Mg-Doped O3-Na[Ni0.6Fe0.25Mn0.15]O2 Cathode for Long-Cycle-Life Na-Ion Batteries
Abstract
1. Introduction
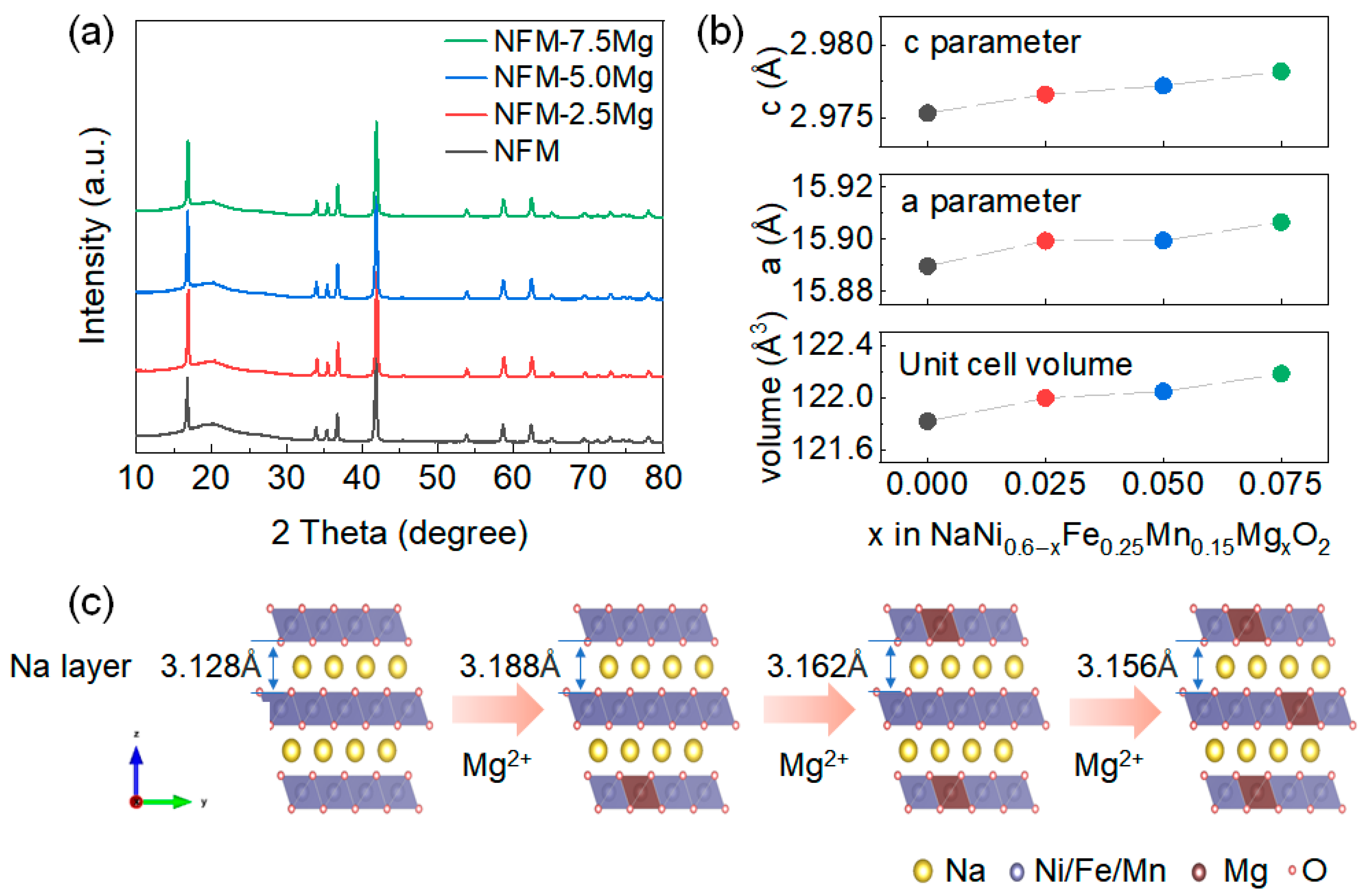
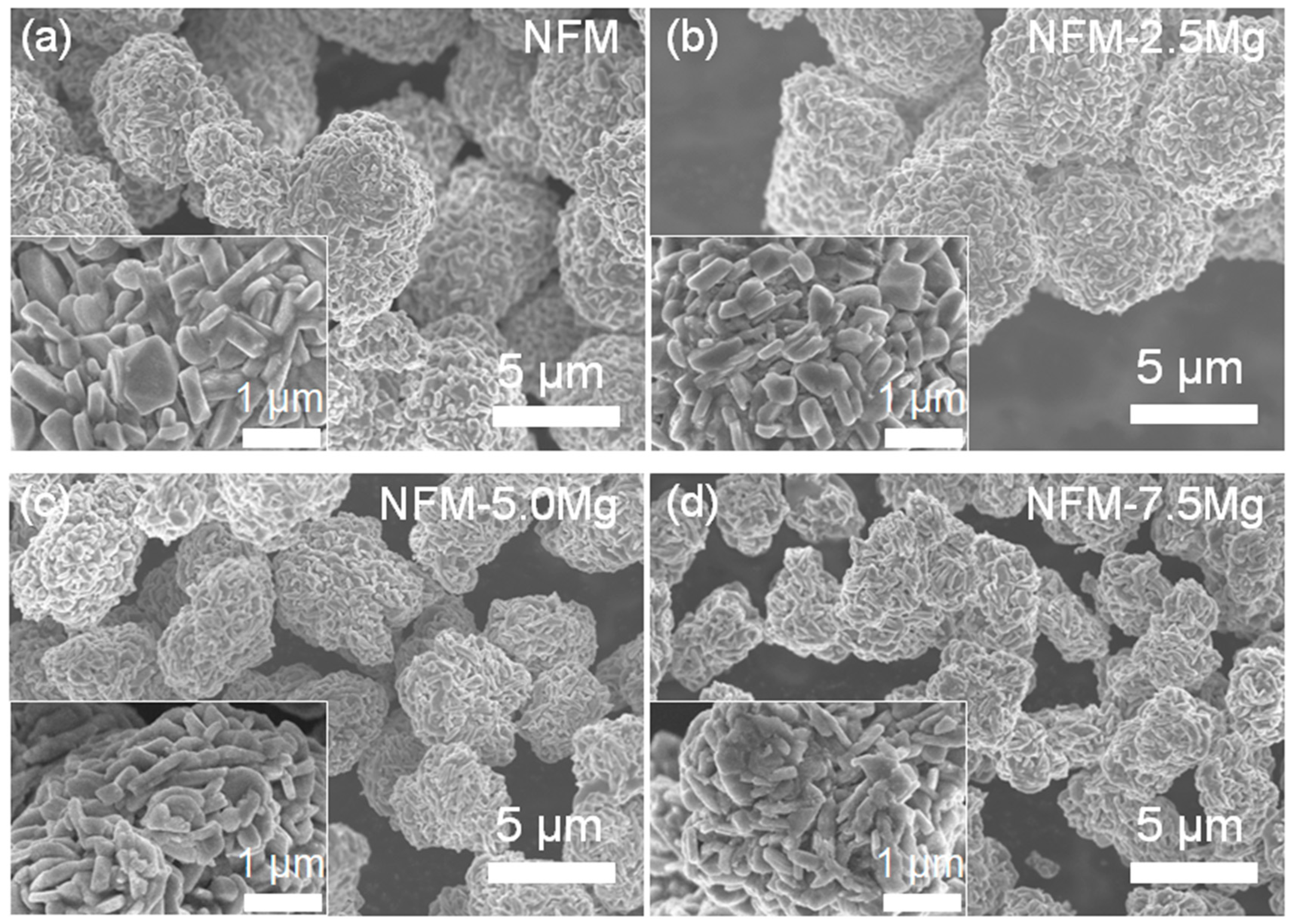
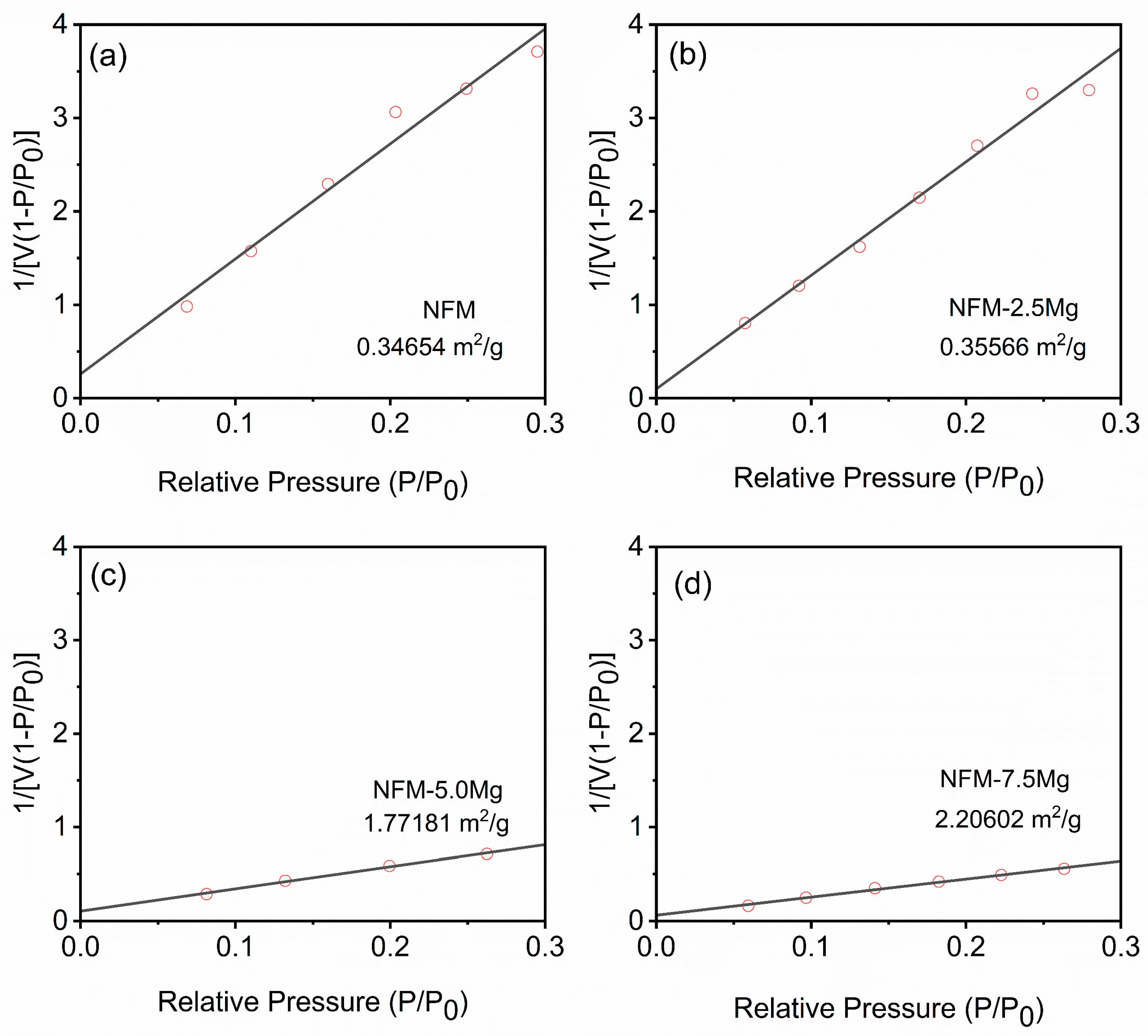
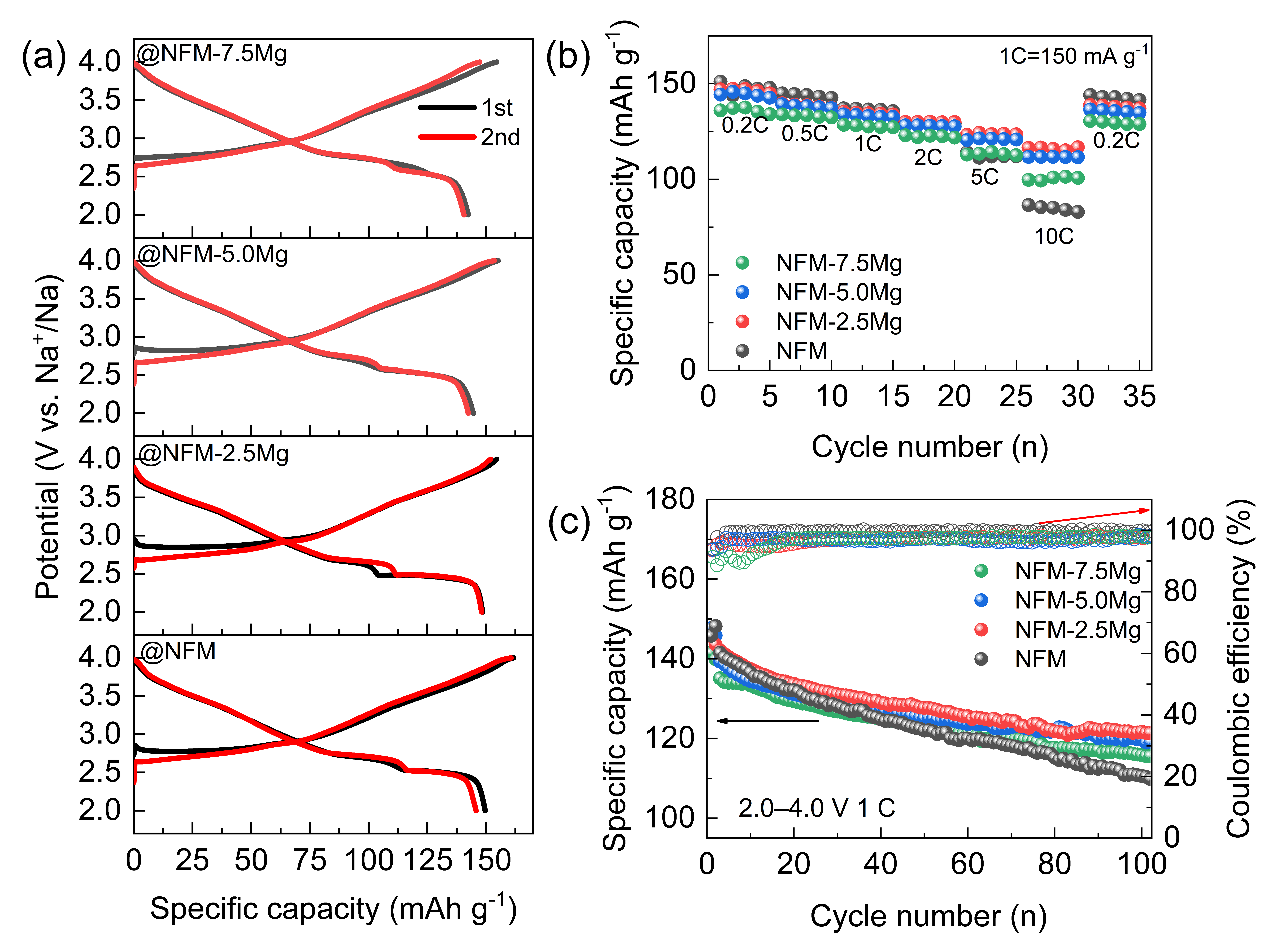
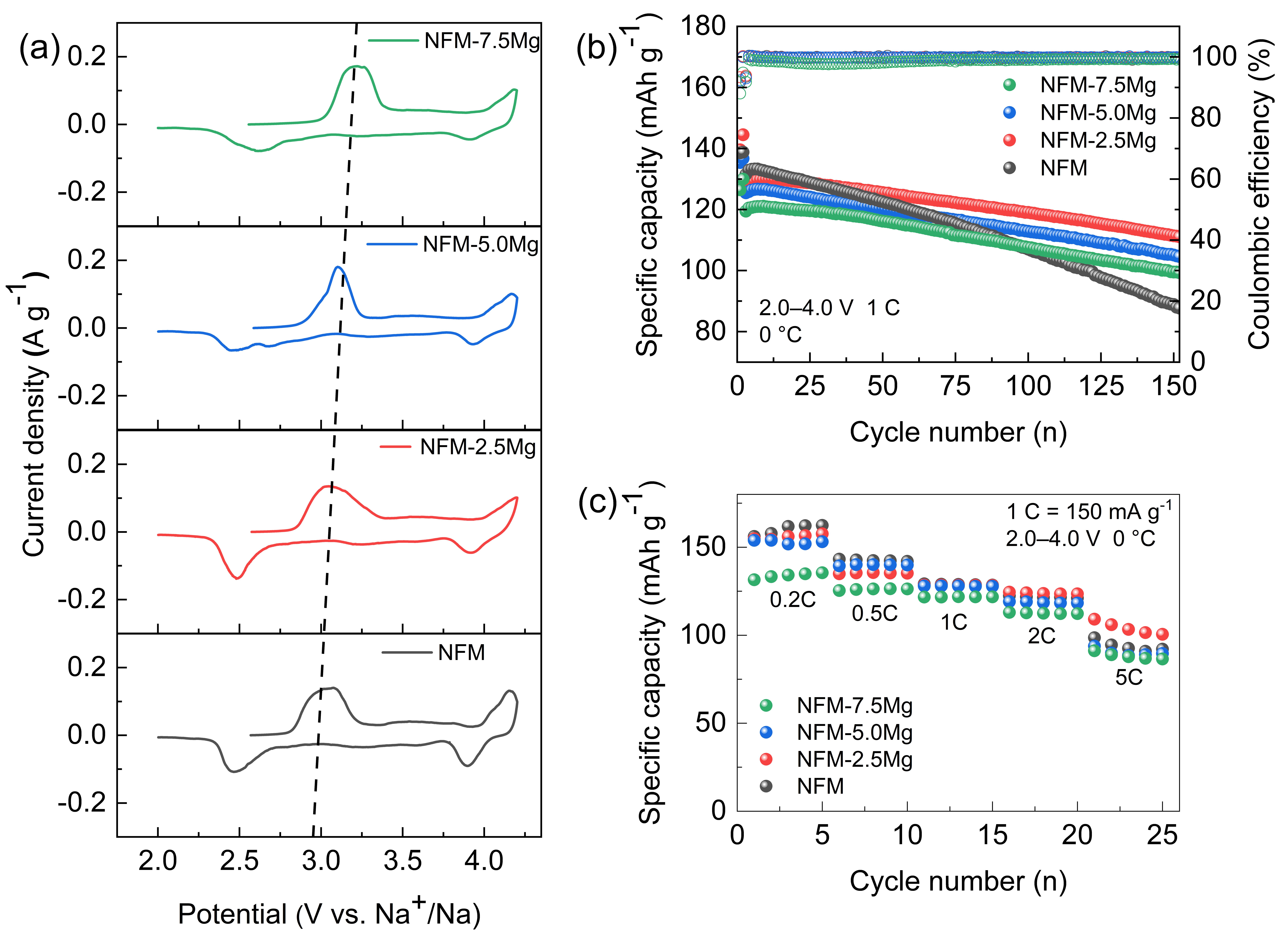
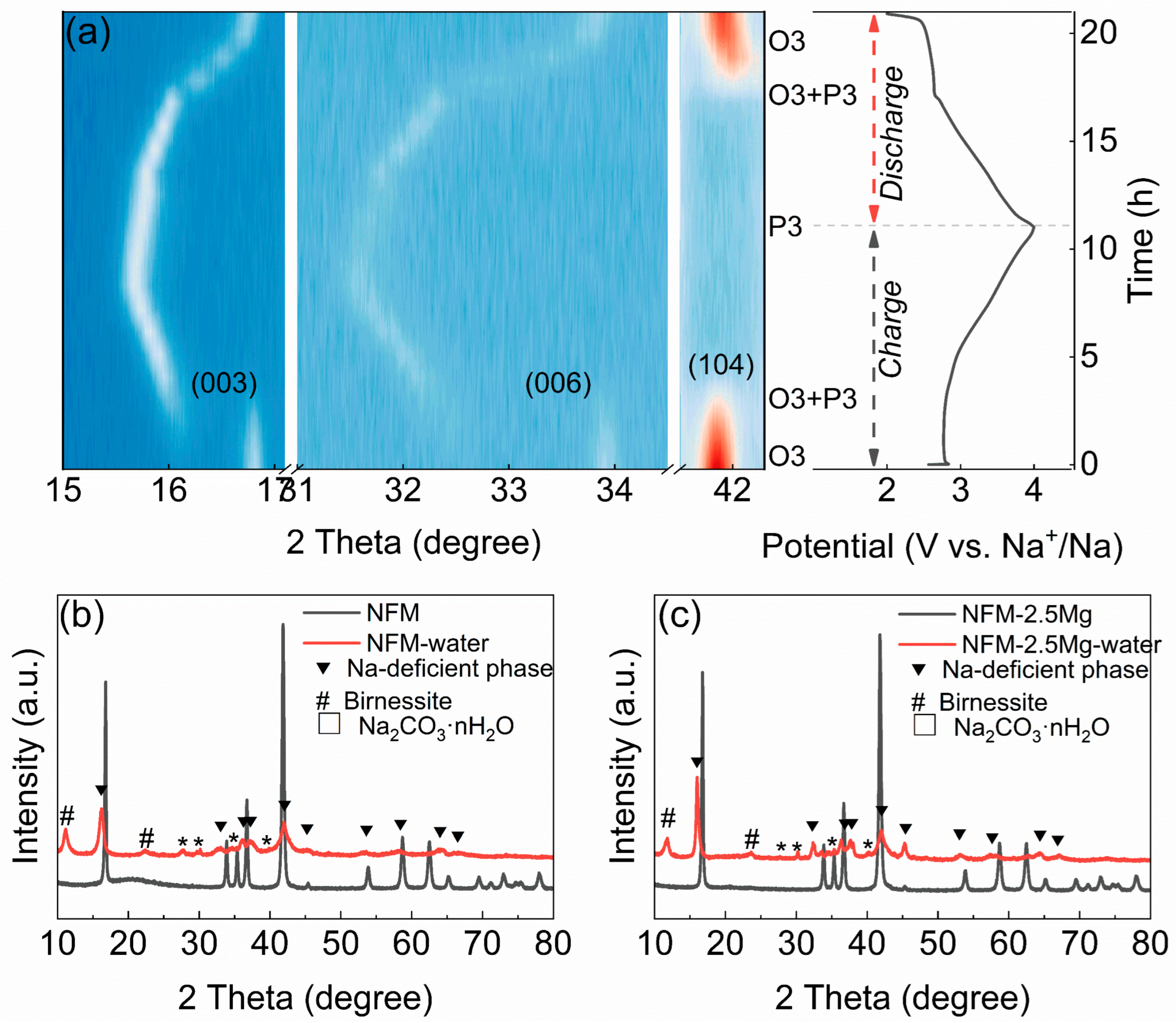
2. Results and Discussions
3. Experimental Section
3.1. Material Preparation
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Wu, Z.; Wang, H.; Niu, X.; Li, H.; Wang, L. Chelating-Type Binders toward Stable Cycling and High-Safety Transition-Metal Sulfide-Based Lithium Batteries. ACS Energy Lett. 2024, 9, 4666–4672. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, G.; Li, S.; Liu, H.; Wang, L.; Li, H. Unlocking cycling longevity in micro-sized conversion-type FeS2 cathodes. Joule 2023, 7, 2609–2621. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Belal, M.A.; Khalil, H.H.; Mahajan, R.L.; Rashed, A.E.; Khattab, S.N.; El-Moneim, A.A. Layered structure design of inkjet-printed graphene/Co3O4 for high-performance flexible microsupercapacitors. J. Energy Storage 2024, 101, 113900. [Google Scholar] [CrossRef]
- Wu, F.; Dong, J.; Chen, L.; Chen, G.; Shi, Q.; Nie, Y.; Lu, Y.; Bao, L.; Li, N.; Song, T. Removing the intrinsic NiO phase and residual lithium for high-performance nickel-rich materials. Energy Mater. Adv. 2023, 4, 0007. [Google Scholar] [CrossRef]
