Light Output Response of a Barium Fluoride (BaF2) Inorganic Scintillator Under X-Ray Radiation
Abstract
1. Introduction
| Units | BaF2 | CeF3 | BGO | GSO:Ce | |
|---|---|---|---|---|---|
| Wavelength of max emission | nm | 310 (slow), 225 (fast) [6,7] | 340 (slow), 300–310 (fast) [6,52,53] | 480 [6,54,55] | 445 [56] |
| Emission wavelength range | nm | 170–460 [8] | 250–425 [53] | 375–650 [55] | 370–560 [56] |
| Decay times | ns | 620–630 (slow), 0.87 (fast) [6,7,8,9,10] | 30 (slow), 8 (fast) [52] | 300 [6,54,56] | 30–60 [9,57,58,59,60] |
| Timing resolution | ps @ FWHM | 80–500 [9,61,62,63,64,65,66,67,68] | 522–2000 [6,69] | 2500–6000 [6,9] | 965 [9,70] |
| Light yield | photons/MeV | 1500 [34] | 4.4 × 103 [52] | 8.2–8.9 × 103 [9,54] | 8–10 × 103 [9,57] |
| Photoelectron yield | % of NaI:Tl | 16 (slow) 3–5 (fast) [6,7,8] | 3–10 [6,7,53,55] | 15–20 [55] | 20 [57,58,59] |
| Radiation length | cm | 2.1 [6,59] | 1.654 [6,53,59] | 1.12 [6,9,59,71] | 1.38 [59] |
