Attachment of Luminescent Neutral “Pt(pq)(C≡CtBu)” Units to Di and Tri N-Donor Connecting Ligands: Solution Behavior and Photophysical Properties
Abstract
:1. Introduction
2. Results and Discussion
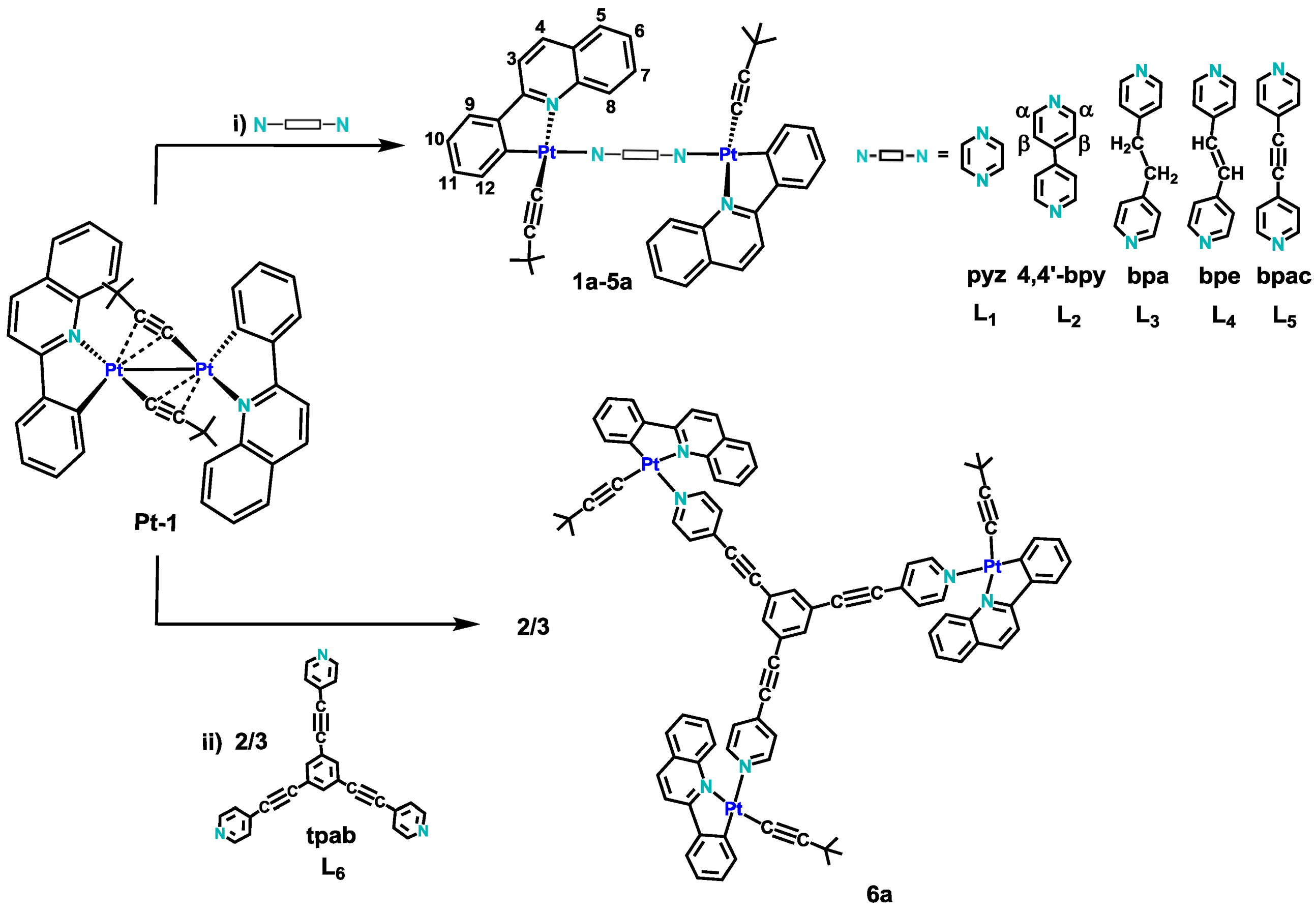
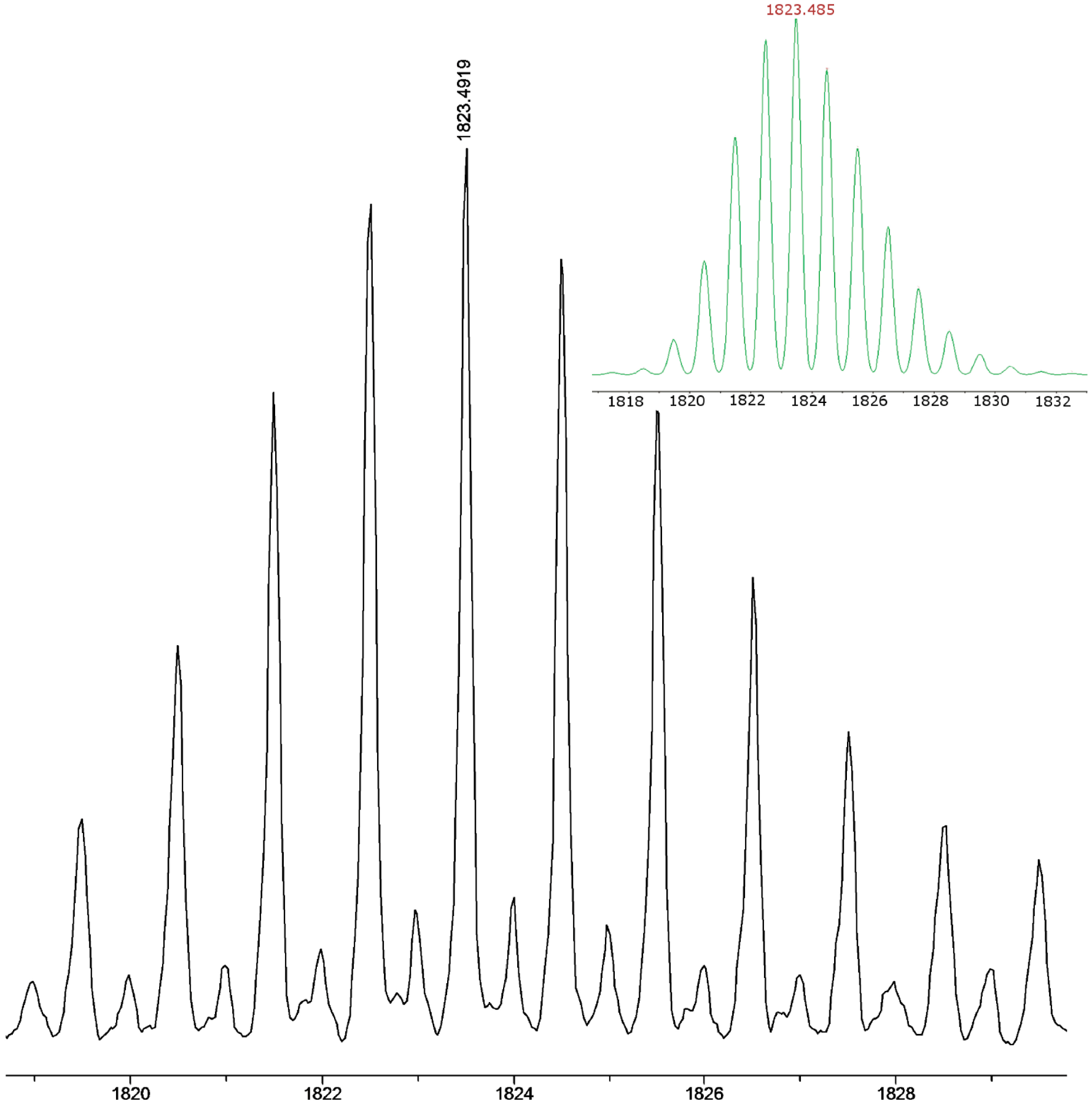
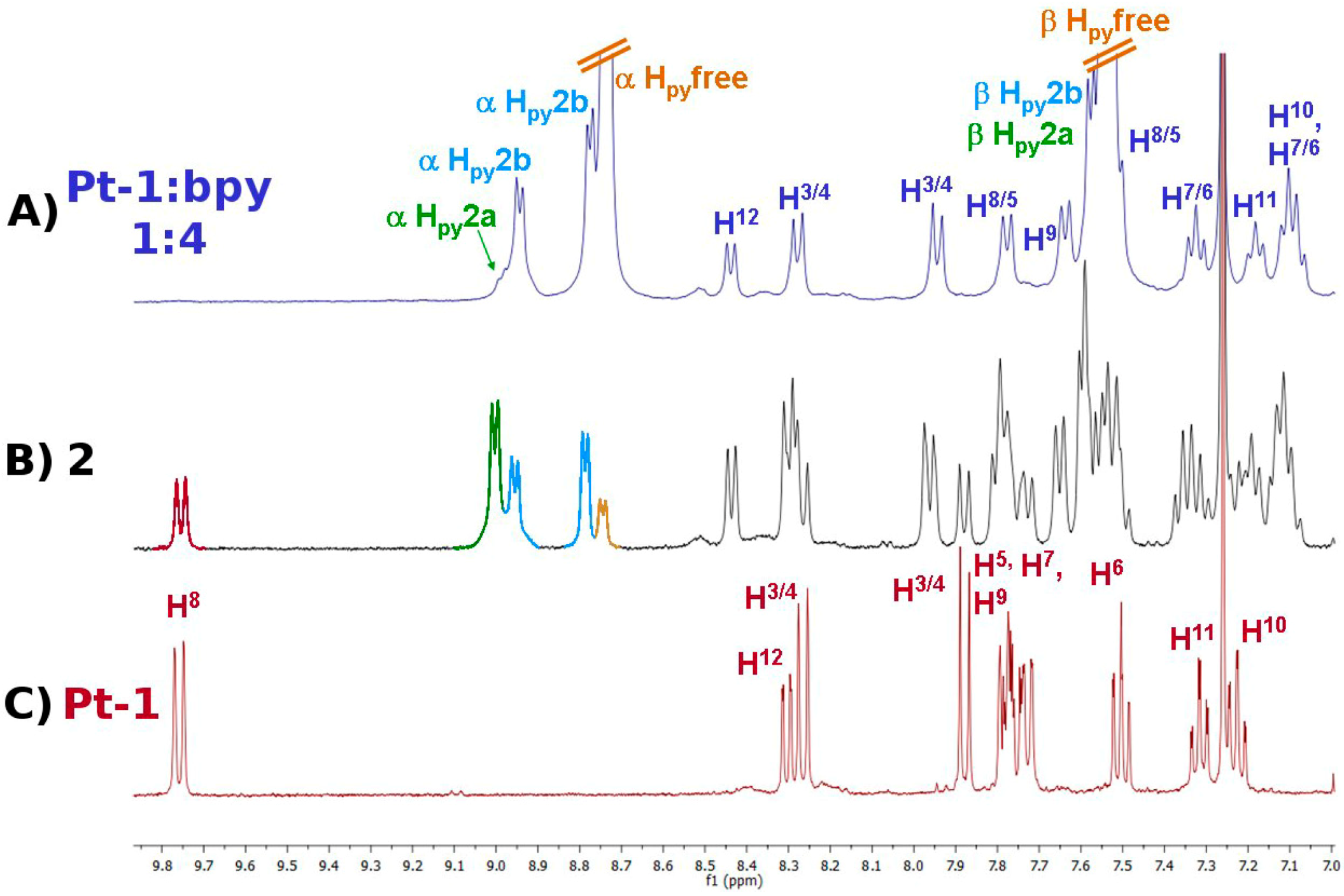
2.1. Synthesis and Characterization
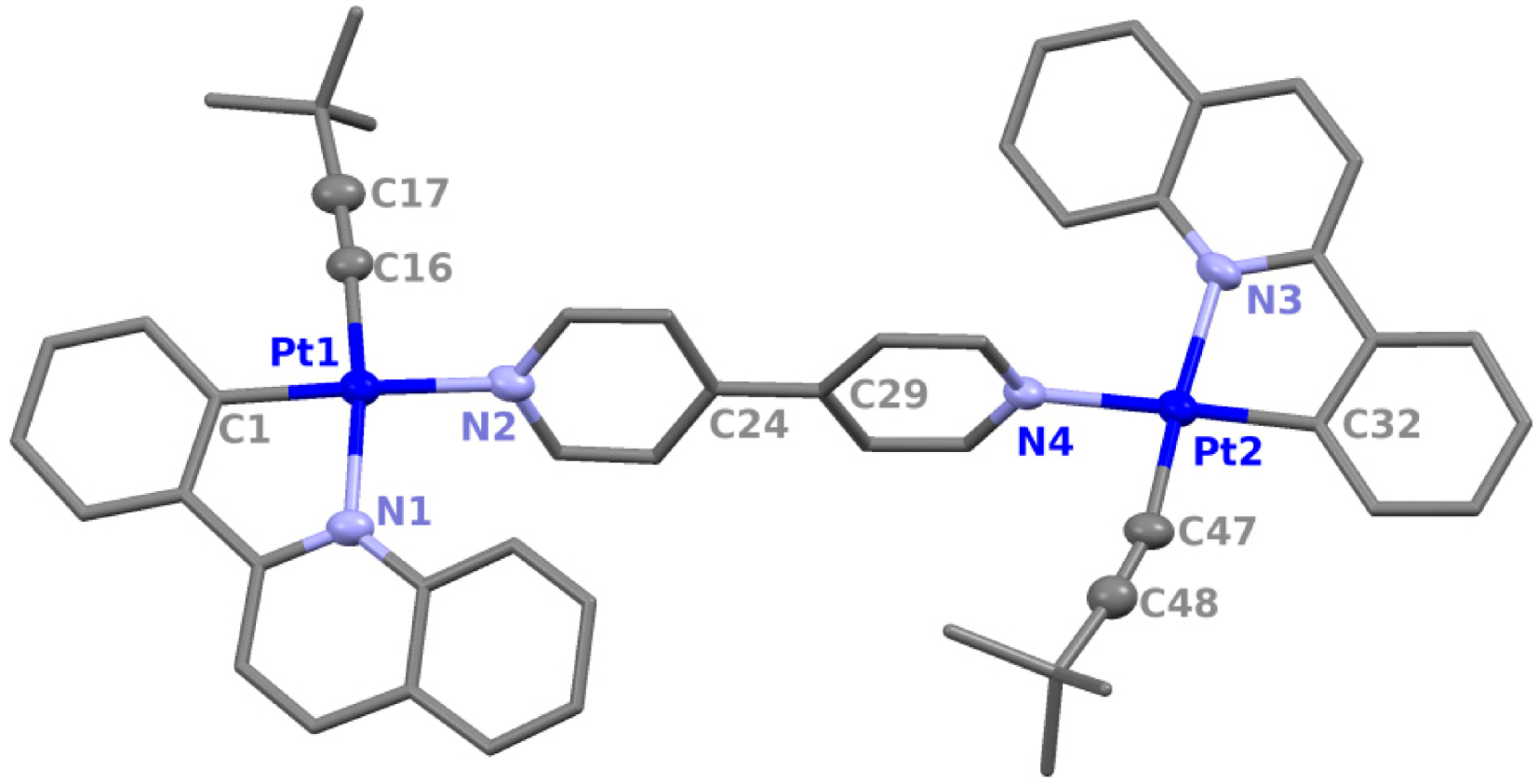

| 2a·2CHCl3·hexane | 4a·4CHCl3 | ||
| Distances (Å) | |||
| Pt(1)-C(1) | 1.994(5) | Pt(1)-C(1) | 1.972(9) |
| Pt(1)-N(1) | 2.115(4) | Pt(1)-N(1) | 2.096(7) |
| Pt(1)-C(16) | 1.967(5) | Pt(1)-C(16) | 1.949(9) |
| Pt(1)-N(2) | 2.113(4) | Pt(1)-N(2) | 2.130(7) |
| C(16)-C(17) | 1.197(7) | C(16)-C(17) | 1.209(12) |
| Pt(2)-C(32) | 1.980(5) | Pt(2)-C(34) | 1.979(8) |
| Pt(2)-N(3) | 2.127(4) | Pt(2)-N(3) | 2.136(7) |
| Pt(2)-C(47) | 1.961(5) | Pt(2)-C(49) | 1.956(9) |
| Pt(2)-N(4) | 2.125(4) | Pt(2)-N(4) | 2.145(7) |
| C(47)-C(48) | 1.209(7) | C(49)-C(50) | 1.195(12) |
| - | - | C(27)-C(28) | 1.290(9) |
| Angles (°) | |||
| N(1)-Pt(1)-C(1) | 80.9(2) | N(1)-Pt(1)-C(1) | 80.3(3) |
| C(16)-Pt(1)-C(1) | 91.7(2) | C(16)-Pt(1)-C(1) | 92.1(4) |
| C(16)-Pt(1)-N(2) | 88.50(19) | C(16)-Pt(1)-N(2) | 87.8(3) |
| N(1)-Pt(1)-N(2) | 98.56(16) | N(1)-Pt(1)-N(2) | 99.4(3) |
| Pt(1)-C(16)-C(17) | 175.2(5) | Pt(1)-C(16)-C(17) | 176.3(9) |
| C(16)-C(17)-C(18) | 176.6(8) | C(16)-C(17)-C(18) | 175.4(11) |
| N(3)-Pt(2)-C(32) | 80.6(2) | N(3)-Pt(2)-C(34) | 80.9(3) |
| C(47)-Pt(2)-C(32) | 95.8(2) | C(49)-Pt(2)-C(34) | 93.0(4) |
| C(47)-Pt(2)-N(4) | 81.36(19) | C(49)-Pt(2)-N(4) | 83.9(3) |
| N(3)-Pt(2)-N(4) | 101.96(16) | N(3)-Pt(2)-N(4) | 102.0(3) |
| Pt(2)-C(47)-C(48) | 167.6(5) | Pt(2)-C(49)-C(50) | 174.7(9) |
| C(47)-C(48)-C(49) | 172.7(6) | C(49)-C(50)-C(51) | 174.6(11) |
| C(27)-C(28)-C(31) | 126.5(14) | C(24)-C(27)-C(28) | 121.4(14) |
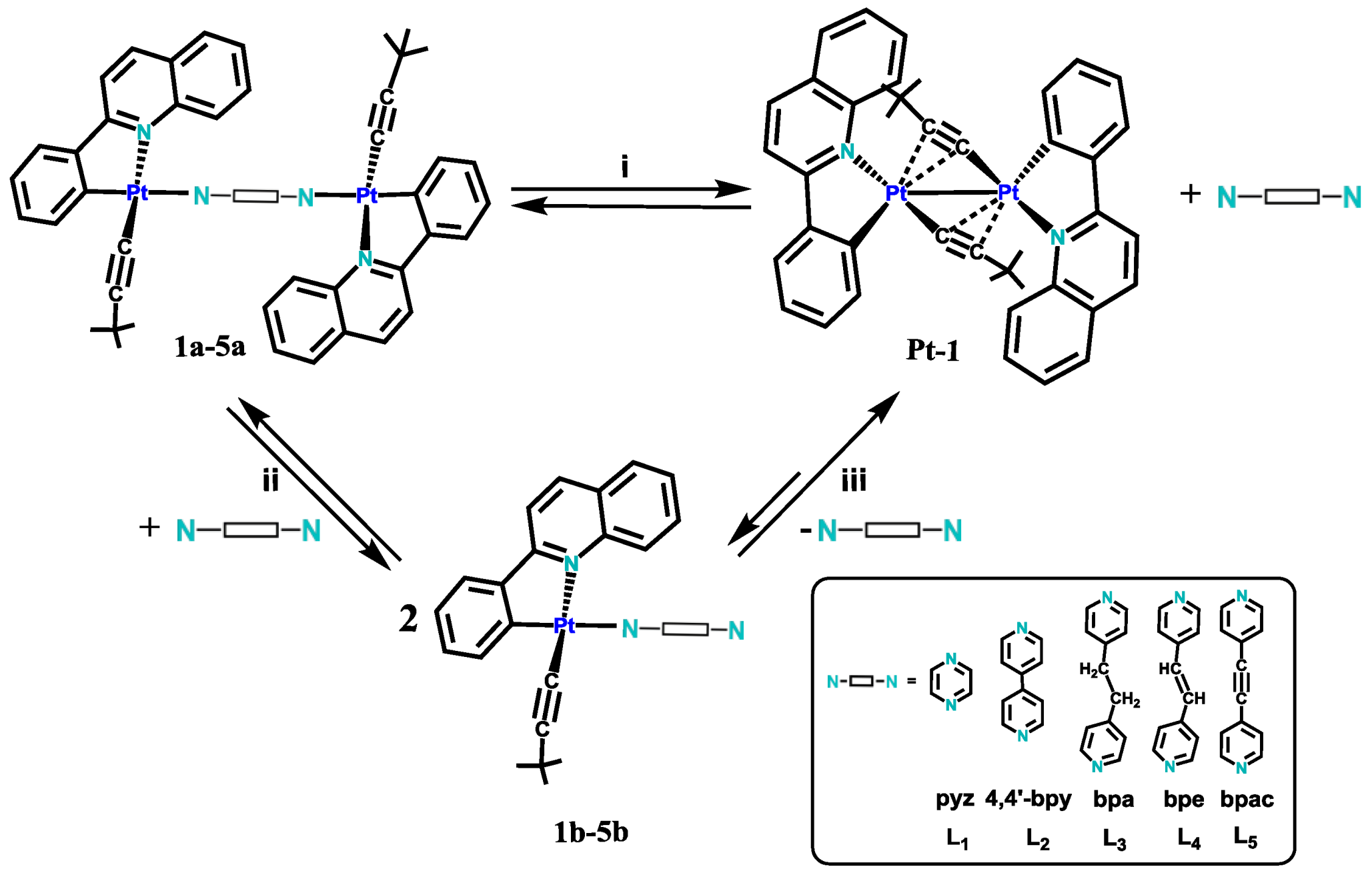
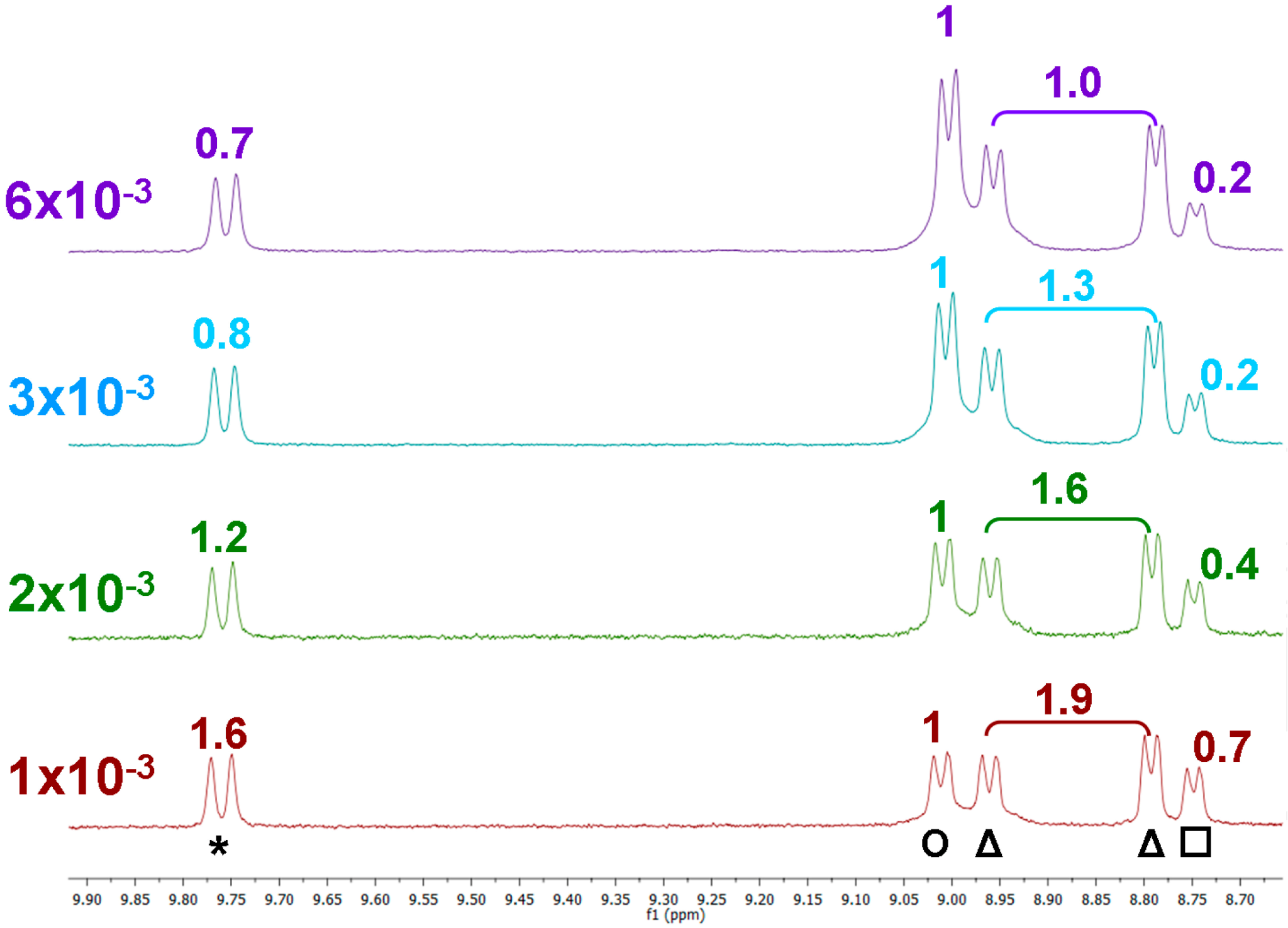
2.2. Photophysical Properties
| Compounds | λabs/nm (103 ε M−1 cm−1) |
|---|---|
| 1 | 242 (65.3), 276 (62.4), 304 (55.9), 353 (40.1), 408 (20.6) CH2Cl2 |
| 300, 330, 365, 390, 430, 530 solid | |
| 2 | 243 (63.8), 270 (59.6), 341 (17.7), 354 (18.7), 410 (13.3) CH2Cl2 |
| 305, 330, 355, 400, 500, 530 solid | |
| 3 | 218 (67.3), 257 (65.1), 299 (42.5), 337 (20.6), 355 (20.2), 413 (17.7) CH2Cl2 |
| 300, 320, 350, 415, 525 solid | |
| 4 | 242 (63.3), 276 (63.9), 314 (58.3), 355 (29.9), 412 (20.7) CH2Cl2 |
| 305, 345, 400, 425, 505, 535 solid | |
| 5 | 243 (63.3), 278 (62.2), 325(60.4), 355 (33.2), 410 (20.8) CH2Cl2 |
| 305, 320, 360, 390, 420, 540 solid | |
