Dehydrogenation of Surface-Oxidized Mixtures of 2LiBH4 + Al/Additives (TiF3 or CeO2)
Abstract
:1. Introduction
2. Results
2.1. Characterization of As-Milled Materials
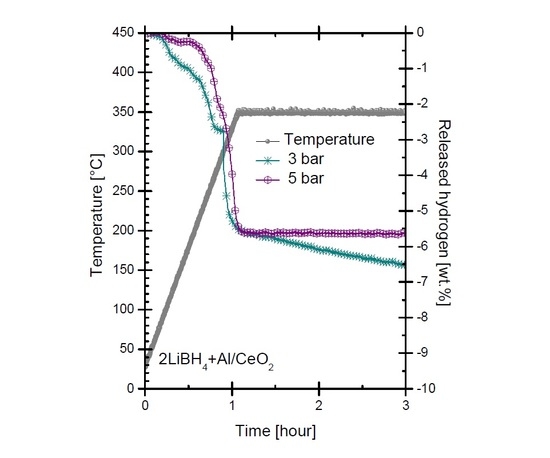
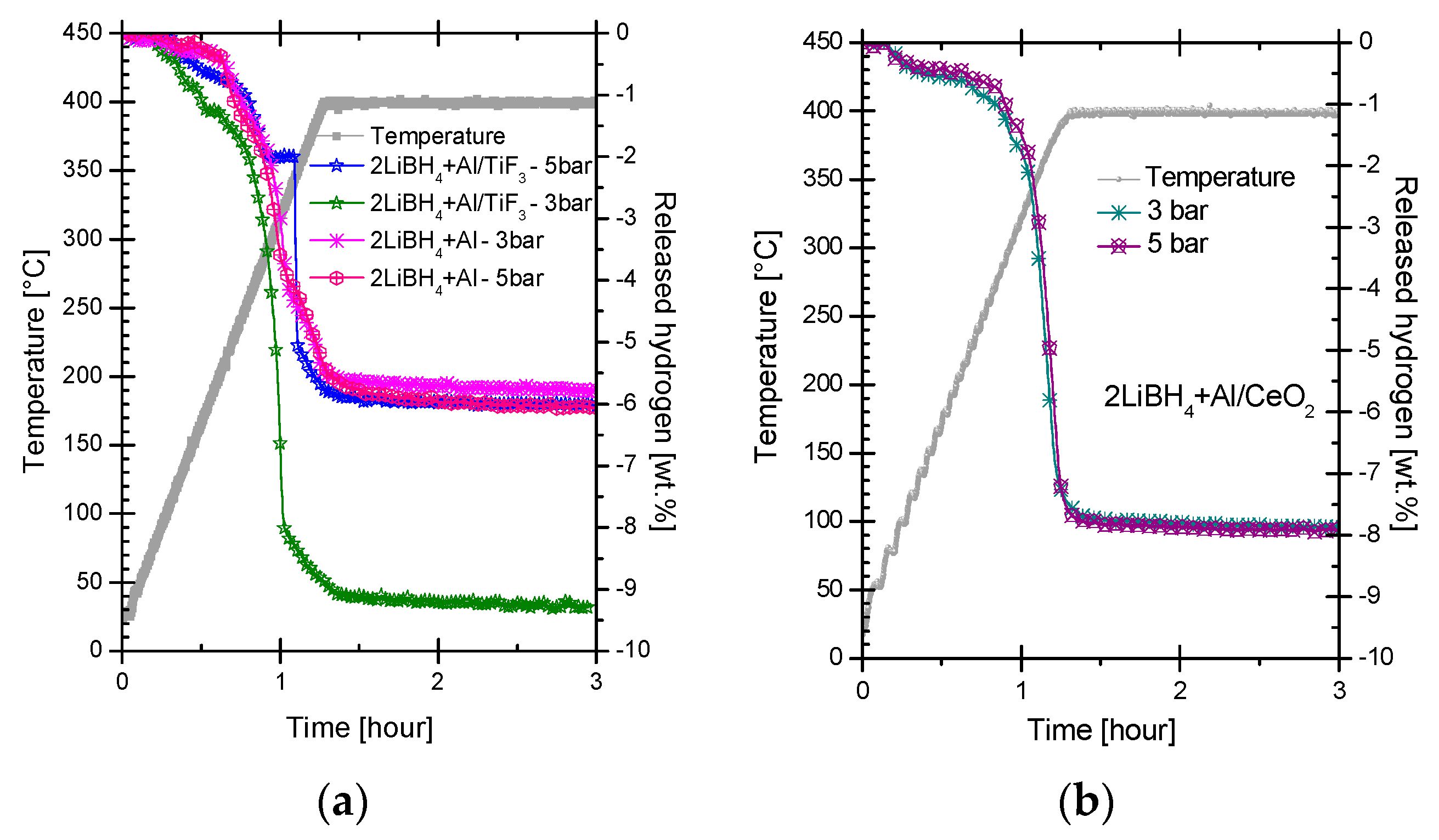
2.2. Dehydrogenation Reactions
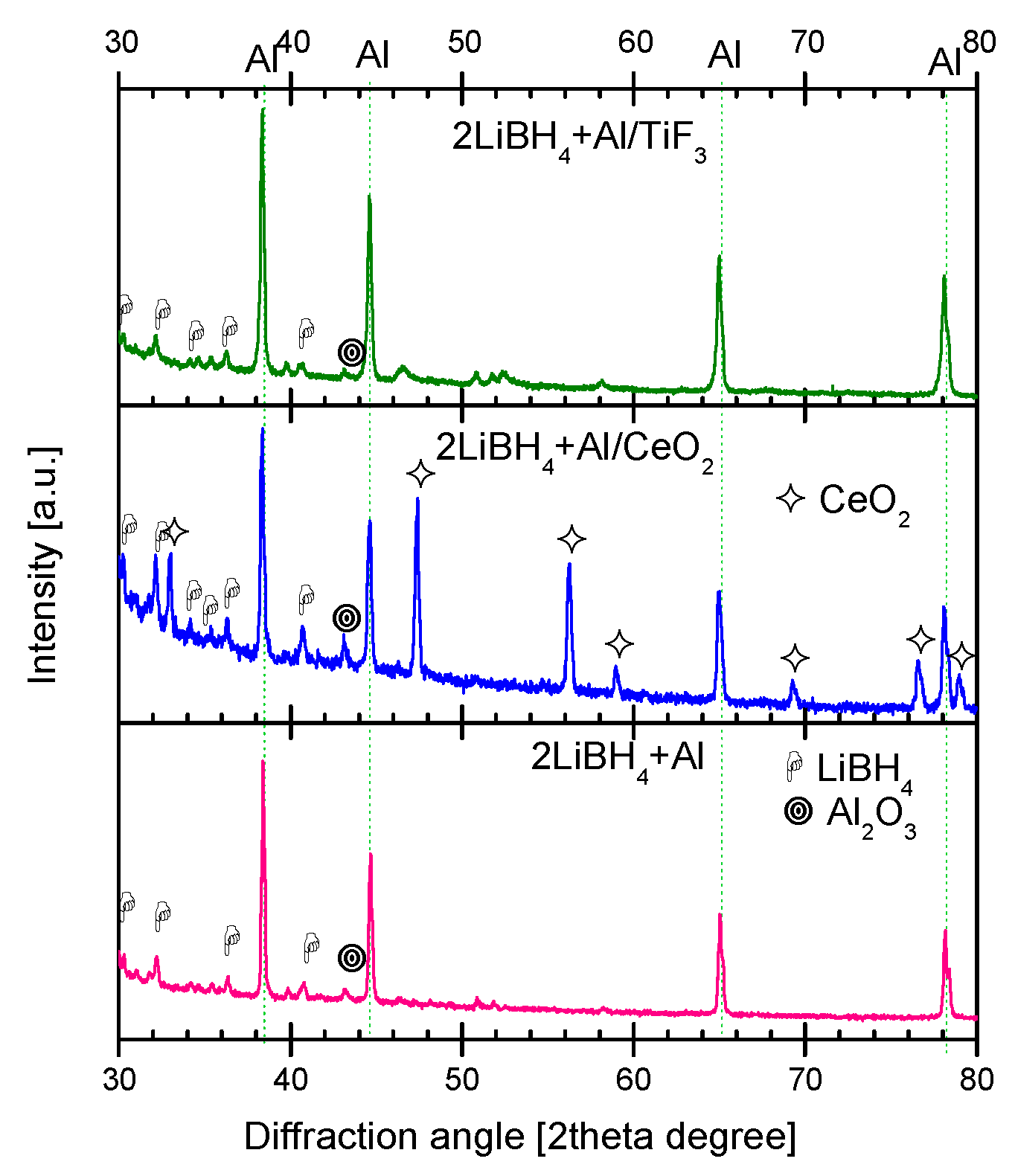
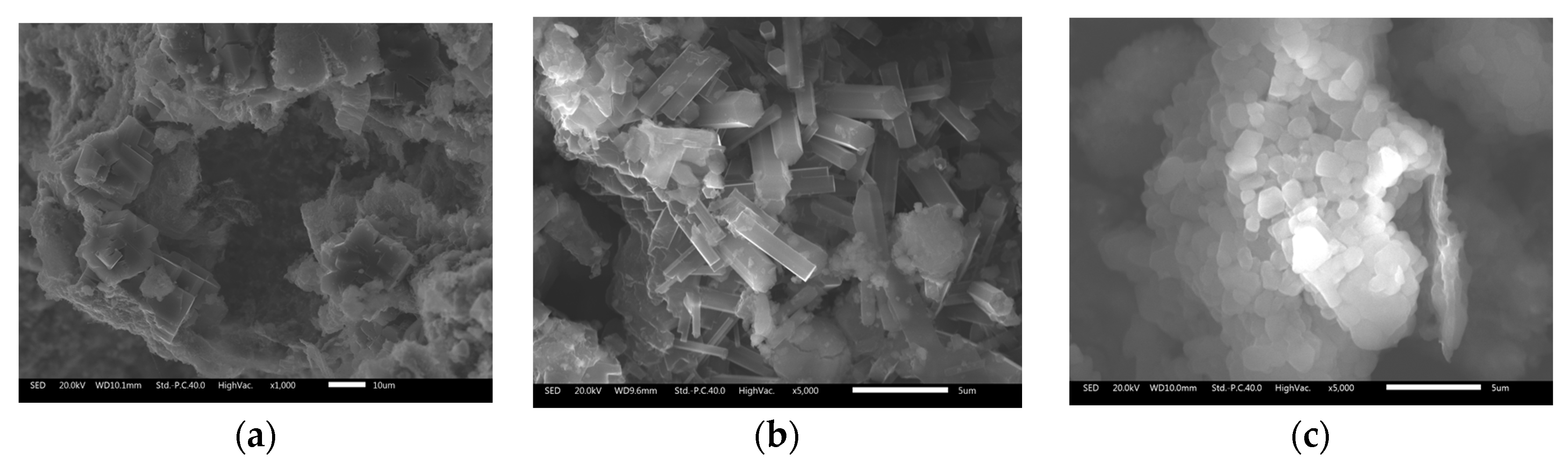
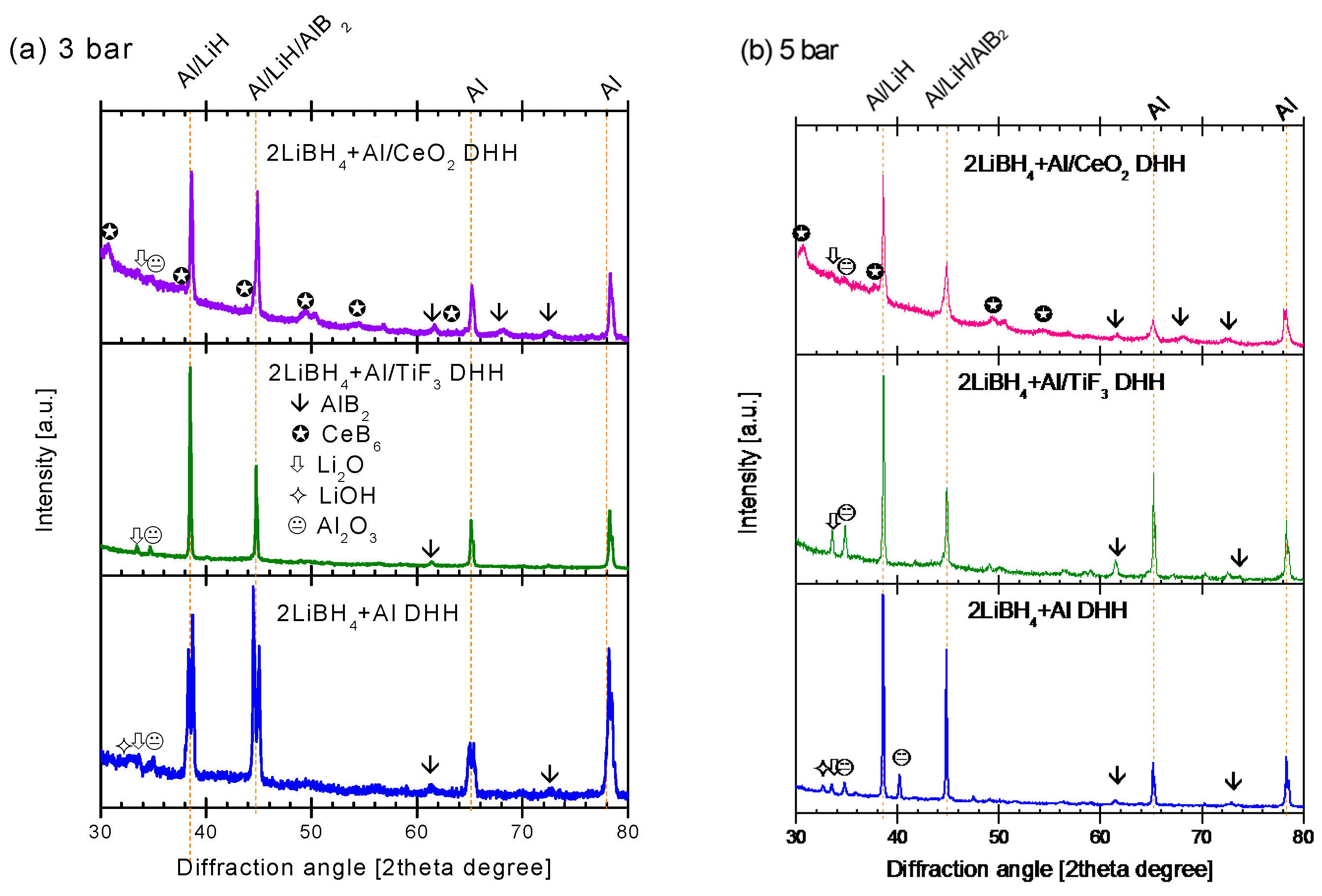
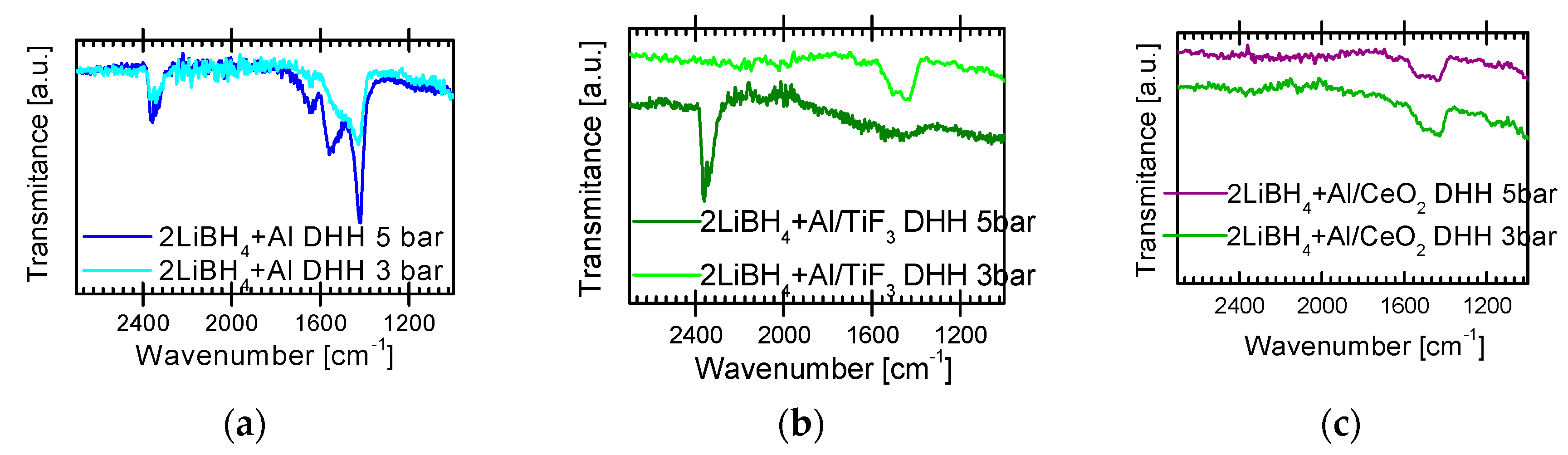
2.3. Characterization of Dehydrogenated Materials
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Characterization of the Ball-Milled and Dehydrogenated Materials
4.3. Dehydrogenation Reaction
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ravnsbæk, D.; Lee, Y.S.; Kim, Y.; Cerenius, Y.; Shim, H.J.; Jensen, T.R.; Hur, N.H.; Cho, Y.W. Decomposition reactions and reversibility of the LiBH4–Ca(BH4)2 composite. J. Phys. Chem. C 2009, 113, 15080–15086. [Google Scholar] [CrossRef]
- Liu, Y.; Reed, D.; Paterakis, C.; Contreras Vasquez, L.; Baricco, M.; Book, D. Study of the decomposition of a 0.62LiBH4–0.38NaBH4 mixture. Int. J. Hydrog. Energy 2017, 42, 22480–22488. [Google Scholar] [CrossRef]
- Gil-Bardají, E.; Zhao-Karger, Z.; Boucharat, N.; Nale, A.; van Setten, M.J.; Lohstroh, W.; Reohm, E.; Catti, M.; Fichtner, M. LiBH4–Mg(BH4)2: A physical mixture of metal borohydrides as hydrogen storage material. J. Phys. Chem. C 2011, 115, 6095–6101. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Orimo, S.; Towata, S. Destabilization of LiBH4 by mixing with LiNH2. Appl. Phys. A 2005, 80, 1409–1412. