Abstract
The aromatic character of silolyl and germolyl anions markedly depends on the substituents in the 2,5-positions; carbon-substituted derivatives are nonaromatic, whereas silyl-substituted ones tend to exhibit an aromatic character. However, only carbon-substituted derivatives have been reported for stannolyl anions. In this study, we present the synthesis and structure of a 2,5-disilylated stannolyl anion. Transmetalation of a 2,5-disilyl-1-zirconacyclopentadiene with SnCl4 gave a dichlorostannole 1, which reacted with potassium tris(trimethylsilyl)silanide to introduce a bulky silyl group on the tin atom. Reduction of the 1-chloro-1-silylstannole 2 with lithium generated the lithium salt of the desired stannolyl anion 3 that adopts an η1-coordination to the lithium atom. We concluded that the stannolyl anion 3 is nonaromatic based on the pyramidalized tin center and the C–C bond alternation in the five-membered ring as well as the NMR properties.
1. Introduction
Heavier congeners of cyclopentadienyl anions, known as metallolyl anions and dianions with general formulas of [C4R5E]− and [C4R4E]2− (E = Si, Ge, Sn, Pb), respectively, have attracted continuous attention as novel ligands in coordination chemistry and unconventional aromatic systems consisting of heavier group 14 elements [1,2,3,4]. Following the seminal works by Tilley on the coordination chemistry of silolyl/germolyl anions and dianions [5,6,7,8,9,10], several groups have contributed to the development of this field. Various transition metal complexes incorporating metallolyl ligands have been reported, exhibiting diverse coordination modes such as μ-η5:η5- [11,12], η5- (I in Figure 1) [13], μ-η1:η1- (II) [14,15], μ-η1:η5- [16], η1- [17], and η4-like fashions [13,18]. Recently, the coordination chemistry of metallolyl dianions towards rare-earth elements and f-block metals has also been investigated [19,20,21,22]. These studies have shown that η5-coordinating metallolyl anions and dianions are aromatic with almost equal C–C bonds in the five-membered ring, whereas η1- and η4-coordinating ones are nonaromatic with a 1,3-diene character [14]. Therefore, coordinating modes have a significant impact on the electronic structure of metallolyl anions and dianions.
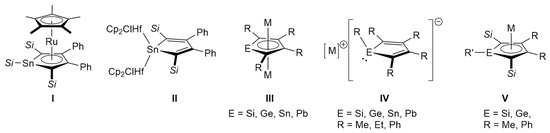
Figure 1.
Selected metallolyl complexes (I,II), alkali metal salts of metallolyl dianions (III), nonaromatic metallolyl anions (IV), and aromatic metallolyl anions (V). The substituents Si and M indicate trialkylsilyl groups and alkali metals, respectively.
The aromaticity of alkali metal salts of metallolyl anions and dianions is also an important topic in this field. The family of group 14 metallole dianions (silole [23,24,25,26], germole [26,27,28], stannole [29,30,31], and plumbole [32,33]) has been concluded to be aromatic (III). In contrast, the situation is more complicated in metallolyl monoanions. In the 1990s, silolyl and germolyl anions with the formula of [C4Me4ER]− (E = Si, Ge; R = Me, Mes (2,4,6-trimethylphenyl), SiMe3) have been structurally characterized, which revealed the pyramidal metal center and bond-altered five-membered ring (IV) [34]. Because this trend is also found in the structures of stannolyl and plumbolyl anions [C4Ph4ER]− (E = Sn, Pb; R = Ph, Mes, SiMe3) [32,35], the family of metallolyl anions was concluded to be nonaromatic. However, more recently, Kovács, Nyulászi, et al. demonstrated theoretically and experimentally that the lithium salt of silolyl anion, which has silyl substituents on the alpha-carbon atoms (Cα: 2,5-positions in the silole ring), is aromatic with an η5-coordination and a planar three-coordinated silicon center (V) [36,37]. Furthermore, Müller’s group reported a potassium salt of a 2,5-disilylgermolyl anion that exhibits an aromatic/nonaromatic switch depending on the coordinating solvent to the potassium ion; the THF-solvated salt has an aromatic character with an η5-coordination, while the corresponding 18-crown-6 ether adduct is nonaromatic with an η1-coordination as well as a pyramidal germanium center [38].
This effect of the silyl substituents, which imparts aromatic character to silolyl and germolyl anions, originates from the effective hyperconjugation (σ*–π interaction) between the trialkylsilyl groups and the anionic π-electron system [38], raising the possibility of realizing aromatic stannolyl anions. To date, only the tetraphenyl- and tetraethyl stannolyl anions [C4Ph4SnR]− and [C4Et4SnR]− have been structurally characterized. These two derivatives are nonaromatic with a highly pyramidal tin center, like IV [30]. In this report, we present the synthesis and structural analysis of a lithium salt of 2,5-disilylated stannolyl anion. X-ray diffraction studies and NMR spectroscopic analysis indicate that the stannolyl anion is nonaromatic rather than aromatic, despite the assistance of the silyl groups.