| 6 | 245 (80.1), 289 (79.0), 308(71.7), 329sh (43.9), 355 (35), 410 (6.0) CH2Cl2 |
| 310, 360, 420, 545 tail to 630 solid |
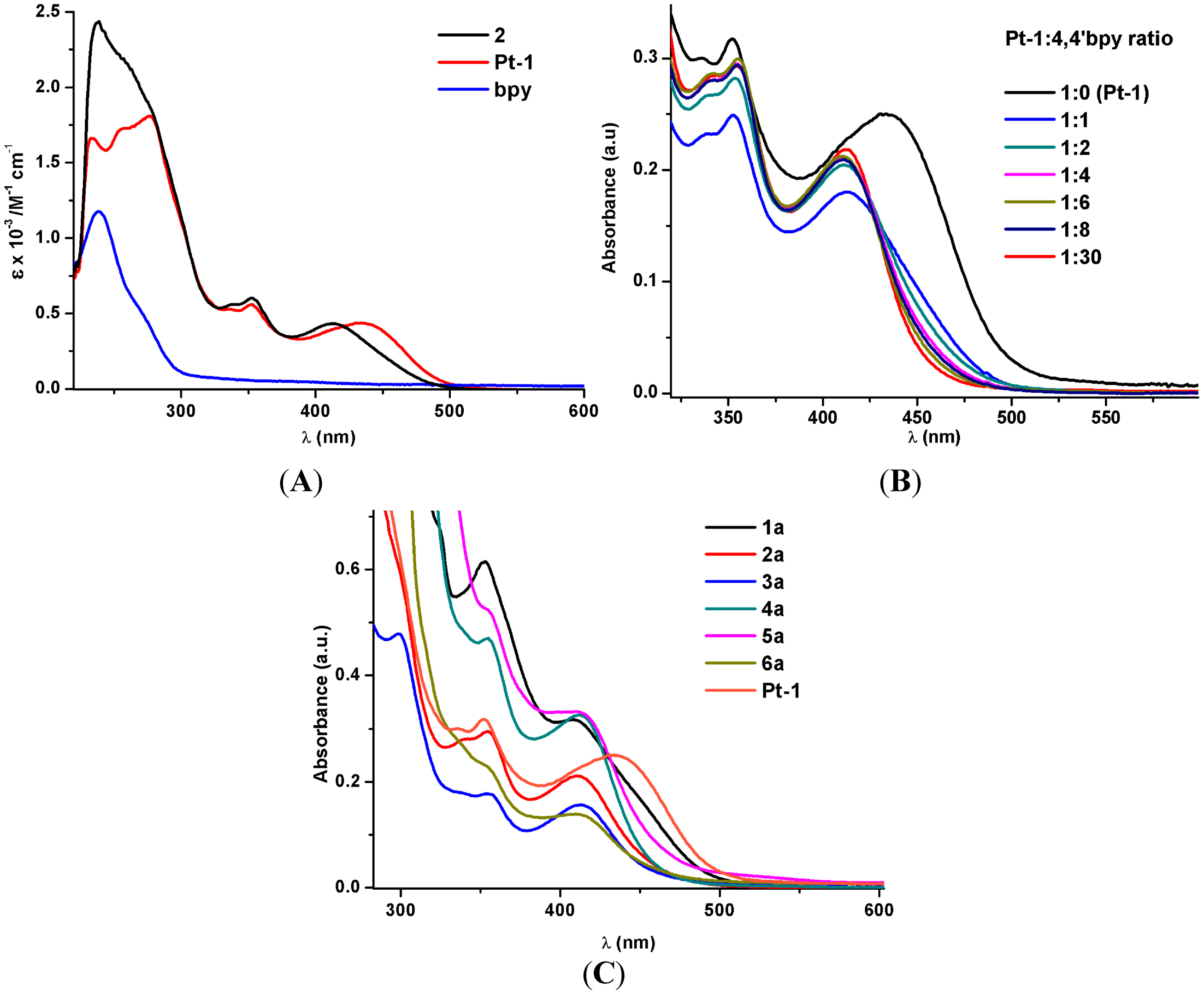
2.2.1. Absorption Spectroscopy
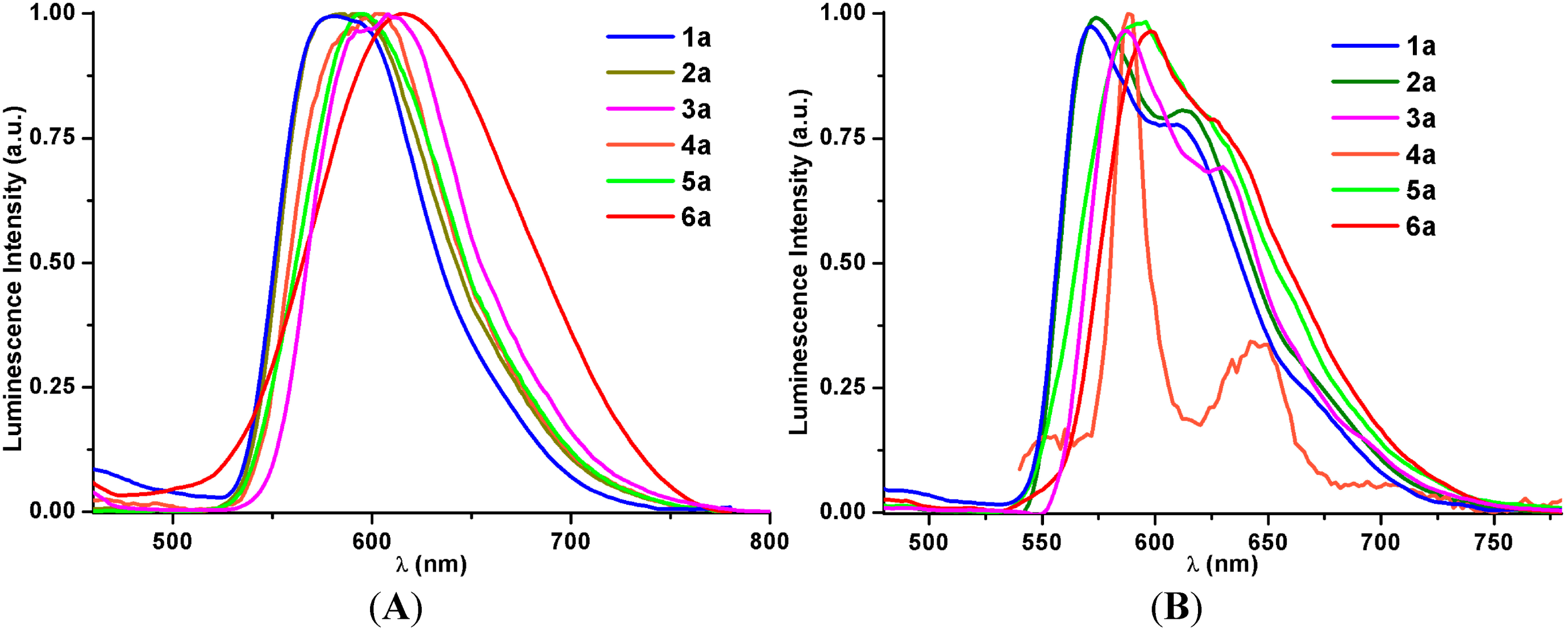
2.2.2. Emission Spectroscopy
| Compound | Medium (T ª/K) | λem/nm (λexc/nm) | τ/μs | ϕ(%) |
|---|---|---|---|---|
| 1 | Solid (298) | 590maxa (365–550) | 8.0 | 13.8 |
| Solid (77) | 572max, 610, 660sh (365–540) | 14.0 | - | |
| 5 × 10−5 M (298) | 595maxa (350–420) | - | - | |
| 5 × 10−5 M (77) | 570max, 610, 660sh (365–450) | - | - | |
| 2 | Solid (298) | 590 a (365–530) | 9.9 | 13.1 |
| Solid (77) | 574max, 612, 650(365–530) | 12.2 | - | |
| 5 × 10−5 M (298) | 590 a (365–410) | - | - | |
| 5 × 10−5 M (77) | 570max, 625, 660sh (365–430) | - | - | |
| 3 | Solid (RT) | 610a (365–540) | 9.9 | 9.4 |
| Solid (77) | 588max, 625 (365–550) | 7.7 | - | |
| 5 × 10−5 M (RT) | 595 (365–420) | - | - | |
| 5 × 10−5 M (77) | 570max, 610, 660sh (365–460) | - | - | |
| 4 | Solid (298) | 605 a (365–540) | 10.4 | 4.9 |
| Solid (77) | 588max, 648, 702(365–540) | 39.2 | - | |
| 5 × 10−5 M (77) b | 578max, 620, 650sh (365–440) | 13.8 | - | |
| 5 | Solid (298) | 596 a (365–540) | 11.4 | 6.8 |
| Solid (77) | 596 a (365–540) | 15.2 | - | |
| 5 × 10−5 M (298) | 595 (365–420) | 9.2 | - | |
| 5 × 10−5 M (77) | 576max, 613, 660sh (365–440) | 14.4 | - | |
| 6 | Solid (298) | 615 a (365–500) | 0.3 | 1.1 |
| Solid (77) | 598 a (365–480) | 7.6 | - | |
| 5 × 10−5 M (298) | 595 (365–415) | - | - | |
| 5 × 10−5 M (77) | 570max, 612, 670sh (365–480) | - | - |
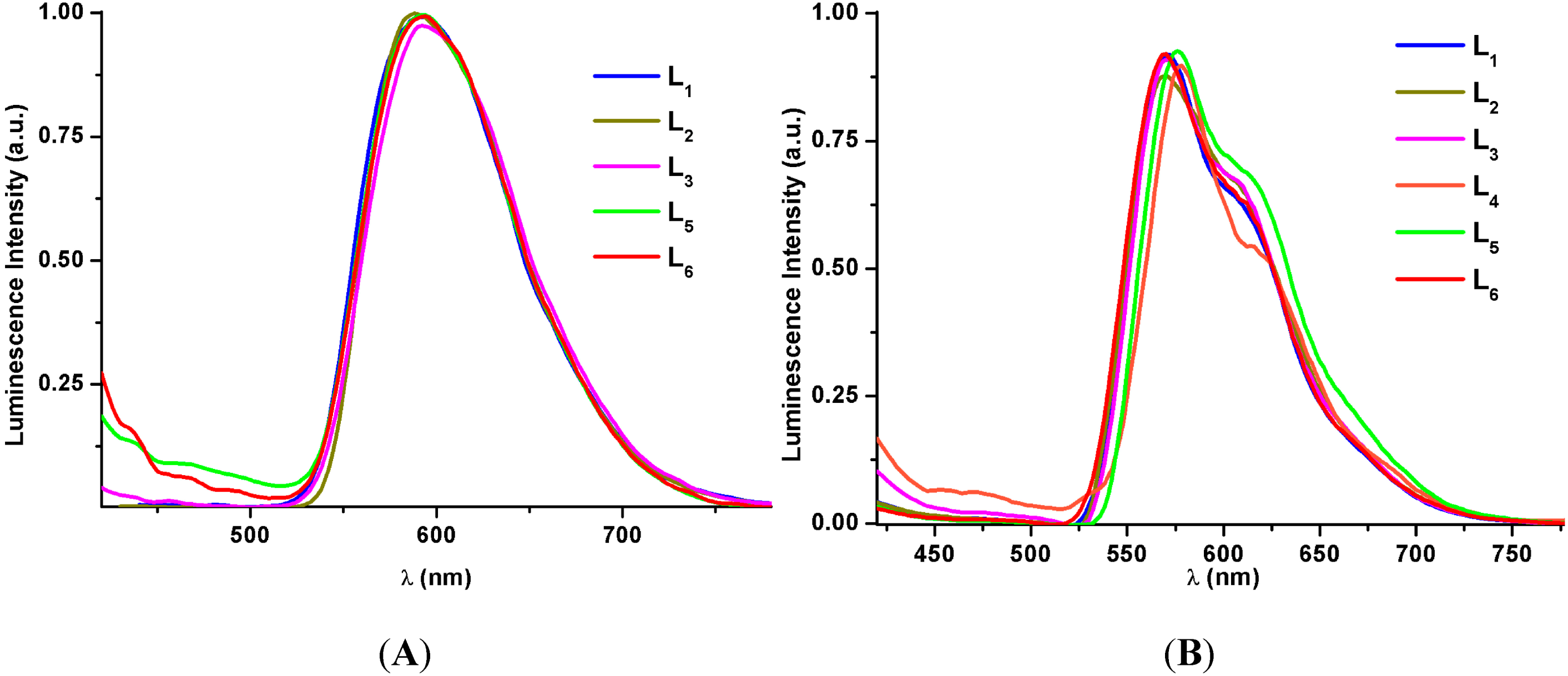
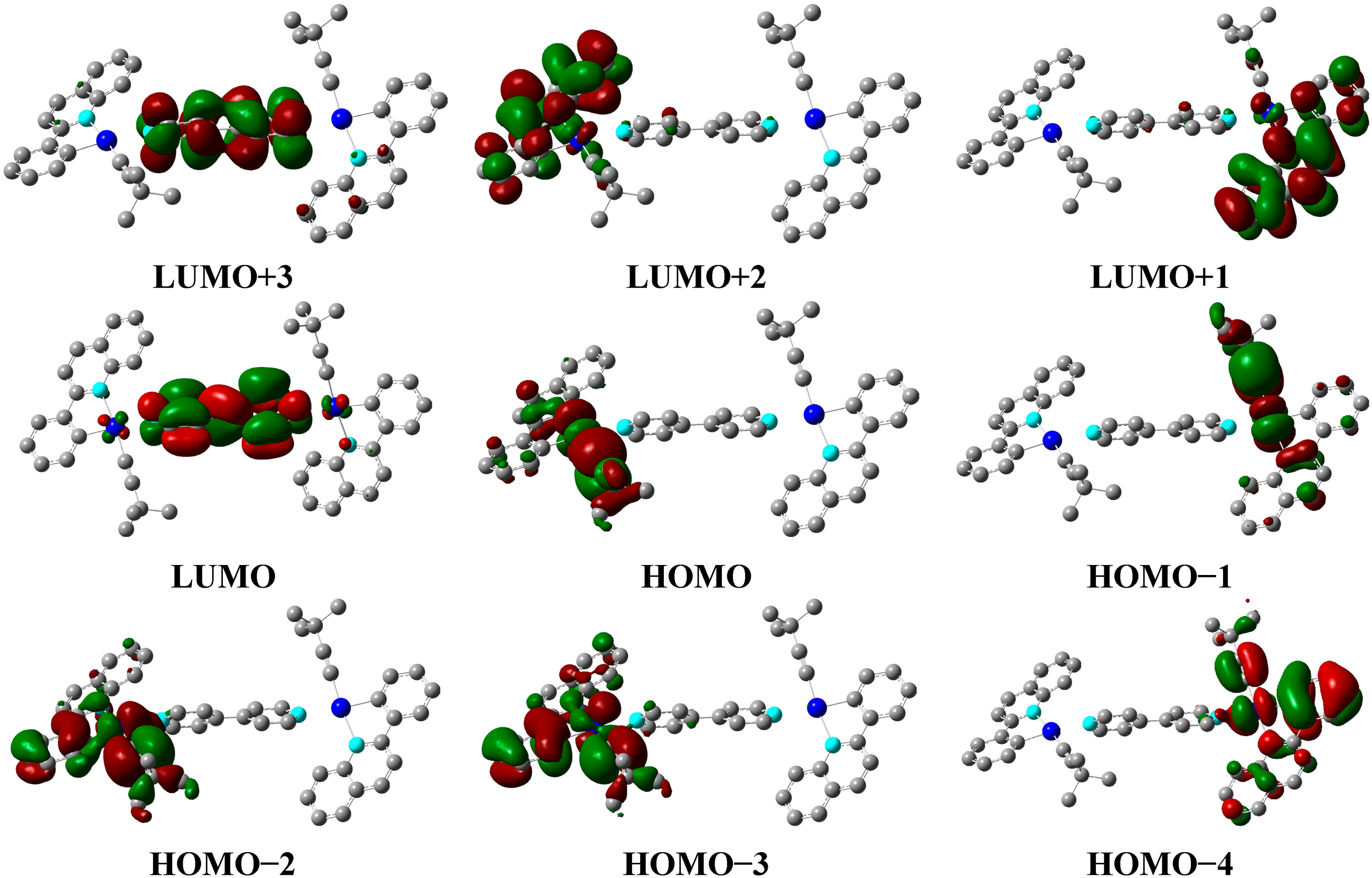
2.3. Theoretical Calculations
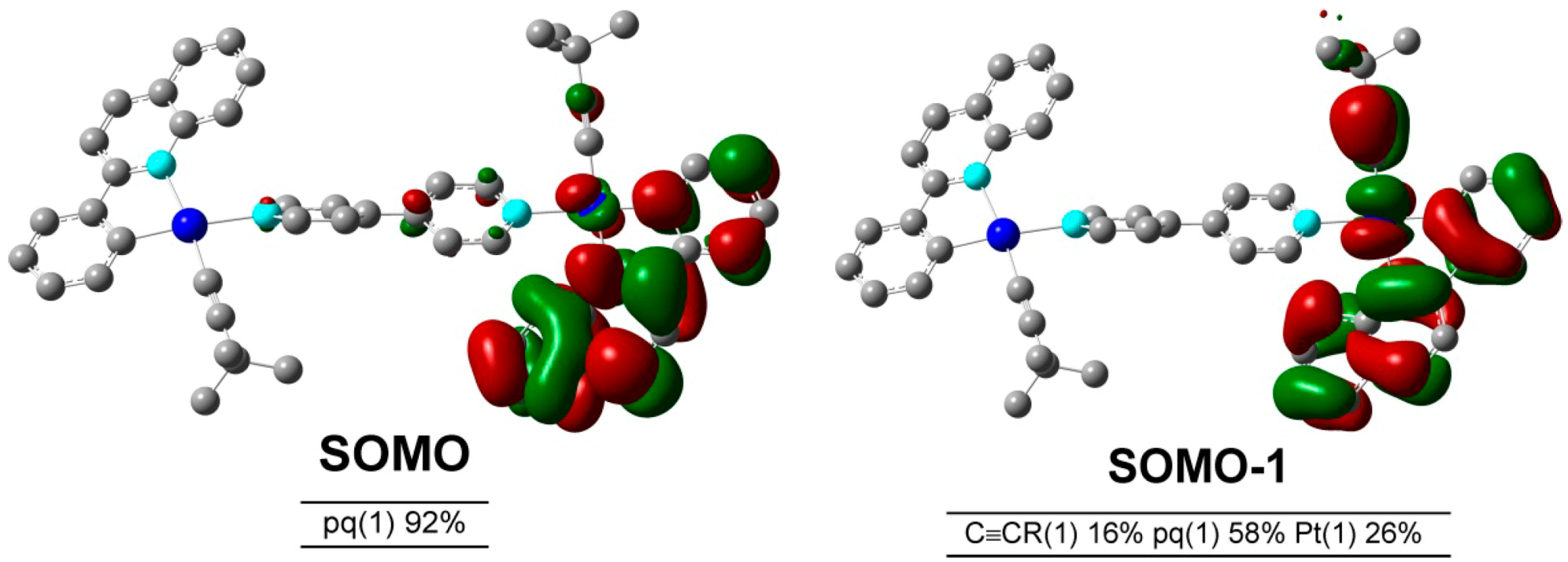
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berenguer, J.R.; Lalinde, E.; Moreno, M.T. An overview of the chemistry of homo and heteropolynuclear platinum complexes containing bridging acetylide (μ-C≡CR) ligands. Coord. Chem. Rev. 2010, 254, 832–857. [Google Scholar] [CrossRef]
- Lang, H.; George, D.S.A.; Rheinwald, G. Bis(alkynyl) transition metal complexes, R1C≡C-[M]-C≡CR2, as organometallic chelating ligands; formation of μ,η1(2)-alkynyl-bridged binuclear and oligonuclear complexes. Coord. Chem. Rev. 2000, 206, 101–197. [Google Scholar]
- Lang, H.; Köhler, K.; Blau, S. η2-Alkyne copper(I) and silver(I) compounds; from polymeric [MIR]n to monomeric [MIR] units (M = Cu, Ag). Coord. Chem. Rev. 1995, 143, 113–168. [Google Scholar] [CrossRef]