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Liua, H.K.; Yu, X.B. Reversible hydrogen storage in titanium-catalyzed LiAlH4–LiBH4 system. J. Alloys Compd. 2009, 487, 434–438. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, X.; Feidenhans, R.; Vegge, T. Destabilized LiBH4–NaAlH4 mixtures doped with titanium based catalysts materials research. J. Phys. Chem. C 2008, 112, 18244–18248. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; et al. Hydrogen sorption properties of MgH2–LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S. Reversible hydrogen storage in the lithium borohydride—Calcium hydride coupled system. J. Alloys Compd. 2008, 464, L1–L4. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with a Metal (M = Mg, Al, Ti, V, Cr, or Sc) or Metal Hydride (MH2 = MgH2, TiH2, or CaH2). J. Phys. Chem. C 2007, 111, 19134–19140. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Qu, X. Investigation on LiBH4–CaH2 composite and its potential for thermal energy storage. Sci. Rep. 2017, 7, 41754–41758. [Google Scholar] [CrossRef] [PubMed]
- Arnbjerg, L.M.; Ravnsbæk, D.B.; Filinchuk, Y.; Vang, R.T.; Cerenius, Y.; Besenbacher, F.; Jørgensen, J.E.; Jakobsen, H.J.; Jensen, T.R. Structure and dynamics for LiBH4–LiCl solid solutions. Chem. Mater. 2009, 21, 5772–5782. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. Dehydrogenation of LiBH4 destabilized with various oxides. J. Phys. Chem. C 2009, 113, 17945–17949. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, S.J.; To, M.; Lee, Y.S.; Whan Cho, Y. Discovery of fluidic LiBH4 on scaffold surfaces and its application for fast co-confinement of LiBH4–Ca(BH4)2 into mesopores. J. Phys. Chem. C 2015, 119, 9025–9035. [Google Scholar] [CrossRef]
