Comparison of Photocatalytic Activity: Impact of Hydrophilic Properties on TiO2 and ZrO2 Thin Films
Abstract
1. Introduction
2. Results and Discussion
2.1. X-Ray Diffraction
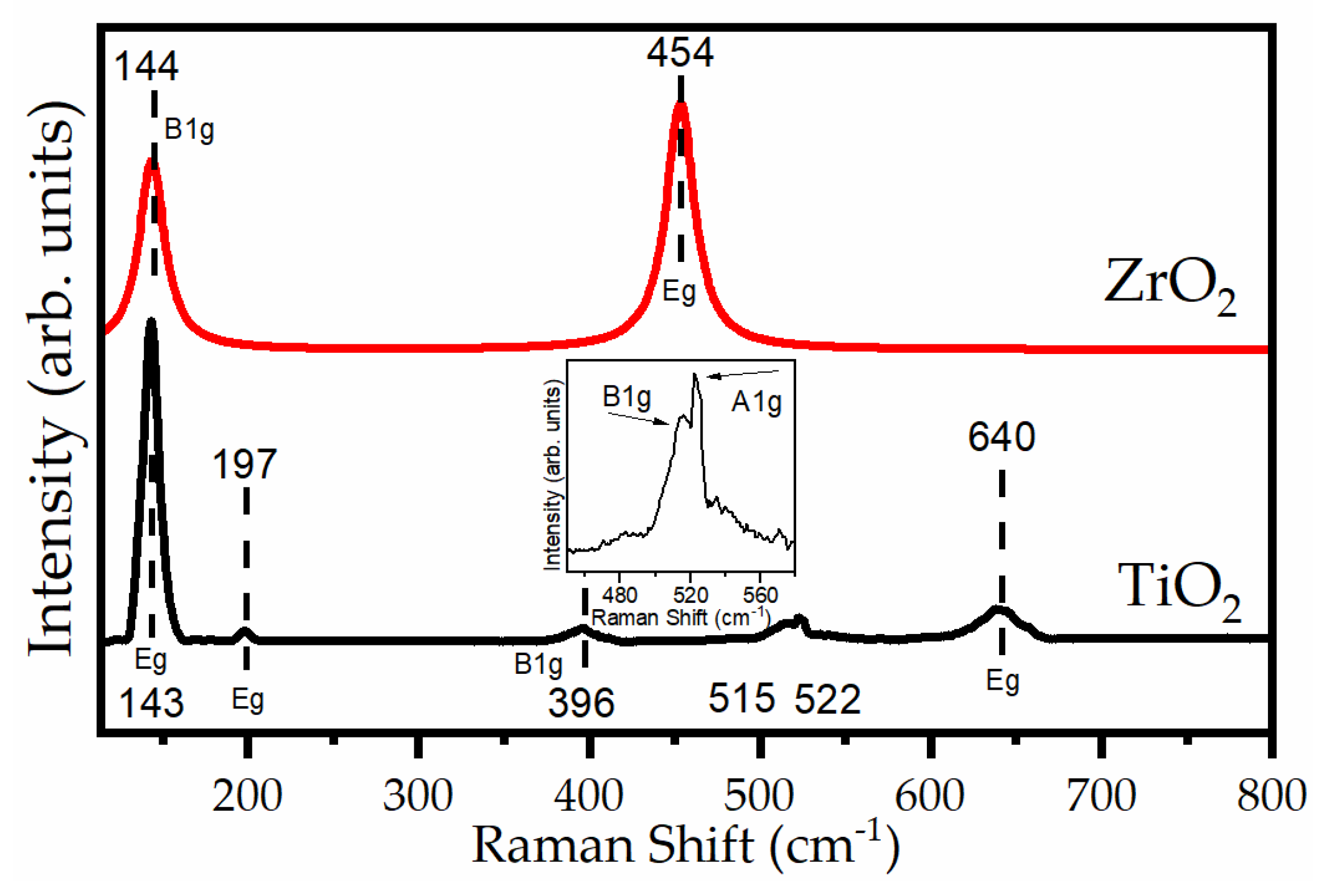
2.2. Raman Spectroscopy
2.3. Scanning Electron Microscopy (SEM)
2.4. UV-Vis Analysis
2.5. Photoluminescence (PL)
2.6. Contact Angle Measurements
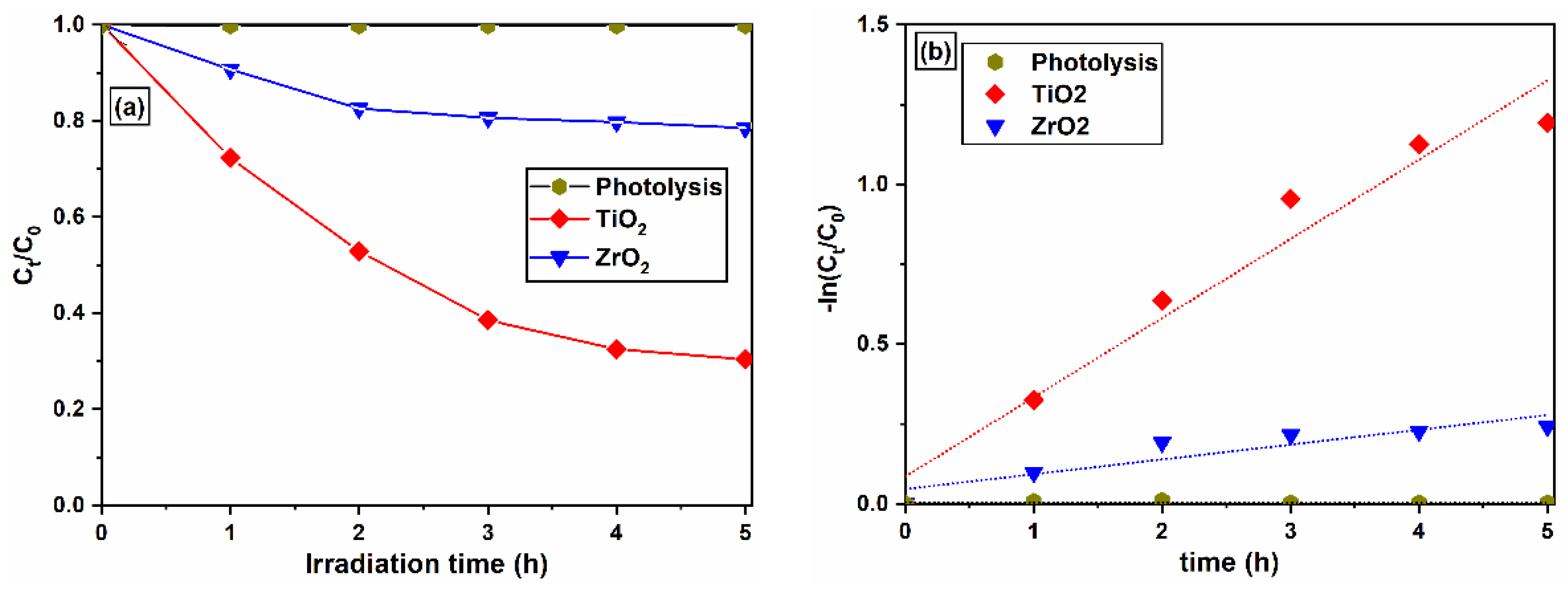
2.7. Photocatalytic Decolorization of MB
3. Experimental Section
3.1. Substrate Cleaning
3.2. TiO2 TFs
3.3. ZrO2 TFs
3.4. Structural, Optical, and Morphological Characterization of the Films
3.5. Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, M.; Augustin, A.; Mohan, S.; Honnappa, B.; Chuaicham, C.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Conducting polymeric nanocomposites: A review in solar fuel applications. Fuel 2022, 325, 124899. [Google Scholar] [CrossRef]
- Raza, R.; Zhu, B.; Rafique, A.; Naqvi, M.R.; Lund, P. Functional ceria-based nanocomposites for advanced low-temperature (300–600 °C) solid oxide fuel cell: A comprehensive review. Materials 2020, 15, 100373. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Zhang, Y.; Wang, R.; Ouyang, Z.; Gou, Z.; Zhang, H. Recent advances in two-dimensional materials and their nanocomposites in sustainable energy conversion applications. Nanoscale 2019, 11, 21622–21678. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Kuppusami, P.; Vengatesh, P. Auto-combustion synthesis and electrochemical studies of La0.6Sr0.4Co0.2Fe0.8O3-δ—Ce0.8Sm0.1Gd0.1O1.9 nanocomposite cathode for intermediate temperature solid oxide fuel cells. Ceram. Int. 2018, 44, 21188–21196. [Google Scholar] [CrossRef]
- UNESCO. United Nations Educational Scientific and Cultural Organization. In The United Nations World Water Development Report 2021: Valuing Water; United Nations: New York, NY, USA, 2021; ISBN 978-92-3-100434-6. [Google Scholar]
- OECD. Groundwater Allocation: Managing Growing Pressures on Quantity and Quality; OECD Publishing: Paris, France, 2017; ISBN 978-92-6-428155-4. [Google Scholar]
- Speight, J.G. Remediation technologies. In Natural Water Remediation—Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–303. [Google Scholar] [CrossRef]
- Hosny, N.M.; Gomaa, I.; Elmahgary, M.G. Adsorption of polluted dyes from water by transition metal oxides: A review. Appl. Surf. Sci. Adv. 2023, 15, 100395. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Barbero, E.J. Introduction to Composite Materials Design, 3rd ed.; CRC Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Choi, S.M.; Awaji, H. Nanocomposites-a new material design concept. Sci. Technol. Adv. Mater. 2005, 6, 2–10. [Google Scholar] [CrossRef]
