Molecular Logic Gates Based on Ferrocene-Containing Compounds
Abstract
1. Introduction
2. Work on Ferrocene-Based MLGs
2.1. Long-Chain Molecules as Logic Gates (LC-MLG)
2.2. Medium-Sized Molecules as Logic Gates (MS-MLG)
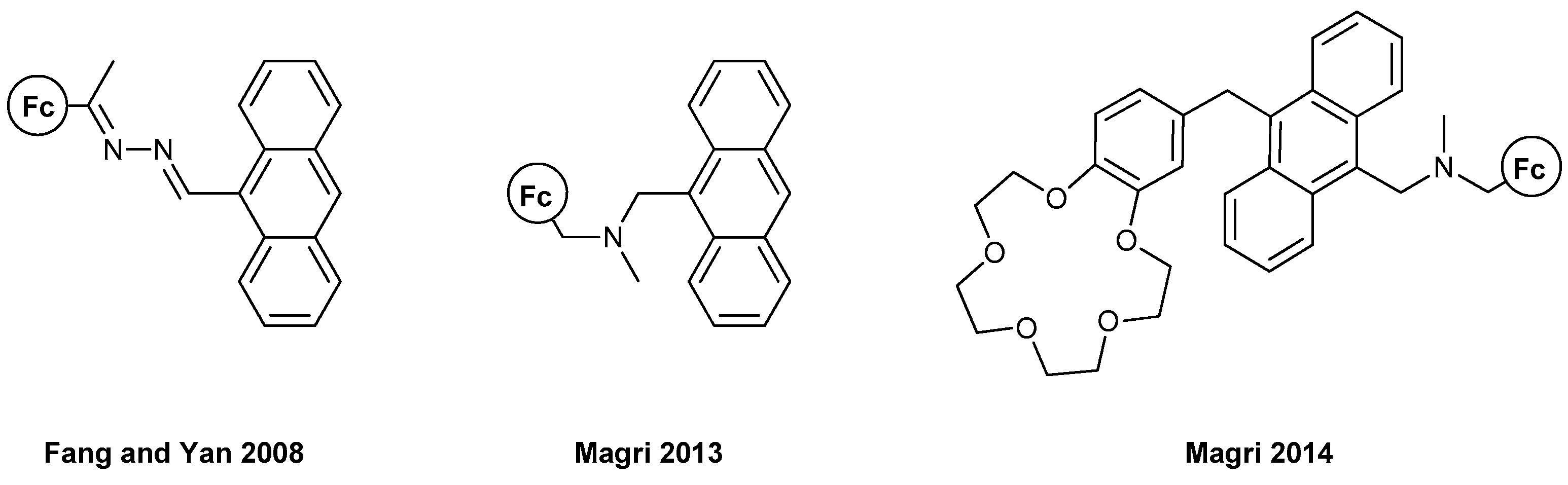
