Lanthanide-Containing Polyoxometalate Crystallized with Bolaamphiphile Surfactants as Inorganic–Organic Hybrid Phosphors
Abstract
1. Introduction
2. Results
2.1. Synthesis of EuW10-Bolaamphiphile Hybrid Crystals
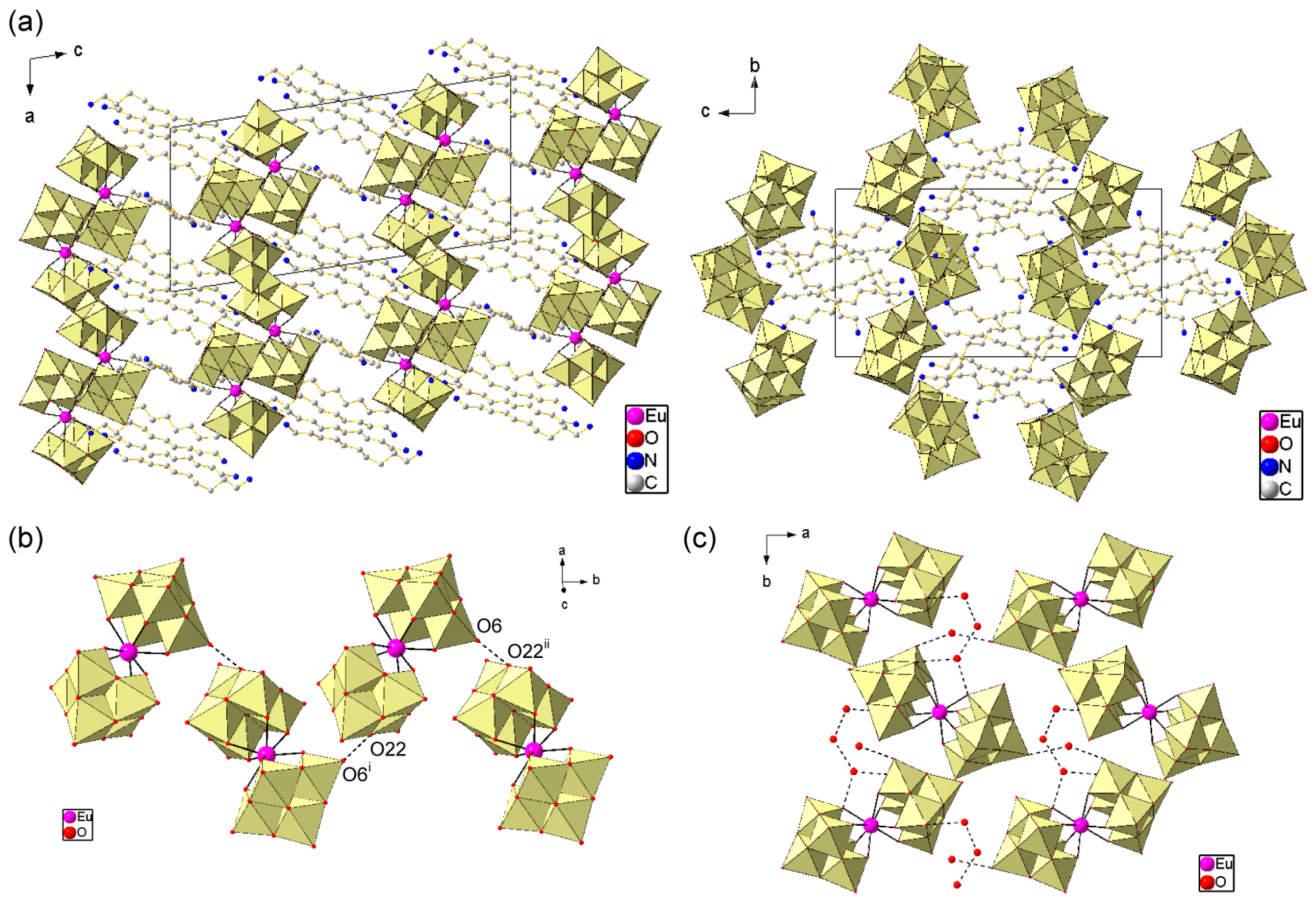
2.2. Crystal Structures of EuW10-Bolaamphiphile Hybrid Crystals
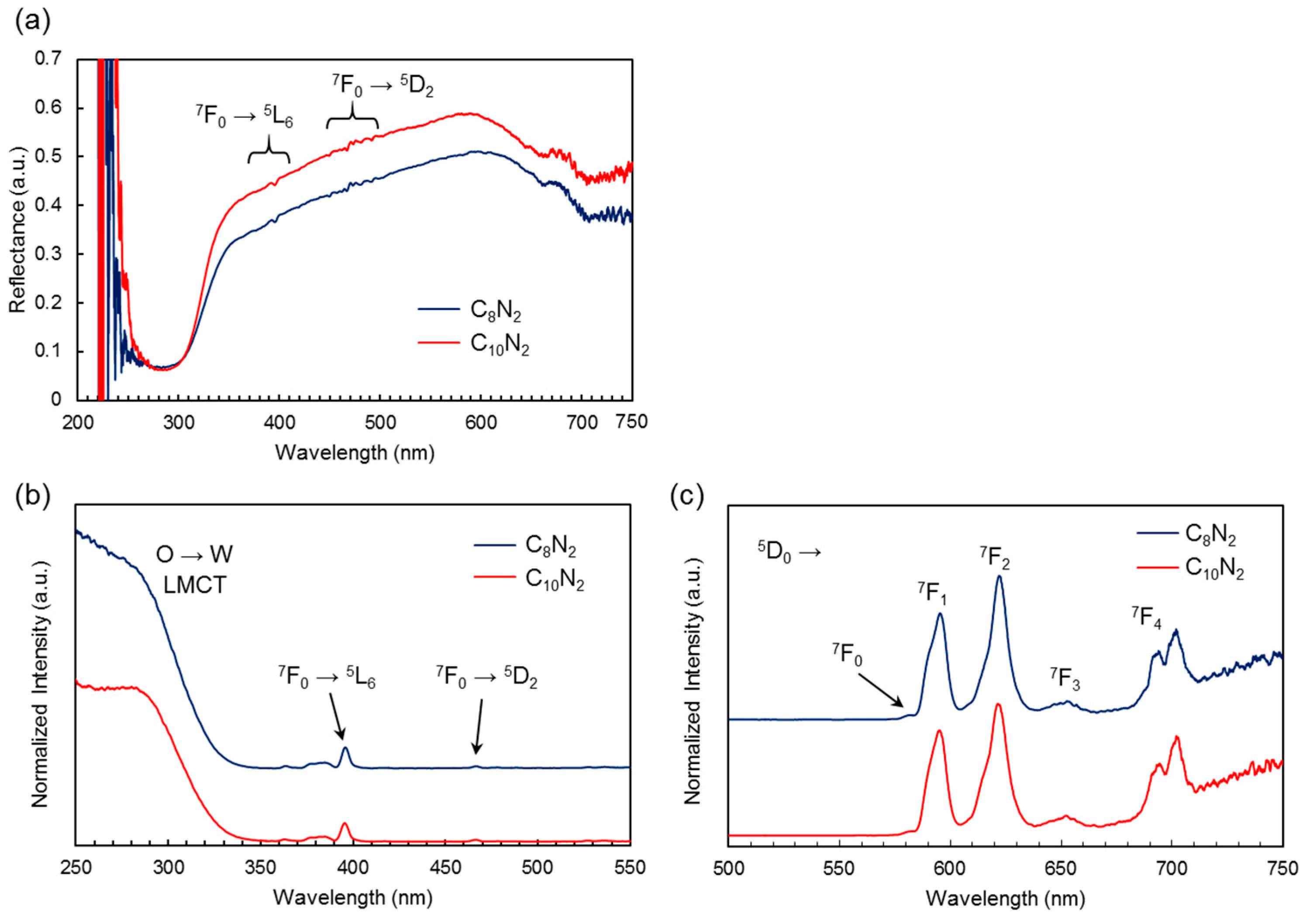
2.3. Photoluminescent Properties of EuW10-Bolaamphiphile Hybrid Crystals
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Measurements
4.3. Synthesis of C8N2-EuW10 Hybrid Crystal
4.4. Synthesis of C10N2-EuW10 Hybrid Crystal
4.5. X-ray Crystallography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adachi, G.; Imanaka, N.; Tamura, S. Ionic Conducting Lanthanide Oxides. Chem. Rev. 2002, 102, 2405–2429. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Aiba, Y.; Sumaoka, J.; Komiyama, M. Artificial DNA cutters for DNA manipulation and genome engineering. Chem. Soc. Rev. 2011, 40, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.D.; Ferreira, R.A.S.; de Zea Bermudez, V.; Julian-Lopez, B.; Escribano, P. Progress on lanthanide-based organic–inorganic hybrid phosphors. Chem. Soc. Rev. 2011, 40, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Nakanishi, T. Luminescent lanthanide coordination polymers for photonic applications. RSC Adv. 2015, 5, 338–353. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar]
- Zhang, R.; Shang, J.; Xin, J.; Xie, B.; Li, Y.; Möhwald, H. Self-assemblies of luminescent rare earth compounds in capsules and multilayers. Adv. Colloid Interface Sci. 2014, 207, 361–375. [Google Scholar] [CrossRef]
- Yamase, T. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–325. [Google Scholar] [CrossRef]
- Pope, M.T. Polyoxometalates. In Handbook on the Physics and Chemistry of Rare Earth; Gschneidner, K.A., Jr., Bunzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 38, pp. 337–382. [Google Scholar]
- Zhao, J.-W.; Li, Y.-Z.; Chen, L.-J.; Yang, G.-Y. Research progress on polyoxometalate-based transition-metal–rare-earth heterometallic derived materials: Synthetic strategies, structural overview and functional applications. Chem. Commun. 2016, 52, 4418–4445. [Google Scholar] [CrossRef]
- Boskovic, C. Rare Earth Polyoxometalates. Acc. Chem. Res. 2017, 50, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T.; Naruke, H.; Sasaki, Y. Crystallographic characterization of the polyoxotungstate [Eu3(H2O)3(SbW9O33)(W5O18)3]18− and energy transfer in its crystalline lattices. J. Chem. Soc. Dalton Trans. 1990, 5, 1687–1696. [Google Scholar] [CrossRef]
