The Tripodal Ligand’s 4f Complexes: Use in Molecular Magnetism
Abstract
1. Introduction
2. Short Theoretical Background
3. Lanthanide Complexes with Tripodal Ligands
3.1. Complexes with Pyrazolyl-Bearing Tripodal Ligand
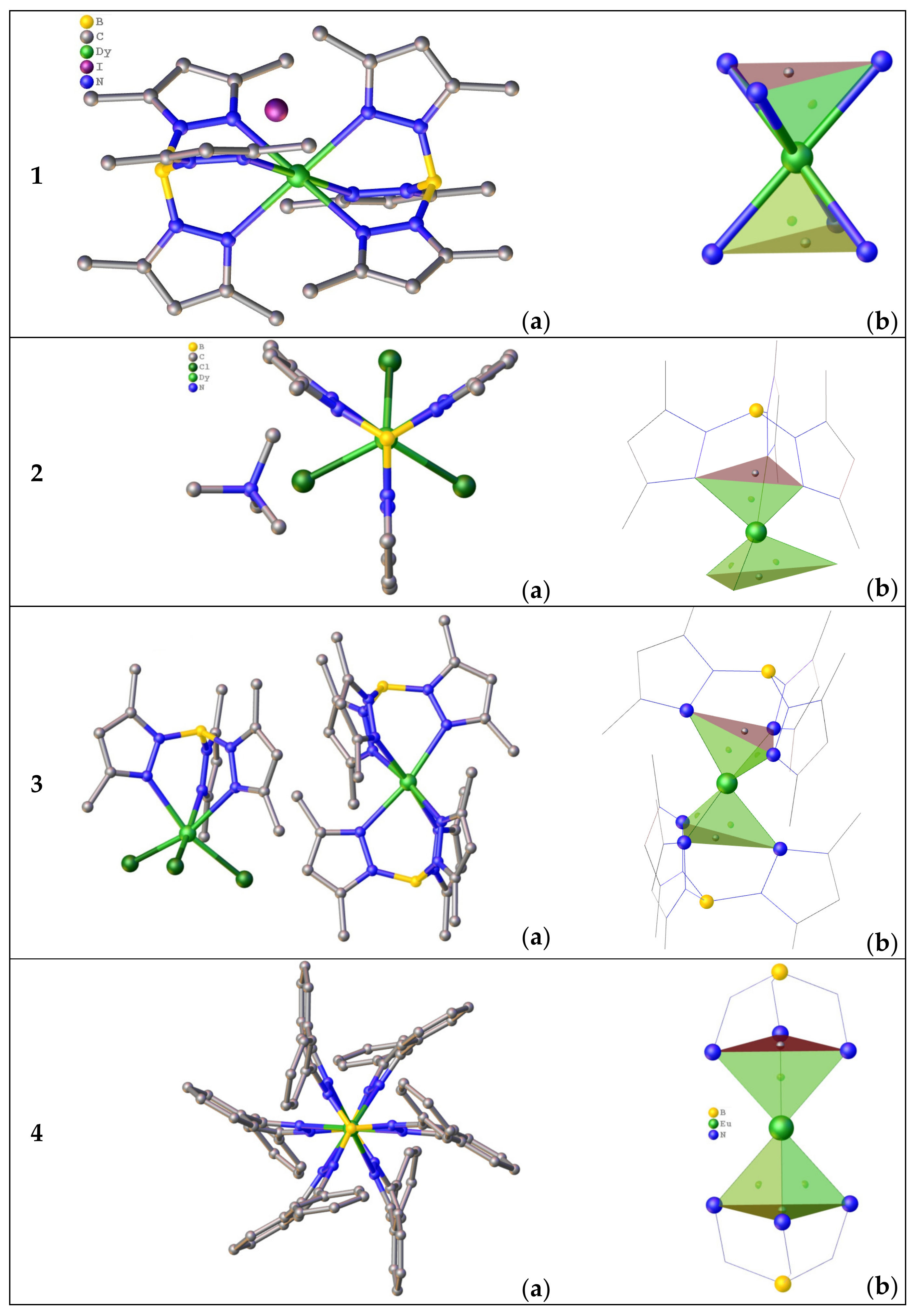
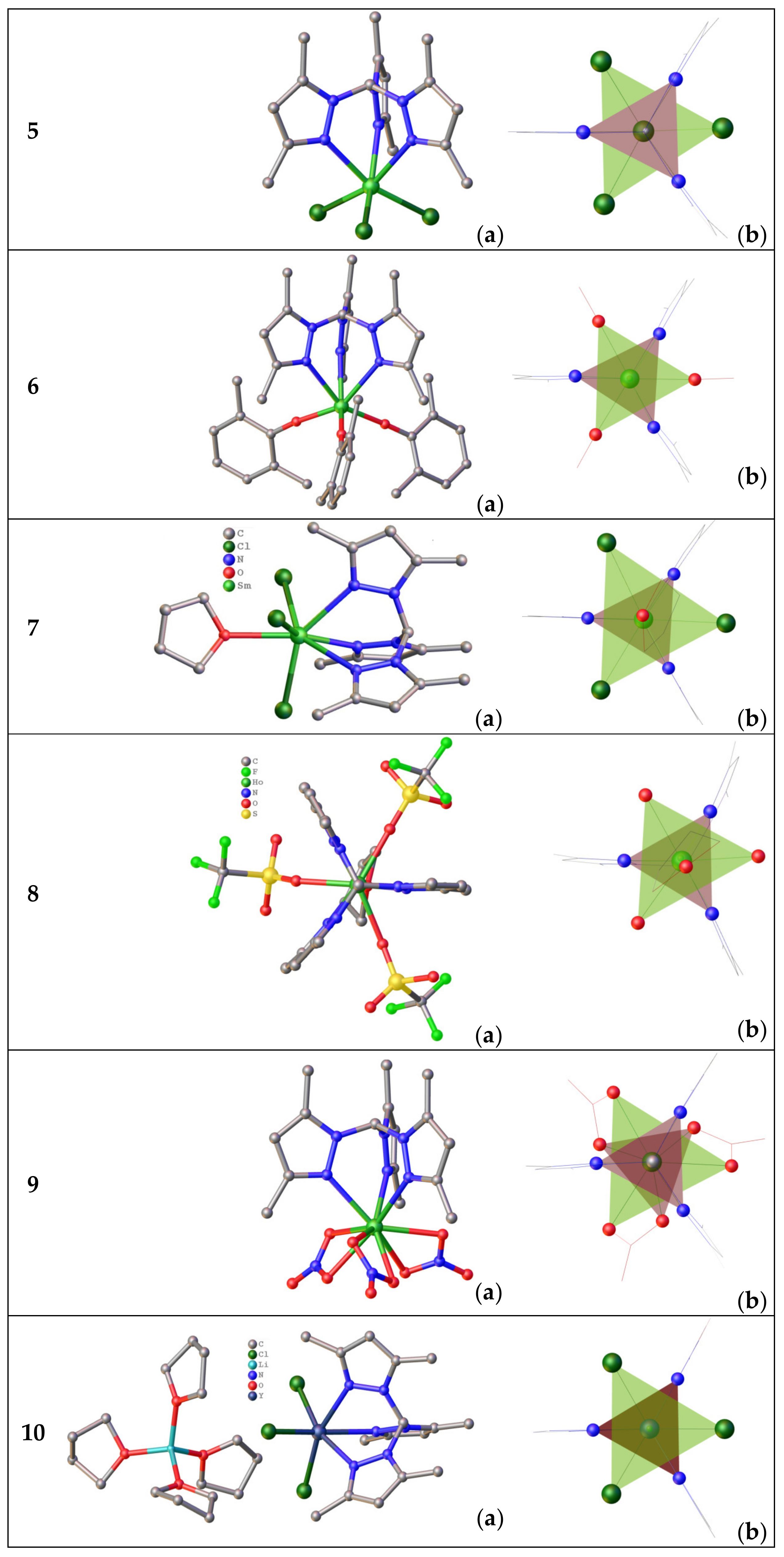
3.1.1. Complexes with Tris(Pyrazolyl)Borates Tripodal Ligands
3.1.2. Complexes of Tris(3,5-dimethylpyrazolyl)methane
3.1.3. Complexes of Anionic Tripodal Ligand: Tris(3,5-dimethylpyrazolyl)-methanide
3.2. Complexes with the Pyridyl-Bearing Tripodal Ligands
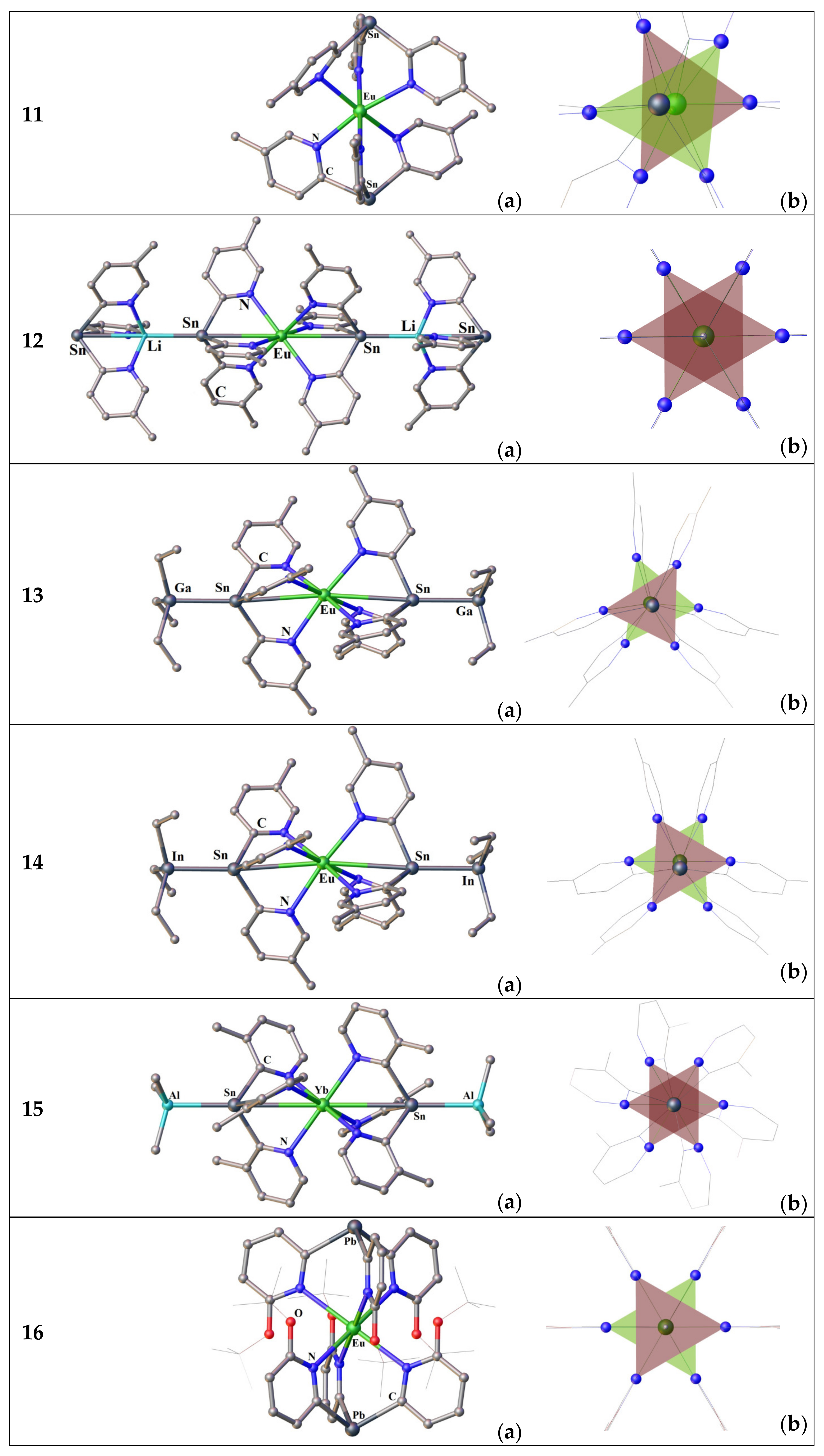
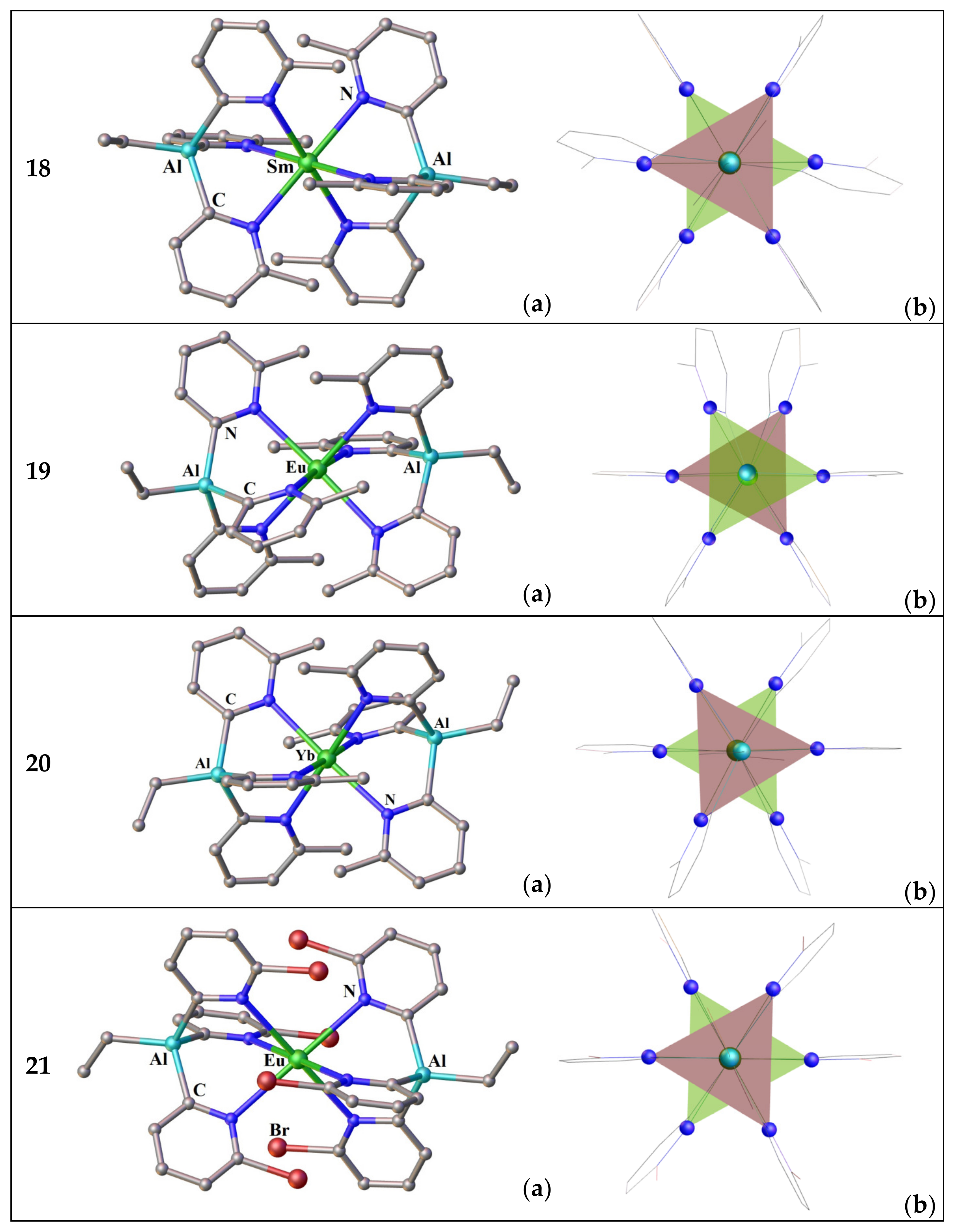
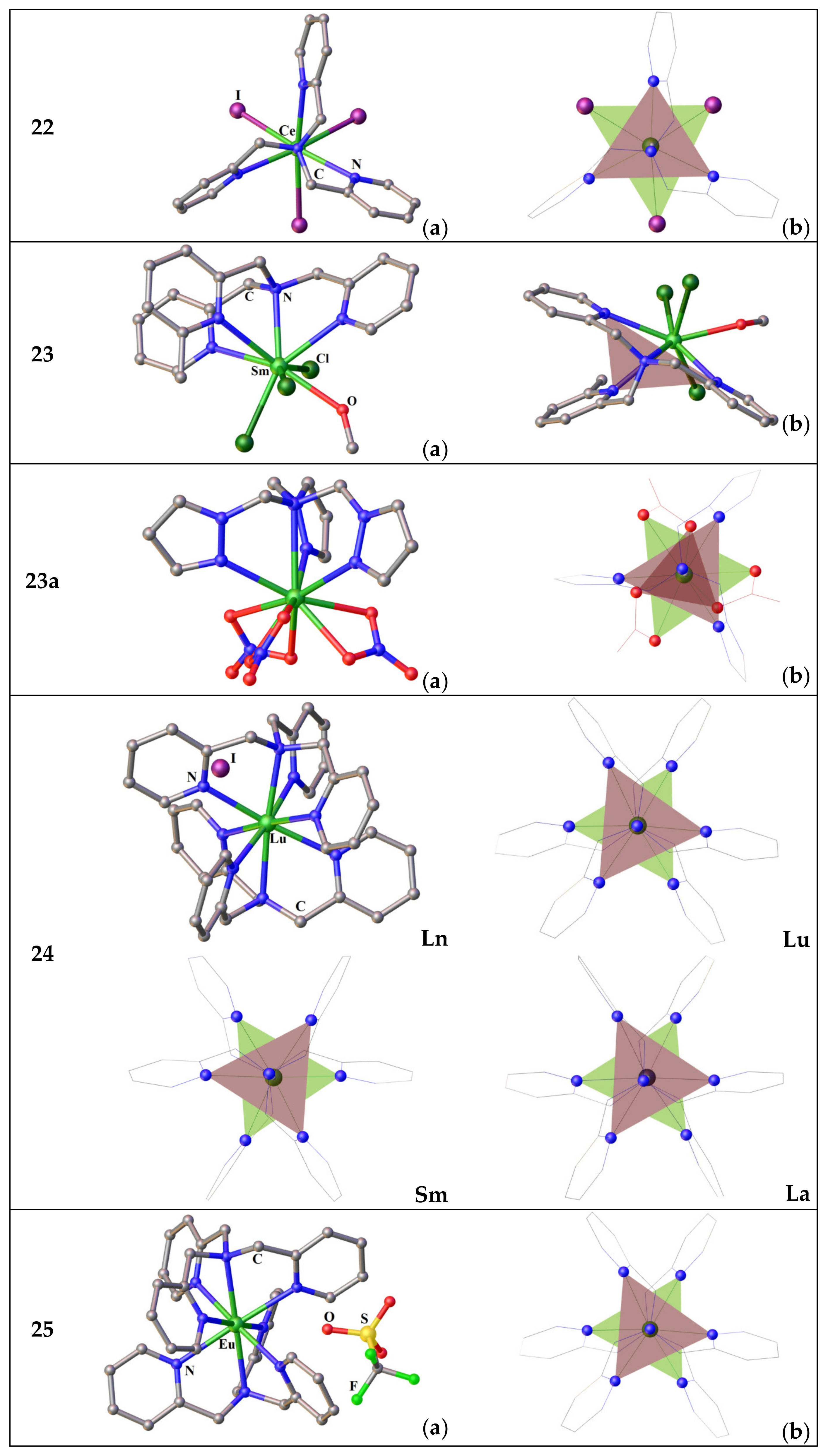
3.2.1. Complexes of Tris(2-Pyridyl)Metalates
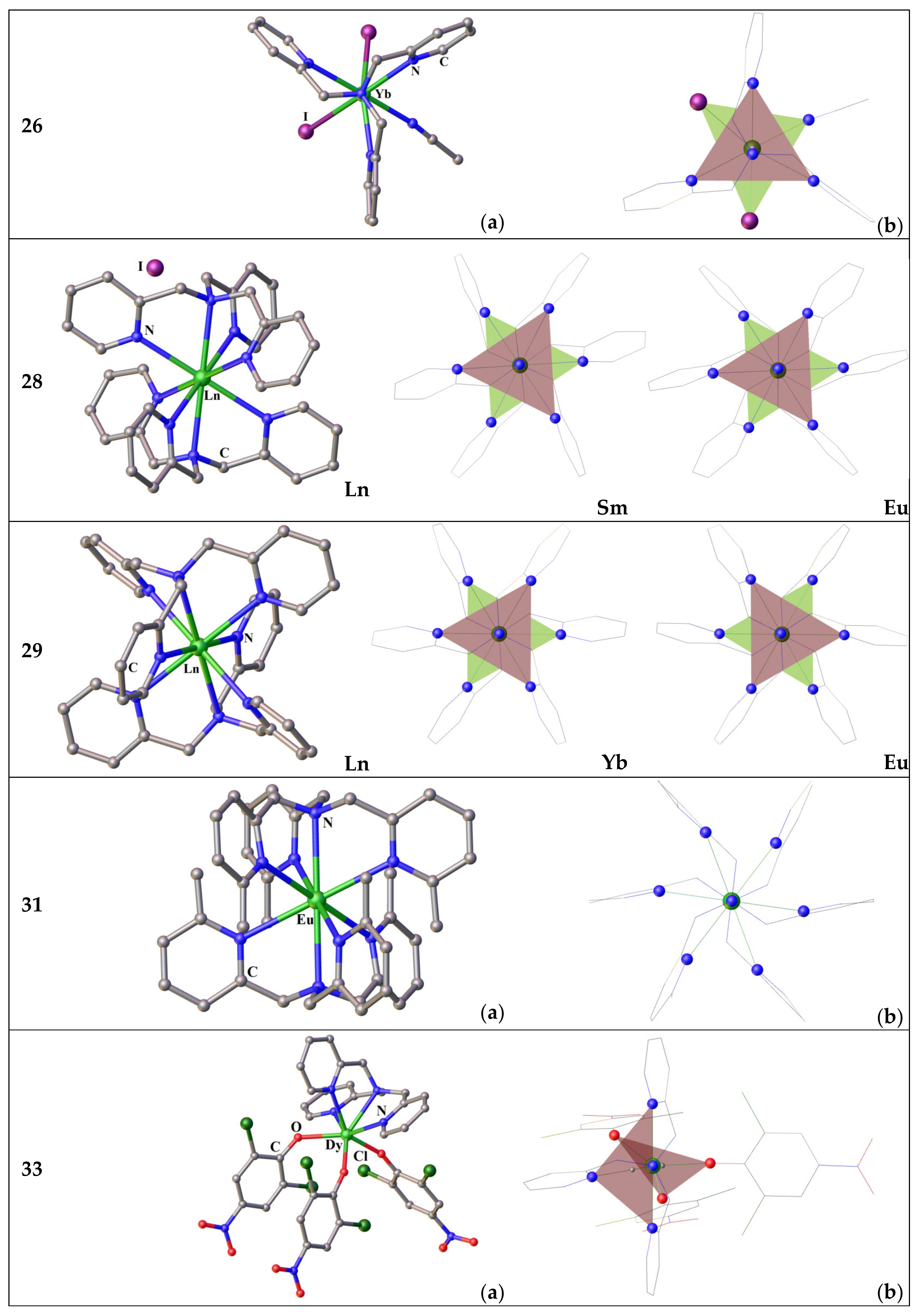
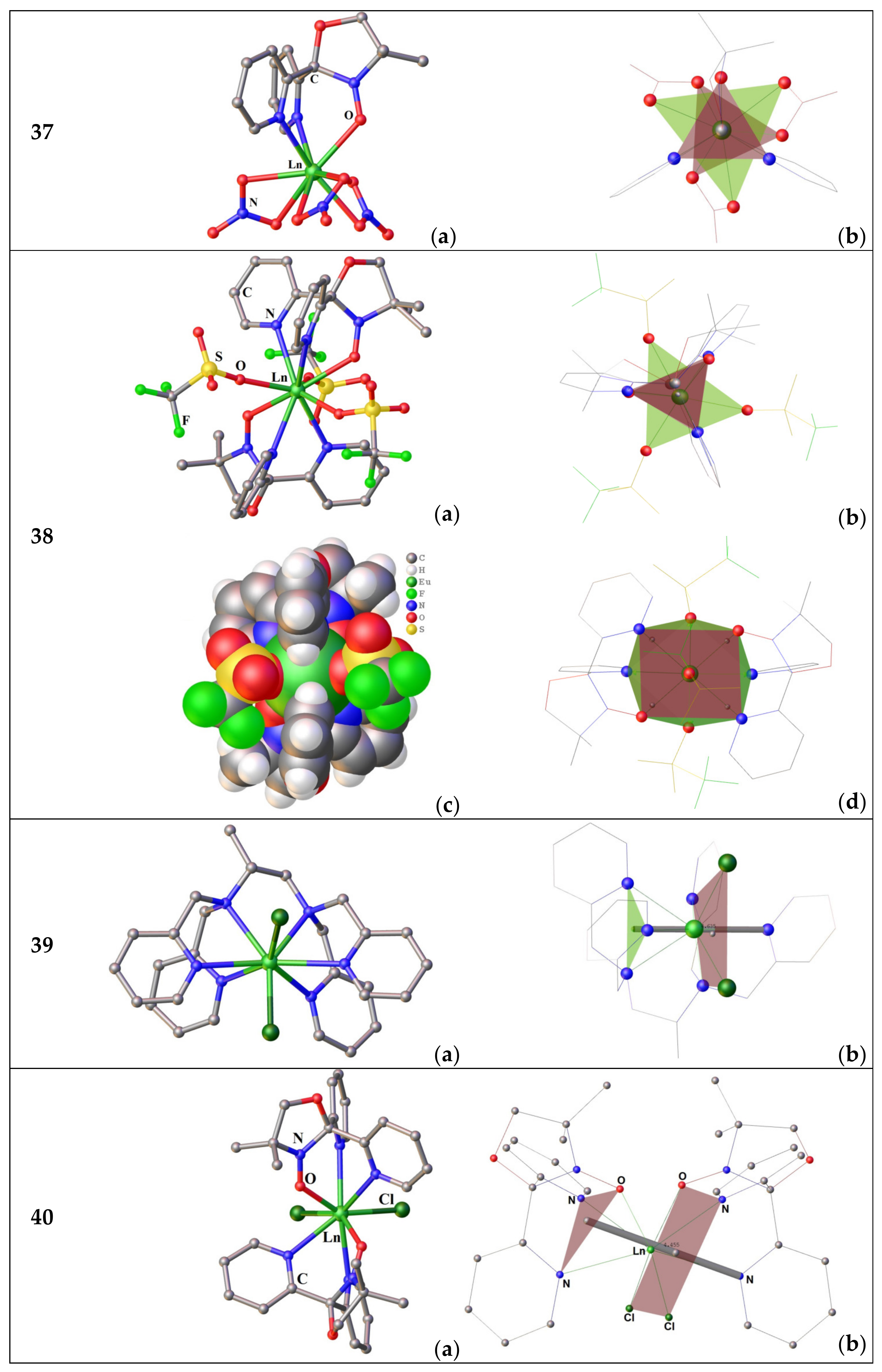
3.2.2. Complexes of Tris(2-Pyridyl)Amines
4. Complexes with Paramagnetic Ligands
4.1. Complexes of the Tripodal Nitroxyl Radicals
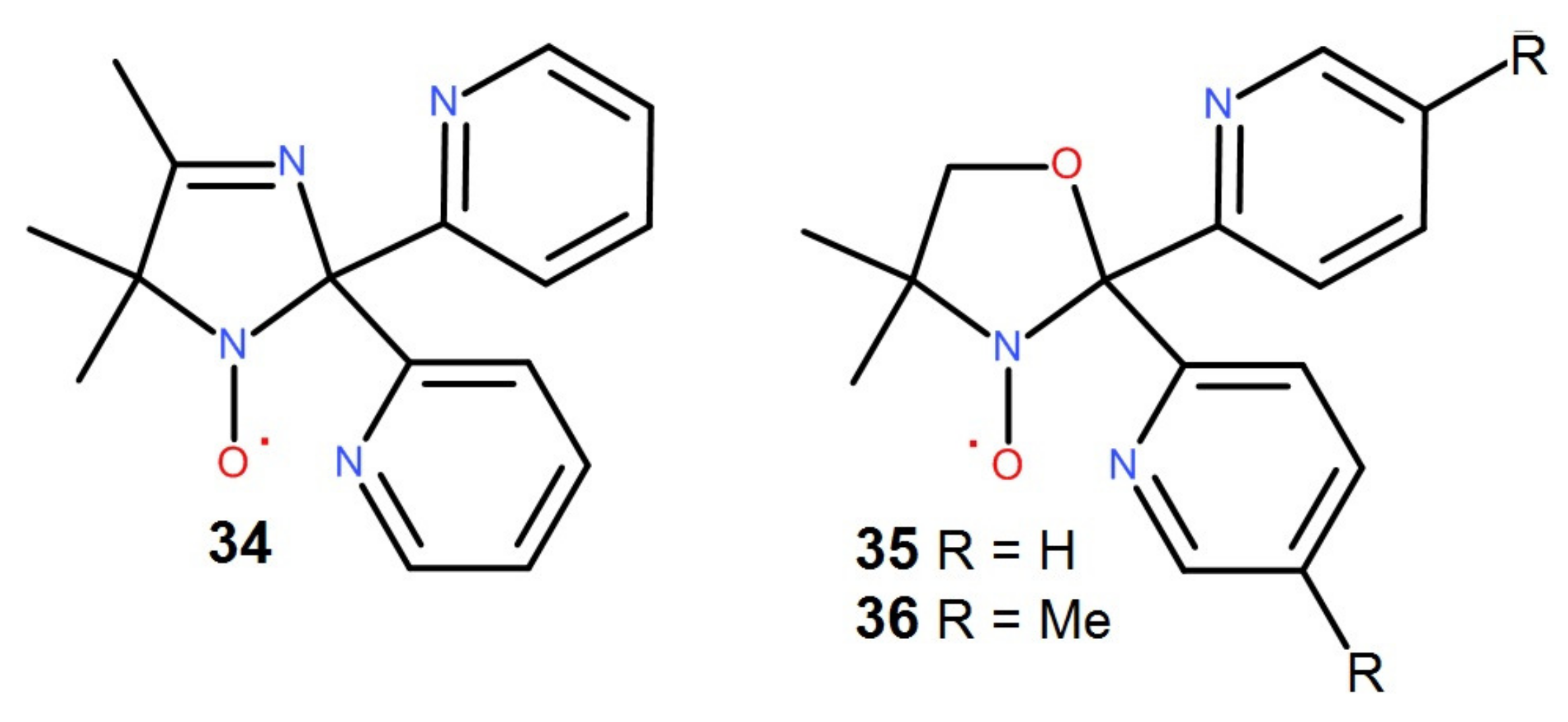
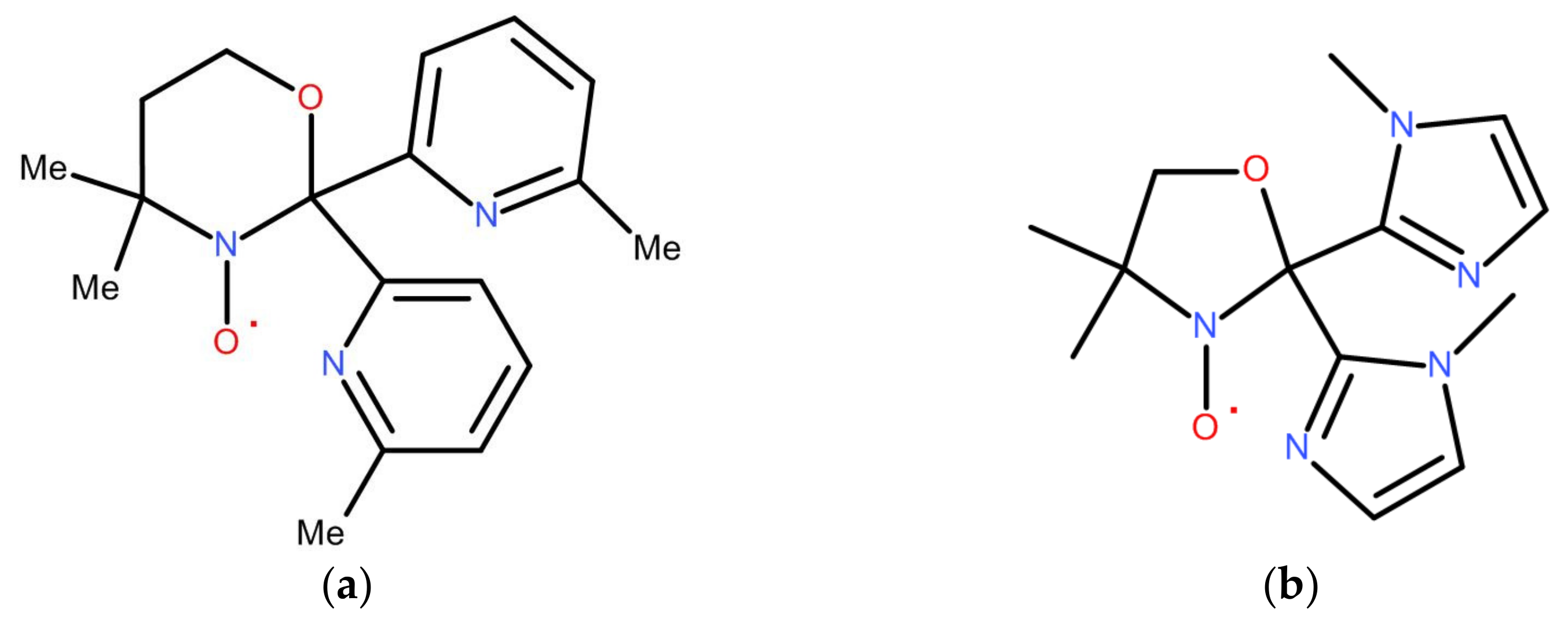
4.1.1. Functionalized by 2-Pyridyl Groups Paramagnetic Tripods and Their Complexes
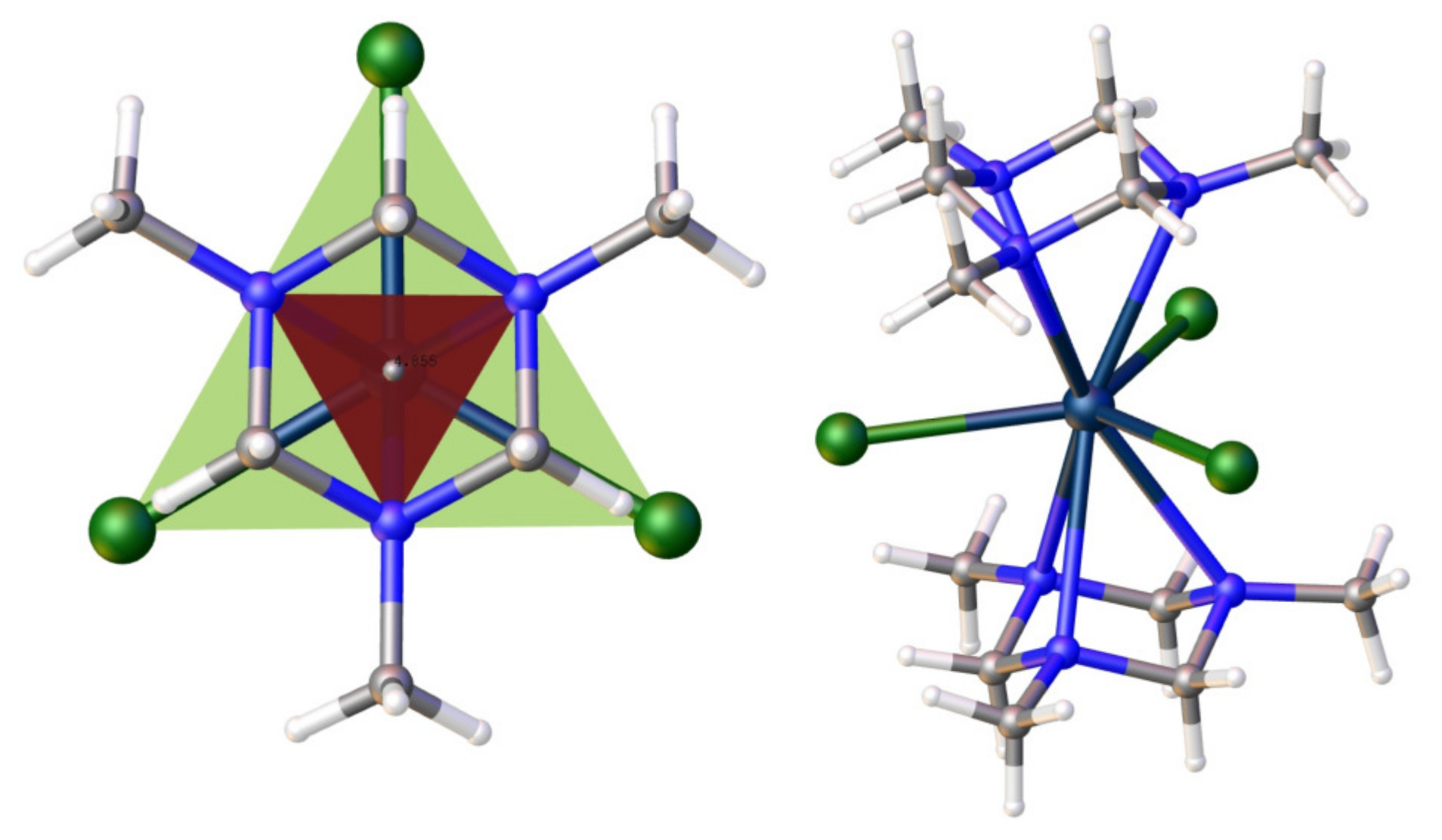
4.1.2. Structural Features of the Paramagnetic Tripod Complexes
4.1.3. Magnetic Properties of the Paramagnetic Tripod Complexes
4.1.4. Rational Design of the Paramagnetic Tripods and Their Complexes
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szczepura, L.F.; Witham, L.M.; Takeuchi, K.J. Tris(2-Pyridyl) Tripod Ligands. Coord. Chem. Rev. 1998, 174, 5–32. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Gade, L.H. A Modular Approach to C1 and C3 Chiral N-Tripodal Ligands for Asymmetric Catalysis. Angew. Chem. Int. Ed. 2002, 41, 3473–3475. [Google Scholar] [CrossRef]
- Natrajan, L.; Pécaut, J.; Mazzanti, M.; LeBrun, C. Controlled Hydrolysis of Lanthanide Complexes of the N-Donor Tripod Tris(2-Pyridylmethyl)Amine versus Bisligand Complex Formation. Inorg. Chem. 2005, 44, 4756–4765. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, H.-H. 1,1,1-Tris(Hydroxymethyl)Ethane as a New, Efficient, and Versatile Tripod Ligand for Copper-Catalyzed Cross-Coupling Reactions of Aryl Iodides with Amides, Thiols, and Phenols. Org. Lett. 2006, 8, 5609–5612. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.; Brethon, A.; Li, Y.-X.; Sheldon, R.A.; Arends, I.W.C.E. Study of the Efficiency of Amino-Functionalized Ruthenium and Ruthenacycle Complexes as Racemization Catalysts in the Dynamic Kinetic Resolution of 1-Phenylethanol. Adv. Synth. Catal. 2007, 349, 2603–2609. [Google Scholar] [CrossRef]