- Wang, M.; Pan, N. Predictions of effective physical properties of complex multiphase materials. Mater. Sci. Eng. R Rep. 2008, 63, 1–30. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Wu, B.; Zhao, X.; Lu, X.; Zhao, Y.; Li, Y. Self-assembled La0.6Sr0.4FeO3−δ-La1.2Sr0.8NiO4+δ composite cathode for protonic ceramic fuel cells. Ceram. Int. 2023, 49, 25381–25388. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hu, W.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef]
- Li, G.; Fu, R.; Agathopoulos, S.; Su, X.; He, Q.; Ji, Y.; Liu, X. Ultra-low thermal expansion coefficient of PZB/β-eucryptite composite glass for MEMS packaging. Ceram. Int. 2020, 46, 8385–8390. [Google Scholar] [CrossRef]
- Martínez-Saucedo, G.; Torres-Delgado, G.; Márquez-Marín, J.; Zelaya-Ángel, O.; Castanedo-Pérez, R. Copper oxide and tin oxide amorphous-thin-film heterojunction diodes obtained via solution-based techniques with increased rectification after rapid thermal annealing treatments. J. Alloys Compd. 2021, 859, 157790. [Google Scholar] [CrossRef]
- Kahveci, O.; Akkaya, A.; Yücel, E.; Aydın, R.; Sahin, B. Production of p-CuO/n-ZnO:Co nanocomposite heterostructure TFs: An optoelectronic study. Ceram. Int. 2023, 49, 16458–16466. [Google Scholar] [CrossRef]
- Dubey, R.S.; Jadkar, S.R.; Bhorde, A.B. Synthesis and characterization of various doped TiO2 nanocrystals for dye-sensitized solar cells. ACS Omega 2021, 6, 3470–3482. [Google Scholar] [CrossRef]
- Jemaa, I.B.; Chaabouni, F.; Ranguis, A. Cr doping effect on the structural, optoelectrical and photocatalytic properties of RF sputtered TiO2 TFs from a powder target. J. Alloys Compd. 2020, 825, 153988. [Google Scholar] [CrossRef]
- DCoronado, R.; Gattorno, G.R.; Pesqueira, M.E.E.; Cab, C.; Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, Brookite and Rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, S.P. The Stability, Electronic Structure, and Optical Property of TiO2 Polymorphs. J. Phys. Chem. C 2014, 118, 11385–11396. [Google Scholar] [CrossRef]
- Zanatta, A.R. Assessing the amount of the Anatase and Rutile phases of TiO2 by optical reflectance measurements. Results Phys. 2021, 22, 103864. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Baran, W.; Masternak, E.; Sapińska, D.; Sobczak, A.; Adamek, E. Synthesis of New Antibiotics Derivatives by the Photocatalytic Method: A Screening Research. Catalysts 2021, 11, 1102. [Google Scholar] [CrossRef]
- Tian, S.; Feng, Y.; Zheng, Z.; He, Z. TiO2-Based Photocatalytic Coatings on Glass Substrates for Environmental Applications. Coatings 2023, 13, 1472. [Google Scholar] [CrossRef]
