Realizing Environmentally Scalable Pre-Lithiation via Protective Coating of LiSi Alloys to Promote High-Energy-Density Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
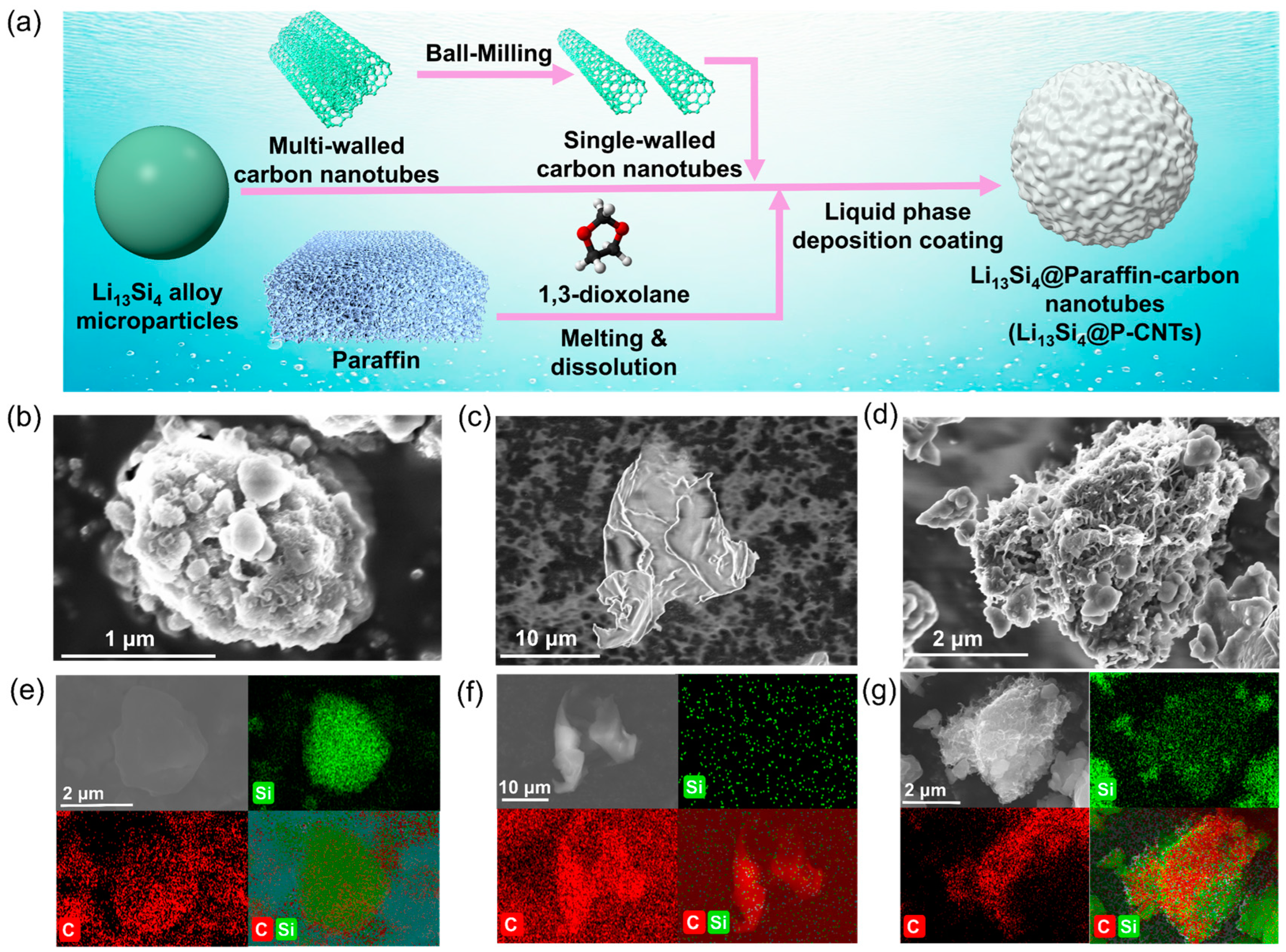
2.1. Synthesis and Morphology of Li13Si4@P-CNTs
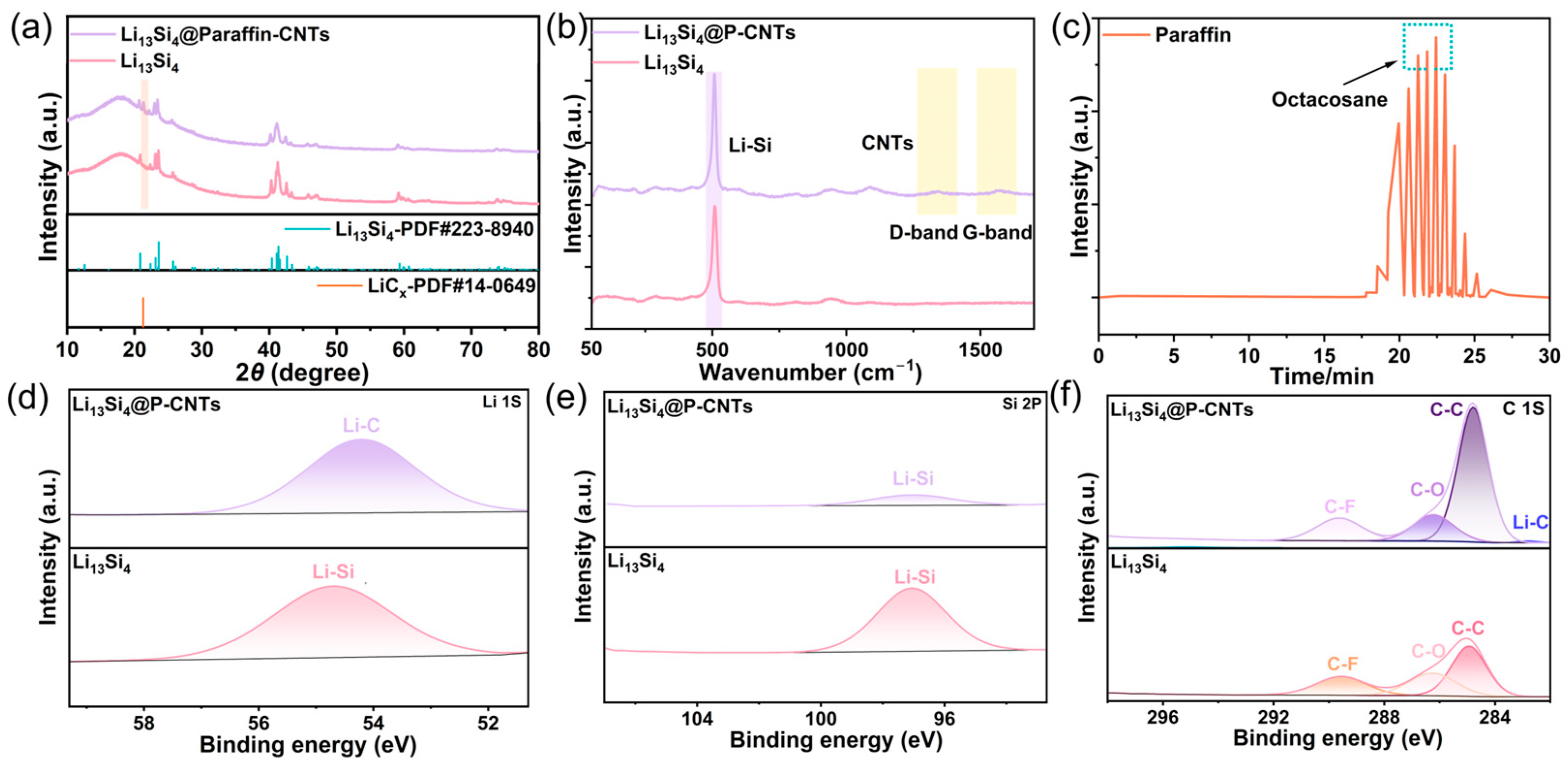
2.2. Crystal Properties and Surface Chemistry Characteristics of Li13Si4@P-CNTs
2.3. Environmental Stability and Structural Evolution of Li13Si4@P-CNTs in Air
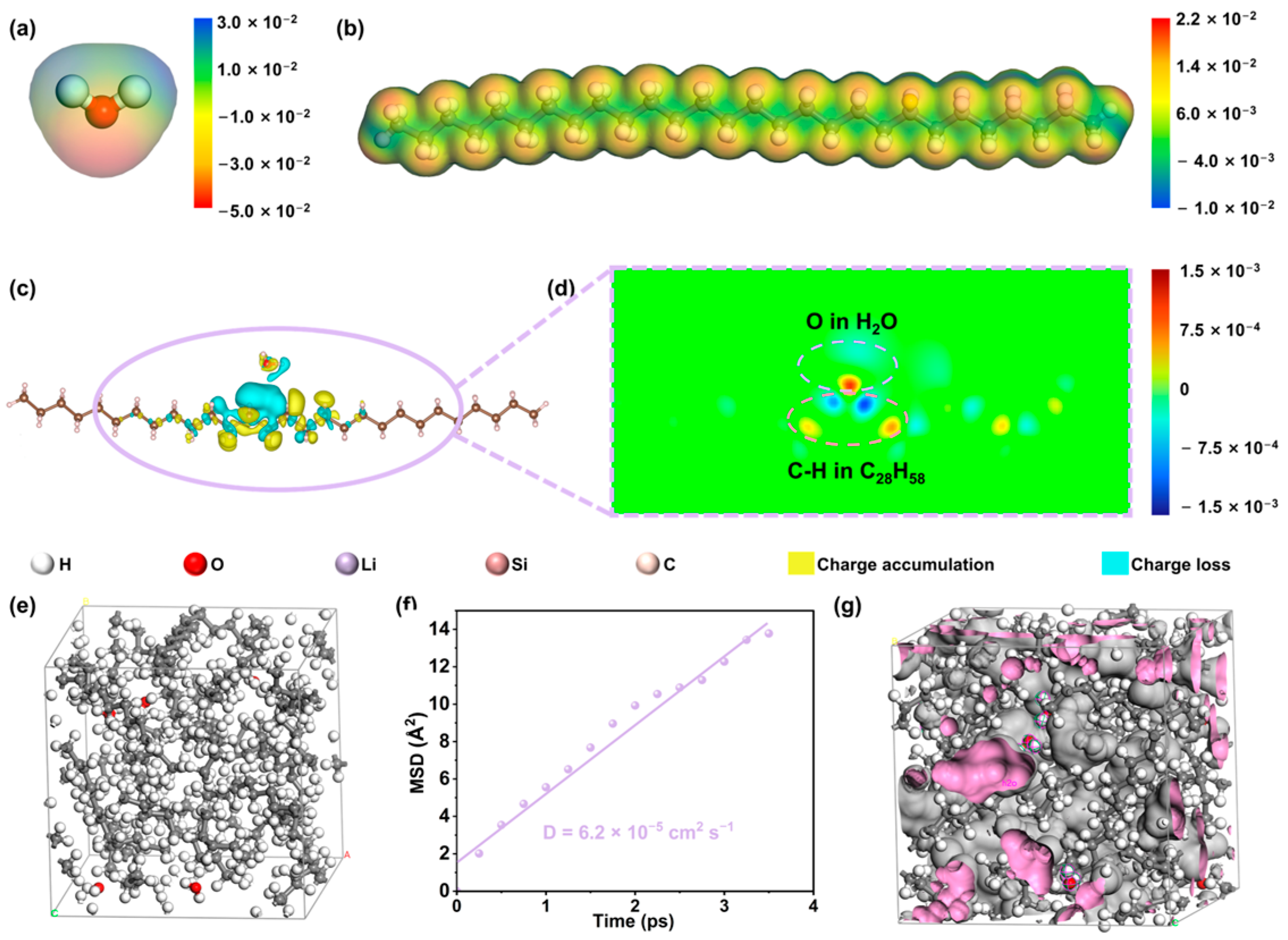
2.4. Theoretical Study on the Hydrophobic Mechanism of Paraffin-Dominant Coating Membrane
2.5. Electrochemical Performance of Li13Si4@PFPE/LiF Pre-Lithiated Anode
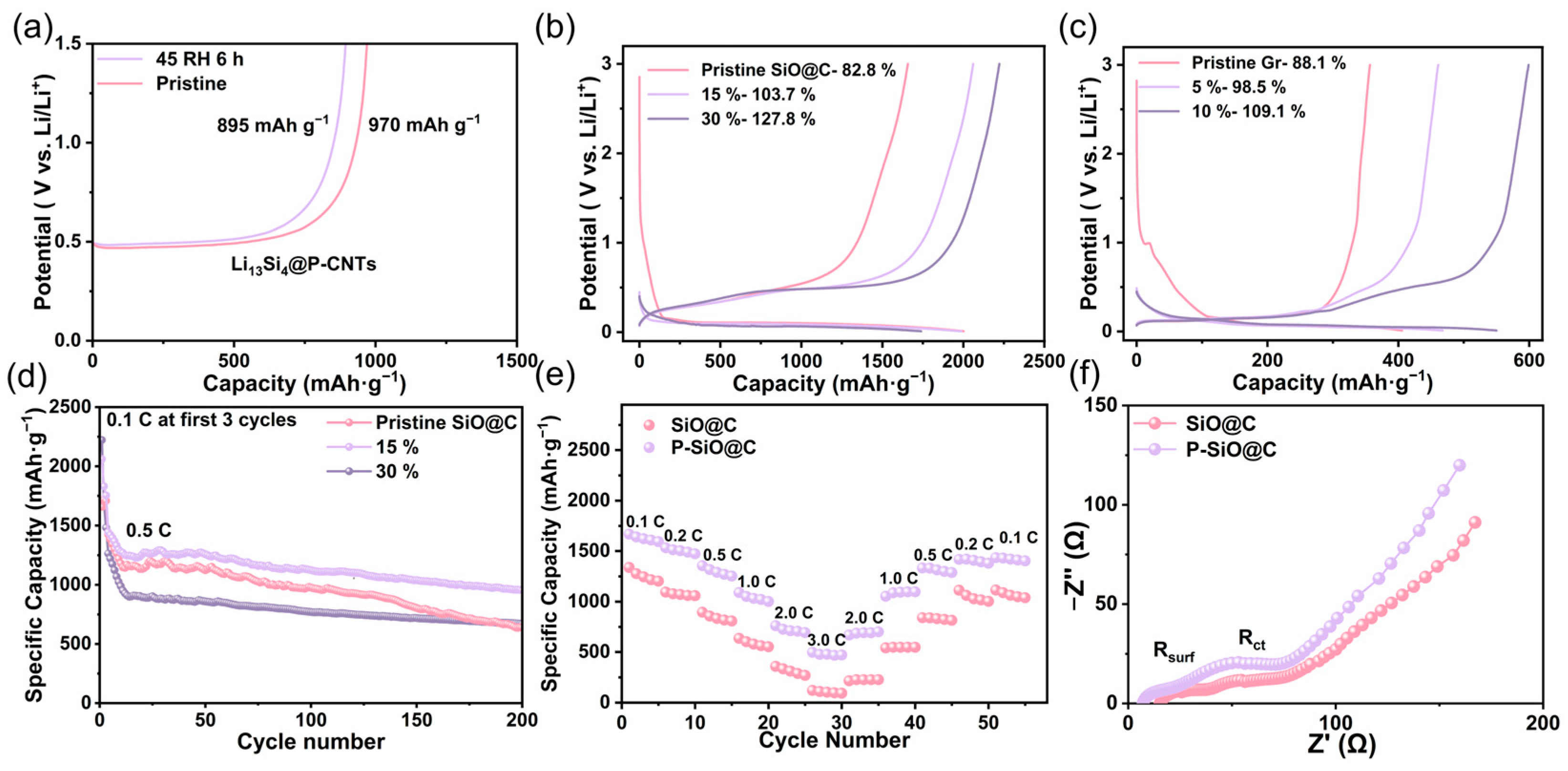
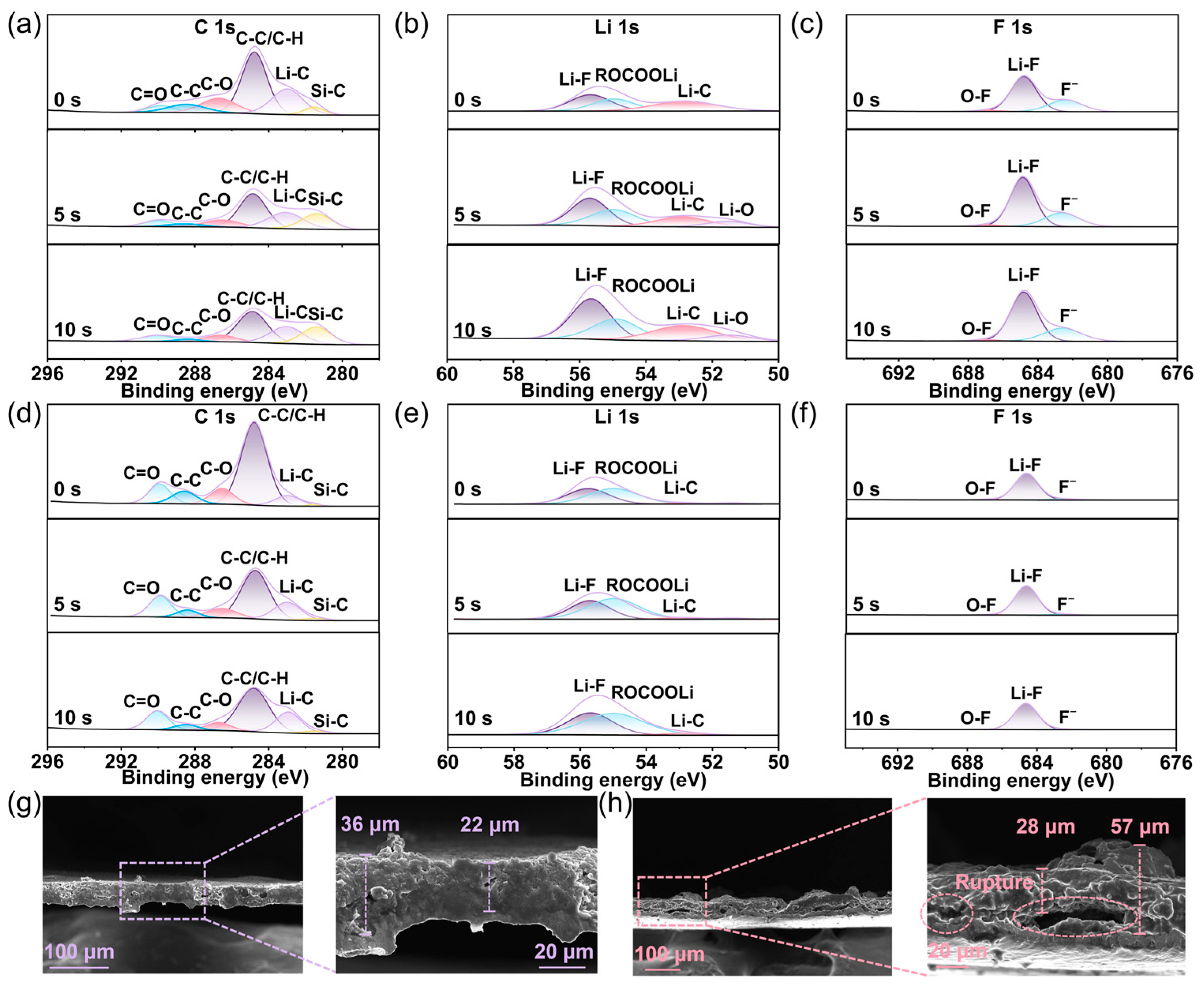
2.6. SEI Regulation of Pre-Lithiation SiO@C via Li13Si4@P-CNTs
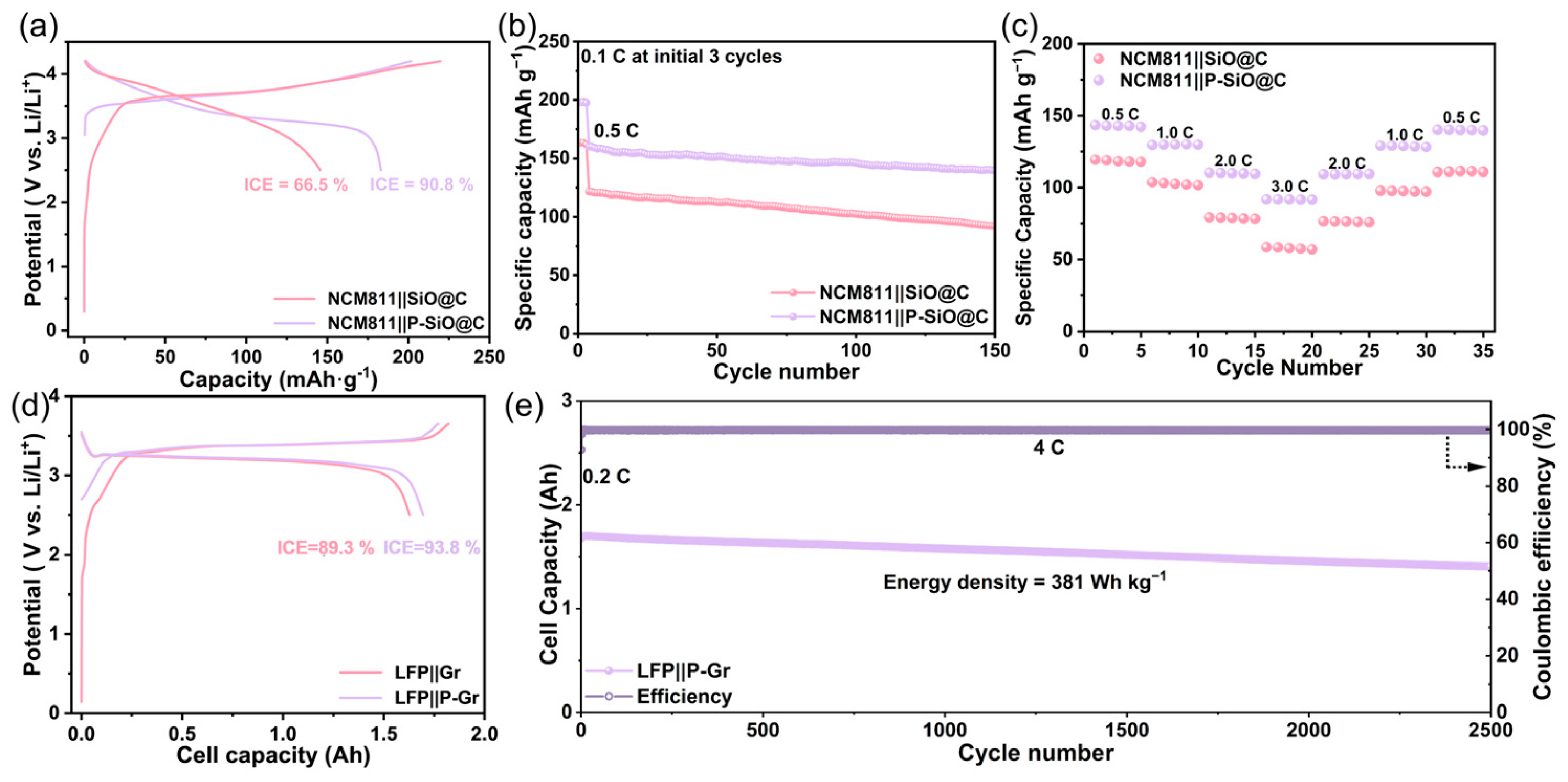
2.7. Full Cell Model Evaluation Based on Li13Si4@PFPE/LiF Pre-Lithiation Strategy
3. Materials and Methods
3.1. Preparation and Synthesis of Li13Si4 and Li13Si4@P-CNTs
3.2. Materials Characterization
3.3. Electrochemical Measurement
3.4. DFT and Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.J.; Behrens, P.; Gasper, P.; Smith, K.; Hu, M.M.; Tukker, A.; Steubing, B. Electric vehicle batteries alone could satisfy short-term grid storage demand by as early as 2030. Nat. Commun. 2023, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar]
- Cano, Z.P.; Banham, D.; Ye, S.Y.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z.W. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Remedies to avoid failure mechanisms of lithium-metal anode in Li-ion batteries. Inorganics 2022, 10, 5. [Google Scholar]
- Roy, J.J.; Phuong, D.; Verma, V.; Chaudhary, R.; Carboni, M.; Meyer, D.; Cao, B.; Srinivasan, M. Direct recycling of Li-ion batteries from cell to pack level: Challenges and prospects on technology, scalability, sustainability and economics. Carbon Energy 2024, 6, 39. [Google Scholar] [CrossRef]
