Cyanoguanidine-Modified Chitosan as an Efficacious Adsorbent for Removing Cupric Ions from Aquatic Solutions: Kinetics, Isotherms, and Mechanisms
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of CCs Adsorbent
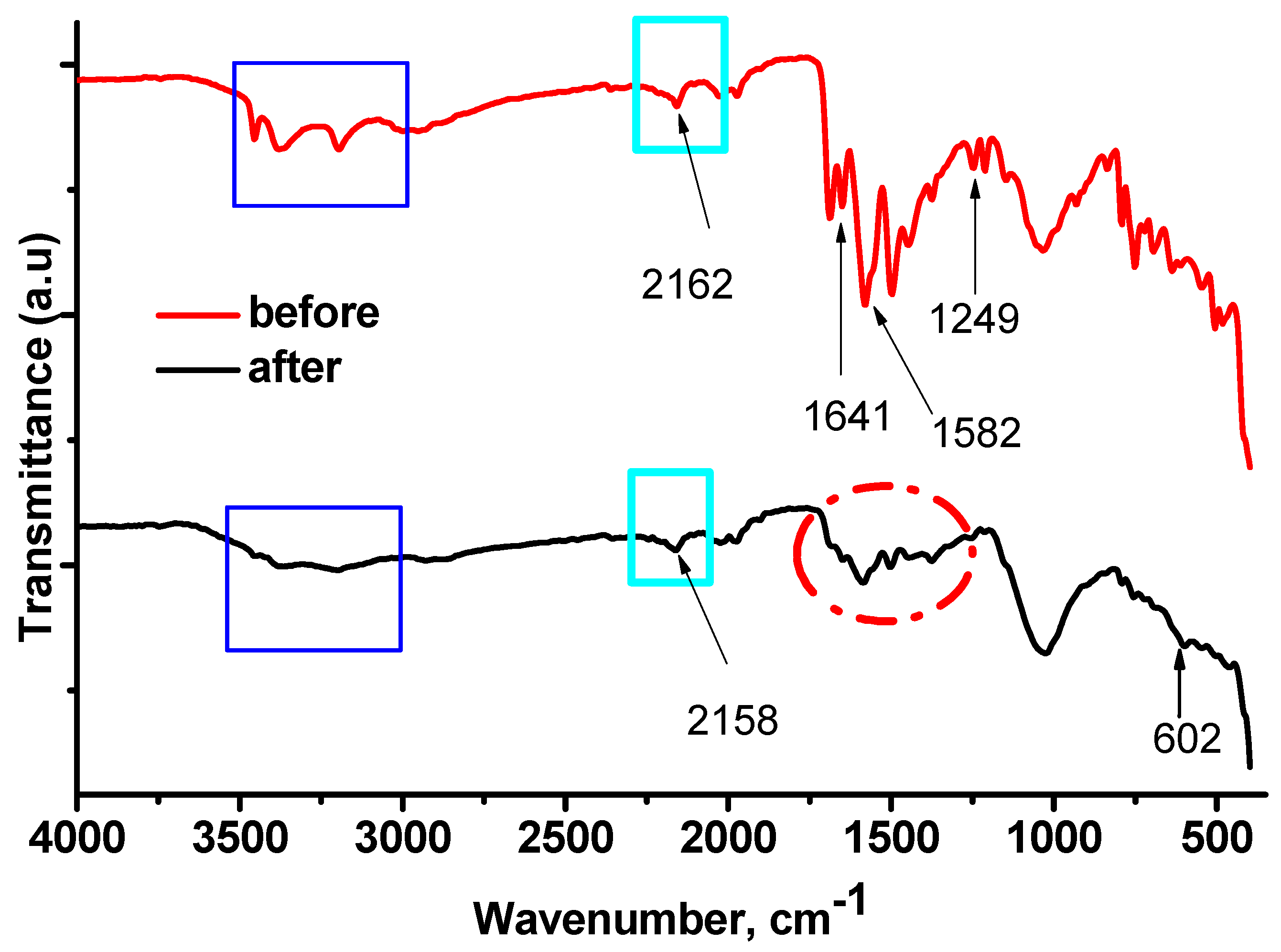
2.1.1. FTIR Analysis
2.1.2. XRD Analysis
2.1.3. SEM Analysis
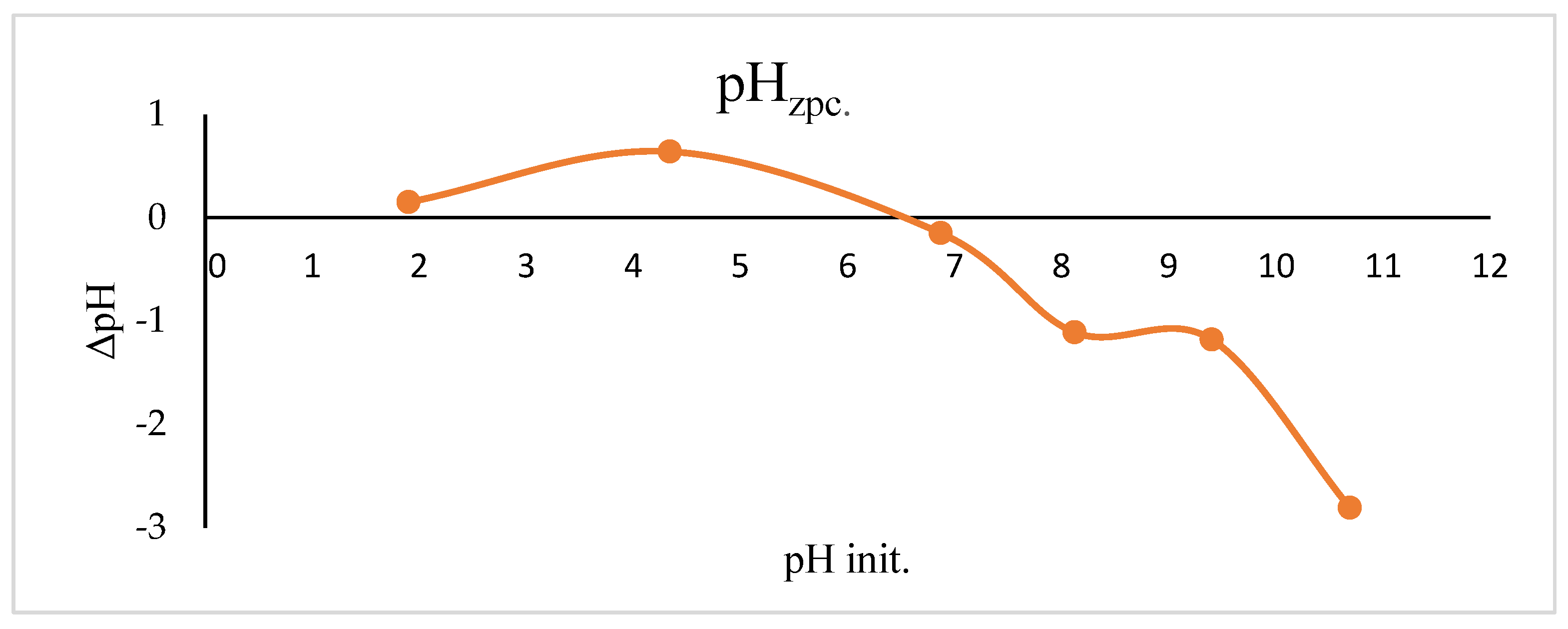
2.2. pHzpc
2.3. Optimizing Adsorption
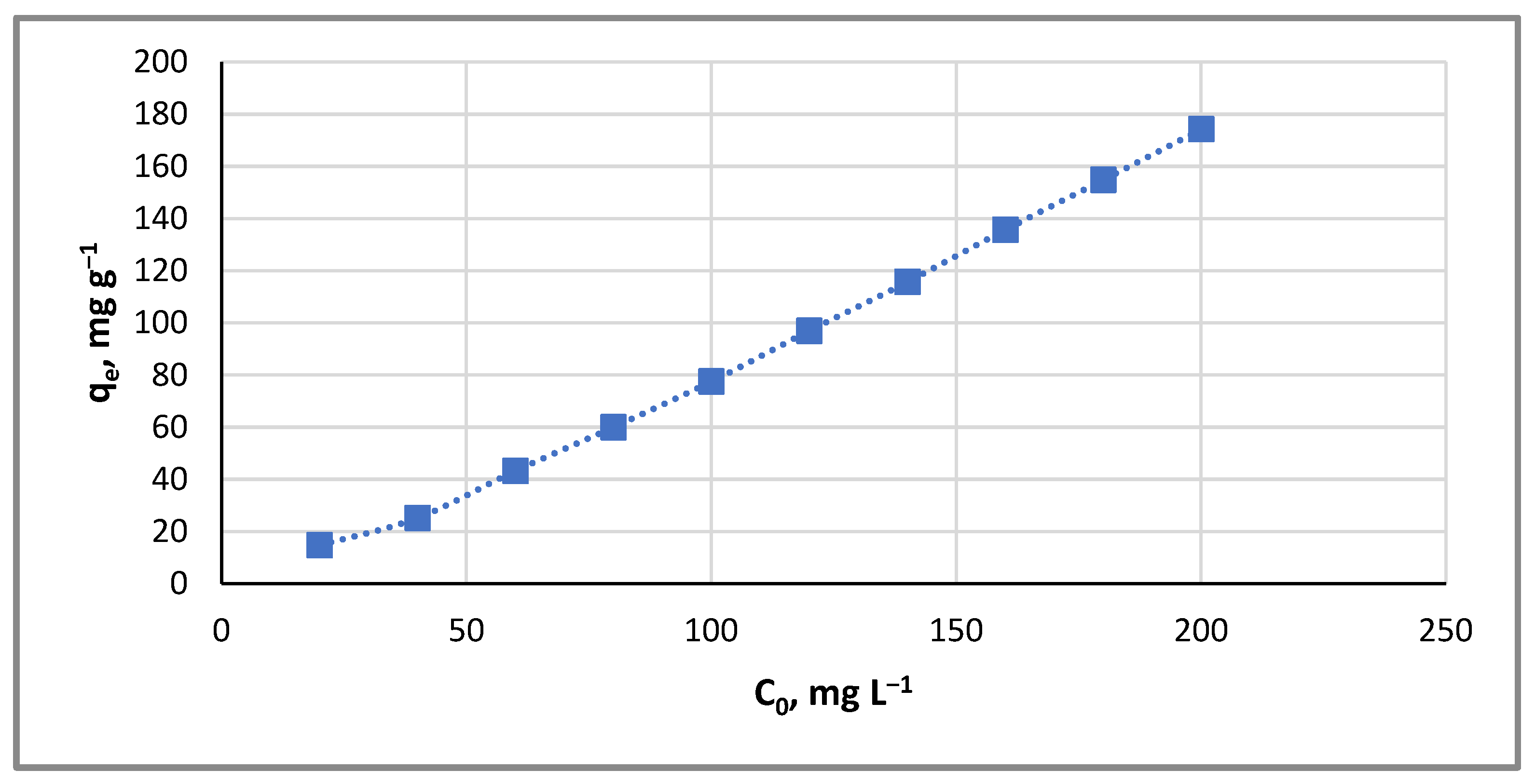
2.3.1. Effect of Cupric Ion Concentration
2.3.2. Effect of Temperature
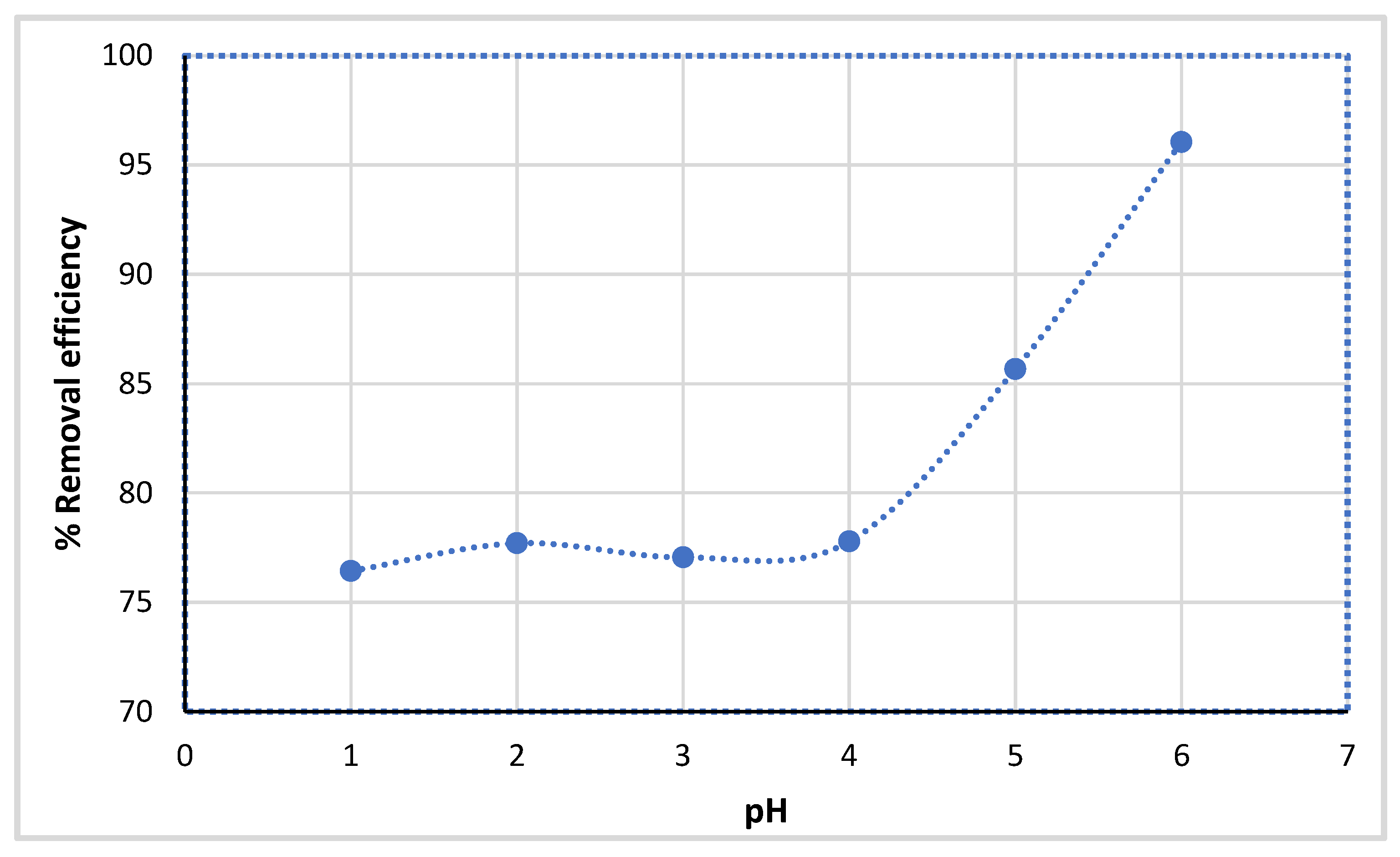
2.3.3. Effect of Solution pH
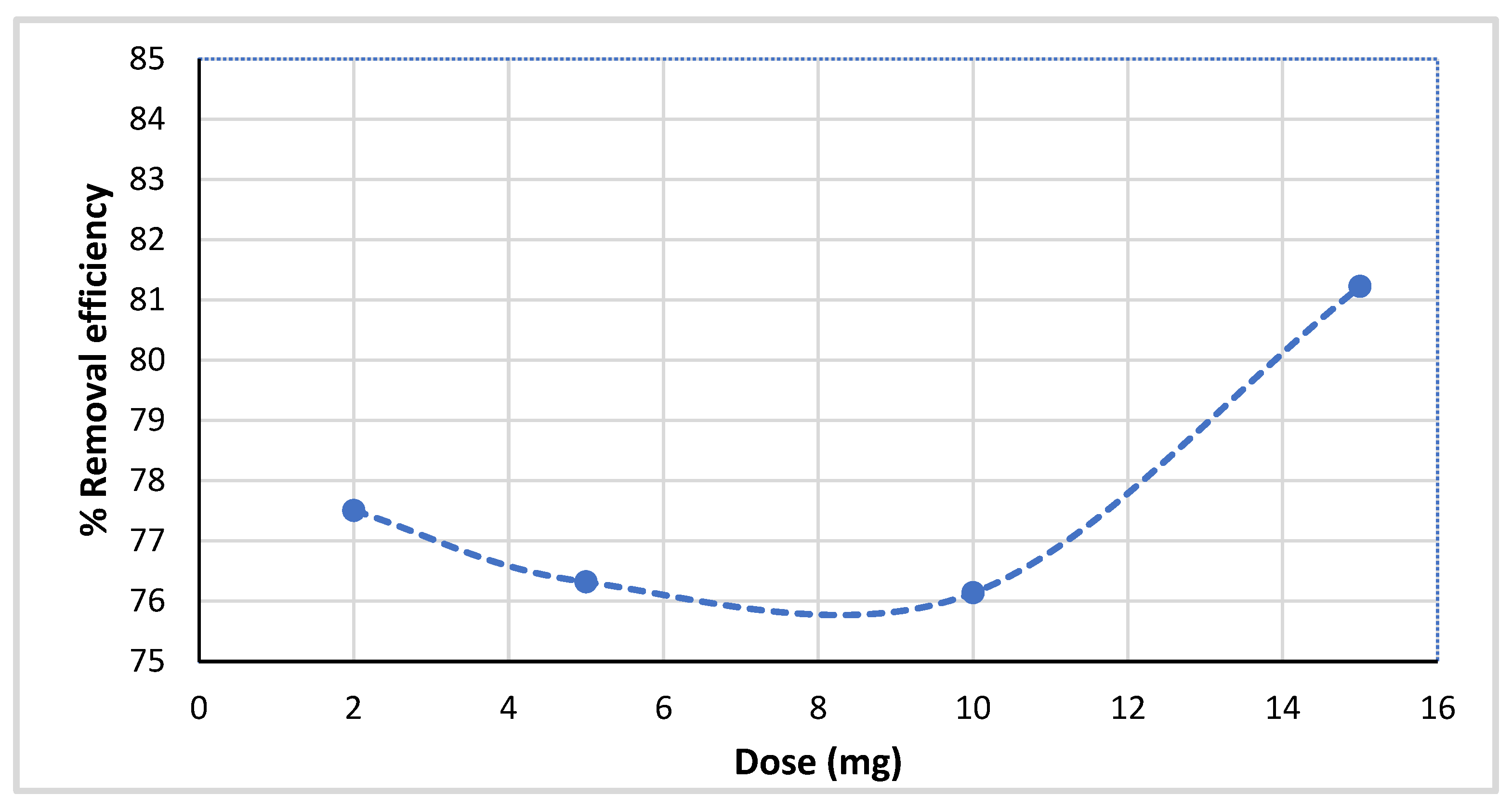
2.3.4. Adsorbent Dosage Effect
