Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials
Abstract
1. Introduction
2. Results
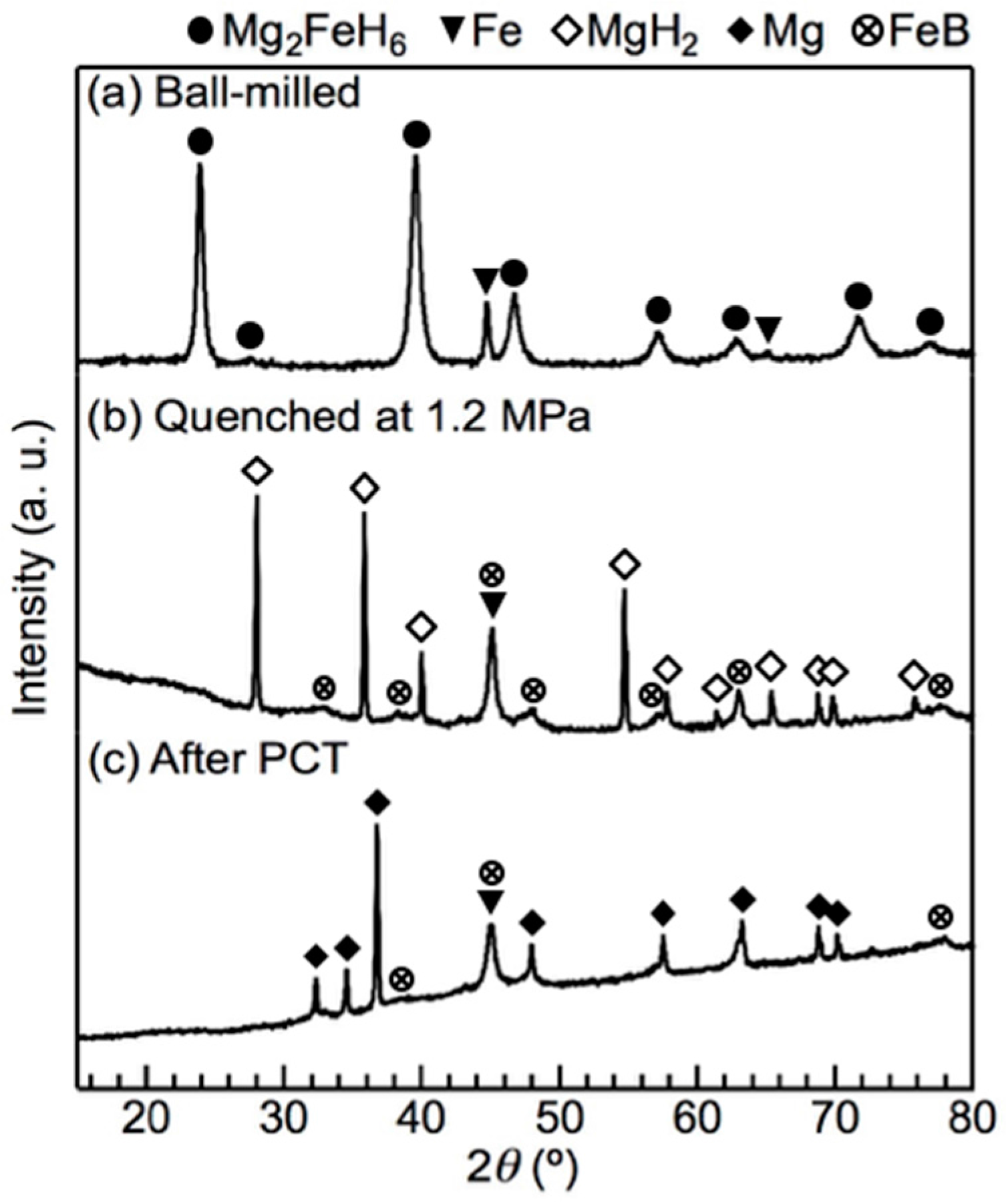
2.1. Optimal Composition Ratio for the Stoichiometric Reaction between LiBH4 and Mg2FeH6
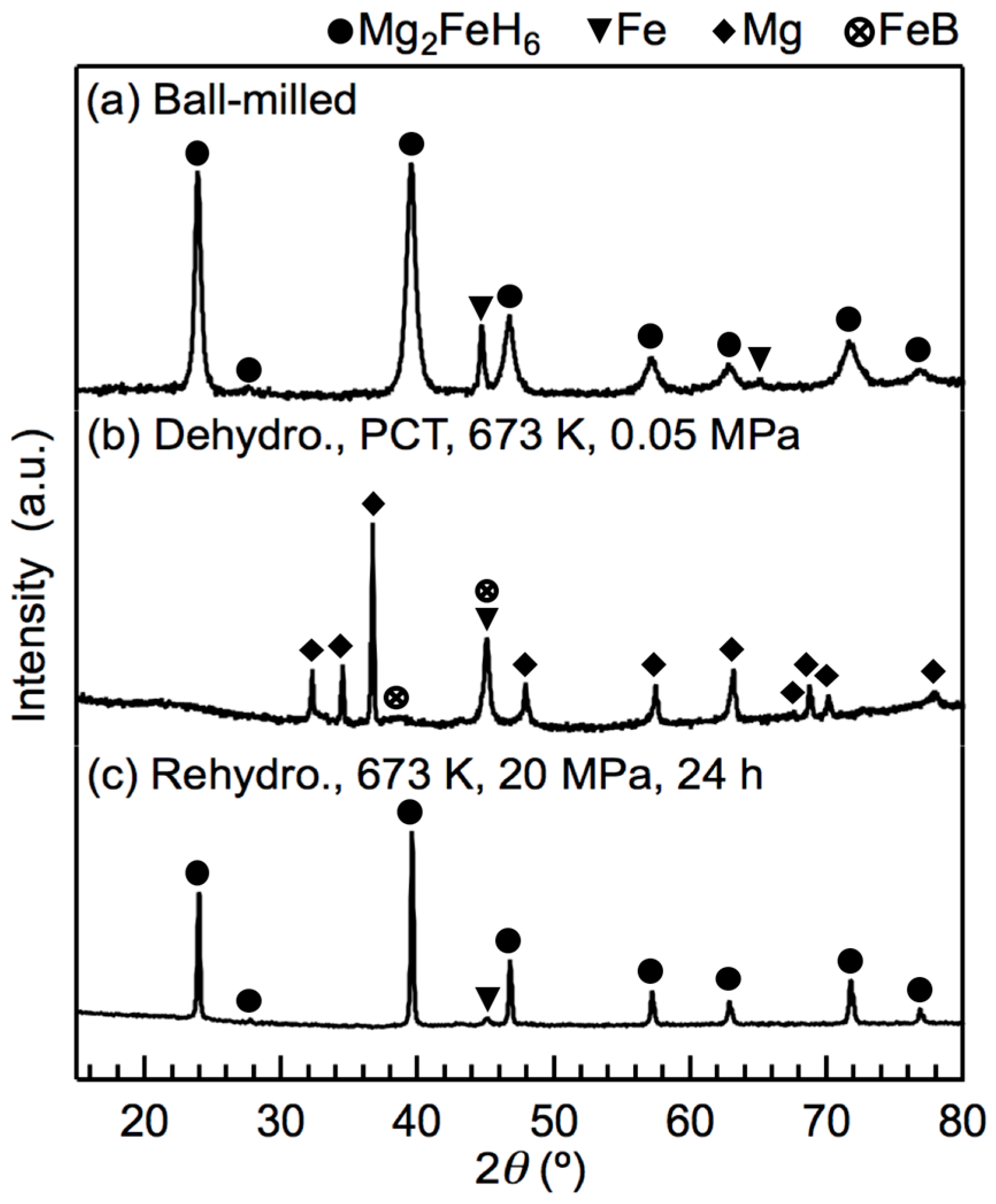
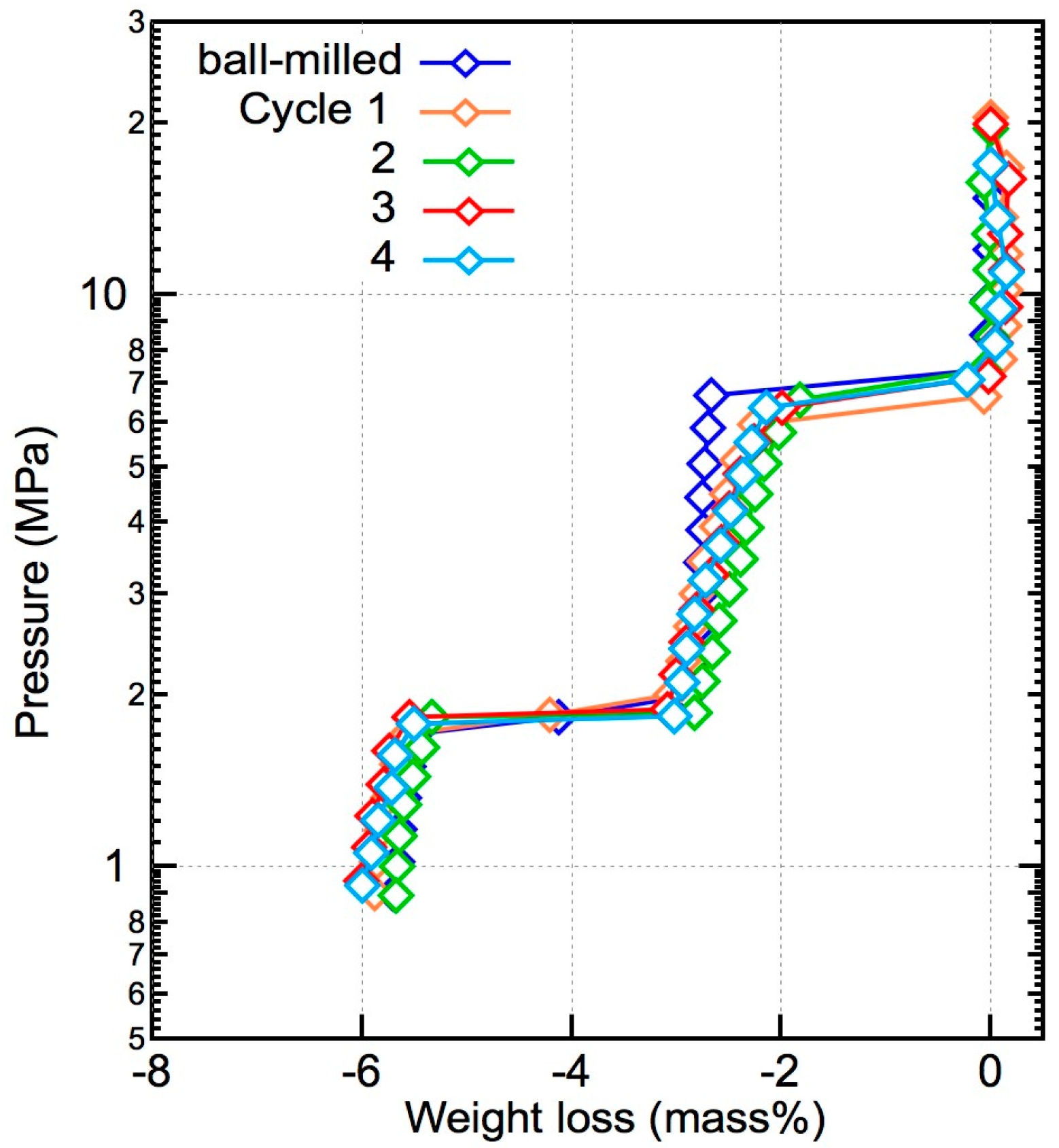
2.2. Reversible Hydrogenation Due to the Optimal Composition Ratio
3. Discussion
4. Materials and Methods
4.1. Synthesis of (1 − x)LiBH4 + xMg2FeH6
4.2. Pressure–Composition–Isothermal (PCT) Measurements
4.3. Phase Identification
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Cerny, R.; Ravnsbaek, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal Borohydrides and Derivatives—Synthesis, Structure and Properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Callini, E.; Atakli, Z.O.K.; Hauback, B.C.; Orimo, S.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjorvarsson, B.; et al. Complex and Liquid Hydrides for Energy Storage. Appl. Phys. A 2016, 122, 353. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Yan, Y.G.; Orimo, S.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; et al. Hydrogen Sorption Properties of MgH2–LiBH4 Composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Vajo, J.J.; Li, W.; Liu, P. Thermodynamic and Kinetic Destabilization in LiBH4–Mg2NiH4: Promise for Borohydride-Based Hydrogen Storage. Chem. Commun. 2010, 46, 6687–6689. [Google Scholar] [CrossRef] [PubMed]
- Javadian, P.; Zlotea, C.; Ghimbeu, C.M.; Latroche, M.; Jensen, T.R. Hydrogen Storage Properties of Nanoconfined LiBH4–Mg2NiH4 Reactive Hydride Composites. J. Phys. Chem. C 2015, 119, 5819–5826. [Google Scholar] [CrossRef]