2. Results and Discussion
Scheme 1 shows the synthetic route to the target stannolyl anion. Although synthetic examples of 1,1-dichlorostannole are limited [39,40], dichlorostannole 1 is expected to be a suitable precursor. Initial attempts to synthesize 1 from the reaction of the corresponding 1,4-dilithiobutadiene, which was prepared by the reduction of 1-(trimethylsilyl)-1-propyne with lithium, and SnCl4 were unsuccessful. Accordingly, CuCl-promoted transmetalation of an in situ generated zirconacycle [41] and SnCl4 was investigated. Although the tin source for this transformation described in the literature has been limited to R2SnX2, where R = alkyl or aryl and X = halogen [42], dichlorostannole 1 was successfully obtained by this method in a 58% yield. Treatment of 1 with a potassium salt of tris(trimethylsilyl)silanide provided chlorostannole 2 with a bulky silyl group on the tin atom. Reduction of 2 with lithium in Et2O, followed by recrystallization from hexane–THF, provided a lithium salt of stannolyl anion 3·(thf)3 as highly air- and moisture sensitive orange crystals. On drying the crystals in vacuo, 3·(thf)3 released two of the three THF molecules to provide 3·(thf) in a 26% isolated yield. These new compounds were characterized using 1H and multinuclear NMR spectroscopy, elemental analysis (excluding 3·(thf)), and X-ray diffraction analysis (excluding 1). Considering that the mono-THF solvated germolyl anion reported by Müller et al. has an aromatic character with a planar germanium atom [38], it is inferred that 3·(thf) may have a potential aromatic character. Unfortunately, our attempts to obtain single crystals of 3·(thf) from its toluene and benzene solution were unsuccessful. However, NMR data of 3·(thf) recorded in C6D6 suggests that 3·(thf) is nonaromatic at least in solution (vide infra).
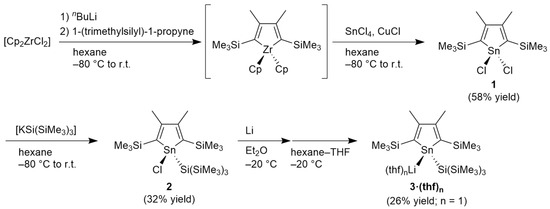
Scheme 1.
Synthetic procedure for lithium salt of stannolyl anion 3.
Figure 2 illustrates the solid-state structures of 2 and 3·(thf)3 determined by X-ray diffraction analysis, and Table 1 highlights the structural differences between 2, 3, and a previously reported stannolyl anion isolated as a solvent-separated ion pair [Li(12-c-4)][C4Ph4Sn(SiMe3)] 4, where 12-c-4 indicates 12-crown-4 ether [35].
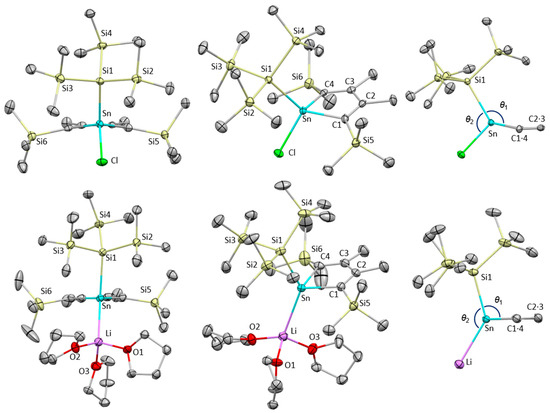
Figure 2.
Molecular structures of one of the three independent molecules of 2 (top) and 3·(thf)3 (bottom) with thermal ellipsoid plots at 50% probability. All hydrogen atoms are omitted for clarity. θ1 and θ2 are the angles of Si1−Sn−Ccenter and Si1−Sn−Cl(Li), respectively (Ccenter is the midpoint of C1 and C4 atoms).

Table 1.
Selected bond lengths [Å] and angles [°].
The asymmetric unit of 2 contains three independent molecules, each with slightly different metrical parameters. The tin and Si5/6 atoms deviate by 0.3 and 0.26–0.41 Å from the least-squares plane defined by C1–C4 atoms due to the steric repulsion between the bulky tris(trimethyl)silyl and trimethylsilyl groups. The average Sn−C1 and Sn−C4 bond lengths are 2.163 and 2.151 Å, which are slightly longer than those in sterically less hindered stannoles (2.1357(18) and 2.134(3) Å) [30,31]. The C−C bonds in the five-membered ring are clearly altered (1.352, 1.521, and 1.352 Å), indicating its 1,3-diene character. In the packing structure, the stannole molecules are well aligned along the b-axis, as shown in Figure S1. At present, we infer that the well-ordered structure is realized by multiple weak hydrogen bonds between the CH groups of the Si(SiMe3)3 and the chlorine atom because the interatomic distances of the CH···Cl (approximately 3.1–3.5 Å) fall into a range of CH···Cl hydrogen bond lengths [43].
