexacyanometallates: Antiferromagnetic ordering, metamagnetism and slow magnetic relaxation. Dalton Trans. 2017, 46, 9088–9096. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-R.; Chen, Z.-W.; Cao, F.; Wang, S.; Huang, X.-Q.; Li, Y.-W.; Lu, J.; Lia, D.-C.; Dou, J.-N. Two 2-D multifunctional cobalt(II) compounds: Field-induced single-ion magnetismand catalytic oxidation of benzylic C–H bonds. Dalton Trans. 2017, 46, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.; Fortea-Perez, F.R.; Pardo, E.; Benmansour, S.; Castro, I.; Krzystek, J.; Armentano, D.; Cano, J. Guest-dependent single-ion magnet behaviour in a cobalt(II) metal-organic framework. Chem. Sci. 2016, 7, 2286–2293. [Google Scholar] [CrossRef]
- Świtlicka-Olszewska, A.; Palion-Gazda, J.; Klemens, T.; Machura, B.; Vallejo, J.; Cano, J.; Lloret, F.; Julve, M. Single-ion magnet behaviour in mononuclear and two-dimensional dicyanamide-containing cobalt(II) complexes. Dalton Trans. 2016, 45, 10181–10193. [Google Scholar]
- Mondal, A.K.; Khatua, S.; Tomar, K.; Konar, S. Field-induced single-ion-magnetic behavior of octahedral CoII in a two-dimensional coordination polymer. Eur. J. Inorg. Chem. 2016, 3545–3552. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Chen, S.-P.; Yin, B.; Sun, Y.-C.; Wang, Z.-X.; Ouyang, Z.-W.; Ren, J.-J.; Wang, W.-Y.; Wei, Q.; et al. A two-dimensional cobalt(II) network with a remarkable positive axial anisotropy parameter exhibiting field-induced single-ion magnet behavior. J. Mater. Chem. C 2016, 4, 7798–7808. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Sun, L.; Zhou, H.-L.; Cen, P.-P.; Jin, X.-Y.; Xie, G.; Chen, S.-P.; Hu, Q.-L. Single-ion-magnet behaviour in a two-dimensional coordination polymer constructed from CoII nodes and a pyridylhydrazone derivative. Inorg. Chem. 2015, 54, 8884–8886. [Google Scholar] [CrossRef] [PubMed]
- Brunet, G.; Safin, D.A.; Jover, J.; Ruiz, E.; Murugesu, M. Single-molecule magnetism arising from cobalt(II) nodes of a crystalline sponge. J. Mater. Chem. C 2017, 5, 835–841. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Chen, L.; Liu, C.-M.; Du, Z.-Y.; Chen, L.-L.; Liu, Q.-Y. 3D chiral and 2D achiral cobalt(II) compounds constructed from a 4-(benzimidazole-1-yl) benzoic ligand exhibiting field-induced single-ion-magnet-type slow magnetic relaxation. Dalton Trans. 2016, 45, 7768–7775. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, X.-J.; Meng, X.-X.; Shi, W.; Cheng, P.; Powell, A.K. Constraining the coordination geometries of lanthanide centers and magnetic building blocks in frameworks: A new strategy for molecular nanomagnets. Chem. Soc. Rev. 2016, 45, 2423–2439. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-C.; Zhou, C.; Shao, D.; Wang, X.-Y. Field-induced slow magnetic relaxation in cobalt(II) compounds with pentagonal bipyramid geometry. Inorg. Chem. 2014, 53, 12671–12673. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhang, S.-L.; Shi, L.; Zhang, Y.-Q.; Wang, X.-Y. Probing the effect of axial ligands on easy-plane anisotropy of pentagonal-bipyramidal cobalt(II) single-ion magnets. Inorg. Chem. 2016, 55, 10859–10869. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013.
