1. Introduction
Several biologically important molecules such as amino acids, alkaloids and vitamins are found in nature as a source of amines. Identification of amines in carbonaceous chondritic meteorites (e.g., the Murchison) has been reported by researchers [
1,
2,
3]. The presence of amines on primitive Earth and elsewhere is reported in electric discharge experiments, e.g., as in Parker [
4]. The role of amines in catalyzing the formation of sugars from formaldehyde and glycoaldehyde was investigated by Weber [
5], whilst the importance of aromatic amines in the formation of polymers containing purines, pyrimidines, amino acids, coenzymes, lipid components and even phosphate was proposed by Nelsestuen [
6]. Friedmann and Miller [
7] also reported the presence of aromatic rings bearing amino acids on primitive Earth. In view of this, the existence of amines on early Earth is speculated, and it is further extrapolated that this may have been important in the “kick starting” of the chemical evolution that led to the emergence of RNAs as described by the “RNA world hypothesis”.
It is generally accepted that the transition metal ions present in primordial seas might have complexed with simple molecules available to them. Cyanide has been reported as a product in several experiments carried out under simulated early Earth conditions; this is relevant in that cyanide was readily available on primitive Earth. It is therefore reasonable to assume that cyanide ions might have complexed with different transition metal ions present in primordial seas, forming a number of soluble and insoluble metal cyanide complexes. As most of the double metal cyanide (DMC) complexes are insoluble in water, it is reasonable to assume that they might have locally settled at the bottom of the sea or at its shores. The presence of transition metal ions in primordial oceans was reported by Kobayashi and Ponnamperuma [
8], while the formation of cyanides in several simulated experiments [
9,
10] showed that cyanide ions might have reacted with the available transition metal ions, thus forming a number of double metal cyanide complexes.
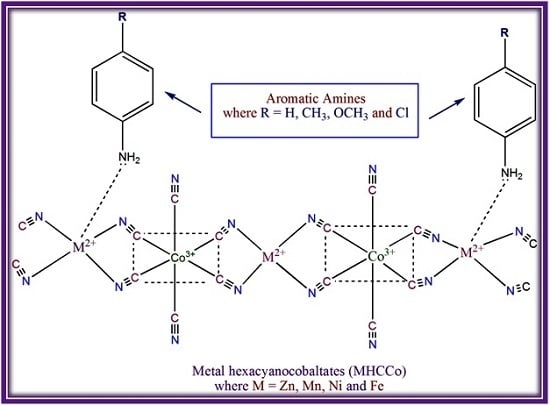
Although several adsorption studies of aromatic amines [