Lithium salt of stannolyl anion 3·(thf)3 crystallizes as a contact ion pair, adopting η1-coordination to the lithium cation instead of an η5-fashion. Note that this represents the first example of a contact ion pair of an alkali metal salt of stannolyl anions. The Sn−Li distance is 2.879(5) Å, which falls within the typical range for Sn−Li bonds [44,45,46]. The 1,3-diene character is confirmed by the C–C bond alternation in the five-membered ring, where the bond lengths of C1–C2, C2–C3, and C3–C4 are 1.362(4), 1.489(4), and 1.363(4) Å, respectively. The Sn–Si bond in 3·(thf)3 (2.6587(8) Å) is longer than that in 2 (2.589(1) Å). The Sn–C1/C4 bonds (2.197(3)/2.202(3) Å) in 3·(thf)3 are also elongated compared to those in 2 (2.148(4)–2.167(4) Å). These structural changes from 2 to 3·(thf)3 are attributed to the negative charge localized on the tin atom; the lone pair with a high s-character increases the p-character of the Sn–Si and Sn–C bonds. Although the bond lengths in 3·(thf)3 and 4 listed in Table 1 resemble each other, the degree of pyramidalization is slightly smaller in 3·(thf)3 (θ1: 103.9 vs. 89.6°) due to the Sn−Li bond in 3·(thf)3. This is also confirmed by the sum of the three angles around the tin atom: 282.3° for 3·(thf)3 and 255.1° for 4. Additionally, the angle θ2 in 2 (Si1−Sn−Cl) is 109.2°, which is an ideal angle for a tetracoordinated atom (109.5°), whereas the angle in 3·(thf)3 (Si1−Sn−Li) is considerably large (134.16(10)°) due to the high s-character of the tin lone pair.
To understand the structure of 3·(thf) in solution, NMR studies were performed. Table 2 summarizes the chemical shift changes upon reduction. The 119Sn{1H} NMR signal shifted from δ 101.3 (2) to −130.6 (3·(thf)), indicating that the negative charge is localized on the tin atom. A similar trend has been reported for related anionic stannacycles [47,48]. On the contrary, the 29Si NMR signal of the aromatic silolyl anion reported by Kovács, Nyulászi, et al. shifted to the lower field compared to that of its neutral precursor (δ 65.7 for the silolyl anion vs. 17.0 for its precursor) due to the π-electron delocalization [36]. A significant downfield shift was observed for the Cα signal [from δ 148.5 (2) to 180.2 (3·(thf)], as was observed in stannolyl and plumbolyl anions, which can be explained by the substantial contribution of the paramagnetic term (σpara) [49]. In the 7Li NMR spectrum, a slightly broadened signal was observed at δ −1.47. Considering that negatively large 7Li NMR chemical shifts (approximately −4 to −6 ppm) for lithiated metalloles indicate the aromaticity of the metallolyl anions due to the diatropic ring currents [50], we concluded that 3·(thf) is also nonaromatic in solution. It is interesting to note that the Me signal of the stannole ring shifted to a lower field upon reduction (from δ 2.01 (2) to 2.34 (3·(thf))), despite the negative charge on the stannole ring in 3·(thf). Therefore, using the chemical shift of the ring Me signal to diagnose aromaticity in metallolyl anions could lead to mischaracterization.

Table 2.
Chemical shift changes upon reduction.
3. Materials and Methods
3.1. General Considerations
All manipulations were carried out under an argon atmosphere using standard Schlenk techniques or a glovebox, unless otherwise stated. Hexane, toluene, Et2O, THF, and C6D6 were distilled over potassium mirror. 1-(Trimethylsilyl)-1-propyne, SnCl4, and [Cp2ZrCl2] were purchased from Tokyo Chemical Industry Co., Ltd. (Fukaya City, Japan), Kanto Chemical Co., Inc. (Tokyo, Japan), and Sigma-Aldrich Chemical Co. (Burlington, MA, USA), respectively. KSi(SiMe3)3 was synthesized according to the literature [51]. 1H (500 MHz), 13C{1H} (125 or 100 MHz), 7Li{1H} (194 MHz), 29Si{1H} (99 MHz), and 119Sn{1H} (186 MHz) NMR spectra were recorded on a JEOL ECA-500 (JEOL Ltd., Tokyo, Japan) or a Varian Mercury 400 spectrometers (International Equipment Trading Ltd., Mundelein, IL, USA) at 20 °C, unless otherwise stated. Chemical shifts are reported in δ and referenced to residual 1H and 13C{1H} signals of deuterated solvents as internal standards or to the 7Li{1H}, 29Si{1H}, and 119Sn{1H} NMR signals of LiCl in D2O (δ = 0.00), SiMe4 in CDCl3 (δ = 0.00), and SnMe4 in C6D6 (δ = 0.00) as external standards. In 13C{1H} NMR data, 1°, 2°, 3°, and 4° represent primary, secondary, tertiary, and quaternary carbons, respectively. Elemental analyses were performed on a Perkin Elmer 2400 series II CHN analyzer (PerkinElmer, Waltham, MA, USA).