- Yan, D.; Xiao, S.; Li, X.; Jiang, J.; He, Q.; Li, H.; Qin, J.; Wu, R.; Niu, X.; Chen, J.S. NiS2/FeS heterostructured nanoflowers for high-performance sodium storage. Energy Mater. Adv. 2023, 4, 0012. [Google Scholar] [CrossRef]
- Kaja, K.R.; Hajra, S.; Panda, S.; Belal, M.A.; Pharino, U.; Khanbareh, H.; Vittayakorn, N.; Vivekananthan, V.; Bowen, C.; Kim, H.J. Exploring liquid-solid interface based triboelectrification, structures, and applications. Nano Energy 2024, 131, 110319. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xia, X.; Ma, H.; Xu, S.; Yao, Y.; Rui, X.; Yu, Y. Sodiophilic V2O3-Inducing Layer for Long Lifespan and Dendrite-Free Sodium Metal Anodes. Energy Mater. Adv. 2023, 4, 0063. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Qian, J.; Gong, Y.; Li, H.; Wu, C.; Bai, Y.; Wu, F. Construct NiSe/NiO heterostructures on NiSe anode to induce fast kinetics for sodium-ion batteries. Energy Mater. Adv. 2023, 4, 0044. [Google Scholar] [CrossRef]
- Berthelot, R.; Carlier, D.; Delmas, C. Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nat. Mater. 2011, 10, 74–80. [Google Scholar] [CrossRef]
- Guignard, M.; Didier, C.; Darriet, J.; Bordet, P.; Elkaïm, E.; Delmas, C. P2-NaxVO2 system as electrodes for batteries and electron-correlated materials. Nat. Mater. 2013, 12, 74–80. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Ceder, G. Electrochemical properties of monoclinic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307. [Google Scholar] [CrossRef]