- Andrez, J.; Bozoklu, G.; Nocton, G.; Pécaut, J.; Scopelliti, R.; Dubois, L.; Mazzanti, M. Lanthanide(II) Complexes Supported by N,O-Donor Tripodal Ligands: Synthesis, Structure, and Ligand-Dependent Redox Behavior. Chem.-Eur. J. 2015, 21, 15188–15200. [Google Scholar] [CrossRef]
- Wietzke, R.; Mazzanti, M.; Latour, J.-M.; Pécaut, J.; Cordier, P.-Y.; Madic, C. Lanthanide(III) Complexes of Tripodal N-Donor Ligands: Structural Models for the Species Involved in Solvent Extraction of Actinides(III). Inorg. Chem. 1998, 37, 6690–6697. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nuriman, N.A.; Verboom, W.; Reinhoudt, D.N. Tripodal Receptors for Cation and Anion Sensors. Sensors 2006, 6, 978–1017. [Google Scholar] [CrossRef]
- Dai, Z.; Canary, J.W. Tailoring Tripodal Ligands for Zinc Sensing. New J. Chem. 2007, 31, 1708–1718. [Google Scholar] [CrossRef]
- Machado, K.; Mukhopadhyay, S.; Videira, R.A.; Mishra, J.; Mobin, S.M.; Mishra, G.S. Polymer Encapsulated Scorpionate Eu3+ Complexes as Novel Hybrid Materials for High Performance Luminescence Applications. RSC Adv. 2015, 5, 35675–35682. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, Y.; Fan, D.; Hong, Z.; Su, W. Preparation, Photo- and Electro-Luminescent Properties of a Novel Complex of Tb(III) with a Tripod Ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Lyubov, D.M.; Mahrova, T.V.; Lyssenko, K.A.; Korlyukov, A.A.; Fedorov, Y.V.; Chernikova, E.Y.; Guari, Y.; Larionova, J.; Trifonov, A.A. Heteroleptic Lanthanide Complexes Coordinated by Tripodal Tetradentate Ligand: Synthesis, Structure, and Magnetic and Photoluminescent Properties. Cryst. Growth Des. 2020, 20, 5184–5192. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, H.; Harima, Y.; Yamashita, K.; Hirayama, D.; Aso, Y.; Otsubo, T. Electrochemical Properties of Self-Assembled Monolayers of Tripod-Shaped Molecules and Their Applications to Organic Light-Emitting Diodes. Chem. Commun. 2001, 1830–1831. [Google Scholar] [CrossRef]
- Zheng, X.-L.; Liu, Y.; Pan, M.; Lü, X.-Q.; Zhang, J.-Y.; Zhao, C.-Y.; Tong, Y.-X.; Su, C.-Y. Bright Blue-Emitting Ce3+ Complexes with Encapsulating Polybenzimidazole Tripodal Ligands as Potential Electroluminescent Devices. Angew. Chem. Int. Ed. 2007, 46, 7399–7403. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Ivashenko, O.; Carroll, G.T.; Robertus, J.; Kistemaker, J.C.M.; London, G.; Browne, W.R.; Rudolf, P.; Feringa, B.L. Control of Surface Wettability Using Tripodal Light-Activated Molecular Motors. J. Am. Chem. Soc. 2014, 136, 3219–3224. [Google Scholar] [CrossRef]
- Kammerer, C.; Rapenne, G. Scorpionate Hydrotris(Indazolyl)Borate Ligands as Tripodal Platforms for Surface-Mounted Molecular Gears and Motors. Eur. J. Inorg. Chem. 2016, 2016, 2214–2226. [Google Scholar] [CrossRef]