11,
12,
13] and amino pyridines [
14,
15,
16] on metal hexacyanoferrate(II) complexes have been carried out, to the best of our knowledge, no studies have been conducted with respect to metal(II) hexacyanocobaltate(III), that is MHCCo. Therefore, in the present study we have explored the adsorption of aromatic amines, namely aniline, 4-chloroaniline, 4-methylaniline and 4-methoxyaniline with MHCCo. On early Earth there would have been a large inventory of all types of molecules, having been delivered there during the heavy bombardment period. The choice of these four aromatic amines was determined due to the fact that they are markedly different from nitrogenous aromatics (e.g., those found in biological systems) and also to demonstrate that it is possible to oligomerize any exotic aromatic compounds, implying that all manner of reactions are possible on metal surfaces, including those that are necessary for the chemical evolution of life.
2. Results and Discussion
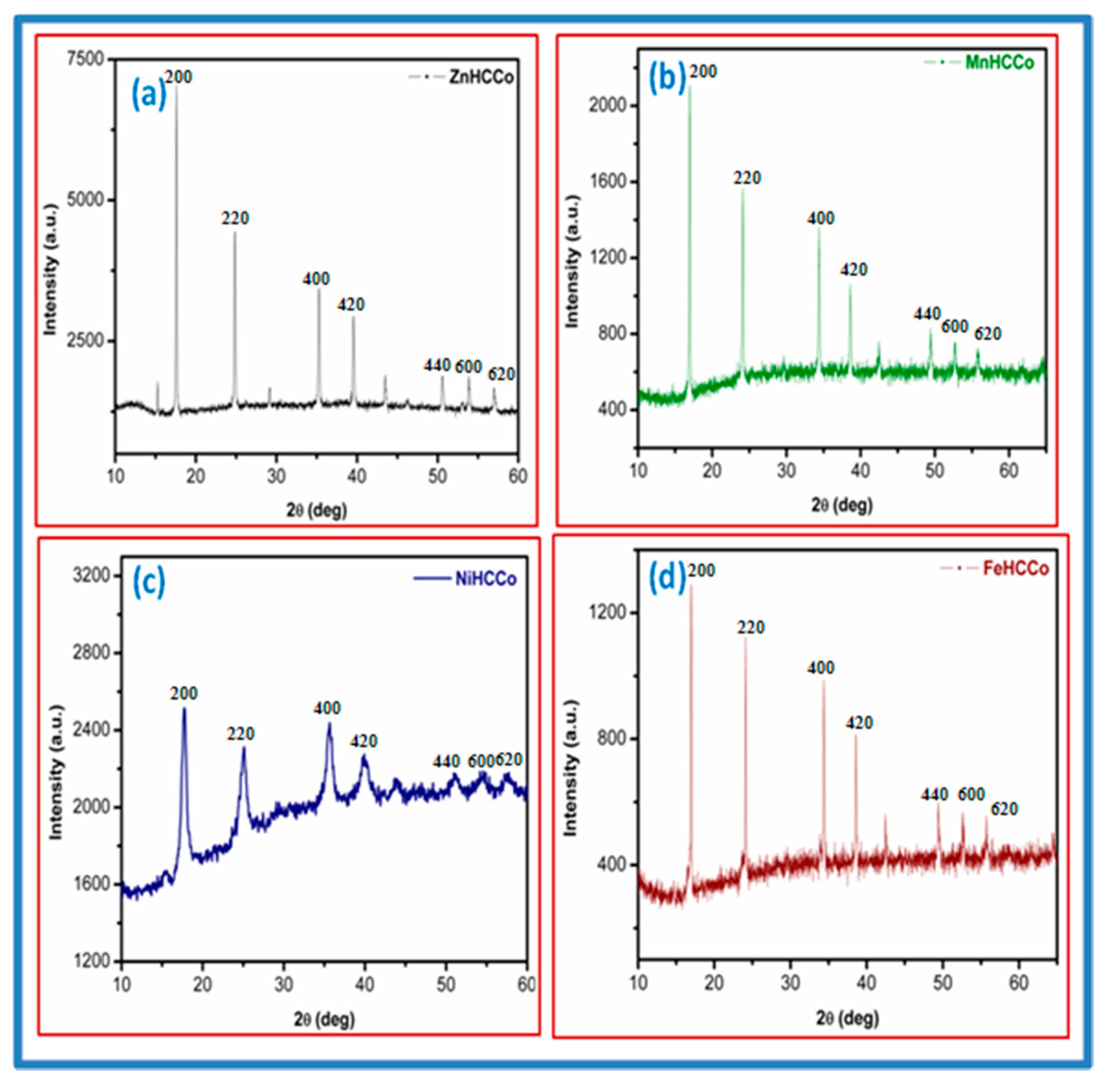
The XRD patterns of ZnHCCo, MnHCCo, NiHCCo and FeHCCo were analyzed using Joint Committee on Powder Diffraction Standards (JCPDS) diffraction files and are shown in