- I. Fc-based MS-MLG containing anthracene as fluorophore.
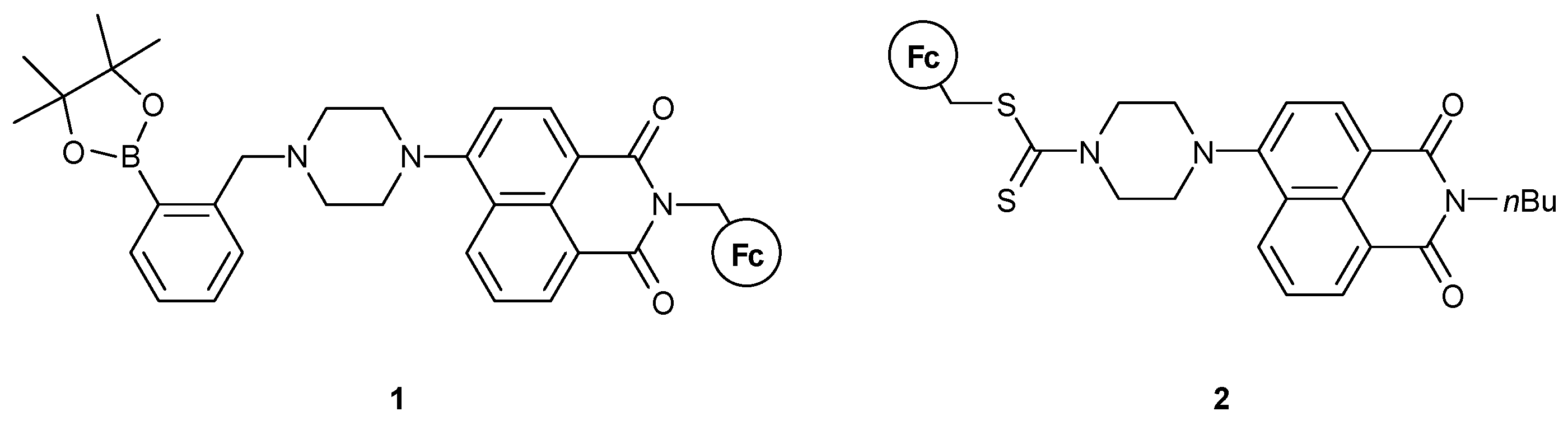
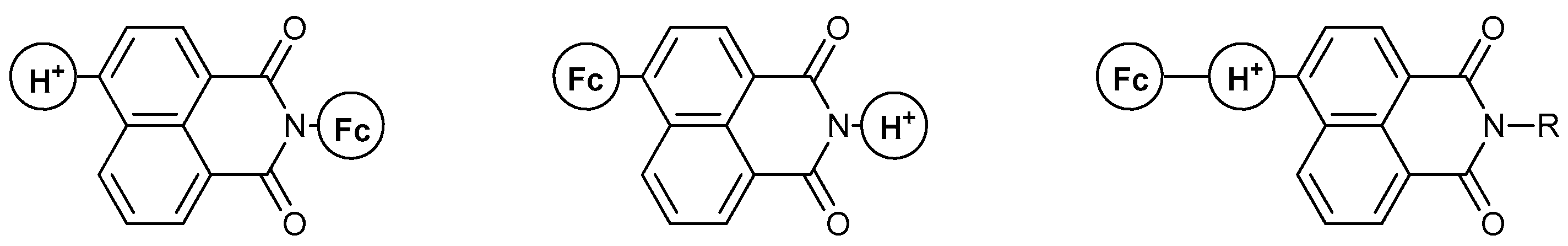
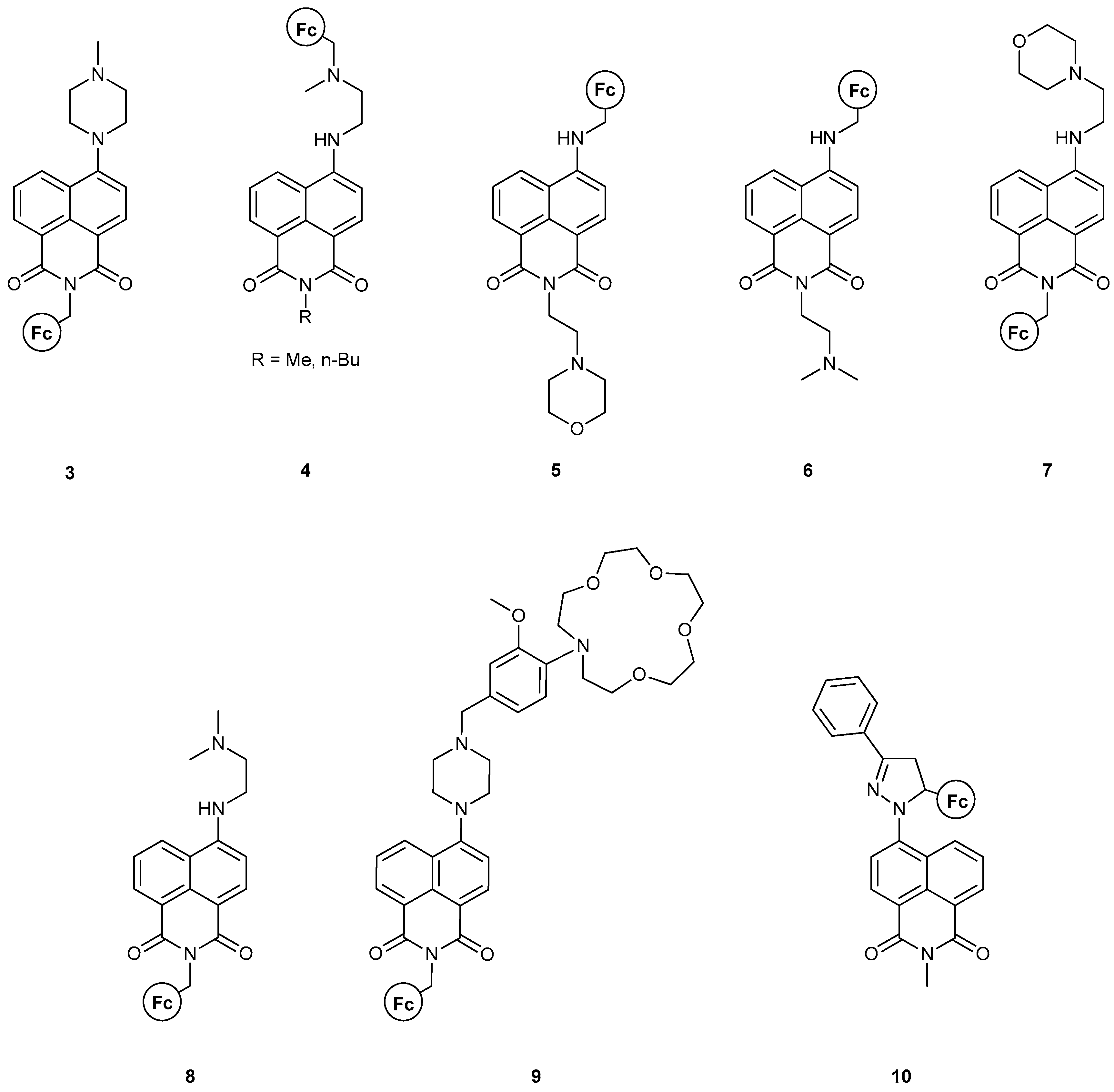
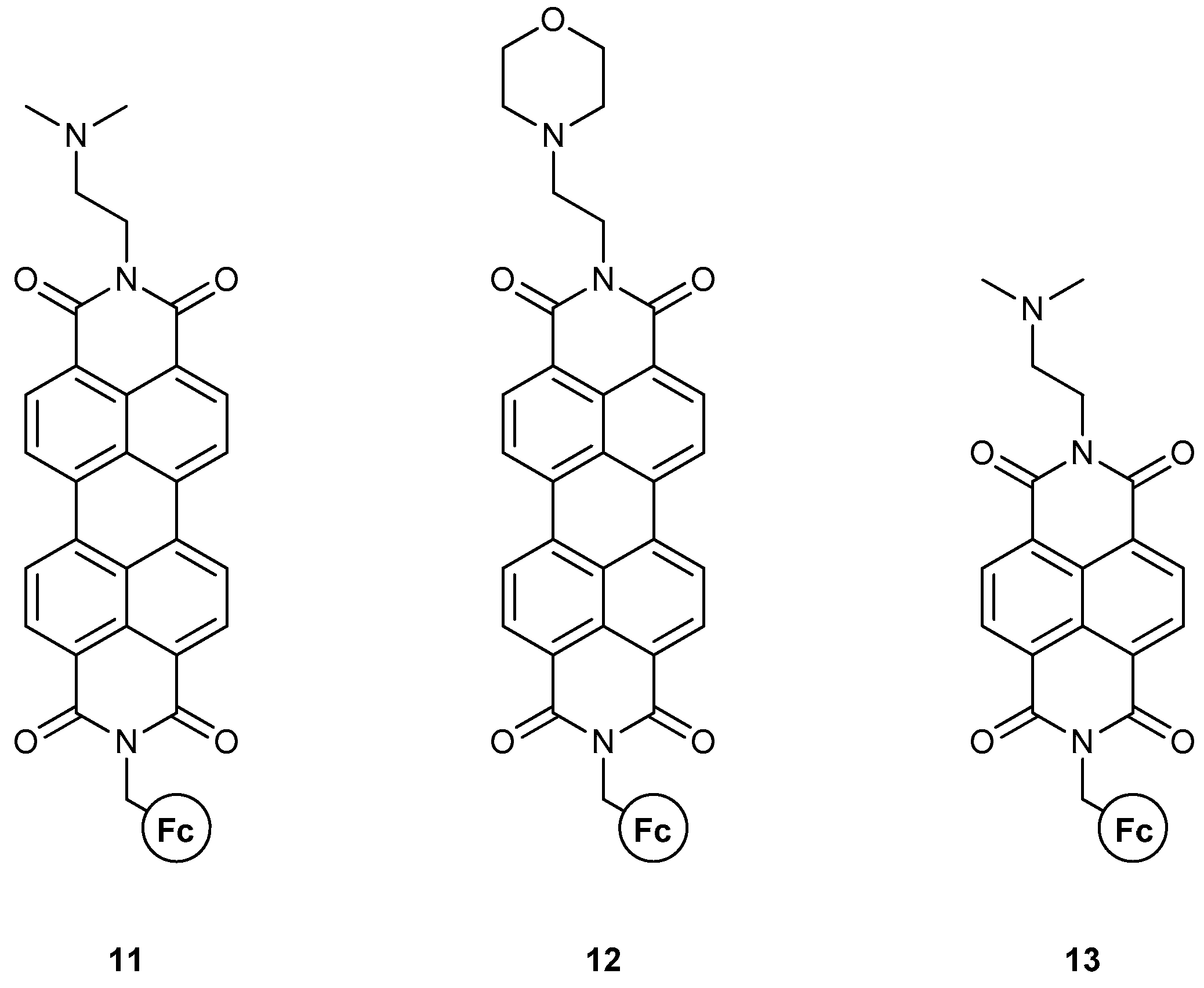
- II. Fc-based MS-MLG containing naphthalimide as fluorophore.
