Mechanochemical Synthesis of Solid-State Electrolytes
Abstract
1. Introduction
2. Results and Discussion
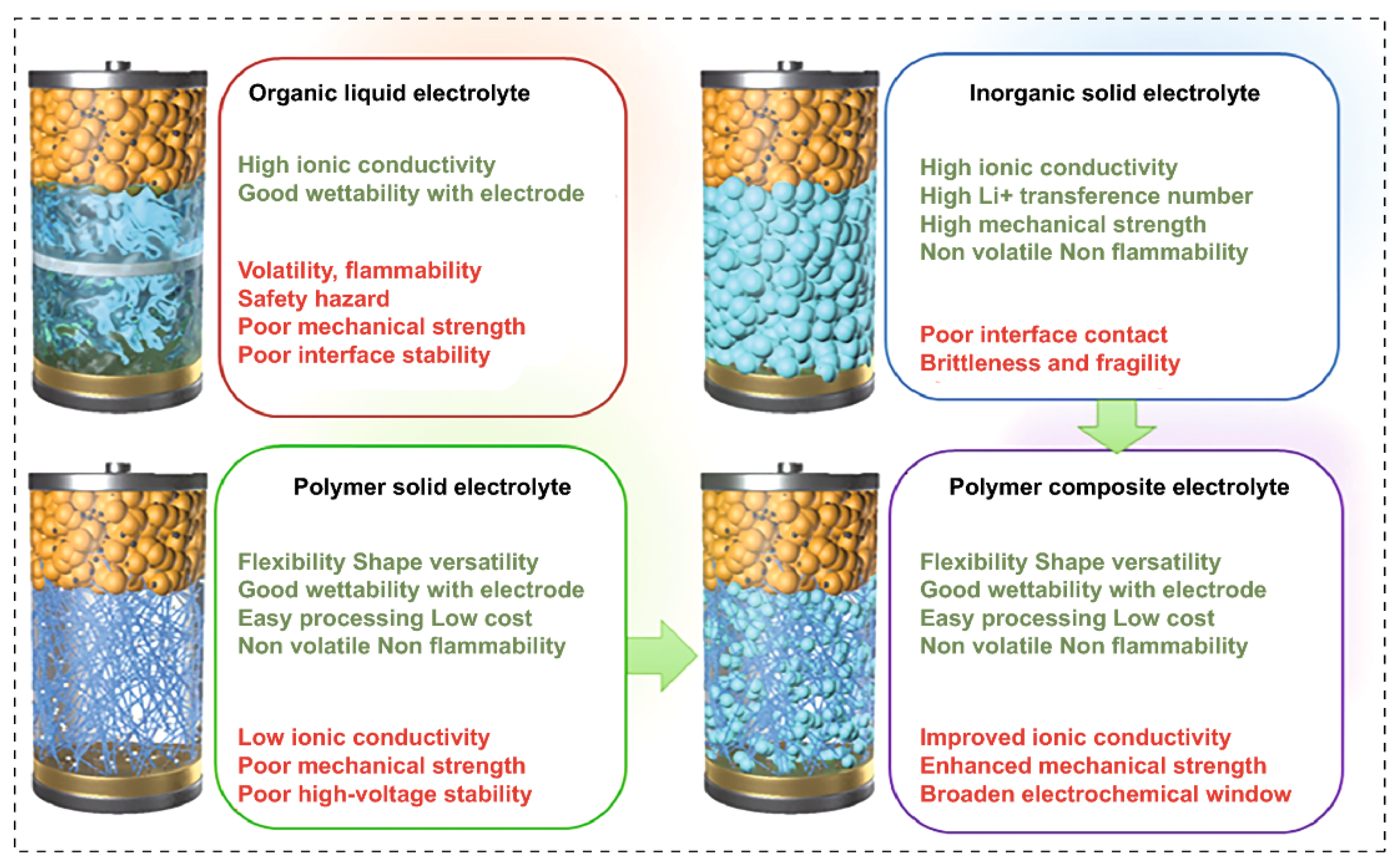
2.1. Difference between Batteries with Liquid and Solid Electrolytes
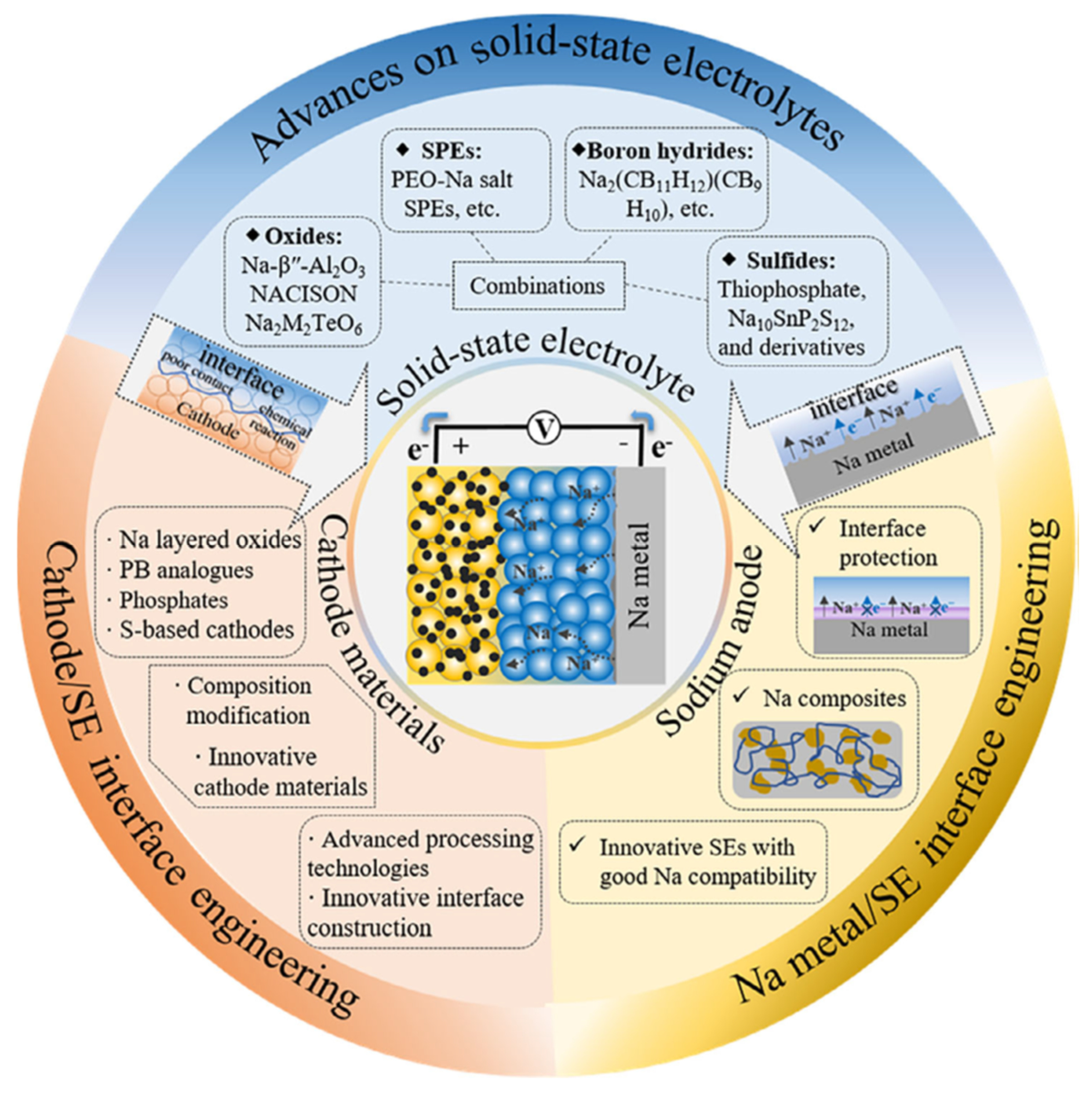
2.2. Types of Solid-State Electrolytes
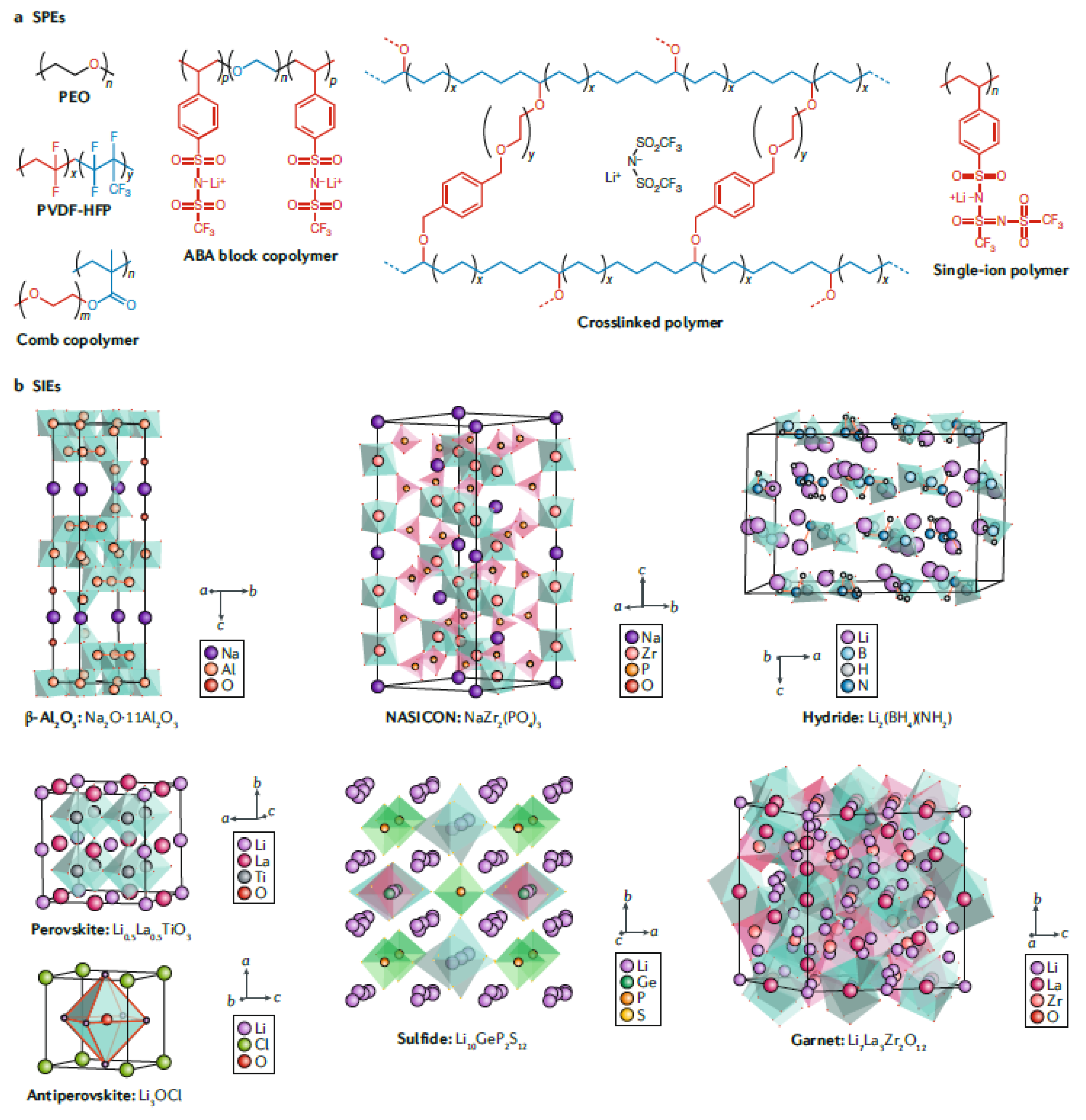
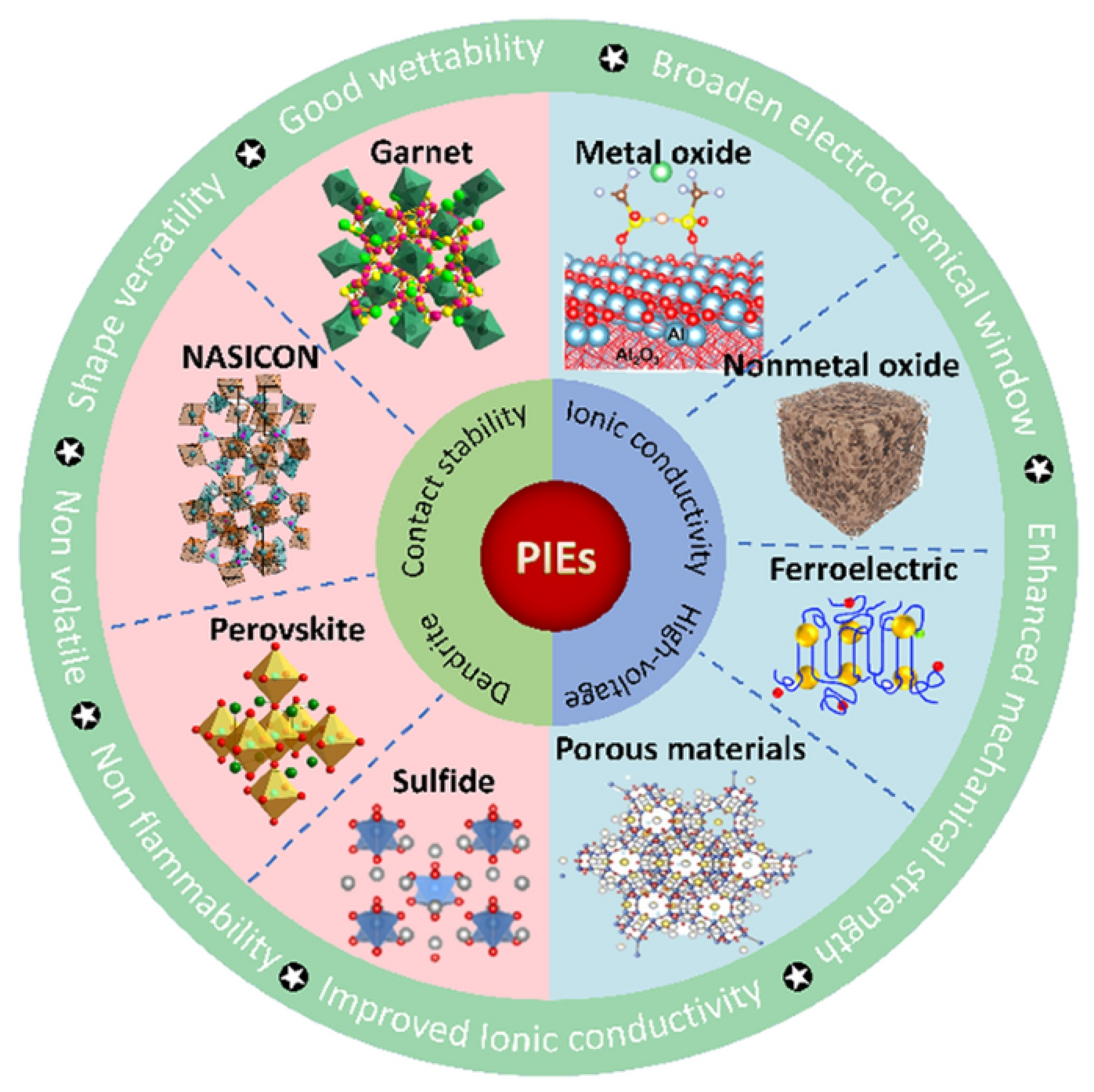
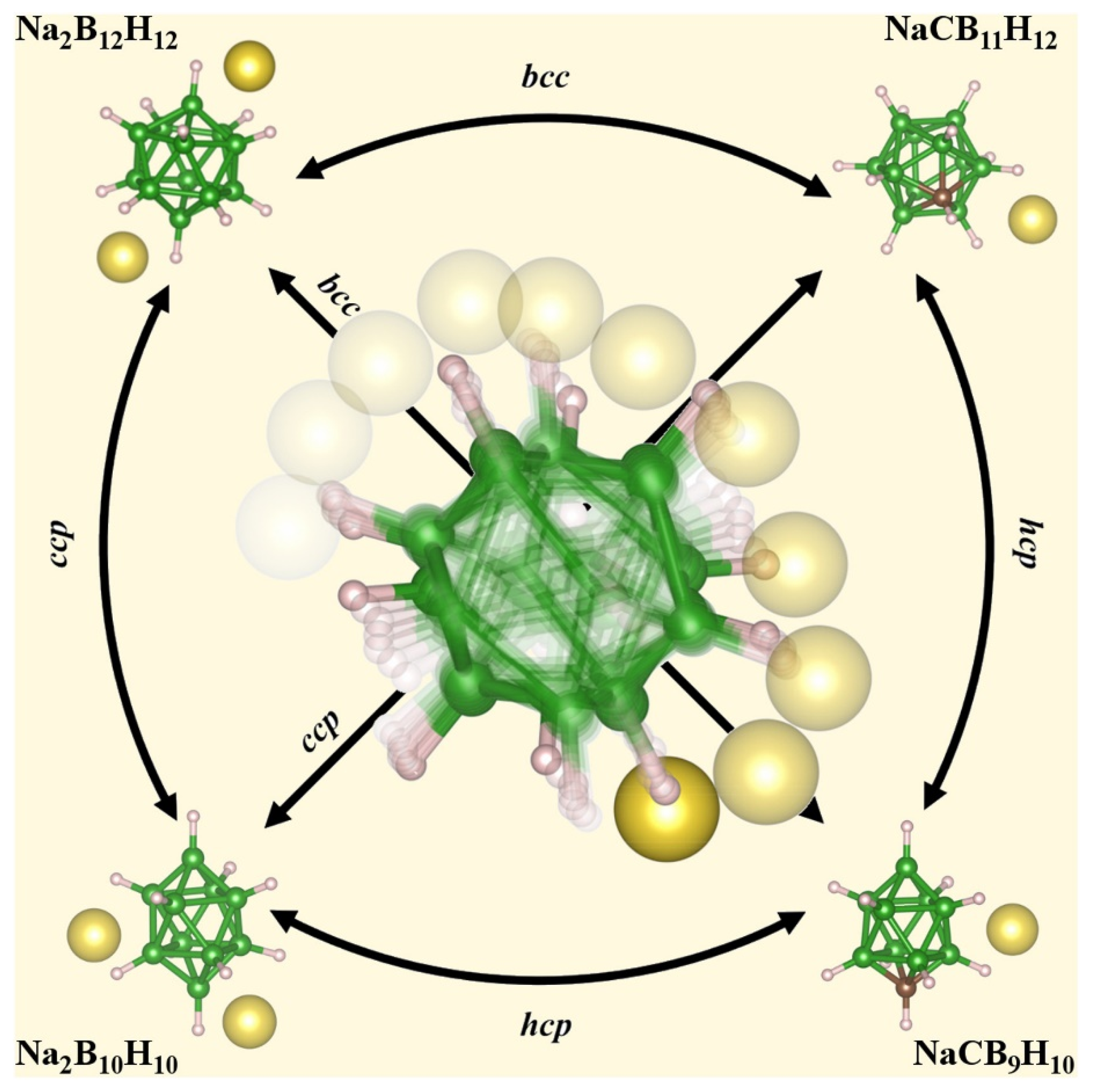
2.2.1. Solid Inorganic Electrolytes
2.2.2. Solid Polymer Electrolytes
2.2.3. Composite Solid Electrolytes
2.3. Problems with Solid Electrolytes and Possible Solutions
2.4. Mechanochemical Techniques
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Copyrights
Abbreviations
| Abbreviation | Definition |
| LiB | lithium-ion batterie |
| EV | electrical vehicle |
| NMC | Lithium nickel manganese cobalt oxide |
| Nb-NCA93 | Li[Ni0.92Co0.06Al0.01Nb0.01]O2 |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| PSS | poly(styrenesulfonate) |
| ASSB | all-solid-state battery |
| SE | solid electrolyte |
| SSE | solid-state electrolyte |
| RT | room temperature |
| SIE | solid inorganic electrolyte |
| SPE | solid polymer electrolyte |
| CSE | composite solid electrolyte |
| LISICON | lithium super ionic conductor |
| LiPON | lithium phosphorus oxynitride |
| NASICON | sodium super ionic conductor |
| NaFSI | sodium bis(fluorosulfonyl)imide |
| TM | transition metal |
| PTO | pyrene-4,5,9,10-tetraone |
| EG | expanded graphite |
| MIB | Mg-ion battery |
| THF | tetrahydrofuran |
| DME | dimethyl ether |
| PEO | poly(ethylene oxide) |