Figure 1.
The JCPDS data of the MHCCos were carefully examined. All the diffraction peaks of the experimental pattern matched with those of the relative intensities of the compounds ZnHCCo (JCPDS file number 32-1468), MnHCCo (JCPDS file number 22-1167), NiHCCo (JCPDS file number 22-1184), and FeHCCo (JCPDS file number 89-3736). Four main peaks are assigned to all the MHCCo complexes. In the case of ZnHCCO, the peaks can be assigned at 2177, 1605, 698 and 450 cm
−1 for strong C≡N
stretching frequency; O–H bending of interstitial water molecules; metal-carbon bending; and metal-cyanide bending, respectively. The peak assignment for the rest of the MHCCo complexes are presented in
Table S1 (
Supplementary Materials).
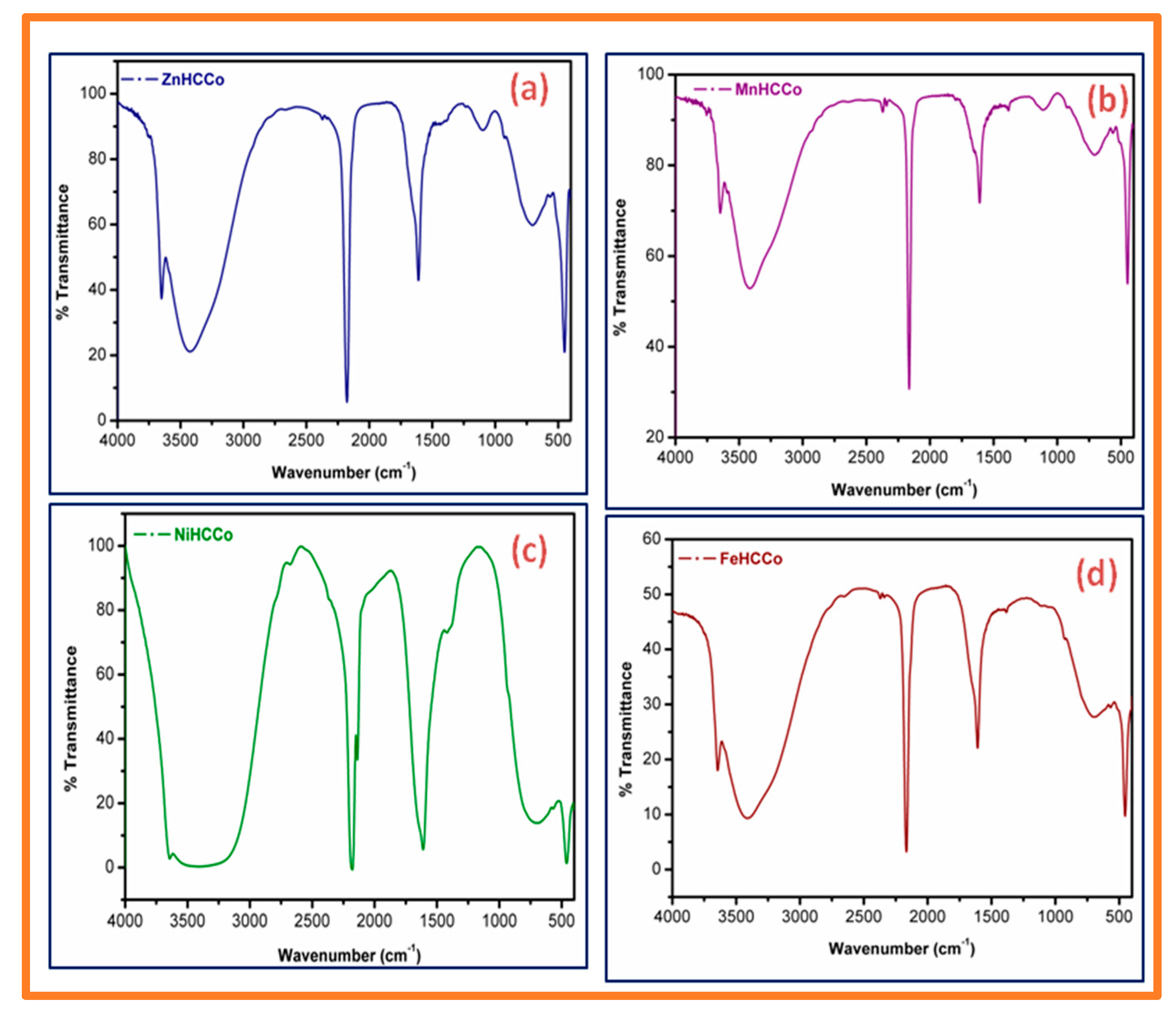
In addition to these peaks, a broad peak at 3600 cm
−1 is also present in all the spectra, attributed to O–H group stretching of water molecules. FT-IR spectra of the respective compounds are presented in
Figure 2.
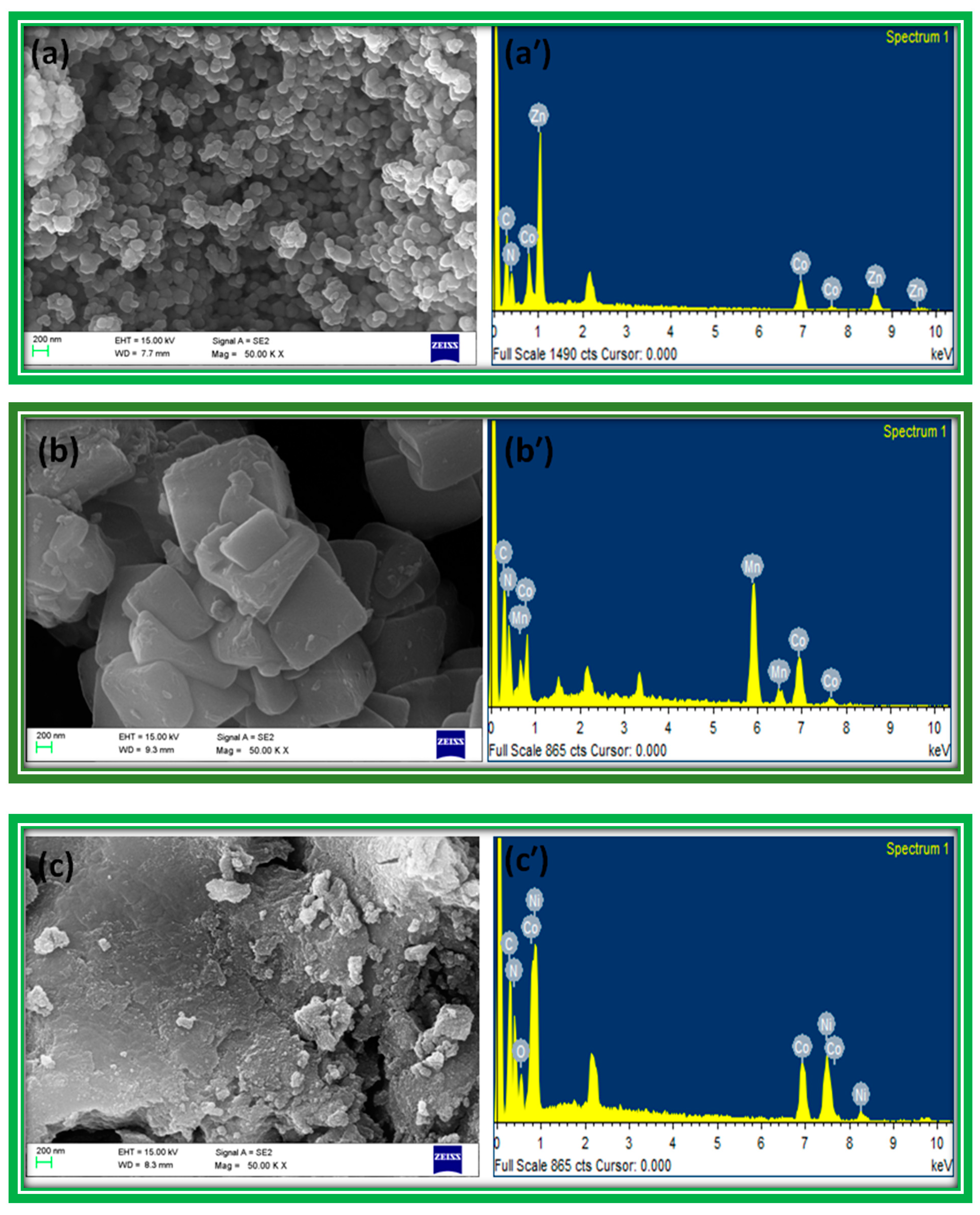