- Yuan, D.; Liang, X.; Wu, L.; Cao, Y.; Ai, X.; Feng, J.; Yang, H. A honeycomb-layered Na3Ni2SbO6: A high-rate and cycle-stable cathode for sodium-ion batteries. Adv. Mater. 2014, 26, 6301–6306. [Google Scholar] [CrossRef]
- Hasa, I.; Buchholz, D.; Passerini, S.; Hassoun, J. A comparative study of layered transition metal oxide cathodes for application in sodium-ion battery. ACS Appl. Mater. Interfaces 2015, 7, 5206–5212. [Google Scholar] [CrossRef]
- Wu, X.; Deng, W.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Single-crystal FeFe(CN)6 nanoparticles: A high capacity and high rate cathode for Na-ion batteries. J. Mater. Chem. A 2013, 1, 10130–10134. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A superior low-cost cathode for a Na-ion battery. Angew. Chem. 2013, 125, 2018–2021. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian blue analogues for sodium-ion batteries: Past, present, and future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, H.; Chung, J.; Lee, J.H.; Kim, B.G.; Choi, J.-J.; Chung, K.Y.; Cho, W.; Kim, S.-J.; Goddard III, W.A. Role of intermediate phase for stable cycling of Na7V4(P2O7)4PO4 in sodium ion battery. Proc. Natl. Acad. Sci. USA 2014, 111, 599–604. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, C.; Wang, Z.; Zhang, Q.; Lv, P.; Yu, K.; Zheng, J.; Wei, W. High-energy Na4MnCr(PO4)3@ C cathode for solid-state sodium metal batteries. Energy Mater. Adv. 2023, 4, 0036. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, X.; Zhou, Q.; Li, K.; Ding, F.; Lu, Y.; Zhao, J.; Chen, L.; Hu, Y.-S. Large scale one-pot synthesis of monodispersed Na3(VOPO4)2F cathode for Na-ion batteries. Energy Mater. Adv. 2022, 2022, 9828020. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Komaba, S.; Yabuuchi, N.; Nakayama, T.; Ogata, A.; Ishikawa, T.; Nakai, I. Study on the reversible electrode reaction of Na1–xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery. Inorg. Chem. 2012, 51, 6211–6220. [Google Scholar] [CrossRef]
- Yoda, Y.; Kubota, K.; Kuroki, K.; Suzuki, S.; Yamanaka, K.; Yaji, T.; Amagasa, S.; Yamada, Y.; Ohta, T.; Komaba, S. Elucidating Influence of Mg-and Cu-Doping on Electrochemical Properties of O3-Nax[Fe, Mn]O2 for Na-Ion Batteries. Small 2020, 16, 2006483. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yano, M.; Yoshida, H.; Kuze, S.; Komaba, S. Synthesis and electrode performance of O3-type NaFeO2-NaNi1/2Mn1/2O2 solid solution for rechargeable sodium batteries. J. Electrochem. Soc. 2013, 160, A3131. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, H.; Yoon, J.; Yoon, W.-S. O3-type NaNi1/3Fe1/3Mn1/3O2 layered cathode for Na-ion batteries: Structural evolution and redox mechanism upon Na (de) intercalation. J. Power Sources 2019, 439, 227064. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, D.; Hu, Z.; Park, M.; Noh, G.; Yang, Y.; Zhang, J.; Lau, V.W.-h.; Chou, S.-L.; Cho, M. Manganese based layered oxides with modulated electronic and thermodynamic properties for sodium ion batteries. Nat. Commun. 2019, 10, 5203. [Google Scholar] [CrossRef]
- Liu, H.; Hong, N.; Bugday, N.; Yasar, S.; Altin, S.; Deng, W.; Deng, W.; Zou, G.; Hou, H.; Long, Z. High Voltage Ga-Doped P2-Type Na2/3Ni0.2Mn0.8O2 Cathode for Sodium-Ion Batteries. Small 2024, 20, 2307225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Liu, Z.; Li, H.; Huang, Y.; Liu, W.; Ruan, D.; Cai, X.; Yu, X. Dual-strategy of Cu-doping and O3 biphasic structure enables Fe/Mn-based layered oxide for high-performance sodium-ion batteries cathode. J. Power Sources 2023, 567, 232930. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, R.; Zheng, L.; Hao, Y.; Liu, X. New insights into the roles of Mg in improving the rate capability and cycling stability of O3-NaMn0.48Ni0.2Fe0.3Mg0.02O2 for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 10819–10827. [Google Scholar] [CrossRef]
- Hou, Y.; Jin, J.; Huo, C.; Liu, Y.; Deng, S.; Chen, J. New insights into the critical role of inactive element substitution in improving the rate performance of sodium oxide cathode material. Energy Storage Mater. 2023, 56, 87–95. [Google Scholar] [CrossRef]
