Metal Complexes with Schiff Bases as Antimicrobials and Catalysts
Abstract
:1. Introduction
2. SBs Complexes with Metals as Antimicrobials
2.1. SBs Complexes with Transition Metals
| Structure | Compd | MIC or IZD | Ref. |
|---|---|---|---|
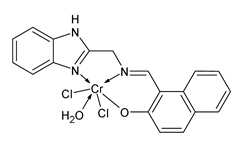 | C19H16O2N3 Cl2Cr (1) | IZD = 23 mm (E. coli) IZD = 24 mm (S. subtilis) IZD = 22 mm (A. niger) | Aroua et al. (2023) [66] |
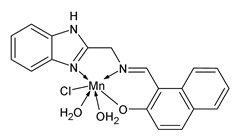 | C19H18O3N3 ClMn (2) | IZD = 26 mm (E. coli) IZD = 24 mm (S. subtilis) IZD = 25 mm (A. niger) | Aroua et al. (2023) [66] |
 | (Co(L)(Cl)2(H2O)2 (3) | IZD = 15 mm (S. enterica ser. thypi at 30 mg/mL) IZD = 19 mm (C. albicans at 30 mg/mL) | Alorini et al. (2023) [67] |
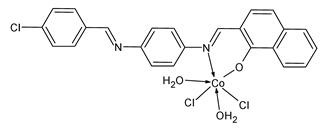
 | 4 | MIC = 25 ± 1.10 mm (S. aureus) MIC = 28 ± 1.10 mm (P. aeruginosa) | Al-Janabi et al. (2023) [68] |
 | 5 | MIC = 0.0225 µmol/mL (S. aureus MTCC 2901) MIC = 0.0112 µmol/mL (B. subtilis NCIM 2063) MIC = 0.0225 µmol/mL (E. coli MTCC 732) MIC = 0.0112 µmol/mL (P. aeruginosa MTCC 424) MIC = 0.0056 µmol/mL (C. albicans MTCC 227) MIC = 0.0112 µmol/mL (A. niger MTCC 9933) | Devi et al. (2022) [70] |
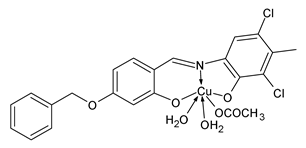 | 6 | MIC = 0.0223 µmol/mL (S. aureus MTCC 2901) MIC = 0.0223 µmol/mL (B. subtilis NCIM 2063) MIC = 0.0223 µmol/mL (E. coli MTCC 732) MIC = 0.0111 µmol/mL (P. aeruginosa MTCC 424) MIC = 0.0055 µmol/mL (C. albicans MTCC 227) MIC = 0.0111 µmol/mL (A. niger MTCC 9933) | Devi et al. (2022) [70] |
 | 7 | MIC = 0.0114 µmol/mL (S. aureus MTCC 2901) MIC = 0.0114 µmol/mL (B. subtilis NCIM 2063) MIC = 0.0228 µmol/mL (E. coli MTCC 732) MIC = 0.0228 µmol/mL (P. aeruginosa MTCC 424) MIC = 0.0056 µmol/mL (C. albicans MTCC 227) MIC = 0.0114 µmol/mL (A. niger MTCC 9933) | Devi et al. (2022) [70] |
 | 8 | MIC = 0.0113 µmol/mL (S. aureus MTCC 2901) MIC = 0.0226 µmol/mL (B. subtilis NCIM 2063) MIC = 0.0226 µmol/mL (E. coli MTCC 732) MIC = 0.0226 µmol/mL (P. aeruginosa MTCC 424) MIC = 0.0055 µmol/mL (C. albicans MTCC 227) MIC = 0.0113 µmol/mL (A. niger MTCC 9933) | Devi et al. (2022) [70] |
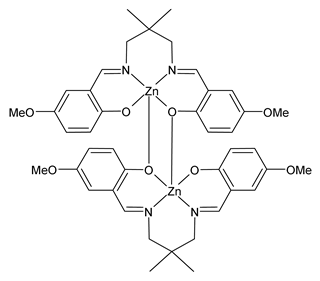
 | Z2Zn (9) | IZD = 15 mm (M. luteus ATCC 934) IZD = 21 mm (S. aureus ATCC 29213) | Al-Shboul et al. (2022) [71] |
 | Z3Zn (10) | IZD = 25 mm (M. luteus ATCC 934) IZD = 18 mm (S. aureus ATCC 29213) | Al-Shboul et al. (2022) [71] |
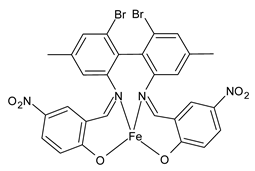 | Z4Fe (11) | IZD = 20 mm (S. aureus ATCC 29213) | Al-Shboul et al. (2022) [71] |
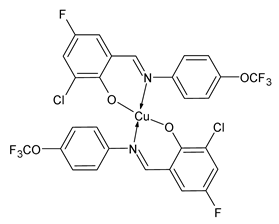
 | Z1Cu (12) | IZD = 10 mm (E. coli ATCC 25922) | Al-Shboul et al. (2022) [71] |
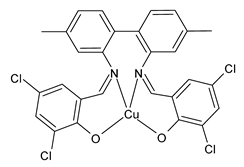
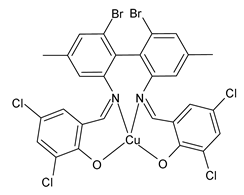 | Z3Cu (13) | IZD = 20 mm (E. coli ATCC 25922) | Al-Shboul et al. (2022) [71] |
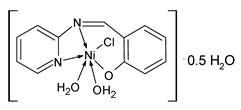 | NiL (14) | IZD = 31.6 ± 0.6 mm (E. coli ATCC 10536) IZD = 20.6 ± 0.6 mm (K. pneumoniae ATCC 10031) IZD = 20.3 ± 0.6 mm (S. aureus ATCC 13565) IZD = 19.6 ± 0.6 mm (S. mutans ATCC 25175) | Abdel-Rahman et al. (2022) [72] |
 | LaL (15) | IZD = 21.3 ± 0.6 mm (E. coli ATCC 10536) IZD = not active (K. pneumoniae ATCC 10031) IZD = 20.3 ± 0.6 mm (S. aureus ATCC 13565) IZD = 17.9 ± 0.5 mm (S. mutans ATCC 25175) | Abdel-Rahman et al. (2022) [72] |
 | 16 | IZD = 20 ± 0.21 mm (B. amyloliquefaciens) IZD = 19 ± 0.16 mm (E. coli) IZD = 18 ± 0.18 mm (S. rolfsii) IZD = 18 ± 0.15 mm (M. phaseolina) | Daravath et al. (2022) [74] |
 | 17 | IZD = 17 ± 0.14 mm (B. amyloliquefaciens) IZD = 16 ± 0.21 mm (E. coli) IZD = 15 ± 0.24 mm (S. rolfsii) IZD = 16 ± 0.16 mm (M. phaseolina) | Daravath et al. (2022) [74] |
 | 18 | IZD = 16 ± 0.18 mm (B. amyloliquefaciens) IZD = 16 ± 0.15 mm (E. coli) IZD = 14 ± 0.15 mm (S. rolfsii) IZD = 15 ± 0.19 mm (M. phaseolina) | Daravath et al. (2022) [74] |
 | Z1 (19) | IZD = 16 mm (S. aureus ATCC 25923) IZD = 15 mm (B. cereus ATCC 11778) IZD = 11 mm (E coli ATCC 25922) IZD = 12 mm (P. aeruginosa ATCC 15442) PMMI = 22.8 mm (A. brasiliensis ATCC 16404) IZD = 22 mm (C. albicans ATCC 10231) | Kargar et al. (2022) [65] |
 | Z2 (20) | IZD = 18 mm (S. aureus ATCC 25923) IZD = 14 mm (B. cereus ATCC 11778) IZD = 13 mm (E coli ATCC 25922) IZD = 12 mm (P. aeruginosa ATCC 15442) PMMI = 22.8 mm (A. brasiliensis ATCC 16404) IZD = 23 mm (C. albicans ATCC 10231) | Kargar et al. (2022) [65] |
 | [ZnLBr]ClO4 (21) | IZD = 14 mm (S. aureus) IZD = 15 mm (B. subtilis) IZD = 19 mm (L. monocytogenes) IZD = 34 mm (E. coli) IZD = 28 mm (K. oxytoca) IZD = 21 (S. thypimurium) | Hajari et al. (2022) [75] |
 | [MnLBr]ClO4 (22) | IZD = 12 mm (S. aureus) IZD = 22 mm (B. subtilis) IZD = 17 mm (L. monocytogenes) IZD = 29 mm (E. coli) IZD = 22 mm (K. oxytoca) IZD = 19 (S. thypimurium) | Hajari et al. (2022) [75] |
 | [CdLBr]ClO4 (23) | IZD = 17 mm (S. aureus) IZD = 17 mm (B. subtilis) IZD = 16 mm (L. monocytogenes) IZD = 24 mm (E. coli) IZD = 18 mm (K. oxytoca) IZD = 21 (S. thypimurium) | Hajari et al. (2022) [75] |
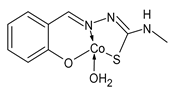 | II (24) | IZD between 11 and 12 mm (B. subtilis) IZD between11 and 12 mm (F.O. Lycopersicum) | Jyothi et al. (2022) [76] |