| PAN | poly(acrylonitrile) |
| PMMA | poly(methyl methacrylate) |
| PVA | poly(vinyl alcohol) |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| Pyr14TFSI | 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide |
| PEGDA | poly(ethyleneglycol)diacrylate |
| PUA | polyurethane acrylate |
| AlF3 | aluminum fluoride |
| NZSP | Na3Zr2Si2PO12 |
| NATFSA | sodium bis(trifluoromethanesulfonyl)amide |
| HT | high temperature |
| NSPSe | Na11Sn2PSe12 |
| NCB | NaCB11H12 |
References
- Schlem, R.; Burmeister, C.F.; Michalowski, P.; Ohno, S.; Dewald, G.F.; Kwade, A.; Zeier, W.G. Energy Storage Materials for Solid-State Batteries: Design by Mechanochemistry. Adv. Energy Mater. 2021, 11, 2101022. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for Solid-State Lithium Rechargeable Batteries: Recent Advances and Perspectives. Chem. Soc. Rev. 2011, 40, 2525. [Google Scholar] [CrossRef]
- Battery2030+—Battery 2030+. Available online: https://battery2030.eu/ (accessed on 10 November 2023).
- Anuradha. Understanding Self-Discharge of a Lithium-Ion Battery. EVreporter. Available online: https://evreporter.com/understanding-self-discharge-of-a-lithium-ion-battery (accessed on 10 November 2023).
- Fast-Charge Lithium Batteries for Electric Vehicles | Flash Battery. Available online: https://www.flashbattery.tech/en/lithium-batteries-electric-vehicles (accessed on 10 November 2023).
- Boll, C. How Long Do Lithium-Ion Batteries Last? Available online: https://www.protoolreviews.com/how-long-do-lithium-ion-batteries-last (accessed on 11 November 2023).