- Rabinovich, D. Synthetic Bioinorganic Chemistry: Scorpionates Turn 50. In 50 Years of Structure and Bonding—The Anniversary Volume; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 139–157. ISBN 978-3-319-35138-4. [Google Scholar]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Klitsche, F.; Ramcke, J.; Migenda, J.; Hensel, A.; Vossmeyer, T.; Weller, H.; Gross, S.; Maison, W. Synthesis of Tripodal Catecholates and Their Immobilization on Zinc Oxide Nanoparticles. Beilstein J. Org. Chem. 2015, 11, 678–686. [Google Scholar] [CrossRef]
- Kurşunlu, A.N.; Özmen, M.; Güler, E. A Fluorescent Sensor-Based Tripodal-Bodipy for Cu (II) Ions: Bio-Imaging on Cells. Turk. J. Chem. 2021, 45, 2024–2033. [Google Scholar] [CrossRef]
- Brechin, E.K. Using Tripodal Alcohols to Build High-Spin Molecules and Single-Molecule Magnets. Chem. Commun. 2005, 5141–5153. [Google Scholar] [CrossRef]
- Milios, C.J.; Manoli, M.; Rajaraman, G.; Mishra, A.; Budd, L.E.; White, F.; Parsons, S.; Wernsdorfer, W.; Christou, G.; Brechin, E.K. A Family of [Mn6] Complexes Featuring Tripodal Ligands. Inorg. Chem. 2006, 45, 6782–6793. [Google Scholar] [CrossRef]
- Domingo, N.; Bellido, E.; Ruiz-Molina, D. Advances on Structuring, Integration and Magnetic Characterization of Molecular Nanomagnets on Surfaces and Devices. Chem. Soc. Rev. 2012, 41, 258–302. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Lyubov, D.M.; Mahrova, T.V.; Cherkasov, A.V.; Fukin, G.K.; Guari, Y.; Larionova, J.; Trifonov, A.A. Synthesis, Structure and Magnetic Properties of Tris(Pyrazolyl)Methane Lanthanide Complexes: Effect of the Anion on the Slow Relaxation of Magnetization. Dalton Trans. 2018, 47, 5153–5156. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Nakano, Y.; Urabe, M.; Tanaka, K.; Shiro, M. Structural and Magnetic Studies of Copper(II) and Zinc(II) Coordination Complexes Containing Nitroxide Radicals as Chelating Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3359–3368. [Google Scholar] [CrossRef]
- Gass, I.A.; Gartshore, C.J.; Lupton, D.W.; Moubaraki, B.; Nafady, A.; Bond, A.M.; Boas, J.F.; Cashion, J.D.; Milsmann, C.; Wieghardt, K.; et al. Anion Dependent Redox Changes in Iron Bis-Terdentate Nitroxide {NNO} Chelates. Inorg. Chem. 2011, 50, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Gass, I.A.; Tewary, S.; Nafady, A.; Chilton, N.F.; Gartshore, C.J.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Boas, J.F.; et al. Observation of Ferromagnetic Exchange, Spin Crossover, Reductively Induced Oxidation, and Field-Induced Slow Magnetic Relaxation in Monomeric Cobalt Nitroxides. Inorg. Chem. 2013, 52, 7557–7572. [Google Scholar] [CrossRef]
