Ti3AlC2 MAX/MXene for Hydrogen Generation via Photocatalytic Hydride Hydrolysis
Abstract
1. Introduction
2. Results and Discussion
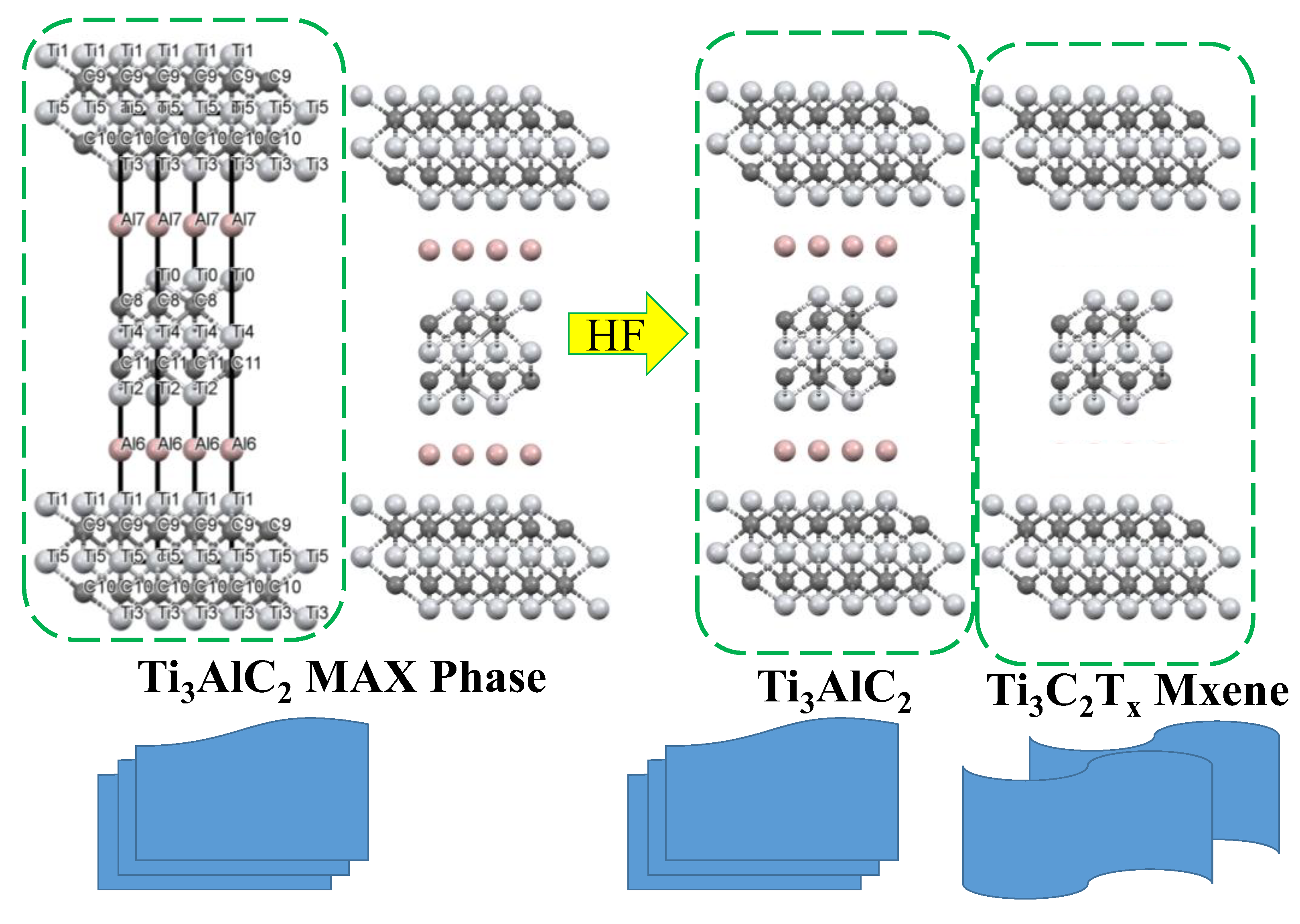
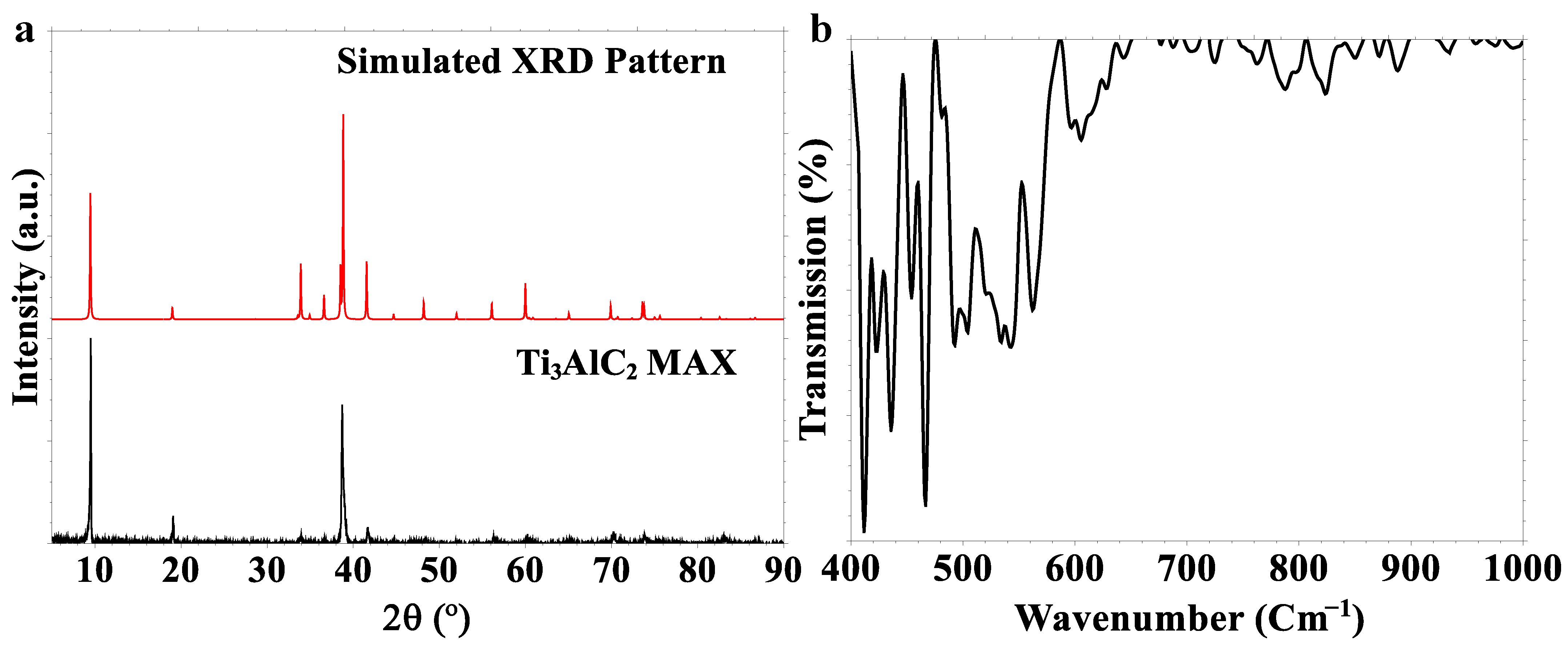
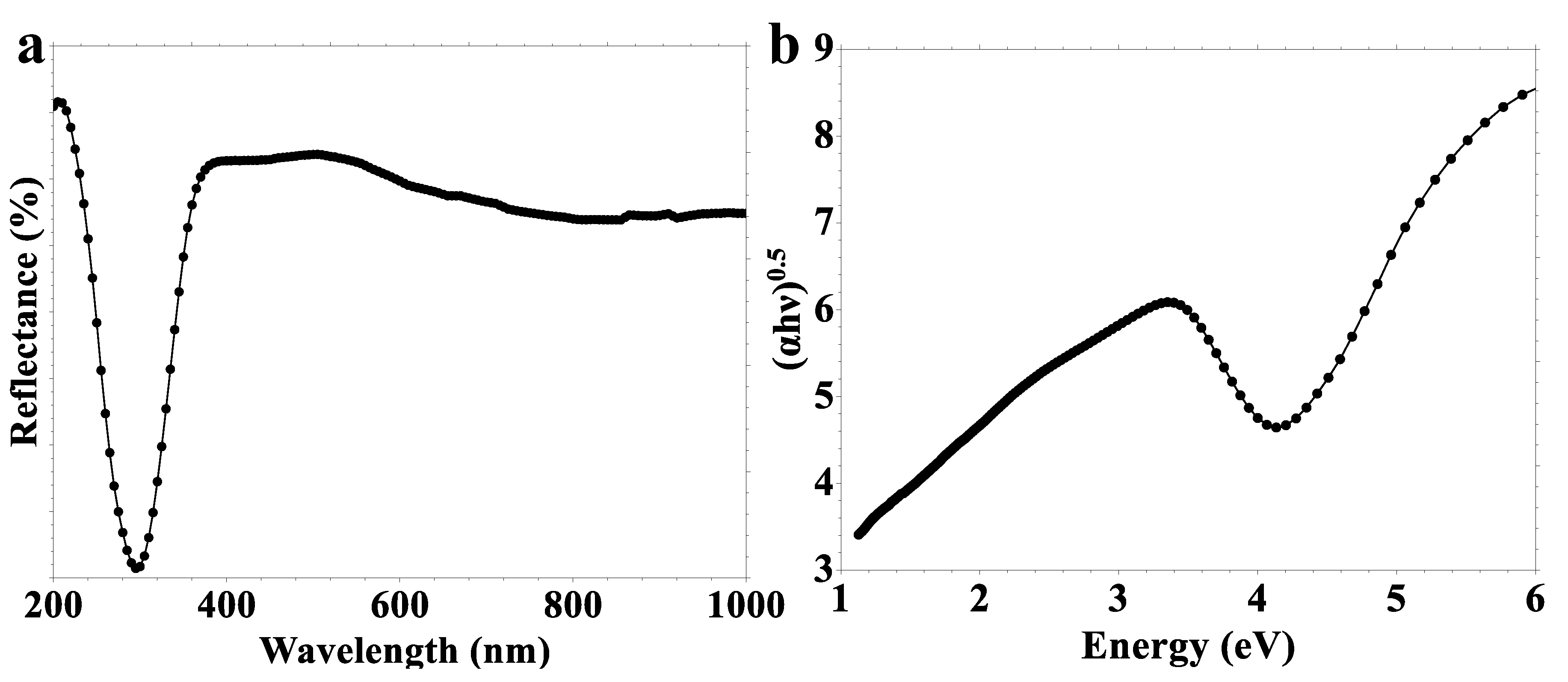
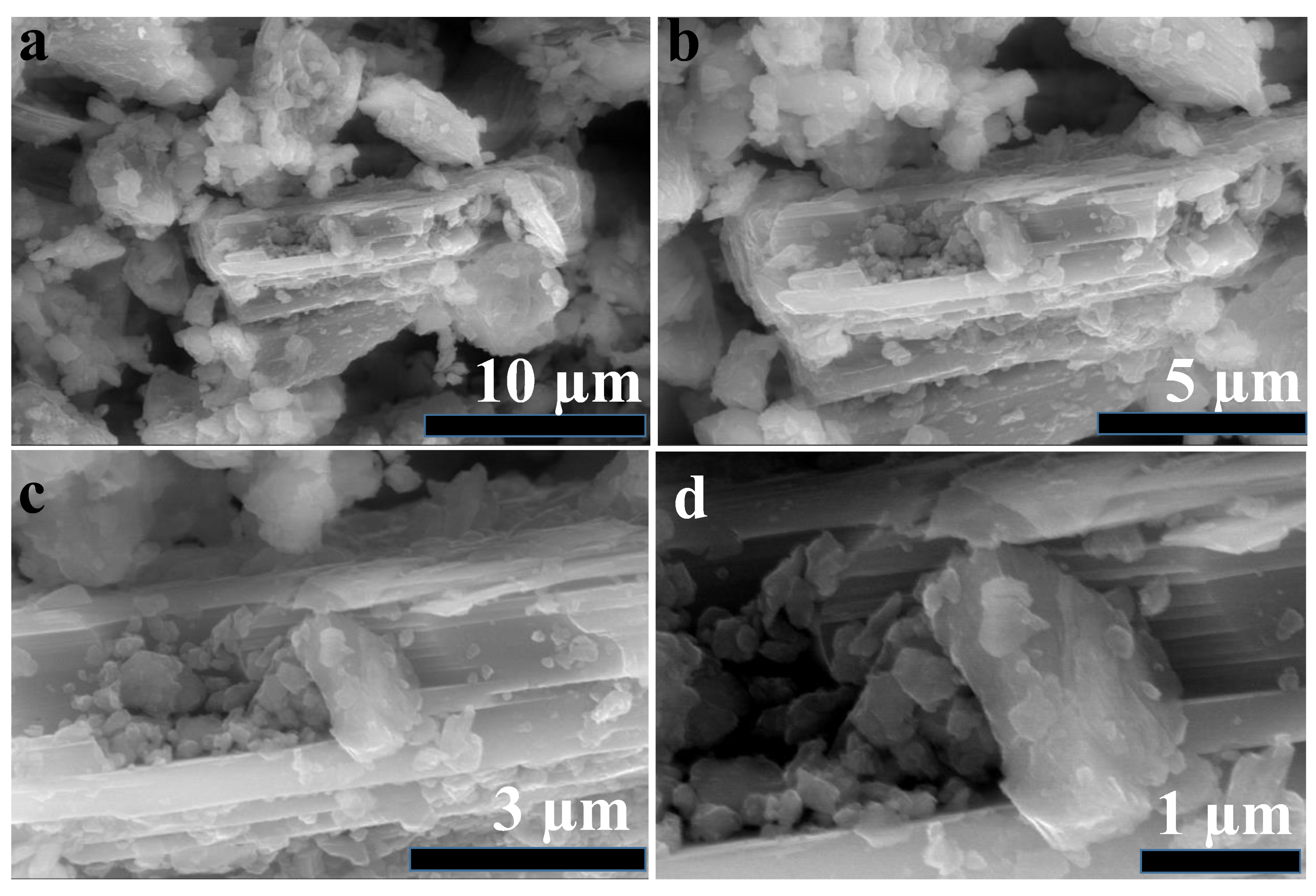
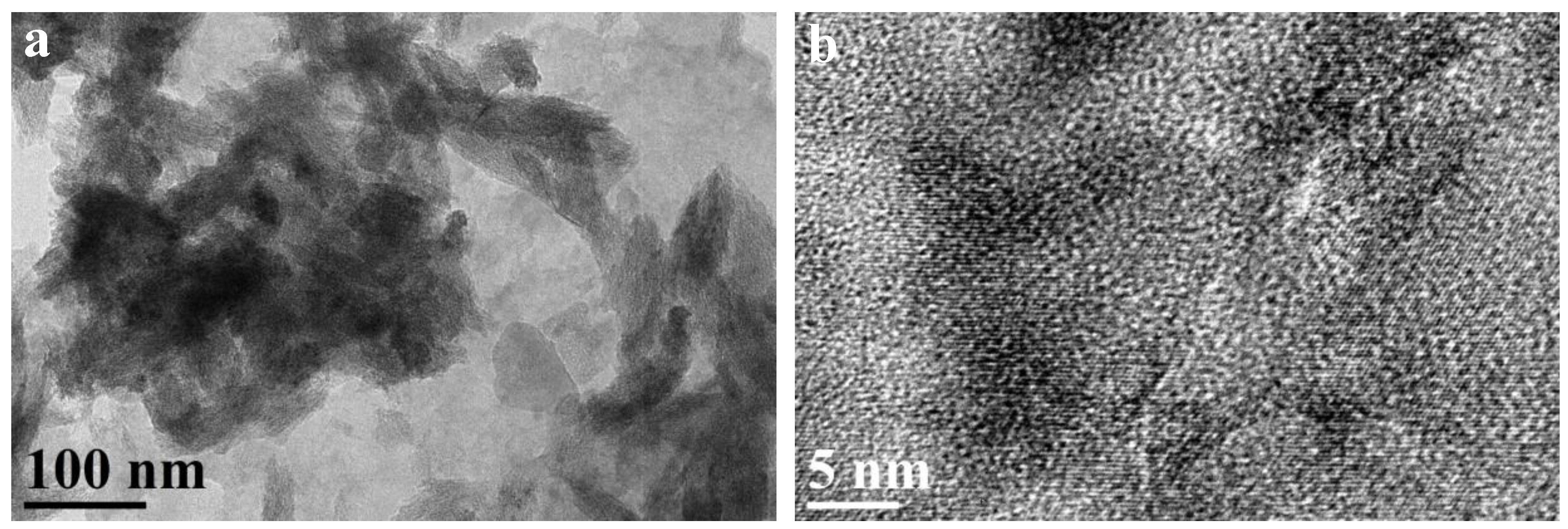
2.1. Characterization
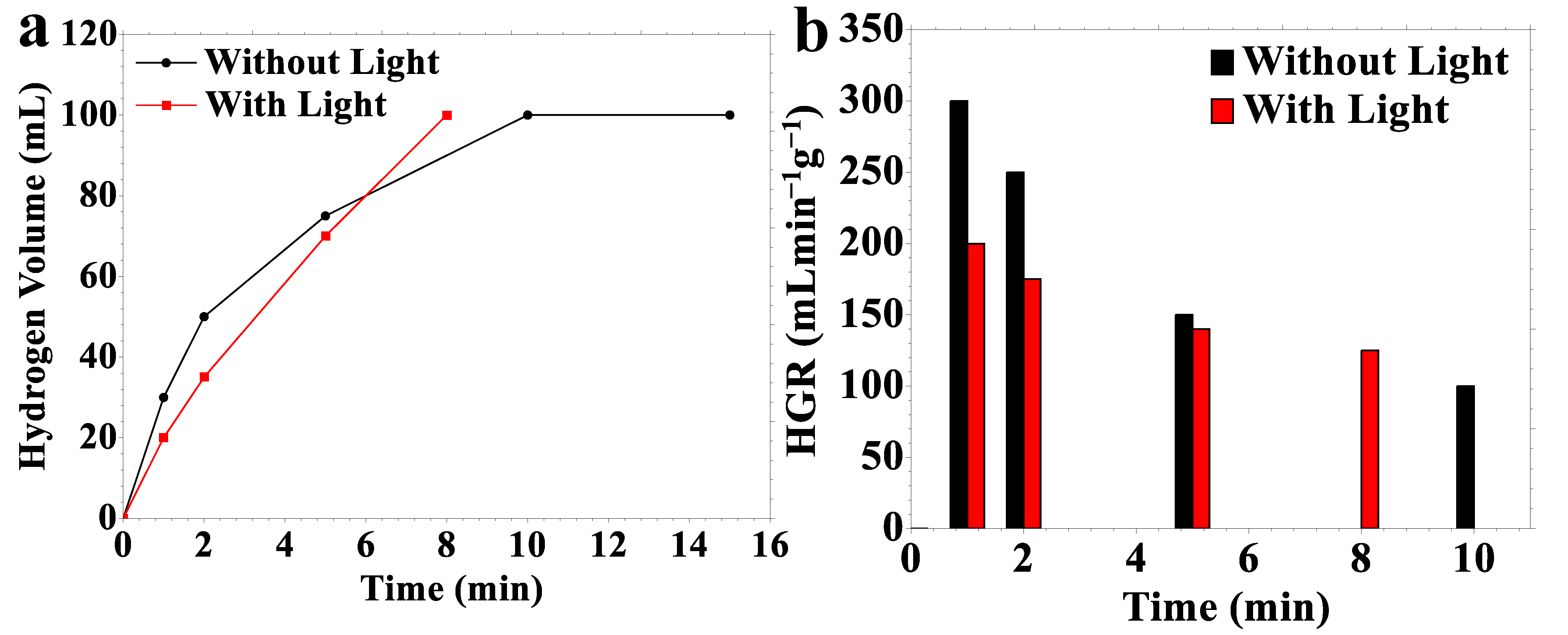
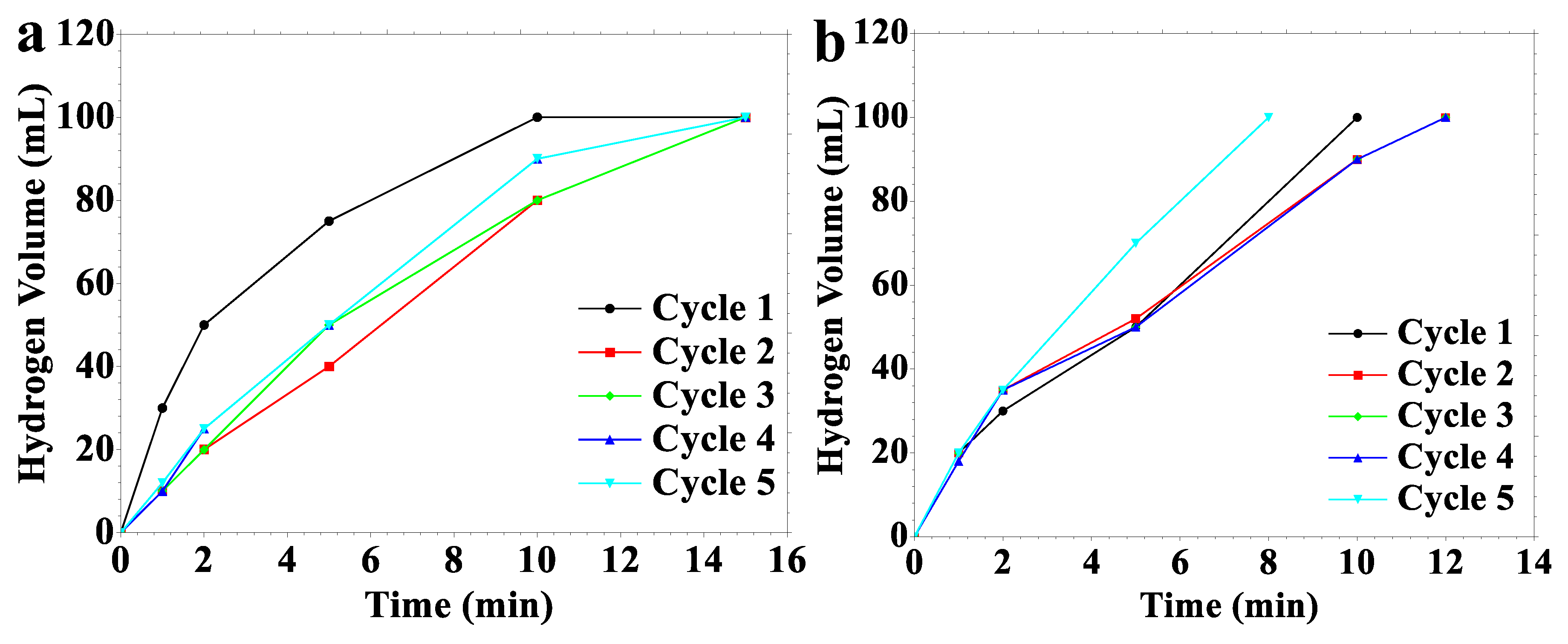
2.2. Hydrogen Generation
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Ti3C2Tx MXene
3.3. Material Characterization
3.4. Hydrogen Generation Measurements
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Kumar, A. (Ed.) Towards Sustainable and Green Hydrogen Production by Photocatalysis: Insights into Design and Development of Efficient Materials (Volume 2); ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; Volume 1468, ISBN 9780841296701. [Google Scholar]
- David Gaima Kafadi, A.; Yusuf Hafeez, H.; Mohammed, J.; Ndikilar, C.E.; Suleiman, A.B.; Isah, A.T. A recent prospective and progress on MXene-based photocatalysts for efficient solar fuel (hydrogen) generation via photocatalytic water-splitting. Int. J. Hydrogen Energy 2024, 53, 1242–1258. [Google Scholar] [CrossRef]
- Thabet, S.M.; Abdelhamid, H.N.; Ibrahim, S.A.; El-Bery, H.M. Boosting photocatalytic water splitting of TiO2 using metal (Ru, Co, or Ni) co-catalysts for hydrogen generation. Sci. Rep. 2024, 14, 10115. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Onajah, S.; Sarkar, R.; Islam, M.S.; Lalley, M.; Khan, K.; Demir, M.; Abdelhamid, H.N.; Farghaly, A.A. Silica-Derived Nanostructured Electrode Materials for ORR, OER, HER, CO 2 RR Electrocatalysis, and Energy Storage Applications: A Review. Chem. Rec. 2024, 24, e202300234. [Google Scholar] [CrossRef]
