Carbon Nanomaterials for Electrochemical Hydrogen Storage: Mechanisms and Advancements
Abstract
1. Introduction
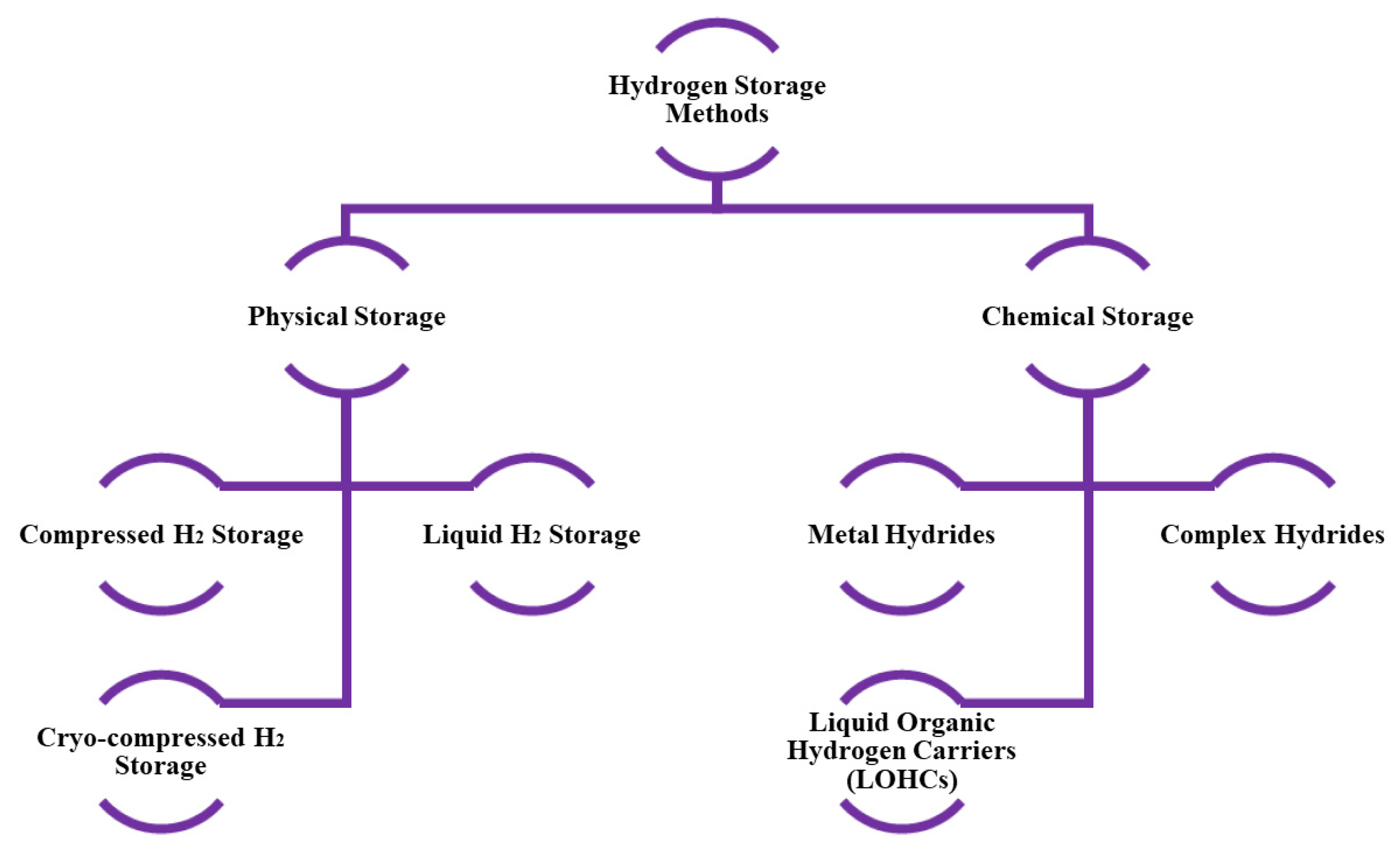
2. Hydrogen Storage Technologies and Mechanisms
- Compressed Gas Storage
- Cryogenic Storage
- Solid Material Storage
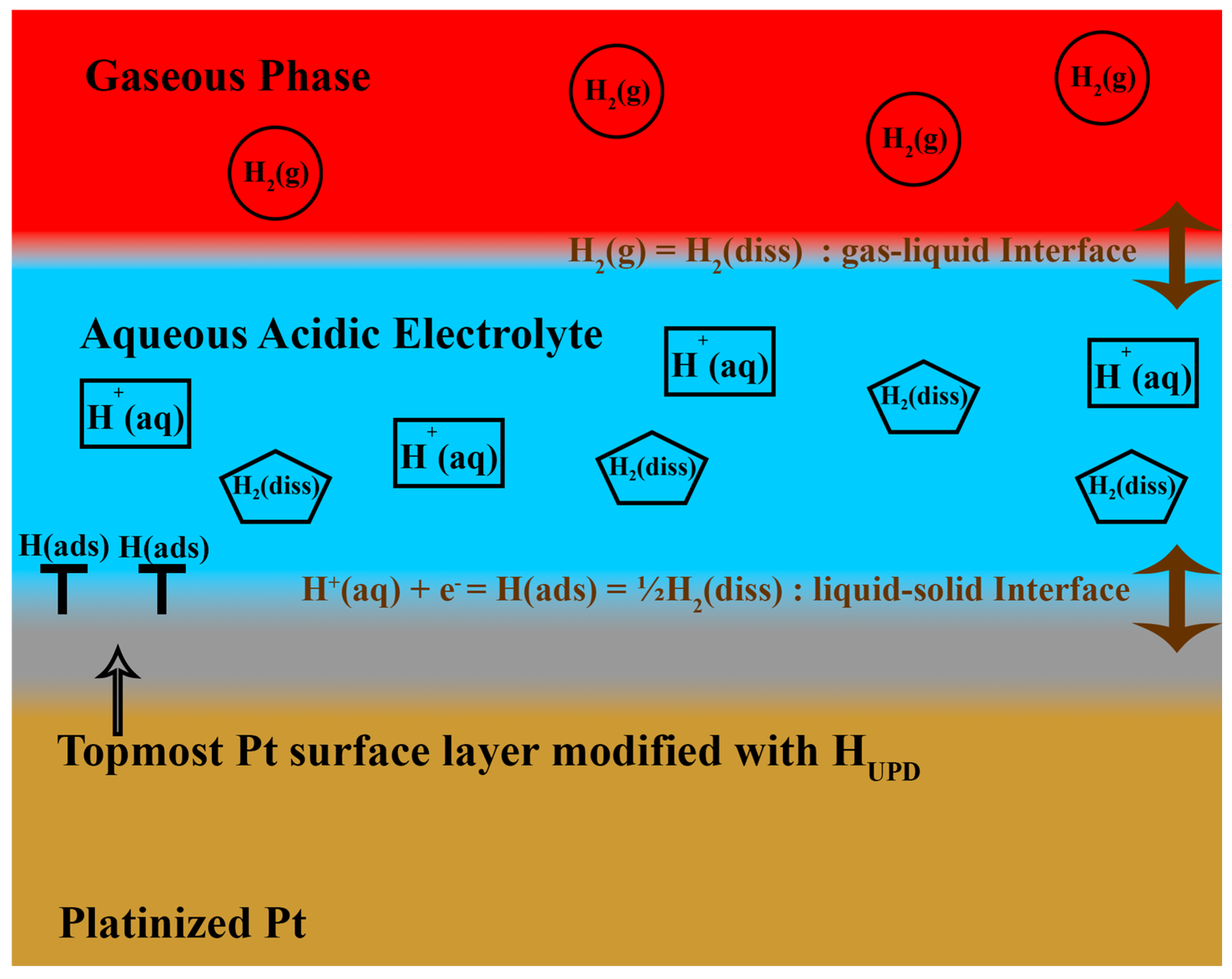
2.1. Electrochemical Hydrogen Storage Mechanism
- The galvanostatic method of charge–discharge (GCD);
- Cyclic voltammetry (CV);
- Electrochemical impedance spectroscopy (EIS).