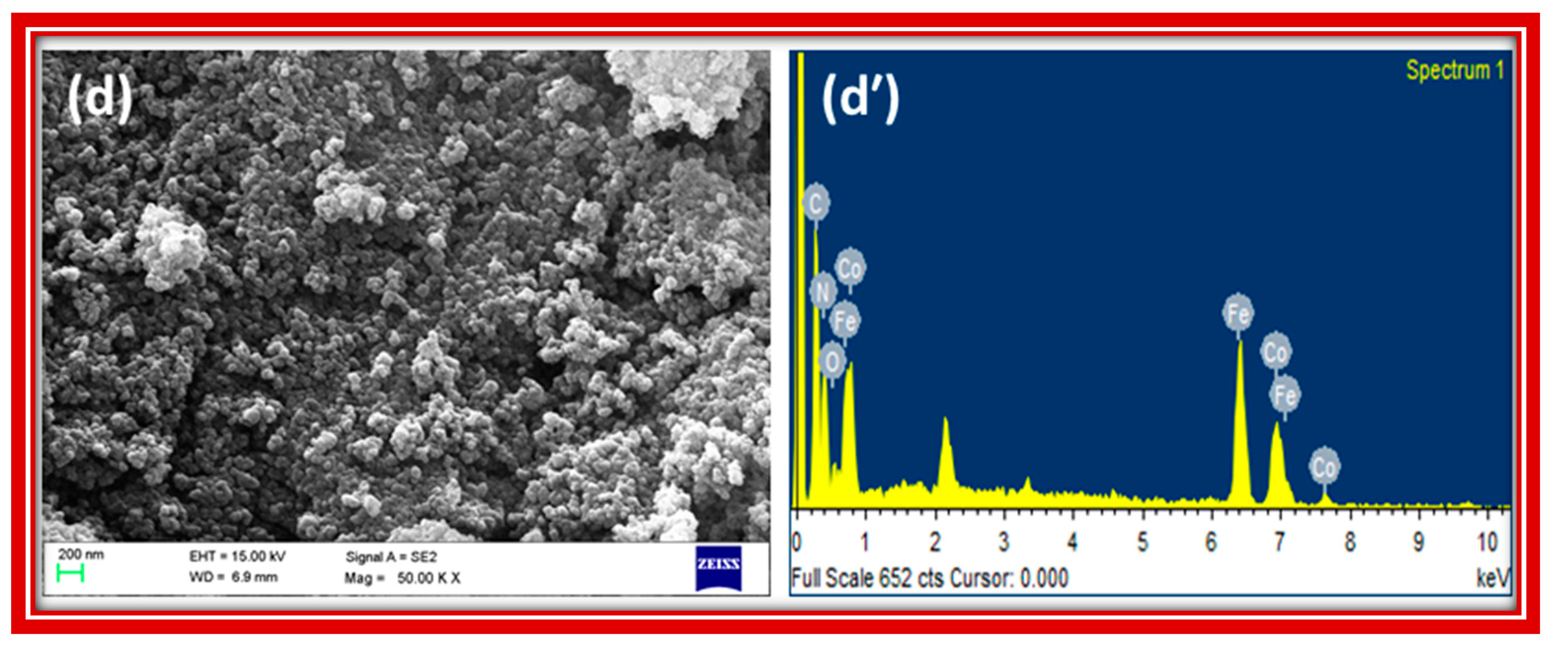
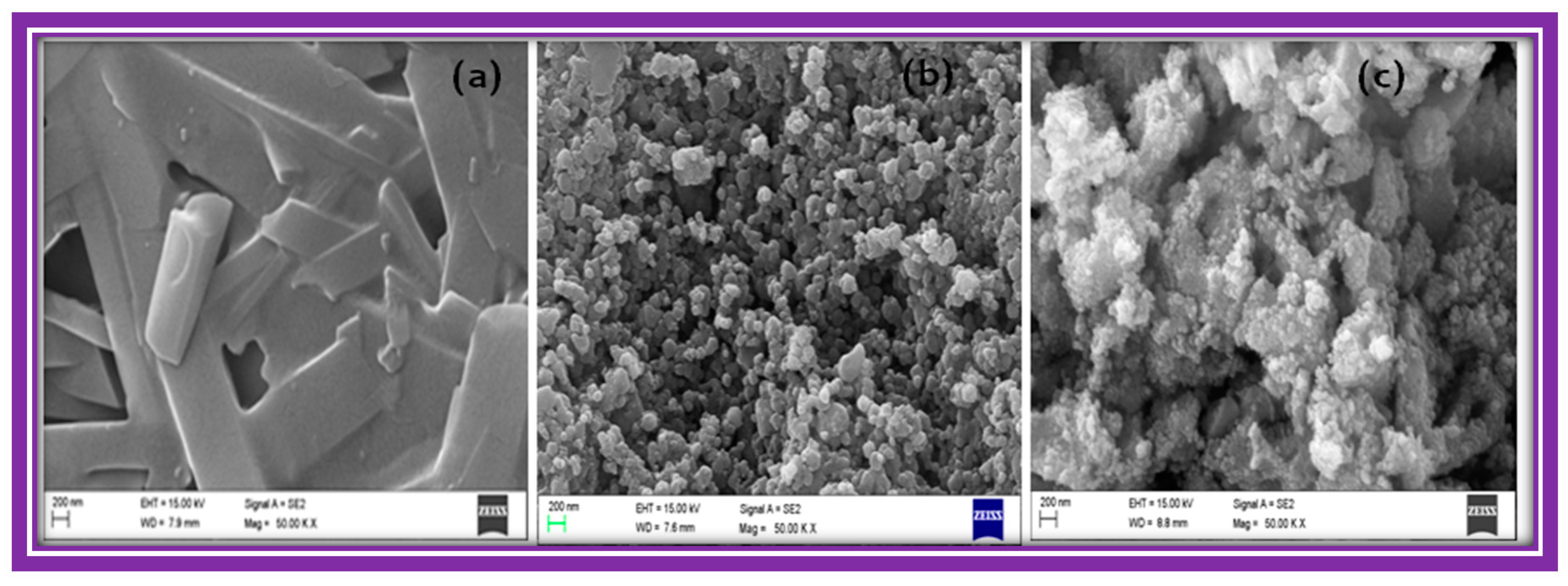
The FE-SEM images in
Figure 3 show that zinc, nickel and FeHCCo particles have uniform, almost spherical, structural morphology with a narrow size distribution, whereas manganese hexacyanocobaltate appeared as nearly square shaped, with a wide size distribution. The EDX (energy dispersive X-ray analysis) spectra presented in
Figure 3 revealed the composition of the materials by identifying the characteristic elements present in the MHCCo complexes.
The surface area, pore volume and pore radius of the newly made materials are presented in
Table 1 and
Table 2.
The initial adsorption studies of amines on MHCCo were performed over a pH range of 4–9. Upon variation of pH of the solution, a noticeable change towards the adsorption affinity of the studied amines was observed. At low pH adsorption, the affinity of aromatic amines towards MHCCo was found to be less, and this probably be due to the protonation of the amine group bearing nitrogen atom, whilst at alkaline pH, the electrostatic interaction between OH