- Teli, A.M.; Mane, S.M.; Mishra, R.K.; Jeon, W.; Shin, J.C. Unveiling the Electrocatalytic Performances of the Pd-MoS2 Catalyst for Methanol-Mediated Overall Water Splitting. Inorganics 2025, 13, 21. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Páez-López, R.D.; Gómez-Soto, M.Á.; Cortés-Hernández, H.F.; Solano-Peralta, A.; Castro, M.; Kroneck, P.M.H.; Sosa-Torres, M.E. Understanding Dioxygen Activation in the Fe(III)-Promoted Oxidative Dehydrogenation of Amines: A Computational Study. Inorganics 2025, 13, 22. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Saleh, M.; Abdelhamid, H.N.; Fouad, D.M.; El-Bery, H.M. Enhancing photocatalytic water splitting: Comparative study of TiO2 decorated nanocrystals (Pt and Cu) using different synthesis methods. Fuel 2023, 354, 129248. [Google Scholar] [CrossRef]
- Davis Cortina, M.; Romero de Terreros Aramburu, M.; Neves, A.M.; Hurtado, L.; Jepsen, J.; Ulmer, U. The Integration of Thermal Energy Storage Within Metal Hydride Systems: A Comprehensive Review. Inorganics 2024, 12, 313. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Salts Induced Formation of Hierarchical Porous ZIF-8 and Their Applications for CO2 Sorption and Hydrogen Generation via NaBH4 Hydrolysis. Macromol. Chem. Phys. 2020, 221, 2000031. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Hierarchical porous ZIF-8 for hydrogen production via the hydrolysis of sodium borohydride. Dalt. Trans. 2020, 49, 4416–4424. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y. MXenes; Jenny Stanford Publishing: New York, NY, USA, 2023; ISBN 9781003306511. [Google Scholar]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Amara, U.; Hussain, I.; Ahmad, M.; Mahmood, K.; Zhang, K. 2D MXene-Based Biosensing: A Review. Small 2023, 19, 2205249. [Google Scholar] [CrossRef]