- Li, Q.; Yang, Y.; Yu, X.; Li, H. A 700 Wh Kg−1 Rechargeable Pouch Type Lithium Battery. Chin. Phys. Lett. 2023, 40, 048201. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N. Maximizing Energy Density of Lithium-Ion Batteries for Electric Vehicles: A Critical Review. Energy Rep. 2023, 9, 11–21. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational Design on Materials for Developing next Generation Lithium-Ion Secondary Battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Kim, U.-H.; Lee, S.-B.; Park, N.-Y.; Kim, S.J.; Yoon, C.S.; Sun, Y.-K. High-Energy-Density Li-Ion Battery Reaching Full Charge in 12 min. ACS Energy Lett. 2022, 7, 3880–3888. [Google Scholar] [CrossRef]
- Sun, Q.; Bijelić, M.; Djurišić, A.B.; Suchomski, C.; Liu, X.; Xie, M.; Ng, A.M.C.; Kong Li, H.; Shih, K.; Burazer, S.; et al. Graphene-Oxide-Wrapped ZnMn2O4 as a High Performance Lithium-Ion Battery Anode. Nanotechnology 2017, 28, 455401. [Google Scholar] [CrossRef] [PubMed]
- Bijelić, M.; Liu, X.; Sun, Q.; Djurišić, A.B.; Xie, M.H.; Ng, A.M.C.; Suchomski, C.; Djerdj, I.; Skoko, Ž.; Popović, J. Long Cycle Life of CoMn2O4 Lithium Ion Battery Anodes with High Crystallinity. J. Mater. Chem. A 2015, 3, 14759–14767. [Google Scholar] [CrossRef]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal Stability and Flammability of Electrolytes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Zhang, H.; Wei, F.; Huang, L.; Ke, S.; Fu, J.; Jing, C.; Cheng, J.; Liu, S. Tuning Electron Delocalization of Hydrogen-Bonded Organic Framework Cathode for High-Performance Zinc-Organic Batteries. Nat. Commun. 2023, 14, 5235. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Xu, H.; Zhang, T.; Li, W.; Huang, L.; Peng, Y.; Guo, H.; Wang, Y.; Guan, S.; Fu, J.; et al. Mesoporous Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) as Efficient Iodine Host for High-Performance Zinc–Iodine Batteries. ACS Nano 2023, 17, 20643–20653. [Google Scholar] [CrossRef] [PubMed]
- Golub, I.E.; Heere, M.; Gounaris, V.; Li, X.; Steenhaut, T.; Wang, J.; Robeyns, K.; Li, H.-W.; Dovgaliuk, I.; Ikeda, K.; et al. Structural Insight into the Magnesium Borohydride—Ethylenediamine Solid-State Mg-Ion Electrolyte System. Dalton Trans. 2023, 52, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Janek, J.; Zeier, W.G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From Nanoscale Interface Characterization to Sustainable Energy Storage Using All-Solid-State Batteries. Nat. Nanotechnol. 2020, 15, 170–180, Correction in Nat. Nanotechnol. 2021, 16, 479. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2020, 121, 1623–1669. [Google Scholar] [CrossRef]
- Černý, R.; Murgia, F.; Brighi, M. Metal Hydroborates: From Hydrogen Stores to Solid Electrolytes. J. Alloys Compd. 2022, 895, 162659. [Google Scholar] [CrossRef]
- Shao, B.; Huang, Y.; Han, F. Electronic Conductivity of Lithium Solid Electrolytes. Adv. Energy Mater. 2023, 13, 2204098. [Google Scholar] [CrossRef]
- Sadikin, Y.; Schouwink, P.; Brighi, M.; Łodziana, Z.; Černý, R. Modified Anion Packing of Na2B12H12 in Close to Room Temperature Superionic Conductors. Inorg. Chem. 2017, 56, 5006–5016. [Google Scholar] [CrossRef]
- Richards, W.D.; Tsujimura, T.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ong, S.P.; Uechi, I.; Suzuki, N.; Ceder, G. Design and Synthesis of the Superionic Conductor Na10SnP2S12. Nat. Commun. 2016, 7, 11009. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single Lithium-Ion Conducting Solid Polymer Electrolytes: Advances and Perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All Solid-State Polymer Electrolytes for High-Performance Lithium Ion Batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, K.; Yu, C.; Chen, X.; Zhang, C.; Yao, Y.; Jiang, B.; Long, H. Recent Progress of Composite Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Chin. Chem. Lett. 2021, 32, 2659–2678. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Liu, H.; Liang, Y.; Fan, L. A Review of Polymer-based Solid-State Electrolytes for Lithium-Metal Batteries: Structure, Kinetic, Interface Stability, and Application. Batter. Supercaps 2023, 6, e202200502, Correction in Batter. Supercaps 2023, 6, e202300177. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO Based Polymer-Ceramic Hybrid Solid Electrolytes: A Review. Nano Converg. 2021, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, D.M.; Frechero, M.A. Strategies for Rational Design of Polymer-Based Solid Electrolytes for Advanced Lithium Energy Storage Applications. Energy Storage Mater. 2022, 52, 430–464. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-State Polymer Electrolytes for High-Performance Lithium Metal Batteries. Nat. Commun. 2019, 10, 4398. [Google Scholar] [CrossRef] [PubMed]
- Popovic, J. Dry Polymer Electrolyte Concepts for Solid-State Batteries. Macromol. Chem. Phys. 2021, 223, 2100344. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-Type Solid-State Fast Li Ion Conductors for Li Batteries: Critical Review. Chem. Soc. Rev. 2014, 43, 4714. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhao, N.; Huo, H.; Guo, X. Comprehensive Investigation into Garnet Electrolytes toward Application-Oriented Solid Lithium Batteries. Electrochem. Energy Rev. 2020, 3, 656–689. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of Solid-State Electrolytes for Sodium-Ion Battery–A Short Review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Singh, B.; Wang, Z.; Park, S.; Gautam, G.S.; Chotard, J.-N.; Croguennec, L.; Carlier, D.; Cheetham, A.K.; Masquelier, C.; Canepa, P. A Chemical Map of NaSICON Electrode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2021, 9, 281–292. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; You, Y.; Vinu, A.; Mai, L. NASICONs-type Solid-state Electrolytes: The History, Physicochemical Properties, and Challenges. Interdiscip. Mater. 2022, 2, 91–110. [Google Scholar] [CrossRef]
- Morimoto, H.; Awano, H.; Terashima, J.; Shindo, Y.; Nakanishi, S.; Ito, N.; Ishikawa, K.; Tobishima, S. Preparation of Lithium Ion Conducting Solid Electrolyte of NASICON-Type Li1+xAlxTi2−x(PO4)3 (x = 0.3) Obtained by Using the Mechanochemical Method and Its Application as Surface Modification Materials of LiCoO2 Cathode for Lithium Cell. J. Power Sources 2013, 240, 636–643. [Google Scholar] [CrossRef]
- Dudney, N.J. Thin Film Micro-Batteries. Electrochem. Soc. Interface 2008, 17, 44–48. [Google Scholar] [CrossRef]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A Review of Lithium and Non-Lithium Based Solid State Batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Wei, R.; Chen, S.; Gao, T.; Liu, W. Challenges, Fabrications and Horizons of Oxide Solid Electrolytes for Solid-state Lithium Batteries. Nano Sel. 2021, 2, 2256–2274. [Google Scholar] [CrossRef]
- Kim, A.; Woo, S.; Kang, M.; Park, H.; Kang, B. Research Progresses of Garnet-Type Solid Electrolytes for Developing All-Solid-State Li Batteries. Front. Chem. 2020, 8, 468. [Google Scholar] [CrossRef]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, Z.; Shi, J.; Han, K.; Wan, Q.; Liu, Y.; Qu, X. Recent Advances and Perspectives of Air Stable Sulfide-Based Solid Electrolytes for All-Solid-State Lithium Batteries. Chem. Rec. 2022, 22, e202200086. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Mizuno, F.; Hayashi, A.; Tatsumisago, M. Lithium Ion Conductivity of the Li2S–P2S5 Glass-Based Electrolytes Prepared by the Melt Quenching Method. Solid State Ion. 2007, 178, 837–841. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A Lithium Superionic Conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2S GeS2 P2S5 System. J. Electrochem. Soc. 2001, 148, A742. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Aswathy, P.; Suriyakumar, S.; Kumar, S.A.; Oliyantakath Hassan, M.S.; Vijayan, V.; Shaijumon, M.M. Microwave-Assisted Synthesis of Sulfide Solid Electrolytes for All-Solid-State Sodium Batteries. ACS Appl. Energy Mater. 2022, 5, 12592–12601. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium Battery Chemistries Enabled by Solid-State Electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Heo, J.W.; Banerjee, A.; Park, K.H.; Jung, Y.S.; Hong, S. New Na-Ion Solid Electrolytes Na4−xSn1−xSbxS4 (0.02 ≤ x ≤ 0.33) for All-Solid-State Na-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702716. [Google Scholar] [CrossRef]
- Bieker, G.; Küpers, V.; Kolek, M.; Winter, M. Intrinsic Differences and Realistic Perspectives of Lithium-Sulfur and Magnesium-Sulfur Batteries. Commun. Mater. 2021, 2, 37. [Google Scholar] [CrossRef]
- Suci, W.G.; Aliwarga, H.K.; Azinuddin, Y.R.; Setyawati, R.B.; Stulasti, K.N.R.; Purwanto, A. Review of Various Sulfide Electrolyte Types for Solid-State Lithium-Ion Batteries. Open Eng. 2022, 12, 409–423. [Google Scholar] [CrossRef]
- Su, H.; Jiang, Z.; Liu, Y.; Li, J.; Gu, C.; Wang, X.; Xia, X.; Tu, J. Recent Progress of Sulfide Electrolytes for All-Solid-State Lithium Batteries. Energy Mater. 2022, 2, 200005. [Google Scholar] [CrossRef]
- Yu, T.; Ke, B.; Li, H.; Guo, S.; Zhou, H. Recent Advances in Sulfide Electrolytes toward High Specific Energy Solid-State Lithium Batteries. Mater. Chem. Front. 2021, 5, 4892–4911. [Google Scholar] [CrossRef]
- Kim, S.; Oguchi, H.; Toyama, N.; Sato, T.; Takagi, S.; Otomo, T.; Arunkumar, D.; Kuwata, N.; Kawamura, J.; Orimo, S. A Complex Hydride Lithium Superionic Conductor for High-Energy-Density All-Solid-State Lithium Metal Batteries. Nat. Commun. 2019, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Orimo, S. Lithium Fast-Ionic Conduction in Complex Hydrides: Review and Prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- Yan, Y.; Kühnel, R.; Remhof, A.; Duchêne, L.; Reyes, E.C.; Rentsch, D.; Łodziana, Z.; Battaglia, C. A Lithium Amide-Borohydride Solid-State Electrolyte with Lithium-Ion Conductivities Comparable to Liquid Electrolytes. Adv. Energy Mater. 2017, 7, 1700294. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Zhou, W.; Talin, A.A.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; et al. Liquid-Like Ionic Conduction in Solid Lithium and Sodium Monocarba-closo-Decaborates Near or at Room Temperature. Adv. Energy Mater. 2016, 6, 1502237, Correction in Adv. Energy Mater. 2016, 6, 1670139. [Google Scholar] [CrossRef]
