Nickel Foam-Supported FeP Encapsulated in N, P Co-Doped Carbon Matrix for Efficient Electrocatalytic Hydrogen Evolution
Abstract
1. Introduction
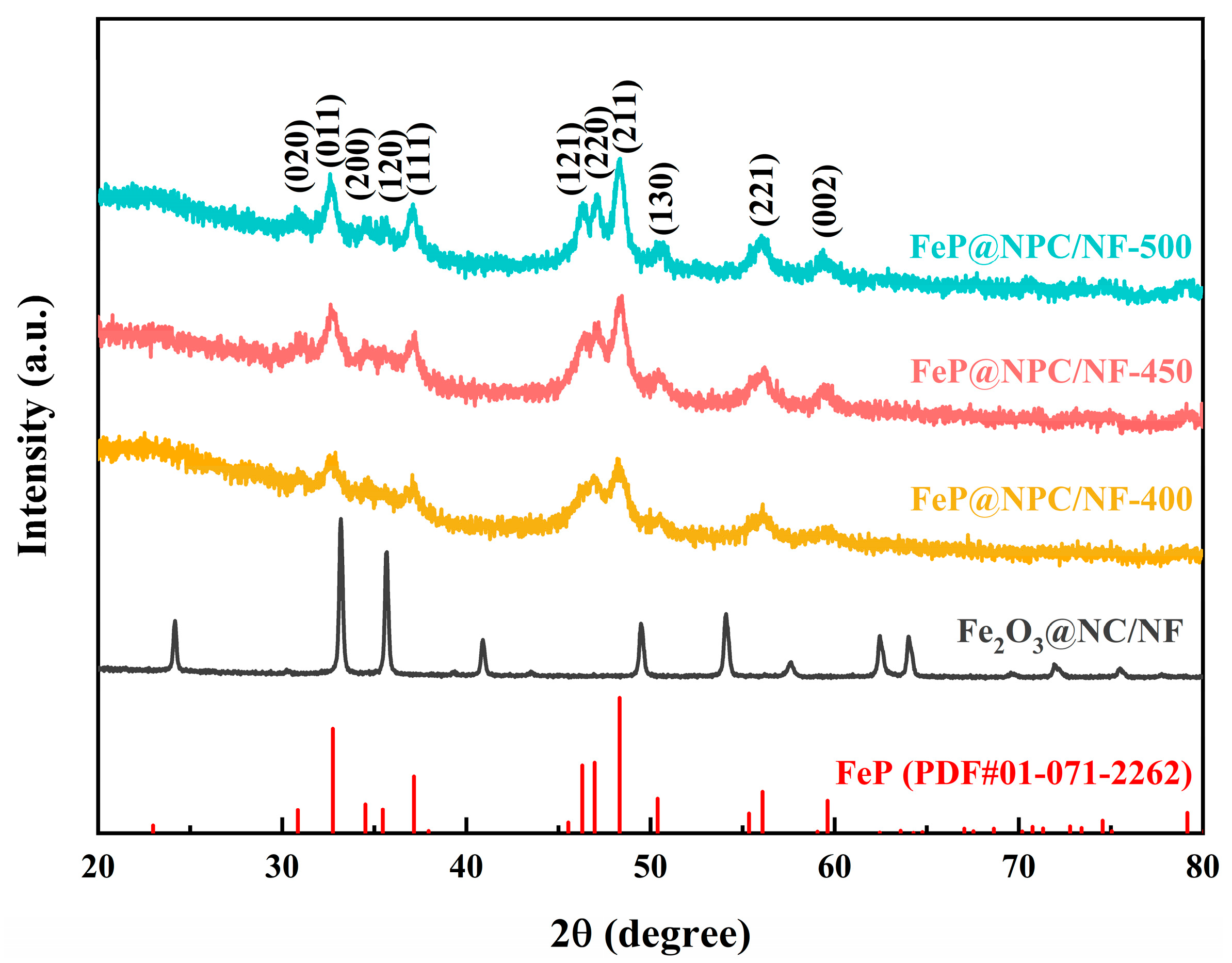
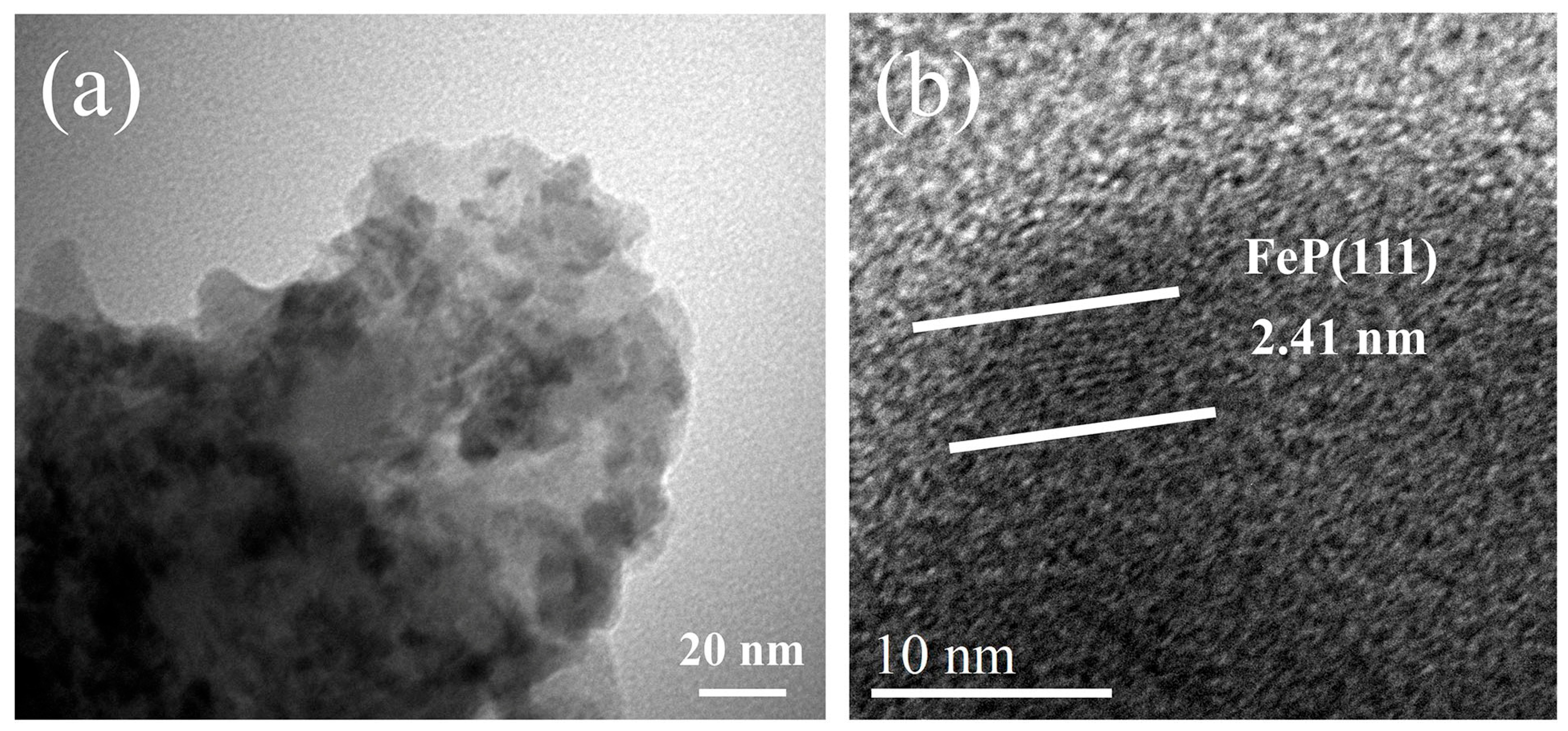
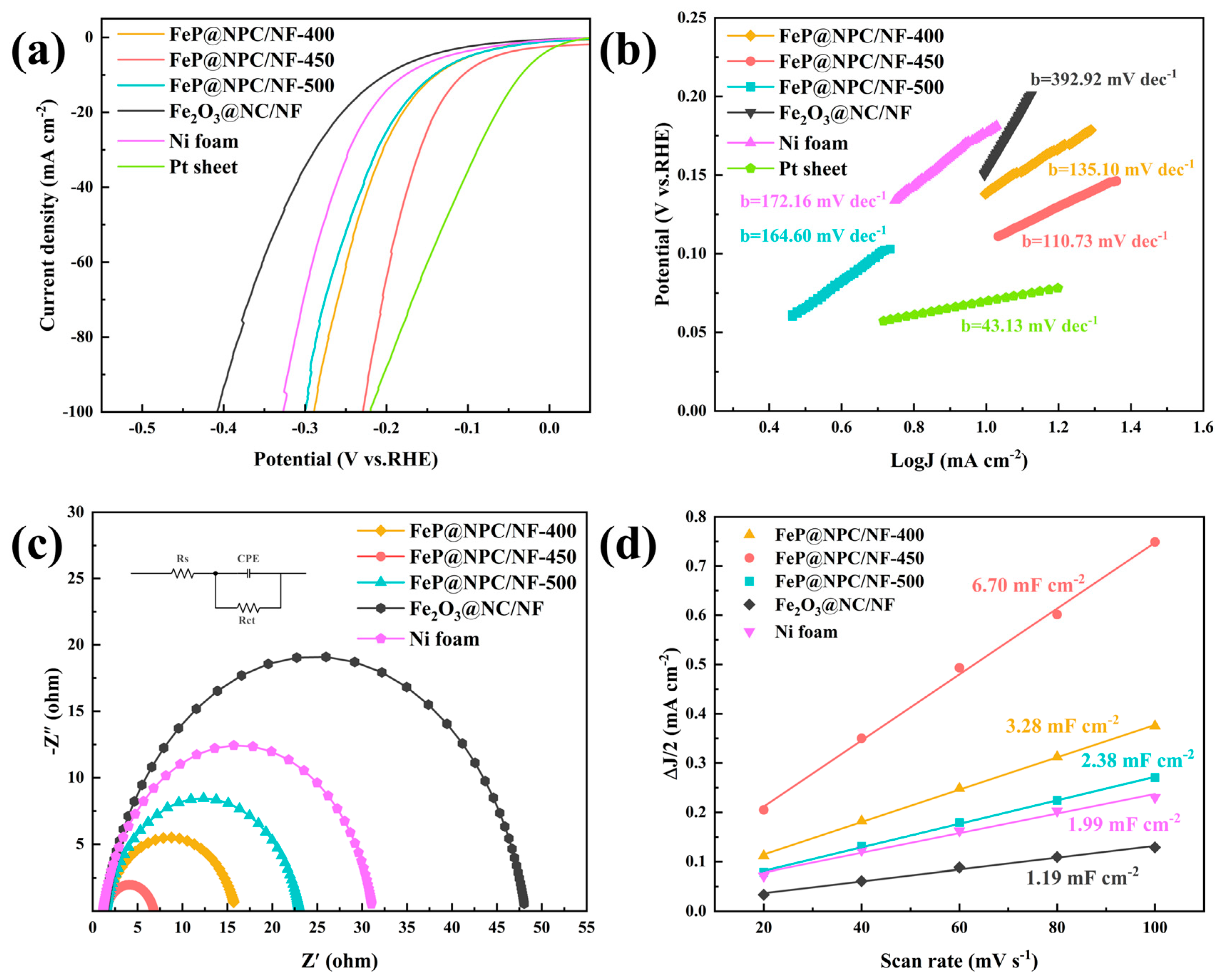
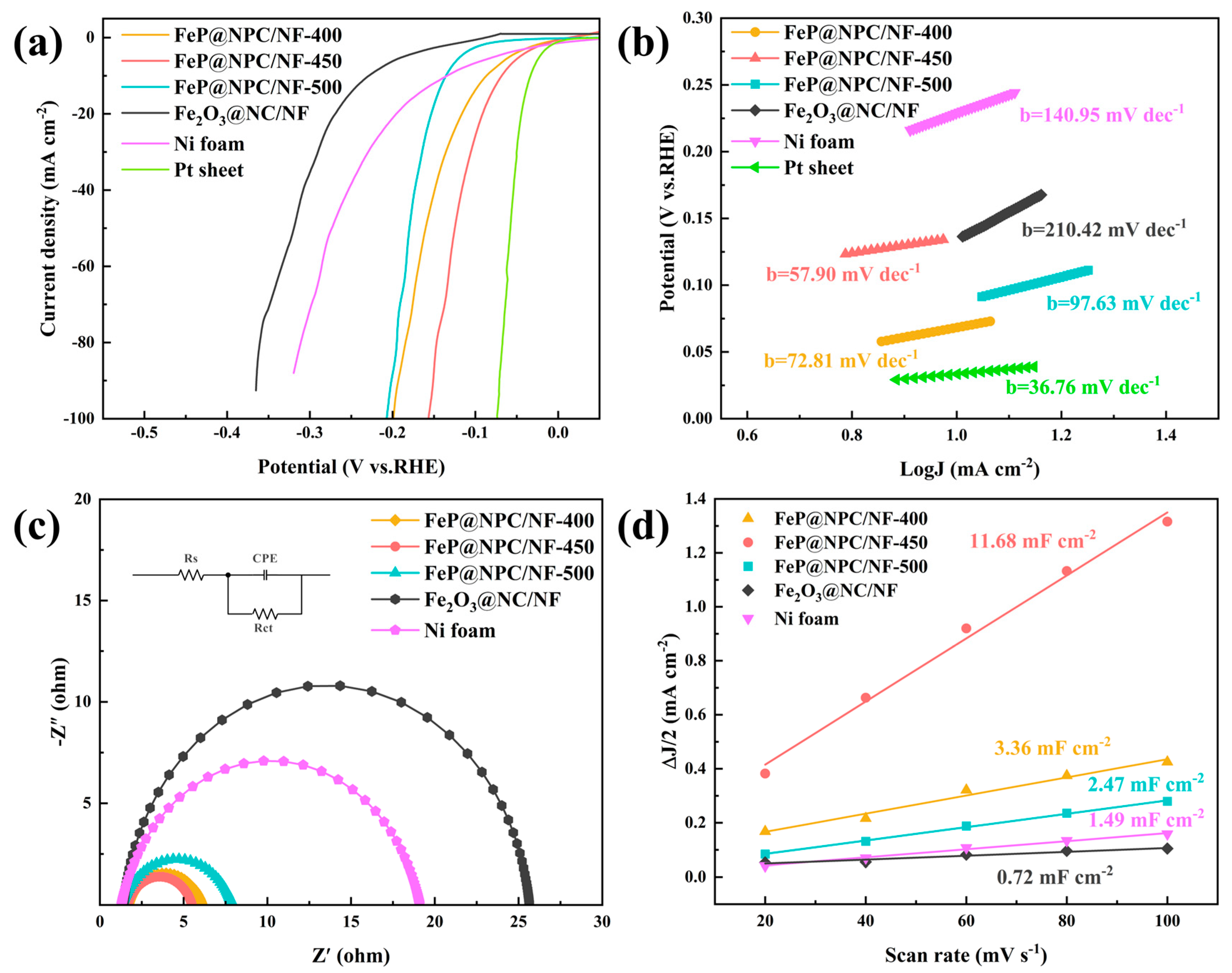
2. Result and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Pretreatment of Nickel Foam
3.3. Preparation of Fe-MOF Precursor on Nickel Foam
3.4. Preparation of FeP@NPC/NF
3.5. Preparation of FeP Powder Material Without Ni Foam
3.6. Material Characterization
3.7. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Wang, R.; Zheng, Y.; Zhang, L.; Jiao, T.; Peng, Q.; Liu, Z. Facile preparation of self-assembled Ni/Co phosphates composite spheres with highly efficient HER electrocatalytic performances. Appl. Surf. Sci. 2020, 509, 145383. [Google Scholar] [CrossRef]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Zhang, T.; He, Z.; Yin, L.; Gao, W.; Wang, Y. CoNi alloy nanoparticles confined by N-doped carbon matrix with tailored d-Band center for electrocatalytic hydrogen evolution. Fuel 2024, 365, 131176. [Google Scholar] [CrossRef]
- Gao, P.; Yue, C.; Zhang, J.; Bao, J.; Wang, H.; Chen, Q.; Jiang, Y.; Huang, S.; Hu, Z.; Zhang, J.J. Construction of unique NiCoP/FeNiCoP hollow heterostructured ellipsoids with modulated electronic structure for enhanced overall water splitting. J. Colloid Interface Sci. 2024, 666, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, E.; Ramakrishnan, S.; Sathiskumar, C.; Yoo, D.J.; Balamurugan, J.; Noh, H.S.; Kwon, D.; Kim, Y.H.; Lee, H. MOF-derived CoP-nitrogen-doped carbon@ NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries. Chem. Eng. J. 2022, 428, 131115. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Y.; Chen, W.; Yin, L.; Hao, J.; Wang, Y. Defects engineering of nickel tellurium electrocatalyst to boost urea oxidation reaction: Electrodeposition mechanism and performance. J. Alloys Compd. 2024, 997, 174944. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Wang, Y.; Cao, E.; Xiao, F.; Chen, S.; Du, S.; Wu, Y.; Ren, Z. Regulating the allocation of N and P in codoped graphene via supramolecular control to remarkably boost hydrogen evolution. Energy Environ. Sci. 2019, 12, 2697–2705. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, J.; Gao, W.; Wang, Y. Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction. Coatings 2024, 14, 817. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, T.; Qu, G.; Huang, S.; Cao, P.; Gao, W. Phase control and stabilization of 1T-MoS2 via black TiO2−x nanotube arrays supporting for electrocatalytic hydrogen evolution. J. Energy Chem. 2022, 68, 71–77. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.; Tackett, B.M.; Denny, S.R.; Tian, B.; Chen, X.; Wang, B.; Chen, J.G. Trends and descriptors of metal-modified transition metal carbides for hydrogen evolution in alkaline electrolyte. ACS Catal. 2019, 9, 2415–2422. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.; Xiang, H.; Guan, Y.; Yang, C.; Zhang, Q.; Li, Y.; Cong, Y.; Li, X. Two-dimensional ordered double-transition metal carbides for the electrochemical nitrogen reduction reaction. ACS Appl. Mater. Interfaces 2023, 15, 6797–6806. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Cui, J.; Chen, C.; Zhang, L.; Sun, D. Metal-organic framework derived transition metal sulfides grown on carbon nanofibers as self-supported catalysts for hydrogen evolution reaction. J. Colloid Interface Sci. 2024, 659, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kapuria, A.; Mondal, T.K.; Shaw, B.K.; Su, Y.