- Forniés, J.; Lalinde, E. Synthesis, structure and reactivity of homo- and hetero-polynuclear complexes of platinum bearing C≡CR groups as unique bridging ligands. Dalton Trans. 1996. [Google Scholar] [CrossRef]
- Wong, K.M.C.; Yam, V.W.W. Self-Assembly of Luminescent Alkynylplatinum(II) Terpyridyl Complexes: Modulation of Photophysical Properties through Aggregation Behavior. Acc. Chem. Res. 2011, 44, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.W. Molecular Design of Transition Metal Alkynyl Complexes as Building Blocks for Luminescent Metal-Based Materials: Structural and Photophysical Aspects. Acc. Chem. Res. 2002, 35, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Hissler, M.; McGarrah, J.E.; Connick, W.B.; Geiger, D.K.; Cummings, S.D.; Eisenberg, R. Platinum diimine complexes: Towards a molecular photochemical device. Coord. Chem. Rev. 2000, 208, 115–137. [Google Scholar]
- Rossi, E.; Colombo, A.; Dragonetti, C.; Roberto, D.; Ugo, R.; Valore, A.; Falciola, L.; Brulatti, P.; Cocchi, M.; Williams, J.A.G. Novel N^C^N-cyclometallated platinum complexes with acetylide co-ligands as efficient phosphors for OLEDs. J. Mater. Chem. 2012, 22, 10650–10655. [Google Scholar] [CrossRef]
- Yam, V.W.W. Luminescent metal alkynyls—From simple molecules to molecular rods and materials. J. Organomet. Chem. 2004, 689, 1393–1401. [Google Scholar] [CrossRef]
- Wong, W.Y. Luminescent organometallic poly(aryleneethynylene)s: Functional properties towards implications in molecular optoelectronics. Dalton Trans. 2007. [Google Scholar] [CrossRef]
- Chen, Z.N.; Zhao, N.; Fan, Y.; Ni, J. Luminescent groups 10 and 11 heteropolynuclear complexes based on thiolate or alkynyl ligands. Coord. Chem. Rev. 2009, 253, 1–20. [Google Scholar] [CrossRef]
- Castellano, F.N.; Pomestchenko, I.E.; Shikhova, E.; Hua, F.; Muro, M.L.; Rajapakse, N. Photophysics in bipyridyl and terpyridyl Platinum(II) acetylides. Coord. Chem. Rev. 2006, 250, 1819–1828. [Google Scholar] [CrossRef]
- Wong, K.M.C.; Yam, V.W.W. Luminescence Platinum(II) terpyridyl complexes—From fundamental studies to sensory functions. Coord. Chem. Rev. 2007, 251, 2477–2488. [Google Scholar] [CrossRef]
- Mei, J.; Ogawa, K.; Kim, Y.G.; Heston, N.C.; Arenas, D.J.; Nasrollahi, Z.; McCarley, T.D.; Tanner, D.B.; Reynolds, J.R.; Schanze, K.S. Low-Band-Gap Platinum Acetylide Polymers as Active Materials for Organic Solar Cells. Appl. Mater. Interfaces 2009, 1, 150–161. [Google Scholar] [CrossRef]