- Sadikin, Y.; Brighi, M.; Schouwink, P.; Černý, R. Superionic Conduction of Sodium and Lithium in Anion-Mixed Hydroborates Na3BH4B12H12 and (Li0.7Na0.3)3BH4B12H12. Adv. Energy Mater. 2015, 5, 1501016. [Google Scholar] [CrossRef]
- Cuan, J.; Zhou, Y.; Zhou, T.; Ling, S.; Rui, K.; Guo, Z.; Liu, H.; Yu, X. Borohydride-Scaffolded Li/Na/Mg Fast Ionic Conductors for Promising Solid-State Electrolytes. Adv. Mater. 2018, 31, e1803533. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.S.; Yoshida, K.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Dimitrievska, M.; Stavila, V.; Orimo, S.; Udovic, T.J. Stabilizing Superionic-Conducting Structures via Mixed-Anion Solid Solutions of Monocarba-Closo-Borate Salts. ACS Energy Lett. 2016, 1, 659–664. [Google Scholar] [CrossRef]
- Andersson, M.S.; Stavila, V.; Skripov, A.V.; Dimitrievska, M.; Psurek, M.T.; Leão, J.B.; Babanova, O.A.; Skoryunov, R.V.; Soloninin, A.V.; Karlsson, M.; et al. Promoting Persistent Superionic Conductivity in Sodium Monocarba-Closo-Dodecaborate NaCB11H12 via Confinement within Nanoporous Silica. J. Phys. Chem. C 2021, 125, 16689–16699. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Unemoto, A.; Orimo, S.; Udovic, T.J. Stabilizing Lithium and Sodium Fast-Ion Conduction in Solid Polyhedral-Borate Salts at Device-Relevant Temperatures. Energy Storage Mater. 2016, 4, 79–83. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic Glass-Ceramic Electrolytes for Room-Temperature Rechargeable Sodium Batteries. Nat. Commun. 2012, 3, 856. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Yang, X.; Adair, K.R.; Wang, C.; Zhao, F.; Sun, X. Progress and Perspectives on Halide Lithium Conductors for All-Solid-State Lithium Batteries. Energy Environ. Sci. 2020, 13, 1429–1461. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Adair, K.R.; Sun, X. Metal Halide Superionic Conductors for All-Solid-State Batteries. Acc. Chem. Res. 2021, 54, 1023–1033. [Google Scholar] [CrossRef]
- Yu, T.; Yang, X.; Yang, R.; Bai, X.; Xu, G.; Zhao, S.; Duan, Y.; Wu, Y.; Wang, J. Progress and Perspectives on Typical Inorganic Solid-State Electrolytes. J. Alloys Compd. 2021, 885, 161013. [Google Scholar] [CrossRef]
- Wang, C.; Liang, J.; Luo, J.; Liu, J.; Li, X.; Zhao, F.; Li, R.; Huang, H.; Zhao, S.; Zhang, L.; et al. A Universal Wet-Chemistry Synthesis of Solid-State Halide Electrolytes for All-Solid-State Lithium-Metal Batteries. Sci. Adv. 2021, 7, eabh1896. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Yu, Z.; Bowden, M.; Miller, Q.R.S.; Chen, P.; Schaef, H.T.; Mueller, K.T.; Lu, D.; Xiao, J.; et al. Wet-Chemical Synthesis of Li7P3S11 with Tailored Particle Size for Solid State Electrolytes. Chem. Eng. J. 2022, 429, 132334. [Google Scholar] [CrossRef]
- Jo, Y.-S.; Hong, J.-W.; Choi, I.-H.; Sung, J.; Park, J.-H.; Park, H.; Kim, D.; Kim, B.G.; Ha, Y.-C.; Seo, J.; et al. Engineering Green and Sustainable Solvents for Scalable Wet Synthesis of Sulfide Electrolytes in High-Energy-Density All-Solid-State Batteries. Green Chem. 2023, 25, 1473–1487. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, W.; Zhu, X.; Ling, M.; Liang, C. Understanding the Synthesis of Inorganic Solid-State Electrolytes for Li Ion Batteries: Features and Progress. Sustain. Mater. Technol. 2022, 33, e00491. [Google Scholar] [CrossRef]
- Martinez, V.; Stolar, T.; Karadeniz, B.; Brekalo, I.; Užarević, K. Advancing Mechanochemical Synthesis by Combining Milling with Different Energy Sources. Nat. Rev. Chem. 2022, 7, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef] [PubMed]
- Beamish-Cook, J.; Shankland, K.; Murray, C.A.; Vaqueiro, P. Insights into the Mechanochemical Synthesis of MOF-74. Cryst. Growth Des. 2021, 21, 3047–3055. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2016, 3, 13–19. [Google Scholar] [CrossRef]
- Ayoub, G.; Karadeniz, B.; Howarth, A.J.; Farha, O.K.; Đilović, I.; Germann, L.S.; Dinnebier, R.E.; Užarević, K.; Friščić, T. Rational Synthesis of Mixed-Metal Microporous Metal–Organic Frameworks with Controlled Composition Using Mechanochemistry. Chem. Mater. 2019, 31, 5494–5501. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward Green Synthesis of Metal–Organic Frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Stolar, T.; Užarević, K. Mechanochemistry: An Efficient and Versatile Toolbox for Synthesis, Transformation, and Functionalization of Porous Metal–Organic Frameworks. CrystEngComm 2020, 22, 4511–4525. [Google Scholar] [CrossRef]
- Užarević, K.; Wang, T.C.; Moon, S.-Y.; Fidelli, A.M.; Hupp, J.T.; Farha, O.K.; Friščić, T. Mechanochemical and Solvent-Free Assembly of Zirconium-Based Metal–Organic Frameworks. Chem. Commun. 2016, 52, 2133–2136. [Google Scholar] [CrossRef]
- Pagola, S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals 2023, 13, 124. [Google Scholar] [CrossRef]
- Kim, J.W.; Shim, J.-H.; Ahn, J.-P.; Cho, Y.W.; Kim, J.-H.; Oh, K.H. Mechanochemical Synthesis and Characterization of TiB2 and VB2 Nanopowders. Mater. Lett. 2008, 62, 2461–2464. [Google Scholar] [CrossRef]
- Halasz, I.; Friščić, T.; Kimber, S.A.J.; Užarević, K.; Puškarić, A.; Mottillo, C.; Julien, P.; Štrukil, V.; Honkimäki, V.; Dinnebier, R.E. Quantitative in Situ and Real-Time Monitoring of Mechanochemical Reactions. Faraday Discuss. 2014, 170, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Halasz, I.; Puškarić, A.; Kimber, S.A.J.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Honkimäki, V.; Dinnebier, R.E.; Patel, B.; Jones, W.; et al. Real-Time In Situ Powder X-ray Diffraction Monitoring of Mechanochemical Synthesis of Pharmaceutical Cocrystals. Angew. Chem. Int. Ed. 2013, 52, 11538–11541. [Google Scholar] [CrossRef] [PubMed]
- Černý, R.; Penin, N.; Hagemann, H.; Filinchuk, Y. The First Crystallographic and Spectroscopic Characterization of a 3d-Metal Borohydride: Mn(BH4)2. J. Phys. Chem. C 2009, 113, 9003–9007, Correction in J. Phys. Chem. C 2009, 113, 14582. [Google Scholar] [CrossRef]
- Ravnsbæk, D.; Filinchuk, Y.; Cerenius, Y.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A Series of Mixed-Metal Borohydrides. Angew. Chem. Int. Ed. 2009, 48, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Llamas Jansa, I.; Kalantzopoulos, G.N.; Nordholm, K.; Hauback, B.C. Destabilization of NaBH4 by Transition Metal Fluorides. Molecules 2020, 25, 780. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.E.; Sørby, M.H.; Hauback, B.C. Chloride-Substitution in Sodium Borohydride. J. Alloys Compd. 2011, 509, L228–L231. [Google Scholar] [CrossRef]
- Doppiu, S.; Schultz, L.; Gutfleisch, O. In Situ Pressure and Temperature Monitoring during the Conversion of Mg into MgH2 by High-Pressure Reactive Ball Milling. J. Alloys Compd. 2007, 427, 204–208. [Google Scholar] [CrossRef]
- Fichtner, M.; Frommen, C.; Fuhr, O. Synthesis and Properties of Calcium Alanate and Two Solvent Adducts. Inorg. Chem. 2005, 44, 3479–3484. [Google Scholar] [CrossRef]
- Brinks, H.W.; Istad-Lem, A.; Hauback, B.C. Mechanochemical Synthesis and Crystal Structure of α’-AlD3 and α-AlD3. J. Phys. Chem. B 2006, 110, 25833–25837. [Google Scholar] [CrossRef]
- Kuziora, P.; Wyszyńska, M.; Polanski, M.; Bystrzycki, J. Why the Ball to Powder Ratio (BPR) Is Insufficient for Describing the Mechanical Ball Milling Process. Int. J. Hydrogen Energy 2014, 39, 9883–9887. [Google Scholar] [CrossRef]
- Sadikin, Y.; Stare, K.; Schouwink, P.; Brix Ley, M.; Jensen, T.R.; Meden, A.; Černý, R. Alkali Metal—Yttrium Borohydrides: The Link between Coordination of Small and Large Rare-Earth. J. Solid State Chem. 2015, 225, 231–239. [Google Scholar] [CrossRef]
- Černý, R.; Severa, G.; Ravnsbæk, D.B.; Filinchuk, Y.; D’Anna, V.; Hagemann, H.; Haase, D.; Jensen, C.M.; Jensen, T.R. NaSc(BH4)4: A Novel Scandium-Based Borohydride. J. Phys. Chem. C 2009, 114, 1357–1364. [Google Scholar] [CrossRef]
- Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Černý, R.; Penin, N.; Sørby, M.H.; Hauback, B.C.; Severa, G.; Jensen, C.M. LiSc(BH4)4: A Novel Salt of Li+ and Discrete Sc(BH4)4− Complex Anions. J. Phys. Chem. A 2008, 112, 7551–7555. [Google Scholar] [CrossRef] [PubMed]
- Solinas, I.; Lutz, H.D. Nonceramic Preparation Techniques for Ternary Halides AB2X4 with A = Mg, Mn, Zn; B = Li, Na; X = Cl, Br. J. Solid State Chem. 1995, 117, 34–38. [Google Scholar] [CrossRef]
- Černý, R.; Schouwink, P.; Sadikin, Y.; Stare, K.; Smrčok, L.; Richter, B.; Jensen, T.R. Trimetallic Borohydride Li3MZn5(BH4)15 (M = Mg, Mn) Containing Two Weakly Interconnected Frameworks. Inorg. Chem. 2013, 52, 9941–9947. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Sørensen, L.H.; Filinchuk, Y.; Reed, D.; Book, D.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. Mixed-Anion and Mixed-Cation Borohydride KZn(BH4)Cl2: Synthesis, Structure and Thermal Decomposition. Eur. J. Inorg. Chem. 2010, 2010, 1608–1612. [Google Scholar] [CrossRef]
- Černý, R.; Ravnsbæk, D.B.; Schouwink, P.; Filinchuk, Y.; Penin, N.; Teyssier, J.; Smrčok, L.; Jensen, T.R. Potassium Zinc Borohydrides Containing Triangular [Zn(BH4)3]− and Tetrahedral [Zn(BH4)xCl4–x]2– Anions. J. Phys. Chem. C 2011, 116, 1563–1571. [Google Scholar] [CrossRef]
- Rude, L.H.; Zavorotynska, O.; Arnbjerg, L.M.; Ravnsbæk, D.B.; Malmkjær, R.A.; Grove, H.; Hauback, B.C.; Baricco, M.; Filinchuk, Y.; Besenbacher, F.; et al. Bromide Substitution in Lithium Borohydride, LiBH4–LiBr. Int. J. Hydrogen Energy 2011, 36, 15664–15672. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Ley, M.B.; Lee, Y.-S.; Hagemann, H.; D’Anna, V.; Cho, Y.W.; Filinchuk, Y.; Jensen, T.R. A Mixed-Cation Mixed-Anion Borohydride NaY(BH4)2Cl2. Int. J. Hydrogen Energy 2012, 37, 8428–8438. [Google Scholar] [CrossRef]
- Ley, M.B.; Boulineau, S.; Janot, R.; Filinchuk, Y.; Jensen, T.R. New Li Ion Conductors and Solid State Hydrogen Storage Materials: LiM(BH4)3Cl, M = La, Gd. J. Phys. Chem. C 2012, 116, 21267–21276. [Google Scholar] [CrossRef]
- Matsuo, M.; Remhof, A.; Martelli, P.; Caputo, R.; Ernst, M.; Miura, Y.; Sato, T.; Oguchi, H.; Maekawa, H.; Takamura, H.; et al. Complex Hydrides with (BH4)− and (NH2)− Anions as New Lithium Fast-Ion Conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar] [CrossRef]
