Bottom-Up, Wet Chemical Technique for the Continuous Synthesis of Inorganic Nanoparticles
Abstract
:1. Introduction
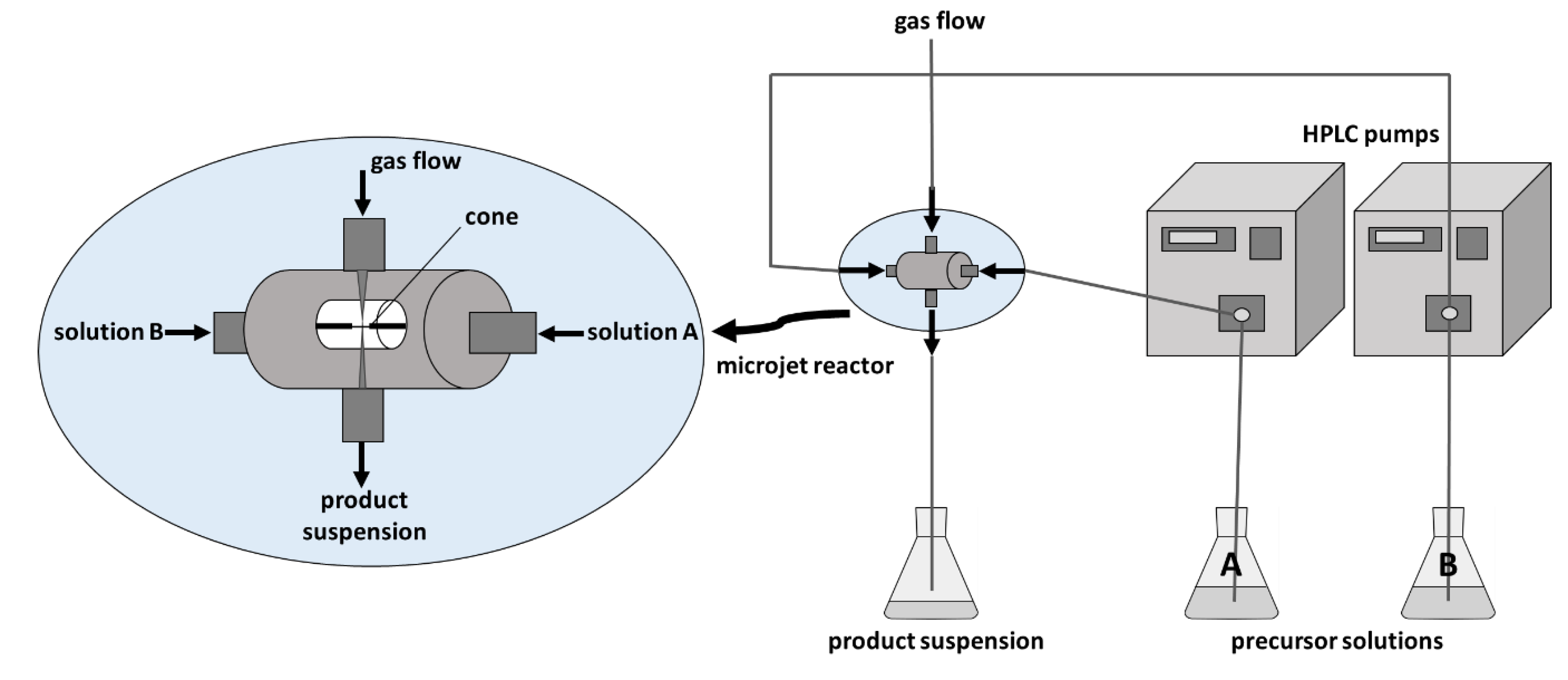
2. Results and Discussion
2.1. Zinc Oxide
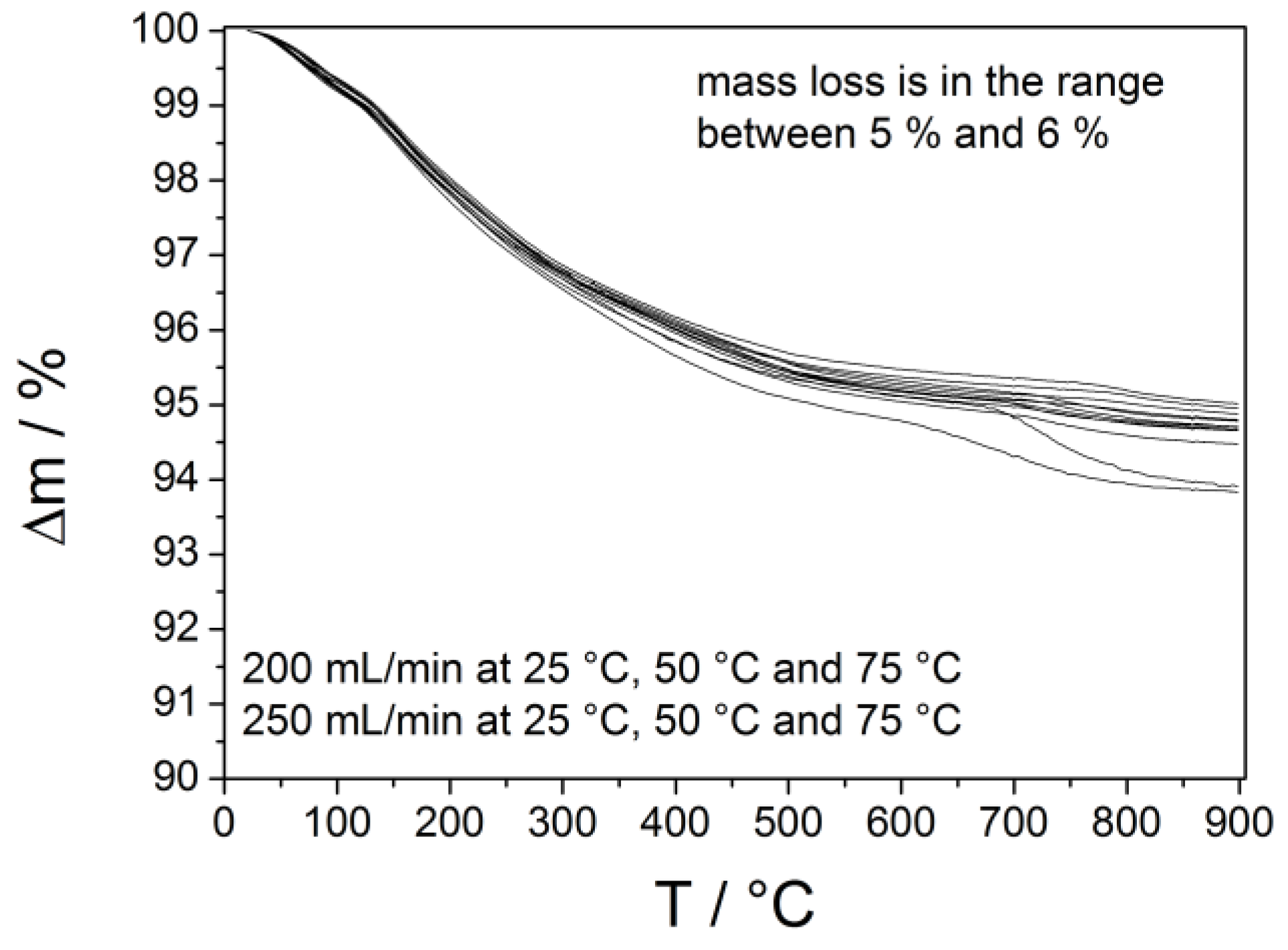
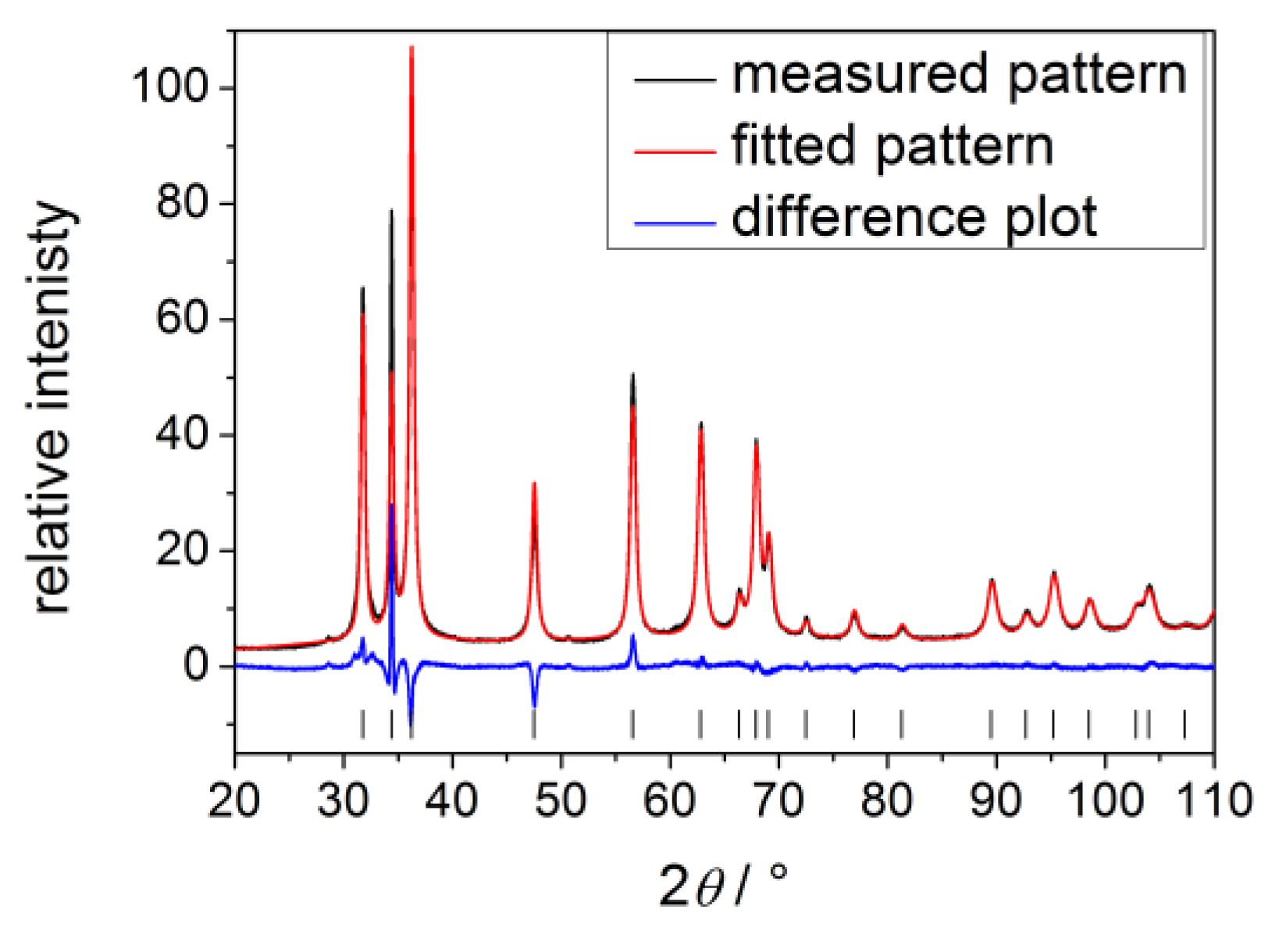
| Process parameters | Crystallite size |
|---|---|
| 200 mL/min/25 °C | 22.2 ± 0.5 |
| 200 mL/min/50 °C | 20.4 ± 0.7 |
| 200 mL/min/75 °C | 16.3 ± 0.3 |
| 250 mL/min/25 °C | 22.7 ± 0.5 |
| 250 mL/min/50 °C | 21.3 ± 0.4 |
| 250 mL/min/75 °C | 16.9 ± 0.3 |
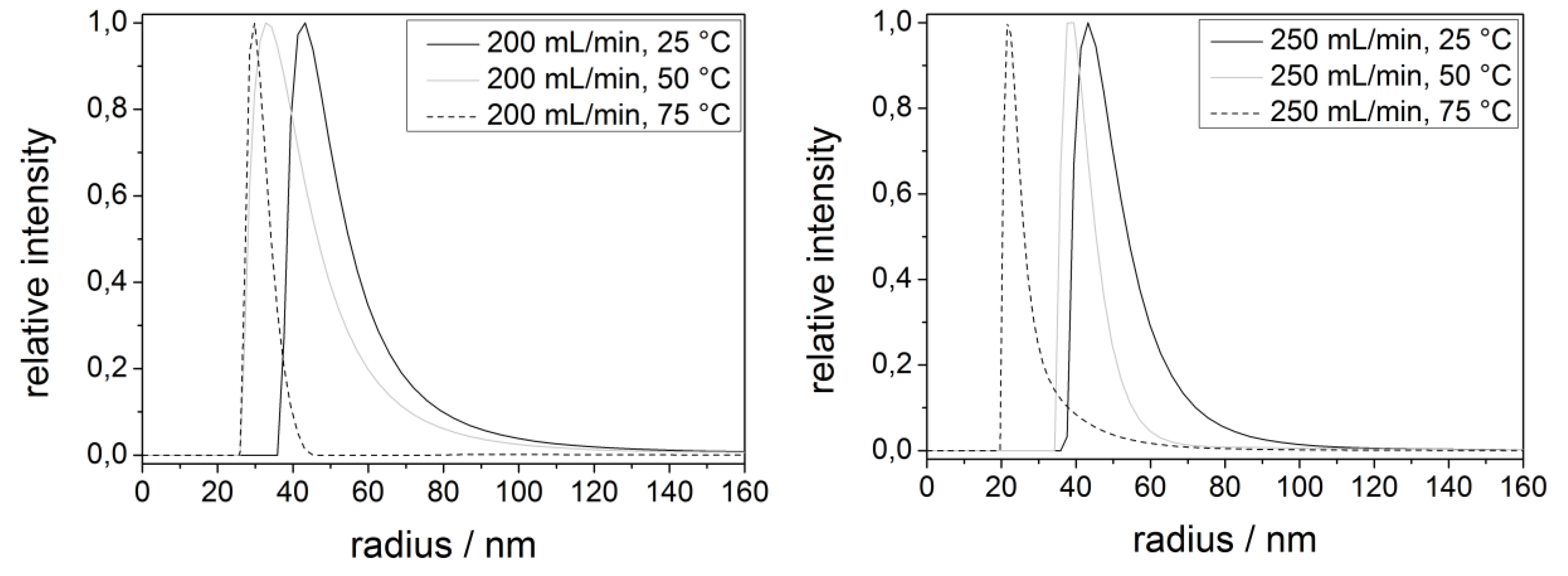
| Process parameters | Radius/nm |
|---|---|
| 200 mL/min/25 °C | 51 ± 7 |
| 200 mL/min/50 °C | 41 ± 8 |
| 200 mL/min/75 °C | 27 ± 3 |
| 250 mL/min/25 °C | 43 ± 6 |
| 250 mL/min/50 °C | 36 ± 4 |
| 250 mL/min/75 °C | 22 ± 2 |
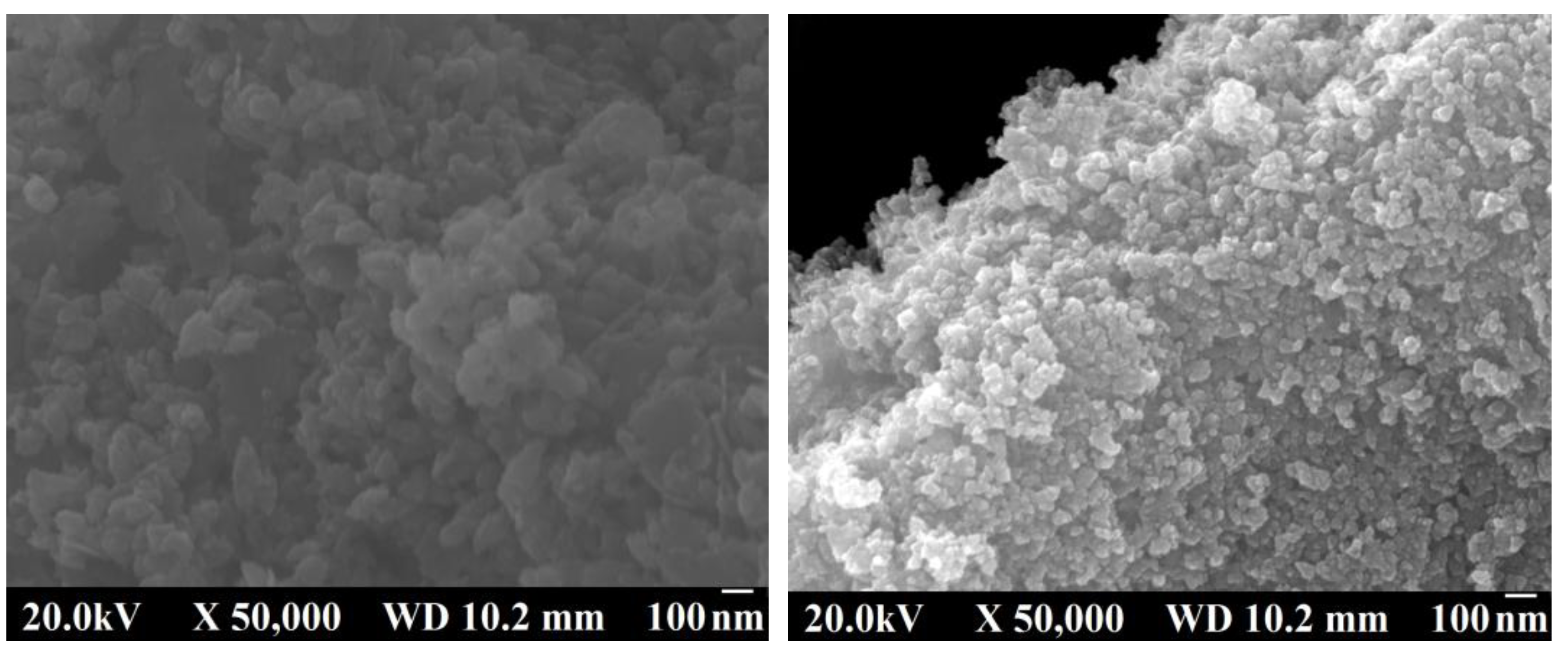
2.2. Magnetite
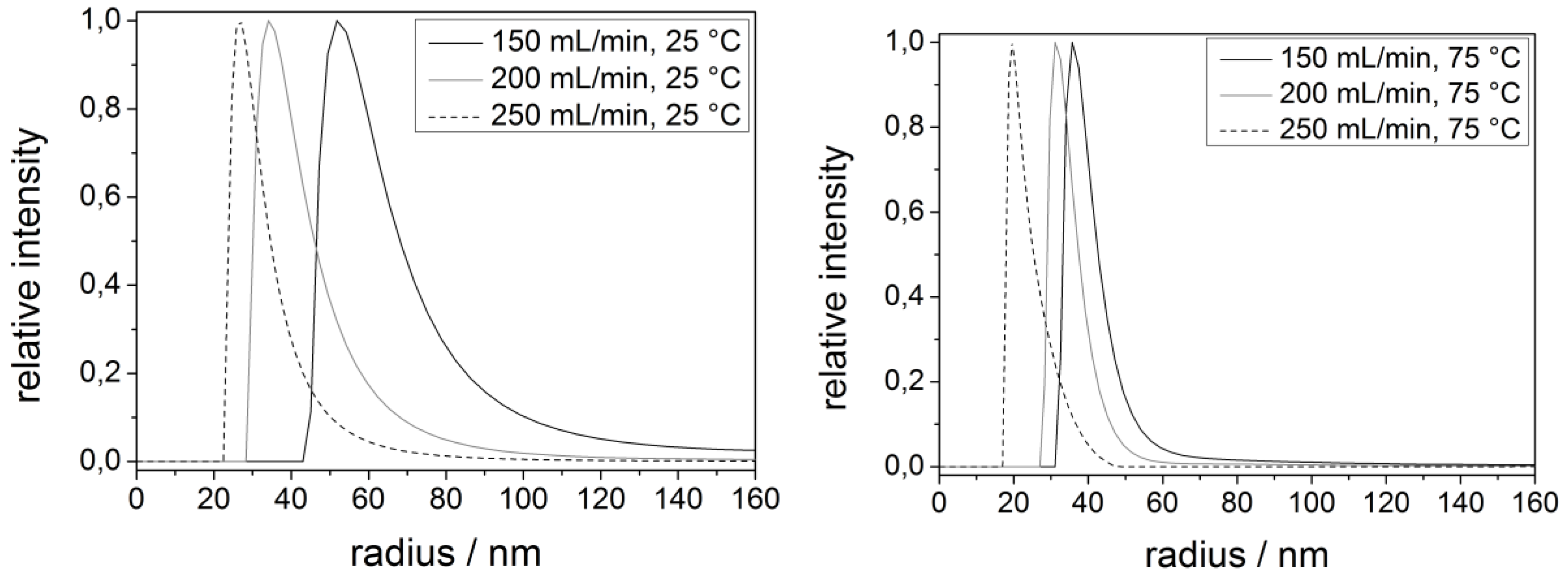
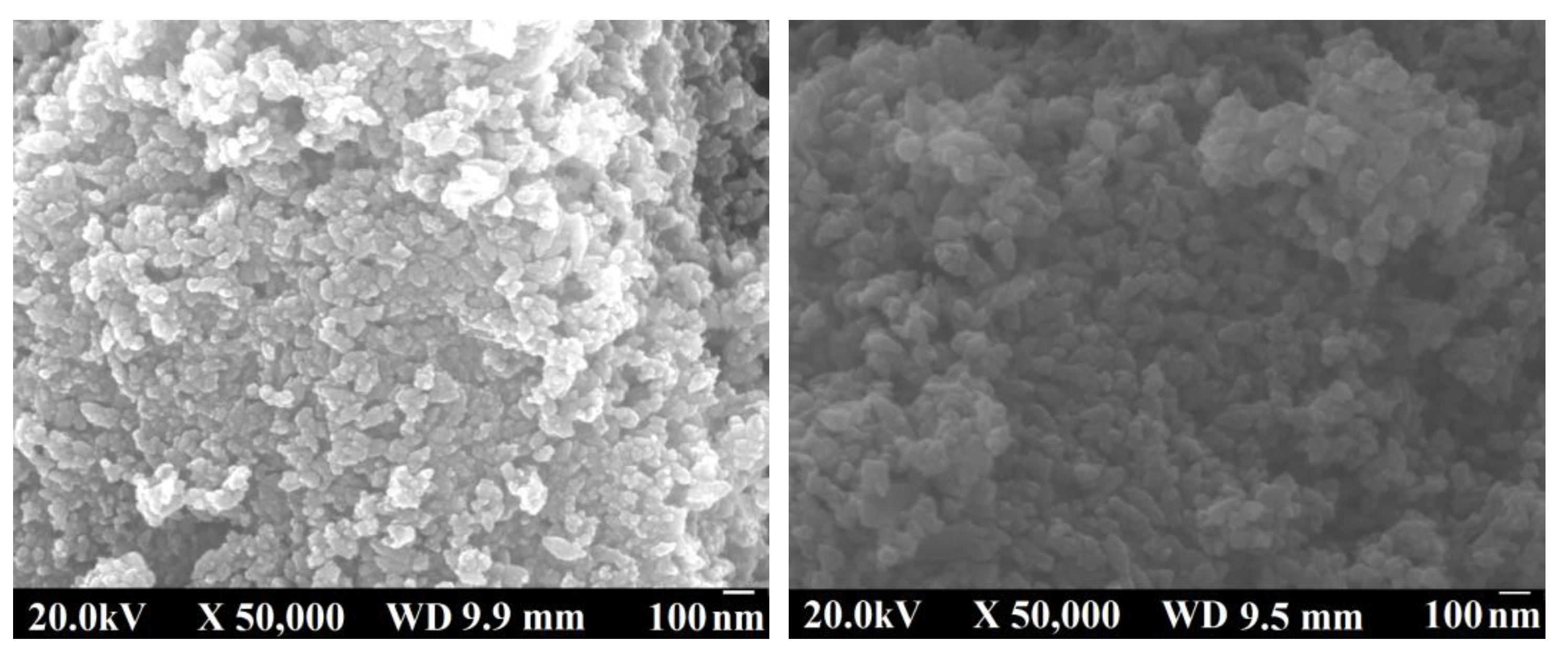
| Process parameters | Radius/nm |
|---|---|
| 150 mL/min/25 °C | 66 ± 9 |
| 200 mL/min/25 °C | 42 ± 7 |
| 250 mL/min/25 °C | 32 ± 5 |
| 150 mL/min/75 °C | 41 ± 4 |
| 200 mL/min/75 °C | 35 ± 4 |