| Refractive index @ max nm | 1.57 @ 310 nm [6,7] | 1.62–1.68 @ 440 nm [7,52] | 2.15 @ 480 nm [6,72] | 1.85 [57] | |
| Density | g/cm3 | 4.87 [6,7,8] | 6.16 [6,7,52] | 7.13 [6,7,54] | 6.7 [57] |
| Melting point | °C | 1386 [8] | 1443–1450 [52,73,74] | 1050 [55] | 1626 [57] |
| Mechanical hardness | mohs | 3 [8] | 4.5–5 [73] | 5 [75] | 5.7 [57] |
| Radiation hardness | Rad | 106–107 [59] | >106 [59,76] | 104–107 [59,77] | 106–109 [57,58,59] |
| Hygroscopic | No [6] | No [52] | No [54] | No [59] |
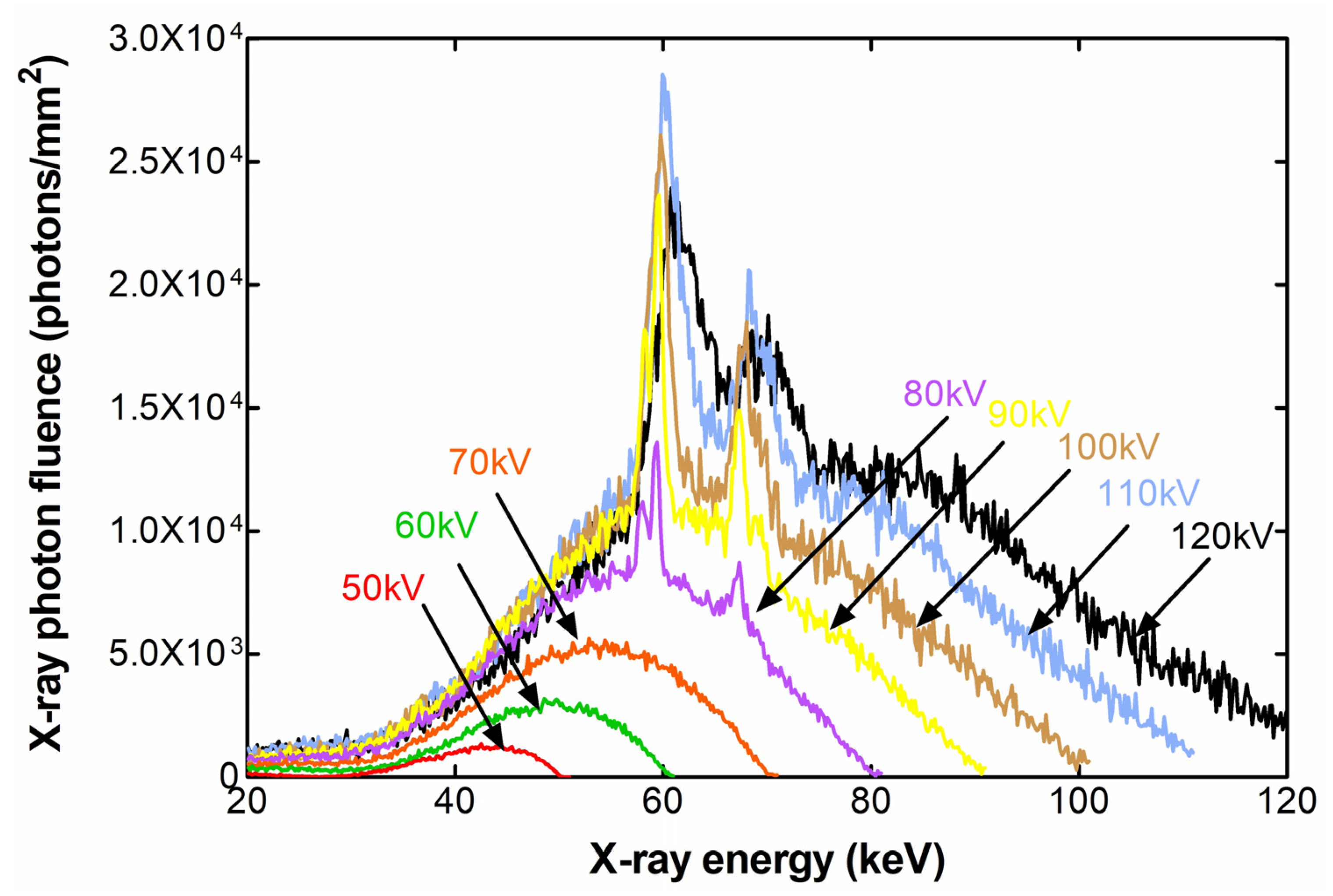
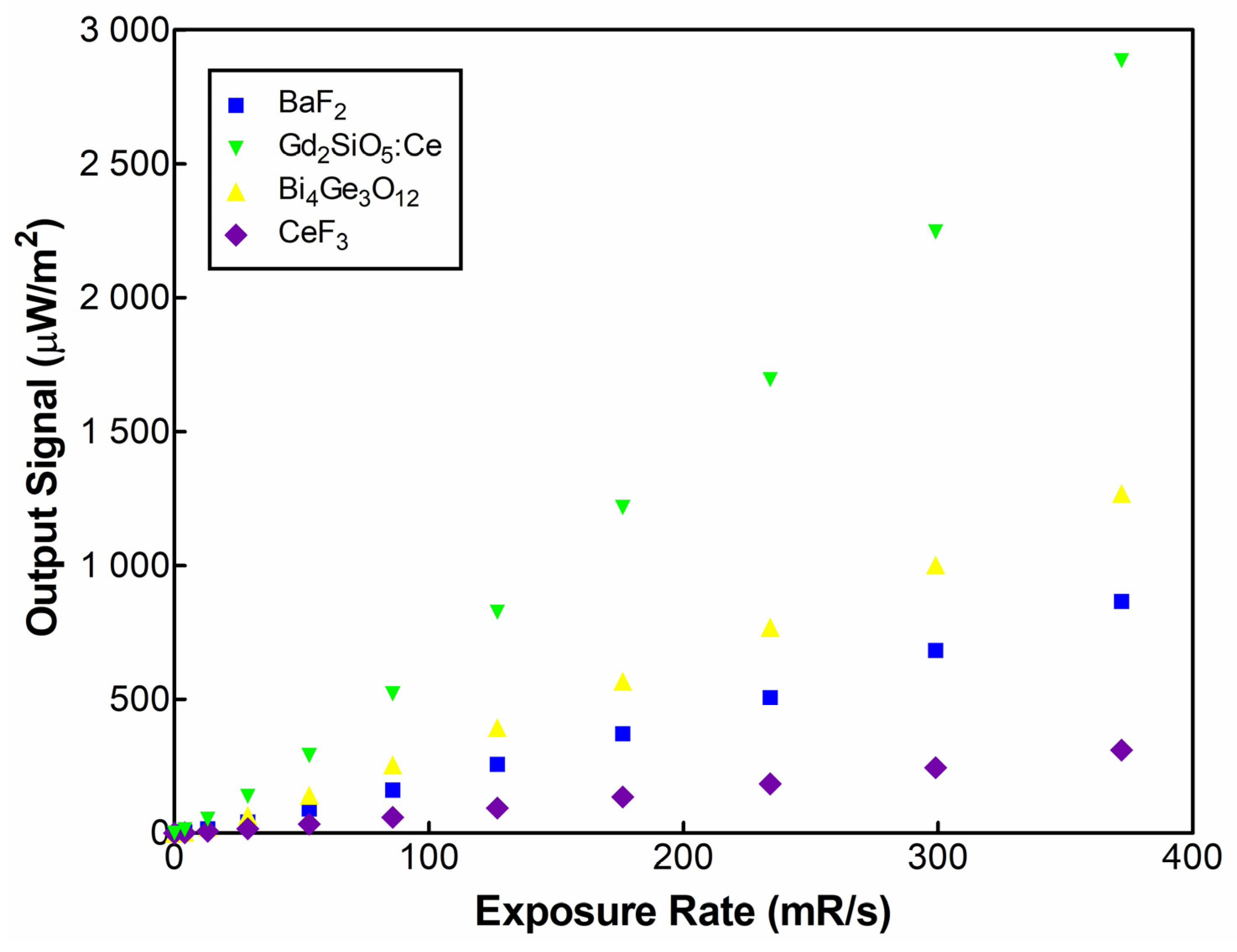
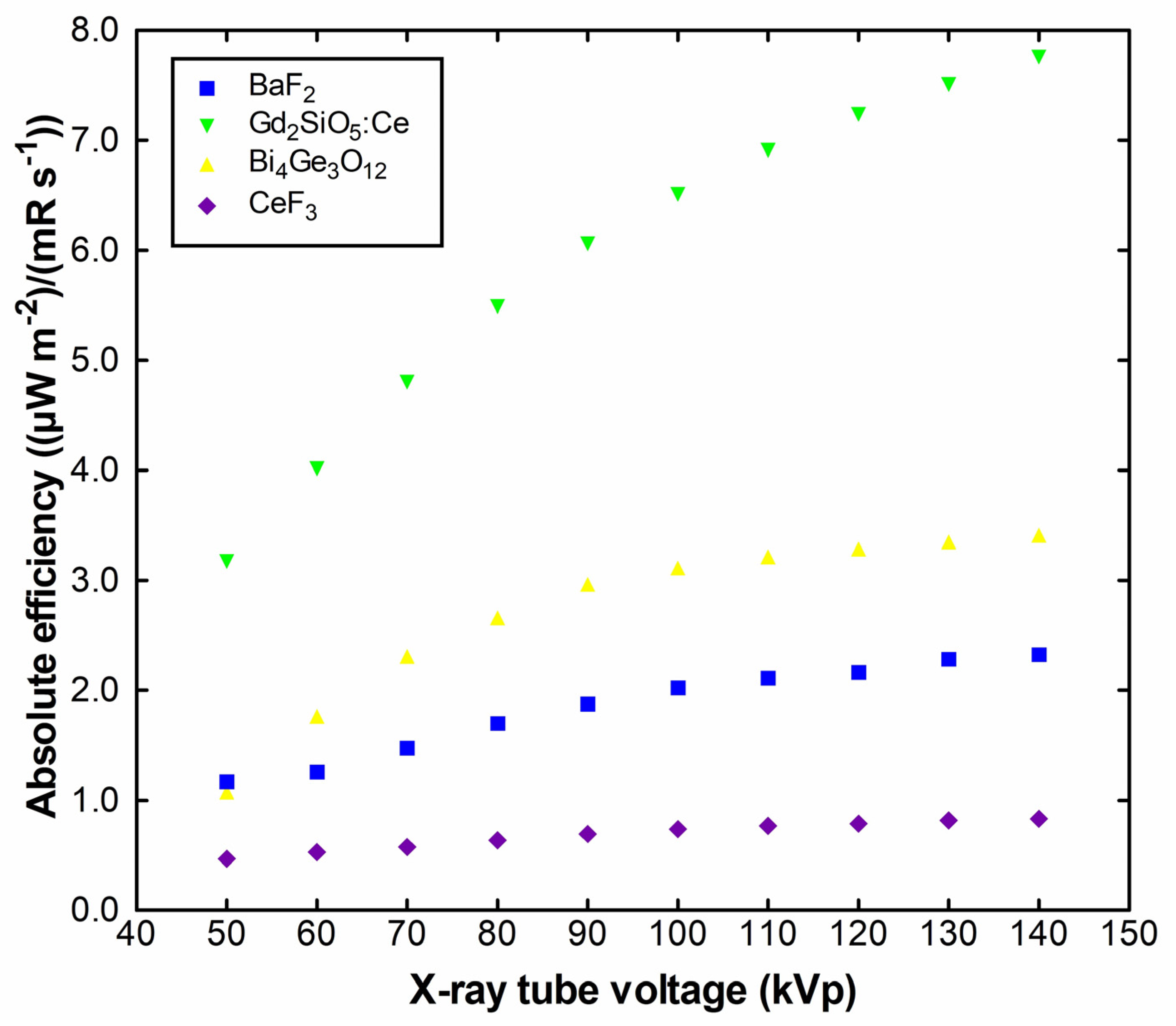
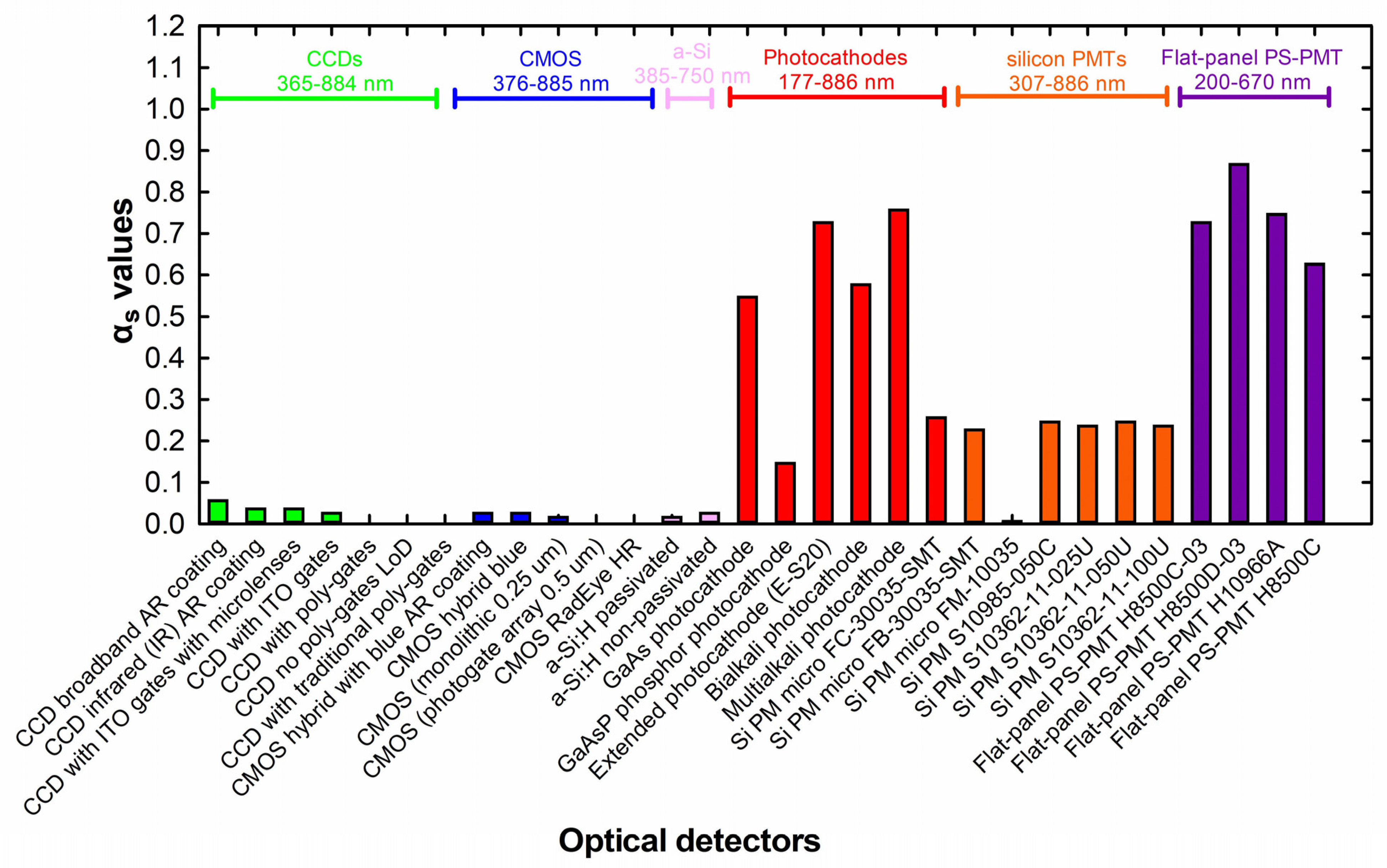
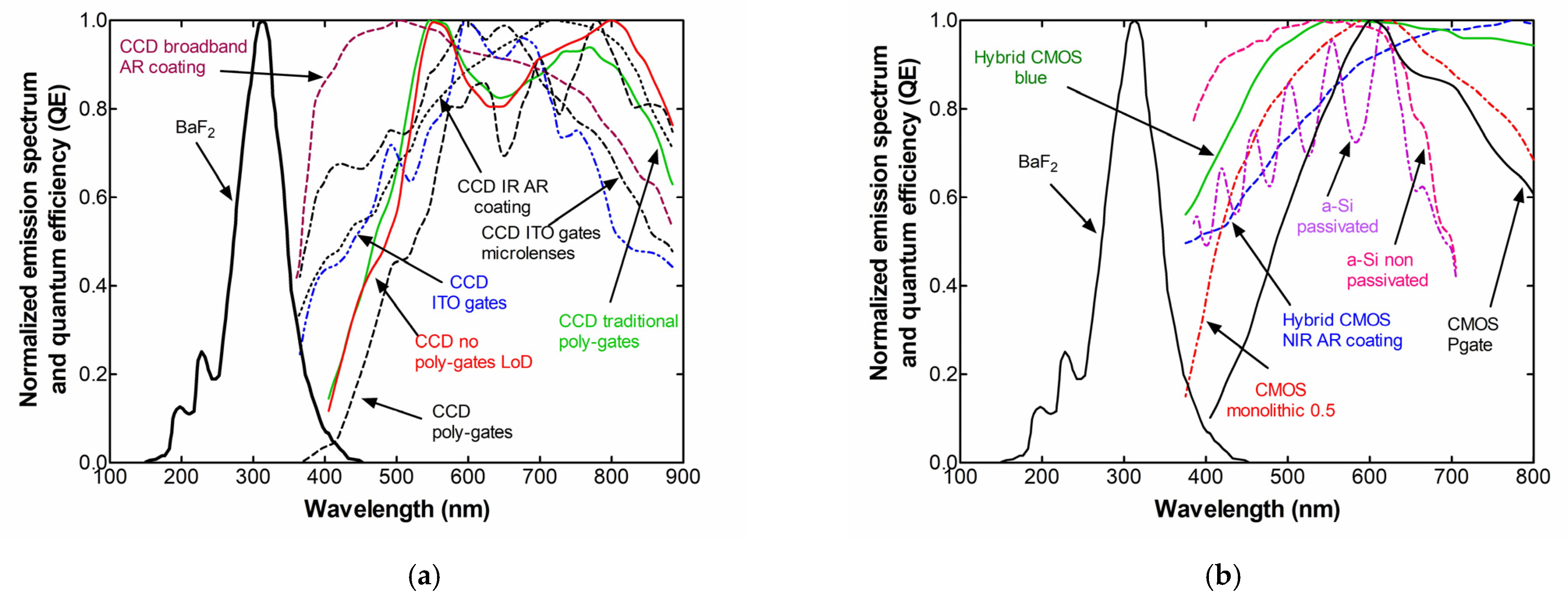
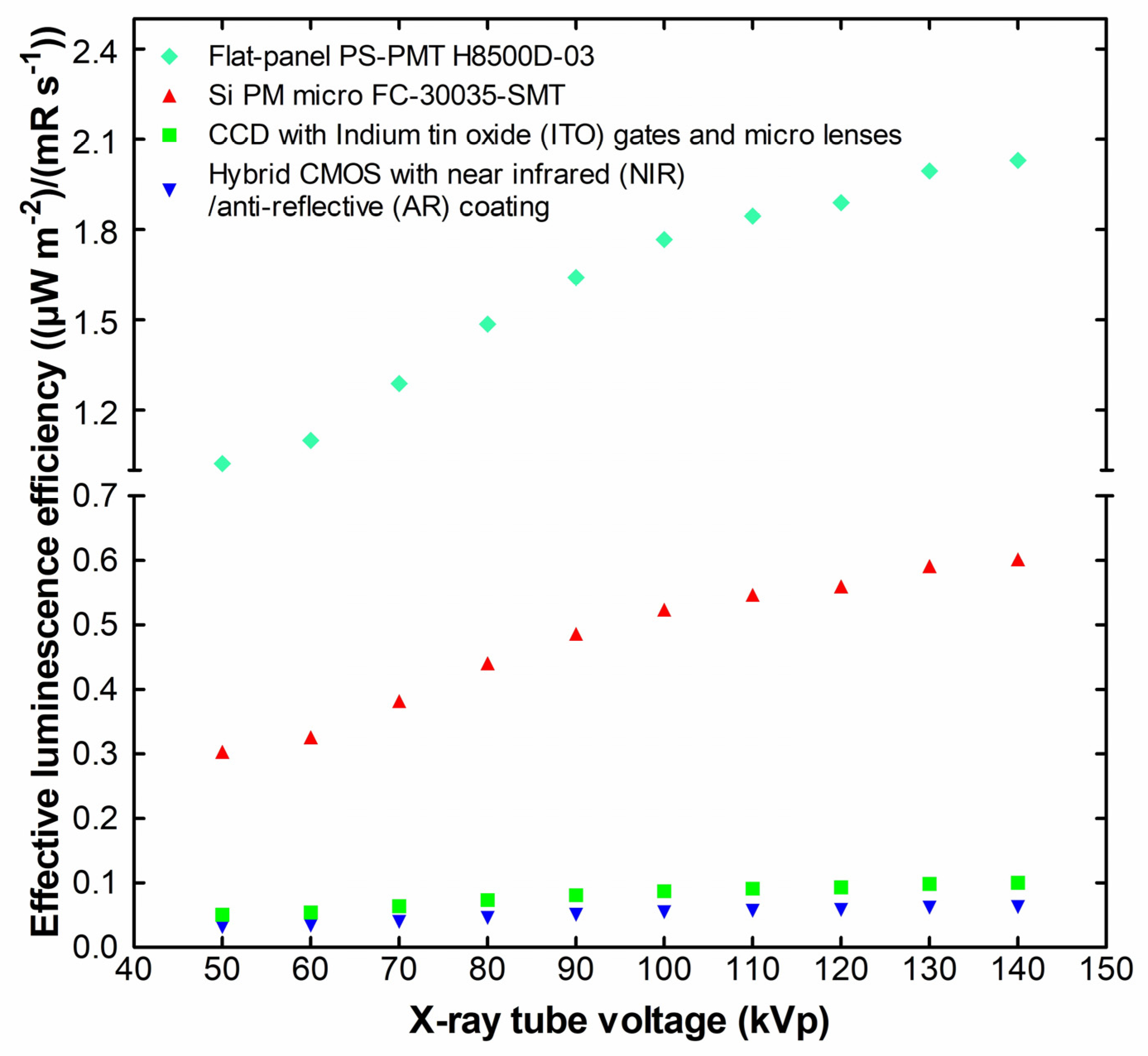
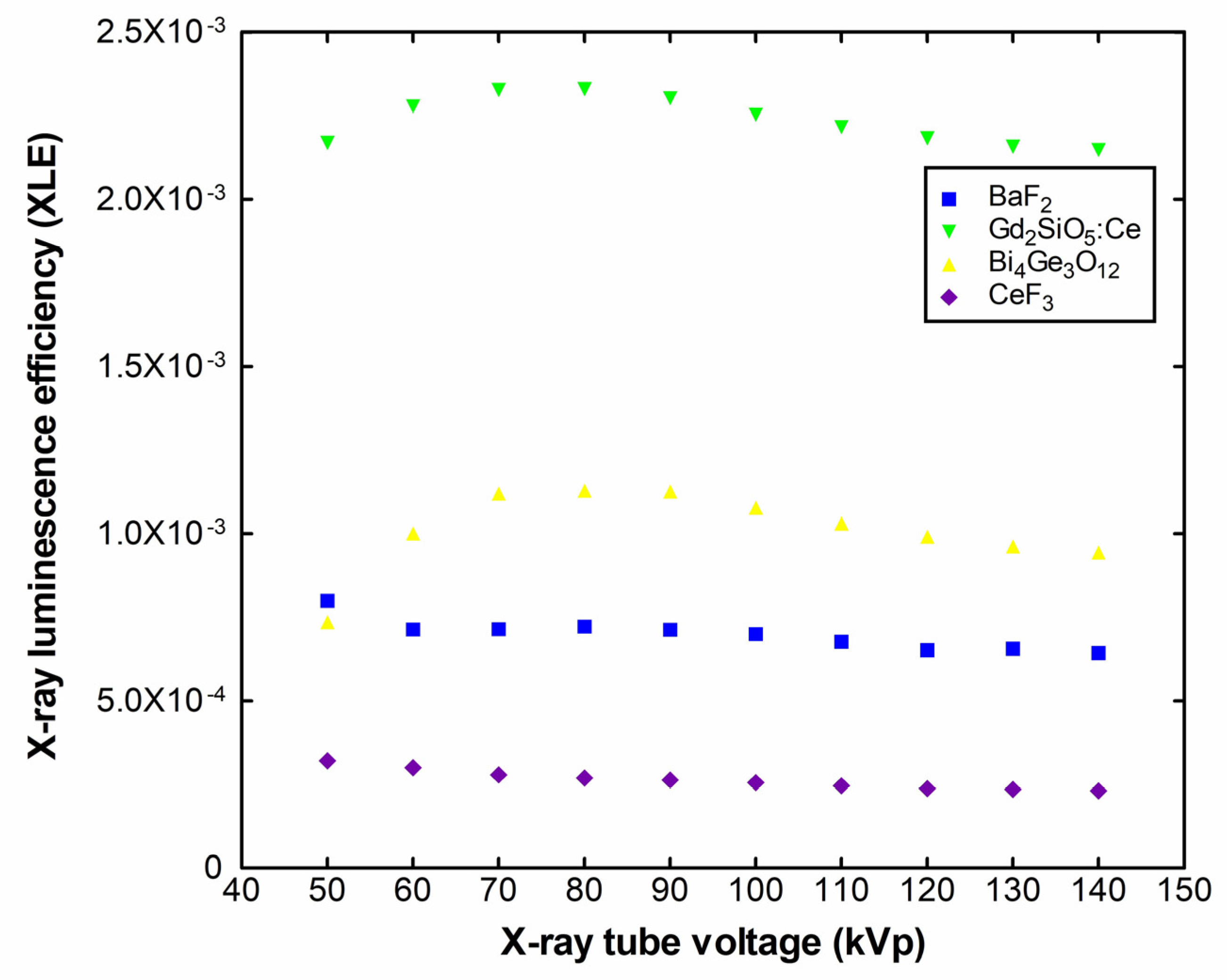
2. Results
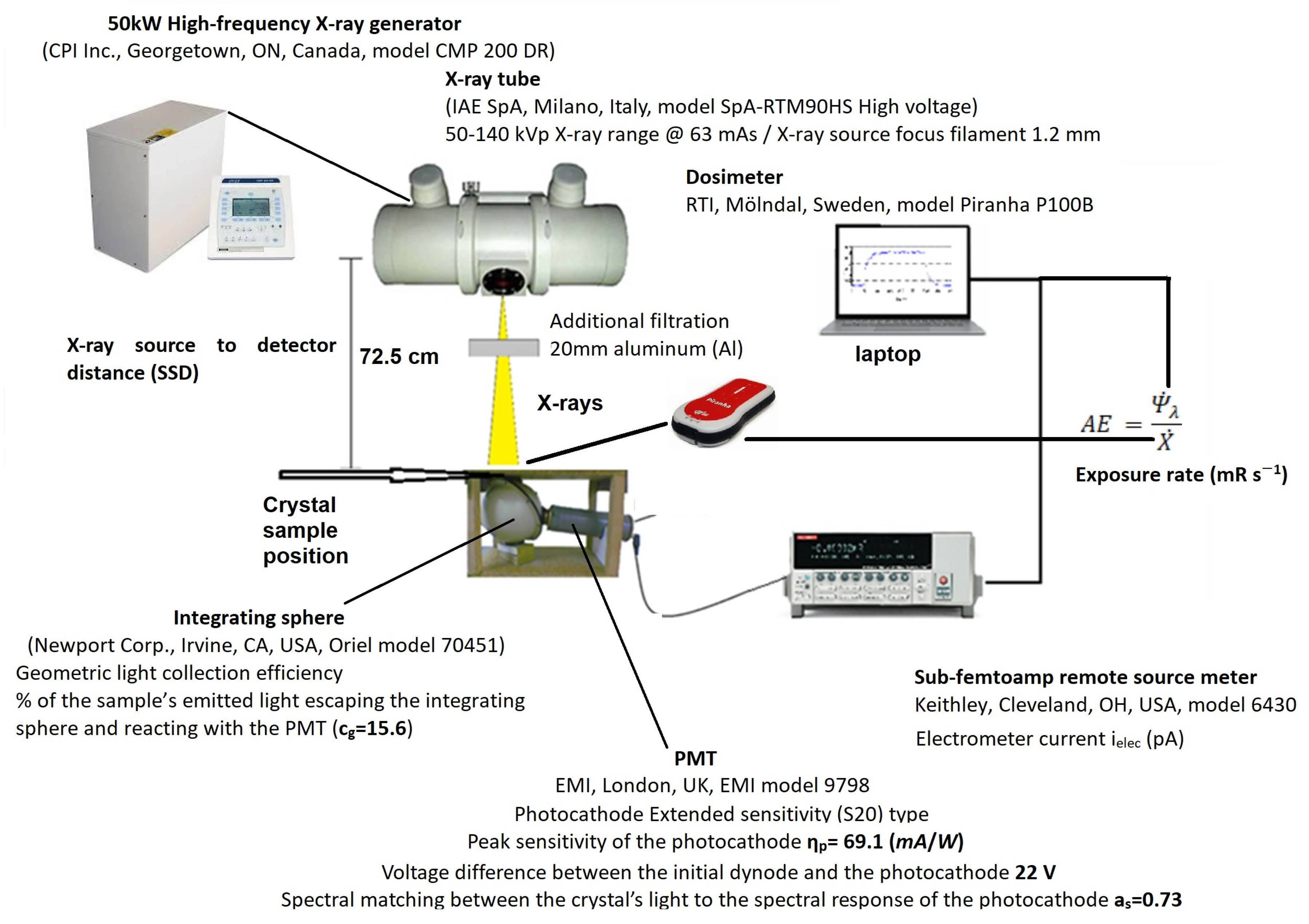
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| AE | Absolute luminescence efficiency |
| AR | Anti-reflective |