- Gass, I.A.; Tewary, S.; Rajaraman, G.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Murray, K.S. Solvate-Dependent Spin Crossover and Exchange in Cobalt(II) Oxazolidine Nitroxide Chelates. Inorg. Chem. 2014, 53, 5055–5066. [Google Scholar] [CrossRef]
- Gass, I.A.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Guo, S.-X.; Murray, K.S. Manganese(II) Oxazolidine Nitroxide Chelates: Structure, Magnetism, and Redox Properties. Aust. J. Chem. 2014, 67, 1618–1624. [Google Scholar] [CrossRef]
- Pedersen, A.H.; Geoghegan, B.L.; Nichol, G.S.; Lupton, D.W.; Murray, K.S.; Martínez-Lillo, J.; Gass, I.A.; Brechin, E.K. Hexahalorhenate(IV) Salts of Metal Oxazolidine Nitroxides. Dalton Trans. 2017, 46, 5250–5259. [Google Scholar] [CrossRef]
- Gass, I.A.; Lu, J.; Asadi, M.; Lupton, D.W.; Forsyth, C.M.; Geoghegan, B.L.; Moubaraki, B.; Cashion, J.D.; Martin, L.L.; Bond, A.M.; et al. Use of the TCNQF42− Dianion in the Spontaneous Redox Formation of [FeIII (L−)2][TCNQF4 −]. Chempluschem 2018, 83, 658–668. [Google Scholar] [CrossRef]
- Gass, I.A.; Lu, J.; Ojha, R.; Asadi, M.; Lupton, D.W.; Geoghegan, B.L.; Moubaraki, B.; Martin, L.L.; Bond, A.M.; Murray, K.S. [FeII(L•)2][TCNQF4•−]2: A Redox-Active Double Radical Salt. Aust. J. Chem. 2019, 72, 769–777. [Google Scholar] [CrossRef]
- Trofimenko, S. Boron-Pyrazole Chemistry. J. Am. Chem. Soc. 1966, 88, 1842–1844. [Google Scholar] [CrossRef]
- Oliver, J.D.; Mullica, D.F.; Hutchinson, B.B.; Milligan, W.O. Iron-Nitrogen Bond Lengths in Low-Spin and High-Spin Iron(II) Complexes with Poly(Pyrazolyl)Borate Ligands. Inorg. Chem. 1980, 19, 165–169. [Google Scholar] [CrossRef]
- Trofimenko, S. Recent Advances in Poly(Pyrazolyl)Borate (Scorpionate) Chemistry. Chem. Rev. 1993, 93, 943–980. [Google Scholar] [CrossRef]
- Wu, L.P.; Yamagiwa, Y.; Ino, I.; Sugimoto, K.; Kuroda-Sowa, T.; Kamikawa, T.; Munakata, M. Unique Tetranuclear Copper(II) Cluster and Monomeric Iron(II), (III) Complexes with a Tris(Imidazolyl) Chelating Ligand. Polyhedron 1999, 18, 2047–2053. [Google Scholar] [CrossRef]
- Pettinari, C. (Ed.) Scorpionates II: Chelating Borate Ligands-Dedicated to Swiatoslaw Trofimenko; Imperial College Press and Distribiuted by World Scientific: London, UK, 2008; ISBN 978-1-86094-876-3. [Google Scholar]
- Dougherty, W.G.; Kassel, W.S. Synthesis of a Series of First-Row Tris-Imidazolylphosphine Sandwich Complexes and Their Potential for Formation of Polymetallic Species. Inorganica Chim. Acta 2010, 364, 120–124. [Google Scholar] [CrossRef]
- Saouma, C.T.; Peters, J.C. ME and ME Complexes of Iron and Cobalt That Emphasize Three-Fold Symmetry (E = O, N, NR). Coord. Chem. Rev. 2011, 255, 920–937. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Strekalova, A.D.; Smolentsev, A.I.; Naumov, D.Y.; Bogomyakov, A.S.; Sheludyakova, L.A.; Vasilevskii, S.F. Mono- and Heteroligand Iron(II) Complexes with Tris(3,5-Dimethylpyrazol-1-Yl)Methane. Russ. J. Coord. Chem. 2016, 42, 711–718. [Google Scholar] [CrossRef]
