Abstract
Perovskite materials are promising for thermochemical energy storage due to their ability to undergo redox cycling over a wide temperature range. Although BaCoO3 exhibits excellent air cycling properties, its heat storage capacity in air remains suboptimal. This study introduces Na into the lattice structure to enhance oxygen vacancy formation and mobility. DFT+U simulations of the surface structure of Na-doped BaCoO3−δ indicate that incorporating Na improves surface stability and facilitates the formation of surface oxygen vacancies. NaxBa1−xCoO3−δ compounds were synthesized using a modified sol–gel method, and their properties were investigated. The experimental results demonstrate that Na doping significantly enhances the redox activity of the material. The heat storage capacity increased by above 50%, with the Na0.0625Ba0.9375CoO3−δ solid solution achieving a heat storage density of up to 341.7 kJ/kg. XPS analysis reveals that Na doping increases the concentration of surface defect oxygen, leading to more active oxygen release sites at high temperatures. This enhancement in redox activity aligns with DFT predictions. During high-temperature cycling, the distribution of Na within the material becomes more uniform, and no performance degradation is observed after 300 cycles. Even after 450 cycles, Na0.0625Ba0.9375CoO3−δ retains over 96% of its initial redox activity, significantly outperforming fresh BaCoO3−δ. These findings elucidate the mechanism by which Na doping enhances the thermochemical heat storage performance of BaCoO3−δ and provide new insights for the design of perovskite-based materials.
1. Introduction
The inherent instability of most renewable energy sources creates an urgent need for effective large-scale energy storage solutions. Thermal energy storage (TES) is a key component of these solutions, offering versatile temperature adaptability, flexible scalability, high safety, and low costs [1]. TES has found wide applications in solar thermal power generation [2,3,4], industrial and building energy efficiency [5], and equipment thermal management [6,7]. By addressing mismatches in the timing, location, or intensity of energy supply and demand, TES significantly enhances thermal energy utilization efficiency and presents considerable market potential. Sensible heat storage technology, such as molten salt, has been effectively utilized in commercial Concentrated Solar Power (CSP) plants [8,9]. However, its application is constrained by a limited storage temperature range and relatively low energy density. Thermochemical energy storage (TCES) operates by storing and releasing heat through reversible chemical reactions, providing high energy density and minimal energy loss during long-term storage [10,11,12]. This makes TCES a highly promising area for development. TCES systems are categorized based on the reactants used, such as metal hydrides, inorganic hydroxides, organic compounds, ammonia-based systems, carbonates, and metal oxides [13]. Metal oxide systems store and release heat through the reversible redox reactions of metal ions, which involve only the absorption and release of oxygen [14,15,16,17,18]. This characteristic makes them particularly well suited for use in air environments. However, despite their simplicity, flexibility, and ease of control, metal oxide systems are constrained by a narrow operational temperature range, posing challenges in identifying suitable materials for specific temperature intervals [19,20].
Perovskite-type metal oxides (ABO3) have garnered significant attention due to their wide thermal energy storage temperature range [21] and favorable doping properties [22]. Unlike single metal oxides, perovskite materials maintain the stability of their crystal structure during redox reactions, with only partial changes occurring within the framework, often accompanied by the adsorption and desorption of oxygen [22]. In the perovskite structure, the A-site is typically occupied by alkaline earth or rare earth metal elements with relatively large ionic radii, which help stabilize the overall crystal framework. The B-site is generally occupied by transition metals with smaller ionic radii, such as Mn, Co, or Fe, which predominantly determine the material’s fundamental chemical characteristics [23]. Incorporating various elements into the A or B sites can modify their reaction properties and enhance their potential applications [22,23,24,25,26]. Doped calcium manganese oxides (CaMnO3), along with La-based, Sr-based, and Ba-based perovskite oxides, are among the most extensively studied in this category [27]. These materials show significant potential for advancing thermochemical energy storage technologies due to their combined stability and adaptability in high-temperature conditions.
Jin et al. [28] discovered that CaCo0.05Mn0.95O3−δ exhibits strong redox capabilities, achieving a high theoretical enthalpy value of approximately 571 kJ/kg through redox cycling between 500 °C/0.2 atm O2 and 1000 °C/10−5 atm O2. However, research by Lucio et al. [29] indicated that actual energy storage density measured by differential scanning calorimetry (DSC) is significantly lower than theoretical predictions, with CaCr0.1Mn0.9O3 showing an energy storage density of 174 kJ/kg. Babiniec et al. [22] focused on La-based perovskites such as LaxSr1−xCoyMn1−yO3 and LaxSr1−xCoyFe1−yO3. These materials demonstrated effective redox cycling between 200 °C/0.9 atm O2 and 1000 °C/0.001 atm O2, with La0.3Sr0.7Co0.9Mn0.1O3−δ achieving a reaction enthalpy of 245 kJ/kg. Zhang et al. [21] extensively studied BaCoO3, revealing that among 12 different types of Ba/Sr-based perovskites, BaCoO3 exhibited the highest reactivity and energy storage capacity. DSC measurements showed its energy storage capability to be 292 kJ/kg during redox cycles between 600 °C/0.2 atm O2 and 1050 °C/10−6 atm O2. These materials’ thermal storage performance, based on oxygen partial pressure-controlled cycles, can achieve ideal thermal storage densities. However, the redox performance of perovskite materials in air is often compromised. Studies by Yuan et al. [30,31] demonstrated that BaCoO3 could maintain stable redox cycles in air and store and release energy over a temperature range of 400–1000 °C, showing a thermal storage density of 208 kJ/kg. Doping with Sr at the A-site can increase the reaction temperature and improve cyclic performance, with Sr0.5Ba0.5CoO3 achieving a reaction enthalpy of 202 kJ/kg. Similarly, Mn doping at the B-site can also elevate the reaction temperature, but the reaction enthalpy tends to decrease due to the limited reaction extent. While BaCoO3 is an ideal material for air-based redox cycles, its thermal storage density is limited, significantly impacting storage efficiency. Traditional A-site and B-site substitution doping primarily affects material properties by adjusting structural stability and microscopic bond energies. This approach has not substantially increased thermal storage density and can sometimes have adverse effects. Therefore, exploring new methods to enhance thermal storage density is essential.