| Ba | Barium |
| BaF2 | Barium fluoride |
| Bi | Bismuth |
| Bi4Ge3O12-BGO | Bismuth germanate |
| CdTe | Cadmium telluride |
| Ce | Cerium |
| CeF3 | Cerium fluoride |
| CCDs | Charge-coupled devices |
| CTR | Coincidence timing resolution |
| CMOS | Complementary metal oxide semiconductors |
| ELE | Effective luminescence efficiency |
| EU | Efficiency units |
| EAE | Energy absorption efficiency |
| F | Fluorine |
| Gd | Gadolinium |
| Gd2SiO5:Ce-GSO:Ce | Gadolinium orthosilicate |
| Ge | Germanium |
| ITO | Indium tin oxide |
| IR | Infrared |
| SI | International system of units |
| LYSO:Ce | Lutetium–yttrium oxyorthosilicate, doped with cerium |
| MRI | Magnetic resonance imaging |
| NIR | Near-infrared |
| NDT | Non-destructive testing |
| OCT | Optical coherence tomography |
| O | Oxygen |
| PMTs | Photomultipliers |
| PS | Position-sensitive |
| PET/CT | Positron emission tomography/computed tomography system |
| QE | Quantum efficiency |
| STE | Self-trapped exciton |
| Si | Silicon |
| SiPM | Silicon photomultipliers |
| ToF | Time-of-flight |
| ToF-CT | Time-of-flight computed tomography |
| UV | Ultraviolet |
| XLE | X-ray luminescence efficiency |
| ZnO | Zinc oxide |
References
- Luo, G.; Peng, M.; Yang, Z.; Chu, C.P.; Deng, Z. Emerging New-Generation Semiconductor Single Crystals of Metal Halide Perovskites for Radiation Detection. Inorganics 2024, 12, 278. [Google Scholar] [CrossRef]
- Kato, T.; Okada, G.; Fukuda, K.; Yanagida, T. Development of BaF2 Transparent Ceramics and Evaluation of the Scintillation Properties. Radiat. Meas. 2017, 106, 140–145. [Google Scholar] [CrossRef]
- Del Zoppo, A.; Agodi, C.; Alba, R.; Bellia, G.; Coniglione, R.; Finocchiaro, P.; Maiolino, C.; Migneco, E.; Peghaire, A.; Piattelli, P.; et al. Response of the BaF2 Scintillator to Light Charged Particles. Nucl. Instrum. Methods Phys. Res. Sect. A 1993, 327, 363–368. [Google Scholar] [CrossRef]