| 250 mL/min/75 °C | 23 ± 3 |

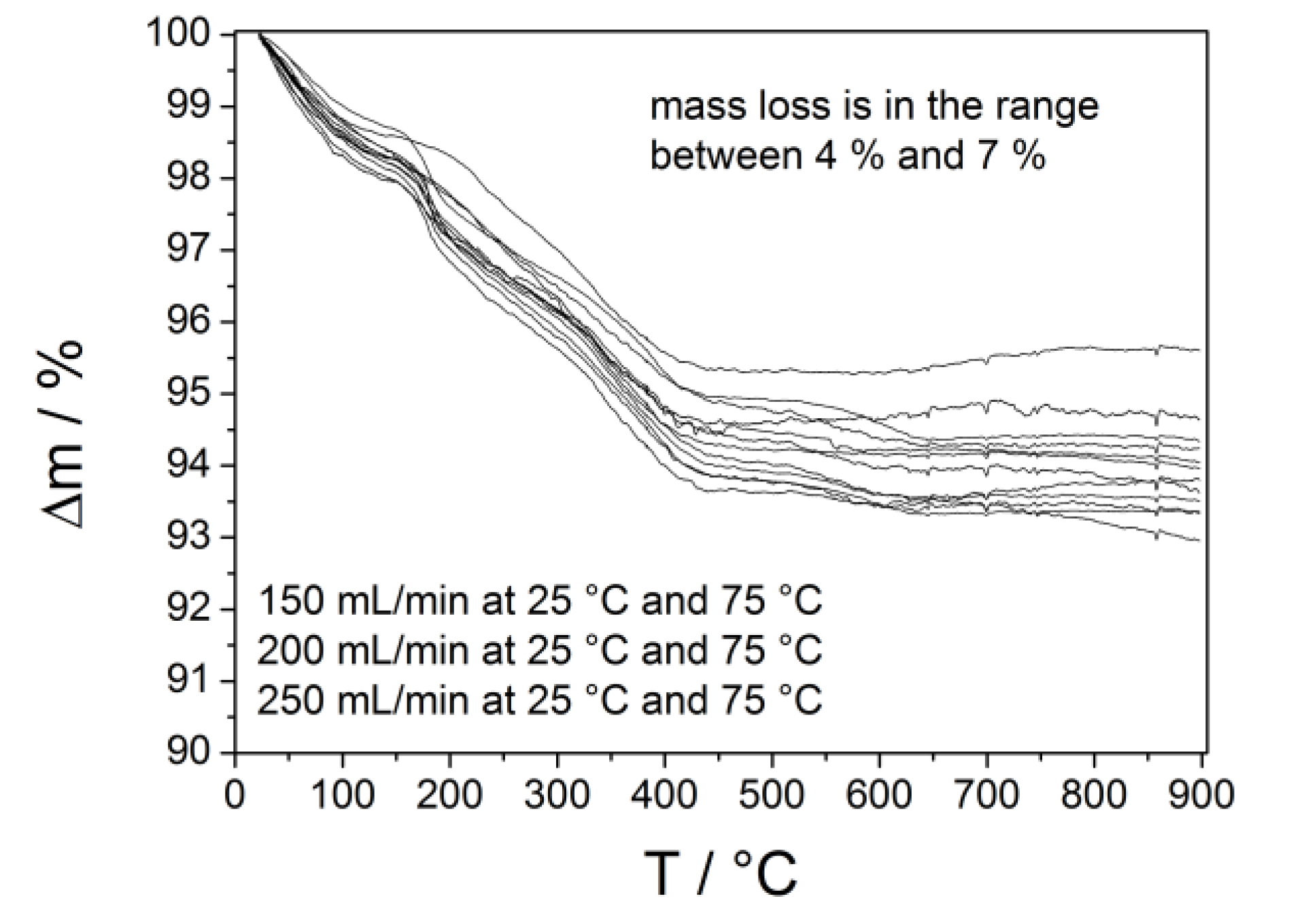
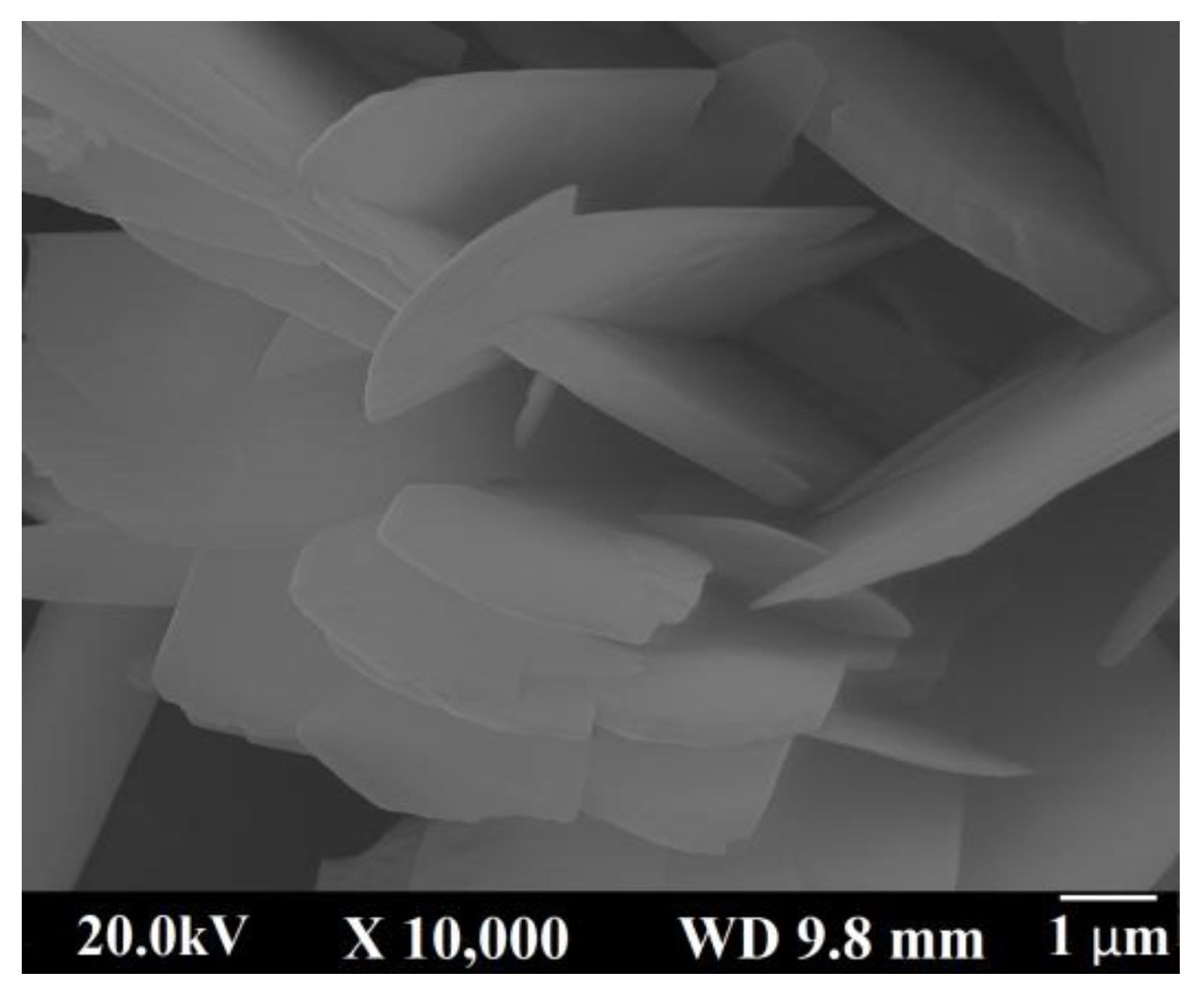
2.3. Calcium Hydrogen Phosphate/Brushite
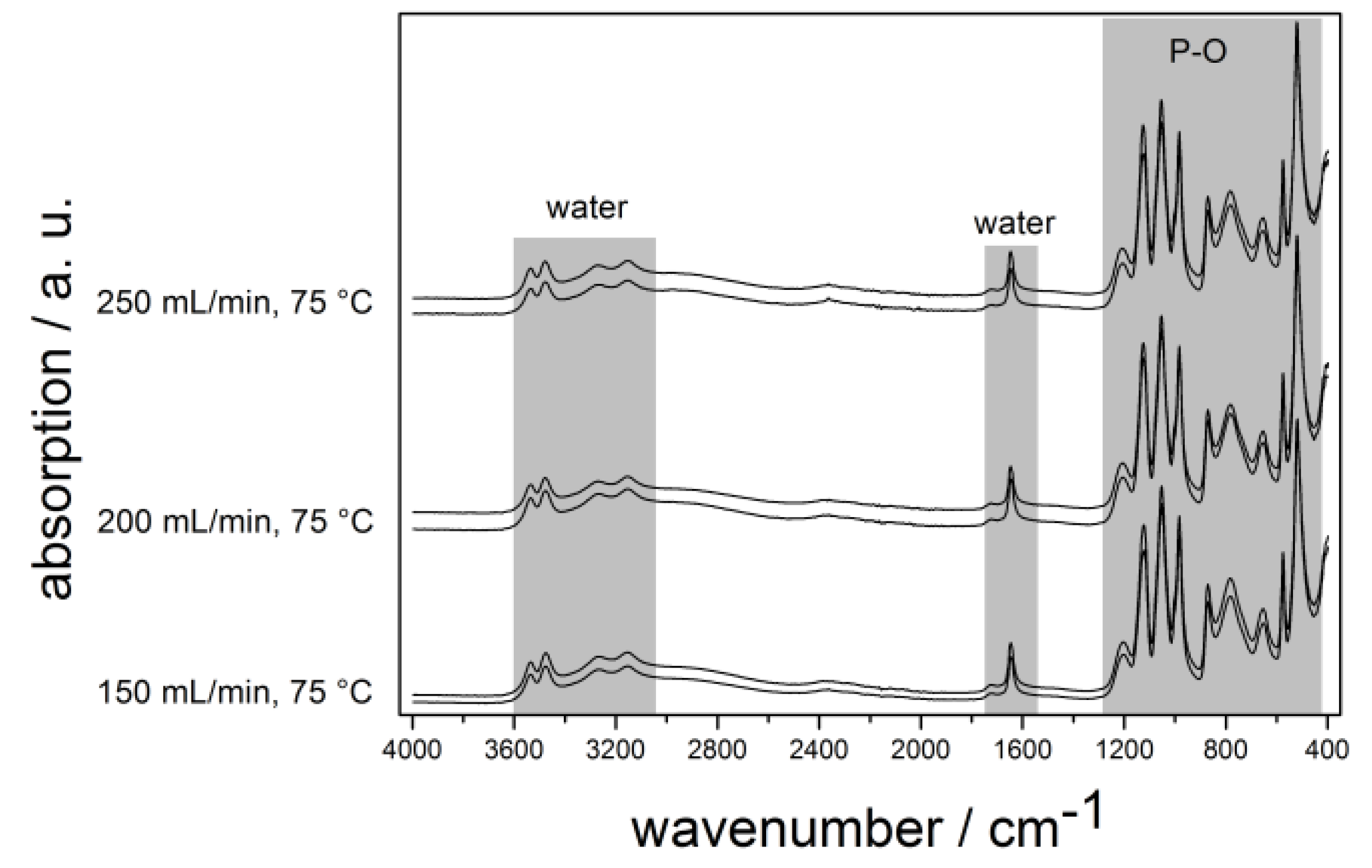
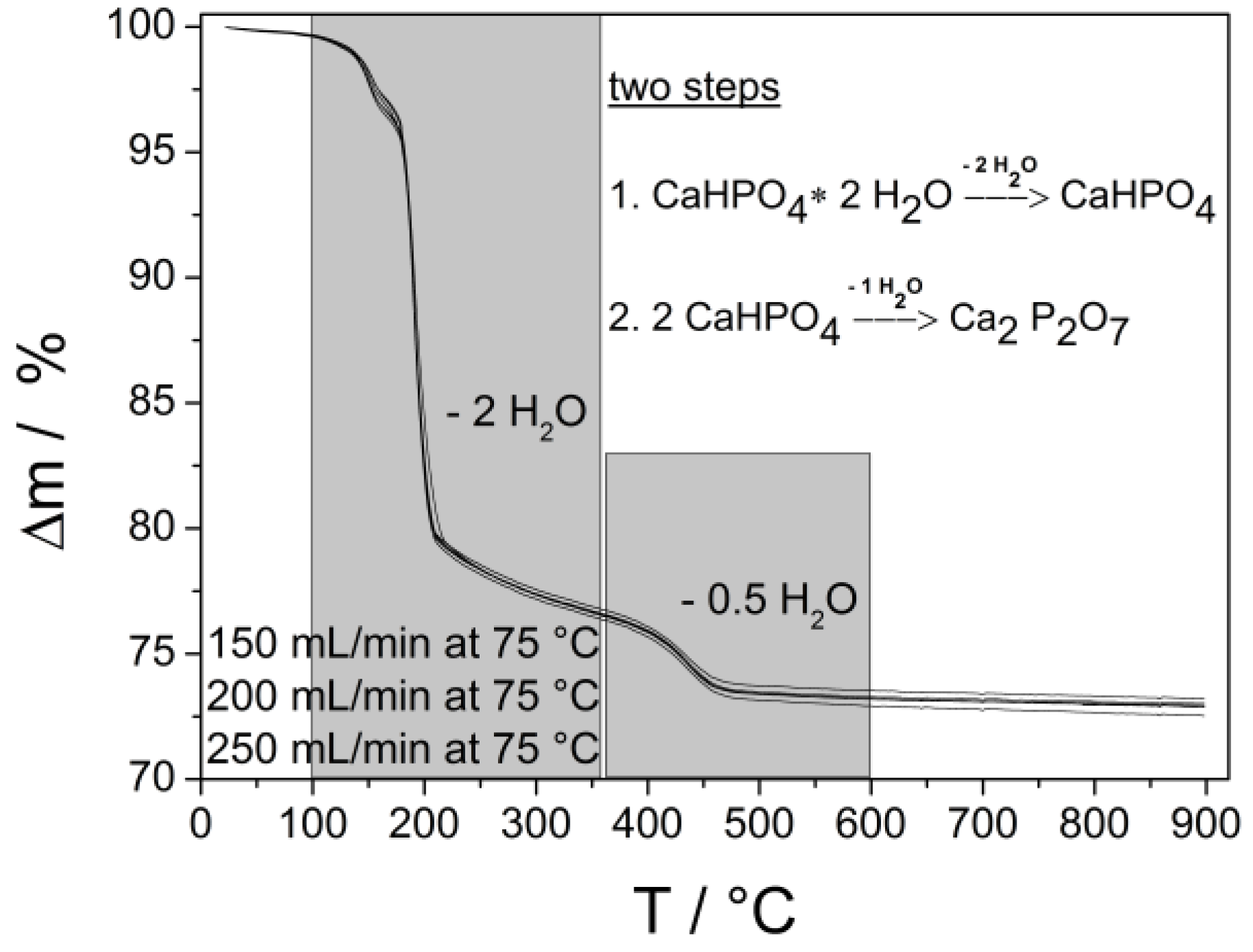
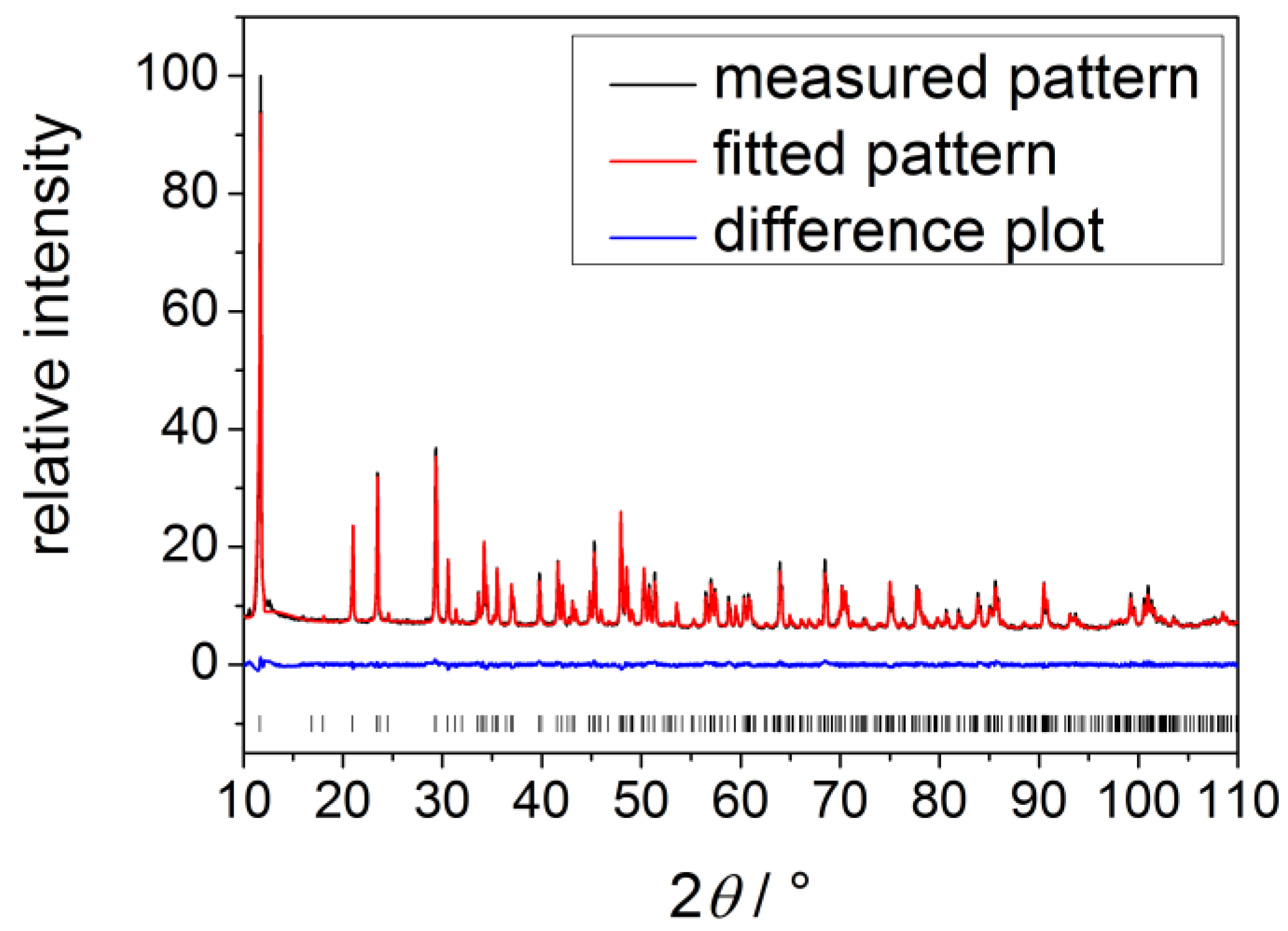
3. Experimental Section
3.1. Materials
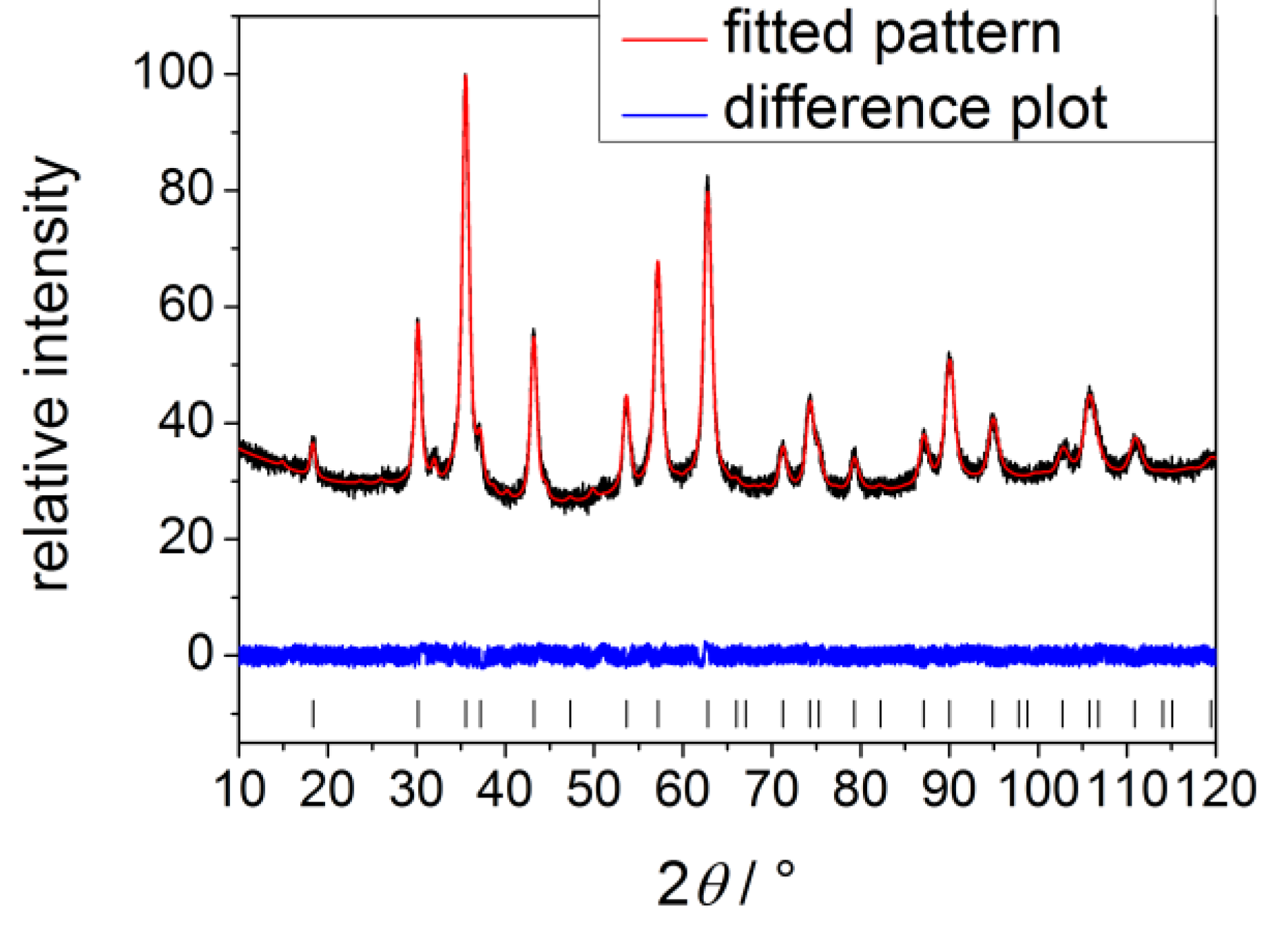
3.2. Instruments and Characterization
3.3. Synthesis
4. Conclusions
Conflicts of Interest
References
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites - A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Castro, F.; Kuhn, S.; Jensen, K.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Process intensification and optimization for hydroxyapatite nanoparticles production. Chem. Eng. Sci. 2013, 100, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; Chen, G.; Zhao, Y.; Li, S.; Yuan, Q. A high throughput methodology for continuous preparation of monodispersed nanocrystals in microfluidic reactors. Chem. Eng. J. 2008, 135, 209–215. [Google Scholar]