- III. Fc-based MS-MLG containing other fused heterocycles as fluorophore.
- IV. Fc-based MS-MLG containing other fluorophores.
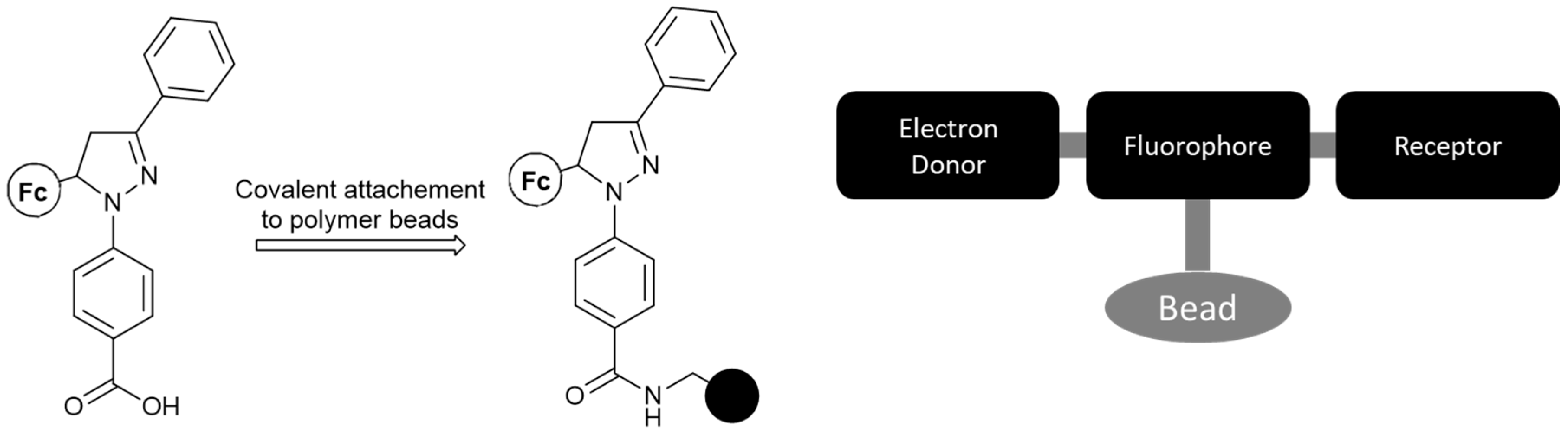
- V. Fc-based MS-MLGs not in the other categories.
2.3. Miscellaneous MLG Systems Using Ferrocene
3. MLGs including a Metal Cation without the Ferrocene Moiety
4. Computational Methodologies Used for MLG Prediction
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balzani, V.; Credi, A.; Venturi, M. Molecular Devices and Machines Concepts and Perspectives for the Nanoworld, 2nd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 259–275. [Google Scholar]
- Tzeliou, C.E.; Tzeli, D. Logic Gates and Molecular Logic Gates; Encyclopedia MDPI: Basel, Switzerland, 2024; Available online: https://encyclopedia.pub/entry/56522 (accessed on 30 March 2024).
- All about Circuits (Multiple-Input Gates). Available online: https://www.allaboutcircuits.com/textbook/digital/chpt-3/multiple-input-gates/ (accessed on 29 March 2024).
- de Silva, P.A.; Gunaratne, N.H.Q.; McCoy, C.P. A molecular photoionic AND gate based on fluorescent signaling. Nature 1993, 364, 42–44. [Google Scholar] [CrossRef]
- de Silva, A.P.; Rupasinghe, R.A.D.D. A new class of fluorescent pH indicators based on photo-induced electron transfer. J. Chem. Soc. Chem. Commun. 1985, 1669–1670. [Google Scholar] [CrossRef]
- Huston, M.E.; Akkaya, E.U.; Czarnik, A.W. Chelation Enhanced Fluorescence Detection of Non-Metal Ions. J. Am. Chem. Soc. 1989, 111, 8735–8737. [Google Scholar] [CrossRef]
- Hosseini, M.W.; Blacker, A.J.; Lehn, J.-M. Multiple molecular recognition and catalysis. A multifunctional anion receptor bearing an anion binding site, an intercalating group, and a catalytic site for nucleotide binding and hydrolysis. J. Am. Chem. Soc. 1990, 112, 3896–3904. [Google Scholar] [CrossRef]
- Van Arman, S.A.; Czarnik, A.W. Chemical communication of enzymatic ATP hydrolysis using a fluorescent chemosensor. Supramolec. Chem. 1993, 1, 99–101. [Google Scholar] [CrossRef]
- Aviram, A. Molecules for Memory, Logic, and Amplification. J. Am. Chem. Soc. 1988, 110, 5687–5692. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Kealy, T.; Pauson, P. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Wilkinson, G.; Rosenblum, M.; Whiting, M.C.; Woodward, R.B. The structure of iron bis-cyclopentadienyl. J. Am. Chem. Soc. 1952, 74, 2125–2126. [Google Scholar] [CrossRef]
- Scott, D.R.; Becker, R.S. Comprehensive Investigation of the Electronic Spectroscopy and Theoretical Treatments of Ferrocene and Nickelocene. J. Chem. Phys. 1961, 35, 516–531, Erratum in Commun. J. Chem. Phys. 1961, 35, 2246–2247. [Google Scholar] [CrossRef]
- Armstrong, A.T.; Smith, F.; Elder, E.; McGlynn, S.P. Electronic Absorption Spectrum of Ferrocene. J. Chem. Phys. 1967, 46, 4321–4328. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Hendrickson, D.N.; Hart Smith, J.; Gray, H.B. Single-crystal electronic spectrum of ferrocene at 4.2 °K. Chem. Phys. Lett. 1970, 6, 499–501. [Google Scholar] [CrossRef]
- Salzner, U. Quantitatively Correct UV-vis Spectrum of Ferrocene with TDB3LYP. J. Chem. Theory Comput. 2013, 9, 4064–4073. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, J.C.; Denisov, S.A.; Jonusauskas, G.; Klejna, S.; Szaciłowski, K.; McClenaghan, N.D.; Magri, D.C. Molecular engineering of logic gate types by module rearrangement in ‘Pourbaix Sensors’: The effect of excited-state electric fields. Org. Biomol. Chem. 2018, 16, 6195–6201. [Google Scholar] [CrossRef] [PubMed]
- Tzeliou, C.E.; Tzeli, D. 3-input AND molecular logic gate with enhanced fluorescence output: The key atom for the accurate prediction of the spectra. J. Chem. Inf. Model. 2022, 62, 6436–6448. [Google Scholar] [CrossRef] [PubMed]
- Tzeliou, C.E.; Tzeli, D. Metallocene-naphthalimide derivatives: The effect of geometry, DFT methodology, and transition metals on absorption spectra. Molecules 2023, 28, 3565. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, Y.; Sun, F.; Khatri, G.; Kwon, J.; Nickle, C.; Wang, L.; Wang, C.-K.; Thompson, D.; Li, Z.-L.; et al. Stable Universal 1- and 2-Input Single-Molecule Logic Gates. Adv. Mater. 2022, 34, 2202135. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Abe, K.; Mitsuishi, M.; Aoki, A.; Miyashita, T. Quasi-Solid-State Optical Logic Devices Based on Redox Polymer Nanosheet Assembly. Langmuir 2009, 25, 11061–11066. [Google Scholar] [CrossRef]