- Colombo, A.; Nisic, F.; Dragonetti, C.; Marinotto, D.; Oliveri, I.P.; Righetto, S.; Lobello, M.G.; De Angelis, F. Unexpectedly high second-order nonlinear optical properties of simple Ru and Pt alkynyl complexes as an analytical springboard for NLO-active polymer films. Chem. Commun. 2014, 50, 7986–7989. [Google Scholar] [CrossRef]
- Rossi, E.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; Valore, A.; Williams, J.A.G.; Lobello, M.G.; De Angelis, F. Tuning the Dipolar Second-Order Nonlinear Optical Properties of Cyclometalated Platinum(II) Complexes with Tridentate N^C^N Binding Ligands. Chem. Eur. J. 2013, 19, 9875–9883. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Cifuentes, M.P.; Samoc, M.; Humphrey, M.G. Metal alkynyl complexes as switchable NLO systems. Coord. Chem. Rev. 2011, 255, 2530–2541. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Forniés, J.; Lalinde, E.; Martín, A.; Serrano, B. Preparation and characterisation of neutral double- and mono-alkynyl bridged diplatinum complexes. Dalton Trans. 2001. [Google Scholar] [CrossRef]
- Ara, I.; Falvello, L.R.; Fernández, S.; Forniés, J.; Lalinde, E.; Martín, A.; Moreno, M.T. Synthesis and Reactivity of σ-Alkynyl/P-Bonded Phosphinoalkyne Platinum Complexes toward cis-[M(C6F5)2(thf)2] (M = Pt, Pd). Organometallics 1997, 16, 5923–5937. [Google Scholar] [CrossRef]
- García, A.; Lalinde, E.; Moreno, M.T. Ethynyltolan Platinum Complexes with (Arylalkynyl)phosphane Ligands. Eur. J. Inorg. Chem. 2007, 2007, 3553–3560. [Google Scholar] [CrossRef]
- Forniés, J.; Gómez-Saso, M.A.; Lalinde, E.; Martínez, F.; Moreno, M.T. Preparation of Doubly Acetylide-Bridged Binuclear Platinum-Platinum and Platinum-Palladium Complexes. Structures of [{(dppe)Pt(C≡CPh)2}Pt(C6F5)2] and (PMePh3)2[(C6F5)2Pt(μ-C≡CPh)2Pt(C6F5)2]. Organometallics 1992, 11, 2873–2883. [Google Scholar] [CrossRef]
- Falvello, L.R.; Forniés, J.; Gómez, J.; Lalinde, E.; Martín, A.; Martínez, F.; Moreno, M.T. Some Platinum(II) complexes containing bis(diphenylphosphino)acetylene PPh2C≡CPPh2: Synthesis, characterisation and crystal structures. Dalton Trans. 2001. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Forniés, J.; Martínez, F.; Cubero, J.C.; Lalinde, E.; Moreno, M.T.; Welch, A.J. Synthesis and reactivity of bimetallic acetylide-bridged Pt-Pt complexes. Crystal and molecular structure of [(PPh3)(C6F5)Pt(μ-C≡CPh)2Pt(C6F5)(PPh3)]. Polyhedron 1993, 12, 1797–1804. [Google Scholar] [CrossRef]
- Falvello, L.R.; Forniés, J.; Martín, A.; Gómez, J.; Lalinde, E.; Moreno, M.T.; Sacristán, J. Synthesis of Heterobridged (μ-C≡CR)(μ-X) (X = PPh2, PPh2O) Platinum−Rhodium or Platinum−Iridium Dimers. Inorg. Chem. 1999, 38, 3116–3125. [Google Scholar] [CrossRef]