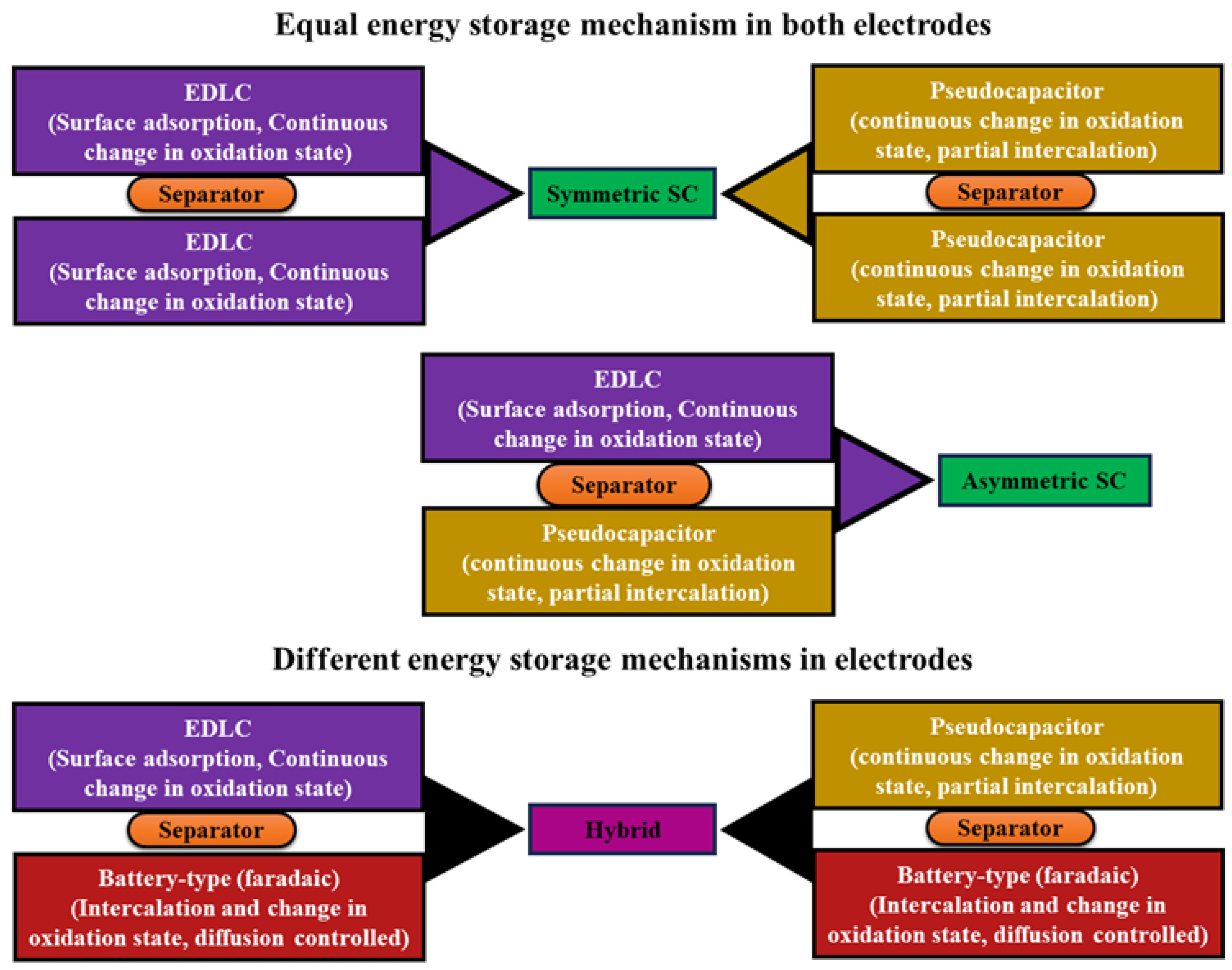
2.1.1. Galvanostatic Charge–Discharge
- In solid electrode materials
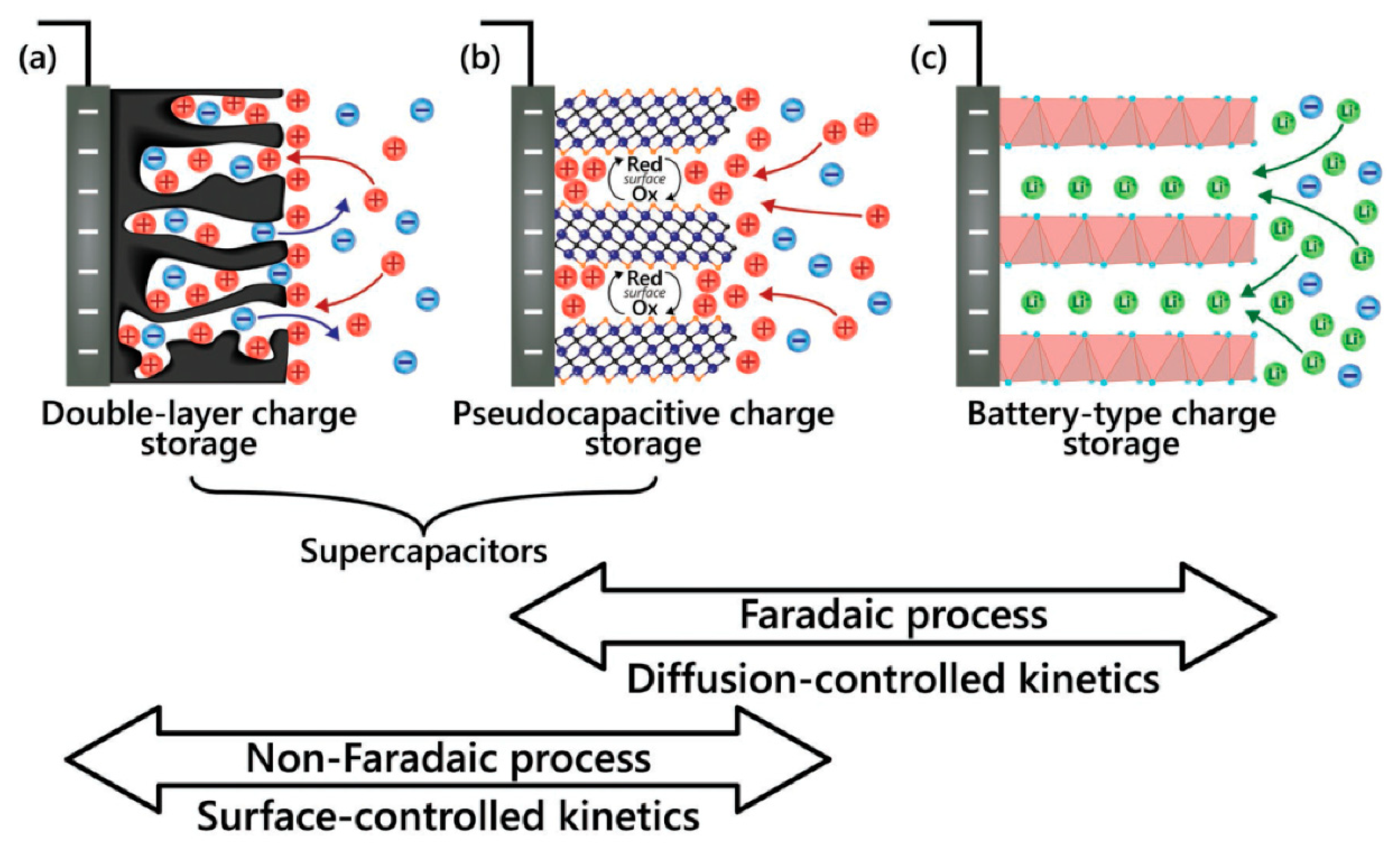
- Double-layer mechanism
- Faradaic process
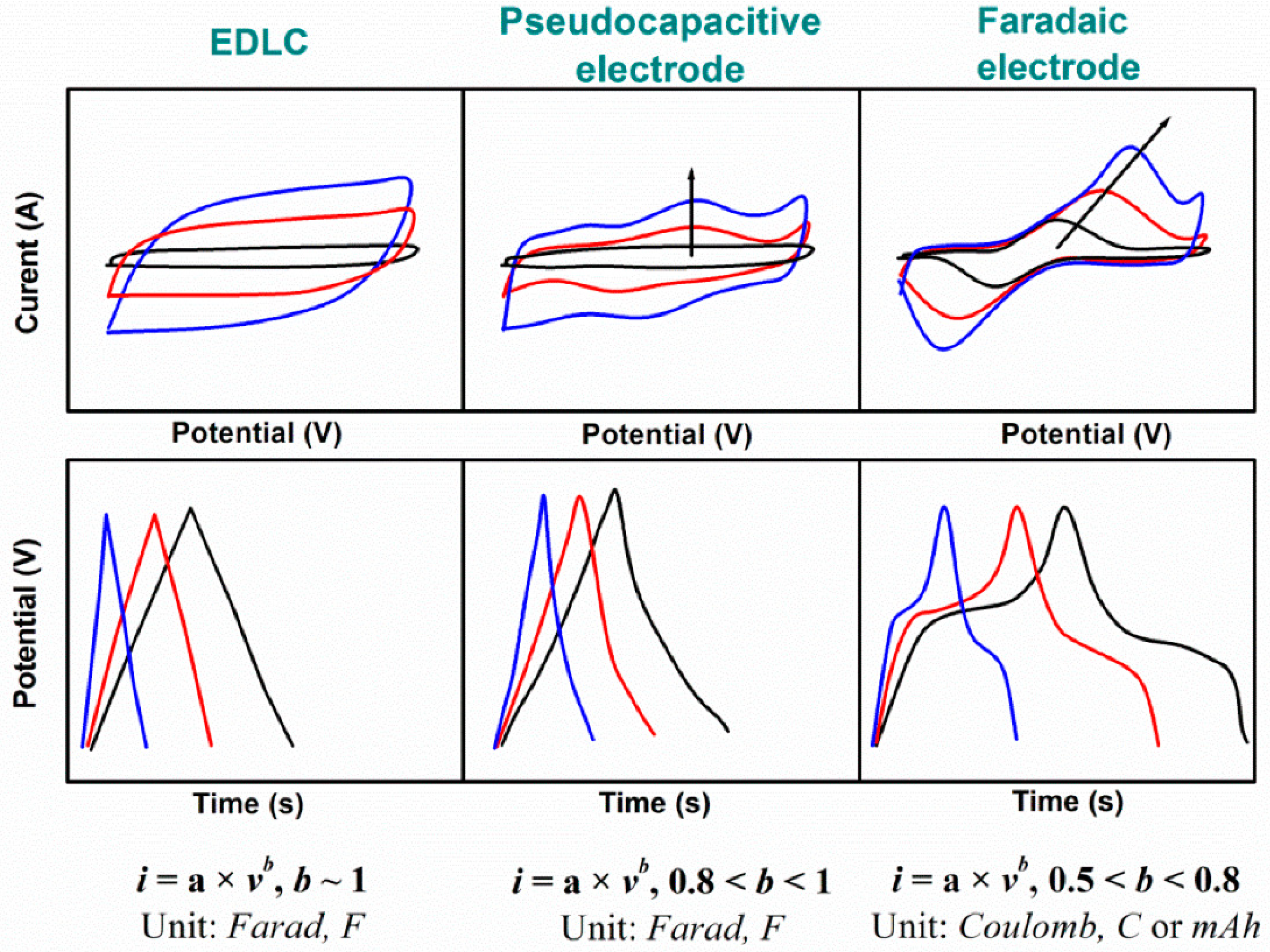
2.1.2. Cyclic Voltammetry
2.1.3. Electrochemical Impedance Spectroscopy
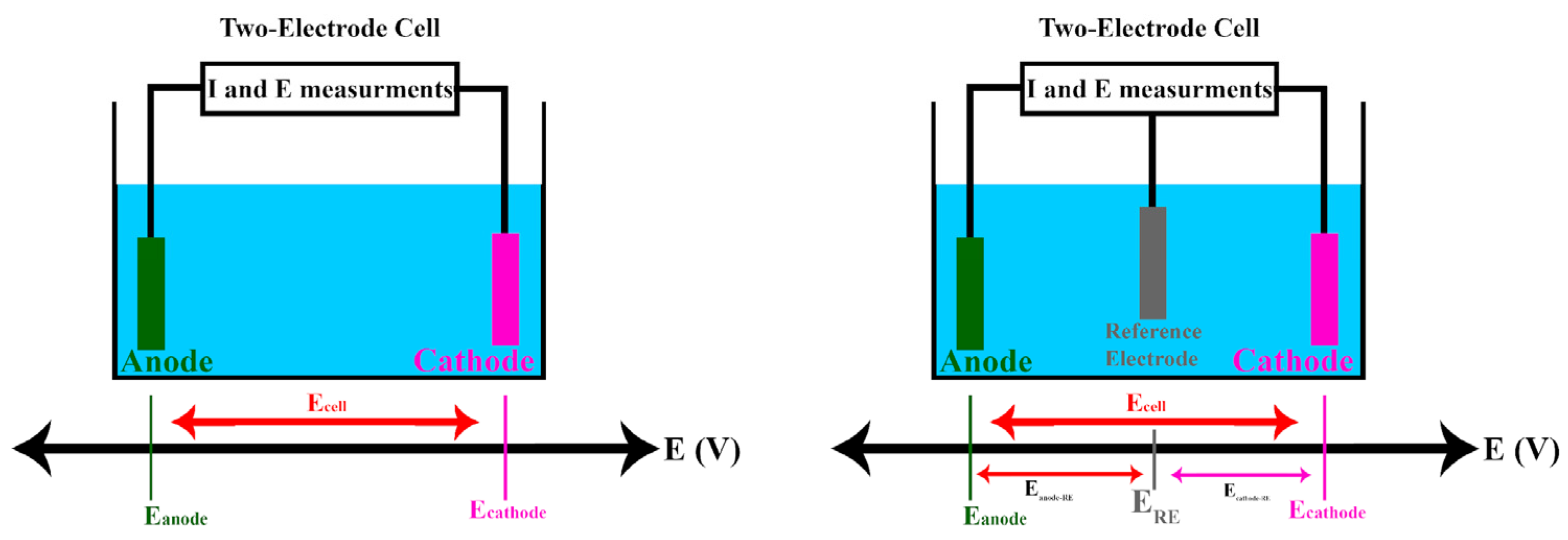
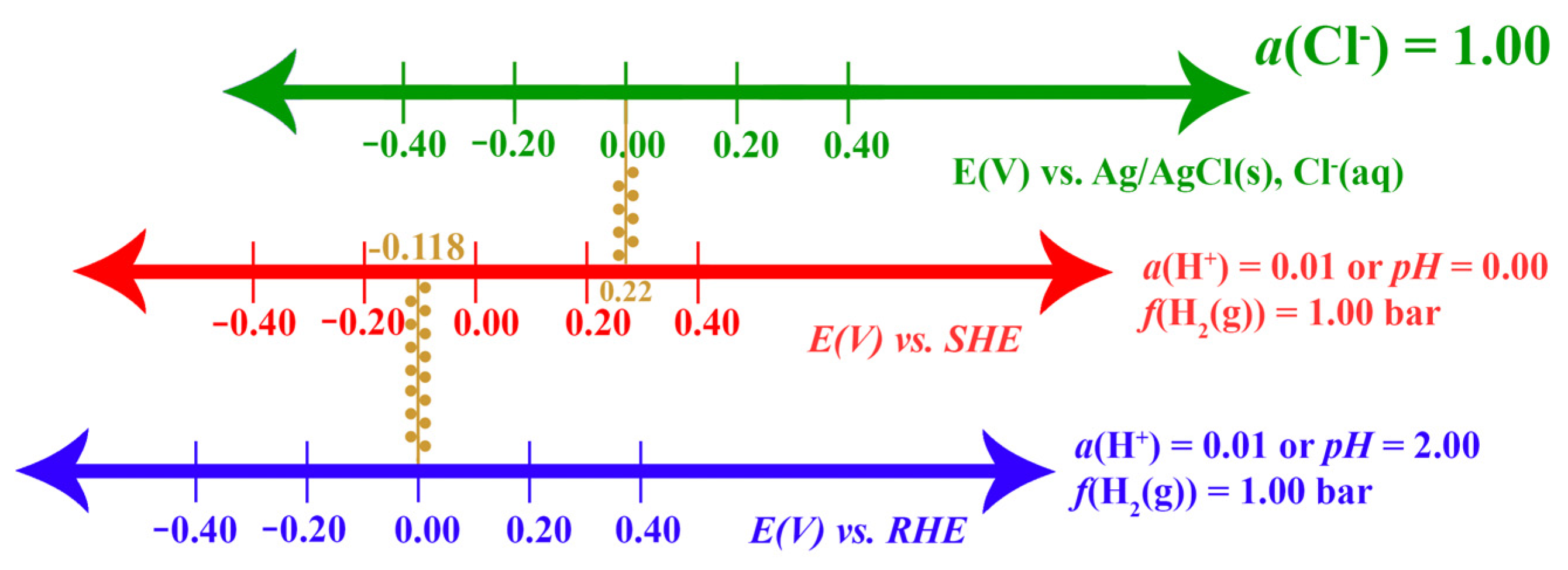
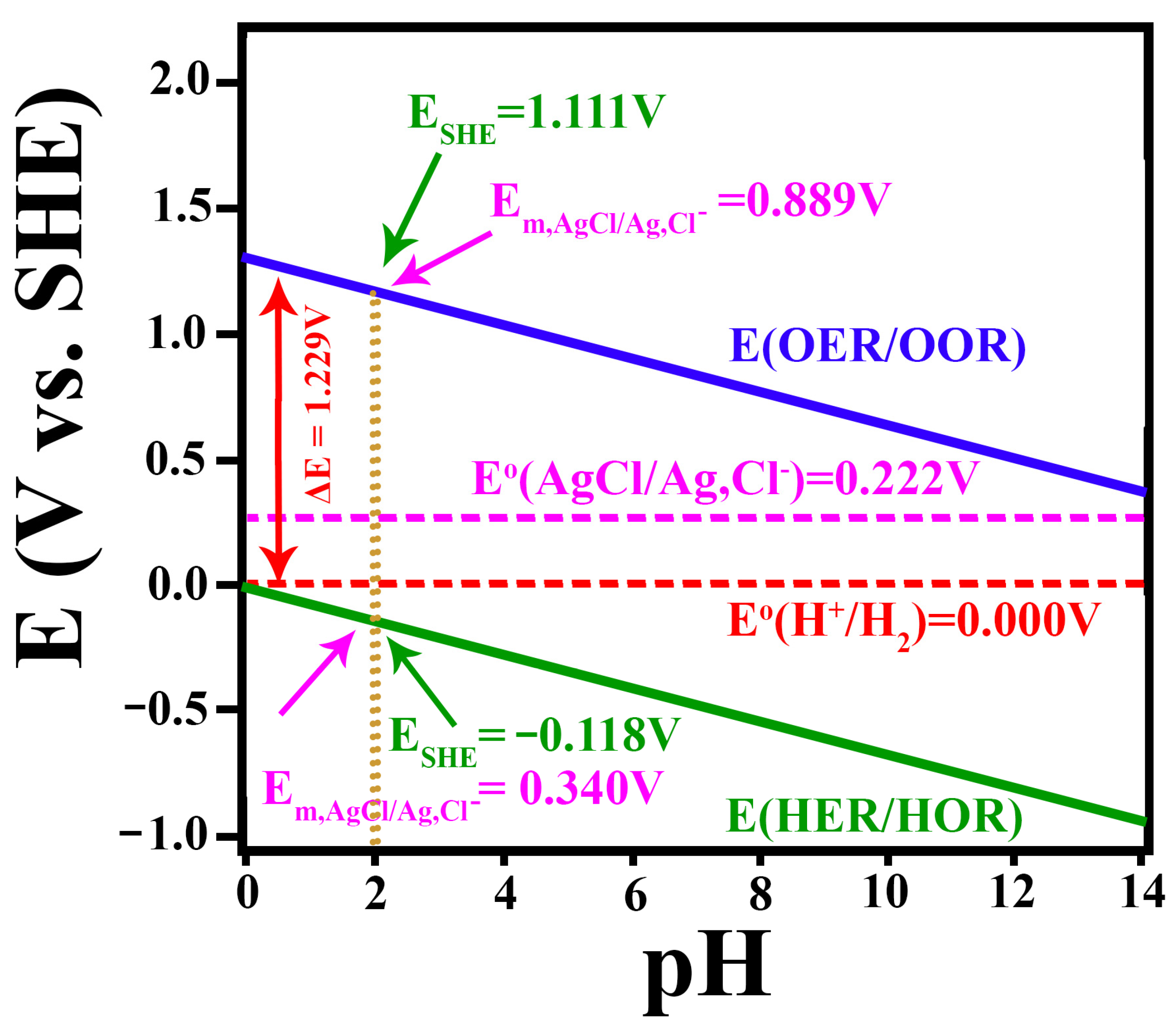
2.1.4. The Standard Hydrogen Electrode and Its Alternative Reference Electrodes
- Using the reversible hydrogen electrode (RHE):
- Using the standard hydrogen electrode (SHE):
- Using the silver–silver chloride electrode (Ag/AgCl):
3. Carbon Materials for Hydrogen Storage
3.1. Carbon Active
3.2. Graphene and Graphene Oxide and Their Properties
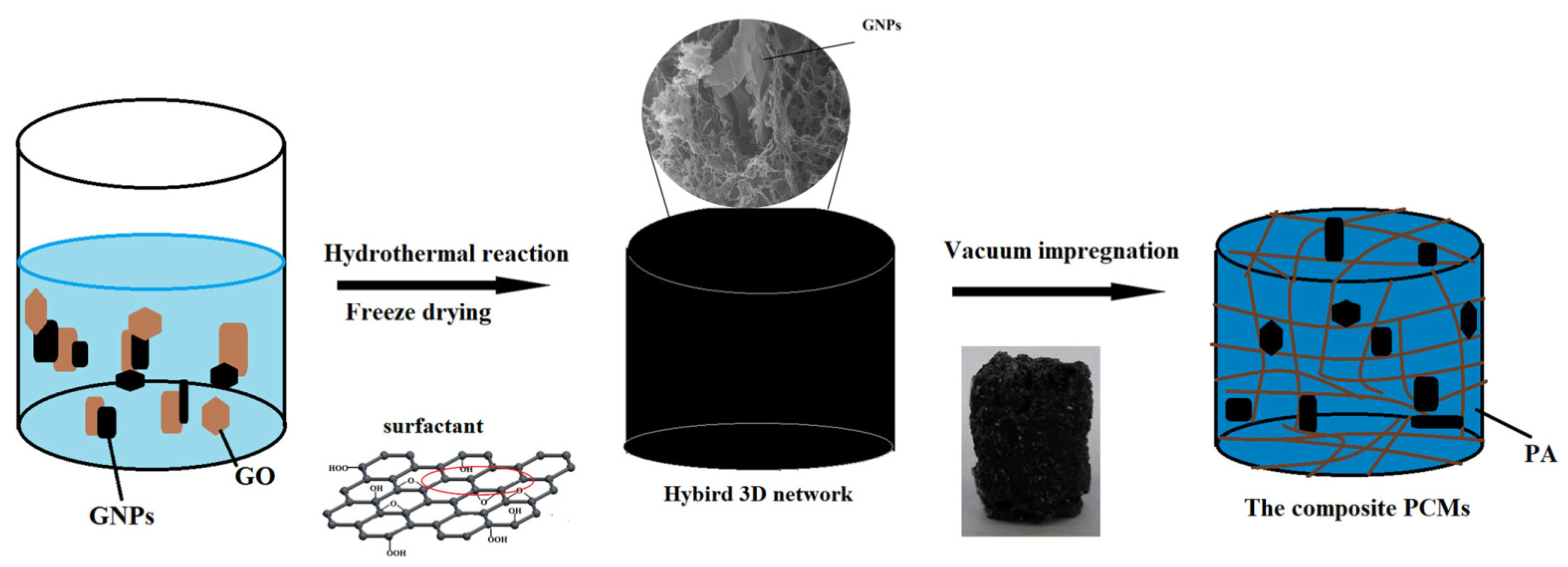
3.3. Three-Dimensional (3D) Graphene Foam
4. Prospects and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Peng, S.; Hu, R.; Ramakrishna, S. Copper Vanadates/Polyaniline Composites as Anode Materials for Lithium-Ion Batteries. RSC Adv. 2015, 5, 20692–20698. [Google Scholar] [CrossRef]
- Ghorbani, R.; Behrangi, S.; Aghajani, H.; Taghizadeh Tabrizi, A.; Abdian, N. Application of Synthesized Porous 3D Graphene Structure for Electrochemical Hydrogen Storage. Mater. Sci. Eng. B 2021, 268, 115139. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Decoration of green synthesized S, N-GQDs and CoFe2O4 on halloysite nanoclay as natural substrate for electrochemical hydrogen storage application. Sci. Rep. 2022, 12, 8103, Erratum: Sci. Rep. 2022, 12, 13241. [Google Scholar]
- Zhang, Y.; Feng, D.; Sun, H.; Bu, W.; Qi, Y.; Guo, S. Structure and Electrochemical Hydrogen Storage Characteristics of Ce-Mg-Ni-Based Alloys Synthesized by Mechanical Milling. J. Rare Earths 2017, 35, 280–289. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen Production, Storage and Transport for Renewable Energy and Chemicals: An Environmental Footprint Assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, X.; Yin, J.; Zhou, C.; Chen, Y.; Li, M.; Wei, A. The Effect of MWNTs with Different Diameters on the Electrochemical Hydrogen Storage Capability. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2005, 339, 370–377. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fang, B. Electrochemical Hydrogen Storage: Opportunities for Fuel Storage, Batteries, Fuel Cells, and Supercapacitors. Int. J. Hydrogen Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Alinavaz, S.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Electrochemical Hydrogen Storage Capacities of Sol-Gel Synthesized Dy3Fe5O12/DyFeO3 Nanocomposites: Schiff-Base Ligand-Assisted Synthesis and Characterization. Fuel 2022, 324, 124600. [Google Scholar] [CrossRef]
- Rubio-Saavedra, D.; Zagal-Padilla, C.K.; Bustos-Terrones, V.; Shirley-Irazoque; Gamboa, S.A. An Insight into the Electrochemical Charge/Discharge Process of Ceramic Hydride Electrodes for Battery Applications. Int. J. Hydrogen Energy 2022, 47, 30225–30233. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H.S.S. Electrochemical Hydrogen-Storage Performance of Copper Sulfide Micro-Hexagons. Int. J. Hydrogen Energy 2021, 46, 5530–5536. [Google Scholar] [CrossRef]
- Salehabadi, A.; Dawi, E.A.; Sabur, D.A.; Al-Azzawi, W.K.; Salavati-Niasari, M. Progress on Nano-Scaled Alloys and Mixed Metal Oxides in Solid-State Hydrogen Storage; an Overview. J. Energy Storage 2023, 61, 106722. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; An, M.; Li, Z. Enhanced Thermal Performance of Composite Phase Change Materials Based on Hybrid Graphene Aerogels for Thermal Energy Storage. Materials 2022, 15, 5380. [Google Scholar] [CrossRef] [PubMed]
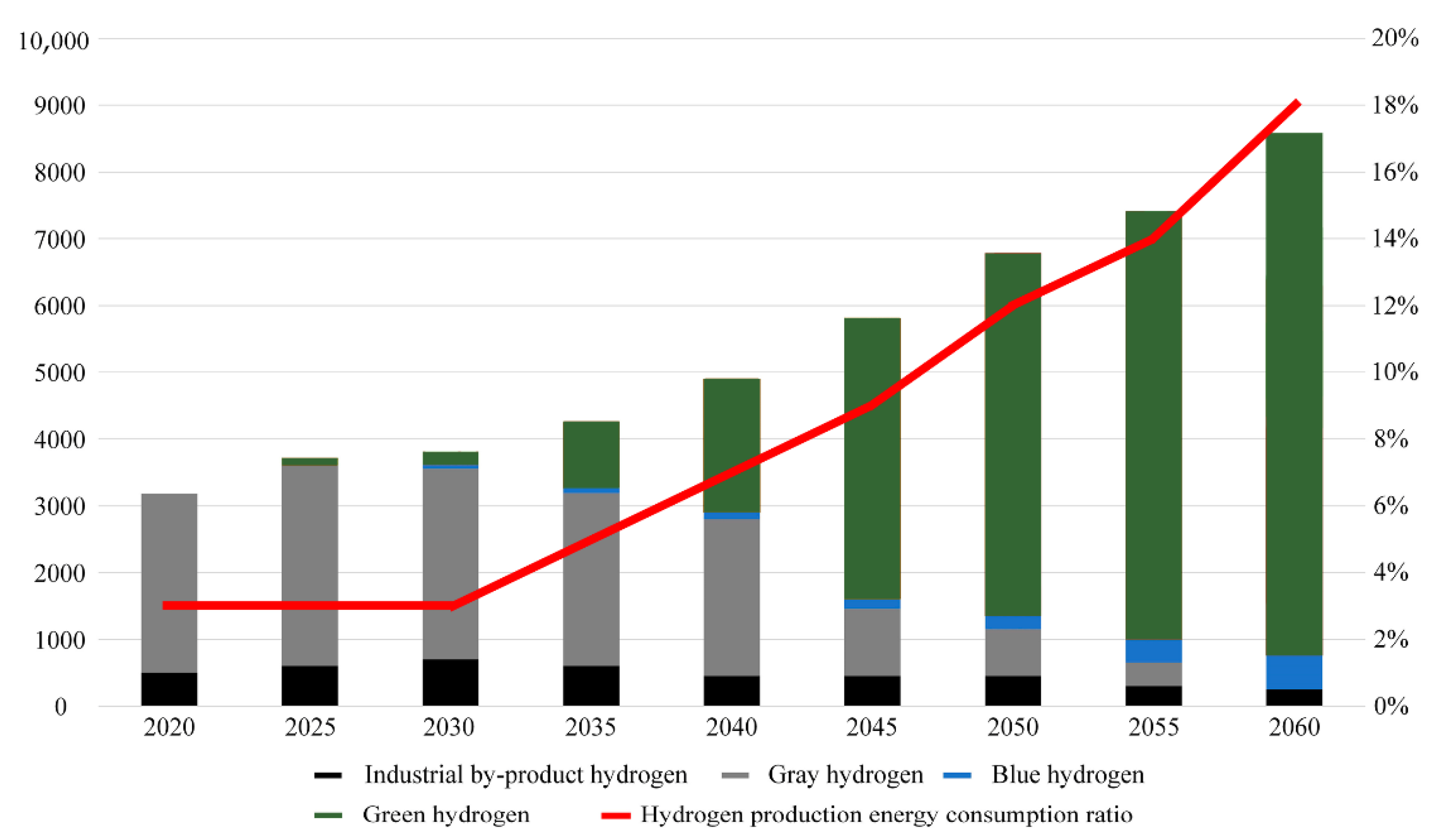
- Tuluhong, A.; Chang, Q.; Xie, L.; Xu, Z.; Song, T. Current Status of Green Hydrogen Production Technology: A Review. Sustainability 2024, 16, 9070. [Google Scholar] [CrossRef]
- Owais, M.; Shiverskii, A.; Pal, A.K.; Mahato, B.; Abaimov, S.G. Recent Studies on Thermally Conductive 3D Aerogels/Foams with the Segregated Nanofiller Framework. Polymers 2022, 14, 4796. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, K. Potential Electrochemical Hydrogen Storage in Nickel and Cobalt Nanoparticles-Induced Zirconia-Graphene Nanocomposite. J. Mater. Sci. Mater. Electron. 2020, 31, 10903–10911. [Google Scholar] [CrossRef]