- Yan, Y.G.; Li, H.W.; Maekawa, H.; Miwa, K.; Towata, S.; Orimo, S. Formation of Intermediate Compound Li2B12H12 during The Dehydrogenation Process of The LiBH4–MgH2 System. J. Phys. Chem. C 2011, 115, 19419–19423. [Google Scholar] [CrossRef]
- Bergemann, N.; Pistidda, C.; Milanese, C.; Emmler, T.; Karimi, F.; Chaudhary, A.L.; Chierotti, M.R.; Klassen, T.; Dornheim, M. Ca(BH4)2–Mg2NiH4: On The Pathway to A Ca(BH4)2 System with A Reversible Hydrogen Cycle. Chem. Commun. 2016, 52, 4836–4839. [Google Scholar] [CrossRef] [PubMed]
- Bosenberg, U.; Kim, J.W.; Gosslar, D.; Eigen, N.; Jensen, T.R.; von Colbe, J.M.B.; Zhou, Y.; Dahms, M.; Kim, D.H.; Gunther, R.; et al. Role of Additives in LiBH4–MgH2 Reactive Hydride Composites for Sorption Kinetics. Acta Mater. 2010, 58, 3381–3389. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with A Metal (M = Mg, Al, Ti, V, Cr, or Sc) or Metal Hydride (MH2, MgH2, TiH2, or CaH2). J. Phys. Chem. C 2007, 111, 19134–19140. [Google Scholar] [CrossRef]
- Li, G.; Matsuo, M.; Deledda, S.; Sato, R.; Hauback, B.C.; Orimo, S. Dehydriding Property of LiBH4 Combined with Mg2FeH6. Mater. Trans. 2013, 54, 1532–1534. [Google Scholar] [CrossRef]
- Li, G.; Matsuo, M.; Aoki, K.; Ikeshoji, T.; Orimo, S. Dehydriding Process and Hydrogen-Deuterium Exchange of LiBH4–Mg2FeD6 Composites. Energies 2015, 8, 5459–5466. [Google Scholar] [CrossRef]
- Chaudhary, A.-L.; Li, G.; Matsuo, M.; Orimo, S.; Deledda, S.; Sørby, M.H.; Hauback, B.C.; Pistidda, C.; Klassen, T.; Dornheim, M. Simultaneous Desorption Behavior of M Borohydrides and Mg2FeH6 Reactive Hydride Composites (M = Mg, then Li, Na, K, Ca). Appl. Phys. Lett. 2015, 107, 073905. [Google Scholar] [CrossRef]