- Feng, M.; Tong, M.-L. Single Ion Magnets from 3d to 5f: Developments and Strategies. Chem.-Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef]
- Landart-Gereka, A.; Quesada-Moreno, M.M.; Palacios, M.A.; Díaz-Ortega, I.F.; Nojiri, H.; Ozerov, M.; Krzystek, J.; Colacio, E. Pushing up the Easy-Axis Magnetic Anisotropy and Relaxation Times in Trigonal Prismatic CoII Mononuclear SMMs by Molecular Structure Design. Chem. Commun. 2023, 59, 952–955. [Google Scholar] [CrossRef]
- Apostolidis, C.; Carvalho, A.; Domingos, A.; Kanellakopulos, B.; Maier, R.; Marques, N.; de Matos, A.P.; Rebizant, J. Chloro-Lanthanide, and Plutonium Complexes Containing the Hydrotris (3,5-Dimethylpyrazol-1-Yl)Borate Ligand: The Crystal and Molecular Structures of [PrCl(μ-Cl)TpMe2(3,5-Me2pzH)]2 and YbCl2TpMe2 (THF). Polyhedron 1998, 18, 263–272. [Google Scholar] [CrossRef]
- Hillier, A.C.; Zhang, X.W.; Maunder, G.H.; Liu, S.Y.; Eberspacher, T.A.; Metz, M.V.; McDonald, R.; Domingos, A.; Marques, N.; Day, V.W.; et al. Synthesis and Structural Comparison of a Series of Divalent Ln(TpR,R)2 (Ln = Sm, Eu, Yb) and Trivalent Sm(TpMe2)2X (X = F, Cl, I, BPh4) Complexes. Inorg. Chem. 2001, 40, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Sella, A.; Brown, S.E.; Steed, J.W.; Tocher, D.A. Synthesis and Solid-State Structures of Pyrazolylmethane Complexes of the Rare Earths. Inorg. Chem. 2007, 46, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Maunder, G.H.; Sella, A.; Stevenson, M.; Tocher, D.A. Synthesis and Molecular Structures of Hydrotris(Dimethylpyrazolyl)Borate Complexes of the Lanthanides. Inorg. Chem. 1996, 35, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.J.; Biros, S.M. Supramolecular Ligands for the Extraction of Lanthanide and Actinide Ions. Org. Chem. Front. 2019, 6, 2067–2094. [Google Scholar] [CrossRef]
- Barraza, R.; Sertage, A.G.; Kajjam, A.B.; Ward, C.L.; Lutter, J.C.; Schlegel, H.B.; Allen, M.J. Properties of Amine-Containing Ligands That Are Necessary for Visible-Light-Promoted Catalysis with Divalent Europium. Inorg. Chem. 2022, 61, 19649–19657. [Google Scholar] [CrossRef]
- Frost, J.M.; Harriman, K.L.M.; Murugesu, M. The Rise of 3-d Single-Ion Magnets in Molecular Magnetism: Towards Materials from Molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular Magnetism of Lanthanide: Advances and Perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-Ac to Dysprosium Metallocenes, a Travel in Time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D. Strangely Attractive: Collaboration and Feedback in the Field of Molecular Magnetism. Int. J. Quantum Chem. 2020, 120, e26248. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, P. Lanthanide Single Molecule Magnets; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-46998-9. [Google Scholar]