Oxygen vacancies on the material’s surface are critical in determining its reaction activity [28]. To enhance the gas–solid reactions on the perovskite surface at lower temperatures, it is essential to generate more oxygen vacancies on the surface. In catalysis, methods such as laser treatment [32], vacuum annealing [33], chemical reduction [34], and plasma treatment [35] are commonly used to generate oxygen vacancies. However, these techniques may face stability challenges under the high-temperature conditions necessary for the cycling processes of our material. In thermochemical energy storage, one effective approach for creating oxygen vacancies in solid metal oxide materials is the introduction of foreign heteroatoms. This method offers greater thermal stability, making it more suitable for high-temperature applications [36]. These vacancies help the movement of lattice oxygen during gas–solid reactions and increase the number of active sites [37]. Alkali metal elements have well-documented self-diffusion properties, making them easy to incorporate into crystal structures. This generates more active sites that promote redox reactions [38]. In this study, the thermochemical energy storage performance of the NaxBa1−xCoO3−δ system was investigated using computational and experimental methods. Density functional theory (DFT) calculations were employed to predict the enhancing effect of sodium (Na) on thermochemical energy storage properties. A modified preparation method was used to introduce Na ions into the BaCoO3−δ crystal, and the material was comprehensively evaluated in terms of its energy storage performance, cycling stability, and changes in physicochemical properties. This research aims to provide more reliable theoretical guidance for new methods of perovskite modification.
2. Results and Discussion
2.1. DFT Calculations
DFT was employed to predict the microscopic properties of different models, guiding subsequent experiments and aiding in a deeper understanding of the mechanisms underlying modifications. The impact of substitution was analyzed by examining the crystal surface structure, as well as the formation and migration of oxygen vacancies. We developed and optimized 2 × 2 × 2 superlattice models for BaCoO3 (BC) and NaxBa1−xCoO3 (NBC). The NBC model achieved its lowest energy configuration when x = 0.125. To characterize surface reactions during gas–solid interactions, a series of SLAB models were then created using these optimized crystal cells. Figure 1a shows the surface energy calculations for various crystalline surfaces of BC and NBC materials. For the BC material, the (100)-Ba surface was found to be the most stable, with a surface energy of 0.42 J/m2. In the NBC material, surface energy calculations were performed for surfaces with different end facets. The Ba facet consistently exhibited lower surface energy compared to the Na facet, with values of 0.36 J/m2 for the (100)-Ba surface and 0.49 J/m2 for the (100)-Na surface. These findings suggest that Na doping reduces surface energy, thereby enhancing the stability of the material’s surfaces. The surface structure of NBC is likely more stable than that of BC, indicating that the introduction of Na may play a critical role in improving the material’s redox activity.

Figure 1.
DFT calculation results: (a) Surface energy calculations for different SLAB models of BC and NBC; (b) oxygen vacancy formation energy, E(Ovac), on various surfaces; (c) step diagram of surface and subsurface oxygen vacancy formation energies for SLAB models.
The thermal storage reaction process in metal oxide materials involves the transfer of oxygen atoms. Previous studies have shown that the energy required to form oxygen vacancies is closely related to the reaction characteristics [30]. Consequently, the calculation of the surface oxygen vacancy formation energy was carried out for the most stable surfaces of BC and NBC. Figure 1b illustrates the oxygen vacancy formation energies. The oxygen vacancy formation energy for A1-O on the BC surface is relatively high at 1.22 eV. In contrast, the same site on the NBC surface exhibits a significantly lower vacancy formation energy of 0.11 eV (a1-O). This indicates that the NBC (100) surface contains more reductively active oxygen than the BC (100) surface. Na doping not only stabilizes the BaCoO3 crystal surface but also reduces the oxygen vacancy formation energy. Figure 1c presents a stepwise diagram of vacancy formation energies for the migration of surface oxygen vacancies to subsurface layers. Compared to BC (100), NBC (100) demonstrates lower surface and subsurface oxygen vacancy formation energies. This suggests that Na doping facilitates the migration of surface oxygen vacancies inward, allowing subsurface oxygen ions to diffuse more easily outward. Given the well-established microscopic oxygen release mechanism [39], it can be anticipated that Na doping will enhance the diffusion and release of oxygen ions to the surface.
2.2. Microstructural Structure and Composition
Figure 2a shows the XRD patterns of BaCoO3−δ samples doped with varying proportions of Na. All synthesized NaxBa1−xCoO3−δ samples (x = 0, 0.0625, 0.1, 0.125, 0.15, 0.2) exhibit the BaCoO3−δ crystal phase (ICCD PDF #97-001-5257) [40]. However, with Na incorporation, the Na0.5CoO2 phase (ICCD PDF #97-005-5674) starts to appear in samples. The standard peak data of BaCoO3−δ and Na0.5CoO2 are listed in Tables S1 and S2. As the Na content increases, the diffraction peaks associated with the Na0.5CoO2 phase intensify, indicating its progressive formation. The Na0.5CoO2 phase could influence the overall performance of NaxBa1−xCoO3−δ, which is crucial for thermochemical energy storage applications. Figure 2b focuses on an enlarged view of the primary diffraction peaks for the BaCoO3−δ phase. The specific peak positions of the main reflections are provided in Table S3. These peaks shift and become more pronounced with increased Na doping, suggesting that Na likely enters the BaCoO3−δ lattice, causing subtle changes in the crystal structure. To evaluate the stability of Na in the NaxBa1−xCoO3−δ samples during high-temperature preparation, the metal element contents were measured using ICP analysis, as shown in Figure 2c. The levels of Ba, Co, and Na are consistent with the intended ratios used during material preparation, indicating no significant Na evaporation during the high-temperature process. This confirms that Na is stably incorporated into the BaCoO3−δ lattice.

Figure 2.
Structure and composition of Na-doped BaCoO3−δ samples: (a) XRD patterns of BaCoO3−δ doped with varying proportions of Na; (b) magnified view of the main XRD diffraction peaks of the BaCoO3−δ phase; (c) ICP results showing the metal element content of NaxBa1−xCoO3−δ samples.
Figure 3a illustrates the microstructure of BaCoO3−δ and two Na-doped BaCoO3−δ samples. The surface of the NBC6.25 sample shows a well-developed porous structure and looser packing compared to the BC sample. In contrast, the NBC12.5 sample exhibits small fragmented particles on its surface. Figure 3b displays the elemental distribution EDS mapping for NBC6.25 and NBC12.5. The NBC6.25 sample shows a uniform distribution of Na, Ba, and Co elements across the surface. However, certain areas on the NBC12.5 surface reveal significant variations in Na concentration, with Na-rich, Ba-deficient, and Co-rich regions indicating the presence of Na0.5CoO2 crystals on the surface. These observations, combined with the XRD results, suggest that Na can be incorporated into the BaCoO3−δ lattice, but as the doping level increases, Na tends to precipitate as Na0.5CoO2 crystals. The effects of lattice-incorporated Na and surface-deposited Na0.5CoO2 on the material’s reactivity will be discussed in the following sections.