- Afrasiabi, R.; Kraatz, H.-B. Rational Design and Application of a Redox-Active, Photoresponsive, Discrete Metallogelator. Chem. Eur. J. 2015, 21, 7695–7700. [Google Scholar] [CrossRef] [PubMed]
- Vella Refalo, M.; Farrugia, N.V.; Johnson, A.D.; Klejna, S.; Szaciłowski, K.; Magri, D.C. Fluorimetric naphthalimide-based polymer logic beads responsive to acidity and oxidizability. J. Mater. Chem. C 2019, 7, 15225–15232. [Google Scholar] [CrossRef]
- Zerafa, N.; Cini, M.; Magri, D.C. Molecular engineering of 1,3,5-triaryl-2-pyrazoline fluorescent logic systems responsive to acidity and oxidisability and attachment to polymer beads. Mol. Syst. Des. Eng. 2021, 6, 93–99. [Google Scholar] [CrossRef]
- Liu, Y.; Offenhäusser, A.; Mayer, D. Molecular rectification in metal–bridge molecule–metal junctions. Phys. Status Solidi A Appl. Mater. Sci. 2010, 207, 891–897. [Google Scholar] [CrossRef]
- Frasconi, M.; Mazzei, F. Electrochemically Controlled Assembly and Logic Gates Operations of Gold Nanoparticle Arrays. Langmuir 2012, 28, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Abe, K.; Mitsuishi, M.; Aoki, A.; Miyashita, T. Molecular Optical Switch Based on Polymer Nanosheet Assembly Operated at Visible Wavelength. Chem. Lett. 2011, 40, 816–817. [Google Scholar] [CrossRef]
- Trasobares, J.; Martín-Romano, J.C.; Khaliq, M.W.; Ruiz-Gómez, S.; Foerster, M.; Niño, M.Á.; Pedraz, P.; Dappe, Y.J.; de Ory, M.C.; García-Pérez, J.; et al. Hybrid molecular graphene transistor as an operando and optoelectronic platform. Nat. Commun. 2023, 14, 1381. [Google Scholar] [CrossRef]
- Hernández-Ortiz, O.J.; Cerón-Castelán, J.E.; Muñoz-Pérez, F.M.; Salazar-Pereda, V.; Ortega-Mendoza, J.G.; Veloz-Rodríguez, M.A.; Lobo-Guerrero, A.; Espinosa-Roa, A.; Rodríguez-Rivera, M.A.; Vázquez-García, R.A. Synthesis, optical, electrochemical, and magnetic properties of new ferrocenyl chalcone semiconductors for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3342–3353. [Google Scholar] [CrossRef]
- Fang, C.-J.; Li, C.-Y.; Fu, X.-F.; Yue, Y.-F.; Yan, C.-H. Redox-Active Fluorescent Molecular Switch to Realize AND Logic Function. Chin. J. Inorg. Chem. 2008, 24, 1832–1836. [Google Scholar]
- Farrugia, T.J.; Magri, D.C. ‘Pourbaix sensors’: A new class of fluorescent pE–pH molecular AND logic gates based on photoinduced electron transfer. New J. Chem. 2013, 37, 148–151. [Google Scholar] [CrossRef]
- Magri, D.C.; Camilleri Fava, M.; Mallia, C.J. A sodium-enabled ‘Pourbaix sensor’: A three-input AND logic gate as a ‘lab-on-a-molecule’ for monitoring Na+, pH and pE. Chem. Commun. 2014, 50, 1009–1011. [Google Scholar] [CrossRef]
- Zhu, W.; Song, L.; Yang, Y.; Tian, H. Novel Bisthienylethene Containing Ferrocenyl-Substituted Naphthalimide: A Photo- and Redox Multi-Addressable Molecular Switch. Chem. Eur. J. 2012, 18, 13388–13394. [Google Scholar] [CrossRef]
- Li, M.; Guo, Z.; Zhu, W.; Marken, F.; James, T.D. A redox-activated fluorescence switch based on a ferrocene–fluorophore–boronic ester conjugate. Chem. Commun. 2015, 51, 1293–1296. [Google Scholar] [CrossRef]
- Dong, J.; Hu, J.; Baigude, H.; Zhang, H. A novel ferrocenyl–naphthalimide as a multichannel probe for the detection of Cu(II) and Hg(II) in aqueous media and living cells. Dalton Trans. 2018, 47, 314–322. [Google Scholar] [CrossRef]
- Spiteri, J.C.; Schembri, J.S.; Magri, D.C. A naphthalimide-based ‘Pourbaix sensor’: A redox and pH driven AND logic gate with photoinduced electron transfer and internal charge transfer mechanisms. New J. Chem. 2015, 39, 3349–3352. [Google Scholar] [CrossRef]
- Scerri, G.J.; Spiteri, J.C.; Mallia, C.J.; Magri, D.C. A lab-on-a-molecule with an enhanced fluorescent readout on detection of three chemical species. Chem. Commun. 2019, 55, 4961–4964. [Google Scholar] [CrossRef]
- Johnson, A.D.; Paterson, K.A.; Spiteri, J.C.; Denisov, S.A.; Jonusauskas, G.; Tron, A.; McClenaghan, N.D.; Magri, D.C. Water-soluble naphthalimide-based ‘Pourbaix sensors’: pH and redox-activated fluorescent AND logic gates based on photoinduced electron transfer. New J. Chem. 2016, 40, 9917–9922. [Google Scholar] [CrossRef]
- Sammut, D.; Bugeja, N.; Szaciłowski, K.; Magri, D.C. Molecular engineering of fluorescent bichromophore 1,3,5-triaryl-Δ2-pyrazoline and 4-amino-1,8-naphthalimide molecular logic gates. New J. Chem. 2022, 46, 15042–15051. [Google Scholar] [CrossRef]
- Scerri, G.J.; Spiteri, J.C.; Magri, D.C. Pourbaix sensors in polyurethane molecular logic-based coatings for early detection of corrosion. Mater. Adv. 2021, 2, 434–439. [Google Scholar] [CrossRef]