- Yamase, T.; Naruke, H. X-ray structural and photoluminescence spectroscopic investigation of the europium octamolybdate polymer Eu2(H2O)12[Mo8O27]·6H2O and intramolecular energy transfer in the crystalline lattice. J. Chem. Soc. Dalton Trans. 1991, 2, 285–292. [Google Scholar] [CrossRef]
- Tewari, S.; Adnan, M.; Balendra; Kumar, V.; Jangra, G.; Prakash, G.V.; Ramanan, A. Photoluminescence properties of two closely related isostructural series based on Anderson-Evans cluster coordinated with lanthanides [Ln(H2O)7{X(OH)6Mo6O18}]·yH2O, X = Al, Cr. Front. Chem. 2019, 6, 631. [Google Scholar] [CrossRef] [PubMed]
- Shitamatsu, K.; Kojima, T.; Waddell, P.G.; Sugiarto; Ooyama, H.E.; Errington, R.J.; Sadakane, M. Structural characterization of cerium-encapsulated Preyssler-type phosphotungstate: Additional evidence of Ce(III) in the cavity. Z. Anorg. Allg. Chem. 2021, 647, 1239–1244. [Google Scholar] [CrossRef]
- Qi, W.; Wu, L. Polyoxometalate/polymer hybrid materials: Fabrication and properties. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Lanthanopolyoxometalates: From the structure of polyanions to the design of functional materials. Polyhedron 2013, 52, 10–24. [Google Scholar] [CrossRef]
- Ritchie, C.; Baslon, V.; Moore, E.G.; Reber, C.; Boskovic, C. Sensitization of lanthanoid luminescence by organic and inorganic ligands in lanthanoid-organic-polyoxometalates. Inorg. Chem. 2012, 51, 1142–1151. [Google Scholar] [CrossRef]
- Wu, H.; Zhi, M.; Chen, H.; Singh, V.; Ma, P.; Wang, J.; Niu, J. Well-tuned white-light-emitting behaviours in multicenter-Ln polyoxometalate derivatives: A photoluminescence property and energy transfer pathway study. Spectrochim. Acta Part A 2019, 223, 117294. [Google Scholar] [CrossRef]
- Stillman, M.J.; Thomson, A.J. Emission spectra of some lanthanoid decatungstate and undecatungstosilicate ions. J. Chem. Soc. Dalton Trans. 1976, 12, 1138–1144. [Google Scholar] [CrossRef]
- Blasse, G.; Dirksen, G.J.; Zonnevijlle, F. The luminescence of some lanthanide decatungstates and other polytungstates. J. Inorg. Nucl. Chem. 1981, 43, 2847–2853. [Google Scholar] [CrossRef]
- Ballardini, R.; Mulazzani, Q.G.; Venturi, M.; Bolletta, F.; Balzani, V. Photophysical characterization of the decatungstoeuropate(9−) anion. Inorg. Chem. 1984, 23, 300–305. [Google Scholar] [CrossRef]
