Abstract
Dental caries is a prevalent global health issue characterized by the progressive demineralization of dental tissues, which occurs when the balance between demineralization and remineralization processes is disrupted at the tooth level. Silver diamine fluoride (SDF) has gained recognition for its ability to arrest caries. However, its interaction with mineralized tissues remains incompletely understood. This study aimed to investigate the chemical interactions between SDF and mineralized bioceramics, using hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP) as analogs for enamel and dentin. Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) were employed to identify functional groups and quantify elemental compositions at varying depths. FTIR analysis revealed structural modifications in HA and β-TCP. XPS demonstrated high retention of fluoride, with limited penetration into deeper layers, while silver exhibited deeper penetration. These findings suggest that SDF primarily acts on superficial layers, forming calcium fluoride and silver phosphate as key reaction products. These findings highlight the potential of SDF in managing deep carious lesions by demonstrating its ability to form a protective CaF2 layer at the surface while allowing deeper penetration of silver ions into mineralized tissues. This dual mechanism may contribute to SDF’s clinical efficacy in arresting caries and preventing further demineralization.
1. Introduction
The inorganic composition of dental enamel constitutes approximately 95% by weight and 87% by volume and is predominantly characterized by hydroxyapatite, which is essential for its remarkable resilience and functionality [1]. The destruction of this inorganic part, called tooth decay, poses a significant public health challenge globally, affecting billions of individuals across various age demographics [2]. According to the Global Oral Health Status Report by the World Health Organization (WHO, 2022), approximately 2 billion people suffer from caries in permanent teeth, while 514 million children are affected in primary teeth.
This condition arises from an imbalance between the processes of demineralization and remineralization [3]. Demineralization occurs when bacterial acids, primarily produced by Streptococcus mutans, dissolve hydroxyapatite crystals, releasing calcium (Ca2+) and phosphate (PO43−) ions into the oral environment [3,4]. In contrast, remineralization involves the restoration of these lost minerals into the hydroxyapatite matrix, relying on the presence of these ions in saliva [3]. Saliva plays a critical role in mitigating this process by acting as a buffer, neutralizing acids, and providing proteins that stabilize mineral ions, thus supporting remineralization [5].
Caries management should be addressed not only from the perspective of treating caries lesions but also as part of a comprehensive approach to tackling caries disease as a whole. To address dental caries in a less invasive and more accessible manner, particularly for patients with special needs or children, various therapeutic strategies have been explored. These include atraumatic restorative treatment and the use of chemical agents such as sodium fluoride (NaF) and silver diamine fluoride (SDF) [6,7]. Among these, SDF has emerged as a highly effective, multifunctional anticaries agent due to its synergistic mechanism of action. Widely used for both prevention and desensitization, SDF combines silver and fluoride ions, arresting carious lesions, inhibiting biofilm formation, preventing collagen degradation in dentin, and increasing the mineral density of demineralized areas [8,9,10,11,12]. These properties make it clinically effective, despite limitations such as the discoloration of treated tissues, which may hinder its widespread acceptance [8,9,10,11,13,14].
The 38% SDF solution has the chemical formula Ag(NH3)2F and consists of 25% (w/v) silver, 8% (w/v) ammonia, and 5.5% (w/v) fluoride, with a pH of 10 and approximately 44,800 ppm fluoride. Studies have shown that SDF penetrates enamel to a depth of approximately 25 μm and dentin between 16 and 200 μm, effectively arresting carious lesions up to 150 μm thick [15]. SDF reacts with hydroxyapatite crystals to form silver phosphate and calcium fluoride as its primary products [15]. Silver acts as an antimicrobial agent and inhibitor of collagenolytic enzymes, while fluoride promotes remineralization and reduces the solubility of apatite [8,9,10,11,12,16]. Additionally, fluoride can precipitate as calcium fluoride, acting as a reservoir of fluoride ions for release in acidic environments, leading to the formation of fluorapatite [17]. The ammonia in SDF stabilizes the solution by forming diamine silver fluoride, a more stable structure than silver fluoride, enhancing its efficacy during application [17]. Together, these mechanisms inhibit biofilm formation, promote remineralization, halt demineralization, prevent collagen degradation, and occlude dentinal tubules [18].
Despite its long history of use since the 1960s and its clinical efficacy with minimal side effects beyond lesion discoloration, several aspects of SDF remain underexplored [17]. Studies, such as those by Sulyanto et al. [19], have shown that SDF increases the mineral density of demineralized dentin lesions, yet its mechanism of action on mineralized tissues is not fully understood. Additionally, SDF has been associated with reversible pulpal inflammation and mild gingival inflammation that does not require treatment [17]. Approved by the FDA in 2014 and Health Canada in 2017, SDF has shown promising results, but its interactions with mineralized tissues and the diffusion of its components toward the dental pulp remain poorly understood [8,20]. These findings highlight the need for further research to clarify how SDF components, particularly silver, affect pulp tissues, depending on their penetration depth. Recent investigations underscore the importance of understanding these interactions to optimize their clinical use and mitigate adverse effects, such as dental discoloration [10,11,13].
This study investigates the mechanisms of action of SDF on mineralized tissues by examining its interaction with two pure bioceramics: hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP). HA was selected as it constitutes the primary crystalline component of both dentin and enamel, while β-TCP serves as a comparative model to understand how different calcium phosphate crystalline structures influence SDF interactions. Through complementary surface analysis techniques—Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS)—we characterized the chemical reactions between SDF and these calcium phosphate crystals at the nanometric level. FTIR analysis enabled the identification of functional groups and their modifications, while XPS provided detailed atomic composition analysis at various depths from the surface [21]. This dual analytical approach offers unprecedented insights into the fundamental mechanisms of SDF interaction with the nano-surfaces of dental structures, potentially guiding the development of more effective therapeutic agents for caries management. Understanding these interactions at the molecular level is crucial for optimizing current treatments and developing next-generation preventive materials for dental caries.
2. Results
2.1. Fourier Transform Infrared Spectroscopy
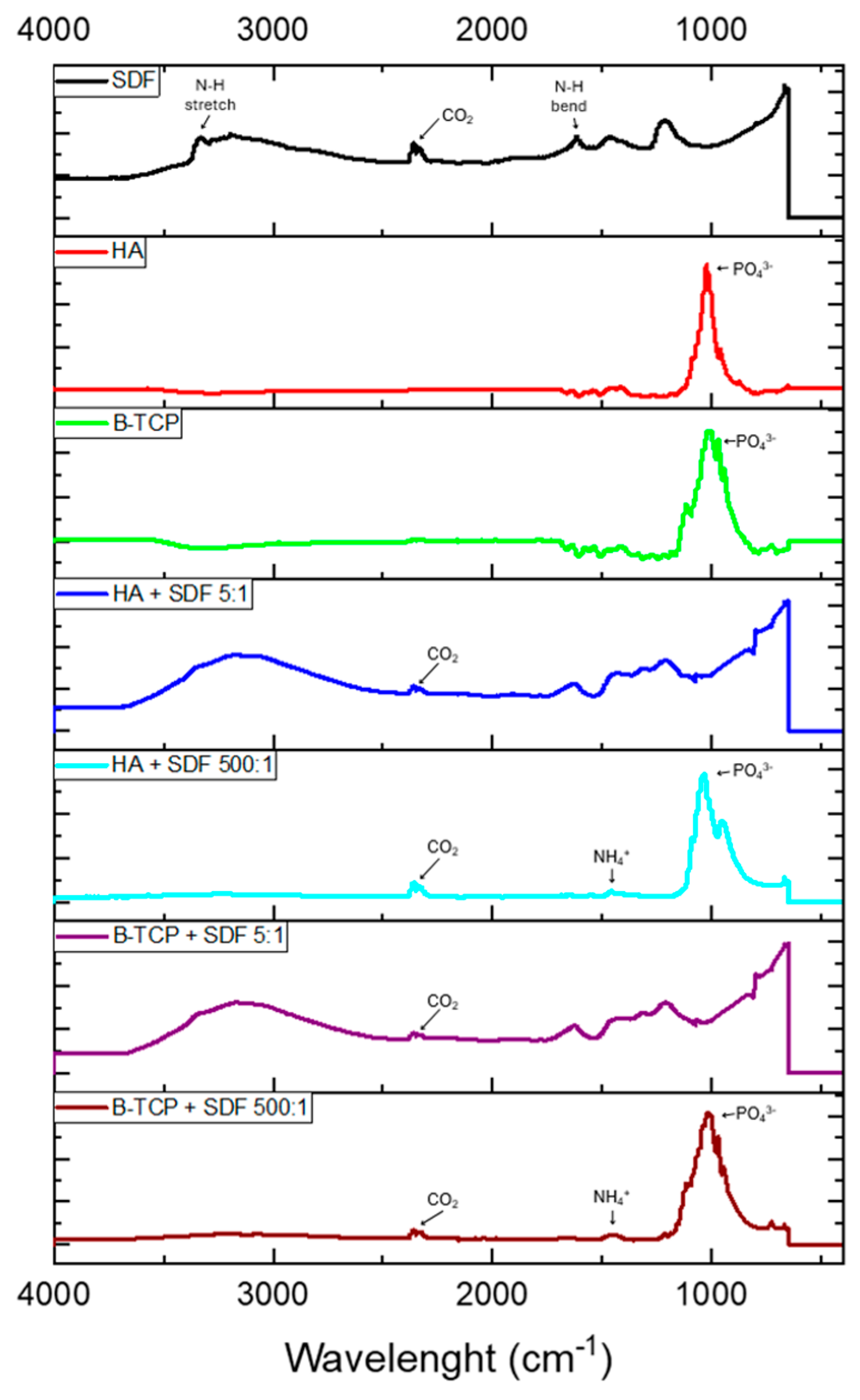
Figure 1 displays the infrared spectra obtained for all control and test groups. For the control SDF sample, a broad absorption band at 3350–3200 cm−1 indicates N-H stretching, while the peak at 1600 cm−1 represents N-H bending. When SDF is combined with HA and β-TCP, these bands are no longer present at 3350–3150 cm−1 or 1600 cm−1. However, a weak band at 1425 cm−1 appears in both test groups, suggesting the presence of ammonium ions (NH4+) which confirms the formation of NH4OH, as previously suggested in this report [14].