Figure 3.
Microscopic surface structure and elemental distribution of samples: (a) SEM images of BC, NBC6.25, and NBC12.5; (b) EDS mapping results for NBC6.25 and NBC12.5.
2.3. Heat Storage Performance
The performance of thermochemical heat storage materials for practical applications is primarily assessed by evaluating reaction extent, reaction enthalpy, reaction kinetics, and cyclic stability [41]. To investigate these properties in the synthesized NaxBa1−xCoO3−δ samples, TG and DSC experiments were conducted. Mass change (Δm) was used as an indicator of the degree of reaction [26]. Figure 4a presents the TG curves for the reduction and oxidation reactions of the NaxBa1−xCoO3−δ samples. The Na-doped samples exhibit significantly enhanced reduction extents compared to the undoped BC sample, demonstrating higher activity and improved kinetics during reduction at 920–980 °C and oxidation at 700–870 °C. The raw TG data and the thermogravimetric hysteresis curve, shown in Figure S1, indicate that Na0.0625Ba0.9375CoO3−δ exhibits exceptional reversibility, with a recovery rate exceeding 94%. However, while materials containing Na0.5CoO2 crystals show greater reduction extents, they also experience irreversible mass loss at high temperatures, a phenomenon that intensifies with increasing Na content. Therefore, the presence of Na0.5CoO2 crystals imposes some limitations on the reversibility of the reaction.

Figure 4.
Thermal reaction characteristics: (a) TG curves of NaxBa1−xCoO3−δ samples (showing reduction reaction during heating and oxidation reaction during cooling); (b) comparison of oxidation/reduction reaction enthalpies for BC, NBC6.25, NBC12.5, NBC15; (c) TG and DSC curves of BC sample (pre-Na doping); (d) TG and DSC curves of NBC6.25 sample (post-Na doping).
To elucidate the enhancement in reaction enthalpy, we conducted a comprehensive analysis of the thermal storage performance. Figure 2b presents the reduction and oxidation enthalpies of BC, NBC6.25, NBC12.5, and NBC15, measured by DSC. The differences between the reduction and oxidation enthalpies for each material are relatively small, indicating a high degree of reversibility in heat storage and release. The heat storage densities for BC, NBC6.25, NBC12.5, and NBC15 are 197.4 kJ/kg, 341.7 kJ/kg, 320.4 kJ/kg, and 337.1 kJ/kg, respectively. Notably, NBC6.25 exhibits the highest heat storage density, which is 75% greater than that of BC. Figure 2c,d illustrate the DSC results for BC and NBC6.25. During heating, the main endothermic peak for BC appears around 950 °C, while the main exothermic peak during cooling occurs around 890 °C. NBC6.25 shows similar peak positions but with significantly enhanced peak intensities. Figure S2 displays the thermal storage performance of NBC10 and NBC12.5. The presence of Na0.5CoO2 leads to increased peak intensities for the endothermic reactions between 920 and 980 °C and exothermic reactions between 700 and 870 °C. This suggests that Na doping enhances the oxygen release capacity at high temperatures, improving both the reaction kinetics and reaction enthalpy, thereby increasing the material’s practical application potential. Table 1 compares the thermochemical performance of various Co-based perovskites. The air cycle exhibited lower redox activity compared to the oxygen-concentration-controlled cycle, and the reaction enthalpies measured via DSC were generally lower than those calculated using the Van’t Hoff and Point-defect model. Notably, the chemical reaction enthalpy (ΔHch) of the Na0.0625Ba0.9375CoO3−δ material demonstrated outstanding performance among air-cycled perovskites, positioning it as a highly promising candidate for thermochemical heat storage applications.

Table 1.
Key parameters of Co-family perovskite oxides for thermochemical energy storage.
2.4. Surface Chemistry and Phase Evolution
To gain a more precise understanding of lattice evolution during the heat storage reaction, XRD was used to characterize the phase changes in BC and NBC6.25 during the reduction process at 800–1050 °C, as shown in Figure 5a,b (see raw data in Figure S3). During heating, both BC and NBC6.25 undergo lattice expansion, forming a uniform solid solution with a hexagonal structure similar to BaCoO3 at 800 °C. As the temperature increases, the main diffraction peaks of the (101) and (110) planes of BaCoO3 shift to varying degrees, indicating significant changes in lattice constants associated with oxygen release. When the temperature exceeds 930 °C, both BC and NBC6.25 experience a drastic phase transition, resulting in the formation of a uniform cubic phase. At 950–1050 °C, the diffraction peaks continue to shift toward lower angles, indicating further oxygen release. The cubic phase of NBC shows more pronounced activity in the (100) diffraction peak at high temperatures compared to BC (100), consistent with predictions from DFT calculations. Figure S4 illustrates the morphological changes in BC and NBC6.25 during redox cycles. Initially, both untreated BC and NBC6.25 exhibit loosely packed grains. After the reduction reaction, the grain morphology of both samples becomes uniform and smooth. Compared to BC, NBC6.25 displays smaller grain sizes and a more developed porous structure, likely due to a more intense oxygen release reaction. Following oxidation, BC and NBC6.25 revert to their initial grain sizes and porous structures.
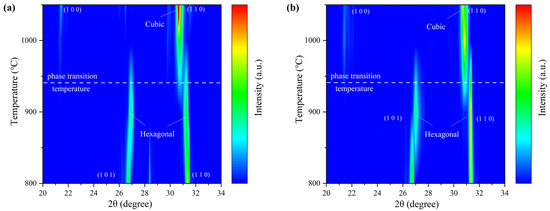
Figure 5.
Phase evolution during one redox cycle: (a) XRD pattern evolution of BC during reduction process (800–1050 °C); (b) XRD pattern evolution of NBC6.25 during reduction process (800–1050 °C).