- Hansen, B.R.S.; Ravnsbæk, D.B.; Reed, D.; Book, D.; Gundlach, C.; Skibsted, J.; Jensen, T.R. Hydrogen storage capacity loss in a LiBH4–Al composite. J. Phys. Chem. C 2013, 117, 7423–7432. [Google Scholar] [CrossRef]
- Siegel, D.J.; Wolverton, C.; Ozoliņš, V. Thermodynamic guidelines for the prediction of hydrogen storage reactions and their application to destabilized hydride mixtures. Phys. Rev. B 2007, 76, 134102–134106. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q.; Zhang, J.; Liu, S.S.; Sun, L.X. The dehydrogenation reactions and kinetics of 2LiBH4–Al composite. J. Phys. Chem. C 2009, 113, 18424–18430. [Google Scholar] [CrossRef]
- Kang, X.D.; Wang, P.; Ma, L.P.; Cheng, H.M. Reversible hydrogen storage in LiBH4 destabilized by milling with Al. Appl. Phys. A 2007, 89, 963–966. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Jensen, T.R. Mechanism for reversible hydrogen storage in LiBH4–Al. J. Appl. Phys. 2012, 111, 112621–112628. [Google Scholar] [CrossRef]
- Bosenberg, U.; Ravnsbæk, D.B.; Hagemann, H.; D’Anna, V.; Bonatto-Minella, C.; Pistidda, C.; van Beek, W.; Jensen, T.R.; Bormann, R.; Dornheim, M. Pressure and temperature influence on the desorption pathway of the LiBH4–MgH2 composite system. J. Phys. Chem. C 2010, 114, 15212–15217. [Google Scholar] [CrossRef]