- Novotny, R.; Riess, R.; Hingmann, R.; Ströher, H.; Fischer, R.D.; Koch, G.; Kühn, W.; Metag, V.; Mühlhans, R.; Kneissl, U.; et al. Detection of Hard Photons with BaF2 Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A 1987, 262, 340–346. [Google Scholar] [CrossRef]
- Pots, R.H.; Auffray, E.; Gundacker, S. Exploiting Cross-Luminescence in BaF2 for Ultrafast Timing Applications Using Deep-Ultraviolet Sensitive HPK Silicon Photomultipliers. Front. Phys. 2020, 8, 592875. [Google Scholar] [CrossRef]
- Anderson, D.F. Properties of the High-Density Scintillator Cerium Fluoride. IEEE Trans. Nucl. Sci. 1989, 36, 137–140. [Google Scholar] [CrossRef]
- Miyaoka, R.S.; Lewellen, T.K. Evaluation of CeF3 as a Scintillator for High Speed Dynamic PET Imaging. IEEE Trans. Nucl. Sci. 1994, 41, 2743–2747. [Google Scholar] [CrossRef]
- BaF2-Barium Fluoride Scintillator Crystal|Advatech UK. Available online: https://www.advatech-uk.co.uk/baf2.html (accessed on 19 December 2024).