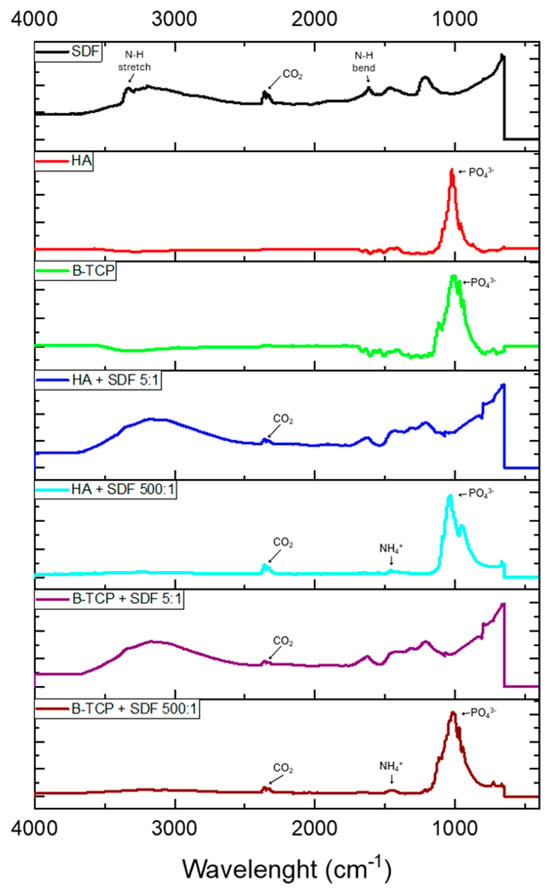
Figure 1.
FTIR analyses of the analyzed groups.
The bioceramic powders, both in isolation and when combined with SDF, show strong vibrational bands between 900 and 1100 cm−1, corresponding to the phosphate (PO43−) group [22]. HA in isolation exhibits a strong, narrow band with the greatest peak at 1024 cm−1, along with weaker peaks at 958 cm−1 and 1078 cm−1. When combined with SDF, the vibrational band broadens, with the greatest peak shifting to 1030 cm−1 and weaker peaks at 947 cm−1 and 1090 cm−1. Along with this shift, there is a more pronounced separation between the peaks at 947 cm−1 and 1030 cm−1 when HA is combined with SDF, suggesting a change in structure and composition. Furthermore, the change in intensity and broadening of the peak may indicate that F− ions have dissociated from the SDF solution and substituted the OH− groups within the HA lattice [23].
The β-TCP in isolation shows a broader band compared to isolated HA, with more pronounced peaks within the band. β-TCP has a weak peak at 945 cm−1, with weaker peaks at 970 cm−1 and 1113 cm−1, and its greatest peak at 1007 cm−1 [22]. In the β-TCP and SDF preparation, there is an upshift in PO43− wavenumbers, indicating a change, albeit to a lesser extent than with HA in isolation and when combined with SDF.