− ion and metal ion is responsible for the considerable decrease in adsorption. In view of the pH effect, all further adsorption experiments were performed at pH~7.0 using the concentration range of aromatic amines (100–400 μM). To better understand the adsorption process, isotherms were plotted as
Xe (amount of amines adsorbed in mg/g) against
Ce (equilibrium concentration in mol·L
−1). The adsorption isotherms of the amines ZnHCCo, MnHCCo and NiHCCo are shown in
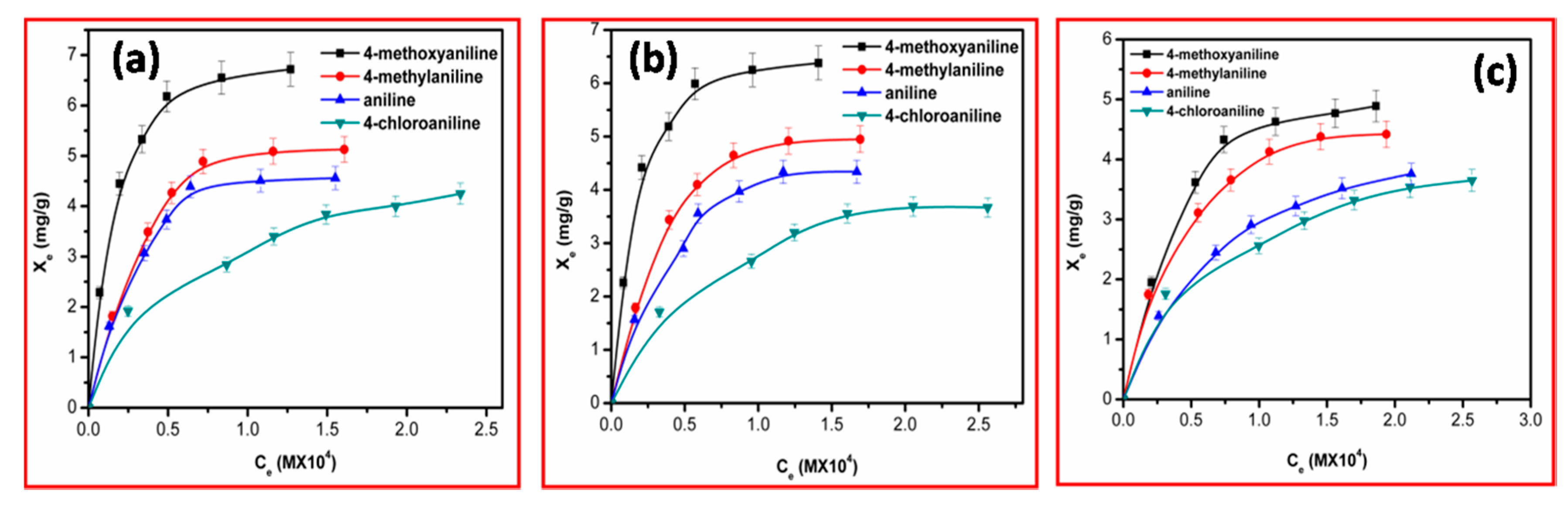
Figure 4.
At the low concentration range, the isotherms rise exponentially with respect to the amount of amine adsorbed and equilibrium concentration of amine; after a certain concentration, the isotherm reaches its saturation point. The percentage saturation point represents the attachment of a given amine, listed in
Table 3.
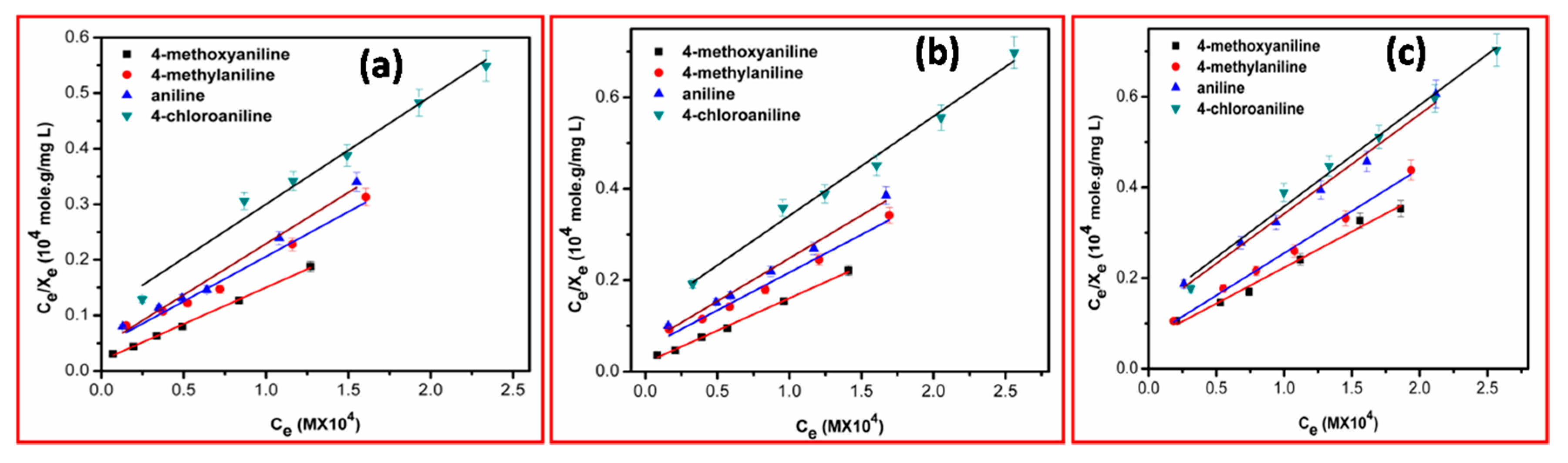
The Langmuir adsorption isotherms method (
Figure 5) was used to calculate the different parameters such as maximum adsorption capacity (
Xm) and Langmuir constant (
KL).
The calculated values of adsorption capacity and Langmuir constant are presented in
Table 4.