-K.; Saha, S.K. Polysulfide functionalized reduced graphene oxide for electrocatalytic hydrogen evolution reaction and supercapacitor applications. Int. J. Hydrogen Energy 2023, 48, 17014–17025. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Palladium oxide decorated transition metal nitride as efficient electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2021, 855, 157511. [Google Scholar] [CrossRef]
- Gujral, H.S.; Singh, G.; Yang, J.H.; Sathish, C.; Yi, J.; Karakoti, A.; Fawaz, M.; Ramadass, K.; Ala’a, H.; Yu, X. Mesoporous titanium carbonitride derived from mesoporous C3N5 for highly efficient hydrogen evolution reaction. Carbon 2022, 195, 9–18. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.G.; Wang, L.; Weng, Y.; Tang, C.S.; Yin, X.; Han, K.; Wu, H.; Yu, X.; Wong, L.M. Electronic-reconstruction-enhanced hydrogen evolution catalysis in oxide polymorphs. Nat. Commun. 2019, 10, 3149. [Google Scholar] [CrossRef]
- Hu, F.; Yu, D.; Ye, M.; Wang, H.; Hao, Y.; Wang, L.; Li, L.; Han, X.; Peng, S. Lattice-Matching formed mesoporous transition metal oxide heterostructures advance water splitting by active Fe–O–Cu bridges. Adv. Energy Mater. 2022, 12, 2200067. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, T.; Li, B.; Wei, S.; Gao, W. MOF-derived formation of ultrafine FeP nanoparticles confined by N/P Co-doped carbon as an efficient and stable electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2022, 597, 153662. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, D. 3D nanoporous NiCoP as a highly efficient electrocatalyst for the hydrogen evolution reaction in alkaline electrolyte. New J. Chem. 2022, 46, 7490–7496. [Google Scholar] [CrossRef]
- Liu, H.; Shi, S.; Wang, Z.; Han, Y.; Huang, W. Recent Advances in Metal–Gas Batteries with Carbon-Based Nonprecious Metal Catalysts. Small 2022, 18, 2103747. [Google Scholar] [CrossRef]