- Si, P.; Zheng, Z.; Gu, Y.; Geng, C.; Guo, Z.; Qin, J.; Wen, W. Nanostructured TiO2 Arrays for Energy Storage. Materials 2023, 16, 3864. [Google Scholar] [CrossRef] [PubMed]
- Bertel, L.; Miranda, D.A.; García-Martín, J.M. Nanostructured Titanium Dioxide Surfaces for Electrochemical Biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zangari, G. TiO2 Nanotubes Architectures for Solar Energy Conversion. Coatings 2021, 11, 931. [Google Scholar] [CrossRef]
- Gnida, P.; Jarka, P.; Chulkin, P.; Drygała, A.; Libera, M.; Tański, T.; Schab-Balcerzak, E. Impact of TiO2 Nanostructures on Dye-Sensitized Solar Cells Performance. Materials 2021, 14, 1633. [Google Scholar] [CrossRef]
- Arifin, Z.; Suyitno, S.; Hadi, S.; Sutanto, B. Improved Performance of Dye-Sensitized Solar Cells with TiO2 Nanoparticles/Zn-Doped TiO2 Hollow Fiber Photoanodes. Energies 2018, 11, 2922. [Google Scholar] [CrossRef]
- Ezeh, I.M.; Enaroseha, O.O.E.; Agbajor, G.K.; Achuba, F.I. Effect of Titanium Oxide (TiO2) on Natural Dyes for the Fabrication of Dye-Sensitized Solar Cells. Eng. Proc. 2024, 63, 25. [Google Scholar] [CrossRef]
- Matsui, M.; Akaogi, M. Molecular Dynamics Simulation of the Structural and Physical Properties of the Four Polymorphs of TiO2. Mol. Simul. 1991, 6, 239–244. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Balog, M.; Schieber, M.; Michman, M.; Patai, S. The chemical vapour deposition and characterization of ZrO2 films from organometallic compounds. Thin Solid Films 1977, 47, 109–120. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Photocatalytic activity of nanostructured ZnO–ZrO2 binary oxide using fluorometric method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Zegtouf, H.; Saoula, N.; Azibi, M.; Bait, L.; Madaoui, N.; Khelladi, M.R.; Kechouane, M. A study of properties of ZrO2 TFs deposited by magnetron sputtering under different plasma parameters: Biomedical application. J. Electr. Eng. 2019, 70, 117. [Google Scholar] [CrossRef]
- Hirano, M.; Watanabe, S.; Kato, E.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Fabrication, electrical conductivity and mechanical properties of Sc2O3-doped tetragonal zirconia ceramics. Solid State Ion. 1998, 111, 161–169. [Google Scholar] [CrossRef]
- Nova, C.V.; Reis, K.A.; Pinheiro, A.L.; Dalmaschio, C.J.; Chiquito, A.J.; Teodoro, M.D.; Rodrigues, A.D.; Longo, E.; Pontes, F.M. Synthesis, characterization, photocatalytic, and antimicrobial activity of ZrO2 nanoparticles and Ag@ZrO2 nanocomposite prepared by the advanced oxidative process/hydrothermal route. J. Sol-Gel Sci. Technol. 2021, 98, 113–126. [Google Scholar] [CrossRef]
- Lamm, B.W.; Mitchell, D.J. Chemical Vapor Deposition of Zirconium Compounds: A Review. Coatings 2023, 13, 266. [Google Scholar] [CrossRef]