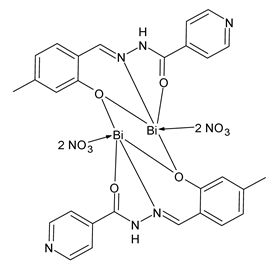 | 4a (25) | MIC = 4 µg/mL (S. aureus) MIC = 8 µg/mL (B. subtilis) MIC = 8 µg/mL (E. coli) MIC = 8 µg/mL (P. aeruginosa) | Li et al. (2022) [77] |
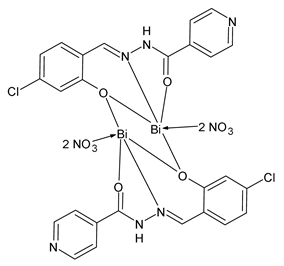 | 5a (26) | MIC = 4 µg/mL (S. aureus) MIC = 4 µg/mL (B. subtilis) MIC = 4 µg/mL (E. coli) MIC = 8 µg/mL (P. aeruginosa) | Li et al. (2022) [77] |
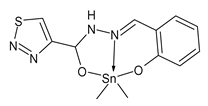 | 27 | MIC = 0.01080 μmol/mL (B. subtilis MTCC 441) MIC = 0.01080 μmol/mL (E. coli MTCC 732) MIC = 0.01080 μmol/mL (P. aeruginosa MTCC 424) MIC = 0.01080 μmol/mL (C. albicans MTCC 227) MIC = 0.01080 μmol/mL (A. niger MTCC 9933) | Saroya et al. (2022) [78] |
2.2. SBs Complexes with Inner Transition Metals (Lanthanides and Actinides) as Antimicrobials
3. Chitosan SBs Complexes as Antimicrobials
4. Metal Complexes with SBs with Catalytic Activity
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff bases and their metal complexes: A review on the history, synthesis, and applications. Inorg. Chem. Commun. 2023, 150, 110451. [Google Scholar] [CrossRef]
- Catalano, A. Schiff bases: A short survey on a promising scaffold in drug discovery. Curr. Med. Chem. 2023, 30, 4170–4180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Afshari, F.; Ghomi, E.R.; Dinari, M.; Ramakrishna, S. Recent advances on the corrosion inhibition behavior of Schiff base compounds on mild steel in acidic media. ChemistrySelect 2023, 8, e202203231. [Google Scholar] [CrossRef]
- Upendranath, K.; Venkatesh, T.; Nayaka, Y.A.; Shashank, M.; Nagaraju, G. Optoelectronic, DFT and current-voltage performance of new Schiff base 6-nitro-benzimidazole derivatives. Inorg. Chem. Commun. 2022, 139, 109354. [Google Scholar] [CrossRef]
- Alam, M.Z.; Khan, S.A. A review on Schiff base as a versatile fluorescent chemo-sensors tool for detection of Cu2+ and Fe3+ metal ion. J. Fluoresc. 2023; in press. [Google Scholar] [CrossRef]
- Aytac, S.; Gundogdu, O.; Bingol, Z.; Gulcin, I. Synthesis of Schiff bases containing phenol ring and investigation of their antioxidant capacity, anticholinesterase, butyrylcholinesterase and carbonic anhydrase inhibition properties. Pharmaceutics 2023, 15, 779. [Google Scholar] [CrossRef]
- Raju, S.K.; Settu, A.; Thiyagarajan, A.; Rama, D.; Sekar, P.; Kumar, S. Biological applications of Schiff bases: An overview. GSC Biol. Pharm. Sci. 2022, 21, 203–215. [Google Scholar] [CrossRef]
- Çelik, F.; Bektaş, K.İ.; Güler, H.İ.; Direkel, Ş.; Ünver, Y. New Schiff bases with thiophene ring: Synthesis, biological activities, and molecular docking study. Russian J. Gen. Chem. 2023, 93, 409–417. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Alyamani, N.M. New Schiff Base–TMB Hybrids: Design, synthesis and antiproliferative investigation as potential anticancer agents. Symmetry 2023, 15, 609. [Google Scholar] [CrossRef]
- Al-Shemary, R.K.; Mohapatra, R.K.; Kumar, M.; Sarangi, A.K.; Azam, M.; Tuli, H.S.; Ansari, A.; Mohapatra, P.K.; Dhama, K. Synthesis, structural investigations, XRD, DFT, anticancer and molecular docking study of a series of thiazole based Schiff base metal complexes. J. Mol. Struct. 2023, 1275, 134676. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Tople, M.S.; Patel, N.B.; Patel, P.P.; Purohit, A.C.; Ahmad, I.; Patel, H. An in silico-in vitro antimalarial and antimicrobial investigation of newer 7-chloroquinoline based Schiff-bases. J. Mol. Struct. 2023, 1271, 134016. [Google Scholar] [CrossRef]
- Yuldasheva, N.; Acikyildiz, N.; Akyuz, M.; Yabo-Dambagi, L.; Aydin, T.; Cakir, A.; Kazaz, C. The synthesis of Schiff bases and new secondary amine derivatives of p-vanillin and evaluation of their neuroprotective, antidiabetic, antidepressant and antioxidant potentials. J. Mol. Struct. 2022, 1270, 133883. [Google Scholar] [CrossRef]
- Hamid, S.J.; Salih, T. Design, synthesis, and anti-inflammatory activity of some coumarin Schiff base derivatives: In silico and in vitro study. Drug Des. Develop. Ther. 2022, 16, 2275–2288. [Google Scholar] [CrossRef]
- Çakmak, R.; Başaran, E.; Şentürk, M. Synthesis, characterization, and biological evaluation of some novel Schiff bases as potential metabolic enzyme inhibitors. Archiv. Pharm. 2022, 355, 2100430. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Abdel-Aziz, A.A.-M.; Ghabbour, H.A.; Bua, S.; Nocentini, A.; Alkahtani, H.M.; Alsaif, N.A.; Al-Agamy, M.H.M.; Supuran, C.T. Carbonic anhydrase inhibition activities of Schiff’s bases based on quinazoline-linked benzenesulfonamide. Molecules 2022, 27, 7703. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Rahim, F.; Zaman, K.; Anouar, E.H.; Uddin, N.; Nawaz, F.; Sajid, M.; Khan, K.M.; Shah, A.A.; Wadood, A.; et al. Synthesis, in vitro biological screening and docking study of benzo[d]oxazole bis Schiff base derivatives as a potent anti-Alzheimer agent. J. Biomol. Struct. Dynam. 2023, 41, 1649–1664. [Google Scholar] [CrossRef]