- Moses, W.W. Recent Advances and Future Advances in Time-of-Flight PET. Nucl. Instrum. Methods Phys. Res. Sect. A 2007, 580, 919–924. [Google Scholar] [CrossRef]
- Shendrik, R.; Radzhabov, E. Absolute Light Yield Measurements on SrF2 and BaF2 Doped with Rare Earth Ions. IEEE Trans. Nucl. Sci. 2014, 61, 406–410. [Google Scholar] [CrossRef]
- Herweg, K.; Nadig, V.; Schulz, V.; Gundacker, S. On the Prospects of BaF2 as a Fast Scintillator for Time-of-Flight Positron Emission Tomography Systems. IEEE Trans. Radiat. Plasma Med. Sci. 2023, 7, 241–252. [Google Scholar] [CrossRef]
- Gundacker, S.; Pots, R.H.; Nepomnyashchikh, A.; Radzhabov, E.; Shendrik, R.; Omelkov, S.; Kirm, M.; Acerbi, F.; Capasso, M.; Paternoster, G.; et al. Vacuum Ultraviolet Silicon Photomultipliers Applied to BaF2 Cross-Luminescence Detection for High-Rate Ultrafast Timing Applications. Phys. Med. Biol. 2021, 66, 114002. [Google Scholar] [CrossRef] [PubMed]
- Herweg, K.; Rutstrom, D.; Nadig, V.; Stand, L.; Melcher, C.L.; Zhuravleva, M.; Schulz, V.; Gundacker, S. Timing Limits of Ultrafast Cross-Luminescence Emission in CsZnCl-Based Crystals for TOF-CT and TOF-PET. EJNMMI Phys. 2024, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, S.; Heering, A. The Silicon Photomultiplier: Fundamentals and Applications of a Modern Solid-State Photon Detector. Phys. Med. Biol. 2020, 65, 17TR01. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, P.; Gundacker, S. SiPM Applications in Positron Emission Tomography: Toward Ultimate PET Time-of-Flight Resolution. Eur. Phys. J. Plus 2021, 136, 292. [Google Scholar] [CrossRef]
- Singh, M.K. A Review of Digital PET-CT Technology: Comparing Performance Parameters in SiPM Integrated Digital PET-CT Systems. Radiography 2024, 30, 13–20. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, M.S. Advancements in Positron Emission Tomography Detectors: From Silicon Photomultiplier Technology to Artificial Intelligence Applications. PET Clin. 2024, 19, 1–24. [Google Scholar] [CrossRef]
- Kratochwil, N.; Gundacker, S.; Auffray, E. A Roadmap for Sole Cherenkov Radiators with SiPMs in TOF-PET. Phys. Med. Biol. 2021, 66, 195001. [Google Scholar] [CrossRef]
- Wang, Z.; Dujardin, C.; Freeman, M.S.; Gehring, A.E.; Hunter, J.F.; Lecoq, P.; Liu, W.; Melcher, C.L.; Morris, C.L.; Nikl, M.; et al. Needs, Trends, and Advances in Scintillators for Radiographic Imaging and Tomography. IEEE Trans. Nucl. Sci. 2023, 70, 1244–1280. [Google Scholar] [CrossRef]
- Shendrik, R.; Radzhabov, E.; Myasnikova, A.; Pankratova, V.; Šarakovskis, A.; Nepomnyashchikh, A.; Bogdanov, A.; Gavrilenko, V.; Pankratov, V. Ultrafast Core-to-Core Luminescence in BaF2-LaF3 Single Crystals. arXiv 2024, arXiv:2412.04303. [Google Scholar]
- Seliverstov, D.M.; Demidenko, A.A.; Garibin, E.A.; Gain, S.D.; Gusev, Y.I.; Fedorov, P.P.; Kosyanenko, S.V.; Mironov, I.A.; Osiko, V.V.; Rodnyi, P.A.; et al. New Fast Scintillators on the Base of BaF2 Crystals with Increased Light Yield of 0.9 Ns Luminescence for TOF PET. Nucl. Instrum. Methods Phys. Res. Sect. A 2012, 695, 369–372. [Google Scholar] [CrossRef]
- Saito, H.; Nagashima, Y.; Kurihara, T.; Hyodo, T. A New Positron Lifetime Spectrometer Using a Fast Digital Oscilloscope and BaF2 Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A 2002, 487, 612–617. [Google Scholar] [CrossRef]
- Nelson, M.A.; Rooney, B.D.; Dinwiddie, D.R.; Brunson, G.S. Analysis of Digital Timing Methods with BaF2 Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A 2003, 505, 324–327. [Google Scholar] [CrossRef]
- Danylenko, Y.; Nepokupna, T. Analysis of Scintillation Materials for Nuclear Medicine on the Basis of Patent Analytics. Sci. Innov. 2023, 19, 43–56. [Google Scholar] [CrossRef]
- Lewellen, T.K. The Challenge of Detector Designs for PET. Am. J. Roentgenol. 2010, 195, 301–309. [Google Scholar] [CrossRef]
- Melcher, C.L. Scintillation Crystals for PET. J. Nucl. Med. 2000, 41, 1051–1055. [Google Scholar]
- Zhu, R.-Y. The Next Generation of Crystal Detectors. J. Phys. Conf. Ser. 2015, 587, 012055. [Google Scholar] [CrossRef]
- Nassalski, A.; Kapusta, M.; Batsch, T.; Wolski, D.; Mockel, D.; Enghardt, W.; Moszynski, M. Comparative Study of Scintillators for PET/CT Detectors. IEEE Trans. Nucl. Sci. 2007, 54, 3–10. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Kuznetsov, S.V.; Smirnov, A.N.; Garibin, E.A.; Gusev, P.E.; Krutov, M.A.; Chernenko, K.A.; Khanin, V.M. Microstructure and Scintillation Characteristics of BaF2 Ceramics. Inorg. Mater. 2014, 50, 738–744. [Google Scholar] [CrossRef]
- Hahn, D. Calcium Fluoride and Barium Fluoride Crystals in Optics. Opt. Photonik 2014, 9, 45–48. [Google Scholar] [CrossRef]
- Andrade, A.B.; Ferreira, N.S.; Valerio, M.E.G. Particle Size Effects on Structural and Optical Properties of BaF2 Nanoparticles. RSC Adv. 2017, 7, 26839–26848. [Google Scholar] [CrossRef]
- Klamra, W.; Sibczynski, P.; Moszynski, M.; Czarnacki, W.; Kozlov, V. Extensive Studies on Light Yield Non-Proportional Response of Undoped CeF3 at Room and Liquid Nitrogen Temperatures. J. Instrum. 2013, 8, P06003. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Zhu, R.-Y.; Chen, A.; Wang, Z.; Ying, L.; Yu, Z. BaF2:Y and ZnO:Ga Crystal Scintillators for GHz Hard X-Ray Imaging. Nucl. Instrum. Methods Phys. Res. Sect. A 2020, 950, 162767. [Google Scholar] [CrossRef]
- Xia, M.; Xie, Z.; Wang, H.; Jin, T.; Liu, L.; Kang, J.; Sang, Z.; Yan, X.; Wu, B.; Hu, H.; et al. Sub-Nanosecond 2D Perovskite Scintillators by Dielectric Engineering. Adv. Mater. 2023, 35, 2211769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, W.; Xiao, J.; Li, A.; Han, X. Low-Dimensional Metal Halide for High Performance Scintillators. Adv. Funct. Mater. 2024, 34, 2402902. [Google Scholar] [CrossRef]
- Fan, J.; Li, W.; Zhou, Q.; Yang, G.; Tang, P.; He, J.; Ma, L.; Zhang, J.; Xiao, J.; Yan, Z.; et al. Metal Halide Perovskites for Direct X-Ray Detection in Medical Imaging: To Higher Performance. Adv. Funct. Mater. 2024, 2401017. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Zhu, R.-Y.; Chen, A.; Wang, Z.; Ying, L.; Yu, Z. Ultrafast Inorganic Scintillators for Gigahertz Hard X-Ray Imaging. IEEE Trans. Nucl. Sci. 2018, 65, 2097–2104. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Ning, Y.; Yang, X.; Huang, Y.; Zhang, Z.; Tang, J.; Zheng, P.; Yan, J.; Zhao, J.; et al. Preparation and Application of Nanostructured ZnO in Radiation Detection. Materials 2024, 17, 3549. [Google Scholar] [CrossRef] [PubMed]
- Fooladchang, F.; Majidiyan Sarmazdeh, M.; Benam, M.R.; Arabshahi, H. First Principles Calculations of Structural, Electronic and Optical Properties of BaF2 Scintillator Crystal at Ambient Conditions. Phys. B Condens. Matter 2013, 427, 47–52. [Google Scholar] [CrossRef]
- Yang, X.; Hao, A.; Wang, X.; Liu, X.; Zhu, Y. First-Principles Study of Structural Stabilities, Electronic and Elastic Properties of BaF2 under High Pressure. Comput. Mater. Sci. 2010, 49, 530–534. [Google Scholar] [CrossRef]
- Turk, T.; Liu, T.; Hung, C.-H.; Billo, R.; Park, J.; Leu, M.C. In-Situ Thermographic Monitoring and Numerical Simulations of Laser-Foil-Printing Additive Manufacturing. Virtual Phys. Prototyp. 2025, 20, e2440609. [Google Scholar] [CrossRef]
- Petersen, C.R.; Rajagopalan, N.; Markos, C.; Israelsen, N.M.; Rodrigo, P.J.; Woyessa, G.; Tidemand-Lichtenberg, P.; Pedersen, C.; Weinell, C.E.; Kiil, S.; et al. Non-Destructive Subsurface Inspection of Marine and Protective Coatings Using Near- and Mid-Infrared Optical Coherence Tomography. Coatings 2021, 11, 877. [Google Scholar] [CrossRef]
- Jiang, H.; Pandey, R.; Darrigan, C.; Rérat, M. First-Principles Study of Structural, Electronic and Optical Properties of BaF2 in Its Cubic, Orthorhombic and Hexagonal Phases. J. Phys. Condens. Matter 2003, 15, 709. [Google Scholar] [CrossRef][Green Version]
- Rodnyi, P.A.; Garibin, E.A.; Venevtsev, I.D.; Davydov, Y.I. The Application of Barium Fluoride Luminescence: Challenges and Prospects. St. Petersburg Polytech. State Univ. J. Phys. Math. 2019, 12, 9–24. [Google Scholar] [CrossRef]
- McGregor, D.; Shultis, J.K. Radiation Detection: Concepts, Methods, and Devices; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-4398-1940-1. [Google Scholar]
- Kandemir, M.; Tiras, E.; Kirezli, B.; Koca, İ. SSLG4: A Novel Scintillator Simulation Library for Geant4. Comput. Phys. Commun. 2025, 306, 109385. [Google Scholar] [CrossRef]
- Li, X.; Deng, M.; Shi, Y.; Qi, X.; Wang, S.; Lu, Y.; Du, Y.; Chen, J. Bulk Polystyrene-BaF2 Composite Scintillators for Highly Efficient Radiation Detection. Crystals 2023, 13, 1334. [Google Scholar] [CrossRef]
- Cerium Bromide (CeBr3) Scintillators|Berkeley Nucleonics. Available online: https://www.berkeleynucleonics.com/cerium-bromide (accessed on 24 March 2022).