- Wei, S.; Xing, P.; Tang, Z.; Wang, Y.; Dai, L. Spindle-shaped cobalt-doped iron phosphide anchored on three-dimensional graphene electrocatalysis for hydrogen evolution reactions in both acidic and alkaline media. J. Power Sources 2023, 555, 232414. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, Z.; Li, G.; Wang, M.; Chen, P.; Liu, X.; Tu, X.; Hu, Y.; Shen, Z.; Wu, Y. Hollow Spherical Heterostructured FeCo-P Catalysts Derived from MOF-74 for Efficient Overall Water Splitting. Adv. Sci. 2024, 11, 2306919. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, S.; Shahrokhian, S. Ruthenium/Ruthenium oxide hybrid nanoparticles anchored on hollow spherical Copper-Cobalt Nitride/Nitrogen doped carbon nanostructures to promote alkaline water splitting: Boosting catalytic performance via synergy between morphology engineering, electron transfer tuning and electronic behavior modulation. J. Colloid Interface Sci. 2022, 626, 1070–1084. [Google Scholar] [PubMed]
- Zheng, Z.; Wu, H.-H.; Liu, H.; Zhang, Q.; He, X.; Yu, S.; Petrova, V.; Feng, J.; Kostecki, R.; Liu, P.; et al. Achieving fast and durable lithium storage through amorphous FeP nanoparticles encapsulated in ultrathin 3D P-doped porous carbon nanosheets. ACS Nano 2020, 14, 9545–9561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhong, J.; Ding, Y.; Gao, W.; Wang, Y. Mo-doped FeP nanoparticles encapsulated in N, P doped carbon matrix with optimized d-band center for highly efficient and stable hydrogen evolution reaction. New J. Chem. 2024, 48, 11302–11309. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, S.; Yang, J.; Du, X. NiMo/NiCo2O4 as synergy catalyst supported on nickel foam for efficient overall water splitting. Mol. Catal. 2022, 518, 112086. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, L.; Zhang, Y.; Xu, Z.; Lu, L.; Huang, J.; Yin, L.; Zhu, W.; Zhuang, Z. Insights into the effect of precursors on the FeP-catalyzed hydrogen evolution reaction. Inorg. Chem. 2022, 61, 2954–2961. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Wang, X.; Li, C.; Xiao, Z.; Liu, Y.; Deng, Y.; Li, Z.; Wang, L. The construction of defect-rich CoP@CoP@(Co/Ni)2P triple-shell hollow nanospheres with boosted electrocatalytic hydrogen evolution performances over a wide pH range. Chem. Eng. J. 2023, 463, 142448. [Google Scholar] [CrossRef]
- Qian, M.; Cui, S.; Jiang, D.; Zhang, L.; Du, P. Highly efficient and stable water-oxidation electrocatalysis with a very low overpotential using FeNiP substitutional-solid-solution nanoplate arrays. Adv. Mater. 2017, 29, 1704075. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Cui, X.; Xu, X.; Wang, Y.; Xiang, G.; Gao, L.; Lin, Z.; Yang, Y. Collaborative integration of ultrafine Fe2P nanocrystals into Fe, N, P-codoped carbon nanoshells for highly-efficient oxygen reduction. Nano Energy 2023, 108, 108179. [Google Scholar] [CrossRef]
- Xu, L.-H.; Che, P.-C.; Zhang, X.-J.; Cosnier, S.; Shan, D. FeP nanoparticles highly dispersed on N, P-doped petaloid carbon nanosheet: Interface engineering and boosted intrinsic ORR activity. Appl. Surf. Sci. 2023, 620, 156770. [Google Scholar] [CrossRef]
- Zhai, W.; Chen, Y.; Liu, Y.; Ma, Y.; Vijayakumar, P.; Qin, Y.; Qu, Y.; Dai, Z. Covalently bonded Ni sites in black phosphorene with electron redistribution for efficient metal-lightweighted water electrolysis. Nanomicro Lett. 2024, 16, 115. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Jing, Z.; Chen, M.; Wang, B.; Wang, L.; Qu, G.; Kong, Y.; Xu, L. A General “In Situ Etch-Adsorption-Phosphatization” Strategy for the Fabrication of Metal Phosphides/Hollow Carbon Composite for High Performance Liquid/Flexible Zn–Air Batteries. Small 2023, 19, 2301985. [Google Scholar] [CrossRef]