- Sugeta, M.; Yamase, T. Crystal structure and luminescence site of Na9[EuW10O36]·32H2O. Bull. Chem. Soc. Jpn. 1993, 66, 444–449. [Google Scholar] [CrossRef]
- Li, H.; Qi, W.; Li, W.; Sun, H.; Bu, W.; Wu, L. A highly transparent and luminescent hybrid based on the copolymerization of surfactant-encapsulated polyoxometalate and methyl methacrylate. Adv. Mater. 2005, 17, 2688–2692. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Ma, Y.; Peng, A.; Fu, H.; Yao, J. Chemically responsive luminescent switching in transparent flexible self-supporting [EuW10O36]9−-agarose nanocomposite thin films. J. Mater. Chem. 2010, 20, 271–277. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Li, Y.; Zhao, H.; Wan, X. Hybrid assemblies of Eu-containing polyoxometalates and hydrophilic block copolymers with enhanced emission in aqueous solution. Angew. Chem. Int. Ed. 2012, 51, 4598–4602. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Granadeiro, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C.; Ferreira, R.A.S.; Carlos, L.D.; Cavaleiro, A.M.V.; Trindade, T.; Nogueira, H.I.S. Luminescent transparent composite films based on lanthanopolyoxometalates and filmogenic polysaccharides. Eur. J. Inorg. Chem. 2013, 2013, 1890–1896. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Fu, L.; Liu, F.; Zhang, H. Study on highly ordered luminescent Langmuir–Blodgett films of heteropolytungstate complexes containing lanthanide. Thin Solid Films 2002, 415, 242–247. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Wang, Z.; Yin, Y.; Liu, F.; Li, H.; Fu, L.; Zhang, H. Luminescent hybrid Langmuir–Blodgett films of polyoxometaloeuropate. J. Alloys Compd. 2004, 365, 102–107. [Google Scholar] [CrossRef]
- Jiang, M.; Zhai, X.; Liu, M. Fabrication and photoluminescence of hybrid organized molecular films of a series of gemini amphiphiles and europium(III)-containing polyoxometalate. Langmuir 2005, 21, 11128–11135. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yashiro, H.; Yamase, T. Two-dimensional photoluminescence behavior of Langmuir-Blodgett monolayers and multilayers composed of decatungstoeuropate. J. Cluster Sci. 2006, 17, 375–387. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; López-Muñoz, A.; Repetto, D.; Ito, T.; Konya, T.; Yamase, T.; Constable, E.C.; Housecroft, C.E.; Doyle, K.; et al. Dual-emissive photoluminescent Langmuir-Blodgett films of decatungstoeuropate and an amphiphilic iridium complex. Langmuir 2010, 26, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.R.; Lu, R.; Zhang, H.Y.; Xue, P.C.; Feng, W.; Liu, X.L.; Zhao, B.; Zhao, Y.Y.; Li, T.J.; Yao, J.N. Highly ordered photoluminescent self-assembled films based on polyoxotungstoeuropate complex Na9[EuW10O36]. J. Mater. Chem. 2003, 13, 580–584. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Hu, C.; Shi, C. Photoluminescent organic–inorganic composite films layer-by-layer self-assembled from the rare-earth-containing polyoxometalate Na9[EuW10O36] and poly(allylamine hydrochloride). J. Mater. Chem. 2002, 12, 703–707. [Google Scholar] [CrossRef]
- Tang, P.; Hao, J. Photoluminescent honeycomb films templated by microwater droplets. Langmuir 2010, 26, 3843–3847. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Jia, G.; Jiang, Z.; Wang, S.; Lu, H.; Song, B.; Li, C. A direct imaging of amphiphilic catalysts assembled at the interface of emulsion droplets using fluorescence microscopy. Chem. Commun. 2008, 3, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gong, Y.; Gao, Y.; Xiao, J.; Wang, T.; Yu, L. Multi-stimuli responsive supramolecular structures based on azobenzene surfactant-encapsulated polyoxometalate. Langmuir 2016, 32, 9293–9300. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, S.; Tan, Y.; Sun, D.; Sun, P.; Cheng, X.; Xin, X. Self-assembly of europium-containing polyoxometalates/tetra-n-alkyl ammonium with enhanced emission for Cu2+ detection. ACS Omega 2018, 3, 14953–14961. [Google Scholar] [CrossRef]
- Liu, H.; Lv, Y.; Li, S.; Yang, F.; Liu, S.; Wang, C.; Sun, J.-Q.; Meng, H.; Gao, G.-G. A solar ultraviolet sensor based on fluorescent polyoxometalate and viologen. J. Mater. Chem. C 2017, 5, 9383–9388. [Google Scholar] [CrossRef]
- Shen, D.-F.; Li, S.; Liu, H.; Jiang, W.; Zhang, Q.; Gao, G.-G. A durable and fast-responsive photochromic and switchable luminescent polyviologen–polyoxometalate hybrid. J. Mater. Chem. C 2015, 3, 12090–12097. [Google Scholar] [CrossRef]
- Song, C.-Y.; Chai, D.-F.; Zhang, R.-R.; Liu, H.; Qiu, Y.-F.; Guo, H.-D.; Gao, G.-G. A silver-alkynyl cluster encapsulating a fluorescent polyoxometalate core: Enhanced emission and fluorescence modulation. Dalton Trans. 2015, 44, 3997–4002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-S.; Su, H.-F.; Wang, Z.; Wang, X.-P.; Chen, W.-X.; Zhao, Q.-Q.; Tung, C.-H.; Sun, D.; Zheng, L.-S. Elimination-fusion self-assembly of a nanometer-scale 72-nucleus silver cluster caging a pair of [EuW10O36]9− polyoxometalates. Chem. Eur. J. 2018, 24, 1998–2003. [Google Scholar] [CrossRef]