The surface chemistry plays a crucial role in determining the nature of gas–solid reactions [44]. To understand how Na doping influences the reaction properties, changes in the material’s surface properties were analyzed. Figure 6a compares the Ba 4d spectra of BC and NBC6.25. The Ba 4d orbital was selected to assess the Na doping effect, as the XPS peaks of Ba 3d and Co 2p overlap. The Ba 4d spectra are divided into two components, corresponding to BaⅡ (~88.7 eV) and BaⅠ (~87.3 eV), reflecting the presence of Ba ions in different coordination environments on the surface of BaCoO3−δ crystals [45,46]. The introduction of Na increases the presence of BaII ions, which have a lower binding energy. This implies a higher concentration of Ba2⁺ ions with greater coordination on the crystal surface, leading to an accumulation of surface oxygen. This effect is likely because Na has a lower charge than Ba, requiring additional BaII ions at the A-site to coordinate with oxygen and preserve electrical neutrality. Consequently, this condition may facilitate the creation of oxygen vacancies on the surface of Na-doped materials.
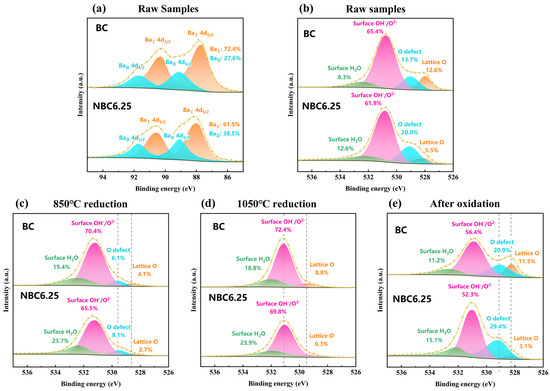
Figure 6.
XPS spectra analysis: (a) comparison of XPS Ba4d spectra for raw BC and NBC6.25; (b) comparison of XPS O1s spectra for raw BC and NBC6.25; (c) comparison of XPS O1s spectra for reduced BC and NBC6.25 at 850 °C; (d) comparison of XPS O1s spectra for reduced BC and NBC6.25 at 1050 °C; (e) comparison of XPS O1s spectra for BC and NBC6.25 after oxidation.
The O1s spectra of BC and NBC6.25 are compared in Figure 6b. The O1s spectra were fitted to four components corresponding to lattice oxygen (lattice O, ~528.3 eV), defective oxygen (O defect, ~529.2 eV), surface-adsorbed OH− and O2− (surface OH−/O2−, ~531.0 eV), and surface-adsorbed H2O species (Surface H2O, ~532.2 eV) [47,48]. Surface-adsorbed species account for the vast majority of the O1s spectra of BC and NBC6.25 samples. Surface defect oxygen and lattice oxygen, although not in high amounts, are the main components affecting the gas-solid reaction activity. Surface defect oxygen is a weakly charged species, and perovskite materials containing this oxygen species are prone to form oxygen vacancies on the surface [30]. The proportion of defect oxygen in NBC6.25 (20.0%) is significantly higher than in BC (15.7%), suggesting that NBC6.25 is more likely to form oxygen vacancies on its surface. To understand the changes in the surface chemical state of oxygen during the reaction, XPS analysis was performed on reduced samples at 850 °C and 1050 °C. Figure 6c shows that at 850 °C, the content of both lattice oxygen and defect oxygen in the reduced hexagonal phases of BC and NBC6.25 decreased significantly. The levels of lattice oxygen and defect oxygen in BC and NBC6.25 were very similar, though NBC6.25 had a slightly higher defect oxygen content. As the temperature increased, defect oxygen on the cubic phase surfaces was progressively released. Figure 6d presents the O1s spectra for BC and NBC6.25 reduced at 1050 °C. The samples were primarily composed of fully reduced cubic phases, where defect oxygen was no longer present, and the electronegativity of oxygen increased substantially. The binding energy of lattice oxygen (~529.4 eV) also increased significantly [49]. NBC6.25 had a slightly lower lattice oxygen content compared to BC, indicating a more complete oxygen release reaction in the NBC materials. As shown in Figure 6e, after re-oxidation, both BC and NBC6.25 regained their defect oxygen, with NBC6.25 still displaying a higher level than BC. This suggests that Na doping enhances the material’s ability to retain defect oxygen. In other words, Na doping facilitates the formation of oxygen vacancies on the sample surface, consistent with the DFT calculation predictions. Consequently, in thermochemical energy storage reactions, NBC6.25 not only demonstrates a stronger potential for oxygen release but also effectively absorbs more defect oxygen, maintaining stable surface chemical properties during oxidation. Electron paramagnetic resonance (EPR) measurements further confirm the increased oxygen vacancy density in the Na-doped samples, as shown in Figure S5. Both the XPS and EPR results confirm that Na doping enhances the activity of surface oxygen vacancies, which is consistent with the findings from DFT calculations.
2.5. Properties Evolution during Cycling
To comprehensively evaluate the impact of Na doping on the material’s properties, cycling tests were conducted on the optimized NBC6.25 material. For comparison, NBC12.5 was selected as a control group to examine the effect of Na0.5CoO2 on reaction characteristics. Figure 7a shows the TG curves of BC, NBC6.25, and NBC12.5 after 10 cycles. NBC6.25 maintained its excellent reaction performance throughout the testing. Notably, NBC12.5 did not exhibit irreversible mass loss after 10 cycles, indicating a significant improvement in reaction reversibility. Moreover, NBC12.5 demonstrated a higher reaction extent compared to both BC and NBC6.25. Figure 7b presents the XRD results of BC, NBC6.25, and NBC12.5 after 10 cycles. The crystal structures of BC and NBC6.25 showed minimal changes after cycling, consistent with the TG test results. However, the Na0.5CoO2 crystals in NBC12.5 disappeared after ten cycles. To investigate the behavior of the Na element during cycling, the elemental distribution in NBC12.5 before and after cycling was analyzed. Figure 7c,d display the TEM-EDS mapping of NBC12.5. Before cycling, NBC12.5 exhibited a uniform distribution of Ba and Co elements, with Na concentrated in certain regions, likely corresponding to Na0.5CoO2 crystals attached to the grain surfaces. After cycling, the distribution of Na became consistent with Ba and Co, with no regions of Na enrichment or Ba depletion. This suggests that Na⁺ ions from Na0.5CoO2 diffused into the BaCoO3−δ lattice during high-temperature cycling, likely facilitated by the Kirkendall effect at the interface between Na0.5CoO2 and BaCoO3−δ [50].
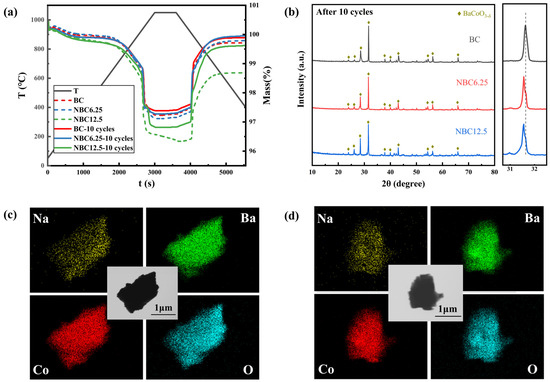
Figure 7.