- Cao, W.; Nie, J.; Cao, Y.; Gao, C.; Wang, M.; Wang, W.; Lu, X.; Ma, X.; Zhong, P. A review of how to improve Ti3C2Tx MXene stability. Chem. Eng. J. 2024, 496, 154097. [Google Scholar] [CrossRef]
- Isa, A.T.; Hafeez, H.Y.; Mohammed, J.; Ndikilar, C.E.; Suleiman, A.B.; Kafadi, A.D.G. Photocatalytic performance of MXenes cocatalyst in hydrogen (H2) production via photocatalytic water splitting: A review. J. Alloys Compd. 2024, 1005, 175951. [Google Scholar] [CrossRef]
- Tie, L.; Li, N.; Yu, C.; Liu, Y.; Yang, S.; Chen, H.; Dong, S.; Sun, J.; Dou, S.; Sun, J. Self-Supported Nonprecious MXene/Ni3S2 Electrocatalysts for Efficient Hydrogen Generation in Alkaline Media. ACS Appl. Energy Mater. 2019, 2, 6931–6938. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Arun, A.; Guduru, R.K.; Velauthapillai, D. Heterostructured two dimensional materials of MXene and graphene by hydrothermal method for efficient hydrogen production and HER activities. Int. J. Hydrogen Energy 2023, 48, 6478–6487. [Google Scholar] [CrossRef]
- Sanna, M.; Ng, S.; Vaghasiya, J.V.; Pumera, M. Fluorinated MAX Phases for Photoelectrochemical Hydrogen Evolution. ACS Sustain. Chem. Eng. 2022, 10, 2793–2801. [Google Scholar] [CrossRef]
- Tahir, M.; Mansoor, R. Constructing a stable 2D Ti3AlC2 MnAXm cocatalyst-modified gC3N4/CoAl-LDH/Ti3AlC2 heterojunction for efficient dry and bireforming of methane for photocatalytic syngas production. J. Alloys Compd. 2023, 947, 169457. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, S.; Wang, C.; Yin, D.; Wang, S.; Wang, Q.; Liang, F.; Li, S.; Wang, L.; Cheng, Y. Heterojunction synergistic catalysis of MXene-supported PrF3 nanosheets for the efficient hydrogen storage of AlH3. Nano Res. 2023, 16, 9546–9552. [Google Scholar] [CrossRef]