As evident from
Table 3, the trend in adsorption (% binding) of aromatic amines for MHCCos was found to increase in the order as shown below:
This trend in adsorption can be explained by considering the basic nature of the amines studied. The pK
a values of 4-methoxyaniline, 4-methylaniline, aniline and 4-chloroaniline are 5.34, 5.08, 4.60 and 3.98 respectively [
17]. These pK
a values indicate that 4-methoxyaniline is the most basic of the amines with 4-chloroaniline being the least basic. Positive divalent metal ions of metal hexacyanocobaltate can interact electrostatically with negatively charged amines that are basic in nature and possess nitrogen bearing an unshared pair of electrons and π electron cloud in an aromatic ring. The greater uptake by 4-methoxyaniline is due to a higher level of availability of electrons than other amines.
Among the studied MHCCo, ZnHCCo with a surface area (SA) of 213.64 m
2/g showed the highest binding of amines, whilst NiHCCo (SA = 100.19 m
2/g) exhibited the lowest. It is evident from the surface area (
Table 1) of the studied MHCCo and their corresponding percentage bindings calculated from the adsorption isotherm that the SA of the synthesized double metal cyanide complexes played an important role in the adsorption of aromatic amines. The adduct obtained after the adsorption process was dried at room temperature and subjected to FT-IR analysis. Electrostatic interaction between aromatic amines and MHCCo was further clarified by means of FT-IR spectroscopy. Typical FT-IR spectra of 4-methoxyaniline before and after interaction with ZnHCCo at neutral pH are illustrated in
Figure 6. Upon interaction with ZnHCCo, the N–H stretching (
N–H) frequencies of 4-methoxyaniline (3420 and 3334 cm
−1) are shifted to 3402 and 3212 cm
−1; N–H bending (δ
N–H) alters from 1631 to 1604 cm
−1. A significant change (from 1234 to 1250 cm
−1) of the C–N stretching (
C–N) frequency of 4-methoxyaniline was also observed.
The FT-IR spectra of the other amines, namely 4-methylaniline, aniline and 4-chloroaniline, upon adsorption onto ZnHCCo are presented in the
Supplementary Materials (
Figures S1–S3).
In all cases, a shift in the spectral frequencies of N–H stretching (
N–H), N–H bending (δ
N–H) and C–N stretching (
C–N) of the studied aromatic amines before and after the adsorption process suggested the involvement of an amino group in the interaction. The detailed spectral shifting of the aromatic amines is presented in
Table 5.
It is interesting to note here that no spectral frequency shifting was observed in the case of all the studied MHCCo after the adsorption process, ruling out the possibility of aromatic amine molecules replacing the cyanide ligand in the inner co-ordination sphere of the materials. This observation would seem obvious as it appears difficult for the amine molecule to replace cyanide into the co-ordination sphere, because strong ligands such as cyanide may be replaced under certain special conditions like irradiation of UV light [
18].
The dried product was further analyzed for observing surface morphological changes of ZnHCCo upon exposure to 4-methoxyaniline by means of FE-SEM studies. FE-SEM images of 4-methoxyaniline, ZnHCCo and amine–ZnHCCo adduct are shown in
Figure 7.
The attachment of 4-methoxyaniline on MHCCo surface is evident from
Figure 7c, along with the change in surface morphology. Morphologically, before interaction, ZnHCCo is globular shaped particles of varied size distribution, which aggregated after adsorption.
Oxidation of Aromatic Amines on Iron(II) Hexacyanocobaltate
The colored dimeric products obtained on the surface of FeHCCo were analyzed by GC-MS analysis. The confirmation of the dimeric products was carried out by comparing the retention time of the standard compounds run under same experimental conditions. Further confirmation was also provided by matching the principal fragmentation patterns of the standard compounds to those of the detected dimers. In all cases, the dimeric compounds were formed as azobenzene, 4,4′-dimethylazobenzene, 4,4′-dichloroazobenzene and 4,4′-dimethoxyazobenzene for aniline, 4-methylaniline, 4-chloroaniline and 4-methoxyaniline, respectively. As evident from
Figure S4, the retention time of 5.75, 7.83, 10.25 and 10.41 min is for aniline, 4-methylaniline, 4-chloroaniline and 4-methoxyaniline, respectively, while the retention time of 15.96, 18.70, 20.06 and 22.02 min represents the detection of their respective dimeric products.
Figure S5 represents the mass spectra of the dimeric products of aniline, 4-methylaniline, 4-chloroaniline and 4-methoxyaniline. In the case of dimer formation, the principal mass spectrum ion peaks (
m/
z) detected are as follows: aniline at 182, 105, 77; 4-methylaniline at 210, 119, 91; 4-chloroaniline at 250, 139, 111 and 4-methoxyaniline at 242, 135 and 107.
The mass ion peaks 182, 250, 210 and 242 represent the masses of azobenzene, 4,4’-dichloroazobenzene, 4,4’-dimethylazobenzene and 4,4’-dimethoxyazobenzene, respectively. In all cases, the observed fragmentation patterns are consistent with the standard compound fragmentation under the same experimental conditions. A schematic mechanism of the fragmentation pattern for the detected dimers is presented in
Figure S6.