- Wei, T.Y.; Lim, K.L.; Tseng, Y.S.; Chan, S.L.I.I. A Review on the Characterization of Hydrogen in Hydrogen Storage Materials. Renew. Sustain. Energy Rev. 2017, 79, 1122–1133. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.J.; Li, S.S.; Wang, Z.; Xiao, T.Y.; Qian, Y.H.; Yu, S.H. Hydrothermal Synthesis of Macroscopic Nitrogen-Doped Graphene Hydrogels for Ultrafast Supercapacitor. Nano Energy 2013, 2, 249–256. [Google Scholar] [CrossRef]
- Esfahani, M.H.; Zinatloo-Ajabshir, S.; Naji, H.; Marjerrison, C.A.; Greedan, J.E.; Behzad, M. Structural Characterization, Phase Analysis and Electrochemical Hydrogen Storage Studies on New Pyrochlore SmRETi2O7 (RE = Dy, Ho, and Yb) Microstructures. Ceram. Int. 2023, 49, 253–263. [Google Scholar] [CrossRef]
- Rezayeenik, M.; Mousavi-Kamazani, M.; Zinatloo-Ajabshir, S. CeVO4/RGO Nanocomposite: Facile Hydrothermal Synthesis, Characterization, and Electrochemical Hydrogen Storage. Appl. Phys. A Mater. Sci. Process. 2023, 129, 47. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, C.; He, G.; Hua, R.; Xie, Z.; Liu, D.; Li, X.; Zhang, R.; Tang, H.; Li, J.; et al. Influence of Structural and Chemical Environmental Factors on Electrochemical Hydrogen Storage in Carbon Materials. Electrochim. Acta 2022, 433, 141223. [Google Scholar] [CrossRef]
- Yousefi Bonab, S.S.; Kouzehgar, H.; Taghizadeh Tabrizi, A.; Aghajani, H. Assessment of the Effect of Electrophoretic Deposition Parameters on Hydrogen Storage Performance of Graphene Oxide Layer Applied on Nickel Foam. Int. J. Hydrogen Energy 2022, 47, 2491–2499. [Google Scholar] [CrossRef]
- Skowroński, J.M.; Urbaniak, J. Nickel Foam/Polyaniline-Based Carbon/Palladium Composite Electrodes for Hydrogen Storage. Energy Convers. Manag. 2008, 49, 2455–2460. [Google Scholar] [CrossRef]
- Cui, P.; Bao, Z.; Liu, Y.; Zhou, F.; Lai, Z.; Zhou, Y.; Zhu, J. Corrosion Behavior and Mechanism of Dual Phase Fe1.125Ni1.06CrAl High Entropy Alloy. Corros. Sci. 2022, 201, 110276. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Behpour, M.; Shoaei, S. Enhancement of Hydrogen Storage by Electrophoresis Deposition of CNTs into Nanoscale Pores of Silver Foams. Int. J. Hydrogen Energy 2012, 37, 2298–2303. [Google Scholar] [CrossRef]
- Gómez, J.A.; Santos, D.M.F. The Status of On-Board Hydrogen Storage in Fuel Cell Electric Vehicles. Designs 2023, 7, 97. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Liu, X.; Wang, X. Electrochemical Hydrogen Storage Properties of Ball-Milled Multi-Wall Carbon Nanotubes. Int. J. Hydrogen Energy 2009, 34, 1437–1443. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Liu, E.; He, C.; Shi, C.; Du, X.; Hauge, R.H.; Zhao, N. Synthesis of Hollow Carbon Nano-Onions and Their Use for Electrochemical Hydrogen Storage. Carbon N. Y. 2012, 50, 3513–3521. [Google Scholar] [CrossRef]
- Liu, E.; Wang, J.; Li, J.; Shi, C.; He, C.; Du, X.; Zhao, N. Enhanced Electrochemical Hydrogen Storage Capacity of Multi-Walled Carbon Nanotubes by TiO2 Decoration. Int. J. Hydrogen Energy 2011, 36, 6739–6743. [Google Scholar] [CrossRef]
- Gao, P.; Chen, Y.; Lv, H.; Li, X.; Wang, Y.; Zhang, Q. Synthesis of CuO Nanoribbon Arrays with Noticeable Electrochemical Hydrogen Storage Ability by a Simple Precursor Dehydration Route at Lower Temperature. Int. J. Hydrogen Energy 2009, 34, 3065–3069. [Google Scholar] [CrossRef]
- Qu, D.; Xu, X.; Zhou, L.; Li, W.; Wu, J.; Liu, D.; Xie, Z.Z.; Li, J.; Tang, H. Electrochemical Hydrogen Storage in Iron–Nitrogen Dual-Doped Ordered Mesoporous Carbon. Int. J. Hydrogen Energy 2019, 44, 7326–7336. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Xie, L.; Li, X.Q. Study on Electrochemical Hydrogen Storage of Multi-Walled Carbon Nanotubes. Adv. Mater. Res. 2007, 24–25, 831–834. [Google Scholar] [CrossRef]
- Young, K.H.; Nei, J. The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Yang, C.C.; Li, Y.J.; Chen, W.H. Electrochemical Hydrogen Storage Behavior of Single-Walled Carbon Nanotubes (SWCNTs) Coated with Ni Nanoparticles. Int. J. Hydrogen Energy 2010, 35, 2336–2343. [Google Scholar] [CrossRef]
- Hu, P.; Cao, Y.; Lu, B. Flowerlike Assemblies of Bi2S3 Nanorods by Solvothermal Route and Their Electrochemical Hydrogen Storage Performance. Mater. Lett. 2013, 106, 297–300. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, K. Synthesis, Characterization and Electrochemical Evaluation of Hydrogen Storage Capacity of Graphitic Carbon Nitride and Its Nanocomposites in an Alkaline Environment. J. Mater. Sci. Mater. Electron. 2021, 32, 12475–12489. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Fang, B.; Zhou, H.; Honma, I. Ordered Porous Carbon with Tailored Pore Size for Electrochemical Hydrogen Storage Application. J. Phys. Chem. B 2006, 110, 4875–4880. [Google Scholar] [CrossRef]