- Desforges, J.; Robichaud, L.; Gauvin, S. Determination of Optical Properties of TFs from Ketteler-Helmholtz Dispersion Relations: Application to the Case of Ultraviolet Irradiated Zirconium Oxide. Adv. Mater. Sci. Eng. 2017, 2017, 8285230. [Google Scholar] [CrossRef]
- Sawka, A. Chemical Vapour Deposition of Scandia-Stabilised Zirconia Layers on Tubular Substrates at Low Temperatures. Materials 2022, 15, 2120. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Q.; Jin, Y.; Guo, X. Low-voltage zinc oxide thin-film transistors with solution-processed channel and dielectric layers below 150 °C. Appl. Phys. Lett. 2012, 101, 222114. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Thermally stable and highly sensitive ethene gas sensor based on polythiophene/zirconium oxide nanocomposites. Mater. Today Commun. 2019, 20, 100574. [Google Scholar] [CrossRef]
- Nishizawa, K.; Miki, T.; Suzuki, K.; Kato, K. Wavelength dependence of crystallization of alkoxy-derived ZrO2 TFs prepared by ultraviolet irradiation. J. Mater. Res. 2005, 20, 3133–3140. [Google Scholar] [CrossRef]
- Lovchinov, K.; Gergova, R.; Alexieva, G. Structural, Morphological and Optical Properties of Nanostructured ZrO2 Films Obtained by an Electrochemical Process at Different Deposition Temperatures. Coatings 2022, 12, 972. [Google Scholar] [CrossRef]
- Ahmad, T.; Shahazad, M.; Phul, R. Hydrothermal synthesis, characterization and dielectric properties of zirconia nanoparticles. Mater. Sci. Eng. Int. J. 2017, 1, 100–104. [Google Scholar] [CrossRef]
- Cao, W.; Kang, J.; Fan, G.; Yang, L.; Li, F. Fabrication of porous ZrO2 nanostructures with controlled crystalline phases and structures via a facile and cost-effective hydrothermal approach. Ind. Eng. Chem. Res. 2015, 54, 12795–12804. [Google Scholar] [CrossRef]
- Boratto, M.H.; Lima, J.V.M.; Scalvi, L.V.A.; Graeff, C.F.O. Low-temperature ZrO2 TFs obtained by polymeric route for electronic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 16065–16072. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Simchen, F.; Winter, L.; Lampke, T. Evaluation of Fracture Toughness of Plasma Electrolytic Oxidized Al2O3-ZrO2 Coatings Utilizing Nano-Scratch Technique. Coatings 2023, 13, 799. [Google Scholar] [CrossRef]
- Liang, L.; Xu, Y.; Wu, D.; Sun, Y. A simple sol–gel route to ZrO2 films with high optical performances. Mater. Chem. Phys. 2009, 114, 252–256. [Google Scholar] [CrossRef]
- Kumar, K.R.; Pridhar, T.; Balaji, V.S.S. Mechanical properties and characterization of zirconium oxide (ZrO2) and coconut shell ash(CSA) reinforced aluminium (Al 6082) matrix hybrid composite. J. Alloys Compd. 2018, 765, 171–179. [Google Scholar] [CrossRef]