- Saldan, I.; Llamas-Jansa, I.; Hino, S.; Frommen, C.; Hauback, B.C. Synthesis and thermal decomposition of Mg(BH4)2–TMO (TMO=TiO2; ZrO2; Nb2O5; MoO3) composites. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012041–012047. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; et al. Hydrogen storage properties of γ-Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrog. Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Sohlberg, K.; Pantelides, S.T.; Pennycook, S.J. Interactions of hydrogen with CeO2. J. Am. Chem. Soc. 2001, 123, 6609–6611. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Alcántara, K.; Palacios-Lazcano, A.F.; Funatsu, T.; Cabañas-Moreno, J.G. Hydriding and dehydriding in air-exposed Mg–Fe powder mixtures. Int. J. Hydrog. Energy 2016, 41, 23380–23387. [Google Scholar] [CrossRef]
- Kato, S.; Bielmann, M.; Borgschulte, A.; Zakaznova-Herzog, V.; Remhof, A.; Orimo, S.I.; Zuttel, A. Effect of the surface oxidation of LiBH4 on the hydrogen desorption mechanism. Phys. Chem. Chem. Phys. 2010, 12, 10950–10955. [Google Scholar] [CrossRef] [PubMed]
- JANAF Thermochemical Tables. Available online: http://kinetics.nist.gov/janaf/ (accessed on 15 July 2017).
- Fang, Z.Z.; Ma, L.P.; Kang, D.; Wang, P.J.; Wang, P.; Cheng, H.M. In situ formation and rapid decomposition of Ti(BH4)3 by mechanical milling LiBH4 with TiF3. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- D’Anna, V.; Spyratou, A.; Sharma, M.; Hagemann, H. FT-IR spectra of inorganic borohydrides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Cao, L.; Wang, J.; Mao, J. Hydrolysis behavior of LiBH4 films. J. Alloys Compd. 2017, 698, 495–500. [Google Scholar] [CrossRef]