- Sorace, L.; Gatteschi, D. Electronic Structure and Magnetic Properties of Lanthanide Molecular Complexes. In Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–26. [Google Scholar]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Vincent, A.H.; Whyatt, Y.L.; Chilton, N.F.; Long, J.R. Strong Axiality in a Dysprosium(III) Bis(Borolide) Complex Leads to Magnetic Blocking at 65 K. J. Am. Chem. Soc. 2023, 145, 1572–1579. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting Single-Ion Anisotropy in the Design of f-Element Single-Molecule Magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Chilton, N.F. Design Criteria for High-Temperature Single-Molecule Magnets. Inorg. Chem. 2015, 54, 2097–2099. [Google Scholar] [CrossRef]
- Harriman, K.L.M.; Errulat, D.; Murugesu, M. Magnetic Axiality: Design Principles from Molecules to Materials. Trends Chem. 2019, 1, 425–439. [Google Scholar] [CrossRef]
- Guo, F.-S.; Bar, A.K.; Layfield, R.A. Main Group Chemistry at the Interface with Molecular Magnetism. Chem. Rev. 2019, 119, 8479–8505. [Google Scholar] [CrossRef]
- Chiesa, A.; Cugini, F.; Hussain, R.; Macaluso, E.; Allodi, G.; Garlatti, E.; Giansiracusa, M.; Goodwin, C.A.P.; Ortu, F.; Reta, D.; et al. Understanding Magnetic Relaxation in Single-Ion Magnets with High Blocking Temperature. Phys. Rev. B 2020, 101, 174402. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-D.; Wang, B.-W.; Gao, S. Advances in Lanthanide Single-Ion Magnets. In Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 111–141. ISBN 978-3-662-45723-8. [Google Scholar]
- Gupta, S.K.; Murugavel, R. Enriching Lanthanide Single-Ion Magnetism through Symmetry and Axiality. Chem. Commun. 2018, 54, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Müntener, T.; Häussinger, D. Intrinsic Anisotropy Parameters of a Series of Lanthanoid Complexes Deliver New Insights into the Structure-Magnetism Relationship. Chem 2021, 7, 3144–3156. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Qin, S.-X. Prediction of the Quantized Axis of Rare-Earth Ions: The Electrostatic Model with Displaced Point Charges. Inorg. Chem. Front. 2015, 2, 613–619. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry Strategies for High Performance Lanthanide-Based Single-Molecule Magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tong, M.-L. Single-Molecule Magnets beyond a Single Lanthanide Ion: The Art of Coupling. Chem. Sci. 2022, 13, 8716–8726. [Google Scholar] [CrossRef]