- Camadan, Y.; Çiçek, B.; Adem, Ş.; Çalişir, Ü.; Akkemik, E. Investigation of in vitro and in silico effects of some novel carbazole Schiff bases on human carbonic anhydrase isoforms I and II. J. Biomol. Struct. Dyn. 2022, 40, 6965–6973. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Jin, Y.; Feng, Y.; Li, J.; Zhang, K. Advances in Schiff base and its coating on metal biomaterials—A review. Metals 2023, 13, 386. [Google Scholar] [CrossRef]
- Mondal, K.; Mistri, S. Schiff base based metal complexes: A review of their catalytic activity on aldol and henry reaction. Comments Inorg. Chem. 2023, 43, 77–105. [Google Scholar] [CrossRef]
- Rakhtshah, J. A comprehensive review on the synthesis, characterization, and catalytic application of transition-metal Schiff-base complexes immobilized on magnetic Fe3O4 nanoparticles. Coord. Chem. Rev. 2022, 467, 214614. [Google Scholar] [CrossRef]
- Meena, R.; Meena, P.; Kumari, A.; Sharma, N.; Fahmi, N. Schiff Bases and Their Metal Complexes: Synthesis, Structural Characteristics and Applications. In Schiff Base in Organic, Inorganic and Physical Chemistry; IntechOpen: London, UK, 2023; ISBN 978-1-80355-679-6. [Google Scholar] [CrossRef]
- Deghadi, R.G.; Elsharkawy, A.E.; Ashmawy, A.M.; Mohamed, G.G. Can one novel series of transition metal complexes of oxy-dianiline Schiff base afford advances in both biological inorganic chemistry and materials science? Comments Inorg. Chem. 2022, 42, 1–46. [Google Scholar] [CrossRef]
- Ashraf, T.; Ali, B.; Qayyum, H.; Haroone, M.S.; Shabbir, G. Pharmacological aspects of Schiff base metal complexes: A critical review. Inorg. Chem. Commun. 2023, 150, 110449. [Google Scholar] [CrossRef]
- Hossain, A.M.S.; Méndez-Arriaga, J.M.; Xia, C.; Xie, J.; Gómez-Ruiz, S. Metal complexes with ONS donor Schiff bases: A review. Polyhedron 2022, 217, 115692. [Google Scholar] [CrossRef]
- Abu-Yamin, A.A.; Abduh, M.S.; Saghir, S.A.M.; Al-Gabri, N. Synthesis, characterization and biological activities of new Schiff base compound and its lanthanide complexes. Pharmaceuticals 2022, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Alezzy, A.A.; Alnahari, H.; Al-horibi, S.A. Short review on metal complexes of Schiff bases containing antibiotic, and bioactivity applications. J. Chem. Nutrit. Biochem. 2022, 3, 44–57. [Google Scholar] [CrossRef]
- Soroceanu, A.; Bargan, A. Advanced and biomedical applications of Schiff-base ligands and their metal complexes: A review. Crystals 2022, 12, 1436. [Google Scholar] [CrossRef]
- Kar, K.; Ghosh, D.; Kabi, B.; Chandra, A. A concise review on cobalt Schiff base complexes as anticancer agents. Polyhedron 2022, 222, 115890. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with Schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Mokhtari, P.; Mohammadnezhad, G. Anti-cancer properties and catalytic oxidation of sulfides based on vanadium(V) complexes of unprotected sugar-based Schiff-base ligands. Polyhedron 2022, 215, 115655. [Google Scholar] [CrossRef]
- Khalil, E.A.; Mohamed, G.G. Synthesis and characterization of some transition and inner transition mixed ligand complexes derived from Schiff base ligand and o-aminophenol. Inorg. Chem. Commun. 2023, 153, 110825. [Google Scholar] [CrossRef]
- Shekhar, S.; Khan, A.; Sharma, S.; Sharma, B.; Sarkar, A. Schiff base metallodrugs in antimicrobial and anticancer chemotherapy applications: A comprehensive review. Emergent Mater. 2022, 5, 279. [Google Scholar] [CrossRef]
- Savcı, A.; Turan, N.; Buldurun, K.; Alkış, M.E.; Alan, Y. Schiff base containing fluorouracil and its M(II) complexes: Synthesis, characterization, cytotoxic and antioxidant activities. Inorg. Chem. Commun. 2022, 143, 109780. [Google Scholar] [CrossRef]
- Turan, N.; Buldurun, K.; Bursal, E.; Mahmoudi, G. Pd(II)-Schiff base complexes: Synthesis, characterization, Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions, enzyme inhibition and antioxidant activities. J. Organomet. Chem. 2022, 970, 122370. [Google Scholar] [CrossRef]
- Deswal, Y.; Asija, S.; Tufail, A.; Dubey, A.; Deswal, L.; Kumar, N.; Saroya, S.; Kirar, J.S.; Gupta, N.M. Instigating the in vitro antidiabetic activity of new tridentate Schiff base ligand appended M(II) complexes: From synthesis, structural characterization, quantum computational calculations to molecular docking, and molecular dynamics simulation studies. Appl. Organometal. Chem. 2023, 37, e7050. [Google Scholar]
- Sudha, A. Investigation of new schiff base transition metal(II) complexes theoretical, antidiabetic and molecular docking studies. J. Mol. Struct. 2022, 1259, 132700. [Google Scholar] [CrossRef]
- Jasińska, A.; Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Mordyl, B.; Głuch-Lutwin, M. V(III) and V(IV) Schiff base complexes as potential insulin-mimetic compounds–Comparison, characterization and biological activity. Polyhedron 2022, 215, 115682. [Google Scholar] [CrossRef]
- Radha, V.P.; Prabakaran, M. Novel thiadiazole-derived Schiff base ligand and its transition metal complexes: Thermal behaviour, theoretical study, chemo-sensor, antimicrobial, antidiabetic and anticancer activity. Appl. Organometal. Chem. 2022, 36, e6872. [Google Scholar] [CrossRef]