- Yuan, T.; Li, S.; Sun, Y.; Wang, J.-H.; Chen, A.-J.; Zheng, Q.; Zhang, Y.; Chen, L.; Nam, G.; Che, H.; et al. A High-Rate, Durable Cathode for Sodium-Ion Batteries: Sb-Doped O3-Type Ni/Mn-Based Layered Oxides. ACS Nano 2022, 16, 18058–18070. [Google Scholar] [CrossRef]
- Ding, F.; Zhao, C.; Zhou, D.; Meng, Q.; Xiao, D.; Zhang, Q.; Niu, Y.; Li, Y.; Rong, X.; Lu, Y.; et al. A Novel Ni-rich O3-Na[Ni0.60Fe0.25Mn0.15]O2 Cathode for Na-ion Batteries. Energy Storage Mater. 2020, 30, 420–430. [Google Scholar] [CrossRef]
- Wang, P.-F.; You, Y.; Yin, Y.-X.; Guo, Y.-G. An O3-type NaNi0.5Mn0.5O2 cathode for sodium-ion batteries with improved rate performance and cycling stability. J. Mater. Chem. A 2016, 4, 17660–17664. [Google Scholar] [CrossRef]
- Yu, T.Y.; Ryu, H.H.; Han, G.; Sun, Y.K. Understanding the capacity fading mechanisms of O3-type Na[Ni0.5Mn0.5]O2 cathode for sodium-ion batteries. Adv. Energy Mater. 2020, 10, 2001609. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Yu, T.-Y.; Sun, Y.-K. Simultaneous MgO coating and Mg doping of Na[Ni0.5Mn0.5]O2 cathode: Facile and customizable approach to high-voltage sodium-ion batteries. J. Mater. Chem. A 2018, 6, 16854–16862. [Google Scholar] [CrossRef]
- Wang, Q.-C.; Meng, J.-K.; Yue, X.-Y.; Qiu, Q.-Q.; Song, Y.; Wu, X.-J.; Fu, Z.-W.; Xia, Y.-Y.; Shadike, Z.; Wu, J. Tuning P2-structured cathode material by Na-site Mg substitution for Na-ion batteries. J. Am. Chem. Soc. 2018, 141, 840–848. [Google Scholar] [CrossRef]