- Klett, M.; Gilbert, J.A.; Pupek, K.Z.; Trask, S.E.; Abraham, D.P. Layered oxide, graphite and silicon-graphite electrodes for lithium-ion cells: Effect of electrolyte composition and cycling windows. J. Electrochem. Soc. 2016, 164, A6095. [Google Scholar]
- Qi, Y.; Harris, S.J. In situ observation of strains during lithiation of a graphite electrode. J. Electrochem. Soc. 2010, 157, A741. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, T.; Chen, J.; Peng, Y.; Fang, Z.; Mo, Y.; Wang, C.; Wang, Y.; Xia, Y.; Dong, X. Uncovering the function of a five-membered heterocyclic solvent-based electrolyte for graphite anode at subzero temperature. Adv. Funct. Mater. 2023, 33, 2215151. [Google Scholar] [CrossRef]
- Chae, C.; Noh, H.J.; Lee, J.K.; Scrosati, B.; Sun, Y.K. A high-energy Li-ion battery using a silicon-based anode and a nano-structured layered composite cathode. Adv. Funct. Mater. 2014, 24, 3036–3042. [Google Scholar] [CrossRef]
- Yeom, S.J.; Lee, C.; Kang, S.; Wi, T.-U.; Lee, C.; Chae, S.; Cho, J.; Shin, D.O.; Ryu, J.; Lee, H.-W. Native void space for maximum volumetric capacity in silicon-based anodes. Nano Lett. 2019, 19, 8793–8800. [Google Scholar]
- Yang, Y.; Ni, C.; Gao, M.; Wang, J.; Liu, Y.; Pan, H. Dispersion-strengthened microparticle silicon composite with high anti-pulverization capability for Li-ion batteries. Energy Storage Mater. 2018, 14, 279–288. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, X.; Zhang, L.; Gao, M.; Liu, Y.; Pan, H. Reaction-ball-milling-driven surface coating strategy to suppress pulverization of microparticle Si anodes. ACS Appl. Mater. Interfaces 2018, 10, 20591–20598. [Google Scholar] [CrossRef]