- Grech, J.; Spiteri, J.C.; Scerri, G.J.; Magri, D.C. Molecular logic with ferrocene-rylene conjugates: A comparison of naphthalenediimide, naphthalimide and perylenediimide Pourbaix sensor designs. Inorganica Chim. Acta 2023, 544, 121176. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Bheemireddy, V.; Thakur, A. A Redox-Driven Fluorescence “Off–On” Molecular Switch Based on a 1,1′-Unsymmetrically Substituted Ferrocenyl Coumarin System: Mimicking Combinational Logic Operation. Organometallics 2017, 36, 829–838. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Mondal, B.; Vijaykumar, G.; Thakur, A. ICT–Isomerization-Induced Turn-On Fluorescence Probe with a Large Emission Shift for Mercury Ion: Application in Combinational Molecular Logic. Inorg. Chem. 2017, 56, 11577–11590. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Bhatta, S.R.; Giri, S.; Thakur, A. Oxidation-Induced Differentially Selective Turn-On Fluorescence via Photoinduced Electron Transfer Based on a Ferrocene-Appended Coumarin–Quinoline Platform: Application in Cascaded Molecular Logic. Inorg. Chem. 2020, 59, 4493–4507. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Han, X.; Zhang, L.-P.; Tung, C.-H.; Wu, L.-Z. Molecular logic circuit based on a multi-state mononuclear platinum(ii) terpyridyl complex. Phys. Chem. Chem. Phys. 2010, 12, 13026–13033. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, W.; Cui, X.; Zhao, J.; Wu, M. Preparation of Bodipy–ferrocene dyads and modulation of the singlet/triplet excited state of bodipy via electron transfer and triplet energy transfer. J. Mater. Chem. C 2016, 4, 2843–2853. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Kang, L.; Zhu, W. A luminescence molecular switch via modulation of PET and ICT processes in DCM system. Sci. China Chem. 2017, 60, 607–613. [Google Scholar] [CrossRef]
- Scerri, G.J.; Cini, M.; Schembri, J.S.; da Costa, P.F.; Johnson, A.D.; Magri, D.C. Redox-Enabled, pH-Disabled Pyrazoline–Ferrocene INHIBIT Logic Gates. Chem. Phys. Chem. 2017, 18, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, S.; Barik, T.; Halder, B.; Mishra, A.; Dhiman, R.; Sasamori, T.; Chatterjee, S. Rhodamine tethered 1,1′-unsymmetrical ferrocene functionalization: Metal sensing, cell imaging and logic gate properties. J. Organomet. Chem. 2021, 948, 121922. [Google Scholar] [CrossRef]
- Tamulis, A.; Tamuliene, J.; Tamulis, V.; Ziriakoviene, A. Quantum Mechanical Design of Molecular Computers Elements Suitable for Self-Assembling to Quantum Computing Living Systems. Solid State Phenom. 2004, 97–98, 173–180. [Google Scholar] [CrossRef]
- Tokunaga, K.; Odate, F.; Asami, D.; Tahara, K.; Sato, M. A Theoretical Procedure Based on Classical Electrostatics and Density Functional Theory for Screening Non-Square-Shaped Mixed-Valence Complexes for Logic Gates in Molecular Quantum-Dot Cellular Automata. Bull. Chem. Soc. Jpn. 2021, 94, 397–403. [Google Scholar] [CrossRef]
- Alzahrani, A.; Omama Khan, K.; Rafique, S.; Hasher, I.; Khadija; Khan, A.M.; Shahzad, S.A. Theoretical and experimental studies on mechanochromic triphenylamine based fluorescent “ON-OFF-ON” sensor for sequential detection of Fe3+ and deferasirox. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 297, 122745. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, M.; Lu, Y.; Yao, H.; Liu, H. An enzymatic calculation system based on electrochemiluminescence and fluorescence of luminol and cyclic voltammetry of ferrocene methanol. Biosens. Bioelectron. 2018, 118, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hou, Y.-J.; Lu, Y.-L.; Fan, Y.-Z.; Fan, Y.-N.; Yu, H.-J.; Li, K.; Pan, M.; Su, C.-Y. Redox-Guest-Induced Multimode Photoluminescence Switch for Sequential Logic Gates in a Photoactive Coordination Cage. Chem. Eur. J. 2019, 25, 11903. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Shan, X.; Li, M.; Liu, Y.; Yang, F.; Wang, F.; Tian, Z.; Shi, K.; Yao, H. Multiple stimuli-switchable electrocatalysis and logic gates of l-cysteine based on P(DEA–co-VPBA) hydrogel films. Mol. Catal. 2023, 546, 113273. [Google Scholar] [CrossRef]