- Noritake, T.; Miwa, K.; Aoki, M.; Matsumoto, M.; Towata, S.; Li, H.-W.; Orimo, S. Synthesis and Crystal Structure Analysis of Complex Hydride Mg(BH4)(NH2). Int. J. Hydrogen Energy 2013, 38, 6730–6735. [Google Scholar] [CrossRef]
- Somer, M.; Acar, S.; Koz, C.; Kokal, I.; Höhn, P.; Cardoso-Gil, R.; Aydemir, U.; Akselrud, L. α- and β-Na2[BH4][NH2]: Two Modifications of a Complex Hydride in the System NaNH2–NaBH4; Syntheses, Crystal Structures, Thermal Analyses, Mass and Vibrational Spectra. J. Alloys Compd. 2010, 491, 98–105. [Google Scholar] [CrossRef]
- Tuan, L.; Nguyen, C.K.; Huan, T.D. First-Principles Prediction for the Stability of LiK(BH4)2. Phys. Status Solidi (B) 2014, 251, 1539–1544. [Google Scholar] [CrossRef]
- Jensen, S.R.H.; Jepsen, L.H.; Skibsted, J.; Jensen, T.R. Phase Diagram for the NaBH4–KBH4 System and the Stability of a Na1−xKxBH4 Solid Solution. J. Phys. Chem. C 2015, 119, 27919–27929. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Ravnsbæk, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical Synthesis of Hydrogen Storage Materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Burazer, S.; Morelle, F.; Filinchuk, Y.; Černý, R.; Popović, J. Mixed-Metal Imidazolates Containing Alkali and Alkaline Earth Metals: Mechanochemical Synthesis and Crystal Structure of AMgIm3 (A = Na or K). Inorg. Chem. 2019, 58, 6927–6933. [Google Scholar] [CrossRef] [PubMed]
- Burazer, S.; Robeyns, K.; Guénée, L.; Mali, G.; Morelle, F.; Ban, V.; Klaser, T.; Filinchuk, Y.; Černý, R.; Popović, J. Quenchable Porous High-Temperature Polymorph of Sodium Imidazolate, NaIm. Cryst. Growth Des. 2021, 21, 770–778. [Google Scholar] [CrossRef]
- Burazer, S.; Horák, L.; Filinchuk, Y.; Černý, R.; Popović, J. Abrupt Change from Moderate Positive to Colossal Negative Thermal Expansion Caused by Imidazolate Composite Formation. J. Mater. Sci. 2022, 57, 11563–11581. [Google Scholar] [CrossRef]
- Fernandez-Diaz, L.; Castillo, J.; Sasieta-Barrutia, E.; Arnaiz, M.; Cabello, M.; Judez, X.; Terry, A.; Otaegui, L.; Morant-Miñana, M.C.; Villaverde, A. Mixing Methods for Solid State Electrodes: Techniques, Fundamentals, Recent Advances, and Perspectives. Chem. Eng. J. 2023, 464, 142469. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Y.; Zhang, H.; Liu, Z.; Zhang, W.; Li, L.; Qiao, Y.; Yang, W.; Wang, J.; Dou, S.; et al. Ball Milling Solid-State Synthesis of Highly Crystalline Prussian Blue Analogue Na2−xMnFe(CN)6 Cathodes for All-Climate Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202205867. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Xu, D.; Wang, Q.; Liu, P. Highly Stretchable Electromagnetic Interference (EMI) Shielding Segregated Polyurethane/Carbon Nanotube Composites Fabricated by Microwave Selective Sintering. J. Mater. Chem. C 2019, 7, 7938–7946. [Google Scholar] [CrossRef]
- Sandu, G.; Ernould, B.; Rolland, J.; Cheminet, N.; Brassinne, J.; Das, P.R.; Filinchuk, Y.; Cheng, L.; Komsiyska, L.; Dubois, P.; et al. Mechanochemical Synthesis of PEDOT:PSS Hydrogels for Aqueous Formulation of Li-Ion Battery Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 34865–34874. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cha, H.; Kostecki, R.; Chen, G. Composite Cathode Design for High-Energy All-Solid-State Lithium Batteries with Long Cycle Life. ACS Energy Lett. 2022, 8, 521–528. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, W.; Bai, C.G.; Luo, X.D.; Pan, F.S. Mechanochemical Synthesis of Nanocrystalline Mg-Based Hydrogen Storage Composites in Hydrogen Alloying Mills. Mater. Sci. Forum 2009, 610–613, 955–959. [Google Scholar] [CrossRef]
- Sakuda, A. Favorable Composite Electrodes for All-Solid-State Batteries. J. Ceram. Soc. Jpn. 2018, 126, 675–683. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Mofa, N.N.; Ketegenov, T.A.; Sadykov, B.S. Mechanochemical Synthesis of Composite Materials; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Zherebtcov, I.S.; Osadchy, A.V.; Savin, V.V.; Savina, L.A.; Chaika, V.A. Synthesis of Powder Functional Composite Materials Containing G-C3N4, β-Si3N4 and Si2N2O Phases. J. Phys. Conf. Ser. 2022, 2388, 012013. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, X.; Zhao, J.; Liu, G.; Yu, W.; Dong, X.; Wang, J. Review on Composite Solid Electrolytes for Solid-State Lithium-Ion Batteries. Mater. Today Sustain. 2023, 21, 100316. [Google Scholar] [CrossRef]
- Gulino, V.; Longo, A.; de Kort, L.M.; Rodenburg, H.P.; Murgia, F.; Brighi, M.; Černý, R.; Sahle, C.J.; Sundermann, M.; Gretarsson, H.; et al. Anomalous Impact of Mechanochemical Treatment on the Na-ion Conductivity of Sodium Closo-Carbadodecaborate Probed by X-Ray Raman Scattering Spectroscopy. Small Methods 2023, 8, e2300833. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, L.; Wang, A.; Song, Y.; Wu, Y.; Yang, Y.; He, X. Tailoring Practically Accessible Polymer/Inorganic Composite Electrolytes for All-Solid-State Lithium Metal Batteries: A Review. Nano-Micro Lett. 2023, 15, 42. [Google Scholar] [CrossRef]
- Chien, Y.; Li, H.; Lampkin, J.; Hall, S.; Garcia-Araez, N.; Brant, W.R.; Brandell, D.; Lacey, M.J. Impact of Compression on the Electrochemical Performance of the Sulfur/Carbon Composite Electrode in Lithium-Sulfur Batteries. Batter. Supercaps 2022, 5, e202200058. [Google Scholar] [CrossRef]
- Kubanska, A.; Castro, L.; Tortet, L.; Dollé, M.; Bouchet, R. Effect of Composite Electrode Thickness on the Electrochemical Performances of All-Solid-State Li-Ion Batteries. J. Electroceramics 2017, 38, 189–196. [Google Scholar] [CrossRef]
- Tran, H.Y.; Greco, G.; Täubert, C.; Wohlfahrt-Mehrens, M.; Haselrieder, W.; Kwade, A. Influence of Electrode Preparation on the Electrochemical Performance of LiNi0.8Co0.15Al0.05O2 Composite Electrodes for Lithium-Ion Batteries. J. Power Sources 2012, 210, 276–285. [Google Scholar] [CrossRef]
- Gonzalez, G.; Gonzalez, R.; Zuniga, L.; Chipara, M.; Alcoutlabi, M. The Effect of Carbon Coatings on the Electrochemical Performance of Composite Electrodes. ECS Trans. 2020, 97, 93–104. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, J.; Ye, C.; Liu, M.; Wang, C.; Wu, S.; Tong, Q.; Zhu, M.; Gao, F. Strategies for Controlling or Releasing the Influence Due to the Volume Expansion of Silicon inside Si–C Composite Anode for High-Performance Lithium-Ion Batteries. Materials 2022, 15, 4264. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, M.; Terauchi, Y.; Kobayashi, Y.; Ikeda, S.; Sakuda, A. Fabrication of Composite Positive Electrode Sheet with High Active Material Content and Effect of Fabrication Pressure for All-Solid-State Battery. J. Ceram. Soc. Jpn. 2017, 125, 391–395. [Google Scholar] [CrossRef]
- Burazer, S.; Sopčić, S.; Mandić, Z. Anodic Deposition of Lead Dioxide at Nafion® Covered Gold Electrode. J. Solid State Electrochem. 2016, 20, 3053–3059. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, H.; Kim, I.T.; Choi, W.; Hur, J. Few-Layer NbSe2@graphene Heterostructures as Anodes in Lithium-Ion Half- and Full-Cell Batteries. Chem. Eng. J. 2020, 382, 122981. [Google Scholar] [CrossRef]
- Université de Genève (UNIGE). Un Nouvel Électrolyte pour des Piles Plus sûres/A New Electrolyte for Greener and Safer Batteries. Available online: https://www.youtube.com/watch?v=xFbNXayYMSE (accessed on 16 November 2023).
- Wang, L.; Li, J.; Lu, G.; Li, W.; Tao, Q.; Shi, C.; Jin, H.; Chen, G.; Wang, S. Fundamentals of Electrolytes for Solid-State Batteries: Challenges and Perspectives. Front. Mater. 2020, 7, 111. [Google Scholar] [CrossRef]
- Yang, H.-L.; Zhang, B.-W.; Konstantinov, K.; Wang, Y.-X.; Liu, H.-K.; Dou, S.-X. Progress and Challenges for All-Solid-State Sodium Batteries. Adv. Energy Sustain. Res. 2021, 2, 2000057. [Google Scholar] [CrossRef]
- Chen, W.; Lei, T.; Wu, C.; Deng, M.; Gong, C.; Hu, K.; Ma, Y.; Dai, L.; Lv, W.; He, W.; et al. Designing Safe Electrolyte Systems for a High-Stability Lithium–Sulfur Battery. Adv. Energy Mater. 2018, 8, 1702348. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuo, S.; Li, Z.; Wang, C. Redox Polymers for Rechargeable Metal-Ion Batteries. EnergyChem 2020, 2, 100030. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Li, W.; Dolocan, A.; Manthiram, A. Interfacial Chemistry in Solid-State Batteries: Formation of Interphase and Its Consequences. J. Am. Chem. Soc. 2017, 140, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Rosero-Navarro, N.C.; Sakuda, A.; Tadanaga, K.; Phuc, N.H.H.; Matsuda, A.; Machida, N.; Hayashi, A.; Tatsumisago, M. Liquid-Phase Syntheses of Sulfide Electrolytes for All-Solid-State Lithium Battery. Nat. Rev. Chem. 2019, 3, 189–198. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating Interfacial Impedance in Garnet-Based Solid-State Li Metal Batteries. Nat. Mater. 2016, 16, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Review—Practical Challenges Hindering the Development of Solid State Li Ion Batteries. J. Electrochem. Soc. 2017, 164, A1731–A1744. [Google Scholar] [CrossRef]
- Strauss, F.; Bartsch, T.; de Biasi, L.; Kim, A.-Y.; Janek, J.; Hartmann, P.; Brezesinski, T. Impact of Cathode Material Particle Size on the Capacity of Bulk-Type All-Solid-State Batteries. ACS Energy Lett. 2018, 3, 992–996. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing Solid-State Electrolytes for Safe, Energy-Dense Batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2015, 116, 140–162. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Huggins, R.A. Lithium-Ion Conductivity in Lithium Nitride. Phys. Lett. A 1976, 58, 231–233. [Google Scholar] [CrossRef]
- Zhao, Y.; Daemen, L.L. Superionic Conductivity in Lithium-Rich Anti-Perovskites. J. Am. Chem. Soc. 2012, 134, 15042–15047. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on Solid Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Hong, H.Y.-P. Crystal Structure and Ionic Conductivity of Li14Zn(GeO4)4 and Other New Li+ Superionic Conductors. Mater. Res. Bull. 1978, 13, 117–124. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Gruzalski, G.R.; Zuhr, R.A.; Choudhury, A.; Luck, C.F.; Robertson, J.D. Fabrication and Characterization of Amorphous Lithium Electrolyte Thin Films and Rechargeable Thin-Film Batteries. J. Power Sources 1993, 43, 103–110. [Google Scholar] [CrossRef]