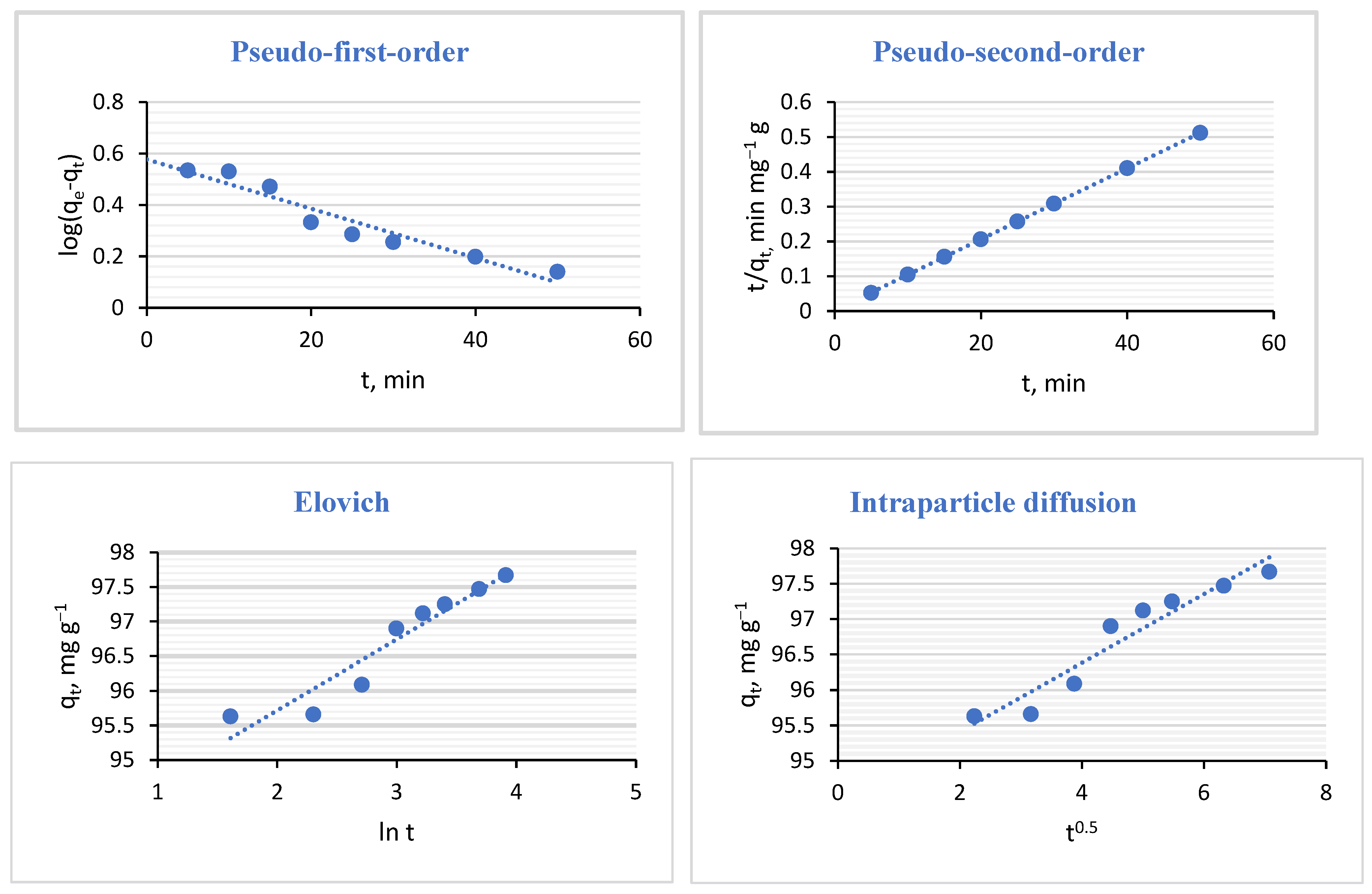
2.4. Adsorption Kinetics
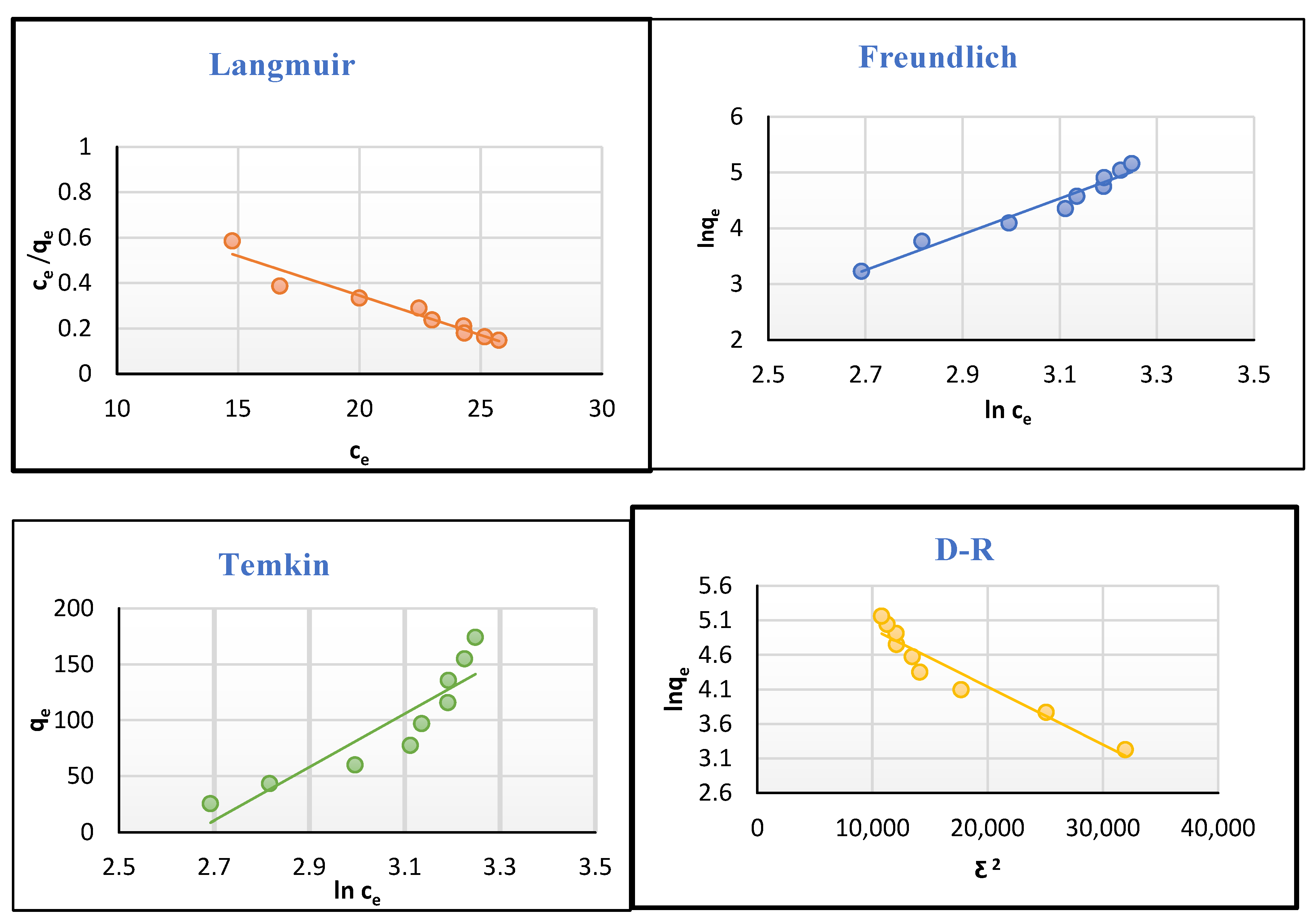
2.5. Isotherms of Adsorption
2.6. Recyclability of CCs
2.7. Comparation of the Efficiency of Various Materials for Adsorbing Cupric Ions
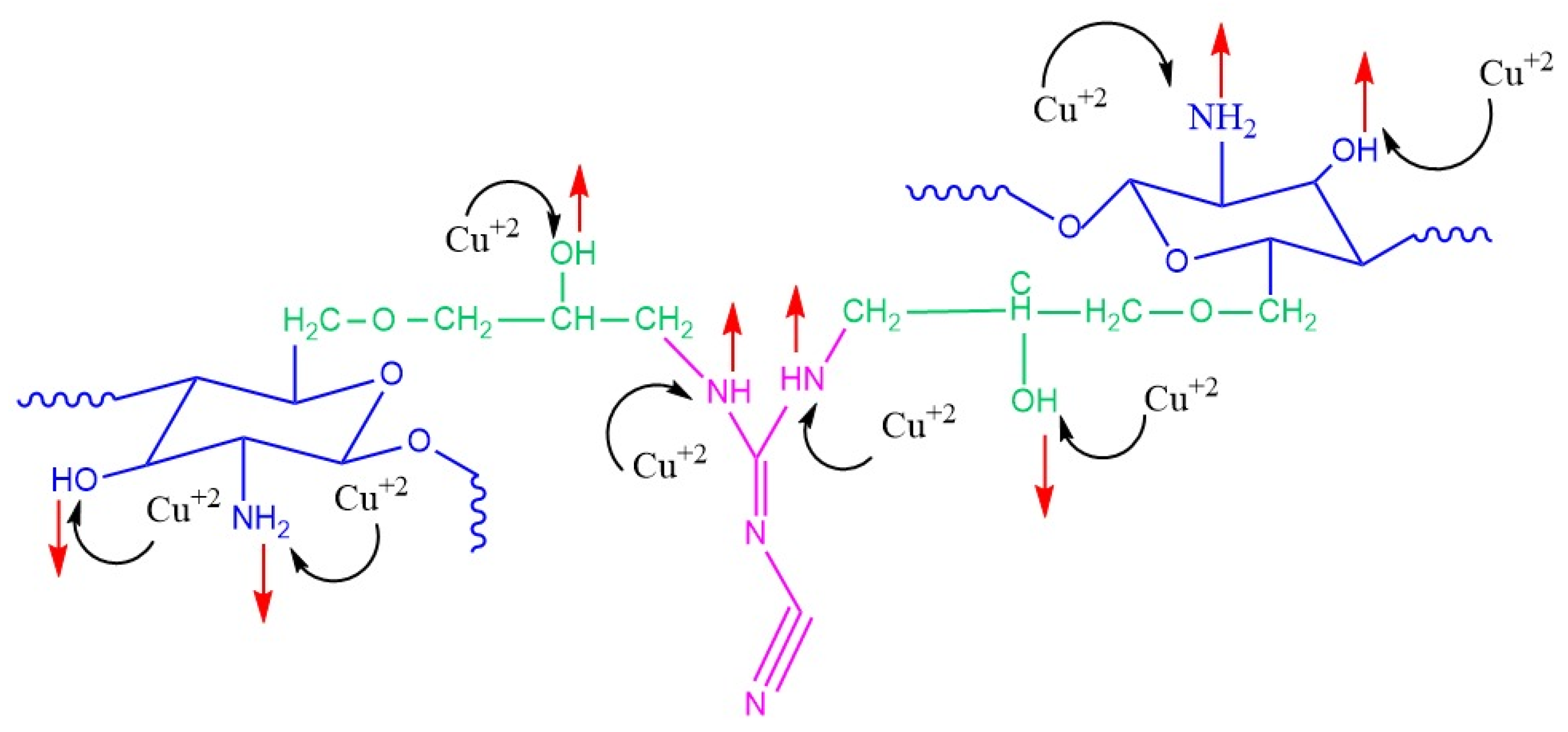
2.8. Adsorption Mechanism
- (i)
- Metal ion complexation is the foremost sorption mechanism to remove Cu2+ via CCs through the ion exchange interactions between the protons of functional groups (such as –NH2, C=N, –OH, –NH, etc.) and the Cu2+ ions in wastewater.
- (ii)
- These nitrogen atoms function as the main active complexation sites. Taking into account, as theoretically proven, the superiority of nitrogen over oxygen in the complexation process, this may be due to its lower electronegativity than oxygen [74].
- (iii)
- Additionally, it is clear that the ability of Cu2+ adsorption by CCs via ion exchange depends on the nature of the functional groups on the adsorbent surface and the pH of the solution. The complex formation occurring between Cu2+ and CCs at optimum pH can be expressed as follows:
- (iv)
- Electrostatic interaction is another pivotal mechanism for removing copper ions using CCs adsorbents. Therefore, to remove Cu+2 ions from wastewater by this mechanism, the adsorbent surface must be negatively charged to cause the attraction of molecules with opposing charges. At pH higher than 6.5, the excessive concentration of negatively charged surface hydroxyl ion functional groups on the adsorbent surface tends to attract copper cations. So, a significant decline in adsorption due to the existence of OH−, which is more dominant on the adsorbent surface, forms insoluble Cu(OH)2 salts that easily precipitate [78].
- (v)
- In the literature, a significant drop in Cu+2 adsorption performance at low pH is reported because the adsorbent surface becomes highly positively charged; so, electrostatic repulsion between cupric ions and the adsorbent surface will occur [70].