- Haeberle, J.; Henkel, K.; Gargouri, H.; Naumann, F.; Gruska, B.; Arens, M.; Tallarida, M.; Schmeißer, D. Ellipsometry and XPS comparative studies of thermal and plasma enhanced atomic layer deposited Al2O3-films. J. Nanotechnol. 2013, 4, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, J.; Sloof, W.G.; Terryn, H.; de Wit, J.H.W. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminum oxide layers. Surf. Interface Anal. 2004, 36, 81–88. [Google Scholar] [CrossRef]
- Ramezani, M.; Neitzert, T. Mechanical milling of aluminum powder using planetary ball milling process. J. Achiev. Mater. Manuf. Eng. 2012, 55, 790–798. [Google Scholar]
- Kang, J.K.; Kim, S.Y.; Han, Y.S.; Muller, R.P.; Goddard, W.A., III. A candidate LiBH4 for hydrogen storage: Crystals structures and reaction mechanisms of intermediate phases. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356–357, 515–520. [Google Scholar] [CrossRef]
- Kim, K.B.; Shim, J.H.; Park, S.H.; Choi, I.S.; Oh, K.H.; Cho, Y.W. Dehydrogenation reaction pathway of the LiBH4–MgH2 composite under various pressure conditions. J. Phys. Chem. C 2015, 119, 9714–9720. [Google Scholar] [CrossRef]
- Friedrichs, O.; Kim, J.W.; Remhof, A.; Buchter, F.; Borgschulte, A.; Wallacher, D.; Cho, Y.W.; Fichtner, M.; Oh, K.H.; Zuttel, A. The effect of Al on the hydrogen sorption mechanism of LiBH4. Phys. Chem. Chem. Phys. 2009, 11, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Tang, J.J.; Yu, Q.; Wang, H.; Ouyang, L.Z.; Zhao, Y.J.; Liu, J.W.; Wang, W.H.; Zu, M. Symbiotic CeH2.73/CeO2 catalyst: A novel hydrogen pump. Nano Energy 2014, 9, 80–87. [Google Scholar] [CrossRef]