- He, L.; Lin, H.; Li, H.-F.; Filinchuk, Y.; Zhang, J.; Liu, Y.; Yang, M.; Hou, Y.; Deng, Y.; Li, H.-W.; et al. Na3NH2B12H12 as High Performance Solid Electrolyte for All-Solid-State Na-Ion Batteries. J. Power Sources 2018, 396, 574–579. [Google Scholar] [CrossRef]
- Ohno, S.; Zeier, W.G. Sodium Is the New Lithium. Nat. Energy 2022, 7, 686–687. [Google Scholar] [CrossRef]
- Pross-Brakhage, J.; Fitz, O.; Bischoff, C.; Biro, D.; Birke, K.P. Post-Lithium Batteries with Zinc for the Energy Transition. Batteries 2023, 9, 367. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Y.; Lotsch, B.; Hu, Y.-S.; Li, H.; Janek, J.; Nazar, L.F.; Nan, C.-W.; Maier, J.; Armand, M.; et al. New Horizons for Inorganic Solid State Ion Conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, Y.; Hao, F.; Kmiec, S.; Dong, H.; Xu, R.; Zhao, K.; Ai, Q.; Terlier, T.; Wang, L.; et al. An Electrochemically Stable Homogeneous Glassy Electrolyte Formed at Room Temperature for All-Solid-State Sodium Batteries. Nat. Commun. 2022, 13, 2854. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Le, P.M.L.; Gao, P.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Cao, X.; Vo, T.D.; Hu, J.; Zhong, L.; et al. Low-Solvation Electrolytes for High-Voltage Sodium-Ion Batteries. Nat. Energy 2022, 7, 718–725. [Google Scholar] [CrossRef]
- Hayashi, A.; Masuzawa, N.; Yubuchi, S.; Tsuji, F.; Hotehama, C.; Sakuda, A.; Tatsumisago, M. A Sodium-Ion Sulfide Solid Electrolyte with Unprecedented Conductivity at Room Temperature. Nat. Commun. 2019, 10, 5266. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Q.; Han, M.; Qi, X.; Li, B.; Zhang, Q.; Zhao, J.; Yang, C.; Liu, H.; Hu, Y.-S. Rapid Mechanochemical Synthesis of Polyanionic Cathode with Improved Electrochemical Performance for Na-Ion Batteries. Nat. Commun. 2021, 12, 2848. [Google Scholar] [CrossRef] [PubMed]
- Ledwoch, D.; Robinson, J.B.; Gastol, D.; Smith, K.; Shearing, P.R.; Brett, D.J.L.; Kendrick, E. Hard Carbon Composite Electrodes for Sodium-Ion Batteries with Nano-Zeolite and Carbon Black Additives. Batter. Supercaps 2020, 4, 163–172. [Google Scholar] [CrossRef]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Yu, H.; Lu, J. Solid Electrolytes and Interfaces in All-Solid-State Sodium Batteries: Progress and Perspective. Nano Energy 2018, 52, 279–291. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.-P.; Kafalas, J.A. Fast Na+-Ion Transport in Skeleton Structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Hao, F.; Chi, X.; Liang, Y.; Zhang, Y.; Xu, R.; Guo, H.; Terlier, T.; Dong, H.; Zhao, K.; Lou, J.; et al. Taming Active Material-Solid Electrolyte Interfaces with Organic Cathode for All-Solid-State Batteries. Joule 2019, 3, 1349–1359. [Google Scholar] [CrossRef]
- Wu, E.A.; Banerjee, S.; Tang, H.; Richardson, P.M.; Doux, J.-M.; Qi, J.; Zhu, Z.; Grenier, A.; Li, Y.; Zhao, E.; et al. A Stable Cathode-Solid Electrolyte Composite for High-Voltage, Long-Cycle-Life Solid-State Sodium-Ion Batteries. Nat. Commun. 2021, 12, 1256. [Google Scholar] [CrossRef]
- Matios, E.; Wang, H.; Wang, C.; Hu, X.; Lu, X.; Luo, J.; Li, W. Graphene Regulated Ceramic Electrolyte for Solid-State Sodium Metal Battery with Superior Electrochemical Stability. ACS Appl. Mater. Interfaces 2019, 11, 5064–5072. [Google Scholar] [CrossRef]
- Ruan, Y.; Guo, F.; Liu, J.; Song, S.; Jiang, N.; Cheng, B. Optimization of Na3Zr2Si2PO12 Ceramic Electrolyte and Interface for High Performance Solid-State Sodium Battery. Ceram. Int. 2019, 45, 1770–1776. [Google Scholar] [CrossRef]
- Karabelli, D.; Birke, K.P.; Weeber, M. A Performance and Cost Overview of Selected Solid-State Electrolytes: Race between Polymer Electrolytes and Inorganic Sulfide Electrolytes. Batteries 2021, 7, 18. [Google Scholar] [CrossRef]
- Duchêne, L.; Remhof, A.; Hagemann, H.; Battaglia, C. Status and Prospects of Hydroborate Electrolytes for All-Solid-State Batteries. Energy Storage Mater. 2020, 25, 782–794. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond. Adv. Mater. 2020, 33, 2000721. [Google Scholar] [CrossRef]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium Superionic Conduction in Lithium Borohydride Accompanied by Structural Transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Yoshida, K.; Unemoto, A.; Oguchi, H.; Orimo, S. Complex Hydride as a Novel Solid Electrolyte and Its Application to an All-Solid-State Battery. Mater. Jpn. 2017, 56, 448–452. [Google Scholar] [CrossRef]
- Paskevicius, M.; Pitt, M.P.; Brown, D.H.; Sheppard, D.A.; Chumphongphan, S.; Buckley, C.E. First-Order Phase Transition in the Li2B12H12 System. Phys. Chem. Chem. Phys. 2013, 15, 15825. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Tang, W.S.; Wu, H.; Stavila, V.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Rush, J.J.; et al. Exceptional Superionic Conductivity in Disordered Sodium Decahydro-closo-decaborate. Adv. Mater. 2014, 26, 7622–7626. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Rush, J.J.; Takamura, H.; Orimo, S. Sodium Superionic Conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750–3752. [Google Scholar] [CrossRef]
- He, L.; Li, H.-W.; Nakajima, H.; Tumanov, N.; Filinchuk, Y.; Hwang, S.-J.; Sharma, M.; Hagemann, H.; Akiba, E. Synthesis of a Bimetallic Dodecaborate LiNaB12H12 with Outstanding Superionic Conductivity. Chem. Mater. 2015, 27, 5483–5486. [Google Scholar] [CrossRef]
- Tang, W.S.; Udovic, T.J.; Stavila, V. Altering the Structural Properties of A2B12H12 Compounds via Cation and Anion Modifications. J. Alloys Compd. 2015, 645, S200–S204. [Google Scholar] [CrossRef]
- Tang, W.S.; Unemoto, A.; Zhou, W.; Stavila, V.; Matsuo, M.; Wu, H.; Orimo, S.; Udovic, T.J. Unparalleled Lithium and Sodium Superionic Conduction in Solid Electrolytes with Large Monovalent Cage-like Anions. Energy Environ. Sci. 2015, 8, 3637–3645. [Google Scholar] [CrossRef]
- de Kort, L.M.; Gulino, V.; de Jongh, P.E.; Ngene, P. Ionic Conductivity in Complex Metal Hydride-Based Nanocomposite Materials: The Impact of Nanostructuring and Nanocomposite Formation. J. Alloys Compd. 2022, 901, 163474. [Google Scholar] [CrossRef]
- Hirose, T.; Mishina, T.; Matsui, N.; Suzuki, K.; Saito, T.; Kamiyama, T.; Hirayama, M.; Kanno, R. Fast Hydride-Ion Conduction in Perovskite Hydrides AELiH3. ACS Appl. Energy Mater. 2022, 5, 2968–2974. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, T.; Unemoto, A.; Matsuo, M.; Ikeshoji, T.; Udovic, T.J.; Orimo, S. Fast Sodium Ionic Conduction in Na2B10H10-Na2B12H12 Pseudo-Binary Complex Hydride and Application to a Bulk-Type All-Solid-State Battery. Appl. Phys. Lett. 2017, 110, 103901. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Y.; Yang, J.; Zheng, S.; Wang, C. Hydrides for Solid-State Batteries: A Review. Mater. Today Nano 2022, 18, 100194. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, W.; Lei, B.; Liu, H.; Li, W. Recent Development of Mg Ion Solid Electrolyte. Front. Chem. 2020, 8, 125. [Google Scholar] [CrossRef]
- Payandeh, S.; Remhof, A.; Battaglia, C. CHAPTER 3. Solid-State Magnesium-Ion Conductors. In Magnesium Batteries Series; Fichtner, M., Ed.; Royal Society of Chemistry Books: London, UK, 2019; pp. 60–78. [Google Scholar] [CrossRef]
- Mohtadi, R.; Tutusaus, O.; Arthur, T.S.; Zhao-Karger, Z.; Fichtner, M. The Metamorphosis of Rechargeable Magnesium Batteries. Joule 2021, 5, 581–617. [Google Scholar] [CrossRef]
- Gregory, T.D.; Hoffman, R.J.; Winterton, R.C. Nonaqueous Electrochemistry of Magnesium: Applications to Energy Storage. J. Electrochem. Soc. 1990, 137, 775–780. [Google Scholar] [CrossRef]
- Aurbach, D.; Cohen, Y.; Moshkovich, M. The Study of Reversible Magnesium Deposition by In Situ Scanning Tunneling Microscopy. Electrochem. Solid-State Lett. 2001, 4, A113. [Google Scholar] [CrossRef]
- Davidson, R.; Verma, A.; Santos, D.; Hao, F.; Fincher, C.; Xiang, S.; Van Buskirk, J.; Xie, K.; Pharr, M.; Mukherjee, P.P.; et al. Formation of Magnesium Dendrites during Electrodeposition. ACS Energy Lett. 2018, 4, 375–376. [Google Scholar] [CrossRef]
- Jäckle, M.; Groß, A. Microscopic Properties of Lithium, Sodium, and Magnesium Battery Anode Materials Related to Possible Dendrite Growth. J. Chem. Phys. 2014, 141, 174710. [Google Scholar] [CrossRef]
- Mohtadi, R.; Matsui, M.; Arthur, T.S.; Hwang, S. Magnesium Borohydride: From Hydrogen Storage to Magnesium Battery. Angew. Chem. Int. Ed. 2012, 51, 9780–9783. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Gil Bardaji, M.E.; Fuhr, O.; Fichtner, M. A New Class of Non-Corrosive, Highly Efficient Electrolytes for Rechargeable Magnesium Batteries. J. Mater. Chem. A 2017, 5, 10815–10820. [Google Scholar] [CrossRef]
- Bockris, J.O.; Reddy, A.K.N. Modern Electrochemistry 1; Kluwer Academic Publishers: New York, NY, USA; Boston, MS, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 1998. [Google Scholar] [CrossRef]
- Takada, K. Progress and Prospective of Solid-State Lithium Batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Xu, Y.; Zhang, G.; Li, S.; Li, C.; Zhang, X.; Sun, X.; Ge, X.; Ma, Y. Strategies to Boost Ionic Conductivity and Interface Compatibility of Inorganic—Organic Solid Composite Electrolytes. Energy Storage Mater. 2021, 36, 291–308. [Google Scholar] [CrossRef]
- Park, H.; Le Mong, A.; Kim, D. Single and Multilayer Composite Electrolytes for Enhanced Li-Ion Conductivity with Restricted Polysulfide Diffusion for Lithium–Sulfur Battery. Mater. Today Energy 2023, 33, 101274. [Google Scholar] [CrossRef]
- Hiraoka, K.; Kato, M.; Kobayashi, T.; Seki, S. Polyether/Na3Zr2Si2PO12 Composite Solid Electrolytes for All-Solid-State Sodium Batteries. J. Phys. Chem. C 2020, 124, 21948–21956. [Google Scholar] [CrossRef]
- Niu, W.; Chen, L.; Liu, Y.; Fan, L.-Z. All-Solid-State Sodium Batteries Enabled by Flexible Composite Electrolytes and Plastic-Crystal Interphase. Chem. Eng. J. 2020, 384, 123233. [Google Scholar] [CrossRef]
- Lim, H.-S.; Liu, L.; Lee, H.-J.; Cha, J.-M.; Yoon, D.; Ryu, B.-K. The Study on the Interface Characteristics of Solid-State Electrolyte. J. Korean Ceram. Soc. 2021, 58, 373–377. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133. [Google Scholar] [CrossRef]
- He, X. Hypes and Hopes of Solid-State Batteries. Available online: https://www.idtechex.com/en/research-article/hypes-and-hopes-of-solid-state-batteries/28621 (accessed on 16 November 2023).