3.2. Synthesis of 1,1-Dichloro-2,5-trimethylsilyl-3,4-dimethylstannole 1
In a Schlenk flask, a suspension of [Cp2ZrCl2] (2.001 g, 6.84 mmol) in hexane (70 mL) was cooled to −80 °C. Then, nBuLi (1.57 M in hexane, 8.9 mL, 14 mmol) was added dropwise, and the mixture was stirred for 30 min at this temperature. After adding 1-(trimethylsilyl)-1-propyne (2.1 mL, 14 mmol), the mixture was stirred at room temperature for 20 h to give a yellow suspension. The mixture was then cooled to −80 °C, and CuCl (135.6 mg, 1.4 mmol) and SnCl4 (1.0 M in hexane, 7.5 mL, 7.5 mmol) were added. The mixture was stirred for 20 h at room temperature to form a white suspension and filtered through Celite®. The solvent of the filtrate was removed in vacuo, and the resulting pale-yellow powder was dissolved in hexane and recrystallized at −20 °C, yielding 1 as colorless crystals (1.645 g, 3.97 mmol, 58%). Because compound 1 gradually decomposes in air, storing it under inert gas is recommended. 1H NMR (C6D6): δ 1.72 (s, 4JSnH = 11 Hz, 6H, CH3), 0.25 (s, 18H, SiMe3); 13C{1H} NMR (C6D6): δ 159.9 (4°, 2JSnC = 159 Hz, Cβ), 131.5 (4°, 1JSnC = 259 Hz, Cα), 21.2 (1°, 3J119SnC = 223 Hz, 3J117SnC = 213 Hz, CH3), 0.6 (1°, 1JSiC = 53 Hz, 3JSnC = 16 Hz, SiMe3); 29Si{1H} NMR (CDCl3): δ −6.01 (SiMe3); 119Sn{1H} NMR (C6D6): δ 53.2; m.p. 111 °C; Elemental analysis calcd (%) for C12H24Si2Cl2Sn: C 34.81, H 5.84; found: C 34.92, H 6.05.
3.3. Synthesis of 1-Chloro-1-tris(trimethylsilyl)silyl-2,5-trimethylsilyl-3,4-dimethylstannole 2
To a hexane solution (10 mL) of dichlorostannole 1 (781 mg, 1.89 mmol), KSi(SiMe3)3 (0.20 M in hexane, 9.4 mL, 1.9 mmol) was added at −80 °C. The resulting mixture was stirred for 18 h at room temperature and then filtered through Celite®. After removing the solvents in vacuo, the resulting yellow powder was dissolved in Et2O. Cooling this solution at −20 °C afforded pale-yellow crystals of 2 (382 mg, 0.610 mmol, 32% yield). Because compound 2 gradually decomposes in air, storing it under inert gas is recommended. 1H NMR (C6D6): δ 2.01 (s, 4JSnH = 5.4 Hz, 6H, CH3), 0.40 (s, 4JSnH = 6.5 Hz, 18H, SiMe3), 0.36 (s, 4JSnH = 6.5 Hz, 27H, Si(SiMe3)3); 13C{1H} NMR (C6D6): δ 162.0 (4°, 2J119SnC = 84 Hz, 2J117SnC = 80 Hz, Cβ), 148.5 (4°, 1J119SnC = 149 Hz, 1J117SnC = 143 Hz, 1JSiC = 63 Hz, Cα), 23.7 (1°, 3J119SnC = 117 Hz, 3J117SnC = 113 Hz, CH3), 3.7 (1°, 1JSiC = 46 Hz, 3JSnC = 14 Hz, Si(SiMe3)3), 2.3 (1°, 1JSiC = 52 Hz, 3JSnC = 10 Hz, SiMe3); 29Si{1H} NMR (CDCl3): δ −7.23 (Si(SiMe3)3), −7.24 (Si(SiMe3)3), −7.88 (SiMe3); 119Sn{1H} NMR (C6D6): δ 101.3; m.p. 150 °C; Elemental analysis calcd (%) for C21H51Si6ClSn: C 40.27, H 8.21; found: C 39.95, H 8.34.