- Biswal, L.; Mohanty, R.; Nayak, S.; Parida, K. Review on MXene/TiO2 nanohybrids for photocatalytic hydrogen production and pollutant degradations. J. Environ. Chem. Eng. 2022, 10, 107211. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M.; Zakaria, Z.Y. Fabricating structured 2D Ti3AlC2 MAX dispersed TiO2 heterostructure with Ni2P as a cocatalyst for efficient photocatalytic H2 production. J. Alloys Compd. 2020, 842, 155752. [Google Scholar] [CrossRef]
- Yang, J.-X.; Yu, W.-B.; Li, C.-F.; Dong, W.-D.; Jiang, L.-Q.; Zhou, N.; Zhuang, Z.-P.; Liu, J.; Hu, Z.-Y.; Zhao, H.; et al. PtO nanodots promoting Ti3C2 MXene in-situ converted Ti3C2/TiO2 composites for photocatalytic hydrogen production. Chem. Eng. J. 2021, 420, 129695. [Google Scholar] [CrossRef]
- Sun, B.; Qiu, P.; Liang, Z.; Xue, Y.; Zhang, X.; Yang, L.; Cui, H.; Tian, J. The fabrication of 1D/2D CdS nanorod@Ti3C2 MXene composites for good photocatalytic activity of hydrogen generation and ammonia synthesis. Chem. Eng. J. 2021, 406, 127177. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Ji, Y.; Bian, R.; Li, J.; Zhang, X.; Tian, J.; Yang, Q.; Shi, F. Ti3C2 MXene coupled with CdS nanoflowers as 2D/3D heterostructures for enhanced photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2022, 47, 22045–22053. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Z.; Li, G.; Cui, X.; Jin, M.; Xie, T.; Liu, L.; Jiang, M.; Zhong, X.; Zhang, Y.; et al. Interfacial Engineering of TiO2/Ti3C2 MXene/Carbon Nitride Hybrids Boosting Charge Transfer for Efficient Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2022, 12, 2102765. [Google Scholar] [CrossRef]