- Rani, V.; Sharma, A.; Kumar, A.; Singh, P.; Thakur, S.; Singh, A.; Le, Q.V.; Nguyen, V.H.; Raizada, P. ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights. Catalysts 2022, 12, 1418. [Google Scholar] [CrossRef]
- Yao, N.Q.; Liu, Z.C.; Gu, G.R.; Wu, B.J. Structural, optical, and electrical properties of Cu-doped ZrO2 films prepared by magnetron co-sputtering. Chin. Phys. B 2017, 26, 106801. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Kern, F.; Dufner, L.; Dubiel, A.; Antonowicz, M.; Matus, K. Effect of Calcination Temperature on the Phase Composition, Morphology, and Thermal Properties of ZrO2 and Al2O3 Modified with APTES (3-aminopropyltriethoxysilane). Materials 2021, 14, 6651. [Google Scholar] [CrossRef]
- Cesaria, M.; Scrimieri, L.; Torrisi, A.; Quarta, G.; Serra, A.; Manno, D.; Caricato, A.P.; Martino, M.; Calcagnile, L.; Velardi, L. Pulsed-laser deposition and photocatalytic activity of pure rutile and anatase TiO2 films: Impact of single-phased target and deposition conditions. Vacuum 2022, 202, 111150. [Google Scholar] [CrossRef]
- Gottmann, J.; Kreutz, E.W. Pulsed laser deposition of alumina and zirconia TFs on polymers and glass as optical and protective coatings. Surf. Coat. Technol. 1999, 116–119, 1189–1194. [Google Scholar] [CrossRef]
- Pore, V.; Kivelä, T.; Ritala, M.; Leskelä, M. Atomic layer deposition of photocatalytic TiO2 TFs from TiF4 and H2O. Dalton Trans. 2008, 45, 6467–6474. [Google Scholar] [CrossRef] [PubMed]
- Cassir, M.; Goubin, F.; Bernay, C.; Vernoux, P.; Lincot, D. Synthesis of ZrO2 TFs by atomic layer deposition: Growth kinetics, structural and electrical properties. Appl. Surf. Sci. 2002, 193, 120–128. [Google Scholar] [CrossRef]
- Iwamura, S.; Motohashi, S.; Mukai, S.R. Development of an efficient CVD technique to prepare TiO2/porous–carbon nanocomposites for high-rate lithium-ion capacitors. RSC Adv. 2020, 10, 38196–38204. [Google Scholar] [CrossRef]
- Flores-Ruiz, F.J.; Diliegros-Godines, C.J.; Hernández-García, F.A.; Castanedo-Pérez, R.; Torres-Delgado, G.; Broitman, E. Mechanical and tribological behavior of sol–gel TiO2–CdO films measured at the microscale levels. J. Sol-Gel Sci. Technol. 2017, 82, 682–691. [Google Scholar] [CrossRef]
- Caracoche, M.C.; Rivas, P.C.; Cervera, M.M.; Caruso, R.; Benavídez, E.; de Sanctis, O.; Escobar, M.E. Zirconium Oxide Structure Prepared by the Sol–Gel Route: I, The Role of the Alcoholic Solvent. J. Am. Ceram. Soc. 2000, 83, 377–384. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Torres-Limiñana, J.; Feregrino-Pérez, A.A.; Vega-González, M.; Escobar-Alarcón, L.; Cervantes-Chávez, J.A.; Esquivel, K. Green Synthesis via Eucalyptus globulus L. Extract of Ag-TiO2 Catalyst: Antimicrobial Activity Evaluation toward Water Disinfection Process. Nanomaterials 2022, 12, 1944. [Google Scholar] [CrossRef]