- Gennari, F.C.; Fernández Albanesi, L.; Puszkiel, J.A.; Arneodo Larochette, P. Reversible hydrogen storage from 6LiBH4–MCl3 (M = Ce, Gd) composites by in-situ formation of MH2. Int. J. Hydrog. Energy 2011, 36, 563–570. [Google Scholar] [CrossRef]
- Liu, B.H.; Zhang, B.J.; Jiang, Y. Hydrogen storage performance of LiBH4 + 1/2MgH2 composites improved by Ce-based additives. Int. J. Hydrog. Energy 2011, 36, 5418–5424. [Google Scholar] [CrossRef]
- Jin, S.A.; Lee, Y.S.; Shim, J.H.; Cho, Y.W. Reversible hydrogen storage in LiBH4–MH2 (M = Ce, Ca) composites. J. Phys. Chem. C 2008, 112, 9520–9524. [Google Scholar] [CrossRef]
- Gennari, F.C. Destabilization of LiBH4 by MH2 (M = Ce, La) for hydrogen storage: Nanostructural effects on the hydrogen sorption kinetics. Int. J. Hydrog. Energy 2011, 36, 15231–15238. [Google Scholar] [CrossRef]
- Zhang, B.J.; Liu, B.H.; Li, Z.P. Destabilization of LiBH4 by (Ce, La)(Cl, F)3 for hydrogen storage. J. Alloys Compd. 2011, 509, 751–757. [Google Scholar] [CrossRef]
- Peschke, M. Wasserstoffspeicherung in Reaktiven Hydrid-Kompositen Einfluss von fluorbasierten Additiven auf das Sorptionsverhalten des MgB2–LiH-Systems. Diplomarbeit (Undergraduate Thesis), GKSS-Forschungszentrum Geesthacht GmbH Helmut-Schmidt-Universität/Universität der Bundeswehr, Hamburg, Germany, April 2009. [Google Scholar]
- Züttel, A.; Borgschulte, A.; Schlapbach, L.; Chorkendorf, I.; Suda, S. Properties if hydrogen. In Hydrogen as A Future Energy Carrier; Züttel, A., Borgschulte, A., Schlapbach, L., Eds.; John Wiley & Sons: Wienheim, Germany, 2008; pp. 71–94. ISBN 978-3-527-30817-0. [Google Scholar]