- Qin, M.; Liu, M.; Zeng, Z.; Wu, Q.; Wu, Y.; Zhang, H.; Lei, S.; Cheng, S.; Xie, J. Rejuvenating propylene carbonate-based electrolyte through nonsolvating interactions for wide-temperature Li-ions batteries. Adv. Energy Mater. 2022, 12, 2201801. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Y.; Zhang, Z.; Yuan, S.; Amine, K.; Battaglia, V.; Liu, G. Application of stabilized lithium metal powder (SLMP) in graphite anode–a high efficient prelithiation method for lithium-ion batteries. J. Power Sources 2014, 260, 57–61. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, X.; Yang, M.; Qian, J.; Cao, Y.; Yang, H.; Luo, Y.; Ai, X. Achieving desirable initial Coulombic efficiencies and full capacity utilization of Li-ion batteries by chemical prelithiation of graphite anode. Adv. Funct. Mater. 2021, 31, 2101181. [Google Scholar] [CrossRef]
- Li, B.; Xiao, Z.; Zai, J.; Chen, M.; Wang, H.; Liu, X.; Li, G.; Qian, X. A candidate strategy to achieve high initial Coulombic efficiency and long cycle life of Si anode materials: Exterior carbon coating on porous Si microparticles. Mater. Today Energy 2017, 5, 299–304. [Google Scholar] [CrossRef]
- Wang, J.; Liao, L.; Lee, H.R.; Shi, F.; Huang, W.; Zhao, J.; Pei, A.; Tang, J.; Zheng, X.; Chen, W. Surface-engineered mesoporous silicon microparticles as high-coulombic-efficiency anodes for lithium-ion batteries. Nano Energy 2019, 61, 404–410. [Google Scholar] [CrossRef]
- Ma, W.; Wu, H.; Long, T.; Cai, Y.; Yu, Z.; Liu, C.; Fang, G.; Zhang, Q.; Jia, X. Bamboo inspired silicon anodes with ultrahigh initial Coulombic efficiency and high capacity for the Li-ion batteries. Small 2024, 20, 2308109. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, B.Y.; Lee, J.S.; Kim, J.S.; Nahm, S. Microstructures and electrochemical performances of nano-sized SiOx (1.18 ≤ x ≤ 1.83) as an anode material for a lithium (Li)-ion battery. J. Power Sources 2013, 244, 115–121. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Liu, Z.; Zheng, J.; Zuo, Y.; Xue, C.; Li, C.; Cheng, B. High-performance ball-milled SiOx anodes for lithium ion batteries. J. Power Sources 2017, 339, 86–92. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, G.; Chen, C.; Jiao, Y.; Li, T.; Chen, X.; Yang, Y.; Yang, D.; Dong, A. An affordable manufacturing method to boost the initial Coulombic efficiency of disproportionated SiO lithium-ion battery anodes. J. Power Sources 2019, 426, 116–123. [Google Scholar]