- Shaikh, I.; Travadi, M.; Jadeja, R.N.; Butcher, R.J.; Pandya, J.H. Crystal feature and spectral characterization of Zn(II) complexes containing Schiff base of Acylpyrazolone ligand with antimalarial action. J. Ind. Chem. Soc. 2022, 99, 100428. [Google Scholar] [CrossRef]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. Exploring novel derivatives of isatin-based Schiff bases as multi-target agents: Design, synthesis, in vitro biological evaluation, and in silico ADMET analysis with molecular modeling simulations. RSC Adv. 2023, 13, 9281–9303. [Google Scholar] [PubMed]
- Aragón-Muriel, A.; Reyes-Márquez, V.; Cañavera-Buelvas, F.; Parra-Unda, J.R.; Cuenú-Cabezas, F.; Polo-Cerón, D.; Colorado-Peralta, R.; Suárez-Moreno, G.V.; Aguilar-Castillo, B.A.; Morales-Morales, D. Pincer complexes derived from tridentate Schiff bases for their use as antimicrobial metallopharmaceuticals. Inorganics 2022, 10, 134. [Google Scholar] [CrossRef]
- Odularu, A.T. Ease to challenges in achieving successful synthesized Schiff base, chirality, and application as antibacterial agent. BioMed Res. Int. 2023, 2023, 1626488. [Google Scholar] [CrossRef]
- Pervaiz, M.; Munir, A.; Riaz, A.; Saeed, Z.; Younas, U.; Imran, M.; Ullah, S.; Bashir, R.; Rashid, A.; Adnan, A. Review article-Amalgamation, scrutinizing, and biological evaluation of the antimicrobial aptitude of thiosemicarbazide Schiff bases derivatives metal complexes. Inorg. Chem. Commun. 2022, 141, 109459. [Google Scholar] [CrossRef]
- Jain, S.; Rana, M.; Sultana, R.; Mehandi, R.; Rahisuddin. Schiff base metal complexes as antimicrobial and anticancer agents. Polycycl. Arom. Comp. 2022; in press. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abdelghani, A.A.; AlObaid, A.A.; El-ezz, D.A.; Warad, I.; Shehata, M.R.; Abdalla, E.M. Novel bromo and methoxy substituted Schiff base complexes of Mn(II), Fe(III), and Cr(III) for anticancer, antimicrobial, docking, and ADMET studies. Sci. Rep. 2023, 13, 3199. [Google Scholar] [CrossRef]
- Çetín, Z.; Bülent, D.E.D.E. A novel Schiff base ligand and its metal complexes: Synthesis, characterization, theoretical calculations, catalase-like and catecholase-like enzymatic activities. J. Mol. Liq. 2023, 380, 121636. [Google Scholar] [CrossRef]
- Jayendran, M.; Kurup, M.P. Structural, spectral, cytotoxic and biocatalytic studies of a dinuclear phenoxo bridged Zn(II) complex from NNO donor tridentate Schiff base. Chem. Data Collect. 2022, 39, 100853. [Google Scholar] [CrossRef]
- Ressler, A.J.; Brandt, O.N.; Weaver, A.; Poor, J.E.; Ream, A.; Summers, N.A.; McMillen, C.D.; Seeram, N.P.; Dougherty, W.G.; Henry, G.E. Chromene-based Schiff base ligand: DNA interaction studies and characterization of tetranuclear zinc, nickel and iron complexes. Inorg. Chim. Acta 2023, 547, 121363. [Google Scholar]
- Shahabadi, N.; Abdoli, Z.; Mardani, Z.; Hadidi, S.; Shiri, F.; Soltani, L. DNA interaction studies of a cobalt(III) complex containing β–amino alcohol ligand by spectroscopic and molecular docking methods. J. Biomol. Struct. Dynam. 2023; in press. [Google Scholar] [CrossRef]
- Aggarwal, N.; Maji, S. Potential applicability of Schiff bases and their metal complexes during COVID-19 pandemic—A review. Rev. Inorg. Chem. 2022; in press. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Maio, A.C.; Basile, G.; Giuzio, F.; Bonomo, M.G.; Aquaro, S.; Walsh, T.J.; Sinicropi, M.S.; et al. Are nutraceuticals effective in COVID-19 and post-COVID prevention and treatment? Foods 2022, 11, 2884. [Google Scholar] [CrossRef]
- Catalano, A. COVID-19: Could irisin become the handyman myokine of the 21st century? Coronaviruses 2020, 1, 32–41. [Google Scholar]
- Iraji, M.; Salehi, M.; Malekshah, R.E.; Khaleghian, A.; Shamsi, F. Liposomal formulation of new arsenic Schiff base complex as drug delivery agent in the treatment of acute promyelocytic leukemia and quantum chemical and docking calculations. J. Drug Deliv. Sci. Technol. 2022, 75, 103600. [Google Scholar] [CrossRef]
- Akitsu, T. Hybrid or component?—Schiff base complexes and laccase. Compounds 2022, 2, 307–310. [Google Scholar] [CrossRef]
- Uehara, D.; Salas, P. Facile synthesis of stilbene-derivatized Schiff base ligands and their Cu(II) complexes. Tetrahedron Lett. 2023, 118, 154406. [Google Scholar] [CrossRef]
- Mehmood, M.; Zafar, A.; Iqbal, A.; Mukhtar, M.; Tahir, M.N. Molecular architecture, characterization, and applications of homoleptic heteronuclear 3d/4f metals’ complexes derived from bi-compartmental Schiff-base. J. Mol. Struct. 2023, 1274, 134547. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Ceramella, J.; Iacopetta, D.; Catalano, A.; Mariconda, A.; Rosano, C.; Saturnino, C.; El-Kashef, H.; Longo, P. Metal complexes with Schiff bases: Data collection and recent studies on biological activities. Int. J. Mol. Sci. 2022, 23, 14840. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2021.