Evolution of the reactivity, crystal structure, and morphology of samples during short-term cycling: (a) TG curves of BC, NBC6.25, and NBC12.5 before and after 10 cycles; (b) XRD results of BC, NBC6.25, and NBC12.5 after 10 cycles; (c) TEM morphology and EDS elemental distribution images of raw NBC12.5; (d) TEM morphology and EDS elemental distribution images of NBC12.5 after 10 cycles.
Figure 8a illustrates the reaction activity of NBC6.25 and NBC12.5 after 150, 300, and 450 cycles (see raw data in Figure S6). Both materials maintained stable performance with no significant degradation observed during the first 300 cycles. Interestingly, NBC12.5 even exhibited an increase in reaction activity during this period. After 450 cycles, a slight reduction in redox activity was noted for both materials, with NBC6.25 retaining 96% of its initial activity and NBC12.5 retaining 93%, which are still significantly higher than that of freshly prepared BC. Figure 8b,c present the XRD results of NBC6.25 and NBC12.5 before and after 150, 300, and 450 cycles. The crystal composition of NBC6.25 remained stable throughout the cycling process, indicating a relatively unchanged structure. Consistent with the short-term cycling results, the Na0.5CoO2 phase in NBC12.5 disappeared during cycling and was undetectable after 150 cycles. Overall, both materials demonstrated good lattice stability during long-term cycling. Figure 9a–f depict the microstructural evolution of NBC6.25 and NBC12.5 during long-term cycling. Before cycling, both NBC6.25 and NBC12.5 exhibited relatively loose microstructures with well-developed porosity. After 150 cycles, the structures of both samples became denser, although NBC12.5 retained more porous structures on its surface, likely due to the diffusion of Na⁺ ions. After 450 cycles, significant sintering occurred in both samples, resulting in increased structural density and reduced porosity. This extensive sintering is likely responsible for the observed decrease in redox activity after 450 cycles [20]. In conclusion, Na doping significantly enhances the thermochemical energy storage performance of these materials, providing reliable stability during cycling. The NaxBa1−xCoO3−δ materials exhibit a comprehensive and stable improvement in heat storage capacity, making them promising candidates for high-performance thermal energy storage applications.

Figure 8.
Evolution of the reactivity and crystal structure of samples during long-term cycling: (a) reversible reaction rates of NBC6.25 and NBC12.5 before and after 150, 300, and 450 cycles; (b) XRD patterns of NBC6.25 before and after 150, 300, and 450 cycles; (c) XRD patterns of NBC12.5 before and after 150, 300, and 450 cycles.
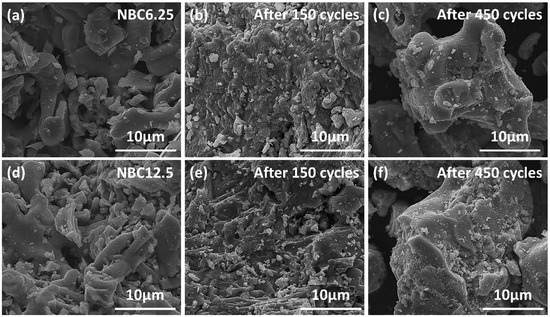
Figure 9.
Morphology evolution of samples during cycling (SEM images): (a) raw NBC6.25; (b) NBC6.25 after 150 cycles; (c) NBC6.25 after 450 cycles; (d) raw NBC12.5; (e) NBC12.5 after 150 cycles; (f) NBC12.5 after 450 cycles.
3. Materials and Methods
3.1. Computational Methods
In this study, density functional theory (DFT) calculations were performed using the Vienna Ab-initio Simulation Package (VASP 5.4.4). The calculations employed the projector augmented wave (PAW) method and the Perdew-Wang 91 functional (a type of generalized gradient approximation, GGA). To improve the description of systems with strongly correlated d or f electrons, the DFT+U method was applied. Initial lattice parameters for the cubic phase of BaCoO3 (a = b = c = 3.98 Å, α = β = γ = 90°) and the U value for the 3d orbitals of cobalt (UCo = 3.32 eV) were obtained from the Materials Project database [51]. Based on convergence test results, a 2 × 2 × 2 supercell model of NaxBa1−xCoO3, consisting of 80 atoms, was constructed, with some Ba atoms replaced by Na atoms. Lattice optimization was performed first, using a 9 × 9 × 9 k-point grid generated by the Monkhorst–Pack method. Spin polarization was considered during the calculations. The kinetic energy cutoff for the plane-wave basis set was set to 520 eV, and the energy convergence criterion was 1 × 10−5 eV. The conjugate gradient algorithm was used to relax the ions to their instantaneous ground state until the force on each ion was less than 0.05 eV/Å. After optimizing the lattice, various SLAB models with different terminations were created by cutting the crystal model along different crystallographic indices. The surface energies were calculated by performing relaxation optimizations using these SLAB models. The SLAB model optimizations used a 3 × 3 × 1 k-point grid with convergence parameters consistent with those used in the initial lattice optimization. The formation energies of oxygen vacancies on the screened stable surfaces and subsurfaces were then calculated. The method for calculating the oxygen vacancy formation energy is given by Equation (1), as shown below:
Here, Edefect represents the total energy of the relaxed SLAB model containing an oxygen vacancy, denotes the chemical potential of a free oxygen molecule, and Etotal is the total energy of the relaxed SLAB model without any vacancies.
3.2. Sample Preparation
The modified Pechini method [52] was used to synthesize the NaxBa1−xCoO3−δ samples, with sample numbers and compositions listed in Table 2. Barium nitrate, cobalt nitrate, and sodium nitrate were dissolved in deionized water according to their stoichiometric ratios. Citric acid, ethylenediaminetetraacetic acid (EDTA), and ethylene glycol were then added to the solution. The molar ratio of citric acid, EDTA, ethylene glycol, and total metal ions was maintained at 1.2:0.6:0.8:1. The pH of the solution was adjusted to 8 using ammonia. The mixture was then stirred in a water bath at 80 °C for 5 h to form a gel. The resulting gel was dried in a forced-air oven at 200 °C for 3 h. The dried precursor was transferred to a muffle furnace and pre-calcined at 300 °C for 3 h to remove organic components, followed by calcination at 1000 °C for 5 h. After cooling, the sample was reheated to 1050 °C, held for 10 min, and then cooled to room temperature. The final product was obtained by grinding the resulting powder. To analyze the evolution of crystal phases and surface chemistry during heating, reduced samples were prepared at different temperatures for further characterization and analysis. The samples were heated in an air atmosphere from room temperature to 800 °C, 850 °C, 900 °C, 1000 °C, and 1050 °C, at a rate of 20 °C per min, holding each sample at the target temperature for 10 min. Subsequently, the samples were cooled to room temperature at a rate of −20 °C per min using nitrogen gas. This process produced reduced samples at each specified temperature.