- Ye, X.; Zhong, H.; Zhang, Y.; Liu, X.; Tian, W.; Ma, L.-A.; Wang, Q. Ti3C2 MXene cocatalyst supported Ti3C2/SrTiO3/g-C3N4 heterojunctions with efficient electron transfer for photocatalytic H2 production. CrystEngComm 2024, 26, 5440–5451. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Sangarimotlagh, Z.; Karbasi, M. Latest progress in photocatalytic hydrogen production using MXene (Ti3C2)/MOFs composite: A review. Int. J. Hydrogen Energy 2024, 79, 771–790. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.; Kang, S.-Z.; Zhang, T.; Qin, L.; Li, X. Facilely in-situ growth of porphyrin metal-organic frameworks on the Ti3C2 MXene/TiO2 for greatly improved photocatalytic hydrogen generation. J. Alloys Compd. 2023, 961, 170929. [Google Scholar] [CrossRef]
- Su, T.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.; Qin, Z.; Wu, Z. Monolayer Ti3C2Tx as an Effective Cocatalyst for Enhanced Photocatalytic Hydrogen Production over TiO2. ACS Appl. Energy Mater. 2019, 2, 4640–4651. [Google Scholar] [CrossRef]
- Mistry, K.; Lakhani, R.; Tripathi, B.; Shinde, S.; Chandra, P. Recent trends in MXene/Metal chalcogenides for electro-/photocatalytic hydrogen evolution reactions. Int. J. Hydrogen Energy 2022, 47, 41711–41732. [Google Scholar] [CrossRef]
- Ghotia, S.; Kumar, A.; Sudarsan, V.; Dwivedi, N.; Singh, S.; Kumar, P. Multilayered Ti3C2Tx MXenes: A prominent materials for hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 100–107. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, D.; Zheng, J.; Xia, A.; Zhang, Q.; Wang, L.; Zhang, L. Heterostructured VF4@Ti3C2 catalyst improving reversible hydrogen storage properties of Mg(BH4)2. Chem. Eng. J. 2023, 460, 141690. [Google Scholar] [CrossRef]
- Karataş, Y.; Çetin, T.; Akinay, Y.; Gülcan, M. Synthesis and characterization of Pd doped MXene for hydrogen production from the hydrolysis of methylamine borane: Effect of cryogenic treatment. J. Energy Inst. 2023, 109, 101310. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Zhang, X. Ti3AlC2/Pd Composites for Efficient Hydrogen Production from Alkaline Formaldehyde Solutions. Nanomaterials 2022, 12, 843. [Google Scholar] [CrossRef]