3.4. Synthesis of 1-Lithio-1-tris(trimethylsilyl)silyl-2,5-trimethylsilyl-3,4-dimethylstannole 3
Lithium metal (3.2 mg, 0.46 mmol) was added to an Et2O solution (2 mL) of chlorostannole 2 (113 mg, 0.180 mmol) at −20 °C. After stirring for 1 h, the remaining lithium was removed by filtration. After removing the solvent in vacuo, the residue was dissolved in hexane/THF. Cooling this solution to −20 °C deposited orange crystals of 3·(thf)3. Drying the crystals under reduced pressure gave off two of the three THF molecules to give 3·thf (31.2 mg, 0.0466 mmol, 26% yield). 1H NMR (C6D6): δ 3.18 (m, 4H, thf), 2.34 (s, 4JSnH = 16 Hz, 6H, CH3), 1.17 (m, 4H, thf), 0.49 (s, 27H, Si(SiMe3)3), 0.48 (s, 18H, Si(CH3)3); 13C{1H} NMR (C6D6): δ 180.2 (4°, Cα), 156.9 (4°, Cβ), 68.9 (2°, thf), 25.9 (1°, 3JSnC = 13 Hz, CH3), 25.2 (2°, thf), 5.0 (1°, 1JSiC = 44 Hz, Si(SiMe3)3), 3.9 (1°, 1JSiC = 51 Hz, 3JSnC = 13 Hz, SiMe3); 29Si{1H} NMR (C6D6): δ −7.6 (Si(SiMe3)3), −8.9 (2JSnSi = 43.7 Hz, Si(SiMe3)3), −10.1 (2JSnSi = 39.3 Hz, SiMe3); 119Sn{1H} NMR (C6D6): δ −130.6; 7Li{1H} NMR (C6D6): δ −1.47; m.p. > 143 °C (decomp.). Although elemental analysis could not be carried out due to the instability towards moisture and oxygen, the purity of 3 was confirmed by the 1H, 13C{1H},119Sn{1H}, 29Si{1H} and 7Li{1H} NMR spectra (Figures S10–S14). Satellite signals caused by Sn−C coupling could not be observed.
3.5. Details for X-ray Diffraction Studies
Diffraction data for 2 and 3·(thf)3 were collected on a VariMax Saturn CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71075 Å) at −180 °C. Intensity data were corrected for Lorenz-polarization effects and for empirical absorption (REQAB). All calculations were performed using the CrystalStructure 4.3 crystallographic software package, except for refinements, which were performed using SHELXL-2018/3 [52]. The positions of the non-hydrogen atoms were determined by SHELXT [53]. All non-hydrogen atoms were refined on Fo2 anisotropically by full-matrix least-squares techniques. All hydrogen atoms were placed at the calculated positions with fixed isotropic parameters.
Crystal data for C21H51ClSi6Sn (2) (M = 626.29 g/mol): monoclinic, space group P21/c (no. 14), a = 18.826(2) Å, b = 28.856(3) Å, c = 18.810(2) Å, α = 90°, β = 97.7770(10)°, γ = 90°, V = 10,124.5(19) Å3, Z = 12, T = 93(2) K, μ(MoKα) = 0.71075 mm−1, Dcalc = 1.233 g/cm3, 82,916 reflections measured (6.0° ≤ 2θ ≤ 53.0°), 23,037 unique reflections (Rint = 0.0729), which were used in all calculations. The final R1 was 0.0619 (I > 2σ(I)), wR2 was 0.1272 (all data), and GOF = 1.128.
Crystal data for C33H75LiO3Si6Sn [3·(thf)3] (M = 814.10 g/mol): monoclinic, space group P21/n (no. 14), a = 13.3363(11) Å, b = 19.6417(18) Å, c = 17.7954(16) Å, α = 90°, β = 91.4530(10)°, γ = 90°, V = 4660.0(7) Å3, Z = 4, T = 93(2) K, μ(MoKα) = 0.71075 mm−1, Dcalc = 1.160 g/cm3, 37,674 reflections measured (6.0° ≤ 2θ ≤ 55.0°), 10,667 unique reflections (Rint = 0.0437), which were used in all calculations. The final R1 was 0.0437 (I > 2σ(I)), wR2 was 0.0952 (all data), and GOF = 1.102.
4. Conclusions
We successfully synthesized novel 1,1-dichloro-2,5-disilylstannole 1, monochlorostannole 2, and lithium salt of stannolyl anion 3·(thf)3 that adopts an η1-coordination in the solid-state. Unlike the aromatic 2,5-disilylated silolyl and germolyl anions, the tin counterpart 3·(thf)3 is concluded to be nonaromatic based on the C−C bond alternation and pyramidal tin center. This conclusion is further supported by 119Sn{1H} and 7Li{1H} NMR spectroscopic properties. This result can be explained by a trend where the energy difference between pyramidalized metallolyl anions and the planar ones increases as the group 14 metal becomes heavier [54].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics12030092/s1, Figure S1: Packing structure of 2; Table S1: Crystallographic data for 2 and 3·(thf)3; Figures S2–S14: NMR spectra of 1, 2, and 3·(thf).