- Liang, J.; Yu, X.; Yang, T.; Li, M.; Shen, L.; Jin, Y.; Liu, H. A complicated biocomputing system based on multi-responsive P(NIPAM-co-APBA) copolymer film electrodes and electrocatalysis of NADH. Phys. Chem. Chem. Phys. 2017, 19, 22472–22481. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, J.; Yao, H.; Shi, K.; Liu, H. A versatile molecular logic system based on Eu(iii) coordination polymer film electrodes combined with multiple properties of NADH. Phys. Chem. Chem. Phys. 2020, 22, 22746–22757. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Yu, X.; Wang, L.; Liu, H. Biomacromolecular Logic Devices Based on Simultaneous Electrocatalytic and Electrochemiluminescence Responses of Ru(bpy)32+ at Molecularly Imprinted Polymer Film Electrodes. J. Phys. Chem. C 2015, 119, 20003–20010. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, L.; Yang, X. Electrochemical-Based DNA Logic Devices Regulated by the Diffusion and Intercalation of Electroactive Dyes. ACS Appl. Mater. Interfaces 2021, 13, 42250–42257. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, L.; Ding, T. Multiplexed sensing of mercury(II) and silver(I) ions: A new class of DNA electrochemiluminescent-molecular logic gates. Biosens. Bioelectron. 2011, 26, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.C. Recent Progress on the Evolution of Pourbaix Sensors: Molecular Logic Gates for Protons and Oxidants. Chemosensors 2018, 6, 48. [Google Scholar] [CrossRef]
- Magri, D.C. Logical sensing with fluorescent molecular logic gates based on photoinduced electron transfer. Coord. Chem. Rev. 2021, 426, 213598. [Google Scholar] [CrossRef]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, S.; Gulseren, G.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective Manipulation of ICT and PET Processes inStyryl-Bodipy Derivatives: Applications in Molecular Logicand Fluorescence Sensing of Metal Ions. J. Am. Chem. Soc. 2010, 132, 8029. [Google Scholar] [CrossRef] [PubMed]
- Tzeli, D.; Petsalakis, I.D.; Theodorakopoulos, G. Theoretical study of the photophysical processes of a styryl-bodipy derivative eliciting an AND molecular logic gate response. Int. J. Quantum Chem. 2019, 119, e25958. [Google Scholar] [CrossRef]
- Tzeli, D.; Petsalakis, I.D.; Theodorakopoulos, G. The solvent effect on a styryl-bodipy derivative functioning as an AND molecular logic gate Int. J. Quantum Chem. 2020, 120, e26181. [Google Scholar] [CrossRef]
- Velmurugan, K.; Vickram, R.; Jipsa, C.V.; Karthick, R.; Prabakaran, G.; Suresh, S.; Prabhu, J.; Velraj, G.; Tang, L.; Nandhakumar, R. Quinoline based reversible fluorescent probe for Pb2+; applications in milk, bioimaging and INHIBIT molecular logic gate. Food Chem. 2021, 348, 129098. [Google Scholar] [CrossRef] [PubMed]
- Bahta, M.; Ahmed, N. A novel 1,8-naphthalimide as highly selective naked-eye and ratiometric fluorescent sensor for detection of Hg2+ ions. J. Photochem. Photobiol. A Chem. 2019, 373, 154–161. [Google Scholar] [CrossRef]
- Fu, Q.-Q.; Hu, J.-H.; Yao, Y.; Yin, Z.-Y.; Gui, K.; Xu, N.; Niu, L.-Y.; Zhang, Y.-Q. A benzimidazole derivative based LMCT sensor for the detection of Cu2+ in DMSO/H2O (2:3 v/v) solution and its application in implication logic gates. J. Photochem. Photobiol. A Chem. 2020, 391, 112358. [Google Scholar] [CrossRef]
- Mukherjee, S.; Betal, S. Sensing phenomena, extraction and recovery of Cu2+ followed by smart phone application using a luminescent pyrene based chemosensor. J. Lumin. 2018, 204, 145–153. [Google Scholar] [CrossRef]
- Ray, A.; Bar, N.; Chowdhury, P.; Biswas, D.; Ghosh, K.; Mandi, A.; Das, G.K. Revaluation of copper(II)-Schiff base complex for sensing of D-penicillamine and development of a molecular logic gate: A combined approach. Polyhedron 2023, 243, 116563. [Google Scholar] [CrossRef]
- Ryu, H.; Park, J.; Kim, H.K.; Park, J.Y.; Kim, S.-T.; Baik, M.-H. Pitfalls in Computational Modeling of Chemical Reactions and how to Avoid Them. Organometallics 2018, 37, 3228–3239. [Google Scholar] [CrossRef]
- Andrada, D.M.; Holzmann, N.; Hamadi, T.; Frenking, G. Direct estimate of the internal π-donation to the carbene centre within N-heterocyclic carbenes and related molecules. Beilstein J. Org. Chem. 2015, 11, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Munz, D. Pushing Electrons—Which Carbene Ligand for Which Application? Organometallics 2018, 37, 275–289. [Google Scholar] [CrossRef]
- Maser, L.; Herritsch, J.; Langer, R. Carbodiphosphorane-based nickel pincer complexes and their (de)protonated analogues: Dimerisation, ligand tautomers and proton affinities. Dalton Trans. 2018, 47, 10544–10552. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Sundermeyer, J. Modular Design Strategy toward Second-Generation Tridentate Carbodiphosphorane N,C,N Ligands with a Central Four-Electron Carbon Donor Motif and Their Complexes. Organometallics 2021, 40, 2090–2099. [Google Scholar] [CrossRef]