- Liu, P.; Lee, S.H.; Edwin Tracy, C.; Turner, J.A.; Pitts, J.R.; Deb, S.K. Electrochromic and Chemochromic Performance of Mesoporous Thin-Film Vanadium Oxide. Solid State Ionics 2003, 165, 223–228. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. Novel Synthesis of Zn2GeO4/Graphene Nanocomposite for Enhanced Electrochemical Hydrogen Storage Performance. Int. J. Hydrogen Energy 2017, 42, 17184–17191. [Google Scholar] [CrossRef]
- Ren, L.; Hui, K.S.N.; Hui, K.S.N.; Liu, Y.; Qi, X.; Zhong, J.; Du, Y.; Yang, J. 3D Hierarchical Porous Graphene Aerogel with Tunable Meso-Pores on Graphene Nanosheets for High-Performance Energy Storage. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Mostafavi, E.; Arzaghi, H.; Davaran, S.; Akbarzadeh, A.; Akhavan, O.; Pazoki-Toroudi, H.; Webster, T.J. Three-Dimensional Graphene Foams: Synthesis, Properties, Biocompatibility, Biodegradability, and Applications in Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Salarizadeh, P.; Rastgoo-Deylami, M.; Askari, M.B.; Hooshyari, K. A Short Review on Transition Metal Chalcogenides/Carbon Nanocomposites for Energy Storage. Nano Futur. 2022, 6, 032005. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Leng, J.; Mei, H.; Zhan, L.; Wang, Y.; Yang, S.; Song, Y. V2O3 Nanoparticles Anchored onto the Reduced Graphene Oxide for Superior Lithium Storage. Electrochim. Acta 2017, 231, 732–738. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, Y.; Shu, D.; Zhong, Y.; Hao, J.; He, C.; Zhong, J.; Song, X. Three-Dimensional Nitrogen-Doped Graphene Hydrogels Prepared via Hydrothermal Synthesis as High-Performance Supercapacitor Materials. Electrochim. Acta 2016, 194, 136–142. [Google Scholar] [CrossRef]
- Deng, L.; Gao, Y.; Ma, Z.; Fan, G. Free-Standing Graphene/Vanadium Oxide Composite as Binder-Free Electrode for Asymmetrical Supercapacitor. J. Colloid Interface Sci. 2017, 505, 556–565. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, X.; Wang, S.-P.; Yin, J.-W.; Zhang, Y.-N.; Chen, J. Theoretical and Experimental Design of Pt-Co(OH)2 Electrocatalyst for Efficient HER Performance in Alkaline Solution. Prog. Nat. Sci. Mater. Int. 2019, 29, 356–361. [Google Scholar] [CrossRef]
- Shojaeinia, A.; Aghajani, H.; Tabrizi, A.T. Evaluation of Electrochemical Hydrogen Storage Capability of Graphene Oxide Multi-Layer Coating. Int. J. Hydrogen Energy 2023, 48, 5836–5849. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M. Effects of Copper:Aluminum Ratio in CuO/Al2O3 Nanocomposite: Electrochemical Hydrogen Storage Capacity, Band Gap and Morphology. Int. J. Hydrogen Energy 2016, 41, 15141–15148. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Niessen, R.A.H.; Notten, P.H.L. Electrochemical Hydrogen Storage in MgSc Alloys: A Comparative Study between Thin Films and Bulk Materials. J. Alloys Compd. 2006, 417, 280–291. [Google Scholar] [CrossRef]
- Adams, B.D.; Ostrom, C.K.; Chen, A. Hydrogen Electrosorption into Pd-Cd Nanostructures. Langmuir 2010, 26, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, Y.; Li, C.; Dang, Y.; Zheng, R.; Wang, Z.; Wang, Y.; Cui, Y.; Arandiyan, H.; Shao, Z.; et al. Recent Advances in Electrochemical Impedance Spectroscopy for Solid-State Batteries. Energy Storage Mater. 2024, 69, 103378. [Google Scholar] [CrossRef]
- Li, M.M.; Yang, C.C.; Wang, C.C.; Wen, Z.; Zhu, Y.F.; Zhao, M.; Li, J.C.; Zheng, W.T.; Lian, J.S.; Jiang, Q. Design of Hydrogen Storage Alloys/Nanoporous Metals Hybrid Electrodes for Nickel-Metal Hydride Batteries. Sci. Rep. 2016, 6, 27601. [Google Scholar] [CrossRef]
- Jerkiewicz, G. Standard and Reversible Hydrogen Electrodes: Theory, Design, Operation, and Applications. ACS Catal. 2020, 10, 8409–8417. [Google Scholar] [CrossRef]
- Feng, Y.; Li, W.; An, J.; Zhao, Q.; Wang, X.; Liu, J.; He, W.; Li, N. Graphene Family for Hydrogen Peroxide Production in Electrochemical System. Sci. Total Environ. 2021, 769, 144491. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Peng, Z.; Liu, Y.; Zhang, J.; Liu, Z.; Li, D. 3D Free-Standing Nitrogen-Doped Reduced Graphene Oxide Aerogel as Anode Material for Sodium Ion Batteries with Enhanced Sodium Storage. Sci. Rep. 2017, 7, 4886. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, G.; Duan, X. Self-Assembled Three-Dimensional Graphene Macrostructures: Synthesis and Applications in Supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef]