- Hemmes, H.; Driessen, A.; Driessen, R. Thermodynamic properties of hydrogen at pressures up to 1 Mbar and Temperatures between 100 and 1000 K. J. Phys. C Solid State Phys. 1986, 19, 3571–3585. [Google Scholar] [CrossRef]


| Material and/or Proposed Reaction, and Reported (If Available) (kJ/mol H2) | Desorption Conditions p (bar) and T (°C) | Comments |
|---|---|---|
| LiBH4 → LiH + B + 3/2H2 [1] | p: not specified T: 320 °C and 500 °C | Multi-step dehydrogenation reaction |
| LiBH4 → Li + B + 2H2 95.1 kJ/mol H2 [28] | p: 1 bar T: 25 °C | From standard formation enthalpy of LiBH4 |
| xLiBH4 + (1 − x) Ca(BH4)2 [2] | p: not specified T: 370 °C for x = 0.4 | x = 0 − 1, eutectic melting at 200 °C |
| 0.62LiBH4-0.38NaBH4 [3] | p: not specified T: onset at 287 °C, peaks at 488 °C and 540 °C | Multi-step dehydrogenation reaction |
| xLiBH4 + (1 − x) Mg(BH4)2 [4] | p: 5 bar T: 170 °C and 215 °C | x = 0 − 1, eutectic melting at 180 °C Multi-step dehydrogenation reaction |
| LiBH4 + 2LiNH2 → Li3BN2 + 4H2 23 kJ/mol H2 [5] | p: 100–0.01 bar T: 430 °C | From pressure composition isotherm. |
| LiBH4 + LiAlH4 [6] | p: 0.2 bar T: 118 °C and 210 °C | 2:1 mixture, two-step dehydrogenation. Dehydrogenation temperature reduced if TiF3 addition. |
| LiBH4 + NaAlH4 [7] | p: 1 bar He T: from room temperature up to 210 °C for the doped systems and 110–250 °C for the undoped systems. | Molar ratios 1:1, 2:3 and 1:3; with and without TiCl3 additive. Multi-step dehydrogenation reaction. |
| 2LiBH4 + MgH2 → 2LiH + MgB2 + 4H2 50.4 kJ/mol H2 [10] | p: 3 bar H2 T: 350–400 °C | Multi-step dehydrogenation reaction |