- Marcum, J.S.; Cervarich, T.N.; Manan, R.S.; Roberts, C.C.; Meek, S.J. (CDC)–Rhodium-Catalyzed Hydroallylation of Vinylarenes and 1,3-Dienes with AllylTrifluoroborates. ACS Catal. 2019, 9, 5881–5889. [Google Scholar] [CrossRef]
- Tzeliou, C.E.; Mermigki, M.A.; Tzeli, D. Review on the QM/MM Methodologies and Their Application to Metalloproteins. Molecules 2022, 27, 2660. [Google Scholar] [CrossRef]
- Ferentinos, E.; Tzeli, D.; Sottini, S.; Groenen, E.J.J.; Ozerov, M.; Poneti, G.; Kaniewska-Laskowska, K.; Krzystek, J.; Kyritsis, P. Magnetic anisotropy and structural flexibility in the field-induced single ion magnets [Co{(OPPh2)(EPPh2)N}2], E = S, Se, explored by experimental and computational methods. Dalton Trans. 2023, 52, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y.; Ishizuka, T.; Kotani, H.; Shiota, Y.; Yoshizawa, K.; Mieda, K.; Ogura, T.; Okajima, T.; Nozawa, S.; Kojima, T. A Ruthenium(III)–Oxyl Complex Bearing Strong Radical Character. Angew. Chem. Int. Ed. 2016, 55, 14041–14045. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-W.; Zong, R.; Muckerman, J.T.; Thummel, R. Mononuclear ruthenium(II) complexes that catalyze water oxidation. Inorg. Chem. 2008, 47, 11763–11773. [Google Scholar] [CrossRef] [PubMed]
- Pushkar, Y.; Moonshiram, D.; Purohit, V.; Yan, L.; Alperovich, I. Spectroscopic Analysis of Catalytic Water Oxidation by [RuII(bpy)(tpy)H2O]2+ Suggests That RuV═O Is Not a Rate-Limiting Intermediate. J. Am. Chem. Soc. 2014, 136, 11938–11945. [Google Scholar] [CrossRef] [PubMed]
- Drosou, M.; Kamatsos, F.; Ioannidis, G.; Zarkadoulas, A.; Mitsopoulou, C.; Papatriantafyllopoulou, C.; Tzeli, D. Reactivity and mechanism of photo- and electrocatalytic hydrogen evolution by a diimine copper(I) complex. Catalysts 2020, 10, 1302. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Steinmetz, M.; Grimme, S. Benchmark study of the performance of density functional theory for bond activations with (ni,pd)-based transition-metal catalysts. ChemistryOpen 2013, 2, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-Row Atoms. J. Comp. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations—Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Peterson, K.A.; Figgen, D.; Dolg, M.; Stoll, H. Energy-consistent relativistic pseudopotentials and correlation consistent basis sets for the 4d elements Y-Pd. J. Chem. Phys. 2007, 126, 124101. [Google Scholar] [CrossRef] [PubMed]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Caricato, M.; Mennucci, B.; Tomasi, J.; Ingrosso, F.; Cammi, R.; Corni, S.; Scalmani, G. Formation and relaxation of excited states in solution: A new time dependent polarizable continuum model based on time dependent density functional theory. J. Chem. Phys. 2006, 124, 124520. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, P.-Å.; Roos, B.O. The CASSCF state interaction method. Chem. Phys. Lett. 1989, 155, 189–194. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.-P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Bickelhaupt, F.; Hommes, N.; Guerra, C.; Baerends, E. The Carbon–Lithium Electron Pair Bond in (CH3Li)n (n = 1, 2, 4). Organometallics 1996, 15, 2923–2931. [Google Scholar] [CrossRef]
- Ishikawa, S.; Madjarova, G.; Yamabe, T.J. First-Principles Study of the Lithium Interaction with Polycyclic Aromatic Hydrocarbons. Phys. Chem. B 2001, 105, 11986–11993. [Google Scholar] [CrossRef]
- Maseras, F.; Morokuma, K. Application of the natural population analysis to transition-metal complexes. Should the empty metal p orbitals be included in the valence space? Chem. Phys. Lett. 1992, 195, 500–504. [Google Scholar] [CrossRef]