- Yao, F.; Guan, S.; Bian, L.; Fan, Y.; Liu, X.; Zhang, H.; Li, B.; Liu, B. Ensemble-Exciting Effect in Pd/alk-Ti3C2 on the Activity for Efficient Hydrogen Production. ACS Sustain. Chem. Eng. 2021, 9, 12332–12340. [Google Scholar] [CrossRef]
- Liang, L.; Bian, L.; Fan, Y.; Guan, S.; Liu, X.; Sun, Q.; Liu, B. Nitrogen doping excited Ru and Ti3C2−xNx support for hydrogen generation from ammonia borane. Fuel 2023, 339, 127445. [Google Scholar] [CrossRef]
- Slot, T.K.; Yue, F.; Xu, H.; Ramos-Fernandez, E.V.; Sepúlveda-Escribano, A.; Sofer, Z.; Rothenberg, G.; Shiju, N.R. Surface oxidation of Ti3C2Tx enhances the catalytic activity of supported platinum nanoparticles in ammonia borane hydrolysis. 2F Mater. 2020, 8, 015001. [Google Scholar] [CrossRef]
- Fan, G.; Li, X.; Ma, Y.; Zhang, Y.; Wu, J.; Xu, B.; Sun, T.; Gao, D.; Bi, J. Magnetic, recyclable PtyCo1−y/Ti3C2X2 (X = O, F) catalyst: A facile synthesis and enhanced catalytic activity for hydrogen generation from the hydrolysis of ammonia borane. New J. Chem. 2017, 41, 2793–2799. [Google Scholar] [CrossRef]
- Guo, F.; Zou, H.; Yao, Q.; Huang, B.; Lu, Z.-H. Monodispersed bimetallic nanoparticles anchored on TiO2-decorated titanium carbide MXene for efficient hydrogen production from hydrazine in aqueous solution. Renew. Energy 2020, 155, 1293–1301. [Google Scholar] [CrossRef]