- Li, G.; Matsuo, M.; Deledda, S.; Hauback, B.C.; Orimo, S. Dehydriding Property of NaBH4 Combined with Mg2FeH6. Mater. Trans. 2014, 55, 1141–1143. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P.; Vajo, J.J. Phase Boundaries and Reversibility of LiBH4–MgH2 Hydrogen Storage Material. J. Phys. Chem. C 2007, 111, 12881–12885. [Google Scholar] [CrossRef]
- Deng, S.S.; Xiao, X.Z.; Han, L.Y.; Li, Y.; Li, S.Q.; Ge, H.W.; Wang, Q.D.; Chen, L.X. Hydrogen Storage Performance of 5LiBH4 + Mg2FeH6 Composite System. Int. J. Hydrog. Energy 2012, 37, 6733–6740. [Google Scholar] [CrossRef]
- Langmi, H.W.; McGrady, G.S.; Newhouse, R.; Rönnebro, E. Mg2FeH6–LiBH4 and Mg2FeH6–LiNH2 Composite Materials for Hydrogen Storage. Int. J. Hydrog. Energy 2012, 37, 6694–6699. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Catti, M.; Nale, A. Thermodynamics of Dehydrogenation of the 2LiBH4–Mg2FeH6 Composite. J. Phys. Chem. C 2012, 116, 26694–26699. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Bohmhammel, K.; Christ, B.; Reiser, A.; Schlichte, K.; Vehlen, R.; Wolf, U. Thermodynamic Investigation of the Magnesium–Hydrogen System. J. Alloys Compd. 1999, 282, 84–92. [Google Scholar] [CrossRef]
- Bohmhammel, K.; Wolf, U.; Wolf, G.; Konigsberger, E. Thermodynamic Optimization of the System Magnesium–Hydrogen. Thermochim. Acta 1999, 337, 195–199. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Qu, J.; Zhao, W.; Xie, L.; Tian, W.; Li, X. The Synthesis and Hydrogen Storage Properties of Pure Nanostructured Mg2FeH6. Nanotechnology 2010, 21, 095706. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, J.A.; Larochette, P.A.; Gennari, F.C. Thermodynamic and Kinetic Studies of Mg–Fe–H after Mechanical Milling Followed by Sintering. J. Alloys Compd. 2008, 463, 134–142. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, F.Y.; Li, C.S.; Tao, Z.L.; Chen, J. Preparation and Characterization of Nanocrystalline Mg2FeH6. J. Alloys Compd. 2010, 508, 554–558. [Google Scholar] [CrossRef]