Table 2.
Samples prepared and abbreviations.
3.3. Characterization
X-ray diffraction (XRD) data were collected using a X’Pert Powder instrument manufactured by PANalytical (Almelo, The Netherlands) with Cu Kα radiation, operating at an extraction voltage of 40 kV and a current of 40 mA. Scans covered a 2θ range from 10° to 90°, with a step size of 0.01° and a scan rate of 10°/min. Changes in the morphology of the samples after redox cycles were observed using a Regulus 8100 scanning electron microscope (SEM) manufactured by Hitachi (Tokyo, Japan). An Energy Dispersive Spectrometer (EDS) was employed to analyze the distribution of elements in the synthesized samples. X-ray Photoelectron Spectroscopy (XPS) was conducted using a K-Alpha spectrometer manufactured by Thermo Fisher Scientific (Waltham, MA, USA), with the C1s peak at 284.8 eV used for energy calibration.
3.4. Thermodynamics Measurements
Perovskite materials can release oxygen during reduction and absorb oxygen during oxidation, resulting in a change in mass. To evaluate these reaction properties, thermogravimetric (TG) experiments were performed using a TGA55 instrument by TA (New Castle, DE, USA). Approximately 10 mg of the sample was used to quickly reach equilibrium in the redox reaction and obtain precise data. The change in mass during testing, denoted as Δm, was used to assess the reactivity of the thermal storage material. The thermogravimetric experiment included a single redox cycle, in which the sample was heated in air from 50 °C to 1050 °C at a rate of 20 °C/min and held at 1050 °C for 10 min, and then cooled to 400 °C at a rate of −20 °C/min to allow re-oxidation. Throughout the process, a constant gas flow of 100 N·mL/min at atmospheric pressure was maintained. A blank run was conducted under identical conditions to correct for buoyancy effects. The TG curves were analyzed to determine parameters such as reaction rate, reoxidation rate, and initial reaction temperature. Differential Scanning Calorimetry (DSC) was performed under the same temperature and gas conditions as TG, and data from the second redox cycle were used to calculate the reaction enthalpy of the sample.
To investigate the cyclic stability of doped BaCoO3−δ, three samples with the best reaction performance were selected, including NBC6.25 and NBC12.5. These samples underwent 450 cycles between 600 °C and 1050 °C in an air atmosphere using a tubular furnace. Performance tests were conducted every 150 cycles. At each test point, TG analysis was used to assess redox performance, while SEM was utilized to observe the microstructural changes in the samples.
4. Conclusions
A novel Na doping method was proposed to enhance the thermochemical energy storage performance of BaCoO3-based materials. The mechanism behind this enhancement was explored using a combination of DFT calculations and experimental approaches. Firstly, DFT+U calculations were performed on the solid solution NBC, revealing that Na doping increases the stability of the BaCoO3 crystal planes, enhances the activity of surface oxygen ions, and facilitates the formation and inward migration of oxygen vacancies on the surface. Subsequently, NaxBa1−xCoO3−δ solid solutions were synthesized using an improved sol–gel method, and the effects of Na doping on the microstructure, heat storage capacity, and cycling performance were comprehensively evaluated. XRD and ICP results confirmed the successful incorporation of Na into the BaCoO3−δ lattice, and that excess Na leads to the formation of a Na0.5CoO2 crystal phase. TG and DSC tests demonstrated that Na doping significantly boosts the material’s redox activity, increasing heat storage density by more than 50%, with the Na0.0625Ba0.9375CoO3−δ solid solution achieving a heat storage density of 341.7 kJ/kg. The XPS analysis indicates that Na doping significantly raises the concentration of surface defect oxygen, which results in an increased number of active sites for oxygen release at elevated temperatures. This improvement in redox activity is consistent with the predictions made by DFT calculations. The TEM results indicate that when excess Na is present, the Na0.5CoO2 crystal phase undergoes the Kirkendall effect with BaCoO3−δ during high-temperature cycling, promoting the formation of a homogeneous solid solution, NaxBa1−xCoO3−δ. Once Na is uniformly distributed within the lattice, the material’s thermal energy storage performance is significantly enhanced. Cycling experiments show that the NBC material exhibits almost no performance degradation after 300 cycles. After 450 cycles, the reaction activity of the Na-doped composite material slightly decreases, but the redox activity still remains significantly superior to that of fresh BaCoO3−δ material. The SEM results indicate that the primary cause of performance degradation is the sintering of crystals. In conclusion, the NaxBa1−xCoO3−δ solid solution not only demonstrates a substantial increase in heat storage density but also exhibits excellent structural stability after multiple cycles, making it a highly promising material for thermochemical energy storage. This study clearly explains the enhancement mechanism of Na doping on the thermochemical heat storage performance of BaCoO3−δ and provides a new idea for designing perovskite materials. In future research, exploring alternative synthesis methods capable of introducing alkali metals, such as plasma treatment, irradiation, or impregnation techniques, may offer new insights into optimizing vacancy formation and enhancing material performance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics12100266/s1: Table S1: The diffraction peak data of BaCoO3−δ (ICCD PDF #97-001-5257); Table S2: The diffraction peak data of Na0.5CoO2 (ICCD PDF #97-005-5674); Table S3: The diffraction peak positions of key crystal planes for each sample; Figure S1: The raw TG data of samples; Figure S2: DSC results of the thermal storage performance; Figure S3: The crystal phase composition changes during reduction at 800–1050 °C characterized by XRD; Figure S4: Phase evolution during one redox cycle: SEM morphology images of BC and NBC6.25 after reduction and oxidation; Figure S5: EPR spectra of BC and NBC samples; Figure S6: The raw TG data of samples before and after 150, 300 and 450 cycles.