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.A.; Ardakani, A.A.; Sedighi-Khavidak, S.; Munawarf, K.S.; Ashfaq, M.; Tahir, M.N. Synthesis, spectral characterization, crystal structures, biological activities, theoretical calculations and substitution effect of salicylidene ligand on the nature of mono and dinuclear Zn(II) Schiff base complexes. Polyhedron 2022, 213, 115636. [Google Scholar] [CrossRef]
- Aroua, L.M.; Alhag, S.K.; Al-Shuraym, L.A.; Messaoudi, S.; Mahyoub, J.A.; Alfaifi, M.Y.; Al-Otaibi, W.M. Synthesis and characterization of different complexes derived from Schiff base and evaluation as a potential anticancer, antimicrobial, and insecticide agent. Saudi J. Biol. Sci. 2023, 30, 103598. [Google Scholar] [CrossRef] [PubMed]
- Alorini, T.; Daoud, I.; Al-Hakimi, A.N.; Alminderej, F.; Albadri, A.E. An experimental and theoretical investigation of antimicrobial and anticancer properties of some new Schiff base complexes. Res. Chem. Intermed. 2023, 49, 1701–1730. [Google Scholar] [CrossRef]
- Al-Janabi, A.S.; Elzupir, A.O.; Abou-Krisha, M.M.; Yousef, T.A. New dual inhibitors of SARS-CoV-2 based on metal complexes with Schiff-base 4-chloro-3-methyl phenyl hydrazine: Synthesis, DFT, antibacterial properties and molecular docking studies. Inorganics 2023, 11, 63. [Google Scholar]
- Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An update. Molecules 2022, 27, 8562. [Google Scholar] [CrossRef]
- Devi, J.; Kumar, S.; Kumar, B.; Asija, S.; Kumar, A. Synthesis, structural analysis, in vitro antioxidant, antimicrobial activity and molecular docking studies of transition metal complexes derived from Schiff base ligands of 4-(benzyloxy)-2-hydroxybenzaldehyde. Res. Chem. Intermed. 2022, 48, 1541–1576. [Google Scholar] [CrossRef]
- Al-Shboul, T.M.; El-khateeb, M.; Obeidat, Z.H.; Ababneh, T.S.; Al-Tarawneh, S.S.; Al Zoubi, M.S.; Alshaer, W.; Abu Seni, A.; Qasem, T.; Moriyama, H.; et al. Synthesis, characterization, computational and biological activity of some Schiff bases and their Fe, Cu and Zn complexes. Inorganics 2022, 10, 112. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Basha, M.T.; Al-Farhan, B.S.; Shehata, M.R.; Abdalla, E.M. Synthesis, characterization, potential antimicrobial, antioxidant, anticancer, DNA binding, and molecular docking activities and DFT on novel Co(II), Ni(II), VO(II), Cr(III), and La(III) Schiff base complexes. Appl. Organomet. Chem. 2022, 36, e6484. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; D’Amato, A.; Lauria, G.; Saturnino, C.; Andreu, I.; Longo, P.; Sinicropi, M.S. Diarylureas: New promising small molecules against Streptococcus mutans for the treatment of dental caries. Antibiotics 2023, 12, 112. [Google Scholar] [CrossRef]
- Daravath, S.; Rambabu, A.; Ganji, N.; Ramesh, G.; Lakshmi, P.A. Spectroscopic, quantum chemical calculations, antioxidant, anticancer, antimicrobial, DNA binding and photo physical properties of bioactive Cu(II) complexes obtained from trifluoromethoxy aniline Schiff bases. J. Mol. Struct. 2022, 1249, 131601. [Google Scholar] [CrossRef]
- Hajari, S.; Keypour, H.; Rezaei, M.T.; Farida, S.H.M.; Gable, R.W. New 15-membered macrocyclic Schiff base ligand; synthesis some Cd(II), Mn(II) and Zn(II) complexes, crystal structure, cytotoxicity, antibacterial and antioxidant activity. J. Mol. Struct. 2022, 1251, 132049. [Google Scholar] [CrossRef]
- Jyothi, P.; Sumalatha, V.; Rajitha, D. Cobalt(II) complexes with N-methyl thio semicarbazide Schiff bases: Synthesis, spectroscopic investigation, cytotoxicity, DNA binding and incision, anti-bacterial and anti-fungal studies. Inorg. Chem. Commun. 2022, 145, 110029. [Google Scholar]
- Li, C.H.; Jiang, J.H.; Lei, Y.H.; Li, X.; Yao, F.H.; Ji, M.H.; Zhang, K.W.; Tao, L.M.; Ye, L.J.; Li, Q.G. Design, synthesis, and biological evaluation of dinuclear bismuth(III) complexes with Isoniazid-derived Schiff bases. J. Inorg. Biochem. 2022, 235, 111931. [Google Scholar] [CrossRef] [PubMed]
- Saroya, S.; Asija, S.; Kumar, N.; Deswal, Y. Organotin (IV) complexes derived from tridentate Schiff base ligands: Synthesis, spectroscopic analysis, antimicrobial and antioxidant activity. J. Indian Chem. Soc. 2022, 99, 100379. [Google Scholar]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.S.; Vikraman, D. Detailed investigations of rare earth (Yb, Er and Pr) based inorganic metal-ion complexes for antibacterial and anticancer applications. Inorg. Chem. Commun. 2023, 150, 110510. [Google Scholar] [CrossRef]