- Li, M.; Sun, G.; Yin, P.; Ruan, C.; Ai, K. Controlling the Formation of Rodlike V2O5 Nanocrystals on Reduced Graphene Oxide for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 11462–11470. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Fu, L.; Liu, S.; Yang, H. A Novel and Improved Hydrophilic Vanadium Oxide-Based Cathode for Aqueous Zn-Ion Batteries. Electrochim. Acta 2020, 354, 136721. [Google Scholar] [CrossRef]
- Deng, W.; Fang, Q.; Zhou, X.; Cao, H.; Liu, Z. Hydrothermal Self-Assembly of Graphene Foams with Controllable Pore Size. RSC Adv. 2016, 6, 20843–20849. [Google Scholar] [CrossRef]
- Miao, P.; He, J.; Sang, Z.; Zhang, F.; Guo, J.; Su, D.; Yan, X.; Li, X.; Ji, H. Hydrothermal Growth of 3D Graphene on Nickel Foam as a Substrate of Nickel-Cobalt-Sulfur for High-Performance Supercapacitors; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 732, ISBN 0086139207. [Google Scholar]
- Ndiaye, N.M.; Ngom, B.D.; Sylla, N.F.; Masikhwa, T.M.; Madito, M.J.; Momodu, D.; Ntsoane, T.; Manyala, N. Three Dimensional Vanadium Pentoxide/Graphene Foam Composite as Positive Electrode for High Performance Asymmetric Electrochemical Supercapacitor. J. Colloid Interface Sci. 2018, 532, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Olszowska, K.; Pang, J.; Wrobel, P.S.; Zhao, L.; Ta, H.Q.; Liu, Z.; Trzebicka, B.; Bachmatiuk, A.; Rummeli, M.H. Three-Dimensional Nanostructured Graphene: Synthesis and Energy, Environmental and Biomedical Applications. Synth. Met. 2017, 234, 53–85. [Google Scholar] [CrossRef]
- Shu, R.; Wan, Z.; Zhang, J.; Wu, Y.; Liu, Y.; Shi, J.; Zheng, M. Facile Design of Three-Dimensional Nitrogen-Doped Reduced Graphene Oxide/Multi-Walled Carbon Nanotube Composite Foams as Lightweight and Highly Efficient Microwave Absorbers. ACS Appl. Mater. Interfaces 2020, 12, 4689–4698. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Sun, Y.; Tan, Y.Z.; Yang, S.; Feng, X.; Müllen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, D.; Li, J.; Tang, H.; Xie, Z.; Qu, D. Electrochemical Hydrogen Storage in a Nitrogen-Doped Uniformed Microporous Carbon. Int. J. Hydrogen Energy 2018, 43, 14096–14102. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Mazaheri, M.; Sedighian, A.; Ghanbari, H.; Simchi, A. Composites of Reduced Graphene Oxide/Nickel Submicrorods for Non-Enzymatic Electrochemical Biosensing: Application to Amperometric Glucose Detection. J. Electrochem. Soc. 2020, 167, 087513. [Google Scholar] [CrossRef]
- Hu, K.; Xie, X.; Szkopek, T.; Cerruti, M. Understanding Hydrothermally Reduced Graphene Oxide Hydrogels: From Reaction Products to Hydrogel Properties. Chem. Mater. 2016, 28, 1756–1768. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Tian, R.; Duan, H.; Guo, Y.; Chen, Y.; Zhou, J.; Zhang, C.; DUGNANI, R.; Liu, H. Three Dimensional Graphene Aerogels as Binder-Less, Freestanding, Elastic and High-Performance Electrodes for Lithium-Ion Batteries. Sci. Rep. 2016, 6, 27365. [Google Scholar] [CrossRef]
- Lv, W.; Tao, Y.; Ni, W.; Zhou, Z.; Su, F.Y.; Chen, X.C.; Jin, F.M.; Yang, Q.H. One-Pot Self-Assembly of Three-Dimensional Graphene Macroassemblies with Porous Core and Layered Shell. J. Mater. Chem. 2011, 21, 12352–12357. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into Renewable Hydrogen Energy: Recent Advances and Prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Sangsefidi, F.S.; Salavati-Niasari, M. Fe2O3-CeO2 Ceramic Nanocomposite Oxide: Characterization and Investigation of the Effect of Morphology on Its Electrochemical Hydrogen Storage Capacity. ACS Appl. Energy Mater. 2018, 1, 4840–4848. [Google Scholar] [CrossRef]
- Aghajani, H.; Tabrizi, A.T.; Ghorbani, R.; Behrangi, S.; Stupavska, M.; Abdian, N. Evaluation of Electrochemical Hydrogen Storage Capability of Three-Dimensional Nano-Structured Nitrogen-Doped Graphene. J. Alloys Compd. 2022, 906, 164284. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Lian, Y. The Electrochemical Performance of the N-Doped Graphene Aerogels and Nickel Foam Composite Electrode Prepared by One-Pot Hydrothermal Method. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 582–590. [Google Scholar] [CrossRef]