Author Contributions
Conceptualization, T.K.; methodology, T.K.; validation, K.K. and T.K.; investigation, K.K.; resources, T.K. and Y.I.; data curation, K.K. and T.K.; writing—original draft preparation, T.K.; writing—review and editing, Y.I.; supervision, T.K.; project administration, T.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant numbers 22K05078 and 18K14203.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request. CCDC 2337714 (2) and 2337715 [3·(thf)3] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif; by emailing data_request@ccdc.cam.ac.uk; or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K., fax: +44-1223-336033.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saito, M. Transition-Metal Complexes Featuring Dianionic Heavy Group 14 Element Aromatic Ligands. Acc. Chem. Res. 2018, 51, 160–169. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.-X.; Xi, Z. The aromatic dianion metalloles. Chem. Sci. 2018, 9, 560–568. [Google Scholar] [CrossRef]
- Kuwabara, T.; Saito, M. 3.17—Siloles, Germoles, Stannoles, and Plumboles. In Comprehensive Heterocyclic Chemistry IV; Black, D.S., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; pp. 798–832. [Google Scholar]
- Sun, X.; Roesky, P.W. Group 14 metallole dianions as η5-coordinating ligands. Inorg. Chem. Front. 2023, 10, 5509–5516. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Rheingold, A.L.; Ostrander, R.L. A Stable η5-Germacyclopentadienyl Complex: [(η5-C5Me5)Ru{η5-C4Me4GeSi(SiMe3)3}]. Angew. Chem. Int. Ed. Engl. 1993, 32, 1744–1745. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Rheingold, A.L. Stable Silacyclopentadienyl Complexes of Ruthenium: (η5-C5Me5)Ru[η5-Me4C4SiSi(SiMe3)3] and X-ray Structure of Its Protonated Form. J. Am. Chem. Soc. 1994, 116, 8428–8429. [Google Scholar] [CrossRef]
- Dysard, J.M.; Tilley, T.D. η5-Silolyl and η5-Germolyl Complexes of d0 Hafnium. Structural Characterization of an η5-Silolyl Complex. J. Am. Chem. Soc. 1998, 120, 8245–8246. [Google Scholar] [CrossRef]
- Dysard, J.M.; Tilley, T.D. Synthesis and Reactivity of η5-Silolyl, η5-Germolyl, and η5-Germole Dianion Complexes of Zirconium and Hafnium. J. Am. Chem. Soc. 2000, 122, 3097–3105. [Google Scholar] [CrossRef]
- Dysard, J.M.; Tilley, T.D. Hafnium−Rhodium and Hafnium−Iridium Heterobimetallic Complexes Featuring the Bridging Germole Dianion Ligand [GeC4Me4]2−. Organometallics 2000, 19, 2671–2675. [Google Scholar] [CrossRef]
- Freeman, W.P.; Dysard, J.M.; Tilley, T.D.; Rheingold, A.L. Synthesis and Reactivity of η5-Germacyclopentadienyl Complexes of Iron. Organometallics 2002, 21, 1734–1738. [Google Scholar] [CrossRef]
- Kuwabara, T.; Guo, J.-D.; Nagase, S.; Sasamori, T.; Tokitoh, N.; Saito, M. Synthesis, Structures and Electronic Properties of Triple- and Double-decker Ruthenocenes Incorporated by A Group 14 Metallole Dianion Ligand. J. Am. Chem. Soc. 2014, 136, 13059–13064. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Nakada, M.; Kuwabara, T.; Owada, R.; Furukawa, S.; Narayanan, R.; Abe, M.; Hada, M.; Tanaka, K.; Yamamoto, Y. Inverted Sandwich Rh Complex Bearing a Plumbole Ligand and Its Catalytic Activity. Organometallics 2019, 38, 3099–3103. [Google Scholar] [CrossRef]
- Nakada, M.; Kuwabara, T.; Furukawa, S.; Hada, M.; Minoura, M.; Saito, M. Synthesis and reactivity of a ruthenocene-type complex bearing an aromatic π-ligand with the heaviest group 14 element. Chem. Sci. 2017, 8, 3092–3097. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Saito, M. Synthesis of a Stannole Dianion Complex Bearing a μ-η1;η1-Coordination Mode: Different Electronic State of Stannole Dianion Ligands Depending on Their Hapticity. Organometallics 2015, 34, 4202–4204. [Google Scholar] [CrossRef]
- Cramer, H.H.; Bührmann, L.; Schmidtmann, M.; Müller, T. A phenyl-substituted germole dianion and its reaction with hafnocene dichloride. Mendeleev Commun. 2022, 32, 46–48. [Google Scholar] [CrossRef]