- Aullón, G.; Álvarez, S. Molecular Structure and Isomerization in Square-Planar Edge-Sharing Dinuclear Complexes with Alkynyl Bridges. Organometallics 2002, 21, 2627–2634. [Google Scholar] [CrossRef]
- Hoogervorst, W.J.; Elsevier, C.J.; Lutz, M.; Spek, A.L. New cis- and trans-ArylPlatinum(II) Acetylide Compounds Containing a Bis(imino)aryl [NCN] Ligand. Organometallics 2001, 20, 4437–4440. [Google Scholar] [CrossRef]
- Casas, J.M.; Forniés, J.; Fuertes, S.; Martín, A.; Sicilia, V. New Mono- and Polynuclear Alkynyl Complexes Containing Phenylacetylide as Terminal or Bridging Ligand. X-ray Structures of the Compounds NBu4[Pt(CH2C6H4P(o-tolyl)2-κC,P)(C≡CPh)2], [Pt(CH2C6H4P(o-tolyl)2-κC,P)(C≡CPh)(CO)], [{Pt(CH2C6H4P(o-tolyl)2-κC,P)(C≡CPh)}2], and [{Pt(CH2C6H4P(o-tolyl)2-κC,P)(C≡CPh)2Cu}2]. Organometallics 2007, 26, 1674–1685. [Google Scholar] [CrossRef]
- Forniés, J.; Lalinde, E.; Martín, A.; Moreno, M.T. Di- and tri-nuclear platinum complexes with double acetylide bridges. Molecular structure of [NBu4]2[Pt3(C6F5)4(µ-C≡CPh)4]4thf (thf = tetrahydrofuran). Dalton Trans. 1994. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Forniés, J.; Lalinde, E.; Martínez, F. Unusual stabilization of cationic M(η3-allyl)+n (M = Pt, Pd) units by a dianionic cis-{Pt(C6F5)2(C≡CSiMe3)2}2− fragment. J. Organomet. Chem. 1994, 470, C15–C18. [Google Scholar] [CrossRef]
- Fujita, M.; Yazaki, J.; Ogura, K. Preparation of a macrocyclic polynuclear complex, [(en)Pd(4,4'-bpy)]4(NO3)8 (en = ethylenediamine, bpy = bipyridine), which recognizes an organic molecule in aqueous media. J. Am. Chem. Soc. 1990, 112, 5645–5647. [Google Scholar] [CrossRef]
- Zangrando, E.; Casanova, M.; Alessio, E. Trinuclear Metallacycles: Metallatriangles and Much More. Chem. Rev. 2008, 108, 4979–5013. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M. Metal-directed self-assembly of two- and three-dimensional synthetic receptors. Chem. Soc. Rev. 1998, 27, 417–425. [Google Scholar] [CrossRef]
- Caulder, D.L.; Raymond, K.N. Supermolecules by Design. Acc. Chem. Res. 1999, 32, 975–982. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef]
- Navarro, J.A.R.; Lippert, B. Simple 1:1 and 1:2 complexes of metal ions with heterocycles as building blocks for discrete molecular as well as polymeric assemblies. Coord. Chem. Rev. 2001, 222, 219–250. [Google Scholar] [CrossRef]