- Li, Y.; Zheng, X.; Cao, Z.; Wang, Y.; Wang, Y.; Lv, L.; Huang, W.; Huang, Y.; Zheng, H. Unveiling the mechanisms into Li-trapping induced (Ir) reversible capacity loss for silicon anode. Energy Storage Mater. 2023, 55, 660–668. [Google Scholar]
- Sun, L.; Liu, Y.; Wu, J.; Shao, R.; Jiang, R.; Tie, Z.; Jin, Z. A review on recent advances for boosting initial coulombic efficiency of silicon anodic lithium ion batteries. Small 2022, 18, 2102894. [Google Scholar]
- Zhan, R.; Wang, X.; Chen, Z.; Seh, Z.W.; Wang, L.; Sun, Y. Promises and challenges of the practical implementation of prelithiation in lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2101565. [Google Scholar]
- Wang, R.; Li, H.; Wu, Y.; Li, H.; Zhong, B.; Sun, Y.; Wu, Z.; Guo, X. How to promote the industrial application of SiOx anode prelithiation: Capability, accuracy, stability, uniformity, cost and safety. Adv. Energy Mater. 2022, 12, 2202342. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, J.; Meng, J.; Li, C. C–F-rich oil drop as a non-expendable fluid interface modifier with low surface energy to stabilize a Li metal anode. Energy Environ. Sci. 2021, 14, 3621–3631. [Google Scholar] [CrossRef]
- Kamma, N.; Kanaphan, Y.; Buakeaw, S.; Kaewmala, S.; Chaikawang, C.; Nash, J.; Srilomsak, S.; Meethong, N. A polymeric coating on prelithiated silicon-based nanoparticles for high capacity anodes used in Li-ion batteries. Suan Sunandha Sci. Technol. J. 2020, 7, 30–36. [Google Scholar]
- Zhao, J.; Lee, H.-W.; Sun, J.; Yan, K.; Liu, Y.; Liu, W.; Lu, Z.; Lin, D.; Zhou, G.; Cui, Y. Metallurgically Lithiated SiOx Anode with High Capacity and Ambient Air Compatibility. Proc. Natl. Acad. Sci. 2016, 113, 7408–7413. [Google Scholar]