- Wang, X.; Sun, P.; Zhao, Z.; Liu, Y.; Zhou, S.; Yang, P.; Dong, Y. Effects of the ZrO2 Crystalline Phase and Morphology on the Thermocatalytic Decomposition of Dimethyl Methylphosphonate. Nanomaterials 2024, 14, 611. [Google Scholar] [CrossRef]
- Stefanov, B.I. Optically Transparent TiO2 and ZnO Photocatalytic TFs via Salicylate-Based Sol Formulations. Coatings 2023, 13, 1568. [Google Scholar] [CrossRef]
- Ji, P.; Wang, Z.; Shang, X.; Zhang, Y.; Liu, Y.; Mao, Z.; Shi, X. Direct observation of enhanced Raman scattering on nano-sized ZrO2 substrate: Charge-transfer contribution. Front. Chem. 2019, 7, 245. [Google Scholar] [CrossRef] [PubMed]
- López, E.F.; Escribano, V.S.; Panizza, M.; Carnasciali, M.M. Vibrational and electronic spectroscopic properties of zirconia powders. J. Mater. Chem. 2001, 11, 1891–1897. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on PD of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Sezgin, A.; Čtvrtlík, R.; Václavek, L.; Tomáštík, J.; Nožka, L.; Menşur, E.; Türküz, S. Optical, structural and mechanical properties of TiO2 and TiO2-ZrO2 TFs deposited on glass using magnetron sputtering. Mater. Today Commun. 2023, 35, 106334. [Google Scholar] [CrossRef]
- Prucnal, S.; Gago, R.; Calatayud, D.G.; Rebohle, L.; Liedke, M.O.; Butterling, M.; Wagner, A.; Helm, M.; Zhou, S. TiO2 Phase Engineering by Millisecond Range Annealing for Highly Efficient Photocatalysis. J. Phys. Chem. C 2023, 127, 12686–12694. [Google Scholar] [CrossRef]
- Mansilla, Y.; Arce, M.D.; González-Oliver, C.; Basbus, J.; Troiani, H.; Serquis, A. Characterization of stabilized ZrO2 TFs obtained by sol-gel method. Appl. Surf. Sci. 2021, 569, 150787. [Google Scholar] [CrossRef]
- Pérez-González, M.; Tomás, S.A.; Morales-Luna, M.; Arvizu, M.A.; Tellez-Cruz, M.M. Optical, structural, and morphological properties of photocatalytic TiO2–ZnO TFs synthesized by the sol–gel process. Thin Solid Films 2015, 594, 304–309. [Google Scholar] [CrossRef]
- Khetta, O.B.; Attaf, A.; Derbali, A.; Saidi, H.; Bouhdjer, A.; Aida, M.S.; Khetta, Y.B.; Messemeche, R.; Nouadji, R.; Rahmane, S.; et al. Precursor concentration effect on the physical properties of transparent titania (Anatase-TiO2) TFs grown by ultrasonic spray process for optoelectronics application. Opt. Mater. 2022, 132, 112790. [Google Scholar] [CrossRef]
- Singh, H.; Sunaina; Yadav, K.K.; Bajpai, V.K.; Jha, M. Tuning the bandgap of m-ZrO2 by incorporation of copper nanoparticles into visible region for the treatment of organic pollutants. Mater. Res. Bull. 2020, 123, 110698. [Google Scholar] [CrossRef]