| 6LiBH4 + CaH2 ↔ 6LiH + CaB6 + 10H2 59 kJ/mol H2 [11] | p: 1.3 bar flowing He T: onset at 150 °C, maximum at 350 °C, finished at 450 °C | - |
| LiBH4 + TiH2 [12] | p: not specified (argon) T: ~410 °C | - |
| LiBH4 + LiCl (1:1) to give Li(BH4)1−xClx (x ≈ 0.23) [14] | p: not specified (argon) T: 300–550 °C | Cl− to BH4− substitution at LiBH4 |
| 2LiBH4 + Al → 2LiH + AlB2 + 3H2 57.9 kJ/mol H2 [18] | 277 °C [18] | Theoretical desorption temperature |
| Dehydrogenation: p: 0.001 bar H2 T: 325 °C to 525 °C [19] | H2 release of about 4 wt % Multi-step dehydrogenation reaction | |
| p: 0.001 bar T: 450 °C [20] | Catalyzed with TiF3 | |
| p: dynamic vacuum T: up to 500 °C [21] | Formation of LixAl1−xB2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Bucio, J.L.; Tena-García, J.R.; Suárez-Alcántara, K. Dehydrogenation of Surface-Oxidized Mixtures of 2LiBH4 + Al/Additives (TiF3 or CeO2). Inorganics 2017, 5, 82. https://doi.org/10.3390/inorganics5040082
Carrillo-Bucio JL, Tena-García JR, Suárez-Alcántara K. Dehydrogenation of Surface-Oxidized Mixtures of 2LiBH4 + Al/Additives (TiF3 or CeO2). Inorganics. 2017; 5(4):82. https://doi.org/10.3390/inorganics5040082
Chicago/Turabian StyleCarrillo-Bucio, Juan Luis, Juan Rogelio Tena-García, and Karina Suárez-Alcántara. 2017. "Dehydrogenation of Surface-Oxidized Mixtures of 2LiBH4 + Al/Additives (TiF3 or CeO2)" Inorganics 5, no. 4: 82. https://doi.org/10.3390/inorganics5040082
APA StyleCarrillo-Bucio, J. L., Tena-García, J. R., & Suárez-Alcántara, K. (2017). Dehydrogenation of Surface-Oxidized Mixtures of 2LiBH4 + Al/Additives (TiF3 or CeO2). Inorganics, 5(4), 82. https://doi.org/10.3390/inorganics5040082