3. Experimental
3.1. Materials
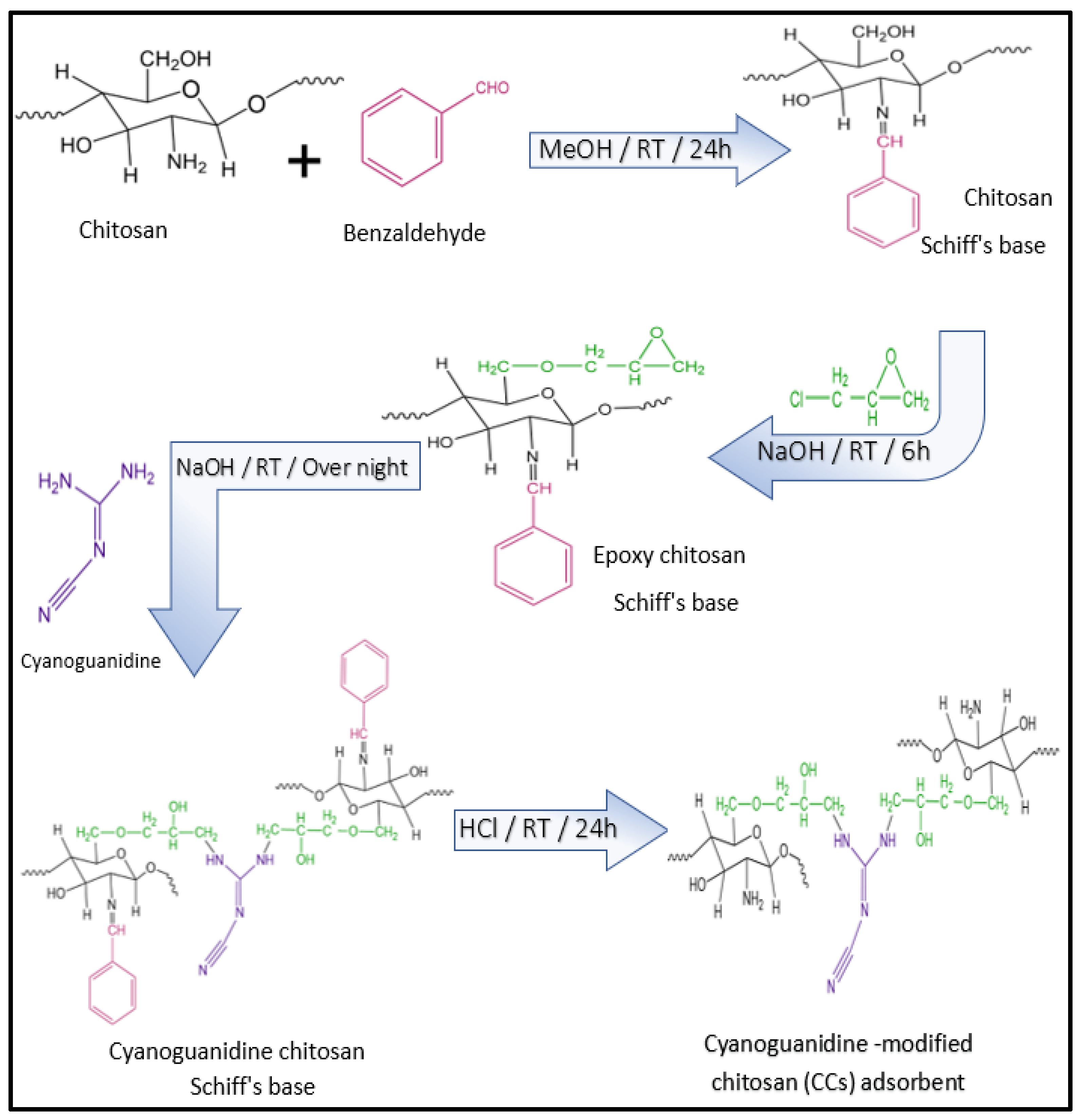
3.2. Synthesis of Cyanoguanidine-Modified Chitosan (CCs) Adsorbent
3.3. Measurements
3.3.1. FTIR Spectroscopy
3.3.2. X-Ray Diffractometry (XRD)
3.3.3. Scanning Electron Microscopy (SEM)
3.4. Zero Point Charge’s pH (pHzpc) Value
3.5. Computational Chemistry Analysis
3.6. Adsorption Investigations
3.7. Adsorption Kinetic Investigations
3.7.1. Pseudo-First-Order Model
3.7.2. Pseudo-Second-Order Model
3.7.3. Model of Elovich
3.7.4. Model of Intraparticle Diffusion
3.8. Adsorption Isotherms
3.8.1. Langmuir Isotherm Model
3.8.2. Freundlich Isotherm Model
3.8.3. Temkin Isotherm Model
3.8.4. Dubinin–Radushkevich (D-R) Isotherm Model
3.9. Desorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uğurlu, E.; Birol, B.; Gencten, M.; Bayrak, Y. Removal of cupric ions from aqueous solutions by ferrochrome ash: Investigation of mechanism and kinetics. Water 2023, 15, 1063. [Google Scholar] [CrossRef]
- Martins, R.J.E.; Rosana, P.; Rui, A.R.B. Cadmium (II) and zinc (II) adsorption by the aquatic moss Fontinalis antipyretica: Effect of temperature, pH and water hardness. Water Res. 2004, 38, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Renu, M.A.; Kailash, S. Methodologies for removal of heavy metal ions from wastewater: An overview. Interdiscip. Environ. Rev. 2017, 18, 124–142. [Google Scholar] [CrossRef]
- Mohamed, N.A. Novel wholly aromatic polyamide-hydrazides: 6. Dependence of membranes reverse osmosis performance on processing parameters and polymer structural variations. Polymer 1997, 38, 4705–4713. [Google Scholar] [CrossRef]
- Tiravanti, G.; Petruzzelli, D.; Passino, R. Pretreatment of tannery wastewaters by an ion exchange process for Cr(III) removal and recovery. Water Sci. Technol. 1997, 36, 197–207. [Google Scholar] [CrossRef]
- Brooks, C.S. Metal Recovery from Industrial Waste; Lewis Publishers Ins.: Portage, MI, USA, 1991; Volume 27. [Google Scholar]
- Hu, Z.; Lei, L.; Li, Y.; Ni, Y. Chromium adsorption on high performance activated carbons from aqueous solution. Sep. Purif. Technol. 2003, 31, 13–18. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.A.; Cadaval, T.R.S., Jr.; Breslin, C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Demirbaş, Ö.; Karadağ, A.; Alkan, M.; Doğan, M. Removal of copper ions from aqueous Solutions by hazelnuts. J. Hazard. Mater. 2008, 153, 677–684. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Synthesis, characterization and antimicrobial activity of novel N-acetyl,N’-chitosanacetohydrazide and its metal complexes. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1369–1379. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, H.J.; Yim, J.H.; Kim, J.M.; Jeon, J.K.; Sohn, J.M.; Yoo, K.S.; Kim, S.S.; Park, Y.K. Removal of cupric-ion over amine-functionalized mesoporous silica materials. J. Ind. Eng. Chem. 2011, 17, 504–509. [Google Scholar] [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N. Extraction of Cu (II) from aqueous solutions by vegetable oil-based organic solvents. J. Hazard. Mater. 2010, 181, 868–872. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Manahan, S.E. Environmental Chemistry, 5th ed.; Lewis Publishers: Chelsea, MI, USA, 1991. [Google Scholar]
- Atalay, E.; Gode, F.; Sharma, Y.C. Removal of selected toxic metals by a modified adsorbent. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2010, 14, 132–138. [Google Scholar] [CrossRef]
- Mohamed, N.A. Synthesis, characterization and evaluation of in vitro potential antimicrobial efficiency of new chitosan hydrogels and their CuO nanocomposites. Int. J. Biol. Macromol. 2024, 276, 133810. [Google Scholar] [CrossRef] [PubMed]
- Reza, B.L.; Hamid, R.Z.; Mohammad, E.B. Removal of cupric ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2014, 45, 1642–1648. [Google Scholar] [CrossRef]
- Uchenna, O.; Mamookho, M.; Lukhanyo, M.; Nkemdinma, U.O.; Tendani, S.; Vuyo, M. Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and United Nations Environment Programme. Health Risks from Marine Pollution in the Mediterranean. Part VII. In Evaluation of Health Risks from Chemically-Contaminated Seafood; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 1995. [Google Scholar]
- The Council of the European Communities. Directive 82/176/EEC—On Pollution Caused by Certain Dangerous Substances Discharged into the Aquatic Environment of the Community; European Union: Brussels, Belgium, 1982. [Google Scholar]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives. a review of the structure-activity relationship. Biomacromolecules 2017, 13, 3846–3868. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Petroudi, S.H.H.; Omagh, S.M.; Romanize, G. Toward understanding the antibacterial mechanism of chitosan: Experimental approach and in silico analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- Ngah, W.S.; Wan, F.S. Adsorption characterization of Pb (II) and Cu (II) ions onto chitosan-tripolyphosphate beads: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2010, 91, 958–969. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Terephthalohydrazido cross-linked chitosan hydrogels: Synthesis, characterization and applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 969–982. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Synthesis and characterization of novel uracil-modified chitosan as a promising adsorbent for efficient removal of Congo red dye. Polymers 2022, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Enhancement of adsorption of Congo red dye onto novel antimicrobial trimellitic anhydride isothiocyanate-cross-linked chitosan hydrogels. Polym. Bull. 2022, 77, 6135–6160. [Google Scholar] [CrossRef]
- Annu, S.; Ahmed, S.; Ikram, S. Chitin and chitosan: History, composition and properties. In Chitosan: Derivatives, Composites and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 3–24. [Google Scholar] [CrossRef]