- Zhao, J.; Lu, Z.; Liu, N.; Lee, H.-W.; McDowell, M.T.; Cui, Y. Dry-air-stable lithium silicide–lithium oxide core–shell nanoparticles as high-capacity prelithiation reagents. Nat. Commun. 2014, 5, 5088. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, H.; Liu, W.; Lee, H.-W.; Yan, K.; Zhuo, D.; Lin, D.; Liu, N.; Cui, Y. Artificial solid electrolyte interphase-protected LixSi nanoparticles: An efficient and stable prelithiation reagent for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 8372–8375. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, L.; Shi, F.; Lei, T.; Chen, G.; Pei, A.; Sun, J.; Yan, K.; Zhou, G.; Xie, J. Surface fluorination of reactive battery anode materials for enhanced stability. J. Am. Chem. Soc. 2017, 139, 11550–11558. [Google Scholar] [CrossRef] [PubMed]
- Paduani, C.; Rappe, A.M. Assemblage of superalkali complexes with ever low-ionization potentials. J. Phys. Chem. A 2016, 120, 6493–6499. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, J.; Jia, J.; Kandasamy, T.; Bobrov, K.; Guillemot, L.; Fuhr, J.D.; Martiarena, M.L.; Esaulov, V.A. Li+-ion neutralization on metal surfaces and thin films. Phys. Rev. A At. Mol. Opt. Phys. 2011, 84, 052901. [Google Scholar] [CrossRef]
- Atta, A.M.; Mohamed, N.H.; Rostom, M.; Al-Lohedan, H.A.; Abdullah, M.M. New hydrophobic silica nanoparticles capped with petroleum paraffin wax embedded in epoxy networks as multifunctional steel epoxy coatings. Prog. Org. Coat. 2019, 128, 99–111. [Google Scholar] [CrossRef]
- Dhand, V.; Rao, M.V.; Mittal, G.; Rhee, K.Y.; Park, S.J. Synthesis of lithium–graphite nanotubes–An in-situ CVD approach using organo-lithium as a precursor in the presence of copper. Curr. Appl. Phys. 2015, 15, 265–273. [Google Scholar]
- Sasrimuang, S.; Chuchuen, O.; Artnaseaw, A. Synthesis, characterization and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth. Green Process. Synth. 2020, 9, 340–348. [Google Scholar] [CrossRef]