Author Contributions
Conceptualization, Z.N.; Data curation, Z.N.; Formal analysis, Z.N.; Funding acquisition, G.X.; Investigation, Z.N.; Methodology, Z.N.; Project administration, G.X.; Resources, G.X.; Software, J.Z.; Supervision, J.Z.; Validation, Y.H.; Visualization, Y.H.; Writing—original draft, Z.N.; Writing—review and editing, Y.H., P.Z., D.C., F.Y. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [No. 52176207], the National Natural Science Foundation of China [No. 52325605], and the Fundamental Research Funds for the Central Universities [2022ZFJH004].
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sadeghi, G. Energy storage on demand: Thermal energy storage development, materials, design, and integration challenges. Energy Storage Mater. 2022, 46, 192–222. [Google Scholar] [CrossRef]
- Woods, J.; Mahvi, A.; Goyal, A.; Kozubal, E.; Odukomaiya, A.; Jackson, R. Rate capability and Ragone plots for phase change thermal energy storage. Nat. Energy 2021, 6, 295–302. [Google Scholar] [CrossRef]
- Sharan, P.; Turchi, C.; Kurup, P. Optimal design of phase change material storage for steam production using annual simulation. Solar Energy 2019, 185, 494–507. [Google Scholar] [CrossRef]
- Amy, C.; Seyf, H.R.; Steiner, M.A.; Friedman, D.J.; Henry, A. Thermal energy grid storage using multi-junction photovoltaics. Energy Environ. Sci. 2019, 12, 334–343. [Google Scholar] [CrossRef]
- Mavrigiannaki, A.; Ampatzi, E. Latent heat storage in building elements: A systematic review on properties and contextual performance factors. Renew. Sust. Energ. Rev. 2016, 60, 852–866. [Google Scholar] [CrossRef]
- Ling, Z.Y.; Zhang, Z.G.; Shi, G.Q.; Fang, X.M.; Wang, L.; Gao, X.N.; Fang, Y.T.; Xu, T.; Wang, S.F.; Liu, X.H. Review on thermal management systems using phase change materials for electronic components, Li-ion batteries and photovoltaic modules. Renew. Sust. Energ. Rev. 2014, 31, 427–438. [Google Scholar] [CrossRef]
- Yang, T.Y.; Kang, J.G.; Weisensee, P.B.; Kwon, B.; Braun, P.V.; Miljkovic, N.; King, W.P. A composite phase change material thermal buffer based on porous metal foam and low-melting-temperature metal alloy. Appl. Phys. Lett. 2020, 116, 071901. [Google Scholar] [CrossRef]
- Li, G. Sensible heat thermal storage energy and exergy performance evaluations. Renew. Sust. Energ. Rev. 2016, 53, 897–923. [Google Scholar] [CrossRef]
- Seyitini, L.; Belgasim, B.; Enweremadu, C.C. Solid state sensible heat storage technology for industrial applications-A review. J. Energy Storage 2023, 62, 106919. [Google Scholar] [CrossRef]
- Prasad, J.S.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energ. 2019, 254, 113733. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.Y.; Markides, C.N.; Wang, H.; Li, W. Medium- and high-temperature latent and thermochemical heat storage using metals and metallic compounds as heat storage media: A technical review. Appl. Energ. 2020, 280, 115950. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhao, C.Y. Prediction of the effective thermal conductivity of packed bed with micro-particles for thermochemical heat storage. Sci. Bull. 2017, 62, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Wang, L.; Ling, H.S.; Ge, Z.W.; Lin, X.P.; Dai, X.J.; Chen, H.S. Critical review of thermochemical energy storage systems based on cobalt, manganese, and copper oxides. Renew. Sust. Energ. Rev. 2022, 158, 112076. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Hosseini, H. A review of material screening in pure and mixed-metal oxide thermochemical energy storage (TCES) systems for concentrated solar power (CSP) applications. Renew. Sust. Energ. Rev. 2018, 98, 9–26. [Google Scholar] [CrossRef]
- Wu, S.K.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energ. Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Andre, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO redox cycle for solar thermochemical energy storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef]
- Pelay, U.; Lu, L.A.; Fan, Y.L.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sust. Energ. Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Pagkoura, C.; Karagiannakis, G.; Zygogianni, A.; Lorentzou, S.; Konstandopoulos, A.G. Cobalt oxide based honeycombs as reactors/heat exchangers for redox thermochemical heat storage in future CSP plants. Enrgy Proced. 2015, 69, 978–987. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Solar Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Andre, L.; Abanades, S. Experimental assessment of oxygen exchange capacity and thermochemical redox cycle behavior of Ba and Sr series perovskites for solar energy storage. Solar Energy 2016, 134, 494–502. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Coker, E.N.; Ambrosini, A.; Miller, J.E. ABO3 (A = La, Ba, Sr, K.; B = Co, Mn, Fe) Perovskites for Thermochemical Energy Storage. Aip Conf. Proc. 2016, 1734, 050006. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Huck, P.; Horton, M.; Guban, D.; Zhu, L.Y.; Lu, Y.J.; Persson, K.A.; Roeb, M.; Sattler, C. Materials design of perovskite solid solutions for thermochemical applications. Energy Environ. Sci. 2019, 12, 1369–1384. [Google Scholar] [CrossRef]
- Sun, C.W.; Alonso, J.A.; Bian, J.J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2021, 11, 2000459. [Google Scholar] [CrossRef]
- Popczun, E.J.; Tafen, D.; Natesakhawat, S.; Marin, C.M.; Nguyen-Phan, T.D.; Zhou, Y.Y.; Alfonso, D.; Lekse, J.W. Temperature tunability in Sr1-xCaxFeO3-δ for reversible oxygen storage: A computational and experimental study. J. Mater. Chem. A 2020, 8, 2602–2612. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Cai, R.X.; Bektas, H.; Wang, X.J.; McClintock, K.; Teague, L.; Yang, K.R.; Li, F.X. Accelerated Perovskite Oxide Development for Thermochemical Energy Storage by a High-Throughput Combinatorial Approach. Adv. Energy Mater. 2023, 13, 2203833. [Google Scholar] [CrossRef]