A carbon dioxide (CO2) impurity is observed in the SDF and test groups, with a weak peak at 2350 cm−1. This suggests that the impurity was incorporated from the atmosphere into the SDF solution when dispensed and when mixed with the HA and β-TCP powders.
2.2. X-Ray Photoelectron Spectroscopy
In the initial experimental design, two powder-to-liquid ratios (5:1 and 500:1) were evaluated using FTIR spectroscopy. However, the FTIR analysis of the 500:1 ratio demonstrated minimal superficial interaction patterns that were fundamentally similar to the untreated material surfaces, without revealing any significant chemical modifications or novel binding patterns. This observation indicated that the higher powder-to-liquid ratio resulted in insufficient material–SDF interaction to warrant further investigation. Consequently, the XPS analysis was conducted exclusively using the 5:1 powder-to-liquid ratio, which had demonstrated more substantial chemical interactions in the FTIR analysis
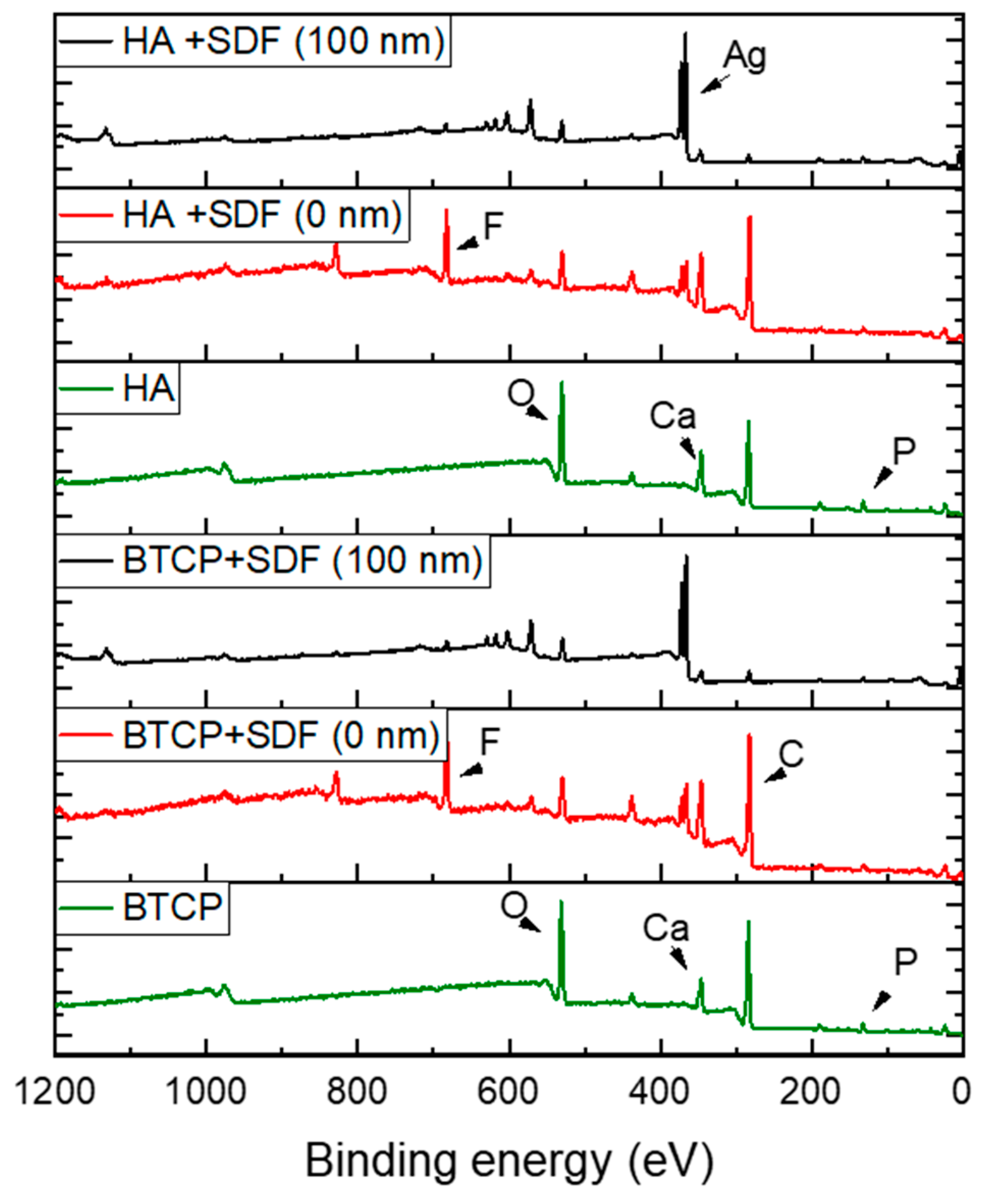
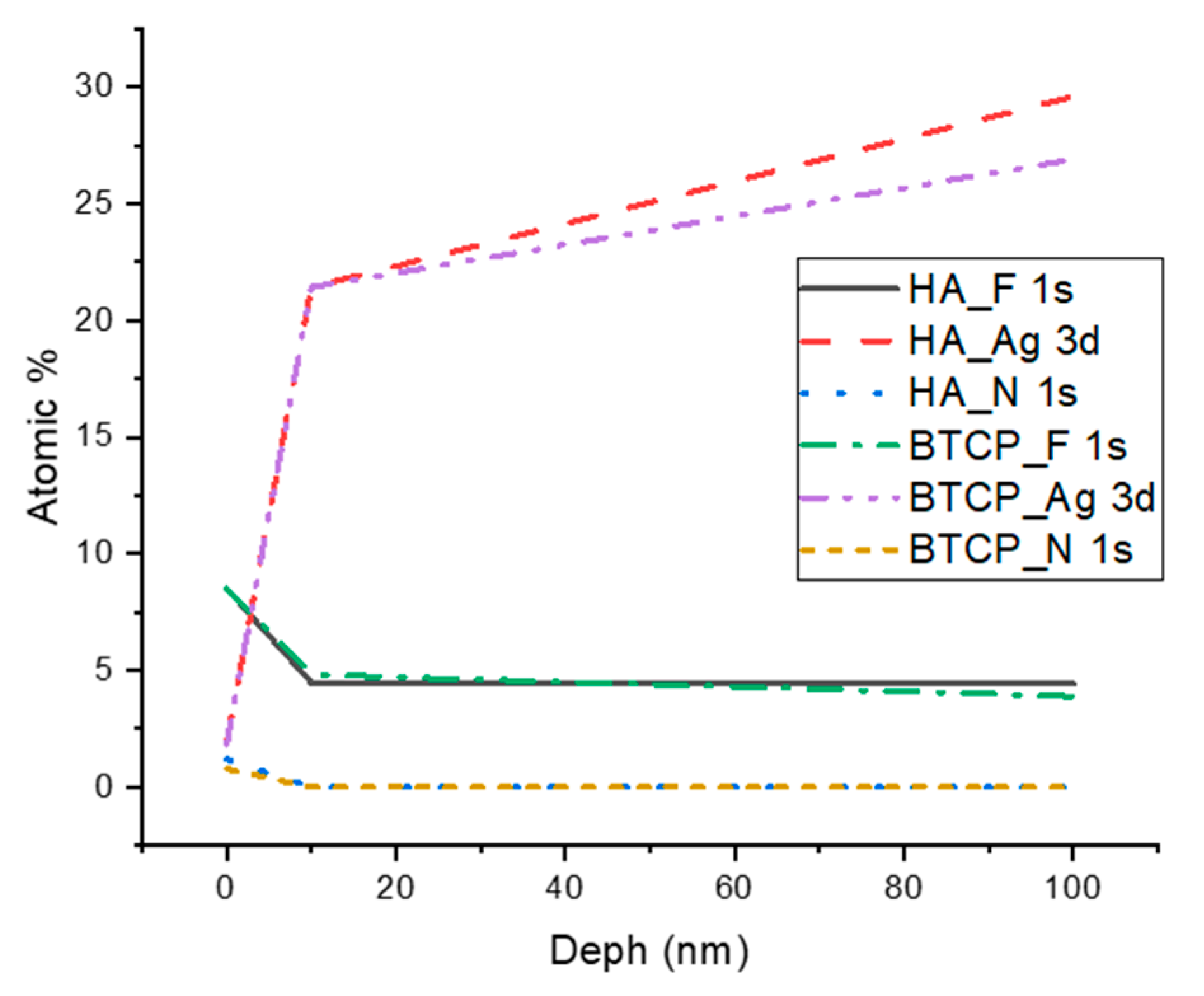
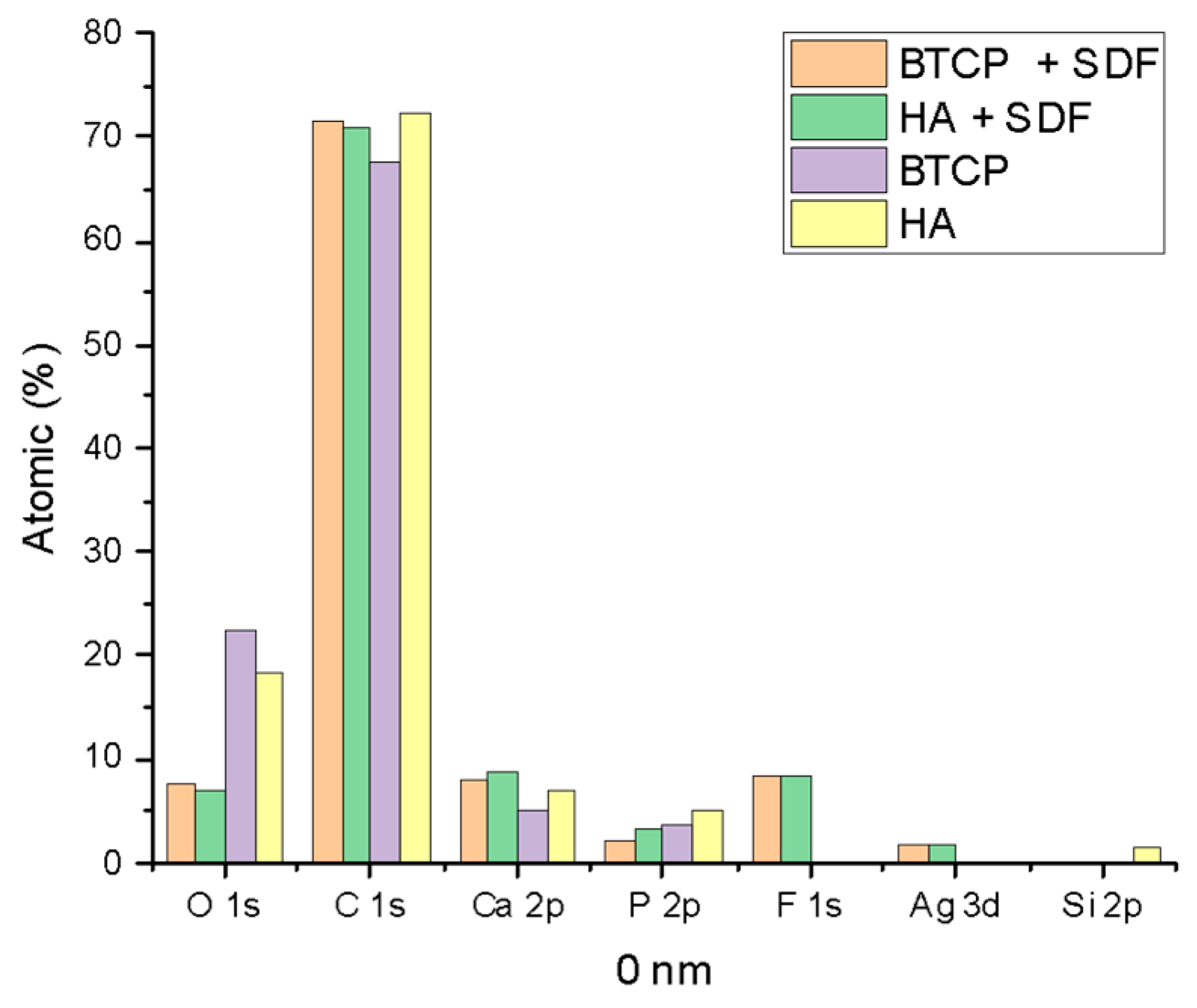
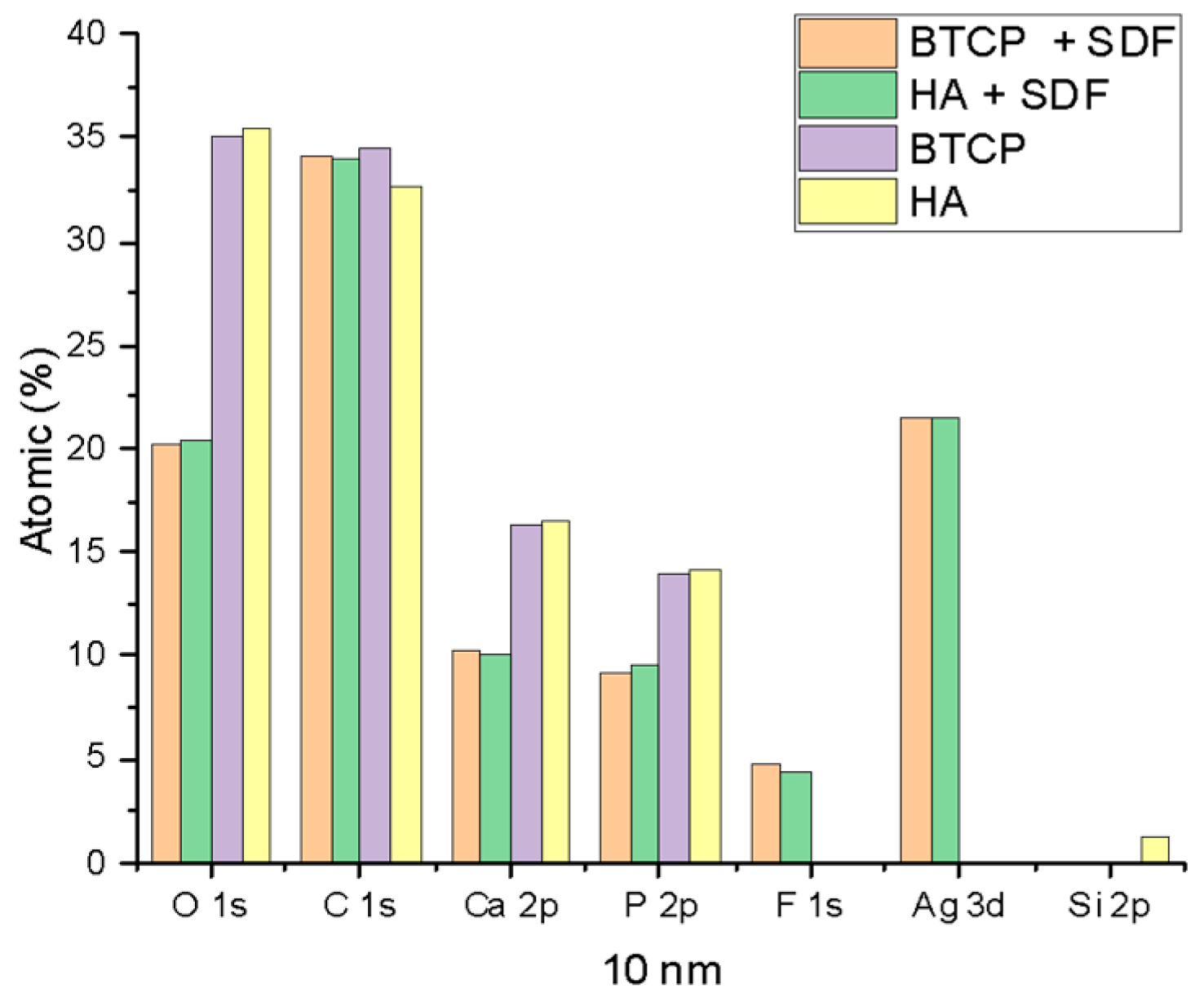
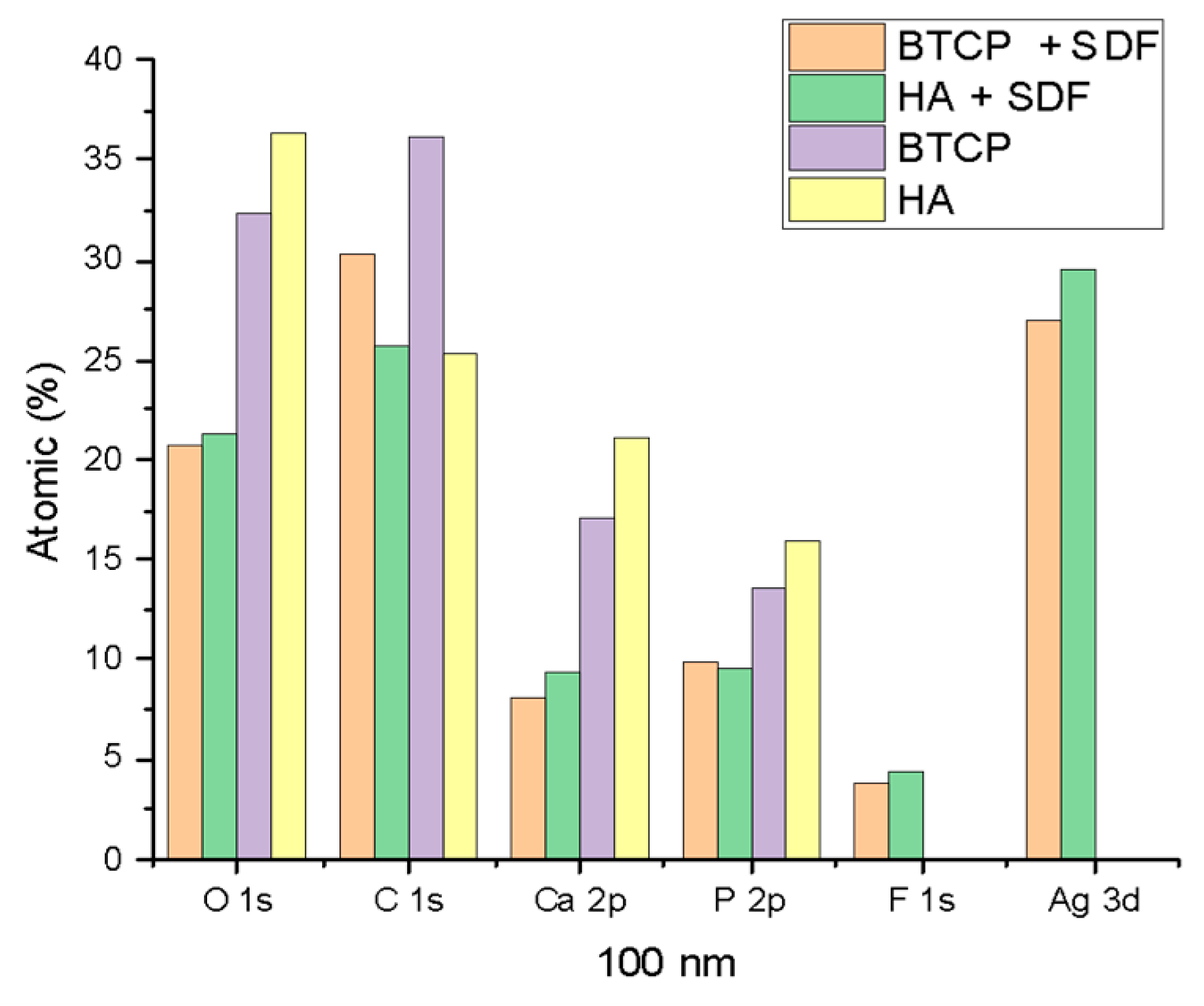