- Han, H.; Zhang, Z.; Weng, X.; Liu, J.; Guan, X.; Zhang, K.; Li, G. Development of a Fast Radiation Detector Based on Barium Fluoride Scintillation Crystal. Rev. Sci. Instrum. 2013, 84, 073503. [Google Scholar] [CrossRef]
- Cohen, D.; Houben, L.; Galisova, A.; Feldman, Y.; Fox, S.; Biton, I.E.; Bar-Shir, A. Cooperative Doping in Ultrasmall BaF2 Nanocrystals for Multimodal 19F-MRI and CT Applications. ACS Appl. Nano Mater. 2023, 6, 13107–13115. [Google Scholar] [CrossRef]
- Michail, C.; Liaparinos, P.; Kalyvas, N.; Kandarakis, I.; Fountos, G.; Valais, I. Phosphors and Scintillators in Biomedical Imaging. Crystals 2024, 14, 169. [Google Scholar] [CrossRef]
- Víllora, E.G.; Yuan, D.; Shimamura, K. CeF3 Single Crystals for UV-VIS-IR Optical Isolators. Int. J. Appl. Ceram. Technol. 2023, 20, 2047–2054. [Google Scholar] [CrossRef]
- CeF3-Cerium Fluoride Scintillator Crystal|Advatech UK. Available online: https://www.advatech-uk.co.uk/cef3.html (accessed on 2 May 2024).
- Lecoq, P. Development of New Scintillators for Medical Applications. Nucl. Instrum. Methods Phys. Res. Sect. A 2016, 809, 130–139. [Google Scholar] [CrossRef]
- Kozma, P.; Kozma, P. Radiation Resistivity of BGO Crystals Due to Low-Energy Gamma-Rays. Nucl. Instrum. Methods Phys. Res. Sect. A 2003, 501, 499–504. [Google Scholar] [CrossRef]
- Valais, I.; Michail, C.; David, S.; Nomicos, C.D.; Panayiotakis, G.S.; Kandarakis, I. A Comparative Study of the Luminescence Properties of LYSO:Ce, LSO:Ce, GSO:Ce and BGO Single Crystal Scintillators for Use in Medical X-Ray Imaging. Phys. Med. 2008, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Gadolinium Orthosilicate-Gd2SiO2(Ce) Scintillator Crystal|Advatech UK. Available online: https://www.advatech-uk.co.uk/gso_ce.html (accessed on 19 December 2024).
- Tanaka, M.; Hara, K.; Kim, S.; Kondo, K.; Takano, H.; Kobayashi, M.; Ishibashi, H.; Kurashige, K.; Susa, K.; Ishii, M. Applications of Cerium-Doped Gadolinium Silicate Gd2SiO5:Ce Scintillator to Calorimeters in High-Radiation Environment. Nucl. Instrum. Methods Phys. Res. Sect. A 1998, 404, 283–294. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishii, M. Effect of Cerium Doping on the Radiation Hardness of Gadolinium Silicate Gd2SiO5. Nucl. Instrum. Methods Phys. Res. Sect. B 1993, 82, 85–90. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Sun, L.; Ren, J.; Zheng, X.; Zhang, J.; Ren, J. UV-C Emitting Phosphor of Pr3+-Doped Fluoroborate Nano-Glass Composites for Disinfection and Fast Scintillator. J. Mater. Chem. C 2025. [Google Scholar] [CrossRef]
- Fraile, L.M.; Mach, H.; Vedia, V.; Olaizola, B.; Paziy, V.; Picado, E.; Udías, J.M. Fast Timing Study of a CeBr3 Crystal: Time Resolution below 120 Ps at 60Co Energies. Nucl. Instrum. Methods Phys. Res. Sect. A 2013, 701, 235–242. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Das, S.; Bhattacharyya, S.; Banik, R.; Dar, S.; Pandit, D.; Choudhury, A.; Banerjee, K.; Mondal, D.; Mukhopadhyay, S. Energy Response and Fast Timing Characteristics of 1.5′′ x 1.5′′ CeBr3 Scintillator. Nucl. Instrum. Methods Phys. Res. Sect. A 2021, 1014, 165737. [Google Scholar] [CrossRef]
- Mach, H.; Fraile, L.M. Fast Life-Time Measurements on Fission Products. Hyperfine Interact. 2014, 223, 147–156. [Google Scholar] [CrossRef]
- Białkowski, J.; Moszyński, M.; Wolski, D.; Klamra, W. Remarks on Constant Fraction Discriminators Applied for BaF2 Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A 1989, 281, 657–659. [Google Scholar] [CrossRef]
- Ziegler, S.I.; Ostertag, H.; Kuebler, W.K.; Lorenz, W.J.; Otten, E.W. Effects of Scintillation Light Collection on the Time Resolution of a Time-of-Flight Detector for Annihilation Quanta. IEEE Trans. Nucl. Sci. 1990, 37, 574–579. [Google Scholar] [CrossRef]
- Ishii, K.; Watanuki, S.; Orihara, H.; Itoh, M.; Matsuzawa, T. Improvement of Time Resolution in a TOF PET System with the Use of BaF2 Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A 1986, 253, 128–134. [Google Scholar] [CrossRef]
- Lewellen, T.K. Time-of-Flight PET. Semin. Nucl. Med. 1998, 28, 268–275. [Google Scholar] [CrossRef]
- Laval, M.; Moszyński, M.; Allemand, R.; Cormoreche, E.; Guinet, P.; Odru, R.; Vacher, J. Barium Fluoride—Inorganic Scintillator for Subnanosecond Timing. Nucl. Instrum. Methods Phys. Res. 1983, 206, 169–176. [Google Scholar] [CrossRef]
- Koshimizu, M.; Kurashima, S.; Kimura, A.; Taguchi, M. Linear Energy Transfer Dependence of Scintillation Properties of CeF3. Nucl. Instrum. Methods Phys. Res. Sect. B 2024, 546, 165158. [Google Scholar] [CrossRef]
- Moszyński, M.; Ludziejewski, T.; Wolski, D.; Klamra, W.; Avdejchikov, V.V. Timing Properties of GSO, LSO and Other Ce Doped Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A 1996, 372, 51–58. [Google Scholar] [CrossRef]
- Yang, F.; Mao, R.; Zhang, L.; Zhu, R.-Y. A Study on Radiation Damage in BGO and PWO-II Crystals. J. Phys. Conf. Ser. 2012, 404, 012025. [Google Scholar] [CrossRef]
- Sasano, M.; Nishioka, H.; Okuyama, S.; Nakazawa, K.; Makishima, K.; Yamada, S.; Yuasa, T.; Okumura, A.; Kataoka, J.; Fukazawa, Y.; et al. Geometry Dependence of the Light Collection Efficiency of BGO Crystal Scintillators Read out by Avalanche Photo Diodes. Nucl. Instrum. Methods Phys. Res. Sect. A 2013, 715, 105–111. [Google Scholar] [CrossRef]
- Karimov, D.N.; Lisovenko, D.S.; Ivanova, A.G.; Grebenev, V.V.; Popov, P.A.; Sizova, N.L. Bridgman Growth and Physical Properties Anisotropy of CeF3 Single Crystals. Crystals 2021, 11, 793. [Google Scholar] [CrossRef]
- Cerium Fluoride Crystal CeF3 Scintillation Crystal|MSE Supplies LLC. Available online: https://www.msesupplies.com/products/cerium-fluoride-cef3-crystal?variant=40204171444282 (accessed on 28 January 2025).