- Gao, S. (Ed.) Molecular Nanomagnets and Related Phenomena—Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-45722-1. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993; ISBN 978-1-56081-566-2. [Google Scholar]
- Boča, R. Theoretical Foundations of Molecular Magnetism; In the Series, Current Methods in Inorganic Chemistry, Vol. 1; Elsevier: Amsterdam, The Netherlands, 1999; Volume 1, p. 874. ISBN 0-444-50229-7. [Google Scholar]
- Chen, Y.-C.; Liu, J.-L.; Wernsdorfer, W.; Liu, D.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Hyperfine-Interaction-Driven Suppression of Quantum Tunneling at Zero Field in a Holmium(III) Single-Ion Magnet. Angew. Chem. Int. Ed. 2017, 56, 4996–5000. [Google Scholar] [CrossRef]
- Skomski, R.; Sellmyer, D.J. Anisotropy of Rare-Earth Magnets. J. Rare Earths 2009, 27, 675–679. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, J. Lanthanide Single-Molecule Magnets with High Anisotropy Barrier: Where to from Here? Natl. Sci. Rev. 2022, 9, nwac194. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Zheng, Q.; Wang, D.; Chen, Y.; Ju, J.; Sun, T.; Cui, Y.; Ding, Y.; Tang, Y. Lanthanide Single-molecule Magnets: Synthetic Strategy, Structures, Properties and Recent Advances. Chem.—Asian J. 2023, 18, e202201297. [Google Scholar] [CrossRef]
- Trofimenko, S. The Coordination Chemistry of Polypyrazolylborate Ligand; Imperial College Press: London, UK, 1999; ISBN 978-1-86094-172-6. [Google Scholar]
- Cheng, J.; Takats, J.; Ferguson, M.J.; McDonald, R. Heteroleptic Tm(II) Complexes: One More Success for Trofimenko’s Scorpionates. J. Am. Chem. Soc. 2008, 130, 1544–1545. [Google Scholar] [CrossRef]
- Dei, A.; Gatteschi, D.; Pécaut, J.; Poussereau, S.; Sorace, L.; Vostrikova, K. Crystal Field and Exchange Effects in Rare Earth Semiquinone Complexes. Comptes Rendus l’Academie Sci.-Ser. IIC-Chem. 2001, 4, 135–141. [Google Scholar] [CrossRef]
- Zhang, P.; Perfetti, M.; Kern, M.; Hallmen, P.P.; Ungur, L.; Lenz, S.; Ringenberg, M.R.; Frey, W.; Stoll, H.; Rauhut, G.; et al. Exchange Coupling and Single Molecule Magnetism in Redox-Active Tetraoxolene-Bridged Dilanthanide Complexes. Chem. Sci. 2018, 9, 1221–1230. [Google Scholar] [CrossRef]
- Xu, G.-F.; Gamez, P.; Tang, J.; Clérac, R.; Guo, Y.-N.; Guo, Y. MIIIDyIII3 (M = FeIII, CoIII) Complexes: Three-Blade Propellers Exhibiting Slow Relaxation of Magnetization. Inorg. Chem. 2012, 51, 5693–5698. [Google Scholar] [CrossRef]
- Xu, G.-F.; Wang, Q.-L.; Gamez, P.; Ma, Y.; Clérac, R.; Tang, J.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. A Promising New Route towards Single-Molecule Magnets Based on the Oxalate Ligand. Chem. Commun. 2010, 46, 1506–1508. [Google Scholar] [CrossRef]