- Xing, G.; Zhang, G.; Wang, B.; Tong, M.; Tian, C.; Wang, L.; Fu, H. Strengthening oxygen reduction activity based on the cooperation of pyridinic-N and graphitic-N for atomically dispersed Fe sites. J. Mater. Chem. A 2023, 11, 9493–9503. [Google Scholar] [CrossRef]
- Shin, C.-H.; Ted, H.Y.; Lee, H.-Y.; Lee, B.-J.; Kwon, S.; Goddard, W.A., III; Yu, J.-S. Ru-loaded pyrrolic-N-doped extensively graphitized porous carbon for high performance electrochemical hydrogen evolution. Appl. Catal. B 2023, 334, 122829. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Zhou, Y.; Lu, J.; Zhou, K.; Yang, L.; Tang, Z.; Li, L.; Chen, S. N-doped carbon-wrapped cobalt nanoparticles on N-doped graphene nanosheets for high-efficiency hydrogen production. Chem. Mater. 2015, 27, 2026–2032. [Google Scholar] [CrossRef]
- Xie, J.; Fu, W.; Zhang, X.; Song, G.; Wu, H.; Zhou, M. Cobalt species immobilized on nitrogen-doped ordered mesoporous carbon for highly efficient catalytic ozonation of atrazine. Sep. Purif. 2024, 335, 126077. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lin, C.-T.; Chen, T.-Y.; Hsu, C.-L.; Lee, Y.-H.; Zhang, W.; Wei, K.-H.; Li, L.-J. Highly efficient electrocatalytic hydrogen production by MoSx grown on graphene-protected 3D Ni foams. Adv. Mater. 2013, 25, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Papiya, F.; Pattanayak, P.; Kumar, P.; Kumar, V.; Kundu, P.P. Development of highly efficient bimetallic nanocomposite cathode catalyst, composed of Ni: Co supported sulfonated polyaniline for application in microbial fuel cells. Electrochim. Acta 2018, 282, 931–945. [Google Scholar] [CrossRef]