- Alqasaimeh, M.; Abu-Yamin, A.A.; Matar, S.; Al Khalyfeh, K.; Rüffer, T.; Lang, H.; Saraerah, I.A.M.; Salman, M.; Figiel, P.; Leniec, G.; et al. Preparation, spectroscopic investigation, biological activity and magnetic properties of three inner transition metal complexes based on (2-((p-tolylimino) methyl) phenol) Schiff base. J. Mol. Struct. 2023, 1274, 134458. [Google Scholar] [CrossRef]
- Hussein, K.A.; Mahdi, S.; Shaalan, N. Synthesis, Spectroscopy of new lanthanide complexes with Schiff base derived from (4-antipyrinecarboxaldehyde with ethylene di-amine) and study the bioactivity. Baghdad Sci. J. 2023, 20, 469–482. [Google Scholar] [CrossRef]
- Awolope, R.O.; Ejidike, I.P.; Clayton, H.S. Schiff base metal complexes as a dual antioxidant and antimicrobial agents. J. Appl. Pharm. Sci. 2023, 13, 132–140. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Aswathy, K.A.; Munuswamy-Ramanujam, G.; Jaisankar, V. Pyridine and isoxazole substituted 3-formylindole-based chitosan Schiff base polymer: Antimicrobial, antioxidant and in vitro cytotoxicity studies on THP-1 cells. Int. J. Biol. Macromol. 2023, 225, 1575–1587. [Google Scholar] [PubMed]
- Adhikari, H.S.; Garai, A.; Yadav, P.N. Synthesis, characterization, and anticancer activity of chitosan functionalized isatin based thiosemicarbazones, and their copper(II) complexes. Carbohydrate Res. 2023, 526, 108796. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Ismail, M.M.; Morsy, J.M.; Hassanin, H.M.; Abdelrazek, M.M. Synthesis, characterization, anticancer, and antioxidant activities of chitosan Schiff bases bearing quinolinone or pyranoquinolinone and their silver nanoparticles derivatives. Polymer Bull. 2023, 80, 4035–4059. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S.; Jena, S.R.; Jena, D.; Nayak, J.; Samanta, L. In situ crosslinked dialdehyde guar gum-chitosan Schiff-base hydrogels for dual drug release in colorectal cancer therapy. Chem. Eng. Sci. 2023, 269, 118482. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Huang, T.; Tian, D.; Gao, R. Characterization and antioxidant properties of chitosan/ethyl-vanillin edible films produced via Schiff-base reaction. Food Sci. Biotechnol. 2023, 32, 157–167. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, L.; Tan, W.; Li, Q.; Guo, Z. The antioxidant and antibacterial activities of the pyridine-4-aldehyde Schiff bases grafted chloracetyl chitosan oligosaccharide derivatives. Starch-Stärke 2023, 75, 2100268. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Omer, A.M.; Baset, W.M.; Abbas, E.; Mohy-Eldin, M.S. Therapeutic potential of two formulated novel chitosan derivatives with prominent antimicrobial activities against virulent microorganisms and safe profiles toward fibroblast cells. Int. J. Pharm. 2023, 634, 122649. [Google Scholar] [CrossRef]
- Ali, E.A.; Abo-Salem, H.M.; Arafa, A.A.; Nada, A.A. Chitosan Schiff base electrospun fabrication and molecular docking assessment for nonleaching antibacterial nanocomposite production. Cellulose 2023, 30, 3505–3522. [Google Scholar] [CrossRef]
- Foroughnia, A.; Khalaji, A.D.; Kolvari, E.; Koukabi, N. Synthesis of new chitosan Schiff base and its Fe2O3 nanocomposite: Evaluation of methyl orange removal and antibacterial activity. Int. J. Biol. Macromol. 2021, 177, 83–91. [Google Scholar] [CrossRef]
- Omer, A.M.; Eltaweil, A.S.; El-Fakharany, E.M.; Abd El-Monaem, E.M.; Ismail, M.M.; Mohy-Eldin, M.S.; Ayoup, M.S. Novel cytocompatible chitosan Schiff base derivative as a potent antibacterial, antidiabetic, and anticancer agent. Arab. J. Sci. Engineer. 2023, 48, 7587–7601. [Google Scholar] [CrossRef]
- Gupta, H.; Kaur, K.; Singh, R.; Kaur, V. Chitosan Schiff base for the spectrofluorimetric analysis of E-waste toxins: Pentabromophenol, Fe3+, and Cu2+ ions. Cellulose 2023, 30, 1381–1397. [Google Scholar]
- Wei, W.; Wu, H.; Chen, Y.; Zhong, K.; Feng, L. Application of new chitosan 2,4-dihydroxyacetophenone Schiff base @SrFe12O19 nanocomposite for remove of Pb(II) ion from aqueous solution. Int. J. Biol. Macromol. 2023, 226, 336–344. [Google Scholar] [CrossRef]