- Li, T.; Xiang, C.; Chu, H.; Xu, F.; Sun, L.; Zou, Y.; Zhang, J. Catalytic effect of highly dispersed ultrafine Ru nanoparticles on a TiO2-Ti3C2 support: Hydrolysis of sodium borohydride for H2 generation. J. Alloys Compd. 2022, 906, 164380. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Yang, J.; Han, B.; Nie, R.; Wang, J.; Wang, J.; Jing, H. In-situ grown nanocrystal TiO2 on 2D Ti3C2 nanosheets for artificial photosynthesis of chemical fuels. Nano Energy 2018, 51, 442–450. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Hu, Q.; Cao, X. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Lin, Z.; Yuan, Z.; Zhang, H.; Guan, S.; Wang, X.; Zhao, S.; Fan, G.; Fan, Y.; Liu, B. Excellent catalytic effect of V2C MXene on dehydrogenation performance of α-AlH3. Int. J. Hydrogen Energy 2024, 56, 998–1006. [Google Scholar] [CrossRef]
- Hussein Hashem, Z.; Abdel-Rahman, L.H.; Gómez-Ruiz, S.; Abdelhamid, H.N. Cerium-Organic Framework (CeOF) for hydrogen generation via the hydrolysis of NaBH4. Results Chem. 2024, 7, 101412. [Google Scholar] [CrossRef]
- Saleh, M.R.; El-Bery, H.M.; Abdelhamid, H.N. Co@ZIF-8/TiO2 Heterojunction for Green Hydrogen Generation. Appl. Organomet. Chem. 2022, 37, e6995. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Dehydrogenation of sodium borohydride using cobalt embedded zeolitic imidazolate frameworks. J. Solid State Chem. 2021, 297, 122034. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Taha, T.A.; Azab, A.A.; Abdelhamid, H.N. ZnO-based nanocomposites for hydrogen generation via hydrolysis of Borohydride. J. Sol-Gel Sci. Technol. 2023, 106, 837–846. [Google Scholar] [CrossRef]
- Abdellatif, A.B.A.; El-Bery, H.M.; Abdelhamid, H.N.; El-Gyar, S.A. ZIF-67 and Cobalt-based@heteroatom–doped carbon nanomaterials for hydrogen production and dyes removal via adsorption and catalytic degradation. J. Environ. Chem. Eng. 2022, 10, 108848. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. In-situ growth of zeolitic imidazolate frameworks into a cellulosic filter paper for the reduction of 4-nitrophenol. Carbohydr. Polym. 2021, 274, 118657. [Google Scholar] [CrossRef]