- Chaiphaksa, W.; Limkitjaroenporn, P.; Kim, H.J.; Kaewkhao, J. Moh’s Hardness Scale and Micro Vicker’s Hardness Study of Bgo and Lyso Inorganic Scintillators. J. Phys. Conf. Ser. 2018, 970, 012005. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishii, M.; Krivandina, E.A.; Litvinov, M.M.; Peresypkin, A.I.; Prokoshkin, Y.D.; Rykalin, V.I.; Sobolev, B.P.; Takamatsu, K.; Vasil’chenko, V.G. Cerium Fluoride, a Highly Radiation-Resistive Scintillator. Nucl. Instrum. Methods Phys. Res. Sect. A 1991, 302, 443–446. [Google Scholar] [CrossRef]
- Kozma, P.; Bajgar, R.; Kozma, P. Radiation Resistivity of PbF2 Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A 2002, 484, 149–152. [Google Scholar] [CrossRef]
- Michail, C.M.; Fountos, G.P.; Valais, I.G.; Kalyvas, N.I.; Liaparinos, P.F.; Kandarakis, I.S.; Panayiotakis, G.S. Evaluation of the Red Emitting Gd2O2S:Eu Powder Scintillator for Use in Indirect X-Ray Digital Mammography Detectors. IEEE Trans. Nucl. Sci. 2011, 58, 2503–2511. [Google Scholar] [CrossRef]
- Ntoupis, V.; Michail, C.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Valais, I. Luminescence Efficiency and Spectral Compatibility of Cerium Fluoride (CeF3) Inorganic Scintillator with Various Optical Sensors in the Diagnostic Radiology X-Ray Energy Range. Inorganics 2024, 12, 230. [Google Scholar] [CrossRef]
- Magnan, P. Detection of Visible Photons in CCD and CMOS: A Comparative View. Nucl. Instrum. Methods Phys. Res. Sect. A 2003, 504, 199–212. [Google Scholar] [CrossRef]
- Schaber, J.; Xiang, R.; Gaponik, N. Review of Photocathodes for Electron Beam Sources in Particle Accelerators. J. Mater. Chem. C 2023, 11, 3162–3179. [Google Scholar] [CrossRef]
- Lishuang, M.; LingYue, C.; Guorui, H.; Jun, H.; Han, X.; Hua, Z.; Huang, X.; Jin, M.; Jiang, X.; Jin, Z.; et al. The Time Resolution Improvement of Cherenkov-Radiator-Window Photomultiplier Tube. J. Instrum. 2023, 18, C12020. [Google Scholar] [CrossRef]
- Ponti, E.D.; Crivellaro, C.; Morzenti, S.; Monaco, L.; Todde, S.; Landoni, C.; Elisei, F.; Musarra, M.; Guerra, L. Clinical Application of a High Sensitivity BGO PET/CT Scanner: Effects of Acquisition Protocols and Reconstruction Parameters on Lesions Quantification. Curr. Radiopharm. 2022, 15, 218–227. [Google Scholar] [CrossRef]
- Sathiakumar, C.; Som, S.; Eberl, S.; Lin, P. NEMA NU 2-2001 Performance Testing of a Philips Gemini GXL PET/CT Scanner. Australas Phys. Eng. Sci. Med. 2010, 33, 199–209. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Zhang, H.; Peng, H.; Ren, Q.; Xu, J.; Peng, Q.; Xie, S. Requirements of Scintillation Crystals with the Development of PET Scanners. Crystals 2022, 12, 1302. [Google Scholar] [CrossRef]
- BGO-Bismuth Germanate Bi4Ge3O12 Scintillation Crystal|MSE Supplies LLC. Available online: https://www.msesupplies.com/products/bgo-bismuth-germanate-bi4ge3o12-scintillation-crystal (accessed on 28 January 2025).
- BaF2-Barium Fluoride Crystal and Substrates|MSE Supplies LLC. Available online: https://www.msesupplies.com/products/mse-pro-barium-fluoride-baf-sub-2-sub-crystal-and-substrates (accessed on 28 January 2025).
- Michail, C.; Karpetas, G.; Kalyvas, N.; Valais, I.; Kandarakis, I.; Agavanakis, K.; Panayiotakis, G.; Fountos, G. Information Capacity of Positron Emission Tomography Scanners. Crystals 2018, 8, 459. [Google Scholar] [CrossRef]
- Storm, L.; Israel, H.I. Photon Cross Sections from 1 keV to 100 MeV for Elements Z = 1 to Z = 100. At. Data Nucl. Data Tables 1970, 7, 565–681. [Google Scholar] [CrossRef]
- Hubbell, J.; Seltzer, S. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1995. [Google Scholar]
- Boone, J.M. X-Ray Production, Interaction, and Detection in Diagnostic Imaging. In Handbook of Medical Imaging. Volume 1: Physics and Psychophysics; Beutel, J., Kundel, H.L., Van Metter, R.L., Eds.; SPIE Press Book: Bellingham, WA, USA, 2000; Volume 1, pp. 1–79. ISBN 978-0-8194-7772-9. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntoupis, V.; Michail, C.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Valais, I. Light Output Response of a Barium Fluoride (BaF2) Inorganic Scintillator Under X-Ray Radiation. Inorganics 2025, 13, 83. https://doi.org/10.3390/inorganics13030083
Ntoupis V, Michail C, Kalyvas N, Bakas A, Kandarakis I, Fountos G, Valais I. Light Output Response of a Barium Fluoride (BaF2) Inorganic Scintillator Under X-Ray Radiation. Inorganics. 2025; 13(3):83. https://doi.org/10.3390/inorganics13030083
Chicago/Turabian StyleNtoupis, Vasileios, Christos Michail, Nektarios Kalyvas, Athanasios Bakas, Ioannis Kandarakis, George Fountos, and Ioannis Valais. 2025. "Light Output Response of a Barium Fluoride (BaF2) Inorganic Scintillator Under X-Ray Radiation" Inorganics 13, no. 3: 83. https://doi.org/10.3390/inorganics13030083
APA StyleNtoupis, V., Michail, C., Kalyvas, N., Bakas, A., Kandarakis, I., Fountos, G., & Valais, I. (2025). Light Output Response of a Barium Fluoride (BaF2) Inorganic Scintillator Under X-Ray Radiation. Inorganics, 13(3), 83. https://doi.org/10.3390/inorganics13030083