Figure 2 displays the XPS spectra for the specimen under treatment and treated with SDF at 0 nm and 100 nm depth. The peaks of the main components are indicated in the figure. Figure 3 points out the variation in the SDF components (Ag, N, F) under the three probed depths (0, 10, and 100 nm). Under XPS analysis, the atomic composition of the β-TCP + SDF and HA + SDF samples was evaluated at depths of 0 nm, 10 nm, and 100 nm, as shown in Figure 4, Figure 5 and Figure 6. At the surface level (0 nm), carbon (C) displayed a higher concentration, approximately 70%; this was likely due to adventitious carbon contamination from the atmosphere. Fluoride (F) was detected at 8.51% in both test groups, indicating its retention on the surface. Oxygen (O), calcium (Ca), phosphorus (P), and silver (Ag) were present in lower concentrations. At deeper levels (10 and 100 nm), after argon ion sputtering, the carbon concentration decreased to about 35%, while the proportions of O, Ca, P, and Ag increased. Fluoride showed a gradual decline with depth, being most concentrated at the surface and nearly absent in the deeper layers. Silver demonstrated consistent penetration across the analyzed depths.
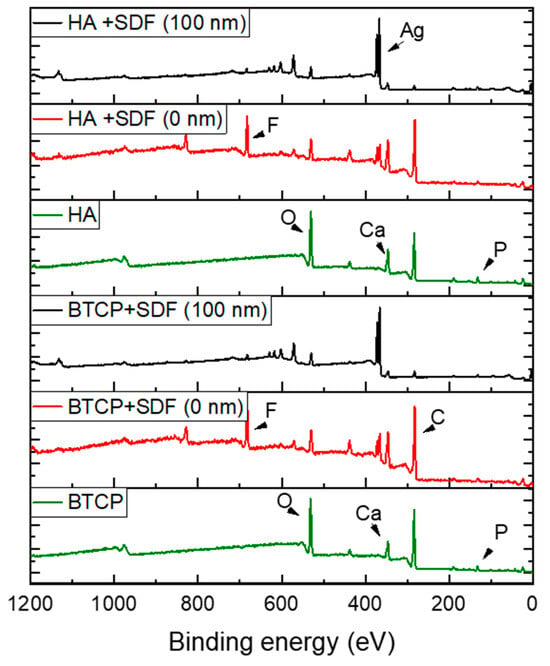
Figure 2.