- Guerra, C.F.; Handgraaf, J.-W.; Baerends, E.J.; Bickelhaupt, F.M. Voronoi deformation density (VDD) charges: Assessment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD methods for charge analysis. J. Comput. Chem. 2004, 25, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Jerome, S.V.; Cramer, C.J.; Truhlar, D.G. Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Willner, B.; Willner, I. Catalytic nucleic acids (DNAzymes) as functional units for logic gates and computing circuits: From basic principles to practical applications. Chem. Commun. 2015, 51, 4144–4160. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Daly, B.; Silverson, V.A.; De Silva, A.P. Taking baby steps in molecular logic-based computation. Chem. Commun. 2015, 40, 8403–8409. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Minko, S. Enzyme-based logic systems interfaced with signal-responsive materials and electrodes. Chem. Commun. 2015, 51, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, J.; Pischel, U. Molecules for security measures: From keypad locks to advanced communication protocols. Chem. Soc. Rev. 2018, 47, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Tzeli, D.; Petsalakis, I.D. Physical insights into molecular sensors, molecular logic gates, and photosensitizers in photodynamic therapy. J. Chem. 2019, 2019, 6793490. [Google Scholar] [CrossRef]
- Yao, C.Y.; Lin, H.Y.; Crory, H.S.; de Silva, A.P. Supra-molecular agents running tasks intelligently (SMARTI): Recent developments in molecular logic-based computation. Mol. Sys. Des. Eng. 2020, 5, 1325–1353. [Google Scholar] [CrossRef]
- Tzeli, D. Molecular logic gates. J. Chem. Tech. App. 2023, 6, 136. [Google Scholar]
- Liu, L.; Liu, P.; Ga, L.; Ai, J. Advances in applications of molecular logic gates. ACS Omega 2021, 6, 30189–30204. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J. (Ed.) Supramolecular Systems in Biomedical Fields; Royal Society Chemistry: London, UK, 2013. [Google Scholar]
- Ozlem, S.; Akkaya, E.U. Thinking outside the silicon box: Molecular and logic as an additional layer of selectivity in singlet oxygen generation for photodynamic therapy. J. Am. Chem. Soc. 2009, 131, 48–49. [Google Scholar] [CrossRef] [PubMed]
- de Silva, A.P.; James, M.R.; McKinney, B.O.F.; Pears, D.A.; Weir, S.M. Molecular computational elements encode large populations of small objects. Nat. Mater. 2006, 5, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Torriero, A.A.J.; Mruthunjaya, A.K.V. Ferrocene-Based Electrochemical Sensors for Cations. Inorganics 2023, 11, 472. [Google Scholar] [CrossRef]
- Fabbrizzi, L. The ferrocenium/ferrocene couple: A versatile redox switch. ChemTexts 2020, 6, 22. [Google Scholar] [CrossRef]


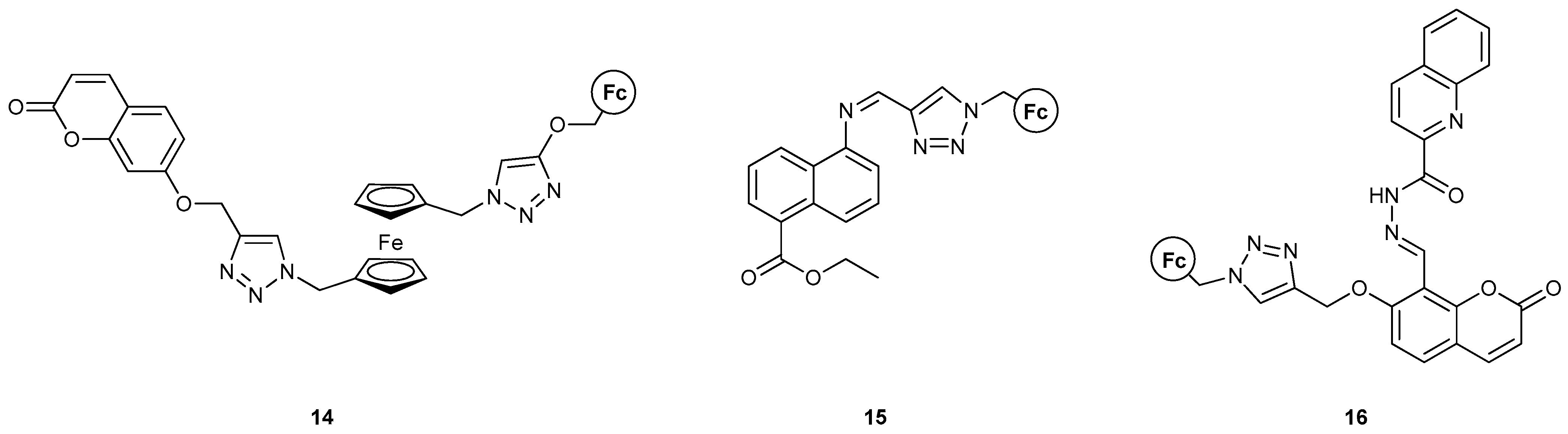
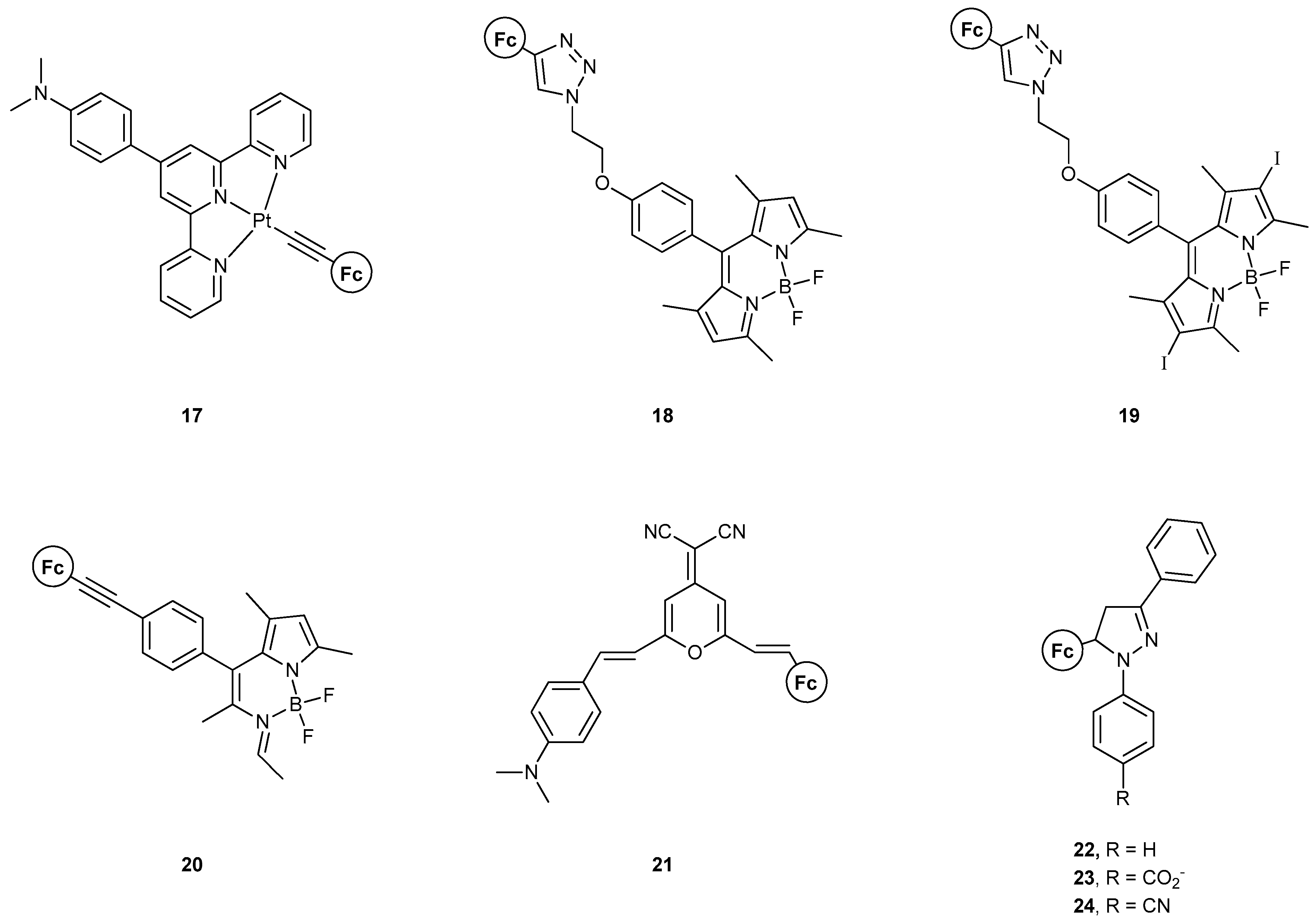
| Logic Operation | MS–MLG [Ref.] a | LC–MLG [Ref.] a | |
|---|---|---|---|
| 1-input | |||
| YES | [52] (expt + th) | [23] (expt) | |
| NOT | [19] (th) | [20] (expt + th); [22] (expt) | |
| PASS | 5 [17] (expt + th); 6 [17] (expt + th); 13 [41] (expt) | [20] (expt + th); [23] (expt + th) | |
| 2-input | |||
| AND | 3 [36] (expt); 4 [38] (expt); 7 [17] (expt + th); 8 [17] (expt + th); 9 [37] (expt); [18] (th); 11 [40] (expt); 12 [40] (expt); [50] (th); [51] (th); [30] (expt); [31] (expt); [32] (expt) | [22] (expt + th); [23] (expt + th); [26] (expt); [28] (expt); [60] (expt) | |
| NAND | [19] (th) | [20] (expt + th); [60] (expt) | |
| OR | 15 [43] (expt); 16 [44] (expt + th); [50] (th); [51] (th) | [20] (expt + th); [21] (expt); [28] (expt); [60] (expt) | |
| NOR | [20] (expt + th); [60] (expt) | ||
| XOR | [20] (expt + th); [21] (expt); [22] (expt) | ||
| XNOR | [20] (expt + th) | ||
| INHIBIT | 1 [34] (expt); 10 [39] (expt + th); 14 [42] (expt + th); 15 [43] (expt + th); 16 [44] (expt + th); 22–24 [48] (expt) | [24] (expt); [49] (expt + th); [60] (expt) | |
| Combinational | |||
| NOR-AND | 1 [34]; 14 [42] (expt + th) | ||
| INHIBIT-OR | 15 [43] (expt + th); [49] (expt + th) | [49] (expt + th) | |
| AND-OR | 16 [44] (expt + th); [50] (expt + th); [51] (th) | ||
| AND-OR-NOT | 17 [45] (expt) | ||
| AND-INHIBIT-YES | [58] (expt) | ||
| AND-INHIBIT-YES-IMPLICATION | [58] (expt) | ||
| ON/OFF | |||
| [33] (expt); [46] (expt); [47] (expt) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeliou, C.E.; Zois, K.P.; Tzeli, D. Molecular Logic Gates Based on Ferrocene-Containing Compounds. Inorganics 2024, 12, 106. https://doi.org/10.3390/inorganics12040106
Tzeliou CE, Zois KP, Tzeli D. Molecular Logic Gates Based on Ferrocene-Containing Compounds. Inorganics. 2024; 12(4):106. https://doi.org/10.3390/inorganics12040106
Chicago/Turabian StyleTzeliou, Christina Eleftheria, Konstantinos P. Zois, and Demeter Tzeli. 2024. "Molecular Logic Gates Based on Ferrocene-Containing Compounds" Inorganics 12, no. 4: 106. https://doi.org/10.3390/inorganics12040106
APA StyleTzeliou, C. E., Zois, K. P., & Tzeli, D. (2024). Molecular Logic Gates Based on Ferrocene-Containing Compounds. Inorganics, 12(4), 106. https://doi.org/10.3390/inorganics12040106