- Polunin, R.A.; Burkovskaya, N.P.; Satska, J.A.; Kolotilov, S.V.; Kiskin, M.A.; Aleksandrov, G.G.; Cador, O.; Ouahab, L.; Eremenko, I.L.; Pavlishchuk, V.V. Solvent-induced change of electronic spectra and magnetic susceptibility of CoII coordination polymer with 2,4,6-Tris(4-pyridyl)-1,3,5-triazine. Inorg. Chem. 2015, 54, 5232–5238. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Nishikiori, S.; Kuroda, R. Construction of supramolecular complexes by use of planar bis(β-diketonato)cobalt(II) complexes as building blocks. Chem. Lett. 2007, 36, 678. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Gan, L.; Zhang, S.-W.; Gao, S. Perovskite-like metal formates with weak ferromagnetism and as precursors to amorphous materials. Inorg. Chem. 2004, 43, 4615–4625. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.P.S.; Bowling, G.; Ariciu, A.-M.; Jailani, N.F.M.; Chilton, N.F.; Waddell, P.G.; Collison, D.; Tuna, F.; Higham, L.J. Evidence of slow magnetic relaxation in Co(AcO)2(py)2(H2O)2. Magnetochemistry 2016, 2, 23. [Google Scholar] [CrossRef]
- Shores, M.P.; Sokol, J.J.; Long, J.R. Nickel(II)−molybdenum(III)−cyanide clusters: Synthesis and magnetic behavior of species incorporating [(Me3tacn)Mo(CN)3]. J. Am. Chem. Soc. 2002, 124, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Coca, S.; Cremades, E.; Aliaga-Alcalde, N.; Ruiz, E. Mononuclear single-molecule magnets: Tailoring the magnetic anisotropy of first-row transition-metal complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Atanasov, M.; Bryan, A.M.; Lin, C.-Y.; Rekken, B.D.; Power, P.P.; Neese, F.; Long, J.R. Slow magnetization dynamics in a series of two-coordinate iron(II) complexes. Chem. Sci. 2013, 4, 125–138. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 1970. [Google Scholar]
- Anderson, H.L.; Anderson, S.; Sanders, J.K.M. Combined NMR spectroscopy and molecular mechanics studies on the stable structures of calix[n]arenes. J. Chem. Soc. Perkin Trans. 1995, 2231–2245. [Google Scholar] [CrossRef]
- SAINT Software Users Guide, Version 7.0; Bruker Analytical X-Ray Systems, Inc.: Madison, WI, USA, 1999.
- SADABS, Version 2.03; Bruker Analytical X-Ray Systems, Inc.: Madison, WI, USA, 2000.
- SHELXTL, Version 6.14; Bruker AXS, Inc.: Madison, WI, USA, 2000–2003.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, D.; Shi, L.; Wei, H.-Y.; Wang, X.-Y. Field-Induced Single-Ion Magnet Behaviour in Two New Cobalt(II) Coordination Polymers with 2,4,6-Tris(4-pyridyl)-1,3,5-triazine. Inorganics 2017, 5, 90. https://doi.org/10.3390/inorganics5040090
Shao D, Shi L, Wei H-Y, Wang X-Y. Field-Induced Single-Ion Magnet Behaviour in Two New Cobalt(II) Coordination Polymers with 2,4,6-Tris(4-pyridyl)-1,3,5-triazine. Inorganics. 2017; 5(4):90. https://doi.org/10.3390/inorganics5040090
Chicago/Turabian StyleShao, Dong, Le Shi, Hai-Yan Wei, and Xin-Yi Wang. 2017. "Field-Induced Single-Ion Magnet Behaviour in Two New Cobalt(II) Coordination Polymers with 2,4,6-Tris(4-pyridyl)-1,3,5-triazine" Inorganics 5, no. 4: 90. https://doi.org/10.3390/inorganics5040090
APA StyleShao, D., Shi, L., Wei, H.-Y., & Wang, X.-Y. (2017). Field-Induced Single-Ion Magnet Behaviour in Two New Cobalt(II) Coordination Polymers with 2,4,6-Tris(4-pyridyl)-1,3,5-triazine. Inorganics, 5(4), 90. https://doi.org/10.3390/inorganics5040090