- Siegel, D.J.; Wolverton, C.; Ozoliņš, V. Thermodynamic Guidelines for the Prediction of Hydrogen Storage Reactions and Their Application to Destabilized Hydride Mixtures. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Price, T.E.C.; Grant, D.M.; Telepeni, I.; Yu, X.B.; Walker, G.S. The Decomposition Pathways for LiBD4–MgD2 Multicomponent Systems Investigated by In Situ Neutron Diffraction. J. Alloys Compd. 2009, 472, 559–564. [Google Scholar] [CrossRef]
- Miwa, K.; Takagi, S.; Matsuo, M.; Orimo, S. Thermodynamical Stability of Complex Transition Metal Hydrides M2FeH6. J. Phys. Chem. C 2013, 117, 8014–8019. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Tesche, B. Thermodynamics and Dynamics of the Mg−Fe−H System and Its Potential for Thermochemical Thermal Energy Storage. J. Alloys Compd. 2002, 345, 77–89. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Corno, M.; Damin, A.; Spoto, G.; Ugliengo, P.; Baricco, M. Vibrational Properties of MBH4 and MBF4 Crystals (M = Li, Na, K): A Combined DFT, Infrared, and Raman Study. J. Phys. Chem. C 2011, 115, 18890–18900. [Google Scholar] [CrossRef]
- Parker, S.F.; Williams, K.P.J.; Bortz, M.; Yvon, K. Inelastic Neutron Scattering, Infrared, and Raman Spectroscopic Studies of Mg2FeH6 and Mg2FeD6. Inorg. Chem. 1997, 36, 5218–5221. [Google Scholar] [CrossRef]
- Kim, K.B.; Shim, J.H.; Park, S.H.; Choi, I.S.; Oh, K.H.; Cho, Y.W. Dehydrogenation Reaction Pathway of the LiBH4−MgH2 Composite under Various Pressure Conditions. J. Phys. Chem. C 2015, 119, 9714–9720. [Google Scholar] [CrossRef]