- Ström, V.; Olsson, R.T.; Rao, K.V. Real-time monitoring of the evolution of the magnetism during precipitation of superparamagnetic nanoparticles for bioscience application. J. Mater. Chem. 2010, 20, 4168–4175. [Google Scholar] [CrossRef]
- Van den Rul, H.; Mondelaers, D.; van Bael, M.K.; Mullens, J. Water-based wet chemical synthesis of (doped) ZnO nanostructures. J. Sol-Gel Sci. Tech. 2006, 39, 41–47. [Google Scholar] [CrossRef]
- Jones, A.; Rigopoulos, S.; Zauner, R. Crystallisation and precipitation engineering. Comput. Chem. Eng. 2005, 29, 1159–1166. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, C.; Chen, G. Interaction of macro- and micromixing on particle size distribution in reactive precipitation. Chem. Eng. Sci. 1996, 51, 1957–1966. [Google Scholar] [CrossRef]
- Mersmann, A. Crystallization and precipitation. Chem. Eng. Process. 1999, 38, 345–353. [Google Scholar] [CrossRef]
- Luo, G.; Du, L.; Wang, Y.; Lu, Y.; Xu, J. Controllable preparation of particles with microfluidics. Particuology 2011, 9, 545–558. [Google Scholar] [CrossRef]
- Chen, G.; Luo, G.; Yang, X.; Sun, Y.; Wang, J. Anatase-TiO2 nano-particle preparation with a micro-mixing technique and its photocatalytic performance. Mater. Sci. Eng. A 2004, 380, 320–325. [Google Scholar] [CrossRef]
- Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Köhler, J.M. Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J. 2004, 101, 251–260. [Google Scholar]
- Zinoveva, S.; De Silva, R.; Louis, R.D.; Datta, P.; Kumar, C.S.S.R.; Goettert, J.; Hormes, J. The wet chemical synthesis of Co nanoparticles in a microreactor system: A time-resolved investigation by X-ray absorption spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. A 2007, 582, 239–241. [Google Scholar] [CrossRef]
- Du, L.; Wang, Y.J.; Lu, Y.C.; Luo, G.S. Preparation of highly purified β-tricalcium phosphate ceramics with a microdispersion process. Chem. Eng. J. 2013, 221, 55–61. [Google Scholar]
- Schwarzer, H.C.; Schwertfirm, F.; Manhart, M.; Schmid, H.J.; Peukert, W. Predictive simulation of nanoparticle precipitation based on the population balance equation. Chem. Eng. Sci. 2006, 61, 167–181. [Google Scholar] [CrossRef]
- Schwarzer, H.C.; Peukert, W. Experimental Investigation into the Influence of Mixing on Nanoparticles Precipitation. Chem. Eng. Technol. 2002, 6, 657–661. [Google Scholar] [CrossRef]
- Lince, F.; Marchisio, D.L.; Barresi, A.A. A comparative study for nanoparticle production with passive mixers via solvent-displacement: Use of CFD models for optimization and design. Chem. Eng. Process. 2011, 50, 356–368. [Google Scholar] [CrossRef]
- Lince, F.; Marchisio, D.L.; Barresi, A.A. Smart mixers and reactors for the production of pharmaceutical nanoparticles: Proof of concept. Chem. Eng. Res. Des. 2009, 87, 543–549. [Google Scholar] [CrossRef]
- Silva, V.M.T.M.; Quadros, P.A.; Laranjeira, P.E.M.S.C.; Dias, M.M.; Lopes, J.C.B. A novel Continuous Industrial Process for Producing Hydroxyapatite Nanoparticles. J. Dispersion Sci. Technol. 2008, 29, 542–547. [Google Scholar] [CrossRef]
- Penth, B. (K)ein Fall für die Fällung. Chem. Tech. 2004, 3, 18–20. [Google Scholar]
- Penth, B. Kontinuierliche Produktion in Mikroreaktoren. German Patent DE 102006004350 Al, August 2006. [Google Scholar]
- Rüfer, A.; Räuchle, K.; Krahl, F.; Reschetilowski, W. Kontinuierliche Darstellung von Bariumsulfat-Nanopartikeln im MicroJet-Reaktor. Chem. Ing. Tech. 2009, 81, 1949–1954. [Google Scholar]