- Wen, L.; Chen, X.; Chen, C.; Yang, R.; Gong, M.; Zhang, Y.; Fu, Q. Ice-templated porous polymer/UiO-66 monolith for Congo red adsorptive removal. Arab. J. Chem. 2020, 13, 5669–5678. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Benhalima, T.; Lerari, D. Sustainable hydrogel nanocomposites based on grafted chitosan and clay for effective adsorption of cationic dye. Int. J. Mater. Metallurgical Eng. 2020, 14, 5–15. [Google Scholar]
- Alfuraydi, R.T.; Alminderej, F.M.; Mohamed, N.A. Evaluation of antimicrobial and anti-biofilm formation activities of novel poly(vinyl alcohol) hydrogels reinforced with crosslinked chitosan and silver nano-particles. Polymers 2022, 14, 1619. [Google Scholar] [CrossRef]
- Shrestha, R.; Thenissery, A.; Khupse, R.; Rajashekara, G. Strategies for the preparation of chitosan derivatives for antimicrobial, drug delivery, and agricultural applications: A Review. Molecules 2023, 28, 7659. [Google Scholar] [CrossRef]
- Alharbi, R.A.; Alminderej, F.M.; Al-harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Design, synthesis, and characterization of novel bis-uracil chitosan hydrogels modified with zinc oxide nanoparticles for boosting their antimicrobial activity. Polymers 2023, 15, 980. [Google Scholar] [CrossRef]
- Woźniak, A.; Biernat, M. Methods for crosslinking and stabilization of chitosan structures for potential medical applications. J. Bioact. Compat. Polym. 2022, 37, 151–167. [Google Scholar] [CrossRef]
- Shanmugapriya, A.; Ramya, R.; Ramasubramaniam, S.; Sudha, P.N. Studies on removal of Cr(VI) and cupric ions using chitosan grafted-polyacrylonitrile. Arch. Appl. Sci. Res. 2011, 3, 424–435. [Google Scholar]
- Xiao, X.; Liang, H.; Chen, W.; Wang, Z. Synthesis and adsorption behavior of chitosan-coated MnFe2O4 nanoparticles for trace heavy metal ions removal. Appl. Surf. Sci. 2013, 285, 498–504. [Google Scholar] [CrossRef]
- Tonetti, C.; Ferrero, F.; Periolatto, M.; Vineis, C.; Varesano, A.; Mazzuchetti, G. Chitosan coated cotton textiles for copper and chromium ions adsorption. In Proceedings of the XIIIth International Izmir Textile and Apparel Symposium, Izmir, Turkey, 2–5 April 2014; pp. 121–126. [Google Scholar]
- Lv, L.; Chen, N.; Feng, C.; Zhanga, J.; Li, M. Heavy metal ions removal from aqueous solution by xanthate-modified cross-linked magnetic chitosan/poly(vinyl alcohol) particle. RSC Adv. 2017, 7, 27992–28000. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Wan, I.W.A.; Hadi, J.B. Equilibrium, kinetic and mechanism studies of cupric and Cd(II) ions adsorption by modified chitosan beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Taleghanib, H.G.; Khanjani, J. Efficient removal of copper ion from aqueous solution using crosslinked chitosan grafted with polyaniline. I.J.E. Trans. B Appl. 2021, 34, 305–312. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, J.; Cai, J. Preparation of guanidinylated carboxymethyl chitosan and its application in the diffusive gradients in thin films (DGT) technique for measuring labile trace metals in water. Int. J. Environ. Anal. Chem. 2018, 98, 1275. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, isotherm and thermodynamic studies for efficient adsorption of Congo Red dye from aqueous solution onto novel cyanoguanidine-modified chitosan adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Ladha, D.G.; Wadhwani, P.M.; Shah, N.K. Comparative study of chitin and chitosan beads for the adsorption of hazardous anionic azo dye Congo red from wastewater. Desalin. Water Treat. 2015, 57, 1–16. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A.-q. Removal of Congo red from aqueous solution using a chitosan/organomontmorillonite nanocomposite. J. Chem. Technol. Biotechnol. 2007, 82, 711–720. [Google Scholar] [CrossRef]
- Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Preparation and characterization of a new bis-uracil chitosan-based hydrogel as efficient adsorbent for removal of anionic Congo red dye. Polymers 2023, 15, 1529. [Google Scholar] [CrossRef]
- Shukla, K.; Verma, A.; Verma, L.; Rawat, S.; Singh, J. A novel approach to utilize disposable paper cups for the development of adsorbent and its application for the malachite green and rhodamine-B dyes removal from aqueous solutions. Nat. Environ. Pollut. Technol. 2020, 19, 57–70. [Google Scholar]
- Antony, V.S.; Shobana, N.; Durga, S.; Chamarthy, S. Utilization of chitosan-coated superparamagnetic iron oxide nanoparticles for chromium removal. Appl. Water Sci. 2018, 8, 192. [Google Scholar] [CrossRef]
- Taha, A.A.; Ahmed, A.M.; Abdel-Rahman, H.H.; Abouzeid, F.M.; Abdel Maksoud, M.O. Removal of nickel ions by adsorption on nano bentonite: Equilibrium, kinetic and thermodynamics. J. Dispersion Sci. Technol. 2017, 38, 757–767. [Google Scholar] [CrossRef]
- Najim, T.S.; Elais, N.J.; Dawood, A.A. Adsorption of copper and iron using low cost material as adsorbent. J. Chem. 2009, 6, 161–168. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Dong, A.; De Castro, L.T.; Ab Rahman, F.B.; Lipp, J.; Blom, D.A.; Regalbuto, J.R. Pushing the limits of electrostatic adsorption: Charge enhanced dry impregnation of SBA-15. Catal. Today 2019, 338, 60–71. [Google Scholar]
- Gosiaco, P.K.L.; Calvo, L.C.A.L.; Bregente, J.C.; Ponce, M.A.; Sanchez, J.M.P. Biosorption ability of starfruit (Averrhoa carambola L.) in removing cadmium and lead in contaminated water samples. Int. J. Commun. Syst. 2019, 7, 919–923. [Google Scholar]
- Esposito, A.; Pagnanelli, F.; Lodi, A.; Solisio, C.; Vegliò, F. Biosorption of heavy metals by sphaerotilus natans: An equilibrium study at different pH and biomass concentrations. Hydrometallurgy 2001, 60, 129–141. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr (VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Effective removal of Basic red 12 dye by novel antimicrobial trimellitic anhydride isothiocyanate-cross linked chitosan hydrogels. Polym. Polym. Compos. 2021, 29, S274. [Google Scholar] [CrossRef]
- Ola, A. Kinetic and isotherm studies of copper (II) removal from wastewater using various adsorbents. Egypt. J. Aquat. Res. 2007, 33, 125–143. [Google Scholar]