- Ma, Y.; Wan, J.; Xu, X.; Sendek, A.D.; Holmes, S.E.; Ransom, B.; Jiang, Z.; Zhang, P.; Xiao, X.; Zhang, W.; et al. Experimental Discovery of a Fast and Stable Lithium Thioborate Solid Electrolyte, Li6+2x[B10S18]Sx (x ≈ 1). ACS Energy Lett. 2023, 8, 2762–2771. [Google Scholar] [CrossRef]
- Hofer, M.; Grube, M.; Burmeister, C.F.; Michalowski, P.; Zellmer, S.; Kwade, A. Effective Mechanochemical Synthesis of Sulfide Solid Electrolyte Li3PS4 in a High Energy Ball Mill by Process Investigation. Adv. Powder Technol. 2023, 34, 104004. [Google Scholar] [CrossRef]
- Banik, A.; Famprikis, T.; Ghidiu, M.; Ohno, S.; Kraft, M.A.; Zeier, W.G. On the Underestimated Influence of Synthetic Conditions in Solid Ionic Conductors. Chem. Sci. 2021, 12, 6238–6263. [Google Scholar] [CrossRef]
- Liu, X.; Kang, W.; Li, X.; Zeng, L.; Li, Y.; Wang, Q.; Zhang, C. Solid-State Mechanochemistry Advancing Two Dimensional Materials for Lithium-Ion Storage Applications: A Mini Review. Nano Mater. Sci. 2023, 5, 210–227. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Adair, K.R.; Li, W.; Goubitz, K.; Zhao, Y.; Willans, M.J.; Thijs, M.A.; Wang, C.; Zhao, F.; et al. Tuning Ionic Conductivity and Electrode Compatibility of Li3YBr6 for High-Performance All Solid-State Li Batteries. Nano Energy 2020, 77, 105097. [Google Scholar] [CrossRef]
- Schlem, R.; Muy, S.; Prinz, N.; Banik, A.; Shao-Horn, Y.; Zobel, M.; Zeier, W.G. Mechanochemical Synthesis: A Tool to Tune Cation Site Disorder and Ionic Transport Properties of Li3MCl6 (M = Y, Er) Superionic Conductors. Adv. Energy Mater. 2019, 10, 1903719. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.-M.; Viallet, V. Mechanochemical Synthesis of Li-Argyrodite Li6PS5X (X = Cl, Br, I) as Sulfur-Based Solid Electrolytes for All Solid State Batteries Application. Solid State Ion. 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid Halide Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef]
- Ohno, S.; Koerver, R.; Dewald, G.; Rosenbach, C.; Titscher, P.; Steckermeier, D.; Kwade, A.; Janek, J.; Zeier, W.G. Observation of Chemomechanical Failure and the Influence of Cutoff Potentials in All-Solid-State Li–S Batteries. Chem. Mater. 2019, 31, 2930–2940. [Google Scholar] [CrossRef]
- Ning, L.J.; Wu, Y.P.; Fang, S.B.; Rahm, E.; Holze, R. Materials Prepared for Lithium Ion Batteries by Mechanochemical Methods. J. Power Sources 2004, 133, 229–242. [Google Scholar] [CrossRef]
- Kwak, H.; Han, D.; Lyoo, J.; Park, J.; Jung, S.H.; Han, Y.; Kwon, G.; Kim, H.; Hong, S.; Nam, K.; et al. New Cost-Effective Halide Solid Electrolytes for All-Solid-State Batteries: Mechanochemically Prepared Fe3+-Substituted Li2ZrCl6. Adv. Energy Mater. 2021, 11, 2003190. [Google Scholar] [CrossRef]
- Rao, R.P.; Zhang, X.; Phuah, K.C.; Adams, S. Mechanochemical Synthesis of Fast Sodium Ion Conductor Na11Sn2PSe12 enables First Sodium–Selenium All-Solid-State Battery. J. Mater. Chem. A 2019, 7, 20790–20798. [Google Scholar] [CrossRef]
- Roos, A.; Schoonman, J. Electronic Conductivity in La1−xBaxF3−x Crystals. Solid State Ion. 1984, 13, 205–211. [Google Scholar] [CrossRef]
- Gschwind, F.; Rodriguez-Garcia, G.; Sandbeck, D.J.S.; Gross, A.; Weil, M.; Fichtner, M.; Hörmann, N. Fluoride Ion Batteries: Theoretical Performance, Safety, Toxicity, and a Combinatorial Screening of New Electrodes. J. Fluor. Chem. 2016, 182, 76–90. [Google Scholar] [CrossRef]
- Ruprecht, B.; Wilkening, M.; Feldhoff, A.; Steuernagel, S.; Heitjans, P. High Anion Conductivity in a Ternary Non-Equilibrium Phase of BaF2 and CaF2 with Mixed Cations. Phys. Chem. Chem. Phys. 2009, 11, 3071. [Google Scholar] [CrossRef]
- Molaiyan, P.; Witter, R. Mechanochemical Synthesis of Solid-State Electrolyte Sm1−xCaxF3−x for Batteries and Other Electrochemical Devices. Mater. Lett. 2019, 244, 22–26. [Google Scholar] [CrossRef]
- Yamanaka, T.; Hayashi, A.; Yamauchi, A.; Tatsumisago, M. Preparation of Magnesium Ion Conducting MgS–P2S5–MgI2 Glasses by a Mechanochemical Technique. Solid State Ion. 2014, 262, 601–603. [Google Scholar] [CrossRef]
- Song, S.; Sheptyakov, D.; Korsunsky, A.M.; Duong, H.M.; Lu, L. High Li Ion Conductivity in a Garnet-Type Solid Electrolyte via Unusual Site Occupation of the Doping Ca Ions. Mater. Des. 2016, 93, 232–237. [Google Scholar] [CrossRef]
- Ghidiu, M.; Ruhl, J.; Culver, S.P.; Zeier, W.G. Solution-Based Synthesis of Lithium Thiophosphate Superionic Conductors for Solid-State Batteries: A Chemistry Perspective. J. Mater. Chem. A 2019, 7, 17735–17753. [Google Scholar] [CrossRef]
- Yoshida, K.; Suzuki, S.; Kawaji, J.; Unemoto, A.; Orimo, S. Complex Hydride for Composite Negative Electrode—Applicable to Bulk-Type All-Solid-State Li-Ion Battery with Wide Temperature Operation. Solid State Ion. 2016, 285, 96–100. [Google Scholar] [CrossRef]
- Unemoto, A.; Matsuo, M.; Orimo, S. Complex Hydrides for Electrochemical Energy Storage. Adv. Funct. Mater. 2014, 24, 2267–2279. [Google Scholar] [CrossRef]
- Duchêne, L.; Kühnel, R.-S.; Stilp, E.; Cuervo Reyes, E.; Remhof, A.; Hagemann, H.; Battaglia, C. A Stable 3 V All-Solid-State Sodium–Ion Battery Based on a Closo-Borate Electrolyte. Energy Environ. Sci. 2017, 10, 2609–2615. [Google Scholar] [CrossRef]
- Sau, K.; Ikeshoji, T.; Kim, S.; Takagi, S.; Orimo, S. Comparative Molecular Dynamics Study of the Roles of Anion–Cation and Cation–Cation Correlation in Cation Diffusion in Li2B12H12 and LiCB11H12. Chem. Mater. 2021, 33, 2357–2369. [Google Scholar] [CrossRef]
- Murgia, F.; Brighi, M.; Černý, R. Room-Temperature-Operating Na Solid-State Battery with Complex Hydride as Electrolyte. Electrochem. Commun. 2019, 106, 106534. [Google Scholar] [CrossRef]
- Brighi, M.; Murgia, F.; Černý, R. Closo-Hydroborate Sodium Salts as an Emerging Class of Room-Temperature Solid Electrolytes. Cell Rep. Phys. Sci. 2020, 1, 100217. [Google Scholar] [CrossRef]
- Li, H.-W.; Yan, Y.; Orimo, S.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Jena, P. Superhalogens: A Bridge between Complex Metal Hydrides and Li Ion Batteries. J. Phys. Chem. Lett. 2015, 6, 1119–1125. [Google Scholar] [CrossRef]
- Brighi, M.; Murgia, F.; Łodziana, Z.; Schouwink, P.; Wołczyk, A.; Cerny, R. A Mixed Anion Hydroborate/Carba-Hydroborate as a Room Temperature Na-Ion Solid Electrolyte. J. Power Sources 2018, 404, 7–12. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Ohba, N.; Miwa, K.; Aoki, M.; Towata, S.; Züttel, A. Experimental Studies on Intermediate Compound of LiBH4. Appl. Phys. Lett. 2006, 89, 021920. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and Rehydriding Processes of Well-Crystallized Mg(BH4)2 Accompanying with Formation of Intermediate Compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman, R.C.; Reiter, J.W.; Rijssenbeek; Soloveichik, G.L.; Zhao, J.-C.; Kabbour, H.; Ahn, C.C. NMR Confirmation for Formation of [B12H12]2− Complexes during Hydrogen Desorption from Metal Borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Friedrichs, O.; Remhof, A.; Hwang, S.-J.; Züttel, A. Role of Li2B12H12 for the Formation and Decomposition of LiBH4. Chem. Mater. 2010, 22, 3265–3268. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-Principles Prediction of Thermodynamically Reversible Hydrogen Storage Reactions in the Li-Mg-Ca-B-H System. J. Am. Chem. Soc. 2008, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a New Hydrogen Storage Material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species. Angew. Chem. Int. Ed. 2011, 50, 11162–11166. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, Ľ.; Černý, R. Structure and Properties of Complex Hydride Perovskite Materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Paskevicius, M.; Brown, D.H.; Sheppard, D.A.; Buckley, C.E. Thermal Stability of Li2B12H12 and Its Role in the Decomposition of LiBH4. J. Am. Chem. Soc. 2013, 135, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Hueso, K.B.; Armand, M.; Rojo, T. High Temperature Sodium Batteries: Status, Challenges and Future Trends. Energy Environ. Sci. 2013, 6, 734. [Google Scholar] [CrossRef]
- Jørgensen, M.; Jensen, S.R.H.; Humphries, T.D.; Rowles, M.R.; Sofianos, M.V.; Buckley, C.E.; Jensen, T.R.; Paskevicius, M. Hydroxylated Closo-Dodecaborates M2B12(OH)12 (M = Li, Na, K, and Cs); Structural Analysis, Thermal Properties, and Solid-State Ionic Conductivity. J. Phys. Chem. C 2020, 124, 11340–11349. [Google Scholar] [CrossRef]
- Li, H.-W.; Orimo, S.; Nakamori, Y.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Materials Designing of Metal Borohydrides: Viewpoints from Thermodynamical Stabilities. J. Alloys Compd. 2007, 446–447, 315–318. [Google Scholar] [CrossRef]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the Decomposition Temperature in Complex Hydrides: Synthesis of a Mixed Alkali Metal Borohydride. Angew. Chem. Int. Ed. 2008, 47, 2817–2819. [Google Scholar] [CrossRef]
- Ley, M.B.; Ravnsbæk, D.B.; Filinchuk, Y.; Lee, Y.-S.; Janot, R.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Roedern, E.; Kühnel, R.-S.; Remhof, A.; Battaglia, C. Magnesium Ethylenediamine Borohydride as Solid-State Electrolyte for Magnesium Batteries. Sci. Rep. 2017, 7, 46189. [Google Scholar] [CrossRef]