- Ding, F.; Zhao, C.; Xiao, D.; Rong, X.; Wang, H.; Li, Y.; Yang, Y.; Lu, Y.; Hu, Y.-S. Using high-entropy configuration strategy to design Na-ion layered oxide cathodes with superior electrochemical performance and thermal stability. J. Am. Chem. Soc. 2022, 144, 8286–8295. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Lu, Y.; Jiang, J.; Zhou, A.; Xu, B.; Xu, X.; Tu, J.; Pan, B.; Zhao, X.; et al. Enhanced cyclability of O3-NaNi0.33Fe0.31Mn0.36O2 by Mg and Cu synergistic doping with strengthened Mn-O bonds. J. Energy Storage 2024, 102, 114046. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Zhou, L.; Cheng, Q.; Jiang, H. Trace Ti/Mg co-doped O3-type layered oxide cathodes with enhanced kinetics and stability for sodium-ion batteries. Appl. Surf. Sci. 2024, 649, 159124. [Google Scholar] [CrossRef]
- Lei, C.; Liu, Y.; Tang, W.; Li, Y.; Cheng, Y.; Huo, G.; He, Z. Zr-doped O3-type NaNi1/3Fe1/3Mn1/3O2 cathodes with enhanced structure stability for sodium-ion batteries. J. Electroanal. Chem. 2024, 970, 118557. [Google Scholar] [CrossRef]
- Yao, H.-R.; Wang, P.-F.; Gong, Y.; Zhang, J.; Yu, X.; Gu, L.; OuYang, C.; Yin, Y.-X.; Hu, E.; Yang, X.-Q.; et al. Designing Air-Stable O3-Type Cathode Materials by Combined Structure Modulation for Na-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 8440–8443. [Google Scholar] [CrossRef]