- Stearns, L.A.; Gryko, J.; Diefenbacher, J.; Ramachandran, G.K.; McMillan, P.F. Lithium monosilicide (LiSi), a low-dimensional silicon-based material prepared by high pressure synthesis: NMR and vibrational spectroscopy and electrical properties characterization. J. Solid State Chem. 2003, 173, 251–258. [Google Scholar] [CrossRef]
- Qin, Z.; Xie, Y.; Meng, X.; Qian, D.; Shan, C.; Mao, D.; He, G.; Zheng, Z.; Wan, L.; Huang, Y. Interface engineering for garnet-type electrolyte enables low interfacial resistance in solid-state lithium batteries. Chem. Eng. J. 2022, 447, 137538. [Google Scholar] [CrossRef]
- Watcharinyanon, S.; Johansson, L.; Zakharov, A.; Virojanadara, C. Studies of Li intercalation of hydrogenated graphene on SiC (0001). Surf. Sci. 2012, 606, 401–406. [Google Scholar] [CrossRef]
- Wang, H.; Shao, A.; Pan, R.; Tian, W.; Jia, Q.; Zhang, M.; Bai, M.; Wang, Z.; Liu, F.; Liu, T. Unleashing the potential of high-capacity anodes through an interfacial prelithiation strategy. ACS Nano 2023, 17, 21850–21864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, L.; Zhao, H.; Fu, Y. Significantly improved electrochemical performance of CFxpromoted by SiO2 modification for primary lithium batteries. J. Mater. Chem. A 2017, 5, 796–803. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Kruse, N. Surface composition and electronic properties of Co-Cu mixed oxalates: A detailed XPS analysis. Appl. Surf. Sci. 2024, 669, 160460. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Dobyns, B.M.; Kim, J.M.; Beckingham, B.S. Multicomponent transport of methanol and sodium acetate in poly (ethylene glycol) diacrylate membranes of varied fractional free volume. Eur. Polym. J. 2020, 134, 109809. [Google Scholar] [CrossRef]