- Amdisen, M.B.; Grinderslev, J.B.; Skov, L.N.; Jensen, T.R. Methylamine Magnesium Borohydrides as Electrolytes for All-Solid-State Magnesium Batteries. Chem. Mater. 2023, 35, 1440–1448. [Google Scholar] [CrossRef]
- Higashi, S.; Miwa, K.; Aoki, M.; Takechi, K. A Novel Inorganic Solid State Ion Conductor for Rechargeable Mg Batteries. Chem. Commun. 2014, 50, 1320–1322. [Google Scholar] [CrossRef]
- Le Ruyet, R.; Fleutot, B.; Berthelot, R.; Benabed, Y.; Hautier, G.; Filinchuk, Y.; Janot, R. Mg3(BH4)4(NH2)2 as Inorganic Solid Electrolyte with High Mg2+ Ionic Conductivity. ACS Appl. Energy Mater. 2020, 3, 6093–6097. [Google Scholar] [CrossRef]
- Yan, Y.; Dononelli, W.; Jørgensen, M.; Grinderslev, J.B.; Lee, Y.-S.; Cho, Y.W.; Černý, R.; Hammer, B.; Jensen, T.R. The Mechanism of Mg2+ Conduction in Ammine Magnesium Borohydride Promoted by a Neutral Molecule. Phys. Chem. Chem. Phys. 2020, 22, 9204–9209. [Google Scholar] [CrossRef]
- Filippov, S.; Grinderslev, J.B.; Andersson, M.S.; Armstrong, J.; Karlsson, M.; Jensen, T.R.; Klarbring, J.; Simak, S.I.; Häussermann, U. Analysis of Dihydrogen Bonding in Ammonium Borohydride. J. Phys. Chem. C 2019, 123, 28631–28639. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ban, V.; Møller, K.T.; Lee, Y.-S.; Cho, Y.W.; Besenbacher, F.; Filinchuk, Y.; Skibsted, J.; Jensen, T.R. Synthesis, Crystal Structure, Thermal Decomposition, and 11B MAS NMR Characterization of Mg(BH4)2(NH3BH3)2. J. Phys. Chem. C 2014, 118, 12141–12153. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Inukai, M.; Oguchi, H.; Takagi, S.; Orimo, S. Magnesium Borohydride Ammonia Borane as a Magnesium Ionic Conductor. ACS Appl. Energy Mater. 2020, 3, 3174–3179. [Google Scholar] [CrossRef]
- Skov, L.N.; Grinderslev, J.B.; Rosenkranz, A.; Lee, Y.; Jensen, T.R. Towards Solid-State Magnesium Batteries: Ligand-Assisted Superionic Conductivity. Batter. Supercaps 2022, 5, e202200163. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Lee, Y.-S.; Jørgensen, M.; Cho, Y.W.; Černý, R.; Jensen, T.R. Ammonia-Assisted Fast Li-Ion Conductivity in a New Hemiammine Lithium Borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 56, 3971–3974. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Dematteis, E.M.; Shinzato, K.; Stevenson, S.C.; Bannenberg, L.J.; Heere, M.; Zlotea, C.; Szilágyi, P.Á.; Bonnet, J.-P.; Grochala, W.; et al. Metal Hydrides and Related Materials. Energy Carriers for Novel Hydrogen and Electrochemical Storage. J. Phys. Chem. C 2020, 124, 7599–7607. [Google Scholar] [CrossRef]
- Bannenberg, L.J.; Heere, M.; Benzidi, H.; Montero, J.; Dematteis, E.M.; Suwarno, S.; Jaroń, T.; Winny, M.; Orłowski, P.A.; Wegner, W.; et al. Metal (Boro-) Hydrides for High Energy Density Storage and Relevant Emerging Technologies. Int. J. Hydrogen Energy 2020, 45, 33687–33730. [Google Scholar] [CrossRef]
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.; Peru, F.; et al. Hadjixenophontos, E.; et al. A Review of the MSCA ITN ECOSTORE—Novel Complex Metal Hydrides for Efficient and Compact Storage of Renewable Energy as Hydrogen and Electricity. Inorganics 2020, 8, 17, Erratum in Inorganics 2020, 8, 63. [Google Scholar] [CrossRef]
- Reynaud, M.; Serrano-Sevillano, J.; Casas-Cabanas, M. Imperfect Battery Materials: A Closer Look at the Role of Defects in Electrochemical Performance. Chem. Mater. 2023, 35, 3345–3363. [Google Scholar] [CrossRef]
- Murgia, F.; Brighi, M.; Piveteau, L.; Avalos, C.E.; Gulino, V.; Nierstenhöfer, M.C.; Ngene, P.; de Jongh, P.; Černý, R. Enhanced Room-Temperature Ionic Conductivity of NaCB11H12 via High-Energy Mechanical Milling. ACS Appl. Mater. Interfaces 2021, 13, 61346–61356. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H. Ionic Motion of Tetrahedrally and Octahedrally Coordinated Lithium Ions in Ternary and Quaternary Halides. Solid State Ion. 1988, 28–30, 1282–1286. [Google Scholar] [CrossRef]
- Zhao, F.; Alahakoon, S.H.; Adair, K.; Zhang, S.; Xia, W.; Li, W.; Yu, C.; Feng, R.; Hu, Y.; Liang, J.; et al. An Air-Stable and Li-Metal-Compatible Glass-Ceramic Electrolyte Enabling High-Performance All-Solid-State Li Metal Batteries. Adv. Mater. 2021, 33, 2006577. [Google Scholar] [CrossRef] [PubMed]
- Sudreau, F.; Petit, D.; Boilot, J.P. Dimorphism, Phase Transitions, and Transport Properties in LiZr2(PO4)3. J. Solid State Chem. 1989, 83, 78–90. [Google Scholar] [CrossRef]
- Nomura, K.; Ikeda, S.; Ito, K.; Einaga, H. Framework Structure, Phase Transition and Ionic Conductivity of MgZr4(PO4)6 and ZnZr4(PO4)6. J. Electroanal. Chem. 1992, 326, 351–356. [Google Scholar] [CrossRef]
- Matsuo, M.; Oguchi, H.; Sato, T.; Takamura, H.; Tsuchida, E.; Ikeshoji, T.; Orimo, S. Sodium and Magnesium Ionic Conduction in Complex Hydrides. J. Alloys Compd. 2013, 580, S98–S101. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Zheng, L.; Xu, F.; Feng, W.; Li, H.; Huang, X.; Armand, M.; Nie, J.; Zhou, Z. Lithium Bis(Fluorosulfonyl)Imide/Poly(Ethylene Oxide) Polymer Electrolyte. Electrochim. Acta 2014, 133, 529–538. [Google Scholar] [CrossRef]
- Vasudevan, S.; Fullerton-Shirey, S.K. Effect of Nanoparticle Shape on the Electrical and Thermal Properties of Solid Polymer Electrolytes. J. Phys. Chem. C 2019, 123, 10720–10726. [Google Scholar] [CrossRef]
- Boschin, A.; Johansson, P. Characterization of NaX (X: TFSI, FSI)—PEO Based Solid Polymer Electrolytes for Sodium Batteries. Electrochim. Acta 2015, 175, 124–133. [Google Scholar] [CrossRef]
- Ni’mah, Y.L.; Cheng, M.-Y.; Cheng, J.H.; Rick, J.; Hwang, B.-J. Solid-State Polymer Nanocomposite Electrolyte of TiO2/PEO/NaClO4 for Sodium Ion Batteries. J. Power Sources 2015, 278, 375–381. [Google Scholar] [CrossRef]

| Type | Materials | Conductivity [S cm−1] | Potential Window (V versus Na+/Na) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Polymer-based | PEO-Na salt PEG, PVDF-HFP, etc. | 10−6−10−4 | ≈4.5 | Stable with sodium metal, lightweight, highly flexible | Low ionic conductivity, limited thermal stability, low oxidation stability |
| Oxides | N-β″-Al2O3 NASICON Na2M2TeO6 | 10−4−10−3 | Up to 7 | High thermal and chemical stability, high mechanical strength, high electrochemical oxidation voltage | High grain boundary resistance, large interfacial resistance |
| Sulfides | Na3PS4 Na11Sn2PS12 and their derivatives | 10−4−10−3 | <4 for Na3PS4 Others up to 5 | Good mechanical strength and mechanical flexibility, low grain-boundary resistance | Sensitive to moisture, low oxidation stability, poor compatibility with sodium metal |
| Boron hydrides | Na2-x(B12H12)x(B10H10)1−x Na2-x(CB11H12)x(B12H12)1−x etc. | 10−4−10−2 | Up to 5 | Wide electrochemical stability windows, stability with sodium metal, high thermal and chemical stability, and stability in air | Limited researches, large interfacial resistance |
| Solid Electrolyte | Ionic Conductivity (mS cm−1) | Reference |
|---|---|---|
| LISICON Li14Zn(GeO4)4 | 125 (300 °C) | [153] |
| LiZr2(PO4)3 | 12 (300 °C) | [264] |
| LiPON | 0.002 | [154] |
| Na2.88Sb0.88W0.12S4 | 32 | [162] |
| Na2−x(CB11H12)x(B12H12)1−x | 2 | [16] |
| Li7P3S11 | 45.66 | [23] |
| Na7P3S11 | 10.97 | [23] |
| NASICON Na3Zr2PSi2O12 | 200 (300 °C) | [166] |
| MgZr4(PO4)6 | 6.1 (800 °C) | [265] |
| ZnZr4(PO4)6 | 3.7 (800 °C) | [265] |
| Composite of LiTFSI/PEGDA/succinonitrile plasticizer | 0.43 | [197] |
| Composite of polyether-based polymer/inorganic Na3Zr2Si2PO12 (NZSP) | 0.01 | [200] |
| Na11Sn2PSe12 (NSPSe) | 1 | [216] |
| Sm1−xCaxF3−x (0 ≤ x ≤ 0.15) | 0.01–0.001 | [217] |
| (100−x)(0.6MgS·0.4P2S5) · xMgI2 (0 ≤ x ≤ 30) | 0.00021 (200 °C) | [221] |
| MgS-P2S5-MgI2 | 0.00021 | [221] |
| Garnet Li6.4Al0.233La3Zr1.95Ca0.05O12 | 0.52 | [223] |
| Na2B12H12 | 0.0001 | [179] |
| β″-Al2O | 0.00024 (300 °C) | [179] |
| LiNaB12H12 | 0.00079 (280 °C) | [179] |
| Mg(en)1(BH4) | 0.06 (70 °C) | [249] |
| Mg(diglyme)0.5(BH4)2 | 0.02 (70 °C) | [247] |
| Mg3(NH2)2(BH4)4 | 0.04 (100 °C) | [250] |
| Mg(BH4)2NH3 | 0.33 (70 °C) | [251] |
| Mg(BH4)22NH3BH3 | 0.1 | [253] |
| NaCB11H12 (NCB) as-prepared | 4 | [256] |
| NaCB11H12 (NCB) milled | 0.00000978 | [261] |
| Li3.06P0.98Zn0.02S3.98O0.02 | 1.12 | [263] |
| Li2(BH4)(NH2) | 0.2 | [107] |
| Na2(BH4)(NH2) | 0.003 | [266] |
| Mg(BH4)(NH2) | 0.003 (100 °C) | [250] |
| LiTFSI-PEO | 0.8 (70 °C) | [267] |
| LiClO4-PEO | 0.2–1 (90 °C) | [268] |
| NaTFSI-PEO | 0.15 (70 °C) | [269] |
| NaClO4-PEO | 0.14 (90 °C) | [270] |
| LiClO4-PEO | 0.5 (90 °C) | [270] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burazer, S.; Popović, J. Mechanochemical Synthesis of Solid-State Electrolytes. Inorganics 2024, 12, 54. https://doi.org/10.3390/inorganics12020054
Burazer S, Popović J. Mechanochemical Synthesis of Solid-State Electrolytes. Inorganics. 2024; 12(2):54. https://doi.org/10.3390/inorganics12020054
Chicago/Turabian StyleBurazer, Sanja, and Jasminka Popović. 2024. "Mechanochemical Synthesis of Solid-State Electrolytes" Inorganics 12, no. 2: 54. https://doi.org/10.3390/inorganics12020054
APA StyleBurazer, S., & Popović, J. (2024). Mechanochemical Synthesis of Solid-State Electrolytes. Inorganics, 12(2), 54. https://doi.org/10.3390/inorganics12020054