- Hao, J.; Zhao, R.; Xu, L.; Chi, C.; Li, H. Conversion of Coal into Graphitized Microcrystalline Carbon with a Hierarchical Porous Structure for Electrochemical Hydrogen Storage. J. Electron. Mater. 2023, 52, 2034–2043. [Google Scholar] [CrossRef]
- Li, G.; Xu, C. Hydrothermal Synthesis of 3D Ni Co1−S2 Particles/Graphene Composite Hydrogels for High Performance Supercapacitors. Carbon N. Y. 2015, 90, 44–52. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Lin, L.; Wu, H.; Su, Y.; Zhang, X.; Liu, A. Controllable Nanoscale Engineering of Vertically Aligned MoS2 Ultrathin Nanosheets by Nitrogen Doping of 3D Graphene Hydrogel for Improved Electrocatalytic Hydrogen Evolution. Carbon N. Y. 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the Specific Surface Area of Monolayer Graphene Oxide in Water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Lin, D.; Richard Liu, C.; Cheng, G.J. Single-Layer Graphene Oxide Reinforced Metal Matrix Composites by Laser Sintering: Microstructure and Mechanical Property Enhancement. Acta Mater. 2014, 80, 183–193. [Google Scholar] [CrossRef]
- Su, Y.; Li, J.J.; Weng, G.J. Theory of Thermal Conductivity of Graphene-Polymer Nanocomposites with Interfacial Kapitza Resistance and Graphene-Graphene Contact Resistance. Carbon N. Y. 2018, 137, 222–233. [Google Scholar] [CrossRef]
- Tabrizi, A.T.; Aghajani, H.; Mashtizadeh, A. Determining the Effect of Porosities on the Hydrogen Adsorption Capacity of 3D Printed PEEK. Int. J. Hydrogen Energy 2023, 49, 1009–1019. [Google Scholar] [CrossRef]
- Nooriha Najafabadi, M.; Ghanbari, H.; Naghizadeh, R. Graphene/Silver-Based Composites and Coating on Dead Coral for Degradation of Organic Pollution Using the Z-Scheme Mechanism. RSC Adv. 2021, 11, 19890–19901. [Google Scholar] [CrossRef]
- Sangani, N.; Eslahi, N.; Varsei, M.; Ghanbari, H. Electrospinning of Cellulose Acetate/Graphene/Nanoclay Nanocomposite for Textile Wastewater Filtration. J. Text. Inst. 2024, 115, 647–655. [Google Scholar] [CrossRef]
- Jabbari, A.; Ghanbari, H.; Naghizadeh, R. Partial Reduction of Graphene Oxide toward the Facile Fabrication of Desalination Membrane. Int. J. Environ. Sci. Technol. 2023, 20, 831–842. [Google Scholar] [CrossRef]
- Afsari, N.; Eslahi, N.; Varsei, M.; Ghanbari, H. Electrospinning of Smart Fibrous Nanocomposite Based on Graphene/Polydiacetylene. J. Appl. Polym. Sci. 2023, 140, 1–10. [Google Scholar] [CrossRef]
- Goodarzi, R.; Ghanbari, H.; Sarpoolaky, H. An Eco-Friendly Polyvinyl Alcohol/Graphene Oxide-Based Hydrogel as a Methylene Blue Adsorbent. ChemistrySelect 2022, 7, 1–10. [Google Scholar] [CrossRef]
- Méndez-Lozano, N.; Pérez-Reynoso, F.; González-Gutiérrez, C. Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method. Materials 2022, 15, 7228. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Zeng, Z.; Shi, W.; Zhu, Y.; Yan, Q.; Liu, H.; Wang, J.; Zhang, H. One-Step Synthesis of Ni3S2 Nanorod@Ni(OH) 2 Nanosheet Core-Shell Nanostructures on a Three-Dimensional Graphene Network for High-Performance Supercapacitors. Energy Environ. Sci. 2013, 6, 2216–2221. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, J.; Zhang, Q.; You, B.; Chen, G. Three Dimensional Ni Foam-Supported Graphene Oxide for Binder-Free Pseudocapacitor. Electrochim. Acta 2015, 152, 216–221. [Google Scholar] [CrossRef]
- Ghanbari, H.; Shafikhani, M.A.; Daryalaal, M. Graphene Nanosheets Production Using Liquid-Phase Exfoliation of Pre-Milled Graphite in Dimethylformamide and Structural Defects Evaluation. Ceram. Int. 2019, 45, 20051–20057. [Google Scholar] [CrossRef]
- Öztürk, A.; Bayrakçeken Yurtcan, A. Nitrogen-Doped Graphene Foam as Carbon Composites Catalysis Catalysts: Preparation, Properties and Applications. Chem. Mater. 2022, 28, 1–40. [Google Scholar] [CrossRef]
- Arthi G, P.B.; BD, L. A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial. J. Nanomed. Nanotechnol. 2015, 06, 1–4. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, S.; Hu, P.; Zhao, G.; Li, Y.; Zhang, X.; Han, W. Enhanced Mechanical, Thermal, and Electric Properties of Graphene Aerogels via Supercritical Ethanol Drying and High-Temperature Thermal Reduction. Sci. Rep. 2017, 7, 1439. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe 3 O 4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef]
- Çögenli, M.S.; Bayrakçeken Yurtcan, A. Heteroatom Doped 3D Graphene Aerogel Supported Catalysts for Formic Acid and Methanol Oxidation. Int. J. Hydrogen Energy 2020, 45, 650–666. [Google Scholar] [CrossRef]