- Fujita, M.; Tominaga, M.; Hori, A.; Therrien, B. Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res. 2005, 38, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, J.R. Construction, Substitution, and Sorting of Metallo-organic Structures via Subcomponent Self-Assembly. Acc. Chem. Res. 2006, 40, 103–112. [Google Scholar] [CrossRef]
- Schmidt, A.; Casini, A.; Kühn, F.E. Self-assembled M2L4 coordination cages: Synthesis and potential applications. Coord. Chem. Rev. 2014, 275, 19–36. [Google Scholar] [CrossRef]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined Nanospaces in Metallocages: Guest Molecules, Weakly Encapsulated Anions, and Catalyst Sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef] [PubMed]
- Desmarets, C.; Ducarre, T.; Rager, M.N.; Gontard, G.; Amouri, H. Self-Assembled M2L4 Nanocapsules: Synthesis, Structure and Host-Guest Recognition Toward Square Planar Metal Complexes. Materials 2014, 7, 287–301. [Google Scholar] [CrossRef]
- Desmarets, C.; Gontard, G.; Cooksy, A.L.; Rager, M.N.; Amouri, H. Encapsulation of a Metal Complex within a Self-Assembled Nanocage: Synergy Effects, Molecular Structures, and Density Functional Theory Calculations. Inorg. Chem. 2014, 53, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Bäuerie, P. Macrocycles and complex three-dimensional structures comprising Pt(II) building blocks. Top. Curr. Chem. 2005, 249, 127–201. [Google Scholar]
- Pollock, J.B.; Schneider, G.L.; Cook, T.R.; Davies, A.S.; Stang, P.J. Tunable Visible Light Emission of Self-Assembled Rhomboidal Metallacycles. J. Am. Chem. Soc. 2013, 135, 13676–13679. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.B.; Cook, T.R.; Stang, P.J. Photophysical and Computational Investigations of Bis(phosphine) OrganoPlatinum(II) Metallacycles. J. Am. Chem. Soc. 2012, 134, 10607–10620. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Zhao, G.J.; Cook, T.R.; Han, K.L.; Stang, P.J. Photophysical Properties of Self-Assembled Multinuclear Platinum Metallacycles with Different Conformational Geometries. J. Am. Chem. Soc. 2013, 135, 6694–6702. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Zhao, G.J.; Cook, T.R.; Sun, X.F.; Yang, S.Q.; Zhang, M.X.; Han, K.L.; Stang, P.J. Experimental and Theoretical Study on the Photophysical Properties of 90° and 60° Bimetallic Platinum Complexes. J. Phys. Chem. A 2012, 116, 9911–9918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Yu, F.; Zhang, M.X.; Northrop, B.H.; Yang, H.; Han, K.L.; Stang, P.J. Substituent Effects on the Intramolecular Charge Transfer and Fluorescence of Bimetallic Platinum Complexes. J. Phys. Chem. A 2011, 115, 6390–6393. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Jude, H.; Krause, B.J.A.; Connick, W.B. Luminescent Platinum(II) Dimers with a Cyclometallating Aryldiamine Ligand. Inorg. Chem. 2005, 44, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Jude, H.; Krause, B.J.A.; Connick, W.B. Tuning the Electronic Structures of Platinum(II) Complexes with a Cyclometalating Aryldiamine Ligand. Inorg. Chem. 2004, 43, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Liao, Y.; Yang, G.C.; Su, Z.M.; Zhao, S.S. Effect of π-Conjugated Length of Bridging Ligand on the Optoelectronic Properties of Platinum(II) Dimers. Inorg. Chem. 2008, 47, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Jude, H.; Krause Bauer, J.A.; Connick, W.B. Synthesis, Structures, and Emissive Properties of Platinum(II) Complexes with a Cyclometallating Aryldiamine Ligand. Inorg. Chem. 2002, 41, 2275–2281. [Google Scholar] [PubMed]
- Fuertes, S.; Woodall, C.H.; Raithby, P.R.; Sicilia, V. Heteropolynuclear Pt(II)–M(I) Clusters with a C^N^C Biscyclometalated Ligand. Organometallics 2012, 31, 4228–4240. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, X.; Khoo, S.B.; Hor, T.S.A. Structure-Dependent Electrochemical Behavior of Thienylplatinum(II) Complexes of N,N-Heterocycles. Eur. J. Inorg. Chem. 2004, 1, 69–77. [Google Scholar] [CrossRef]
- Rao, Y.L.; Wang, S. Impact of Constitutional Isomers of (BMes2)phenylpyridine on Structure, Stability, Phosphorescence, and Lewis Acidity of Mononuclear and Dinuclear Pt(II) Complexes. Inorg. Chem. 2009, 48, 7698–7713. [Google Scholar] [CrossRef] [PubMed]
- Meijer, M.D.; de Wolf, E.; Lutz, M.; Spek, A.L.; van Klink, G.P.M.; van Koten, G. C,N-2-[(Dimethylamino)methyl]phenylplatinum Complexes Functionalized with C60 as Macromolecular Building Blocks. Organometallics 2001, 20, 4198–4206. [Google Scholar] [CrossRef]
- Lalinde, E.; Moreno, M.T.; Ruiz, S.; Sánchez, S. Synthesis, Structural and Photophysical Studies of Phenylquinoline and Phenylquinolinyl Alkynyl Based Pt(II) Complexes. Organometallics 2014, 33, 3078–3090. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Díez, A.; Lalinde, E.; Moreno, M.T.; Ruiz, S.; Sánchez, S. Luminescent Cycloplatinated Complexes Containing Poly(pyrazolyl)-borate and -methane Ligands. Organometallics 2011, 30, 5776–5792. [Google Scholar] [CrossRef]
- Dattelbaum, D.M.; Itokazu, M.K.; Murakami, I.N.Y.; Meyer, T.J. Mechanism of Metal-to-Ligand Charge Transfer Sensitization of Olefin trans-to-cis Isomerization in the fac-[ReI(phen)(CO)3(1,2-bpe)]+ Cation. J. Phys. Chem. A 2003, 107, 4092–4095. [Google Scholar] [CrossRef]
