Metal–Organic Frameworks and Their Derivatives for Photocatalytic Water Splitting
Abstract
:1. Introduction
2. Photocatalytic H2 Evolution Utilizing MOF-based Photocatalysts
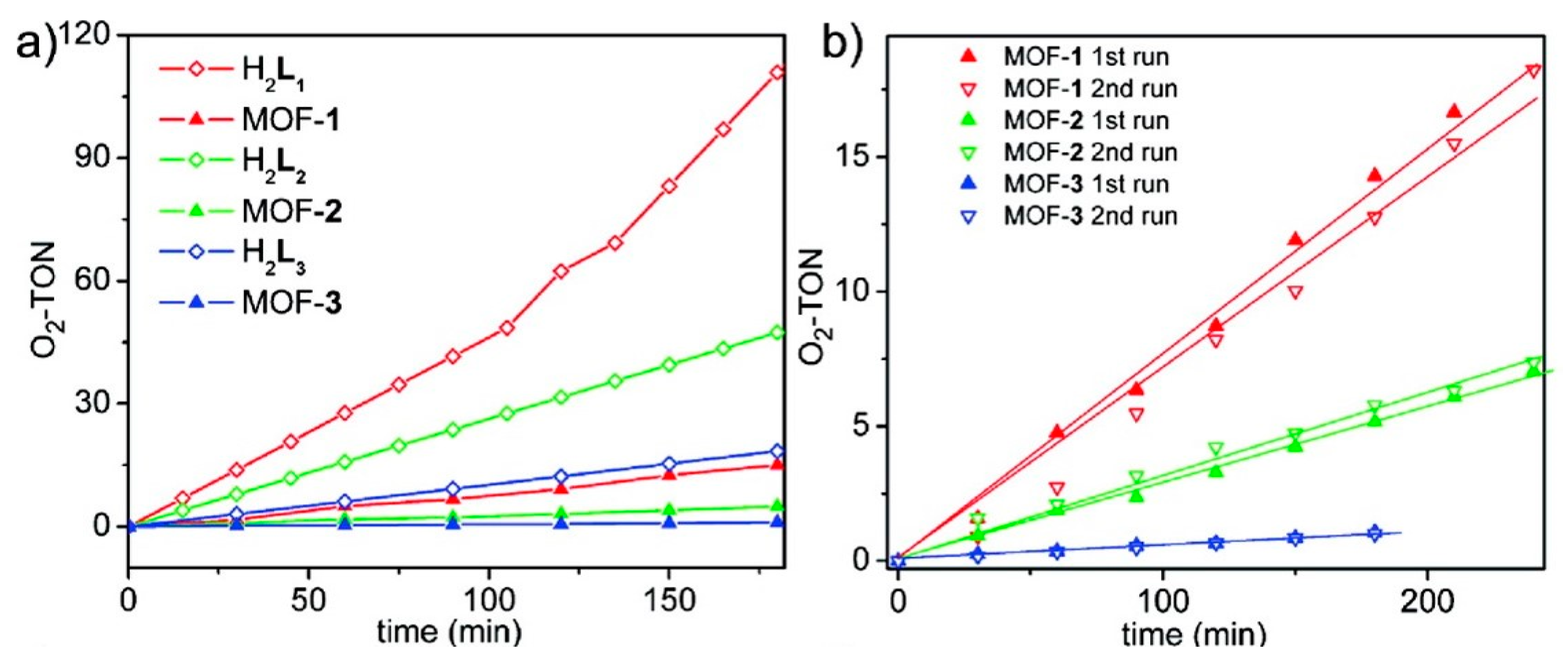
3. O2 Evolution Catalyzed by MOF-based Hybrid Systems
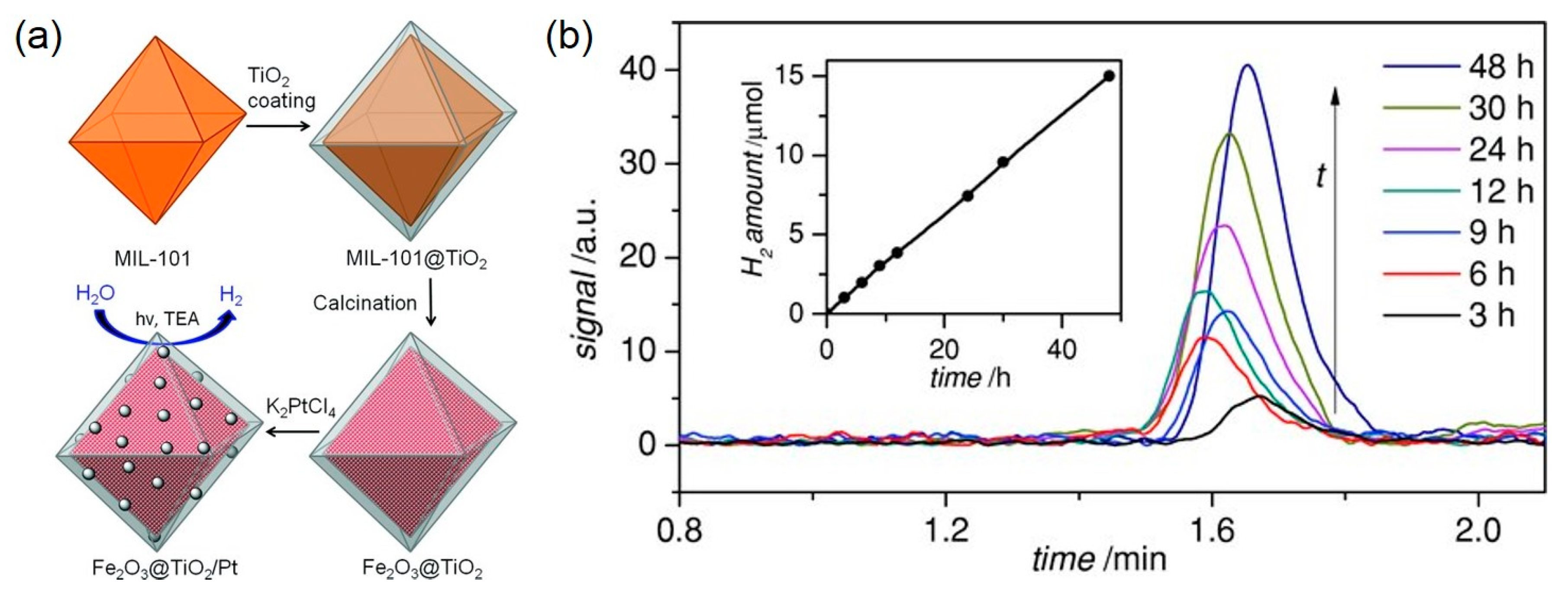
4. Photocatalytic Water Splitting Utilizing MOF-derived Photocatalysts
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Bigi, J.P.; Piro, N.A.; Tang, M.L.; Long, J.R.; Chang, C.J. Molecular cobalt pentapyridine catalysts for generating hydrogen from water. J. Am. Chem. Soc. 2011, 133, 9212–9215. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source-recent developments and future trends. Energy Environ. Sci. 2010, 5, 8171–8181. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Xiong, D.; Liu, L. Efficient and durable electrochemical hydrogen evolution using cocoon-like MoS2 with preferentially exposed edges. Int. J. Hydrogen Energy 2016, 41, 9344–9354. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sun, Y.J. Morphology–activity correlation in hydrogen evolution catalyzed by cobalt sulfides. Inorg. Chem. Front. 2016, 3, 279–285. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. New supported Pd and Pt alloy catalysts for steam reforming and dehydrogenation of methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Kuo, C.H.; Tang, Y.; Chou, L.Y.; Sneed, B.T.; Brodsky, C.N.; Zhao, Z.P.; Tsung, C.K. Yolk-shell nanocrystal@ZIF-8 nanostructures for gas-phase heterogeneous catalysis with selectivity control. J. Am. Chem. Soc. 2012, 134, 14345–14348. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; You, B.; Sheng, M.L.; Sun, Y.J. Electrodeposited cobalt-phosphorous-derived films as competent bifunctional catalysts for overall water splitting. Angew. Chem. Int. Ed. 2015, 54, 6251–6254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; You, B.; Jiang, N.; Sun, Y.J. Facile surface modification of ubiquitous stainless steel led to competent electrocatalysts for overall water splitting. ACS Sustain. Chem. Eng. 2017, 5, 4778–4784. [Google Scholar] [CrossRef]
- Song, F.Z.; Zhu, Q.L.; Tsumori, N.; Xu, Q. Diamine-alkalized reduced graphene oxide: Immobilization of sub-2 nm palladium nanoparticles and optimization of catalytic activity for dehydrogenation of formic acid. ACS Catal. 2015, 5, 5141–5144. [Google Scholar] [CrossRef]
- Song, F.Z.; Zhu, Q.L.; Xu, Q. Monodispersed PtNi nanoparticles deposited on diamine-alkalized graphene for highly efficient dehydrogenation of hydrous hydrazine at room temperature. J. Mater. Chem. A 2015, 3, 23090–23094. [Google Scholar] [CrossRef]
- Song, F.Z.; Zhu, Q.L.; Xu, Q. Monodispersed CuCo nanoparticles supported on diamine-functionalized graphene as a non-noble metal catalyst for hydrolytic dehydrogenation of ammonia borane. ChemNanoMat 2016, 2, 942–945. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Jiang, N.; Sun, Y.J. A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization. J. Am. Chem. Soc. 2016, 138, 13639–13646. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Zou, Z.G.; Matsushita, A. A novel series of water splitting photocatalysts NiM2O6 (M = Nb, Ta) active under visible light. Int. J. Hydrogen Energy 2003, 28, 651–655. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Liu, X.; Dong, Z.; Hng, H.H.; Chen, Z.; Sum, T.C.; Chen, X. Three-dimensional CdS-titanate composite nanomaterials for enhanced visible-light-driven hydrogen evolution. Small 2013, 9, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Keeffe, M.O.