- Witit-anun, N.; Chaiyakun, S. Structural and optical properties of ZrO2 TFs deposited by reactive DC unbalanced magnetron sputtering. Adv. Mater. Res. 2014, 979, 374–377. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the BG Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Murillo, V.N.; Rajput, D.; Manriquez, J.; Bustos, E.; Bueno, J.J.P.; Singh, D.; Dubey, H.; Vazquez, C.E.F.; Godavarthi, S.; Zarhri, Z.; et al. Modulating temperature for Cu2ZnSnS4 (CZTS) synthesis via hot injection method and studying the photocatalytic efficiencies for the degradation of rhodamine 6G and methylene blue pollutants. Environ. Res. 2024, 258, 119371. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Argaez, U.; Cedeño-Caero, L.; Cadena-Nava, R.D.; Ramirez-Acosta, K.; Fuentes-Moyado, S.; Sánchez-López, P.; Alonso-Núñez, G. Photocatalytic Activity and Biocide Properties of Ag–TiO2 Composites on Cotton Fabrics. Materials 2023, 16, 4513. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.S.; Madras, G. Structure and Photocatalytic Activity of Ti1−xMxO2±δ (M = W, V, Ce, Zr, Fe, and Cu) Synthesized by Solution Combustion Method. J. Phys. Chem. B 2004, 108, 20204–20212. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence Mechanisms in Anatase and Rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Prakashbabu, D.; Krishna, R.H.; Nagabhushana, B.M.; Nagabhushana, H.; Shivakumara, C.; Chakradar, R.P.S.; Ramalingam, H.B.; Sharma, S.C.; Chandramohan, R. Low temperature synthesis of pure cubic ZrO2 nanopowder: Structural and luminescence studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 216–222. [Google Scholar] [CrossRef]
- Ashraf, S.; Irfan, M.; Kim, D.; Jang, J.H.; Han, W.T.; Jho, Y.D. Optical influence of annealing in nano- and submicron-scale ZrO2 powders. Ceram. Int. 2014, 40, 8513–8518. [Google Scholar] [CrossRef]
- Gnanamoorthi, K.; Balakrishnan, M.; Mariappan, R.; Kumar, E.R. Effect of Ce doping on microstructural, morphological and optical properties of ZrO2 nanoparticles. Mater. Sci. Semicond. Process. 2015, 30, 518–526. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.I.; Nakata, K.; Furudate, M.; et al. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Al-Shomar, S.M.; Barakat, M.A.Y.; Abdallah, A.W. Ellipsometric and ultrasonic studies of nano titanium dioxide specimens doped with Erbium. Mater. Res. Express 2020, 7, 106413. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Dumitru, A.; Nicolae, A.; Frone, A.N.; Anastasescu, M.; Rădiţoiu, A.; Raduly, M.F.; Gabor, R.A.; Căprărescu, S. Antireflective coating based on TiO2 nanoparticles modified with coupling agents via acid-catalyzed sol-gel method. Appl. Surf. Sci. 2019, 487, 819–824. [Google Scholar] [CrossRef]