- Dong, Z.; Janka, O.; Kösters, J.; Schmidtmann, M.; Müller, T. A Dimeric η1,η5-Germole Dianion Bridged Titanium(III) Complex with a Multicenter Ti−Ge−Ge−Ti Bond. Angew. Chem. Int. Ed. 2018, 57, 8634–8638. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Mokrai, R.; Bombicz, P.; Nyulászi, L.; Kovács, I. η1-silolyl-FeCp(CO)2 complexes. Is there a way to sila-ferrocene? J. Organomet. Chem. 2015, 799–800, 291–298. [Google Scholar] [CrossRef]
- Kuwabara, T.; Nakada, M.; Guo, J.D.; Nagase, S.; Saito, M. Diverse coordination modes in tin analogues of a cyclopentadienyl anion depending on the substituents on the tin atom. Dalton Trans. 2015, 44, 16266–16271. [Google Scholar] [CrossRef]
- Liu, J.; Singh, K.; Dutta, S.; Feng, Z.; Koley, D.; Tan, G.; Wang, X. Yttrium germole dianion complexes with Y–Ge bonds. Dalton Trans. 2021, 50, 5552–5556. [Google Scholar] [CrossRef]
- Sun, X.; Münzfeld, L.; Jin, D.; Hauser, A.; Roesky, P.W. Silole and germole complexes of lanthanum and cerium. Chem. Commun. 2022, 58, 7976–7979. [Google Scholar] [CrossRef]
- Münzfeld, L.; Sun, X.; Schlittenhardt, S.; Schoo, C.; Hauser, A.; Gillhuber, S.; Weigend, F.; Ruben, M.; Roesky, P.W. Introduction of plumbole to f-element chemistry. Chem. Sci. 2022, 13, 945–954. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Mondal, A.; Ruan, Z.-Y.; Tong, M.-L.; Layfield, R.A. Dynamic Magnetic Properties of Germole-ligated Lanthanide Sandwich Complexes. Chem. Eur. J. 2023, 29, e202300567. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Yap, G.P.A.; Rheingold, A.L. Silolyl Anions and Silole Dianions: Structure of [K([18]crown-6)+]2[C4Me4Si2−]. Angew. Chem. Int. Ed. Engl. 1996, 35, 882–884. [Google Scholar] [CrossRef]
- West, R.; Sohn, H.; Bankwitz, U.; Calabrese, J.; Apeloig, Y.; Mueller, T. Dilithium Derivative of Tetraphenylsilole: An η1-η5 Dilithium Structure. J. Am. Chem. Soc. 1995, 117, 11608–11609. [Google Scholar] [CrossRef]
- Szathmári, B.; Fekete, C.; Kelemen, Z.; Holczbauer, T.; Nyulászi, L.; Kovács, I. Synthesis of Silolide Dianions via Reduction of Dichlorosiloles: Important Role of the Solvent. Eur. J. Inorg. Chem. 2023, 26, e202300316. [Google Scholar] [CrossRef]
- Dong, Z.; Reinhold, C.R.W.; Schmidtmann, M.; Müller, T. Trialkylsilyl-Substituted Silole and Germole Dianions. Organometallics 2018, 37, 4736–4743. [Google Scholar] [CrossRef]
- West, R.; Sohn, H.; Powell, D.R.; Müller, T.; Apeloig, Y. The Dianion of Tetraphenylgermole is Aromatic. Angew. Chem. Int. Ed. Engl. 1996, 35, 1002–1004. [Google Scholar] [CrossRef]
- Choi, S.-B.; Boudjouk, P.; Hong, J.-H. Unique Bis-η5/η1 Bonding in a Dianionic Germole. Synthesis and Structural Characterization of the Dilithium Salt of the 2,3,4,5-Tetraethyl Germole Dianion. Organometallics 1999, 18, 2919–2921. [Google Scholar] [CrossRef]
- Saito, M.; Haga, R.; Yoshioka, M.; Ishimura, K.; Nagase, S. The Aromaticity of the Stannole Dianion. Angew. Chem. Int. Ed. 2005, 44, 6553–6556. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kuwabara, T.; Kambayashi, C.; Yoshioka, M.; Ishimura, K.; Nagase, S. Synthesis, Structure, and Reaction of Tetraethyldilithiostannole. Chem. Lett. 2010, 39, 700–701. [Google Scholar] [CrossRef]
- Kuwabara, T.; Guo, J.-D.; Nagase, S.; Minoura, M.; Herber, R.H.; Saito, M. Enhancement of Stannylene Character in Stannole Dianion Equivalents Evidenced by NMR and Mössbauer Spectroscopy and Theoretical Studies of Newly Synthesized Silyl-Substituted Dilithiostannoles. Organometallics 2014, 33, 2910–2913. [Google Scholar] [CrossRef]
- Saito, M.; Sakaguchi, M.; Tajima, T.; Ishimura, K.; Nagase, S.; Hada, M. Dilithioplumbole: A Lead-Bearing Aromatic Cyclopentadienyl Analog. Science 2010, 328, 339–342. [Google Scholar] [CrossRef]
- Saito, M.; Nakada, M.; Kuwabara, T.; Minoura, M. A reversible two-electron redox system involving a divalent lead species. Chem. Commun. 2015, 51, 4674–4676. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.P.; Tilley, T.D.; Liable-Sands, L.M.; Rheingold, A.L. Synthesis and Study of Cyclic π-Systems Containing Silicon and Germanium. The Question of Aromaticity in Cyclopentadienyl Analogues. J. Am. Chem. Soc. 1996, 118, 10457–10468. [Google Scholar] [CrossRef]