- Dittert, B.; Gavrilovic, A.; Schwarz, S.; Angerer, P.; Steiner, H.; Schöftner, R. Phase content controlled TiO2 nanoparticles using the MicroJetReactor technology. J. Eur. Ceram. Soc. 2011, 31, 2475–2480. [Google Scholar] [CrossRef]
- Djutisic, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar]
- Sugawara, A.; Asaoka, K.; Ding, S.J. Calcium phosphate-based cements: Clinical needs and recent progress. J. Mater. Chem. B 2013, 1, 1081–1089. [Google Scholar] [CrossRef]
- Vékás, L.; Tombácz, E.; Turcu, R.; Morjan, I.; Avdeev, M.V.; Krasia-Christoforou, T.; Socoliuc, V. Synthesis of Magnetite Nanoparticles and Magnetic Fluids for Biomedical Applications. Nanomedicine-Basic and Clinical Applications in Diagnostics and Therapy. Else Kröner-Fresenius Symp. Basel Karger 2011, 2, 35–52. [Google Scholar] [CrossRef]
- Samanta, P.K.; Mishra, S. Wet chemical growth and optical property of ZnO nanodiscs. Optik 2013, 124, 2871–2873. [Google Scholar] [CrossRef]
- RuizMoreno, R.G.; Martínez, A.I.; Falcony, C.; Castro-Rodriguez, R.; Bartolo-Pérez, P.; Castro-Román, M. One pot synthesis of water compatible and monodisperse magnetite nanoparticles. Mater. Lett. 2013, 92, 181–183. [Google Scholar] [CrossRef]