- Vijayaraghavan, K.; Teo, T.T.; Balasubramanian, R.; Joshi, U.M. Application of sargassum biomass to remove heavy metal ions from synthetic multimetal solutions and urban storm water runoff. J. Hazard. Mater. 2009, 164, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Yu, X.Y.; Luo, T.; Jia, Y.; Liu, J.H.; Huang, X.J. Γ-Fe2O3 nanoparticles encapsulated millimeter-sized magnetic chitosan beads for removal of Cr (VI) from water: Thermodynamics, kinetics, regeneration, and uptake mechanisms. J. Chem. Eng. Data. 2013, 58, 3142–3149. [Google Scholar] [CrossRef]
- Vakili, M.; Zwain, H.M.; Mojiri, A.; Wang, W.; Gholami, F.; Gholami, Z.; Giwa, A.S.; Wang, B.; Cagnetta, G.; Salamatinia, B. Effective Adsorption of Reactive Black 5 onto Hybrid Hexadecylamine Impregnated Chitosan-Powdered Activated Carbon Beads. Water 2020, 12, 2242. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siéwé, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of cupric ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Yang, L.W.; Qi, P.Y.; Feng, Q.C.; Hua, X.G.; Jie, H.J.; Liang, Z.C.; Lai, B. Enhanced adsorption/photocatalytic removal of cupric from wastewater by a novel magnetic chitosan and bismuth tungstate coated by silver (MCTS-Ag/Bi2WO6) composite. Chemosphere 2021, 263, 128120. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Hu, J.; Wang, J.L. Competitive adsorption of Pb(II), cupric and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221–222, 155–161. [Google Scholar] [CrossRef]
- Kannamba, B.; Reddy, K.L.; AppaRao, B.V. Removal of cupric from aqueous solutions using chemically modified chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef]
- Wahid, F.; Mohammadzai, I.U.; Khan, A.; Shah, Z.; Hassan, W.; Ali, N. Removal of toxic metals with activated carbon prepared from Salvadora persica. Arab. J. Chem. 2017, 10, 2205–2212. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.; Muñiz-Valencia, B.P.A.A.R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Alrasheedi, M.; Mohammed, A.e.M.E.; Soliman, S.M.A.; Mohamed, N.A. Effective removal of Cu(II) ions from aqueous solution by cross-linked chitosan-based hydrogels. Water 2024, 16, 2324. [Google Scholar] [CrossRef]
- Alrasheedi, M.; Mohammed, A.e.M.E.; Al-harby, N.F.; Khedr, G.E.; Mohamed, N.A. Adsorptive elimination of Cu(II) ions from aqueous solution onto chitosan modified with uracil. Water 2024, 16, 3695. [Google Scholar] [CrossRef]
- Yin, H.; Xiong, Q.; Zhang, M.; Wang, B.; Zhang, F. Multi-principles analysis of cupric adsorption in water on magnetic microspheres and modified chitosan. J. Environ. Chem. Eng. 2023, 11, 111285. [Google Scholar] [CrossRef]
- Vafakish, B.; Wilson, L.D. Cu (II) ion adsorption by aniline grafted chitosan and its responsive fluorescence properties. Molecules 2020, 25, 1052. [Google Scholar] [CrossRef]
- Zhang, H.; Dang, Q.; Liu, C.; Cha, D.; Yu, Z.; Zhu, W.; Fan, B. Uptake of Pb(II) and Cd (II) on chitosan microsphere surface successively grafted by methyl acrylate and diethylenetriamine. ACS Appl. Mater. Interfaces 2017, 9, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.K.; Kabir, S.M.F.; Rahman, F.B.A.; Sakib, M.N.; Efty, S.S.; Rahman, M.M. Cupric removal from wastewater using chitosan-based adsorbents: A review. J. Environ. Chem. Eng. 2022, 10, 108048. [Google Scholar] [CrossRef]
- Ibrahim, E.L.S.; Moustafa, H.; El-Molla, S.A.; Abdel Halim, S.; Ibrahim, S.M. Integrated experimental and theoretical insights for Malachite green dye adsorption from wastewater using low cost adsorbent. Water Sci. Technol. 2021, 84, 3833–3858. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Al-Harby, N.F.; El-Molla, S.A.; Ibrahim, E. New combined Experimental and DFT studies for adsorption of sole Azo- dye and a mixture of two cationic dyes from water on a cheap adsorbent. Sci. Rep. 2024, 14, 14756. [Google Scholar] [CrossRef]
- Huang, J.; Ye, M.; Qu, Y.Q.; Chu, L.F.; Chen, R.; He, Q.Z.; Xu, D.F. Pb (II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J. Colloid Interface Sci. 2012, 385, 137–146. [Google Scholar] [CrossRef]
- Sun, X.; Yin, S.; Zhao, L.; Yang, W.; You, Y. Adsorption properties of methylene blue and cupric on magnetically oxidized tannic acid cross-linked carboxymethyl chitosan gels. Int. J. Biol. Macromol. 2024, 278, 134709. [Google Scholar] [CrossRef]
- Hangan, A.C.; Lucaciu, R.L.; Turza, A.; Dican, L.; Sevastre, B.; Páll, E.; Oprean, L.S.; Borodi, G. New copper complexes with antibacterial and cytotoxic activity. Int. J. Mol. Sci. 2023, 24, 13819. [Google Scholar] [CrossRef] [PubMed]
- Valach, F. A bond-valence approach to the semicoordination of copper-oxygen and copper–nitrogen complexes. Polyhedron 1999, 18, 699–706. [Google Scholar] [CrossRef]
- Dev, V.V.; Baburaj, G.; Antony, S.; Arun, V.; Krishnan, K.A. Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and cupric from multi-metal aqueous systems. J. Clean. Prod. 2020, 255, 120309. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A.; Abd El-Ghany, N.A. Evaluation of the antimicrobial and anti-biofilm of novel salicylhydrazido chitosan derivatives impregnated with titanium dioxide nanoparticles. Int. J. Biol. Macromol. 2022, 205, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Elmehbad, N.Y.; Mohamed, N.A.; Abd El-Ghany, N.A.; Abdel-Aziz, M.M. Reinforcement of the antimicrobial activity and biofilm inhibition of novel chitosan-based hydrogels utilizing zinc oxide nanoparticles. Int. J. Biol. Macromol. 2023, 246, 125582. [Google Scholar] [CrossRef]
- Soon, C.Y.; Rahman, N.A.; Tee, Y.B.; Talib, R.A.; Tan, C.H.; Abdan, K.; Chan, E.W.C. Electrospun biocomposite: Nanocellulose and chitosan entrapped within a poly (hydroxyalkanoate) matrix for Congo red removal. J. Mater. Res. Technol. 2019, 8, 5091–5102. [Google Scholar] [CrossRef]
- Alves, D.C.; Coseglio, B.B.; Pinto, L.A.; Cadaval, T.R., Jr. Development of spirulina/chitosan foam adsorbent for phenol adsorption. J. Mol. Liq. 2020, 309, 113256. [Google Scholar] [CrossRef]


 C;
C;  Cu;
Cu;  O;
O;  H;
H;  N.
N.
 C;
C;  Cu;
Cu;  O;
O;  H;
H;  N.
N.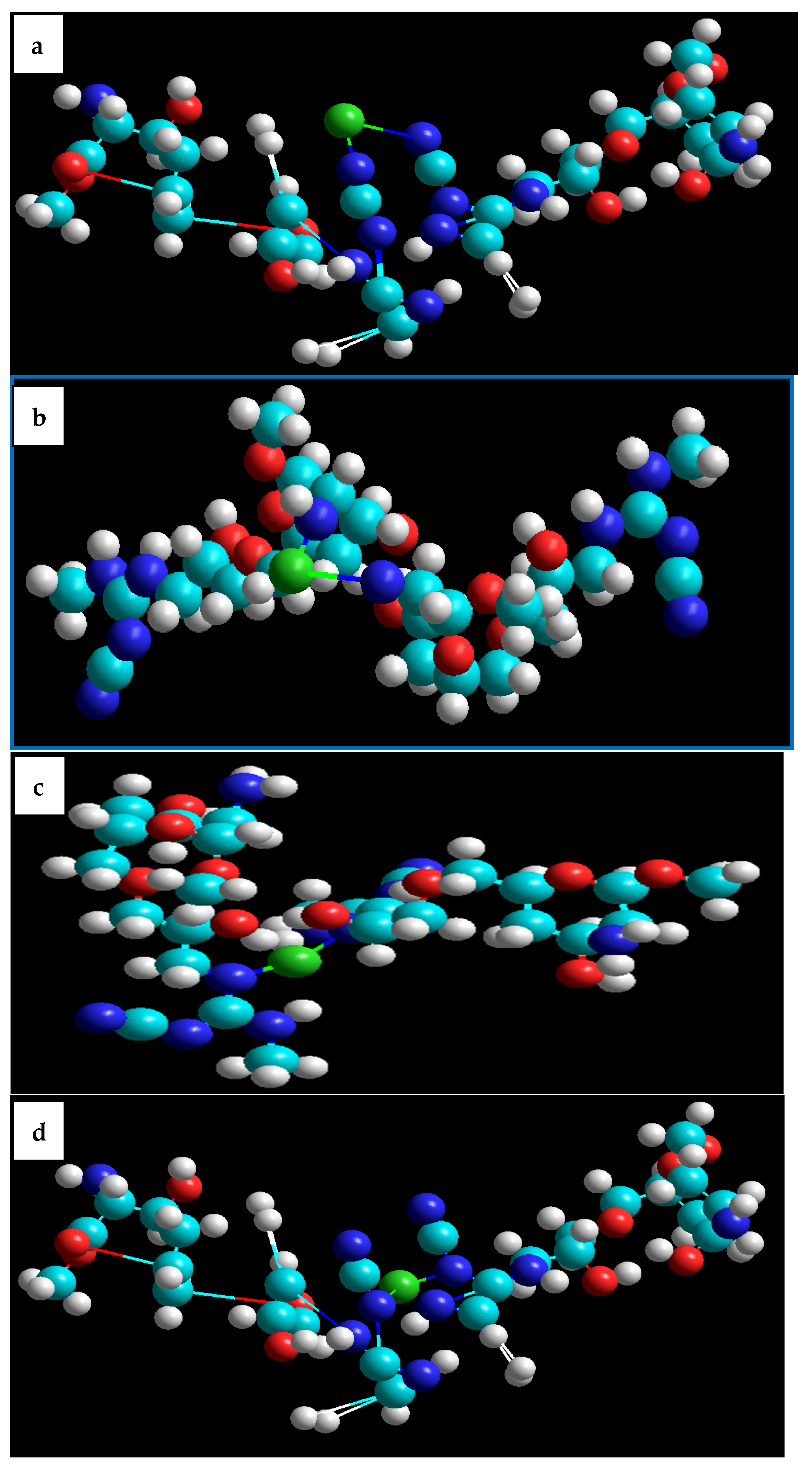
 C;
C;  Cu;
Cu;  O;
O;  H;
H;  N.