- Jin, F.; Xu, C.; Yu, H.Y.; Xia, X.; Ye, F.; Li, X.; Du, X.Z.; Yang, Y.P. CaCo0.05Mn0.95O3-δ: A Promising Perovskite Solid Solution for Solar Thermochemical Energy Storage. Acs Appl. Mater. Inter. 2021, 13, 3856–3866. [Google Scholar] [CrossRef] [PubMed]
- Lucio, B.; Romero, M.; Gonzalez-Aguilar, J. Analysis of solid-state reaction in the performance of doped calcium manganites for thermal storage. Solid. State Ion. 2019, 338, 47–57. [Google Scholar] [CrossRef]
- Yuan, P.; Xu, H.R.; Ning, Z.Y.; Xiao, G. Understanding thermochemical energy storage performance of Ba1-xSrxCoO3-δ perovskite system: A computational and experimental study. J. Energy Storage 2023, 61, 106695. [Google Scholar] [CrossRef]
- Yuan, P.; Gu, C.D.; Xu, H.R.; Ning, Z.Y.; Cen, K.F.; Xiao, G. Regulating thermochemical redox temperature via oxygen defect engineering for protection of solar molten salt receivers. Iscience 2021, 24, 103039. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.B.; Chen, Q.; Wang, D.; Guo, W.; Cheng, D.X.; Sha, Y.; Mokhtar, M.Z.; Jia, Z.Y.; Jacobs, J.; Thomas, A.G.; et al. Laser processing of Li-doped mesoporous TiO for ambient-processed mesoscopic perovskite solar cells. J. Mater. Chem. C 2024, 12, 2025–2036. [Google Scholar] [CrossRef]
- Yan, F.B.; Wu, J.R.; Ning, S.; Luo, F. Orienting Oxygen Vacancy Channels in Brownmillerite Strontium Ferrite Thin Films Using Strain: Implications for Facile Oxygen Ion Transport. Acs Appl. Nano Mater. 2024, 7, 7703–7708. [Google Scholar] [CrossRef]
- Jain, N.; Roy, A.; De, A. Ba-addition induced enhanced surface reducibility of SrTiO: Implications on catalytic aspects. Nanoscale Adv. 2019, 1, 4938–4946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Wang, C.C.; Zhao, S.; Xue, F.; Li, L.; Cui, M.F.; Qiao, X.; Fei, Z.Y. Plasma-reconstructed LaMnO nanonetwork supported palladium catalyst for methane catalytic combustion. J. Environ. Chem. Eng. 2023, 11, 109825. [Google Scholar] [CrossRef]
- Peña, J.A.; Lorente, E.; Romero, E.; Herguido, J. Kinetic study of the redox process for storing hydrogen reduction stage. Catalysis Today 2006, 116, 439–444. [Google Scholar] [CrossRef]
- Wang, H.M.; Liu, G.C.; Veksha, A.; Dou, X.M.; Giannis, A.; Lim, T.T.; Lisak, G. Iron ore modified with alkaline earth metals for the chemical looping combustion of municipal solid waste derived syngas. J. Clean. Prod. 2021, 282, 124467. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Li, Y.L.; Guo, Q.; Zhang, L.; Qi, T. Promoting the reduction reactivity of magnetite by introducing trace-K-ions in hydrogen direct reduction. Int. J. Hydrogen Energy 2023, 48, 18177–18186. [Google Scholar] [CrossRef]
- Jackson, G.S.; Imponenti, L.; Albrecht, K.J.; Miller, D.C.; Braun, R.J. Inert and Reactive Oxide Particles for High-Temperature Thermal Energy Capture and Storage for Concentrating Solar Power. J. Sol. Energ.-T Asme 2019, 141, 021016. [Google Scholar] [CrossRef]
- Zhou, D.H.; Le, F.H.; Jia, W.; Chen, X.H. In Situ Exsolution of Ba(VO) Nanoparticles on a V-Doped BaCoO Perovskite Oxide with Enhanced Activity for Electrocatalytic Hydrogen Evolution. Inorg. Chem. 2023, 62, 8001–8009. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Understanding Redox Kinetics of Iron-Doped Manganese Oxides for High Temperature Thermochemical Energy Storage. J. Phys. Chem. C 2016, 120, 27800–27812. [Google Scholar] [CrossRef]
- Chen, X.Y.; Kubota, M.; Yamashita, S.; Kita, H. Investigation of Sr-based perovskites for redox-type thermochemical energy storage media at medium-high temperature. J. Energy Storage 2021, 38, 102501. [Google Scholar] [CrossRef]
- Gokon, N.; Yawata, T.; Bellan, S.; Kodama, T.; Cho, H.S. Thermochemical behavior of perovskite oxides based on LaSr(Mn, Fe, Co)O and BaySrCoO redox system for thermochemical energy storage at high temperatures. Energy 2019, 171, 971–980. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at surfaces: From atoms to complexity (Nobel lecture). Angew. Chem. Int. Edit 2008, 47, 3524–3535. [Google Scholar] [CrossRef]
- Mcintyre, N.S.; Cook, M.G. X-ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel, and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Vasquez, R.P.; Rupp, M.; Gupta, A.; Tsuei, C.C. Electronic-Structure of Hgba2cacu2o6+Delta Epitaxial-Films Measured by X-ray Photoemission. Phys. Rev. B 1995, 51, 15657–15660. [Google Scholar] [CrossRef]
- Jia, C.H.; Xiang, X.P.; Zhang, J.; He, Z.Y.; Gong, Z.H.; Chen, H.J.; Zhang, N.; Wang, X.W.; Zhao, S.J.; Chen, Y. Shifting Oxygen Evolution Reaction Pathway via Activating Lattice Oxygen in Layered Perovskite Oxide. Adv. Funct. Mater. 2023, 33, 2301981. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, I.; Ahn, S.; Oh, S.; Im, H.; Bae, H.; Song, S.J.; Lee, C.W.; Jung, W.C.; Lee, K.T. On the Role of Bimetal-Doped BaCoO Perovskites as Highly Active Oxygen Electrodes of Protonic Ceramic Electrochemical Cells. Adv. Energy Mater. 2024, 14, 2304059. [Google Scholar] [CrossRef]
- Xiang, D.; Gu, C.D.; Xu, H.R.; Xiao, G. Self-Assembled Structure Evolution of Mn-Fe Oxides for High Temperature Thermochemical Energy Storage. Small 2021, 17, 2101524. [Google Scholar] [CrossRef]
- Pegios, N.; Bliznuk, V.; Theofanidis, S.A.; Galvita, V.V.; Marin, G.B.; Palkovits, R.; Simeonov, K. Ni nanoparticles and the Kirkendall effect in dry reforming of methane. Appl. Surf. Sci. 2018, 452, 239–247. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Shen, Q.W.; Zheng, Y.; Li, S.A.; Ding, H.R.; Xu, Y.Q.; Zheng, C.G.; Thern, M. Optimize process parameters of microwave-assisted EDTA method using orthogonal experiment for novel BaCoO3-delta perovskite. J. Alloys Compd. 2016, 658, 125–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).