- Fang, M.; Ström, V.; Olsson, R.T.; Belova, L.; Rao, K.V. Particle size and magnetic properties dependence on growth temperature for rapid mixed co-precipitated magnetite nanoparticles. Nanotechnology 2012, 23, 145601. [Google Scholar] [CrossRef]
- Arifuzzaman, S.M.; Rohani, S. Experimental study of brushite precipitation. J. Cryst. Growth 2004, 267, 624–634. [Google Scholar] [CrossRef]
- Petrov, I.; Soptrajanov, B.; Fuson, N.; Lawson, J.R. Infra-red investigation of dicalcium phosphates. Spectrochim. Acta 1967, 23A, 2637–2646. [Google Scholar]
- Trpkovska, M.; Soptrajanov, B.; Malkov, P. FTIR reinvestigation of the spectra of synthetic brushite and its partially deuterated analogues. J. Mol. Struct. 1999, 480–481, 661–666. [Google Scholar] [CrossRef]
- Topas, V4.2; General profile and structure analysis software for powder diffraction data, User Manual; Bruker AXS: Karlsruhe, Germany, 2008.
- Khan, A.A. X-ray determination of thermal expansion of zinc oxide. Acta Cryst. A 1968, 24, 403. [Google Scholar] [CrossRef]
- Okudera, H.; Kihara, K.; Matsumoto, T. Temperature Dependence of Structure Parameters in Natural Magnetite: Single Crystal X-ray Studies from 126 to 773 K. Acta Cryst. B 1996, 52, 450–457. [Google Scholar] [CrossRef]
- Beevers, C.A. The Crystal Structure of Dicalcium Phosphate Dihydrate, CaHPO4·2H2O. Acta Cryst. 1958, 11, 273–277. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Betke, A.; Kickelbick, G. Bottom-Up, Wet Chemical Technique for the Continuous Synthesis of Inorganic Nanoparticles. Inorganics 2014, 2, 1-15. https://doi.org/10.3390/inorganics2010001
Betke A, Kickelbick G. Bottom-Up, Wet Chemical Technique for the Continuous Synthesis of Inorganic Nanoparticles. Inorganics. 2014; 2(1):1-15. https://doi.org/10.3390/inorganics2010001
Chicago/Turabian StyleBetke, Annika, and Guido Kickelbick. 2014. "Bottom-Up, Wet Chemical Technique for the Continuous Synthesis of Inorganic Nanoparticles" Inorganics 2, no. 1: 1-15. https://doi.org/10.3390/inorganics2010001
APA StyleBetke, A., & Kickelbick, G. (2014). Bottom-Up, Wet Chemical Technique for the Continuous Synthesis of Inorganic Nanoparticles. Inorganics, 2(1), 1-15. https://doi.org/10.3390/inorganics2010001