XPS survey spectra under various experimental conditions, highlighting the main atomic elements.
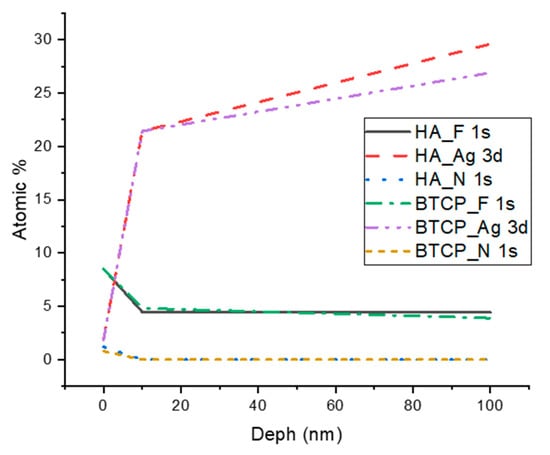
Figure 3.
XPS results of F, Ag, and N atomic concentration according to the analyzed depth.
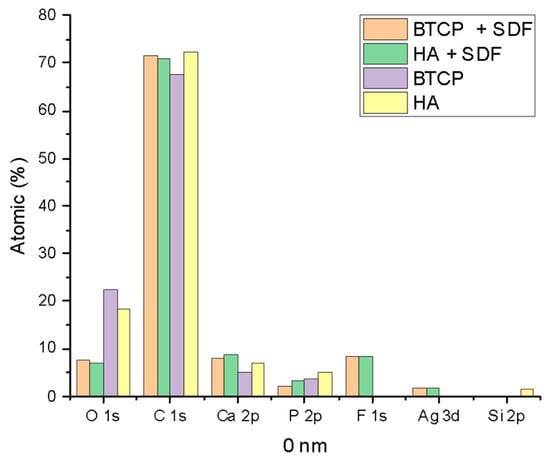
Figure 4.
XPS results of HA, BTCP, HA + SDF, and BTCP + SDF at 0 nm.
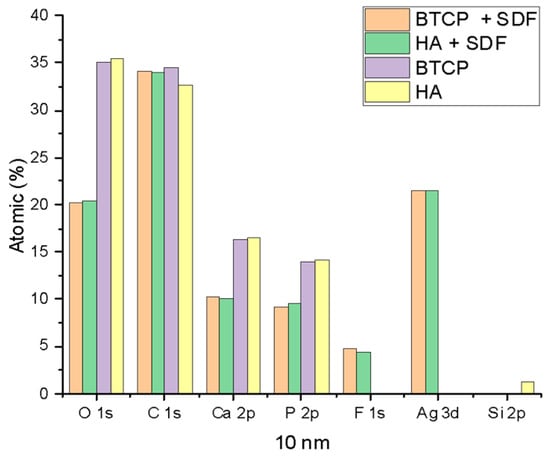
Figure 5.
XPS results of HA, BTCP, HA + SDF, and BTCP + SDF at 10 nm.
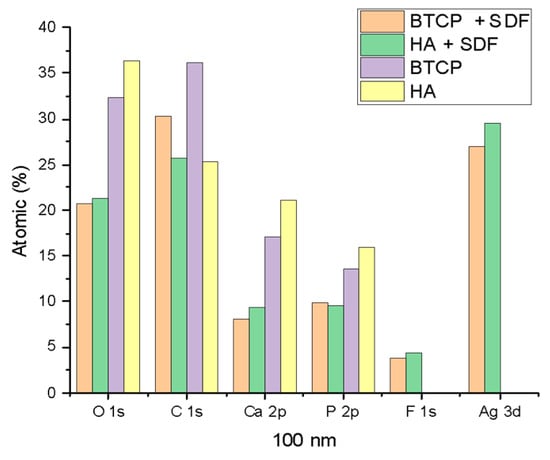
Figure 6.
XPS results of HA, BTCP, HA + SDF, and BTCP + SDF at 100 nm.
Figure 4, Figure 5 and Figure 6 display the atomic percentages of all the detected elements categorized by depths of 0 nm, 10 nm, and 100 nm, respectively. These figures are intended to compare the average atomic percentages across all samples analyzed. Standard deviations, which were consistently very low (from 0.5% to 0.1%), were omitted from the figures for clarity.
3. Discussion
The present study aimed to investigate the interactions of SDF with the main constituent of dental tissues by analyzing the chemical reaction products formed before and after its application. The results provide valuable insights into the efficacy of SDF as a preventive and caries-arresting agent. Advanced analytical techniques, including Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy, were employed to characterize the functional groups and quantify the atomic percentages of key elements, such as silver (Ag+) and fluoride (F−), in the reaction products. An in vitro model was developed using bioceramic powders—hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP)—as analogs for mineralized dental tissues. These powders were combined with a 38% SDF solution to investigate their chemical interactions.
XPS analysis demonstrated significant surface retention of fluoride at 0 nm, with limited penetration into deeper layers (10 and 100 nm). At the surface, fluoride accounted for 8.51% of the atomic composition in both HA and β-TCP groups, decreasing progressively with depth. This observation suggests that the interaction of SDF with calcium in the bioceramic powders led to the formation of calcium fluoride (CaF2), which predominantly acts on the surface and restricts fluoride availability for hydroxyapatite conversion into fluorapatite (FAP) [18]. Over time, CaF2 may transition into fluoride-substituted hydroxyapatite (FSHA), where F− ions partially replace OH− groups in the HA structure [16]. However, FSHA does not penetrate deeply and is less effective for caries prevention compared to fully substituted fluorapatite, which offers greater depth of action and long-term benefits by lowering the critical pH of enamel and dentin [24].
The distinct structural characteristics of HA and β-TCP help explain the subtle differences observed in their interactions with SDF. HA crystallizes in the hexagonal system (space group P63/m) with unit cell parameters a = b = 9.432 Å and c = 6.881 Å, where calcium ions are arranged in two distinct crystallographic sites, and hydroxyl groups are aligned along the c-axis channels. This arrangement creates a more stable and less soluble structure with a Ca/P ratio of 1.67. In contrast, β-TCP belongs to the rhombohedral system (space group R3c) with unit cell parameters a = b = 10.439 Å and c = 37.375 Å, featuring a more complex arrangement with calcium ions distributed across five different crystallographic sites and a lower Ca/P ratio of 1.5. These structural differences explain the variations observed in the FTIR spectra, where HA exhibited a strong, narrow band with the greatest peak at 1024 cm−1, while β-TCP showed a broader band with more pronounced peaks within the band. The broader band in β-TCP can be attributed to its rhombohedral structure, which creates larger spaces between ions and more pathways for ionic exchange. Furthermore, the absence of hydroxyl groups in β-TCP’s structure and its lower calcium content contribute to its increased reactivity, as evidenced by the different patterns of interaction with SDF components. This is particularly notable in the XPS results, where although both materials showed similar surface retention of fluoride (8.51% at 0 nm), the penetration patterns and chemical interactions varied slightly between the two materials, reflecting their inherent structural differences. The more stable hexagonal structure of HA may explain its more pronounced peak separation when combined with SDF, suggesting a more ordered interaction with the fluoride ions compared to the more complex rhombohedral structure of β-TCP.
Under oral conditions, SDF reacts with calcium and phosphate in saliva, facilitated by its high pH, to form calcium fluoride, which can release fluoride ions under acidic conditions to form fluorapatite [17]. Several factors may explain the absence of fluorapatite in this study. β-TCP is less soluble in neutral or alkaline environments, limiting the release of phosphate ions required for the reaction [25,26]. Additionally, its stable crystalline structure hinders the dissociation of phosphate necessary for interaction with fluoride. This interaction may also be less efficient compared to the soluble phosphates present in saliva [5]. Furthermore, the limited detection of fluoride in deeper layers can be attributed to the low atomic number and concentration of fluoride ions [27]. In alkaline conditions, fluoride remains stable and less reactive, whereas its reactivity increases under acidic environments, such as carious lesions [28]. This stability likely accounts for the observed surface retention of fluoride, with minimal penetration into deeper layers.