- Bathula, C.; Nissimagoudar, A.S.; Kumar, S.; Jana, A.; Sekar, S.; Lee, S.; Kim, H.S. Enhanced photodegradation of methylene blue by Zr-doped TiO2: A combined DFT and experimental investigation. Inorg. Chem. Commun. 2024, 164, 112442. [Google Scholar] [CrossRef]
- Pinton, J.H.B.; Oliveira, A.F.; Huanca, D.R.; Mohallem, N.D.S. Development of an automated and cost-effective apparatus for sol-gel solution deposition using spray coating technique and its application for TiO-based photocatalytic films. Mater. Chem. Phys. 2024, 318, 129213. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Florez, J.; Vallejo, W.; Duran, F.; Puello, E.; Roa, V.; Schott, E.; Zarate, X. Removal and PD of methylene blue on ZrO2 TFs modified with Anderson-Polioxometalates (Cr3+, CO3+, Cu2+): An experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2024, 454, 115689. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Jeyakumar, S.J.; Praveen, P.; Panneerdoss, I.J. Photocatalytic activity of spray deposited ZrO2 nano-TFs on methylene blue decolouration. J. Mater. Sci. Mater. Electron. 2016, 27, 5851–5859. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Silva, Y.d.J.; Lugo-Arredondo, M.I.; Gallardo-Hernández, S.; Garcia-Trejo, J.F.; Matsumoto, Y.; Rivas, S.; Feregrino-Pérez, A.A.; Godínez, L.A.; Méndez-López, A. Comparison of Photocatalytic Activity: Impact of Hydrophilic Properties on TiO2 and ZrO2 Thin Films. Inorganics 2024, 12, 320. https://doi.org/10.3390/inorganics12120320
Acosta-Silva YdJ, Lugo-Arredondo MI, Gallardo-Hernández S, Garcia-Trejo JF, Matsumoto Y, Rivas S, Feregrino-Pérez AA, Godínez LA, Méndez-López A. Comparison of Photocatalytic Activity: Impact of Hydrophilic Properties on TiO2 and ZrO2 Thin Films. Inorganics. 2024; 12(12):320. https://doi.org/10.3390/inorganics12120320
Chicago/Turabian StyleAcosta-Silva, Yuliana de Jesús, Misael Ian Lugo-Arredondo, Salvador Gallardo-Hernández, Juan Fernando Garcia-Trejo, Yasuhiro Matsumoto, Sandra Rivas, Ana Angélica Feregrino-Pérez, Luis A. Godínez, and Arturo Méndez-López. 2024. "Comparison of Photocatalytic Activity: Impact of Hydrophilic Properties on TiO2 and ZrO2 Thin Films" Inorganics 12, no. 12: 320. https://doi.org/10.3390/inorganics12120320
APA StyleAcosta-Silva, Y. d. J., Lugo-Arredondo, M. I., Gallardo-Hernández, S., Garcia-Trejo, J. F., Matsumoto, Y., Rivas, S., Feregrino-Pérez, A. A., Godínez, L. A., & Méndez-López, A. (2024). Comparison of Photocatalytic Activity: Impact of Hydrophilic Properties on TiO2 and ZrO2 Thin Films. Inorganics, 12(12), 320. https://doi.org/10.3390/inorganics12120320