- Lis, S. Applications of spectroscopic methods in studies of polyoxometalates and their complexes with lanthanide(III) ions. J. Alloys Compd. 2000, 300–301, 88–94. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999; pp. 12–16. [Google Scholar]
- Svelto, O. Principles of Lasers, 5th ed.; Springer: New York, NY, USA, 2010; Chapter 7. [Google Scholar]
- Fuhrhop, J.-H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2937. [Google Scholar] [CrossRef]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1443. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, Y.; Kojima, T.; Kawahara, R.; Taira, M.; Naruke, H.; Kawano, M.; Uchida, S.; Ito, T. Porous layered inorganic-organic hybrid frameworks constructed from polyoxovanadate and bolaamphiphiles. Cryst. Growth Des. 2021, 21, 7230–7239. [Google Scholar] [CrossRef]
- Ikuma, H.; Aoki, S.; Kawahara, K.; Ono, S.; Iwamatsu, H.; Kobayashi, J.; Kiyota, Y.; Okamura, Y.; Higuchi, M.; Ito, T. An inorganic–organic hybrid framework composed of polyoxotungstate and long-chained bolaamphiphile. Int. J. Mol. Sci. 2023, 24, 2824. [Google Scholar] [CrossRef]
- Misawa, T.; Kobayashi, J.; Kiyota, Y.; Watanabe, M.; Ono, S.; Okamura, Y.; Koguchi, S.; Higuchi, M.; Nagase, Y.; Ito, T. Dimensional control in polyoxometalate crystals hybridized with amphiphilic polymerizable ionic liquids. Materials 2019, 12, 2283. [Google Scholar] [CrossRef]
- Mihara, A.; Kojima, T.; Suda, Y.; Maezawa, K.; Sumi, T.; Mizoe, N.; Watanabe, A.; Iwamatsu, H.; Oda, Y.; Okamura, Y.; et al. Photoluminescent layered crystal consisting of Anderson-type polyoxometalate and surfactant toward a potential inorganic–organic hybrid laser. Int. J. Mol. Sci. 2024, 25, 345. [Google Scholar] [CrossRef]
- Horrocks, W.D., Jr.; Sudnick, D.R. Lanthanide ion luminescence probes of the structure of biological macromolecules. Acc. Chem. Res. 1981, 14, 384–392. [Google Scholar] [CrossRef]
- Yamase, T.; Kobayashi, T.; Sugeta, M.; Naruke, H. Europium(III) luminescence and intramolecular energy transfer studies of polyoxometalloeuropates. J. Phys. Chem. A 1997, 101, 5046–5053. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic semiconductor lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.J.C.; Gather, M.C. Organic lasers: Recent developments on materials, device geometries, and fabrication techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro; Rigaku Oxford Diffraction: Tokyo, Japan, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]