N.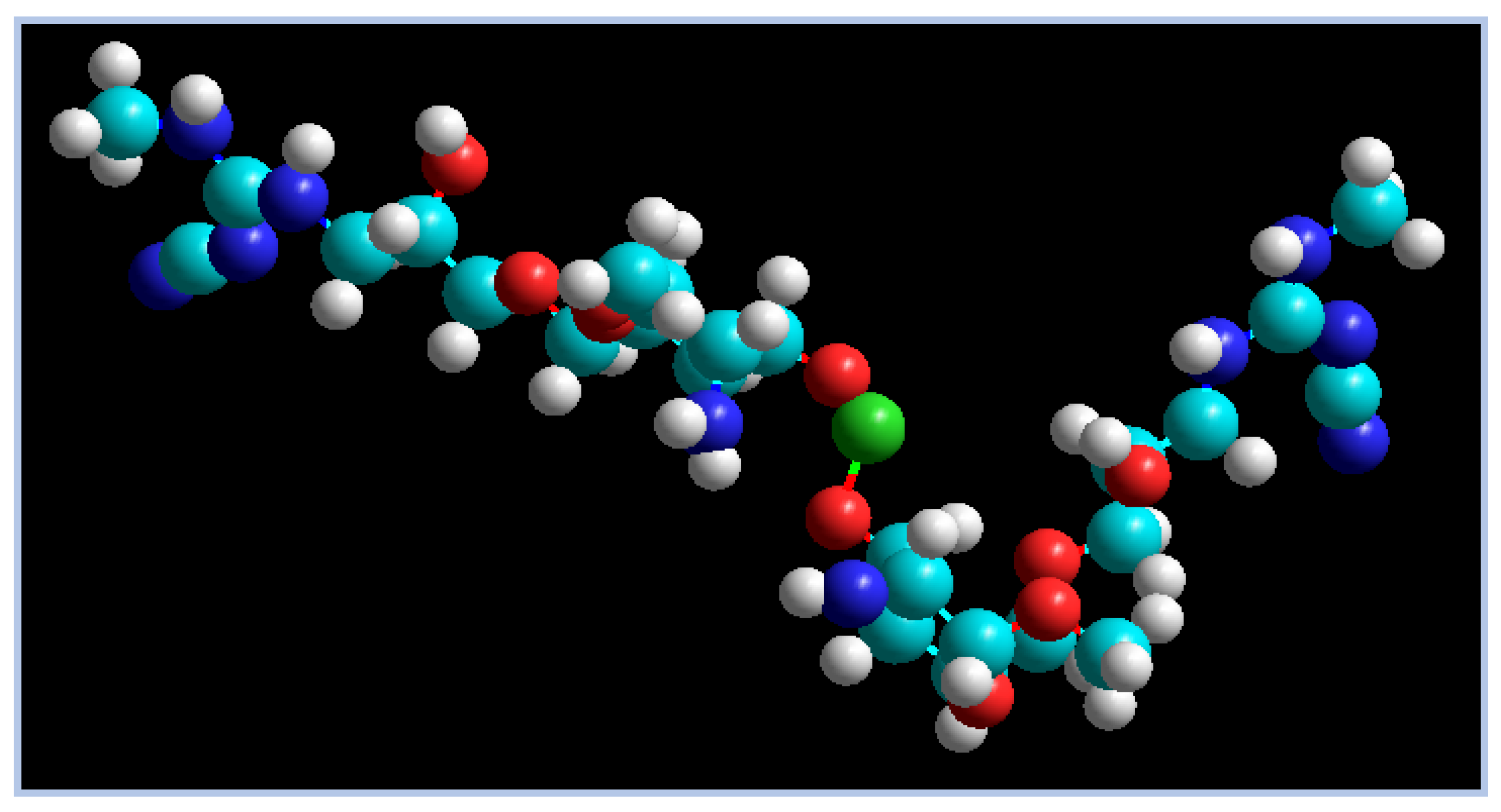
| Kinetic Models | Parameters | |
|---|---|---|
| qe.exp (mg g−1) | 99.05 | |
| Pseudo- first-order | R2 | 0.924 |
| qe.cal (mg g−1) | 1.781 | |
| k1 (min−1) | 0.023 | |
| Pseudo- second-order | R2 | 1 |
| qe,cal (mg g−1) | 100 | |
| k2 (10−5) (g mg−1 min−1) | ||
| 0.050 | ||
| Elovich | R2 | 0.91 |
| β (g mg−1) | 0.975 | |
| α (1039) (mg g−1 min−1) | ||
| 4.706 | ||
| Intraparticle diffusion | R2 | 0.919 |
| k (mg g−1 min−1/2) | 0.485 | |
| Models | Parameter | |
|---|---|---|
| Langmuir | qmax(mg g−1) | 28.571 |
| RL | 1.471–0.147 | |
| KL (L mg−1) | 0.034 | |
| R2 | 0.935 | |
| Freundlich | 1/n | 3.21 |
| Kf (mg g−1) | 0.004 | |
| R2 | 0.96 | |
| Temkin | B (kJ mol−1) | 238.291 |
| KT (L g−1) | 0.07 | |
| R2 | 0.825 | |
| D-R | qm (mg g−1) | 334.655 |
| EkJ mol−1 | 12.504 | |
| B × 10−4 | 1 | |
| R2 | 0.919 |
| Adsorbent | qmax (mg g−1) | Temperature (°C) | Cupric Ion Conc. (mg L−1) | Adsorbent Dose (g) | pH | Ref. |
|---|---|---|---|---|---|---|
| Crosslinked chitosan-g-poly(aniline) | 131.58 | 20–40 | 100 | 0.05 | 6 | [40] |
| Quaternary chitosan microsphere | 687.6 | 25 | 0–2000 | 0.075 | 5 | [62] |
| Calcined horn core | 99.98 | 25 | 100–500 | 0.02 | 5 | [63] |
| Magnetized chitosan/Ag-coated Bi2WO6 | 181.8 | 20–40 | 20–120 | 0.02 | 6 | [64] |
| Magnetized chitosan modified with xanthate | 34.5 | 25 | 100 | - | 5 | [65] |
| Crosslinked chitosan/xanthate with epichlorohydrin | 43.47 | 50 | 100 | - | 5 | [66] |
| Medical Salvadora persica plant | 74.30 | 25 | 100 | - | 4 | [67] |
| Nutshell of pecan | 23.37 | 30 | - | - | 5 | [68] |
| H1 | 96.20 | 25 | 100 | 0.01 | 6 | [69] |
| H2 | 97.59 | 25 | 100 | 0.01 | 6 | [69] |
| UCs | 99.65 | 25 | 100 | 0.01 | 6 | [70] |
| CCs | 99.05 | 25 | 100 | 0.01 | 6 | Present study |
| Active Site | ET (Kcal/mol) | Eads (Kcal/mol) | EHOMO (ev) | ELUMO (ev) | * Energy Gap (ev) |
|---|---|---|---|---|---|
| C=N | −221,601 | −9254 | −8.09 | −0.09 | 8.00 |
| C≡N | −221,599 | −9250 | −7.69 | −0.68 | 7.10 |
| –NH2 | −220,130 | −8989 | −8.23 | −0.45 | 7.78 |
| –NH | −220,103 | −8961 | −8.55 | −0.47 | 8.10 |
| –OH | −220,069 | −8928 | −5.72 | −1.57 | 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.e.M.E.; Al-Harby, N.F.; Alrasheedi, M.; Ibrahim, S.M.; Mohamed, N.A. Cyanoguanidine-Modified Chitosan as an Efficacious Adsorbent for Removing Cupric Ions from Aquatic Solutions: Kinetics, Isotherms, and Mechanisms. Inorganics 2025, 13, 116. https://doi.org/10.3390/inorganics13040116
Mohammed AeME, Al-Harby NF, Alrasheedi M, Ibrahim SM, Mohamed NA. Cyanoguanidine-Modified Chitosan as an Efficacious Adsorbent for Removing Cupric Ions from Aquatic Solutions: Kinetics, Isotherms, and Mechanisms. Inorganics. 2025; 13(4):116. https://doi.org/10.3390/inorganics13040116
Chicago/Turabian StyleMohammed, Ard elshifa M. E., Nouf F. Al-Harby, Muneera Alrasheedi, Shaimaa M. Ibrahim, and Nadia A. Mohamed. 2025. "Cyanoguanidine-Modified Chitosan as an Efficacious Adsorbent for Removing Cupric Ions from Aquatic Solutions: Kinetics, Isotherms, and Mechanisms" Inorganics 13, no. 4: 116. https://doi.org/10.3390/inorganics13040116
APA StyleMohammed, A. e. M. E., Al-Harby, N. F., Alrasheedi, M., Ibrahim, S. M., & Mohamed, N. A. (2025). Cyanoguanidine-Modified Chitosan as an Efficacious Adsorbent for Removing Cupric Ions from Aquatic Solutions: Kinetics, Isotherms, and Mechanisms. Inorganics, 13(4), 116. https://doi.org/10.3390/inorganics13040116