- Bosenberg, U.; Ravnsbaek, D.B.; Hagemann, H.; D’Anna, V.; Minella, C.B.; Pistidda, C.; van Beek, W.; Jensen, T.R.; Bormann, R.; Dornheim, M. Pressure and Temperature Influence on the Desorption Pathway of the LiBH4−MgH2 Composite System. J. Phys. Chem. C 2010, 114, 15212–15217. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Zuttel, A. Dehydriding and Rehydriding Reactions of LiBH4. J. Alloys Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Gosselin, C.; Deledda, S.; Hauback, B.C.; Huot, J. Effect of Synthesis Route on the Hydrogen Storage Properties of 2MgH2–Fe Compound Doped with LiBH4. J. Alloys Compd. 2015, 645, S304–S307. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Matsuo, M.; Takagi, S.; Chaudhary, A.-L.; Sato, T.; Dornheim, M.; Orimo, S.-i. Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials. Inorganics 2017, 5, 81. https://doi.org/10.3390/inorganics5040081
Li G, Matsuo M, Takagi S, Chaudhary A-L, Sato T, Dornheim M, Orimo S-i. Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials. Inorganics. 2017; 5(4):81. https://doi.org/10.3390/inorganics5040081
Chicago/Turabian StyleLi, Guanqiao, Motoaki Matsuo, Shigeyuki Takagi, Anna-Lisa Chaudhary, Toyoto Sato, Martin Dornheim, and Shin-ichi Orimo. 2017. "Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials" Inorganics 5, no. 4: 81. https://doi.org/10.3390/inorganics5040081
APA StyleLi, G., Matsuo, M., Takagi, S., Chaudhary, A.-L., Sato, T., Dornheim, M., & Orimo, S.-i. (2017). Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials. Inorganics, 5(4), 81. https://doi.org/10.3390/inorganics5040081