| Compound | C8N2-EuW10 | C10N2-EuW10 |
|---|---|---|
| Chemical formula | C32H88N8EuW10O46 | C35H91N7EuW10O42 |
| Formula weight | 3311.53 | 3272.59 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c (No. 14) | P21/c (No. 14) |
| a (Å) | 15.5117 (6) | 15.2827 (2) |
| b (Å) | 15.3803 (5) | 16.2555 (2) |
| c (Å) | 31.3536 11) | 32.0125 (6) |
| α (°) | 90.0000 | 90.0000 |
| β (°) | 99.655 (4) | 98.8002(15) |
| γ (°) | 90.0000 | 90.0000 |
| V (Å3) | 7374.2 (5) | 7859.2 (2) |
| Z | 4 | 4 |
| ρcalcd (g cm−3) | 2.983 | 2.766 |
| T (K) | 93 (2) | 93 (2) |
| Wavelength (Å) | 0.71073 | 1.54184 |
| μ (mm−1) | 16.478 | 32.553 |
| No. of reflections measured | 100,838 | 65,068 |
| No. of independent reflections | 19,633 | 15,570 |
| Rint | 0.0911 | 0.0596 |
| No. of parameters | 852 | 535 |
| R1 (I > 2σ(I)) | 0.0533 | 0.0716 |
| wR2 (all data) | 0.1016 | 0.1980 |
| Compound | 15 K | 300 K |
|---|---|---|
| C8N2-EuW10 | 3.0 ± 0.1 | 2.5 ± 0.1 |
| C10N2-EuW10 | 0.94 ± 0.1 + 3.1 ± 0.1 1 | 1.1 ± 0.1 + 1.8 ± 0.1 1 |
| Na-EuW10 | 3.5 2 | 2.6 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, R.; Koike, R.; Suda, Y.; Kojima, T.; Sumi, T.; Misawa, T.; Kizu, K.; Okamura, Y.; Ito, T. Lanthanide-Containing Polyoxometalate Crystallized with Bolaamphiphile Surfactants as Inorganic–Organic Hybrid Phosphors. Inorganics 2024, 12, 146. https://doi.org/10.3390/inorganics12060146
Ishibashi R, Koike R, Suda Y, Kojima T, Sumi T, Misawa T, Kizu K, Okamura Y, Ito T. Lanthanide-Containing Polyoxometalate Crystallized with Bolaamphiphile Surfactants as Inorganic–Organic Hybrid Phosphors. Inorganics. 2024; 12(6):146. https://doi.org/10.3390/inorganics12060146
Chicago/Turabian StyleIshibashi, Rieko, Ruka Koike, Yoriko Suda, Tatsuhiro Kojima, Toshiyuki Sumi, Toshiyuki Misawa, Kotaro Kizu, Yosuke Okamura, and Takeru Ito. 2024. "Lanthanide-Containing Polyoxometalate Crystallized with Bolaamphiphile Surfactants as Inorganic–Organic Hybrid Phosphors" Inorganics 12, no. 6: 146. https://doi.org/10.3390/inorganics12060146
APA StyleIshibashi, R., Koike, R., Suda, Y., Kojima, T., Sumi, T., Misawa, T., Kizu, K., Okamura, Y., & Ito, T. (2024). Lanthanide-Containing Polyoxometalate Crystallized with Bolaamphiphile Surfactants as Inorganic–Organic Hybrid Phosphors. Inorganics, 12(6), 146. https://doi.org/10.3390/inorganics12060146