- Georgouvelas, D.; Abdelhamid, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef]
| Catalysts | Synthesis | Hydrogen Source | HGR | Reaction Conditions | Ref. |
|---|---|---|---|---|---|
| Pd@cryo-MXene |
| CH3NH2-BH3 | 159.4 min−1 | 50 mg of catalyst; 23 mg MeAB; 318 K | [39] |
| PtyCo1−y/Ti3C2Tx | In situ reduction of Pt and Co | NH3BH3 | 100.7 L H2 (min gPt)−1 | 10 mg of Ti3C2Tx; 0.115 mL of H2PtCl6 aqueous solution and CoCl2; AB (34.2 mg); 318 K | [44] |
| Ru/TiO2/Ti3C2Tx |
| NaBH4 | 60 L·min−1·gRu−1 | 0.1 g of catalyst; 5 wt.% NaOH; 1.5 wt.% NaBH4 solution; Ru loading 0.33 wt.%; 303 K | [46] |
| Cerium-based MOF | Hydrothermal method | 1800 mLH2·gcat−1·min−1 | 50 mg catalyst; 1 g NaBH4; 333 K | [52] | |
| ZnO | Sol–gel method | 3000 mLH2·gcat−1·min−1 | 10 mg catalyst; 1 wt.% NaBH4; 303 K | [55] | |
| Ti3AlC2/Ti3C2 | HF etching Ultrasonication | 200–300 mLH2·gcat−1·min−1 | 100 mg catalyst; 0.2 wt.%NaBH4; 298 K, UV-light | Herein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, H.N. Ti3AlC2 MAX/MXene for Hydrogen Generation via Photocatalytic Hydride Hydrolysis. Inorganics 2025, 13, 44. https://doi.org/10.3390/inorganics13020044
Abdelhamid HN. Ti3AlC2 MAX/MXene for Hydrogen Generation via Photocatalytic Hydride Hydrolysis. Inorganics. 2025; 13(2):44. https://doi.org/10.3390/inorganics13020044
Chicago/Turabian StyleAbdelhamid, Hani Nasser. 2025. "Ti3AlC2 MAX/MXene for Hydrogen Generation via Photocatalytic Hydride Hydrolysis" Inorganics 13, no. 2: 44. https://doi.org/10.3390/inorganics13020044
APA StyleAbdelhamid, H. N. (2025). Ti3AlC2 MAX/MXene for Hydrogen Generation via Photocatalytic Hydride Hydrolysis. Inorganics, 13(2), 44. https://doi.org/10.3390/inorganics13020044