- Kashinath, L.; Byrappa, K. Ceria boosting on in situ nitrogen-doped graphene oxide for efficient bifunctional ORR/OER activity. Front. Chem. 2022, 10, 889579. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; He, J.; Qi, S.; Wu, M.; Wang, H.; Jiang, G.; Huang, J.; Wu, D.; Li, F. Separator-wetted, acid-and water-scavenged electrolyte with optimized Li-ion solvation to form dual efficient electrode electrolyte interphases via hexa-functional additive. Adv. Sci. 2022, 9, 2201297. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Luo, L.; Pang, Y.; Yang, J.; Huang, Y.; Zheng, S. Highly stable and ultrahigh-rate Li metal anode enabled by fluorinated carbon fibers. Small 2021, 17, 2006002. [Google Scholar] [CrossRef]
- Lou, P.; Li, C.; Cui, Z.; Guo, X. Job-sharing cathode design for Li–O2 batteries with high energy efficiency enabled by in situ ionic liquid bonding to cover carbon surface defects. J. Mater. Chem. A 2016, 4, 241–249. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, H.; Quan, K.; Chen, H.; Zhang, S.; Yi, H.; Zhang, J. Chemical anchor of lithium polysulfide through sulfur copolymers for high-performance lithium-sulfur batteries. Electrochim. Acta 2024, 474, 143503. [Google Scholar] [CrossRef]
- Guo, J.; Jin, S.; Sui, X.; Huang, X.; Xu, Y.; Li, Y.; Kristensen, P.K.; Wang, D.; Pedersen, K.; Gurevich, L. Unravelling and quantifying the aging processes of commercial Li(Ni0.5Co0.2Mn0.3)O2/graphite lithium-ion batteries under constant current cycling. J. Mater. Chem. A 2023, 11, 41–52. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.H.; Roh, K.C. Functionalized interfacial cover design toward pure silicon anode for high power density lithium–ion capacitor. Chem. Eng. J. 2024, 490, 151635. [Google Scholar]
- Zhao, W.; Wei, Z.; Lu, C.; Tong, Y.; Huang, J.; Cao, X.; Shi, D.; Li, R.K.; Wu, W. Construction of all-organic low dielectric polyimide hybrids via synergistic effect between covalent organic framework and cross-linking structure. Nano Mater. Sci. 2023, 5, 429–438. [Google Scholar]