- Hachem, K.; Jasim, S.A.; Al-Gazally, M.E.; Riadi, Y.; Yasin, G.; Turki Jalil, A.; Abdulkadhm, M.M.; Fenjan, M.N.; Mustafa, Y.F.; Khalaji, A.D.; et al. Retracted: Adsorption of Pb(II) and Cd(II) by magnetic chitosan-salicylaldehyde Schiff base: Synthesis, characterization, thermal study and antibacterial activity. J. Chinese Chem. Soc. 2022, 69, 512–521. [Google Scholar]
- Mahmoud, R.K.; Mohamed, F.; Gaber, E.; Abdel-Gawad, O.F. Insights into the synergistic removal of copper(II), cadmium(II), and chromium(III) ions using modified chitosan based on Schiff bases-g-poly(acrylonitrile). ACS Omega 2022, 7, 42012–42026. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guo, W.; Huang, B.; Chen, Y.; Ren, X.; Shen, Y.; Zhou, Y.; Cheng, R.; Zhang, J.; Qiu, M.; et al. Efficient removal of Cr(VI) by the modified biochar with chitosan Schiff base and MnFe2O4 nanoparticles: Adsorption and mechanism analysis. J. Environm. Chem. Engineer. 2023, 11, 109432. [Google Scholar]
- Eltaweil, A.; Hashem, O.; Abdel-Hamid, H.; El-Monaem, E.; Ayoup, M. Synthesis of a new magnetic sulfacetamide-ethylacetoacetate hydrazone-chitosan Schiff-base for Cr(VI) removal. Int. J. Biol. Macromol. 2022, 222, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, R.E.; Shakeri, F.; Khaleghian, A.; Salehi, M. Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni(II) and Zn(II) complexes and biological evaluation as pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. [Google Scholar] [CrossRef]
- Hamed, A.A.; Saad, G.R.; Abdelhamid, I.A.; Elwahy, A.H.; Abdel-Aziz, M.M.; Elsabee, M.Z. Chitosan Schiff bases-based polyelectrolyte complexes with graphene quantum dots and their prospective biomedical applications. Int. J. Biol. Macromol. 2022, 208, 1029–1045. [Google Scholar] [CrossRef]
- Ignatova, M.; Anastasova, I.; Manolova, N.; Rashkov, I.; Markova, N.; Kukeva, R.; Stoyanova, R.; Georgieva, A.; Toshkova, R. Bio-based electrospun fibers from chitosan Schiff base and polylactide and their Cu2+ and Fe3+ complexes: Preparation and antibacterial and anticancer activities. Polymers 2022, 14, 5002. [Google Scholar]
- Tao, R.; Zhang, N.; Zhang, L.; Habumugisha, T.; Chen, Y.; Lu, Y.; Wang, Y.; Wang, K.; Wang, Y.; Jiang, J. Characterization and antivibrio activity of chitosan-citral Schiff base calcium complex for a calcium citrate sustained release antibacterial agent. Int. J. Biol. Macromol. 2023, 239, 124355. [Google Scholar] [CrossRef] [PubMed]
- Amirthaganesan, K.; Vadivel, T.; Dhamodaran, M.; Chandraboss, V.L. In vitro antifungal studies of ruthenium(III) complex derived from chitosan Schiff bases. Mater. Today Proc. 2022, 60, 1716–1720. [Google Scholar] [CrossRef]
- Bikas, R.; Rashvand, M.H.; Heydari, N.; Kozakiewicz-Piekarz, A. Dinuclear Zn(II) complexes with Schiff base ligands derived from 4-aminoantipyrine; crystal structure and catalytic activity in the synthesis of tetrazoles. J. Mol. Struct. 2023, 1283, 135278. [Google Scholar] [CrossRef]
- Neshat, A.; Cheraghi, M.; Kucerakova, M.; Dusek, M.; Mobarakeh, A.M. A Cu(II) complex based on a Schiff base ligand derived from Ortho-vanillin: Synthesis, DFT analysis and catalytic activities. J. Mol. Struct. 2023, 1274, 134545. [Google Scholar]
- Rabiei, K.; Mohammadkhani, Z.; Keypour, H.; Kouhdareh, J. Palladium Schiff base complex-modified Cu (BDC-NH 2) metal–organic frameworks for C–N coupling. RSC Adv. 2023, 13, 8114–8129. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Nikoorazm, M.; Moradi, P. AV (O)-Schiff-base complex on MCM-41 as an efficient, reusable, and chemoselective nanocatalyst for the oxidative coupling of thiols and oxidation of sulfides. Res. Chem. Intermed. 2023, 49, 1485–1505. [Google Scholar] [CrossRef]
- Hasan, K.; Joseph, R.G.; Patole, S.P.; Al-Qawasmeh, R.A. Development of magnetic Fe3O4-chitosan immobilized Cu(II) Schiff base catalyst: An efficient and reusable catalyst for microwave assisted one-pot synthesis of propargylamines via A3 coupling. Catal. Comm. 2023, 174, 106588. [Google Scholar]
| Structure | Compd | MIC or IZD | Ref. |
|---|---|---|---|
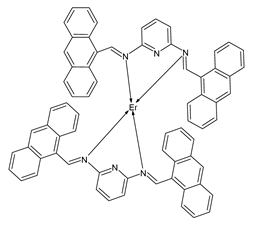 | Schiff-Er (28) | IZD = 21 mm (P. aeruginosa) IZD = 23 mm (S. aureus) | Andiappan et al. (2023) [79] |
 | Schiff-Pr (29) | IZD = 24 mm (P. aeruginosa) IZD = 24 mm (S. aureus) | Andiappan et al. (2023) [79] |
 | Schiff-Yb (30) | IZD = 22 mm (P. aeruginosa) IZD = 20 mm (S. aureus) | Andiappan et al. (2023) [79] |