The penetration depth of SDF in natural dental tissues has been widely studied, with reported values ranging from 25 μm to 2516 μm in tooth structures [19,29,30]. It is important to recognize, however, that natural enamel and dentin exhibit a complex hierarchical structure, incorporating significant organic components, such as type I collagen fibrils, non-collagenous proteins, proteoglycans, and approximately 10% water by volume. These organic elements create a dynamic microenvironment that can influence the diffusion and interaction of SDF within mineralized tissues. In our study, we specifically focused on the interaction between SDF and the pure crystalline phases of HA and β-TCP, intentionally excluding the biological variables present in natural dental tissues. This approach was chosen to investigate the fundamental physicochemical mechanisms underlying the reactions of silver and fluoride ions with calcium phosphate crystal structures, which represent the primary inorganic components of dental hard tissues. Future studies will aim to explore these interactions at the ex vivo dentin level to better understand the penetration depth and clinical relevance of SDF in natural tooth structures.
FTIR analysis revealed a broad absorption band at 3350–3200 cm−1 in the SDF control sample corresponding to N-H stretching vibrations, indicating the presence of ammonia (NH3) or ammonium (NH4+) in the initial state of the solution. After interaction with the bioceramics, this band disappeared at 3350–3150 cm−1 or 1600 cm−1, suggesting that ammonia was consumed or transformed during the chemical reaction. A new band at 1425 cm−1 emerged in the test groups, attributed to ammonium ions (NH4+), indicating that SDF undergoes chemical reactions that alter its composition, likely forming NH4OH and other related products. This is because nitrogen and sulfur have a high affinity for silver ions, reacting and facilitating the penetration of silver particles [31].
Additionally, the analysis of phosphate groups (PO43−) highlighted significant alterations. Bioceramics in isolation exhibited intense and well-defined vibrational bands characteristic of their crystalline structures. When HA was combined with SDF, peak broadening and shifting occurred, which can be attributed to the substitution of hydroxyl groups by fluoride ions, leading to the formation of FSHA [16]. This substitution improves the chemical stability and resistance of HA to demineralization [16]. These alterations may also be attributed to the interaction between phosphate groups and collagenous proteins due to the alkaline environment that SDF provides, promoting apatite nucleation on collagen, contributing to dentin remineralization [31].
The high fluoride concentration in SDF favors the formation of CaF2, which predominantly occurs on the HA surface, particularly when phosphate availability is limited [17]. CaF2 acts as a reservoir of fluoride for subsequent reactions, while the vibrational band alterations indicate structural modifications in the HA matrix. Conversely, silver ions exhibit deeper penetration, suggesting potential incorporation into the mineral matrix. Literature indicates that silver ions can penetrate dentinal tubules to depths ranging from 50–200 microns to 1 nm, forming silver phosphate, an insoluble compound that deposits on the surface or within dentinal tubules, and can transform into other compounds, releasing phosphate ions to initiate apatite formation [18]. The other part is reduced by proteins (collagen), resulting in metallic silver attached to the protein (silver–protein complex) [31]. This confers antimicrobial properties and causes black discoloration [16,32]. Additionally, silver ions may integrate into the HA structure, forming silver-containing hydroxyapatite, which enhances bactericidal effects and resistance to demineralization [33].
In an in vivo study conducted by Seto et al. [34], human carious teeth treated with SDF were analyzed using electron microscopy. The results showed the formation of silver microwires inside the dentinal tubules, observed at a depth of up to 2100 µm from the surface, extending through the previously decayed tissue. This highlights the importance of silver as a component of SDF, not only due to its antimicrobial action but also for its ability to increase the hardness of the lesion and block fluid flow through the dentinal tubules, creating a barrier that helps interrupt the progression of the caries.
Moreover, other studies have investigated the depth of silver penetration into the tooth structure following the application of SDF. For example, a study by Manuschai et al. [31] on permanent teeth reported silver penetration ranging from 629 µm to 2516 µm, depending on the extent of the lesion and the treatment applied. In contrast, a study by Chu et al. [35] observed a more superficial penetration, with depths ranging from 25 µm to 200 µm. These discrepancies can be attributed to variations in experimental conditions, such as the application time of SDF and the concentration of the solution used.
These findings corroborate the results of our study, which demonstrated that silver reaches deeper layers of dentin, suggesting that the effectiveness of SDF is not limited to the superficial layers of the lesion. This effect may be relevant for controlling caries progression in deeper lesions, highlighting the versatility of SDF in treating dental caries.
The study’s findings also underscore the importance of considering the duration of SDF application. The reactivity of silver and fluoride ions, as well as their ability to penetrate the dental substrate, is influenced by the reaction time. Longer exposure times may allow greater interaction between the ions and the dental tissue, potentially enhancing the therapeutic effects. However, it is essential to optimize the application protocols to avoid potential issues such as discoloration, which can occur due to the reduction of silver ions to metallic silver. The metallic silver formed during this process can lead to black discoloration of the treated dental tissue, which is a known side effect of SDF application. More recently, some solutions for intrafibrillar remineralization have been proposed. One of them is the polymer-induced liquid precursor (PILP) process, which represents a critical mechanism for restoring the structural and mechanical integrity of decayed dentin. This process facilitates the infiltration of amorphous calcium phosphate precursors into the collagen fibril network, enabling mineral deposition within the fibrils and the re-establishment of the hierarchical structure of dentin.
Overall, the study provides valuable insights into the chemical interactions of SDF with dental tissues. However, it is important to note that the experimental design does not aim to mimic the complex hierarchical structure of natural dental tissues, such as enamel and dentin. Instead, the study focuses on the interactions of SDF with the crystalline structures of HA and β-TCP as simplified analogs. This approach allows the isolation and investigation of fundamental physicochemical mechanisms, while acknowledging the limitations in replicating the natural organic–inorganic composite nature of dental tissues. The formation of CaF2 on the surface of the bioceramics and the limited penetration of fluoride and silver ions into deeper layers suggest that SDF primarily acts at the surface level, providing a protective barrier against caries. However, the inability of SDF to form fluorapatite in this study highlights the need for further research to optimize its effectiveness in promoting deeper remineralization. The antimicrobial properties of silver ions, coupled with the long-term fluoride release from CaF2, suggest that SDF remains a promising tool for caries prevention and arrest [36]. Future studies should focus on using human enamel and dentin samples under more realistic clinical conditions, including the presence of saliva and biofilm, to better assess the full potential of SDF in preventing and treating dental caries. Additionally, integrating molecular-level findings with clinical outcomes is essential to understanding how the depth of silver ion penetration correlates with caries arrest in vivo, which could inform the development of optimized SDF formulations tailored for specific clinical scenarios.
4. Materials and Methods
The materials used in this study were hydroxyapatite powder (Sigma-Aldrich, St. Louis, MI, USA), β-tricalcium phosphate powder (Sigma-Aldrich, St. Louis, MO, USA), and 38% SDF solution (Elevate Oral Care, West Palm Beach, FL, USA), as seen in Table 1 [6,10,11]. Four test groups were used: HA + SDF and β-TCP + SDF, prepared at 5:1 and 500:1 powder-to-liquid ratios [5]. The 5:1 and 500:1 powder-to-liquid ratios were selected to evaluate both concentrated (5:1) and diluted (500:1) SDF interactions with the bioceramics, where the 5:1 ratio simulates clinical application conditions while the 500:1 ratio represents trace-level interactions that might occur at deeper tissue layers. There were three control groups: HA, β-TCP, and SDF. The test and control groups were both analyzed using Fourier transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS).

Table 1.
Materials required to prepare test and control groups.