- Öner, E.; Öztürk, A.; Yurtcan, A.B. Utilization of the Graphene Aerogel as PEM Fuel Cell Catalyst Support: Effect of Polypyrrole (PPy) and Polydimethylsiloxane (PDMS) Addition. Int. J. Hydrogen Energy 2020, 45, 34818–34836. [Google Scholar] [CrossRef]
- Wang, H.; Yi, H.; Chen, X.; Wang, X. One-Step Strategy to Three-Dimensional Graphene/VO2 Nanobelt Composite Hydrogels for High Performance Supercapacitors. J. Mater. Chem. A 2014, 2, 1165–1173. [Google Scholar] [CrossRef]
- Xu, C.; Su, Y.; Liu, D.; He, X. Three-Dimensional N,B-Doped Graphene Aerogel as a Synergistically Enhanced Metal-Free Catalyst for the Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2015, 17, 25440–25448. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, B.; Cheng, J.; Li, J.; Zhang, H.; Tang, Z.; Yang, J. Hydrothermal Synthesis of Ni(OH)2 Nanoflakes on 3D Graphene Foam for High-Performance Supercapacitors. Electrochim. Acta 2015, 173, 399–407. [Google Scholar] [CrossRef]
- Karimizadeh, N.; Babamoradi, M.; Azimirad, R.; Khajeh, M. Synthesis of Three-Dimensional Multilayer Graphene Foam/ZnO Nanorod Composites and Their Photocatalyst Application. J. Electron. Mater. 2018, 47, 5452–5457. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. Facile Precipitation Synthesis and Electrochemical Evaluation of Zn2SnO4 Nanostructure as a Hydrogen Storage Material. Int. J. Hydrogen Energy 2017, 42, 12420–12429. [Google Scholar] [CrossRef]
- Xu, D.W.; Yang, S.; Chen, P.; Yu, Q.; Xiong, X.H.; Wang, J. 3D Nitrogen-Doped Porous Magnetic Graphene Foam-Supported Ni Nanocomposites with Superior Microwave Absorption Properties. J. Alloys Compd. 2019, 782, 600–610. [Google Scholar] [CrossRef]
- Khamlich, S.; Bello, A.; Fabiane, M.; Ngom, B.D.; Manyala, N. Hydrothermal Synthesis of Simonkolleite Microplatelets on Nickel Foam-Graphene for Electrochemical Supercapacitors. J. Solid State Electrochem. 2013, 17, 2879–2886. [Google Scholar] [CrossRef]
- He, S.; Chen, W. 3D Graphene Nanomaterials for Binder-Free Supercapacitors: Scientific Design for Enhanced Performance. Nanoscale 2015, 7, 6957–6990. [Google Scholar] [CrossRef]
- Fang, Q.; Shen, Y.; Chen, B. Synthesis, Decoration and Properties of Three-Dimensional Graphene-Based Macrostructures: A Review. Chem. Eng. J. 2015, 264, 753–771. [Google Scholar] [CrossRef]
- Mao, L.; Guan, C.; Huang, X.; Ke, Q.; Zhang, Y.; Wang, J. 3D Graphene-Nickel Hydroxide Hydrogel Electrode for High-Performance Supercapacitor. Electrochim. Acta 2016, 196, 653–660. [Google Scholar] [CrossRef]
- Zhao, T.; Ji, X.; Bi, P.; Jin, W.; Xiong, C.; Dang, A.; Li, H.; Li, T.; Shang, S.; Zhou, Z. In Situ Synthesis of Interlinked Three-Dimensional Graphene Foam/Polyaniline Nanorod Supercapacitor. Electrochim. Acta 2017, 230, 342–349. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Yang, X.; Wei, Y.; Xiao, M.; Yang, Z.; Yang, B.R.; Liu, C.; Lu, X.; et al. Three-Dimensional-Structured Boron- and Nitrogen-Doped Graphene Hydrogel Enabling High-Sensitivity NO2 Detection at Room Temperature. ACS Sensors 2019, 4, 1889–1898. [Google Scholar] [CrossRef]





| Working Electrode | Reference Electrode | Counter Electrode | Electrolyte | Specific Surface m2 g−1 | Hydrogen Storage Capacity | Max Discharge Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| SWCNT + 8 wt.%Ni | Ag/AgCl | Ni(OH)2/NiOOH | 6 M KOH | 478.6 | 5.25 wt% | 1404 | [34] |
| SWCNT + 12 wt.%Ni | Ag/AgCl | Ni(OH)2/NiOOH | 6 M KOH | 436.0 | 2.99 wt% | 800 | [34] |
| SWCNTs | Ag/AgCl | Ni(OH)2/NiOOH | 6 M KOH | 584.8 | 1.61 wt% | 431 | [34] |
| VO2@S-rGO composites | Ag/AgCl | Pt | 0.5 M K2SO4 | 12.6 | 18.5 wt% | 173 | [74] |
| Mesoporous carbon | Hg/HgO | Pt | 3 M KOH | 2116 | - | 61 | [20] |
| N-doped mesoporous carbon | Hg/HgO | Pt | 3 M KOH | 1752 | - | 111 | [20] |
| Zn2GeO4/graphene | Ag/AgCl | Pt | 6 M KOH | 2695 | 9.54 wt% | 221 | [40] |
| Fe-N-ordered mesoporous carbon | Hg/HgO | Pt | 3 M KOH | 853 | - | 120 | [30] |
| Graphene oxide-Ni foam | Hg/HgO | Pt | 6 M KOH | - | - | 50 | [21] |
| N-doped graphene | Hg/HgO | Pt | 6 M KOH | - | - | 1916 | [75] |
| Co@NMC | Hg/HgO | Pt | 3 M KOH | 223 | 72.8 wt% | 318 | [17] |
| 2%Pt-Co@NMC | Hg/HgO | Pt | 3 M KOH | 151 | 69.8 wt% | 364 | [17] |
| 3D GO + Ni foam | Hg/HgO | Pt | 6 M KOH | - | 7.7 wt% | 217 | [76] |
| Porous carbon | Hg/HgO | Pt | 6 M KOH | 200 | - | 1050 | [77] |
| Microcrystalline porous carbon | Hg/HgO | Pt | 6 M KOH | 988 | - | 200 | [77] |