- Schanze, K.S.; Lucia, L.A.; Cooper, M.; Walters, K.A.; Ji, H.F.; Sabina, O. Intramolecular Energy Transfer to trans-Stilbene. J. Phys. Chem. A 1998, 102, 5577–5584. [Google Scholar] [CrossRef]
- Saltiel, J.; Marchand, G.R.; Kirkor-Kaminska, E.; Smothers, W.K.; Mueller, W.B.; Charlton, J.L. Nonvertical triplet excitation transfer to cis- and trans-stilbene. J. Am. Chem. Soc. 1984, 106, 3144–3151. [Google Scholar] [CrossRef]
- Coe, B.J.; Harries, J.L.; Harris, J.A.; Brunschwig, B.S.; Coles, S.J.; Light, M.E.; Hursthouse, M.B. Syntheses, spectroscopic and molecular quadratic nonlinear optical properties of dipolar ruthenium(II) complexes of the ligand 1,2-phenylenebis(dimethylarsine). Dalton Trans. 2004. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Cargill, T.A.M.W.; Maher, J.P.; McCleverty, J.A.; Ward, M.D. Di-, Tri-, and Tetranucleating Pyridyl Ligands Which Facilitate Multicenter Magnetic Exchange between Paramagnetic Molybdenum Centers. Inorg. Chem. 1995, 34, 4828–4835. [Google Scholar] [CrossRef]
- Otwinowsky, Z.; Minor, W. Methods Enzymol.; Academic Press: New York, NY, USA, 1997; Volume 276A, pp. 307–326. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. App. Crystallogr. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- SHELX-97; University of Göttingen: Göttingen, Germany, 1997.
- Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 11 October 2014).
- Gaussian 03; Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalinde, E.; Moreno, M.T.; Ruiz, S.; Sánchez, S. Attachment of Luminescent Neutral “Pt(pq)(C≡CtBu)” Units to Di and Tri N-Donor Connecting Ligands: Solution Behavior and Photophysical Properties. Inorganics 2014, 2, 565-590. https://doi.org/10.3390/inorganics2040565
Lalinde E, Moreno MT, Ruiz S, Sánchez S. Attachment of Luminescent Neutral “Pt(pq)(C≡CtBu)” Units to Di and Tri N-Donor Connecting Ligands: Solution Behavior and Photophysical Properties. Inorganics. 2014; 2(4):565-590. https://doi.org/10.3390/inorganics2040565
Chicago/Turabian StyleLalinde, Elena, M. Teresa Moreno, Santiago Ruiz, and Sergio Sánchez. 2014. "Attachment of Luminescent Neutral “Pt(pq)(C≡CtBu)” Units to Di and Tri N-Donor Connecting Ligands: Solution Behavior and Photophysical Properties" Inorganics 2, no. 4: 565-590. https://doi.org/10.3390/inorganics2040565
APA StyleLalinde, E., Moreno, M. T., Ruiz, S., & Sánchez, S. (2014). Attachment of Luminescent Neutral “Pt(pq)(C≡CtBu)” Units to Di and Tri N-Donor Connecting Ligands: Solution Behavior and Photophysical Properties. Inorganics, 2(4), 565-590. https://doi.org/10.3390/inorganics2040565