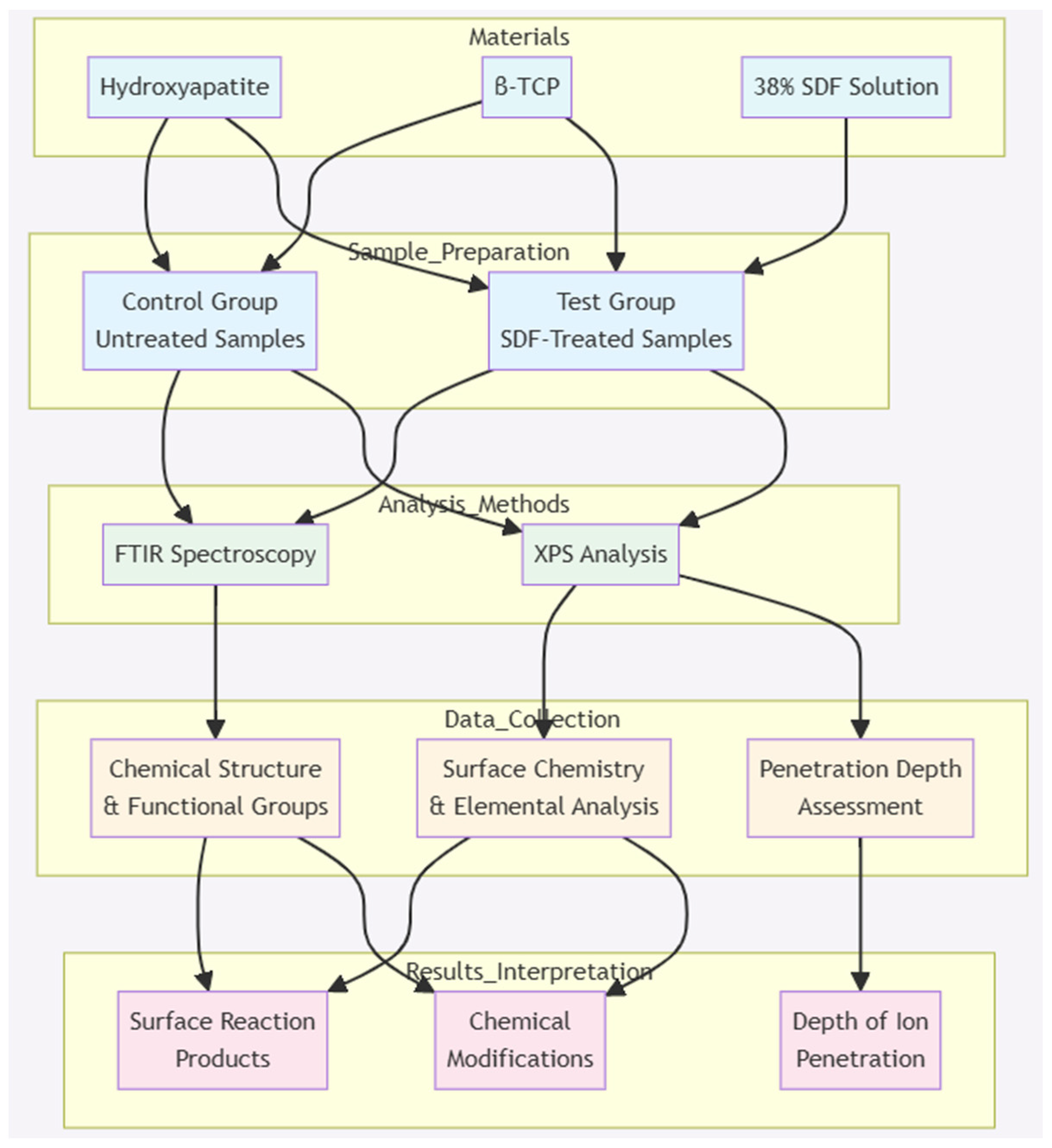
According to the manufacturer, SDF is corrosive to metal and glass [6]. Measures were taken to avoid unwanted reactions with and contamination of the experimental instruments during the storage and preparation of the test and control samples, including the use of plastic well plates, spatulas, and air-tight containers. Figure 7 displays the schematic view of the methodology.
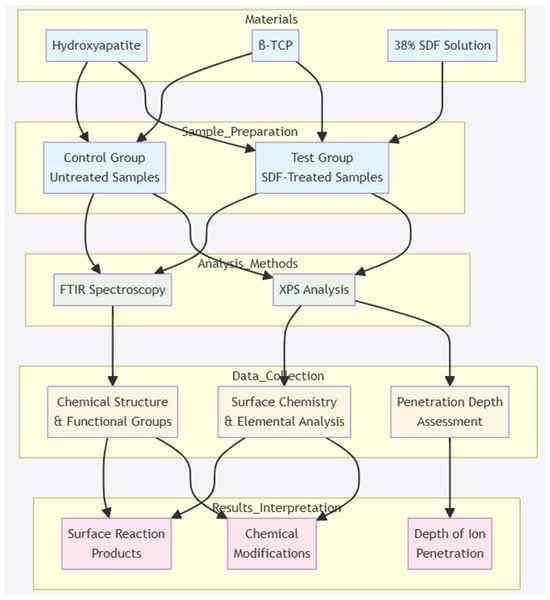
Figure 7.
Schematic representation of the methodology employed in this project.
4.1. X-Ray Fourier Transform Infrared Spectroscopy and Sample Preparation
The Nicolet 6700 FT-IR spectrometer from Thermo Scientific (Waltham, MA, USA) was used with OMNIC software (version 9.8). Each sample was analyzed through 64 scans to create an average spectrum for each analysis. Between each scan, debris or solution was removed using a Kim wipe, and the diamond crystal platform and compression arm were cleaned using a Kim wipe and an ethanol solution.
The HA and β-TCP powder control groups required no preparation before analysis; the materials were dispensed and analyzed immediately. Due to the corrosive nature of SDF on metal and glass, the SDF control group was dried prior to analysis [6]. This step was necessary to prevent corrosion reactions between the SDF sample and the diamond crystal platform of the FTIR machine. To dry the SDF control solution, two drops of SDF, approximately 0.064 mL, were dispensed into a clean plastic well plate. The well plate was sealed with Saran Wrap, placed inside a clean plastic container, and stored at 37 °C in the dark.
To prepare the 5:1 test group samples, 0.16 mg of HA and β-TCP powders was measured and combined with 0.032 mL of SDF solution. For the 500:1 test group samples, 1.6 mg of HA and β-TCP powders was measured and combined with 0.032 mL of SDF solution. The same storage conditions as for the SDF control group were applied. After preparation, the samples were transferred to airtight plastic containers. Five samples were analyzed from each test and control group. Before analysis, the test group samples were stirred using a plastic spatula to ensure an even representation of the entire sample.
4.2. X-Ray Photoelectron Spectroscopy and Sample Preparation
The Kratos Axis Ultra X-ray Photoelectron Spectroscopy (XPS) machine (Manchester, UK) was used with the following parameters: a vacuum of 2 × 10−⁹ torr, an X-ray gun emission set to 15 mA, and an X-ray gun anode HT set to 15 kV, corresponding to a power setting of 225 W and 0° takeoff angle. XPS survey spectra were acquired to characterize the enamel nano-surface by determining the atomic percentages of the elemental composition of each experimental sample. Depth profiles were obtained by applying argon ion sputtering for 75 s. Analysis was performed on the test and powdered control groups. Atomic percentages were measured at the following depths: 0 nm, 10 nm, and 100 nm.
The HA and β-TCP powder control groups required no preparation before analysis; the materials were dispensed onto double-sided carbon tape, approximately 1.5 cm × 1.5 cm in size. The SDF liquid control group required desiccation prior to analysis. SDF was dispensed onto a plastic watch glass, placed inside a glass desiccator, and stored in a desiccation oven with silica gel at 37 °C, protected from light. Once the sample was thoroughly dried, the residue was transferred using a sterile plastic spatula onto a 1.5 cm × 1.5 cm piece of double-sided carbon tape.
For the test groups, 2.5 mg of HA and β-TCP powders was measured and mixed with 0.5 mL of SDF, maintaining a 5:1 powder-to-liquid ratio. The samples were stirred twice a day for one minute until completely dry. Once dried, the residue was transferred using a sterile plastic spatula onto 1.5 cm × 1.5 cm pieces of double-sided carbon tape. The test and control samples (n = 5) were then placed into clean, airtight plastic containers and arranged onto a 10 cm × 1 cm XPS sample holder for analysis.
5. Conclusions
The effectiveness of SDF can be attributed to its unique composition and the actions of its components: silver, fluoride, and ammonia. SDF interacts with affected tooth structure to prevent and arrest decay, with increasing amounts of silver detected at depths up to 100 nm. This is a significant finding and warrants further investigation, as the depth and quantity of silver penetration within tooth structure after application remain unclear. Understanding this could lead to more precise clinical guidelines on the extent of decay for which SDF is suitable and its potential as a long-term conservative treatment option. Additionally, knowledge of the silver penetration depths in affected tooth structure could aid the future development of other dental materials containing silver and provide a better understanding of how various formulations affect silver penetration. However, fluoride was found in high concentrations only at the surface, suggesting limited penetration. This could be due to the use of pure mineralized powders in the study, the reaction of fluoride with calcium to form CaF2, or a combination of both. The study design may have influenced factors that favor the formation of fluorapatite, such as the high pH environment and the mineralized tooth structure.
Thus, improvements in future study designs could offer greater insight into the exact mechanism of fluoride after SDF application and how it contributes to the remarkable clinical success of SDF.