3. Experimental
3.1. Materials and Methods
Manganese(II) nitrate, Mn(NO3)2; iron(II) nitrate Fe(NO3)2; nickel(II) nitrate, Ni(NO3)2; zinc(II) nitrate, Zn(NO3)2; Aniline, 4-methoxyaniline; 4-chloroaniline; and 4-methylaniline were purchased from E. Merck (Kenilworth, NJ, USA) while Potassium(I) hexacyanocobaltate(III) was purchased from Fluka (St. Louis, MO, USA). Deionized water was used throughout the studies.
3.2. Synthesis and Characterization of the Metal Hexacyanocobaltate(III)
A series of metal(II) hexacyanocobaltate(III), namely zinc hexacyanocobaltate (ZnHCCo), Zn
3[Co(CN)
6]
2·14H
2O; manganese hexacyanocobaltate (MnHCCo), Mn
3[Co(CN)
6]
2·14H
2O; nickel hexacyanocobaltate (NiHCCo), Ni
3[Co(CN)
6]
2·15H
2O; and iron hexacyanocobaltate (FeHCCo), Fe
3[Co(CN)
6]
2·15H
2O have been synthesized from potassium hexacyanocobaltate(III)—K
3[Co(CN)
6], following the method of Kaye and Long [
19]. A solution of K
3[Co(CN)
6] (10 mmol) in 100 mL of water was added drop-wise to a solution of the respective metal(II) nitrate (18 mmol) in 100 mL of water with constant stirring. The resulting mixture was kept overnight at room temperature, filtered through a Buchner funnel and then washed thoroughly with Millipore water. The precipitate obtained was dried in air. The dried product was powdered and sieved through a 100 mesh size sieve. Characterization of the materials was carried out by powder X-Ray Diffractometry (XRD), FT-IR spectroscopy, FE-SEM microscopy, elemental analysis of C, H and N with Atomic Absorption Spectroscopy (AAS), Thermogravimetric (TGA/DTA) and surface area analysis. A Brucker AXS D8 (Bremen, Germany) advance X-ray powder diffractometer was used to characterize the samples for crystal structure and the purity of the materials. A Perkin-Elmer FT-IR spectrometer (Waltham, MA, USA) was used for recording the spectra of the samples. Surface morphological images and the elemental composition of the samples was obtained using FEI Quanta (FE-SEM, Hillsboro, MR, USA), with a 20 kV capacity instrument equipped with an elemental analysis probe (EDX). The surface area of the samples was determined using a Nova 2200e (Quantachrome) instrument (Boynton Beach, FL, USA). Only a short description regarding the material characterization is presented in this paper; further details can be found in [
20].
3.3. Spectral Studies
UV-Visible spectra of aniline, 4-chloroaniline, 4-methylaniline and 4-methoxyaniline were determined using a Shimadzu spectrometer (UV-1800, Kyoto, Japan) at characteristic values of λmax 280, 238, 286 and 295 nm, respectively, and FT-IR studies were performed using a Perkin Elmer-1600 series spectrometer (Waltham, MA, USA).
3.4. Adsorption Protocol
Adsorption of aromatic amines on MHCCo was performed by varying the concentration of amines (100–400 μM); amount of adsorbent (25–100 mg); effect of contact time (1–12 h) and influence of pH (4–9) in order to evaluate the important parameters relating to the adsorption process. Maximum adsorption of aromatic amines took place at pH~7.0 with 50 mg of adsorbent within 6 h. A suitable concentration range of aromatic amines (100–400 μM) was chosen, so that the absorbance range could easily be measured on a UV spectrometer. Approximately 50 mg of the adsorbent was mixed with 10.0 mL of aromatic amine solution having a concentration range of 100–400 μM, followed by shaking for 30 min on a Vortex shaker (Spinix) (Bangalore, India). After reaching the equilibration time, the adsorbents were separated by centrifugation and the supernatant was directly measured for the concentration of aromatic amines using a UV spectrometer. The percentage binding of aromatic amines was calculated from the adsorption peaks. The amines-MHCCo adduct was washed thoroughly with water, dried and subjected to FT-IR and FE-SEM studies.
3.5. Oxidation of Aromatic Amines on Iron(II) Hexacyanocobaltate
During the adsorption of aromatic amines on FeHCCo, it was observed that a colored solution was formed within 6 h. These colored soluble products were extracted using dichloromethane as a solvent for GC–MS analysis. A Perkin Elmer GC-MS system with a fused silica capillary column was used for analysis of the reaction products. The product analysis conditions were set as follows: 40 °C for 2 min; 40–220 °C at 20 °C·min−1 and 220–280 °C at 4 °C·min−1. The carrier gas used was helium, having a flow rate of 1 mL·min−1, while the ionization potential was 50 eV, and ion sources were at 250 °C.