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Yaghi, O.M.; Keeffe, M.O.; Ockwing, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zhao, Y.G.; Gong, Q.H.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: the effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as adsorbents for low temperature heating and cooling applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, G.K.H.; Taylor, J.M.; Kim, S. Proton conduction with metal–organic frameworks. Science 2013, 341, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cui, Y.J.; Chen, B.L.; Qian, G.D. A luminescent nanoscale metal–organic framework for sensing of nitroaromatic explosives. Chem. Commun. 2011, 47, 3153–3155. [Google Scholar] [CrossRef] [PubMed]
- Xamena, F.X.L.; Corma, A.; Garcia, H. MOFs as catalysts: Activity, reusability and shape-selectivity of a Pd-containing MOF. J. Catal. 2007, 250, 294–298. [Google Scholar]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regi, M.; Sebban, M.; Taulelle, F.; Ferey, G. Flexible porous metal–organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal–organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Kim, T.; Im, J.H.; Kim, Y.S.; Lee, K.; Jung, H.; Park, C.R. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 2012, 24, 464–470. [Google Scholar] [CrossRef]
- Sun, J.K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.Y.; He, C.; Duan, C.Y. Metal–organic frameworks: Versatile materials for heterogeneous photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic hydrogen production from water using porous material [Ru2(p-BDC)2]n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Kataoka, Y.; Miyazaki, Y.; Sato, K.; Saito, T.; Nakanishi, Y.; Kiatagwa, Y.; Kawakami, T.; Okumura, M.; Yamaguchi, K.; Mori, W. Modification of MOF catalysts by manipulation of counter-ions: Experimental and theoretical studies of photochemical hydrogen production from water over microporous diruthenium (II, III) coordination polymers. Supramol. Chem. 2011, 23, 287–299. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Suzuki, Y.; Tanaka, H.; Kitagawa, Y.; Kawakami, T.; Okumura, M.; Mori, W. Photocatalytic hydrogen production from water using heterogeneous two-dimensional rhodium coordination polymer [Rh2( p-BDC)2]n. Chem. Lett. 2010, 39, 358–359. [Google Scholar] [CrossRef]
- Silva, C.G.; Luz, I.; Xamena, F.X.L.; Corma, A.; Garcia, H. Water stable Zr-benzenedicarboxylate metal–organic frameworks as photocatalysts for hydrogen generation. Chem. Eur. J. 2010, 16, 11133–11138. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti(IV) metal–organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kamegawa, T.; Yamashita, H. Amine-functionalized MIL-101(Cr) with imbedded platinum nanoparticles as a durable photocatalyst for hydrogen production from water. Chem. Commun. 2014, 50, 11645–11648. [Google Scholar] [CrossRef] [PubMed]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A water-stable porphyrin-based metal–organic framework active for visible-light photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Krafft, K.E.; Lin, W. Pt nanoparticles@photoactive metal–organic frameworks: Efficient hydrogen evolution via synergistic photoexcitation and electron injection. J. Am. Chem. Soc. 2012, 134, 7211–7214. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Shen, L.; Ren, Z.; Wu, W.; Tan, Y.; Fu, H.; Zhang, J.; Wu, L. Enhanced photocatalytic hydrogen production activity via dual modification of MOF and reduced graphene oxide on CdS. Chem. Commun. 2014, 50, 8533–8535. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized Pt@UiO-66(Zr) metal–organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. 2014, 50, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Du, Y.; Borgna, A.; Hong, J.; Wang, Y.; Han, J.; Zhang, W.; Xu, R. Post-synthesis modification of a metal–organic framework to construct a bifunctional photocatalyst for hydrogen production. Energy Environ. Sci. 2013, 6, 3229–3234. [Google Scholar] [CrossRef]
- Thoi, V.S.; Sun, Y.; Long, J.R.; Chang, C.J. Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem. Soc. Rev. 2013, 42, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.M.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kraft, M.; Xu, R. Metal-free carbonaceous electrocatalysts and photocatalysts for water splitting. Chem. Soc. Rev. 2016, 45, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Nasalevich, M.A.; Becker, R.; Ramos-Fernandez, E.V.; Castellanos, S.; Veber, S.L.; Fedin, M.V.; Kapteijn, F.; Reek, J.N.H.; Vlugt, J.I.; Gascon, J. Co@NH2-MIL-125(Ti): Cobaloxime-derived metal–organic framework-based composite for lightdriven H2 production. Energy Environ. Sci. 2015, 8, 364–375. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W.B. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ai, L.; Jiang, J. Solvothermal synthesis of MIL-53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J. Mater. Chem. A 2015, 3, 3074–3081. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, X.C. Multifunctional metal–organic frameworks for photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Z.G.; Krafft, K.E.; Lin, W.B. Doping metal organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.L.; Lin, W.B. Elucidating molecular iridium water oxidation catalysts using metal–organic frameworks: A comprehensive structural, catalytic, spectroscopic, and kinetic study. J. Am. Chem. Soc. 2012, 134, 19895–19908. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Jiang, N.; Sheng, M.; Gul, S.; Yano, J.; Sun, Y. High-performance overall water splitting electrocatalysts derived from cobalt-based metal–organic frameworks. Chem. Mater. 2015, 27, 7636–7642. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Sheng, M.; Drisdell, W.S.; Yano, J.; Sun, Y. Bimetal–organic framework self-adjusted synthesis of support-free nonprecious electrocatalysts for efficient oxygen reduction. ACS Catal. 2015, 5, 7068–7076. [Google Scholar] [CrossRef]
- Liu, X.; Dong, J.; You, B.; Sun, Y. Competent overall water-splitting electrocatalysts derived from ZIF-67 grown on carbon cloth. RSC Adv. 2016, 6, 73336–73342. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent progress in metal–organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wang, C.; Lin, W.B. Metal–organic frameworks for light harvesting and photocatalysis. ACS Catal. 2012, 2, 2630–2640. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Pougin, A.; Medishetty, R.; Rosler, C.; Wiktor, C.; Strunk, J.; Fischer, R.A. Fabrication of Gold/Titania photocatalyst for CO2 reduction based on pyrolytic conversion of the metal–organic rramework NH2-MIL-125(Ti) loaded with Gold nanoparticles. Chem. Mater. 2015, 27, 7248–7257. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhai, X.P.; Gao, L.F.; Chen, P.; Zhao, M.; Yang, H.B.; Cao, D.F.; Wang, Q.; Zhang, H.L. In situ preparation of a MOF-derived magnetic carbonaceous catalyst for visible-light-driven hydrogen evolution. RSC Adv. 2016, 6, 2011–2018. [Google Scholar] [CrossRef]
- Krafft, K.E.; Wang, C.; Lin, W.B. Metal–organic framework templated synthesis of Fe2O3/TiO2 nanocomposite for hydrogen production. Adv. Mater. 2012, 24, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Dinh, C.T.; Vuong, G.T.; Ta, N.D.; Do, T.O. Visible light induced hydrogen generation using a hollow photocatalyst with two cocatalysts separated on two surface sides. Phys. Chem. Chem. Phys. 2014, 16, 5937–5941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Giu, L.G.; Yuan, Y.P.; Zhu, Y.J.; Jiang, X.; Xiao, J.D. Magnetic Fe3O4@C/Cu and Fe3O4@CuO core–shell composites constructed from MOF-based materials and their photocatalytic properties under visible light. Appl. Catal. B Environ. 2014, 144, 863–869. [Google Scholar] [CrossRef]
- Bala, S.; Mondal, I.; Goswami, A.; Pal, U.; Mondal, R. Co-MOF as a sacrificial template: manifesting a new Co3O4/TiO2 system with a p–n heterojunction for photocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 20288–20296. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Toyao, T.; Ranc, V.; Rosler, C.; Petr, M.; Zboril, R.; Horiuchi, Y.; Matsuoka, M.; Fischer, R.A. An in situ porous cuprous oxide/nitrogen-rich graphitic carbon nanocomposite derived from a metal–organic framework for visible light driven hydrogen evolution. J. Mater. Chem. A 2016, 4, 18037–18042. [Google Scholar] [CrossRef]
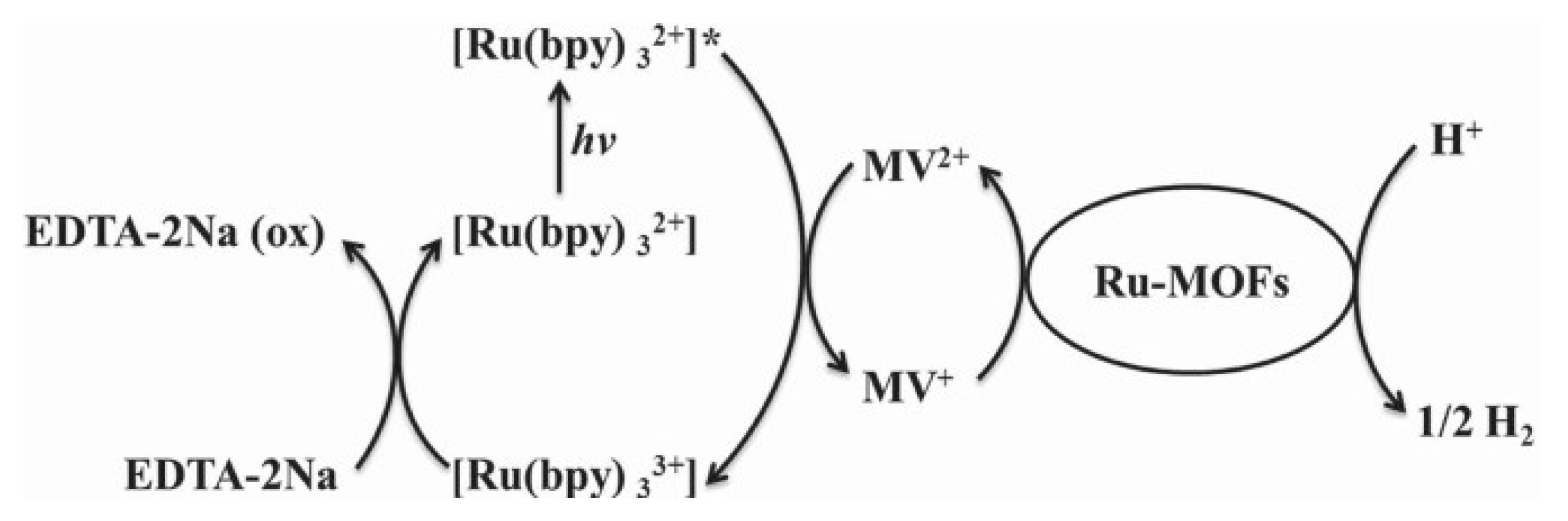