Author Contributions
Conceptualization, R.F.; methodology, D.B.; formal analysis, D.B. and A.C.B.C.J.F.; investigation, D.B. and B.d.S.N.S.; resources, R.F.; data curation, B.d.S.N.S.; writing—original draft preparation, D.B. and B.d.S.N.S. writing—review and editing, D.B., B.d.S.N.S., A.C.B.C.J.F. and R.F.; supervision, R.F.; project administration, R.F.; funding acquisition, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to express our sincere gratitude to the College of Dentistry of the University of Manitoba for their generous financial support for this research project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to extend our heartfelt thanks to Cristina Fiuza for her invaluable assistance during the sample preparation phase of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klimuszko, E.; Orywal, K.; Sierpinska, T.; Sidun, J.; Golebiewska, M. Evaluation of Calcium and Magnesium Contents in Tooth Enamel without Any Pathological Changes: In Vitro Preliminary Study. Odontology 2018, 106, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s Global Oral Health Status Report 2022: Actions, Discussion and Implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.A.; Tenuta, L.M.A. Enamel Remineralization: Controlling the Caries Disease or Treating Early Caries Lesions? Braz. Oral Res. 2009, 23, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mubaraki, H.; Ingle, N.A.; Baseer, M.A.; AlMugeiren, O.M.; Mubaraki, S.; Cicciù, M.; Minervini, G. Effect of Silver Diamine Fluoride on Bacterial Biofilms—A Review Including In Vitro and In Vivo Studies. Biomedicines 2023, 11, 1641. [Google Scholar] [CrossRef]
- Farooq, I.; Bugshan, A. The Role of Salivary Contents and Modern Technologies in the Remineralization of Dental Enamel: A Review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Van Strijp, G.; Van Loveren, C. No Removal and Inactivation of Carious Tissue: Non-Restorative Cavity Control. In Monographs in Oral Science; Schwendicke, F., Frencken, J., Innes, N., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 27, pp. 124–136. ISBN 978-3-318-06368-4. [Google Scholar]
- Wajahat, M.; Abbas, B.; Tariq, K.; Imran, E.; Aslam, S.; Khurshid, Z. Parental Perception of Silver Diamine Fluoride for the Management of Dental Caries. J. Taibah Univ. Med. Sci. 2022, 17, 408–414. [Google Scholar] [CrossRef]
- Mei, M.L.; Nudelman, F.; Marzec, B.; Walker, J.M.; Lo, E.C.M.; Walls, A.W.; Chu, C.H. Formation of Fluorohydroxyapatite with Silver Diamine Fluoride. J. Dent. Res. 2017, 96, 1122–1128. [Google Scholar] [CrossRef]
- Mei, M.L.; Lo, E.C.M.; Chu, C.H. Arresting Dentine Caries with Silver Diamine Fluoride: What’s Behind It? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef]
- Seifo, N.; Robertson, M.; MacLean, J.; Blain, K.; Grosse, S.; Milne, R.; Seeballuck, C.; Innes, N. The Use of Silver Diamine Fluoride (SDF) in Dental Practice. Br. Dent. J. 2020, 228, 75–81. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Niederman, R. Evidence-Based Dentistry Update on Silver Diamine Fluoride. Dent. Clin. N. Am. 2019, 63, 45–68. [Google Scholar] [CrossRef]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Li, Q.-L.; Lo, E.C.-M.; Chu, C.-H. Remineralisation of Enamel with Silver Diamine Fluoride and Sodium Fluoride. Dent. Mater. 2018, 34, e344–e352. [Google Scholar] [CrossRef]
- Seifo, N.; Cassie, H.; Radford, J.R.; Innes, N.P.T. Silver Diamine Fluoride for Managing Carious Lesions: An Umbrella Review. BMC Oral Health 2019, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.L.; Botelho, M.G.; Darvell, B.W. Reaction of Silver Diamine Fluoride with Hydroxyapatite and Protein. J. Dent. 2011, 39, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Rajakumari, T.N.; Thiruvenkadam, G.; Vinola, D.; Kruthika, M. Silver Diamine Fluoride—A Review. J. Acad. Dent. Educ. 2020, 6, 5–10. [Google Scholar] [CrossRef]
- Kaur, M.; Shahid, S.; Karpukhina, N.; Anderson, P.; Wong, F.S.L. Characterization of Chemical Reactions of Silver Diammine Fluoride and Hydroxyapatite under Remineralization Conditions. Front. Oral Health 2024, 5, 1332298. [Google Scholar] [CrossRef]
- Zheng, F.M.; Yan, I.G.; Duangthip, D.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Silver Diamine Fluoride Therapy for Dental Care. Jpn. Dent. Sci. Rev. 2022, 58, 249–257. [Google Scholar] [CrossRef]
- Surendranath, P.; Krishnappa, S.; Srinath, S. Silver Diamine Fluoride in Preventing Caries: A Review of Current Trends. Int. J. Clin. Pediatr. Dent. 2022, 15, S247–S251. [Google Scholar] [CrossRef]
- Sulyanto, R.M.; Kang, M.; Srirangapatanam, S.; Berger, M.; Candamo, F.; Wang, Y.; Dickson, J.R.; Ng, M.W.; Ho, S.P. Biomineralization of Dental Tissues Treated with Silver Diamine Fluoride. J. Dent. Res. 2021, 100, 1099–1108. [Google Scholar] [CrossRef]
- Zhao, I.S.; Gao, S.S.; Hiraishi, N.; Burrow, M.F.; Duangthip, D.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Mechanisms of silver diamine fluoride on arresting caries: A literature review. Int. Dent. J. 2018, 68, 67–76. [Google Scholar] [CrossRef]
- Bubnowicz, L.; França, R. Calcium and Fluoride Interaction in Human Enamel at Nanoscale: An XPS Assessment. Appl. Surf. Sci. Adv. 2022, 11, 100306. [Google Scholar] [CrossRef]
- Popa, C.L.; Ciobanu, C.S.; Voicu, G.; Vasile, E.; Chifiriuc, M.C.; Iconaru, S.L.; Predoi, D. Influence of Thermal Treatment on the Antimicrobial Activity of Silver-Doped Biological Apatite. Nanoscale Res. Lett. 2015, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier Transform Infrared Spectroscopy of Hydroxyapatite Co-Doped with Zinc and Fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Robinson, C. Fluoride and the Caries Lesion: Interactions and Mechanism of Action. Eur. Arch. Paediatr. Dent. 2009, 10, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Almulhim, K.S.; Syed, M.R.; Alqahtani, N.; Alamoudi, M.; Khan, M.; Ahmed, S.Z.; Khan, A.S. Bioactive Inorganic Materials for Dental Applications: A Narrative Review. Materials 2022, 15, 6864. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaître, J.; Ring, T.A. Kinetics of Dissolution of β-Tricalcium Phosphate. J. Colloid Interface Sci. 1997, 190, 37–48. [Google Scholar] [CrossRef]
- Soares-Yoshikawa, A.L.; Cury, J.A.; Tabchoury, C.P.M. Fluoride Concentration in SDF Commercial Products and Their Bioavailability with Demineralized Dentine. Braz. Dent. J. 2020, 31, 257–263. [Google Scholar] [CrossRef]
- Saxegaard, E.; Rölla, G. Fluoride Acquisition on and in Human Enamel during Topical Application in Vitro. Eur. J. Oral Sci. 1988, 96, 523–535. [Google Scholar] [CrossRef]
- Mulder, R.; Potgieter, N.; Noordien, N. Penetration of SDF and AgF from the Infected Dentine towards the Unaffected Tooth Structure. Front. Oral Health 2023, 4, 1298211. [Google Scholar] [CrossRef]
- Kiesow, A.; Menzel, M.; Lippert, F.; Tanzer, J.M.; Milgrom, P. Dentin Tubule Occlusion by a 38% Silver Diamine Fluoride Gel: An in Vitro Investigation. BDJ Open 2022, 8, 1. [Google Scholar] [CrossRef]
- Manuschai, J.; Talungchit, S.; Naorungroj, S. Penetration of Silver Diamine Fluoride in Deep Carious Lesions of Human Permanent Teeth: An In Vitro Study. Int. J. Dent. 2021, 2021, 3059129. [Google Scholar] [CrossRef]
- Hafiz, Z.; Allam, R.; Almazyad, B.; Bedaiwi, A.; Alotaibi, A.; Almubrad, A. Effectiveness of Silver Diamine Fluoride in Arresting Caries in Primary and Early Mixed Dentition: A Systematic Review. Children 2022, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Sayed, M.; Takahashi, M.; Nikaido, T.; Tagami, J. Clinical and Primary Evidence of Silver Diamine Fluoride on Root Caries Management. Jpn. Dent. Sci. Rev. 2022, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Horst, J.A.; Parkinson, D.Y.; Frachella, J.C.; DeRisi, J.L. Enhanced Tooth Structure Via Silver Microwires Following Treatment with 38 Percent Silver Diamine Fluoride. Pediatr. Dent. 2020, 42, 226–231. [Google Scholar] [PubMed]
- Chu, C.H.; Lo, E.C.M. Microhardness of Dentine in Primary Teeth after Topical Fluoride Applications. J. Dent. 2008, 36, 387–391. [Google Scholar] [CrossRef]
- López-García, S.; Sanz, J.L.; Oñate-Sánchez, R.E.; Forner, L.; García-Bernal, D.; Murcia, L.; Rodríguez-Lozano, F.J.; Llena, C. In vitro biocompatibility of ammonia-free silver fluoride products on human dental pulp stem cells. Tissue Cell 2024, 86, 102283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).