- Ye, J.; Zang, Y.; Wang, Q.; Zhang, Y.; Sun, D.; Zhang, L.; Wang, G.; Zheng, X.; Zhu, J. Nitrogen doped FeS2 nanoparticles for efficient and stable hydrogen evolution reaction. J. Energy Chem. 2021, 56, 283–289. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Z.; Wang, Y.; Yin, L.; He, Z.; Zhang, Z.; Hayat, M.D.; Zang, Q.; Lian, J. Cyclic voltammetric deposition of binder-free Ni-Se film on Ni foams as efficient bifunctional electrocatalyst for boosting overall urea-water electrolysis. J. Alloys Compd. 2023, 937, 168460. [Google Scholar] [CrossRef]
- Cheng, C.; Ao, W.; Ren, H.; Fan, Z.; Xu, T.; Dai, L.; Yin, P. Amorphous versus crystalline CoSx anchored on CNTs as heterostructured electrocatalysts toward hydrogen evolution reaction. Sci. China Mater. 2023, 66, 1383–1388. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Zhang, T.; Tian, J.; Gao, W.; Wang, Y. Nickel Foam-Supported FeP Encapsulated in N, P Co-Doped Carbon Matrix for Efficient Electrocatalytic Hydrogen Evolution. Inorganics 2024, 12, 291. https://doi.org/10.3390/inorganics12110291
Zhong J, Zhang T, Tian J, Gao W, Wang Y. Nickel Foam-Supported FeP Encapsulated in N, P Co-Doped Carbon Matrix for Efficient Electrocatalytic Hydrogen Evolution. Inorganics. 2024; 12(11):291. https://doi.org/10.3390/inorganics12110291
Chicago/Turabian StyleZhong, Jianguo, Ting Zhang, Jianqiang Tian, Wei Gao, and Yuxin Wang. 2024. "Nickel Foam-Supported FeP Encapsulated in N, P Co-Doped Carbon Matrix for Efficient Electrocatalytic Hydrogen Evolution" Inorganics 12, no. 11: 291. https://doi.org/10.3390/inorganics12110291
APA StyleZhong, J., Zhang, T., Tian, J., Gao, W., & Wang, Y. (2024). Nickel Foam-Supported FeP Encapsulated in N, P Co-Doped Carbon Matrix for Efficient Electrocatalytic Hydrogen Evolution. Inorganics, 12(11), 291. https://doi.org/10.3390/inorganics12110291