- Bai, R.; Wang, H.; Zhang, P.; Xiao, B.; Jiang, B.; Zhou, G. Molecular dynamics simulation of the diffusion behavior of water in poly (vinylidene fluoride)/silica hybrid membranes. RSC Adv. 2015, 5, 57147–57154. [Google Scholar]
- Allaire, R.H.; Dhakane, A.; Emery, R.; Ganesh, P.; Rack, P.D.; Kondic, L.; Cummings, L.; Fuentes-Cabrera, M. Surface, interface and temperature effects on the phase separation and nanoparticle self assembly of Bi-metallic Ni0.5Ag0.5: A molecular dynamics study. Nanomaterials 2019, 9, 1040. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, W.; Zhang, C.; Jia, P.; Zhang, Z.; Zheng, Y.; Yan, K.; Wang, J.; Qian, Y.; Guo, J.; et al. Realizing Environmentally Scalable Pre-Lithiation via Protective Coating of LiSi Alloys to Promote High-Energy-Density Lithium-Ion Batteries. Inorganics 2025, 13, 115. https://doi.org/10.3390/inorganics13040115
Liu Y, Jiang W, Zhang C, Jia P, Zhang Z, Zheng Y, Yan K, Wang J, Qian Y, Guo J, et al. Realizing Environmentally Scalable Pre-Lithiation via Protective Coating of LiSi Alloys to Promote High-Energy-Density Lithium-Ion Batteries. Inorganics. 2025; 13(4):115. https://doi.org/10.3390/inorganics13040115
Chicago/Turabian StyleLiu, Yinan, Wei Jiang, Congcong Zhang, Pingshan Jia, Zhiyuan Zhang, Yun Zheng, Kunye Yan, Jun Wang, Yunxian Qian, Junpo Guo, and et al. 2025. "Realizing Environmentally Scalable Pre-Lithiation via Protective Coating of LiSi Alloys to Promote High-Energy-Density Lithium-Ion Batteries" Inorganics 13, no. 4: 115. https://doi.org/10.3390/inorganics13040115
APA StyleLiu, Y., Jiang, W., Zhang, C., Jia, P., Zhang, Z., Zheng, Y., Yan, K., Wang, J., Qian, Y., Guo, J., Chen, R., Huang, Y., Shen, Y., Long, L., Zheng, B., & Shao, H. (2025). Realizing Environmentally Scalable Pre-Lithiation via Protective Coating of LiSi Alloys to Promote High-Energy-Density Lithium-Ion Batteries. Inorganics, 13(4), 115. https://doi.org/10.3390/inorganics13040115