- Saito, M.; Kuwabara, T.; Ishimura, K.; Nagase, S. Synthesis and Structures of Lithium Salts of Stannole Anions. Bull. Chem. Soc. Jpn. 2010, 83, 825–827. [Google Scholar] [CrossRef]
- Fekete, C.; Kovács, I.; Nyulászi, L.; Holczbauer, T. Planar lithium silolide: Aromaticity, with significant contribution of non-classical resonance structures. Chem. Commun. 2017, 53, 11064–11067. [Google Scholar] [CrossRef]
- Fekete, C.; Kovács, I.; Könczöl, L.; Benkő, Z.; Nyulászi, L. Substituent effect on the aromaticity of the silolide anion. Struct. Chem. 2014, 25, 377–387. [Google Scholar] [CrossRef]
- Dong, Z.; Schmidtmann, M.; Müller, T. Potassium Salts of 2,5-Bis(trimethylsilyl)-Germolide: Switching between Aromatic and Non-Aromatic States. Chem. Eur. J. 2019, 25, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.D.; Wei, P.; Beck, B.C.; Su, J.; Robinson, G.H. Synthesis and Molecular Structure of Germanium and Tin Tetraphenylbutadienyl Based Heterocyclic Halides. Main Group Chem. 2000, 3, 137–141. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Kehr, G.; Willbold, S.; Ali, S. Novel organotin halides. Organometallic substituted stannoles and alkene derivatives with tin–chlorine and tin–bromine bonds—Exceptionally small magnitude of coupling constants ∣1J(119Sn,13C)∣. J. Organomet. Chem. 2002, 646, 125–133. [Google Scholar] [CrossRef]
- Negishi, E.-i.; Cederbaum, F.E.; Takahashi, T. Reaction of zirconocene dichloride with alkyllithiums or alkyl grignard reagents as a convenient method for generating a “zirconocene” equivalant and its use in zirconium-promoted cyclization of alkenes, alkynes, dienes, enynes, and diynes. Tetrahedron Lett. 1986, 27, 2829–2832. [Google Scholar] [CrossRef]
- Ura, Y.; Li, Y.; Xi, Z.; Takahashi, T. Cu(I) catalyzed or promoted metallacycle transfer of zirconacycles to stannacycles. Tetrahedron Lett. 1998, 39, 2787–2790. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Evans, T.A.; Seddon, K.R.; Pálinkó, I. The C–H···Cl hydrogen bond: Does it exist? New J. Chem. 1999, 23, 145–152. [Google Scholar] [CrossRef]
- Reed, D.; Stalke, D.; Wright, D.S. Observation of a Direct Sn–Li Bond; The Crystal and Molecular Structure of Monomeric [Ph3SnLi · PMDETA] and the Detection of 119, 117Sn–7Li NMR Coupling in Solution. Angew. Chem. Int. Ed. Engl. 1991, 30, 1459–1460. [Google Scholar] [CrossRef]
- Fukawa, T.; Nakamoto, M.; Lee, V.Y.; Sekiguchi, A. Structural Diversity of the Tris(di-tert-butylmethylsilyl)stannyl Anion: Monomeric vs Dimeric, Lithium Coordinated vs Lithium Free. Organometallics 2004, 23, 2376–2381. [Google Scholar] [CrossRef]
- Nanjo, M.; Nanjo, E.; Mochida, K. Tris(trimethylsilyl)-Substituted Heavy Group 14-Element-Centered Anions: Unsolvated Trimeric Germyllithium and Solvated Dimeric Silyl- and Stannyllithiums. Eur. J. Inorg. Chem. 2004, 2004, 2961–2967. [Google Scholar] [CrossRef]
- Ito, S.; Kuwabara, T.; Ishii, Y. A Tin Analogue of the Cycloheptatrienyl Anion: Synthesis, Structure, and Further Reduction to Form a Dianionic Species. Organometallics 2020, 39, 640–644. [Google Scholar] [CrossRef]
- Haga, R.; Saito, M.; Yoshioka, M. Synthesis and Reactions of Stannole Anions. Eur. J. Inorg. Chem. 2007, 2007, 1297–1306. [Google Scholar] [CrossRef]
- Narayanan, R.; Nakada, M.; Abe, M.; Saito, M.; Hada, M. 13C and 207Pb NMR Chemical Shifts of Dirhodio- and Dilithioplumbole Complexes: A Quantum Chemical Assessment. Inorg. Chem. 2019, 58, 14708–14719. [Google Scholar] [CrossRef]
- Saito, M.; Yoshioka, M. The anions and dianions of group 14 metalloles. Coord. Chem. Rev. 2005, 249, 765–780. [Google Scholar] [CrossRef]
- Marschner, C. A New and Easy Route to Polysilanylpotassium Compounds. Eur. J. Inorg. Chem. 1998, 1998, 221–226. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystllogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Goldfuss, B.; Schleyer, P.v.R. Aromaticity in Group 14 Metalloles: Structural, Energetic, and Magnetic Criteria. Organometallics 1997, 16, 1543–1552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).