| 3D graphene foam | Hg/HgO | Pt | 6 M KOH | - | - | 321 | [2] |
| 3D N-doped GO foam | Hg/HgO | Pt | 6 M KOH | 531 | 7 wt% | 387 | [46] |
| N-doped Go 3D foam | Hg/HgO | Pt | 5 M KOH | - | - | 51 | [17] |
| Ni0.31Co0.69S2/GO foam | Hg/HgO | Pt | 6 M KOH | - | - | 1166 | [78] |
| 3D N-graphene foam | Hg/HgO | Pt | 6 M KOH | - | - | 387 | [46] |
| N-doped-3D GO using amino acids | Hg/HgO | Pt | 6 M KOH | 367 | - | 388 | [17] |
| 3D graphene hydrogel | - | - | - | - | - | 175 | [67] |
| MoS2/reduced graphene oxide | Hg/HgO | Pt | 6 M KOH | - | - | 226 | [79] |
| MoS2/N-reduced graphene oxide | Hg/HgO | Pt | 6 M KOH | - | - | 119 | [79] |
| Material | Go Solution | First Additive | Second Additive | Specific Surface M2·g−1 | Synthesis Method | Heat Treatment | Ref. |
|---|---|---|---|---|---|---|---|
| 3D Fe3O4/N-GA | 6 mL (1.5 mg/mL) | Iron Acetate (1–40 mg) | Polypyrrole 20 mg | 110 | Hydrothermal 180 °C 12 h | 600 °C Ar Atm | [97] |
| 3D RGO Aerogel | 5 mg/mL (100 mL) | - | - | 206 | Hydrothermal 150 C 20 h | - | [57] |
| 3D N-doped RGO Foam | 5 mg/mL (100 mL) | NH4HCO3 | - | 316 | Hydrothermal 150 C 20 h | 800 C N2 | [57] |
| Boron-doped GO foam | 5 mg/mL (100 mL) | 2.5 g H3BO3 | 69.92 | Hydrothermal 180 °C 12 h | - | [98] | |
| Nitrogen-doped GO foam | 5 mg/mL (100 mL) | 20 mL NH3.H2O | 379 | Hydrothermal 180 °C 12 h | - | [98] | |
| Pt/GA | 5 mg/mL (100 mL) | H2PtCl6 solution (0.5 M) | 0.63 g Pyrrole | 265 | Freeze-Drying | - | [99] |
| Pt/PPy-GA | 5 mg/mL (100 mL) | H2PtCl6 solution (0.5 M) | 0.63 g Pyrrole | 12.13 | Freeze-Drying | - | [99] |
| RGO hydrogel | 4 mg/mL (10 mL) | - | - | - | Hydrothermal 180 °C 6 h | [70] | |
| Nitrogen-RGO hydrogel | 4 mg/mL (10 mL) | Ammonia 290 µl | - | - | Hydrothermal 180 °C 6 h | [70] | |
| 3D macroporous graphene | 80 mg/ml | Hydrazine Hydrate 1250 µL | - | 263 | Sonication- Freeze Drying | [72] | |
| Co3O4/3D graphene hydrogel | 3 mg/mL (30 mL) | Co3O4 0.5 mmol (20 nm) | - | 266 | Hydrothermal 180 °C 12 h | 900 C Ar | [41] |
| Co3O4/3D graphene hydrogel | 3 mg/mL (30 mL) | Co3O4 0.5 mmol (50 nm) | - | 383 | Hydrothermal 180 °C 12 h | 900 C Ar | [41] |
| Self-Assembled graphene hydrogel | 2 mg/mL (10 mL) | - | - | - | Hydrothermal 180 °C 12 h | - | [100] |
| B-doped graphene aerogels | 2 mg/mL (10 mL) | H3BO3 (30 mg) | - | 100 | Hydrothermal 180 °C 12 h | [101] | |
| N-doped graphene aerogels | 2 mg/mL (10 mL) | 50 mg chitosan | 1 g urea | 545 | Hydrothermal 180 °C 12 h | 1000 C Ar | [101] |
| Ni(OH)2 nanoflakes on 3D graphene foam | - | C2H4 | - | - | APCVD | [102] | |
| 3D graphene foam/ZnO nanorod | - | CH4 | - | - | CVD | [103] | |
| 3D graphene on nickel foam | 2 mg/mL (10 mL) | - | - | - | Hydrothermal 180 C 36 h | - | [62] |
| Nitrogen-doped 3D graphene foam | 2 mg/mL (50 mL) | 2 g/L Ammonia | - | - | Hydrothermal 180 °C 12 h | [104] | |
| Porous CoO on 3D graphene foams | 2 mg/mL (20 mL) | - | - | - | Hydrothermal 180 °C 12 h | [61] | |
| Self-assembled 3D graphene | 2 mg/mL (15 mL) | - | - | - | Hydrothermal 180 °C 12 h | [58] | |
| Graphene hydrogels | 2 mg/mL (17 mL) | - | Hydrothermal 180 °C 12 h | [17] | |||
| Nitrogen-doped graphene hydrogels | 2 mg/mL (17 mL) | Ammonia | - | - | Hydrothermal 180 °C 12 h | [17] | |
| 3D N-doped porous magnetic GO foam supported with Ni nanocomposites | 4 mg/mL (20 mL) | 9 mmol NiCl2·6H2O | 0.5 g CTAB 0.3 g PVA | Hydrothermal 180 C 8 h | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashtizadeh, A.R.; Asl, S.K.; Aghajani, H.; Masoudpanah, S.M.; Wojnicki, M. Carbon Nanomaterials for Electrochemical Hydrogen Storage: Mechanisms and Advancements. Inorganics 2025, 13, 125. https://doi.org/10.3390/inorganics13040125
Mashtizadeh AR, Asl SK, Aghajani H, Masoudpanah SM, Wojnicki M. Carbon Nanomaterials for Electrochemical Hydrogen Storage: Mechanisms and Advancements. Inorganics. 2025; 13(4):125. https://doi.org/10.3390/inorganics13040125
Chicago/Turabian StyleMashtizadeh, Amir Reza, Shahab Khameneh Asl, Hossein Aghajani, Seyed Morteza Masoudpanah, and Marek Wojnicki. 2025. "Carbon Nanomaterials for Electrochemical Hydrogen Storage: Mechanisms and Advancements" Inorganics 13, no. 4: 125. https://doi.org/10.3390/inorganics13040125
APA StyleMashtizadeh, A. R., Asl, S. K., Aghajani, H., Masoudpanah, S. M., & Wojnicki, M. (2025). Carbon Nanomaterials for Electrochemical Hydrogen Storage: Mechanisms and Advancements. Inorganics, 13(4), 125. https://doi.org/10.3390/inorganics13040125