- Zuo, W.; Yang, Y. Synthesis, structure, electrochemical mechanisms, and atmospheric stability of Mn-based layered oxide cathodes for sodium ion batteries. Acc. Mater. Res. 2022, 3, 709–720. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Kim, J.; Oh, G.; Alfaruqi, M.H.; Hwang, J.-Y.; Sun, Y.-K. High-voltage stability of O3-type sodium layered cathode enabled by preferred occupation of Na in the OP2 phase. Energy Storage Mater. 2023, 61, 102908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Zhou, H.; Zhang, Y.; Ji, H.; Wang, L.; Niu, X.; Gao, J. Mg-Doped O3-Na[Ni0.6Fe0.25Mn0.15]O2 Cathode for Long-Cycle-Life Na-Ion Batteries. Inorganics 2025, 13, 261. https://doi.org/10.3390/inorganics13080261
Song Z, Zhou H, Zhang Y, Ji H, Wang L, Niu X, Gao J. Mg-Doped O3-Na[Ni0.6Fe0.25Mn0.15]O2 Cathode for Long-Cycle-Life Na-Ion Batteries. Inorganics. 2025; 13(8):261. https://doi.org/10.3390/inorganics13080261
Chicago/Turabian StyleSong, Zebin, Hao Zhou, Yin Zhang, Haining Ji, Liping Wang, Xiaobin Niu, and Jian Gao. 2025. "Mg-Doped O3-Na[Ni0.6Fe0.25Mn0.15]O2 Cathode for Long-Cycle-Life Na-Ion Batteries" Inorganics 13, no. 8: 261. https://doi.org/10.3390/inorganics13080261
APA StyleSong, Z., Zhou, H., Zhang, Y., Ji, H., Wang, L., Niu, X., & Gao, J. (2025). Mg-Doped O3-Na[Ni0.6Fe0.25Mn0.15]O2 Cathode for Long-Cycle-Life Na-Ion Batteries. Inorganics, 13(8), 261. https://doi.org/10.3390/inorganics13080261