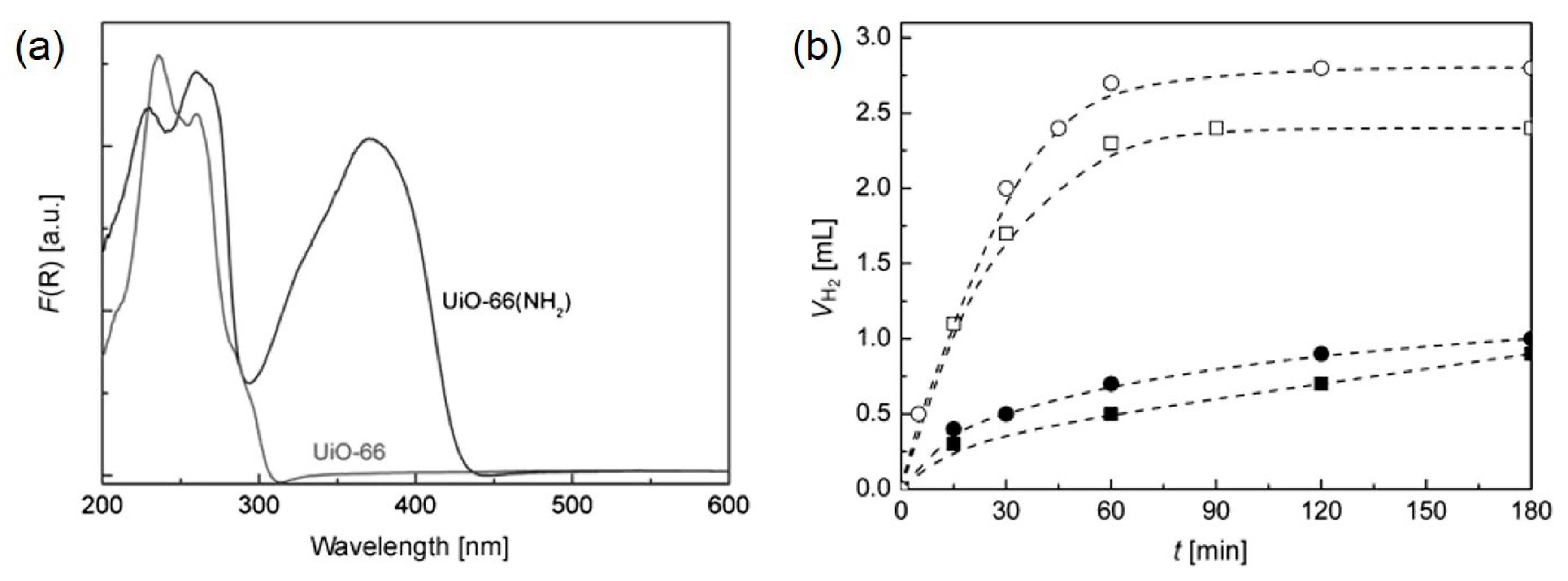
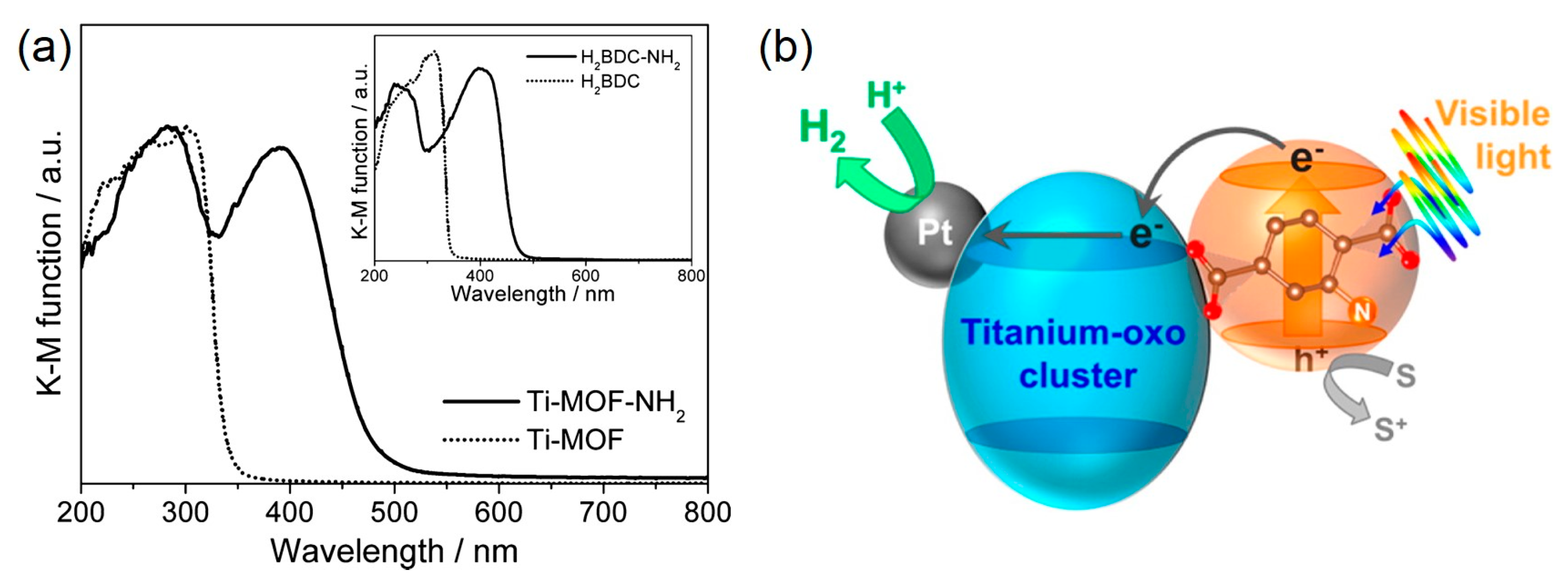
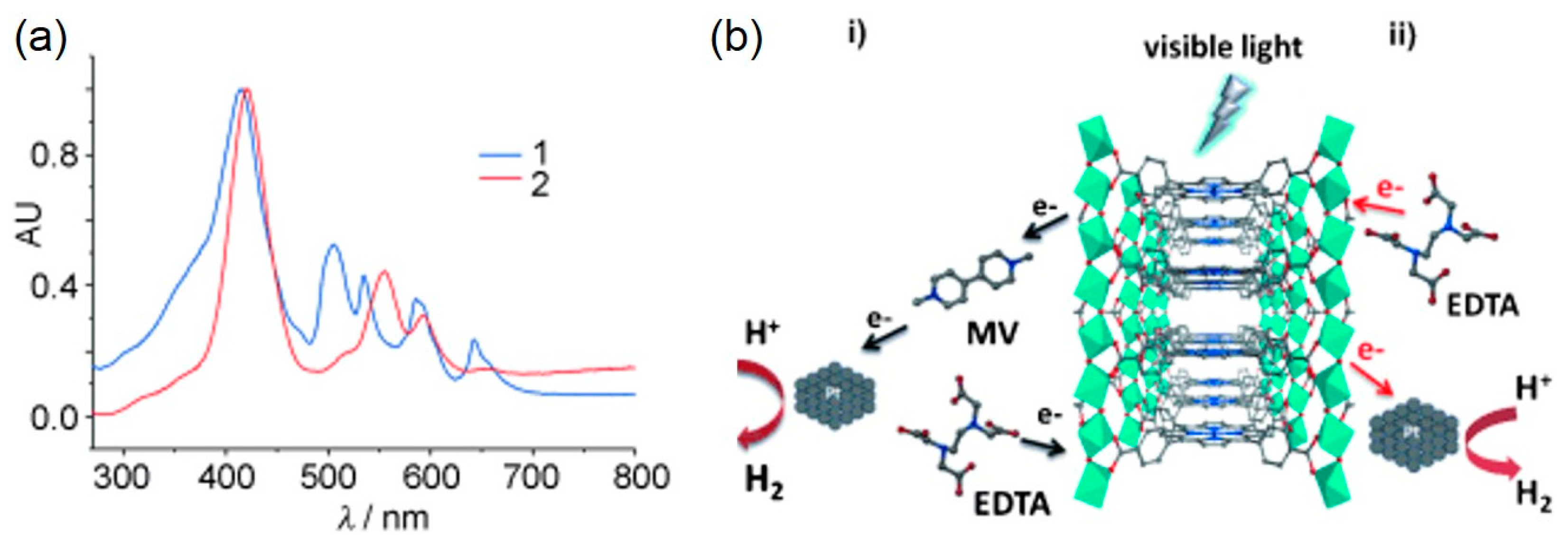
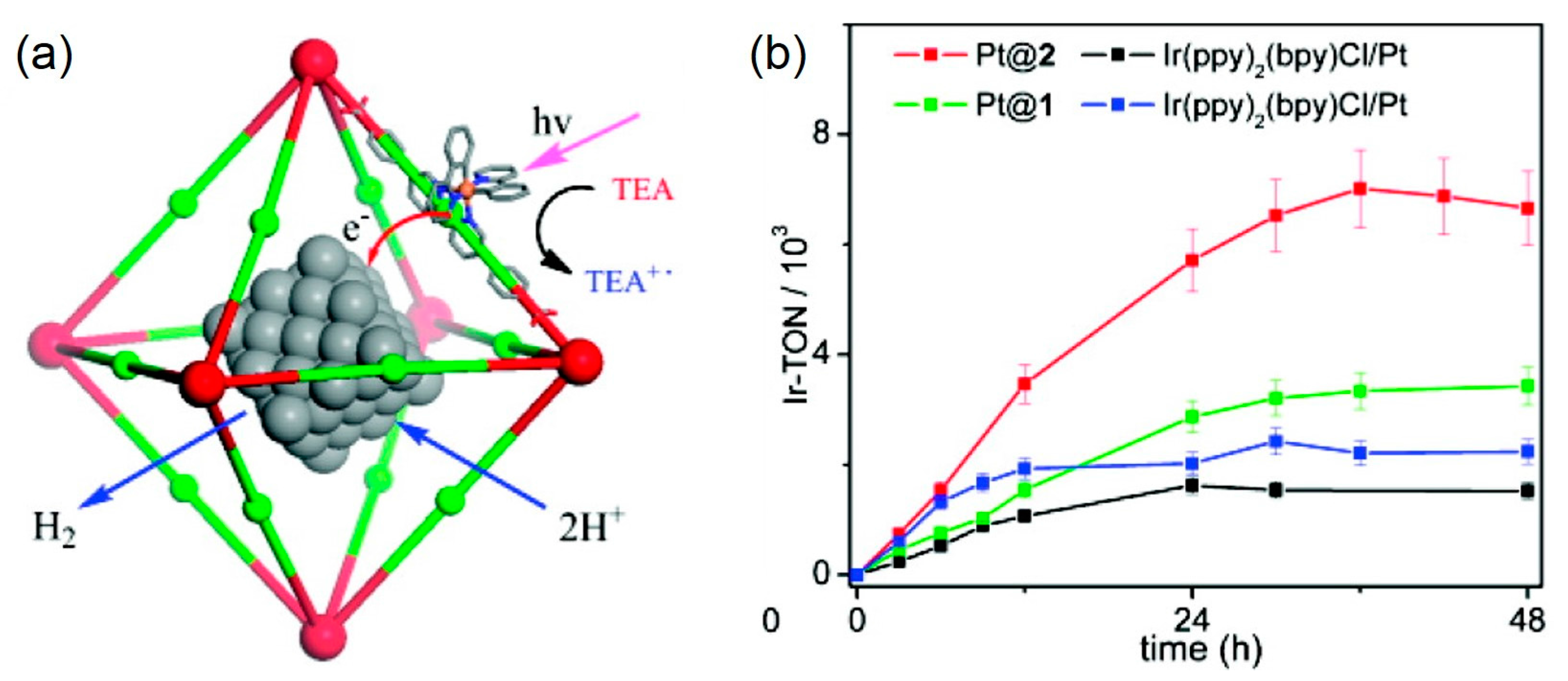
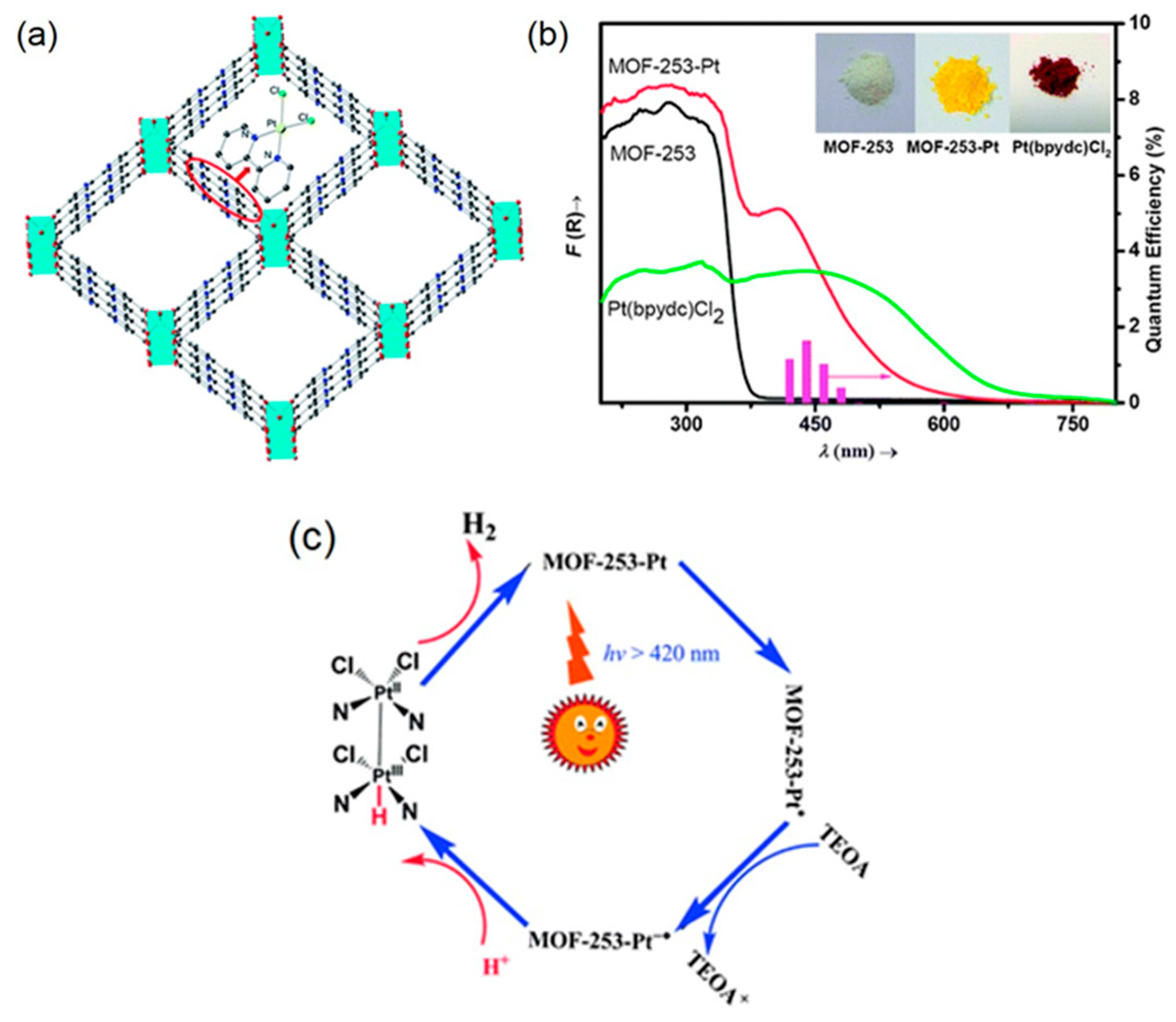
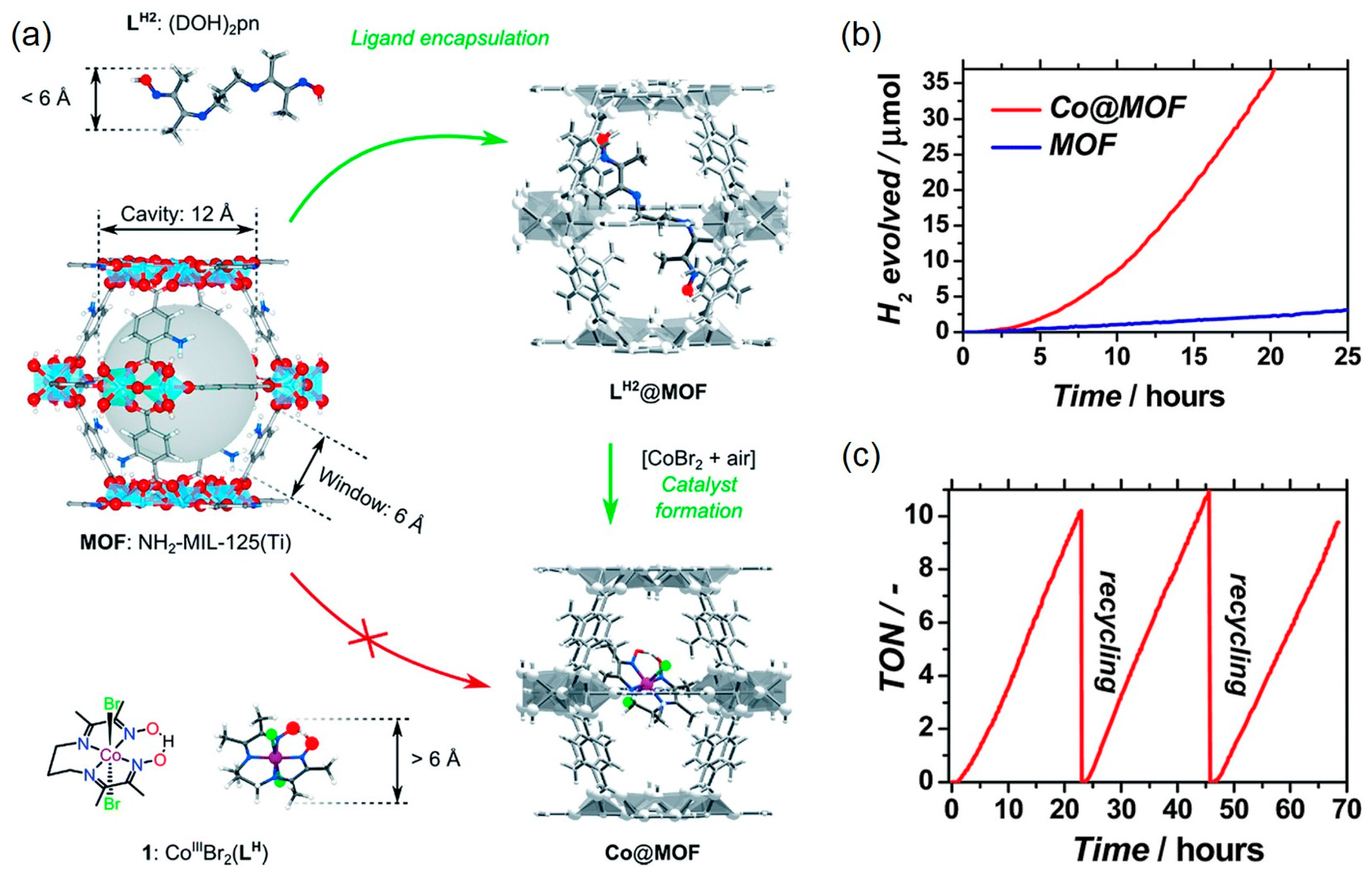
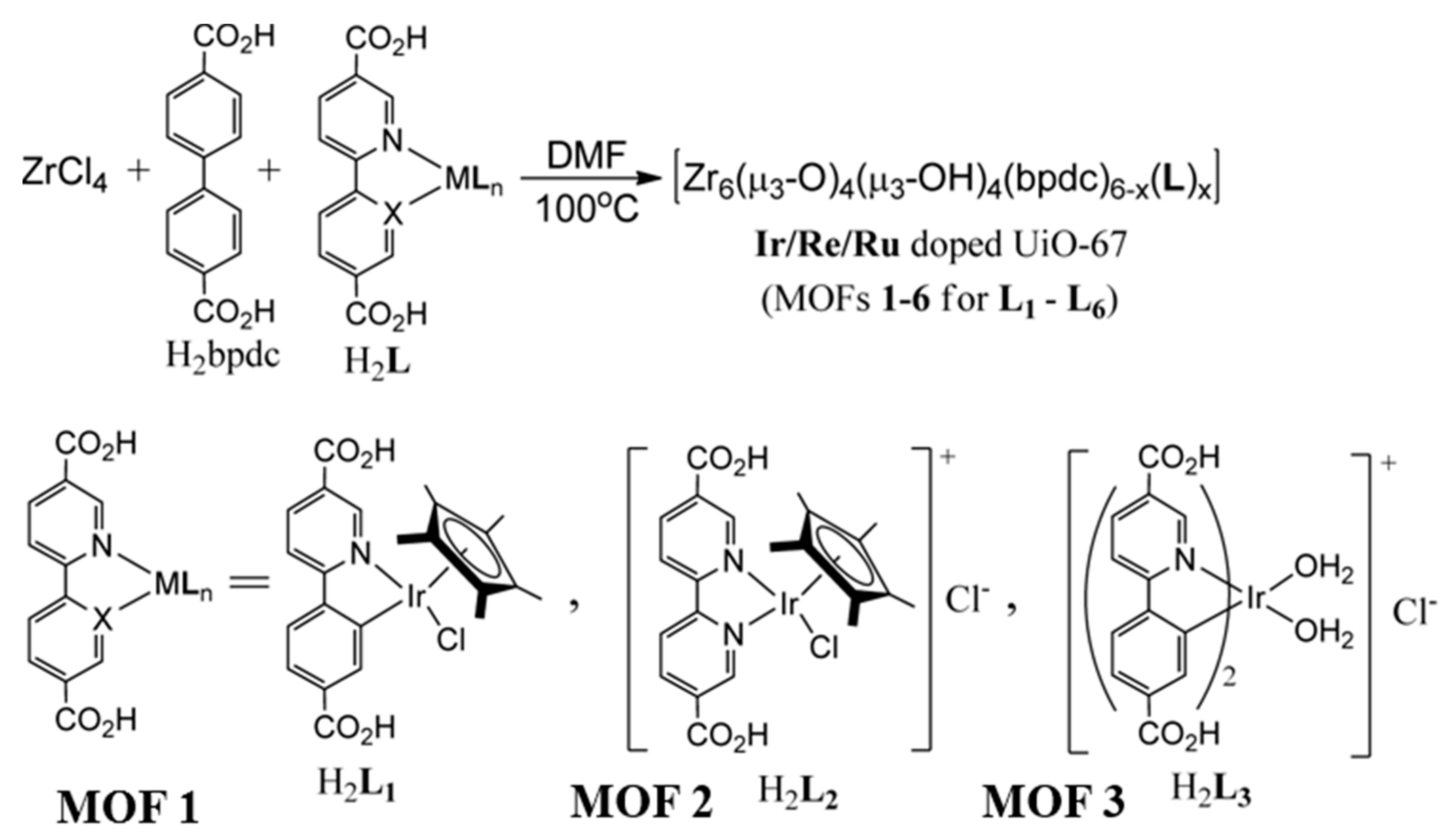
| MOF | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Space Groups | Reference |
|---|---|---|---|---|
| Ru-MOF | 516.5 | 0.26 | P213 | [42] |
| Ti-MOF | 1202 | I4/mmm | [46] | |
| Ti-MOF-NH2 | 1101 | I4/mmm | [46] | |
| MIL-101-NH2 | 1436 | 0.98 | Fd-3m:2 | [47] |
| Al-MOF | 1400 | 0.62 | Cmmm | [48] |
| Al/Zn-MOF | 1200 | Cmm2 | [48] | |
| Zr-MOF | 1194 | F23 | [49] | |
| UiO-66 | 972 | Fm-3m | [50] | |
| Co@NH2MIL-125 | 0.46 | I4/mmm | [60] | |
| Doped-UiO-67 (1) | 1254 | [64] | ||
| Doped-UiO-67 (2) | 1947 | [64] | ||
| Doped-UiO-67 (3) | 1410 | [64] | ||
| Co-MOF | 520 | C2/c | [79] | |
| Cu-MOF | 1182 | Fm3 m | [80] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Li, W.; Sun, Y. Metal–Organic Frameworks and Their Derivatives for Photocatalytic Water Splitting. Inorganics 2017, 5, 40. https://doi.org/10.3390/inorganics5030040
Song F, Li W, Sun Y. Metal–Organic Frameworks and Their Derivatives for Photocatalytic Water Splitting. Inorganics. 2017; 5(3):40. https://doi.org/10.3390/inorganics5030040
Chicago/Turabian StyleSong, Fuzhan, Wei Li, and Yujie Sun. 2017. "Metal–Organic Frameworks and Their Derivatives for Photocatalytic Water Splitting" Inorganics 5, no. 3: 40. https://doi.org/10.3390/inorganics5030040
APA StyleSong, F., Li, W., & Sun, Y. (2017). Metal–Organic Frameworks and Their Derivatives for Photocatalytic Water Splitting. Inorganics, 5(3), 40. https://doi.org/10.3390/inorganics5030040