 | La (31) | MIC = 0.75 mg/mL (S. aureus ATCC 29213) MIC = 3 mg/mL (S. aureus ATCC 33591) MIC = 3 mg/mL (E. coli ATCC 25922) MIC = 1.5 mg/mL (P. aeruginosa ATCC 27853) MIC = 1.5 mg/mL (C. albicans ATCC 10231) | Alqasaimeh et al. (2023) [80] |
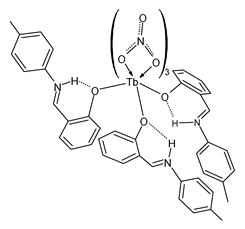 | Lb (32) | MIC = 0.75 mg/mL (S. aureus ATCC 29213) MIC = 3 mg/mL (S. aureus ATCC 33591) MIC = 3 mg/mL (E. coli ATCC 25922) MIC = 1.5 mg/mL (P. aeruginosa ATCC 27853) MIC = 1.5 mg/mL (C. albicans ATCC 10231) | Alqasaimeh et al. (2023) [80] |
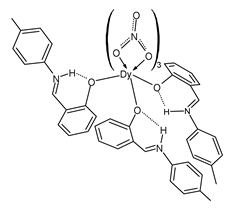 | Lc (33) | MIC = 0.75 mg/mL (S. aureus ATCC 29213) MIC = 3 mg/mL (S. aureus ATCC 33591) MIC = 3 mg/mL (E. coli ATCC 25922) MIC = 1.5 mg/mL (P. aeruginosa ATCC 27853) MIC = 0.75 mg/mL (C. albicans ATCC 10231) | Alqasaimeh et al. (2023) [80] |
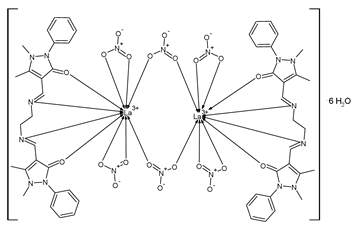 | [La2(C26H28O2N6)2(NO3)6]·6H2O (34) | IZD = 32–35 mm (S. aureus) IZD = 24–28 mm (S. subtilis) IZD = 18–20 mm (E. coli) IZD = 18–20 mm (K. pneumoniae) | Hussein et al. (2023) [81] |
 | [Gd2(C26H28O2N6)2(NO3)6]·6H2O (35) | IZD = 21–35 mm (S. aureus) IZD = 24–28 mm (S. subtilis) IZD = 21–24 mm (E. coli) IZD = 21–24 mm (K. pneumoniae) | Hussein et al. (2023) [81] |
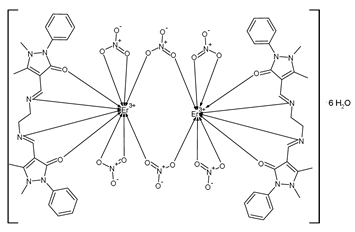 | [Er2(C26H28O2N6)2(NO3)6]·6H2O (36) | IZD = 28–32 mm (S. aureus) IZD = 28–32 mm (S. subtilis) IZD = 18–20 mm (E. coli) IZD = 24–28 mm (K. pneumoniae) | Hussein et al. (2023) [81] |
 | UrO2SV (37) | IZD = 18 mm (S. aureus) IZD = 15 mm (E. faecalis) IZD = 20 mm (K. pneumoniae) IZD = 15 mm (P. aeruginosa) | Awolope et al. (2023) [82] |
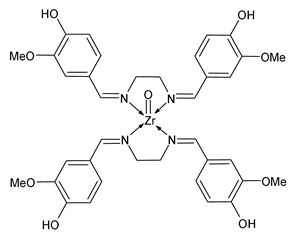 | ZrOSV (38) | IZD = 15 mm (S. aureus) IZD = 17 mm (E. faecalis) IZD = 17 mm (K. pneumoniae) IZD = 16 mm (P. aeruginosa) | Awolope et al. (2023) [82] |
| Structure | Compd | MIC or IZD | Ref. |
|---|---|---|---|
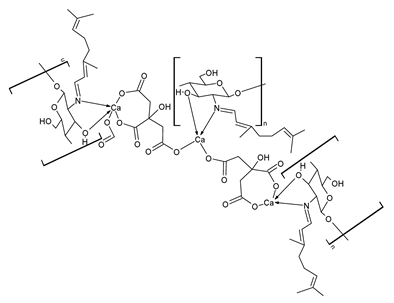 | CS-CT-CCa (39) | MIC = 128 μg/mL (V. parahaemolyticus ATCC 17802) | Tao et al. (2023) [103] |
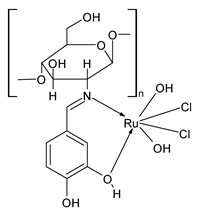 | Ru(CVSB)(H2O)2]Cl2 (40) | IZD = 11 mm (A. flavus) IZD = 12 mm (A. niger) IZD = 11 mm (P. chryogenum) IZD = 10 mm (F. oxysporum) IZD = 12 mm (T. viride) | Amirthaganesan et al. (2022) [104] |
 | Ru(CSSB)(H2O)2]Cl2 (41) | IZD = 14 mm (A. flavus) IZD = 12 mm (A. niger) IZD = 10 mm (P. chryogenum) IZD = 11 mm (F. oxysporum) IZD = 10 mm (T. viride) | Amirthaganesan et al. (2022) [104] |
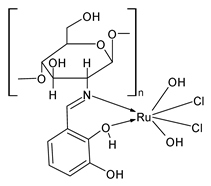 | Ru(COSB)(H2O)2]Cl2 (42) | IZD = 12 mm (A. flavus) IZD = 12 mm (A. niger) IZD = 11 mm (P. chryogenum) IZD = 10 mm (F. oxysporum) IZD = 12 mm (T. viride) | Amirthaganesan et al. (2022) [104] |
| Structure | Compd | Catalyzed Reactions | Ref. |
|---|---|---|---|
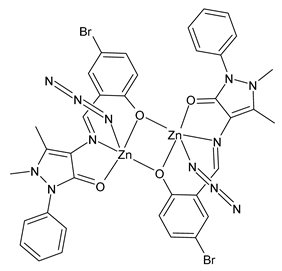 | [Zn2(L1)2(N3)2] (43) | Synthesis of tetrazoles | Bikas et al. (2023) [105] |
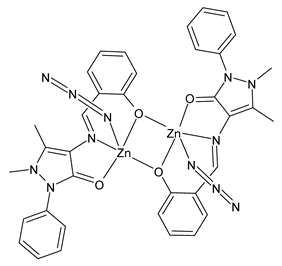 | [Zn2(L2)2(N3)2] (44) | Synthesis of tetrazoles | Bikas et al. (2023) [105] |
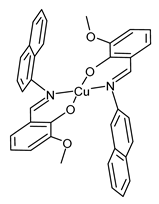 | CuL2 (45) | Oxidation of secondary alcohols | Neshat et al. (2023) [106] |
 | Cu(BDC-NH2)@Schiff base Pd(II) (46) | C–N coupling reactions | Rabiei et al. (2023) [107] |
 | V(O)-5NSA-MCM-41 (47) | Oxidative coupling of thiols and oxidation of sulfides | Jabbari et al. (2023) [108] |
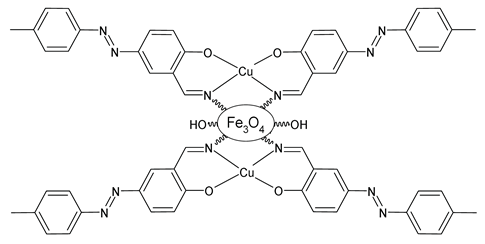 | Fe3O4@CS@Schiffbase@Cu (48) | A3 coupling reaction under microwave irradiation | Hasan et al. (2023) [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Giuzio, F.; Saturnino, C.; Longo, P.; Sinicropi, M.S. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics 2023, 11, 320. https://doi.org/10.3390/inorganics11080320
Iacopetta D, Ceramella J, Catalano A, Mariconda A, Giuzio F, Saturnino C, Longo P, Sinicropi MS. Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics. 2023; 11(8):320. https://doi.org/10.3390/inorganics11080320
Chicago/Turabian StyleIacopetta, Domenico, Jessica Ceramella, Alessia Catalano, Annaluisa Mariconda, Federica Giuzio, Carmela Saturnino, Pasquale Longo, and Maria Stefania Sinicropi. 2023. "Metal Complexes with Schiff Bases as Antimicrobials and Catalysts" Inorganics 11, no. 8: 320. https://doi.org/10.3390/inorganics11080320
APA StyleIacopetta, D., Ceramella, J., Catalano, A., Mariconda, A., Giuzio, F., Saturnino, C., Longo, P., & Sinicropi, M. S. (2023). Metal Complexes with Schiff Bases as Antimicrobials and Catalysts. Inorganics, 11(8), 320. https://doi.org/10.3390/inorganics11080320