- Mikhalyova, E.A.; Zeller, M.; Jasinski, J.P.; Butcher, R.J.; Carrella, L.M.; Sedykh, A.E.; Gavrilenko, K.S.; Smola, S.S.; Frasso, M.; Cazorla, S.C.; et al. Combination of Single-Molecule Magnet Behaviour and Luminescence Properties in a New Series of Lanthanide Complexes with Tris(Pyrazolyl)Borate and Oligo(β-Diketonate) Ligands. Dalton Trans. 2020, 49, 7774–7789. [Google Scholar] [CrossRef]
- Kandel, A.V.; Mikhalyova, E.A.; Zeller, M.; Addison, A.W.; Pavlishchuk, V.V. Influence of the Structure of 3-Arylacetylacetonate Ligands on the Luminescence Properties of Eu3+ and Tb3+ Complexes. Theor. Exp. Chem. 2017, 53, 180–186. [Google Scholar] [CrossRef]
- Depperman, E.C.; Bodnar, S.H.; Vostrikova, K.E.; Shultz, D.A.; Kirk, M.L. Spin Robustness of a New Hybrid Inorganic−Organic High-Spin Molecule. J. Am. Chem. Soc. 2001, 123, 3133–3134. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, J.-L.; Zhou, H.-C.; Choi, H.J.; Ke, Y.; Long, J.R.; You, X.-Z. [(Tp)8(H2O)6CuII6FeIII8(CN)24]4+: A Cyanide-Bridged Face-Centered-Cubic Cluster with Single-Molecule-Magnet Behavior. Angew. Chem. Int. Ed. 2004, 43, 5940–5943. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, J.-L.; Gao, S.; Song, Y.; Zhou, H.-C.; Zhang, Y.-Z.; You, X.-Z. The Observation of Superparamagnetic Behavior in Molecular Nanowires. J. Am. Chem. Soc. 2004, 126, 8900–8901. [Google Scholar] [CrossRef]
- Alexandropoulos, D.I.; Vignesh, K.R.; Xie, H.; Dunbar, K.R. Switching on Single-Molecule Magnet Properties of Homoleptic Sandwich Tris(Pyrazolyl)Borate Dysprosium(III) Cations via Intermolecular Dipolar Coupling. Dalton Trans. 2019, 48, 10610–10618. [Google Scholar] [CrossRef] [PubMed]
- Kühling, M.; Wickleder, C.; Ferguson, M.J.; Hrib, C.G.; McDonald, R.; Suta, M.; Hilfert, L.; Takats, J.; Edelmann, F.T. Investigation of the “Bent Sandwich-like” Divalent Lanthanide Hydro-Tris(Pyrazolyl)Borates Ln(TpiPr2)2 (Ln = Sm, Eu, Tm, Yb). New J. Chem. 2015, 39, 7617–7625. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, Z.; Zhan, G.; Sun, B.; Yan, W.; Wang, C.; Wang, L.; Liu, Z.; Bian, Z.; Huang, C. Air Stable and Efficient Rare Earth Eu(Ii) Hydro-Tris(Pyrazolyl)Borate Complexes with Tunable Emission Colors. Inorg. Chem. Front. 2020, 7, 4593–4599. [Google Scholar] [CrossRef]
- Takats, J.; Zhang, X.W.; Day, V.W.; Eberspacher, T.A. Synthesis and Structure of Bis[Hydrotris(3,5-Dimethylpyrazolyl)Borato]Samarium(II), Sm[HB(3,5-Me2pz)3]2, and the Product of Its Reaction with Azobenzene. Organometallics 1993, 12, 4286–4288. [Google Scholar] [CrossRef]
- Maunder, G.H.; Sella, A.; Tocher, D.A. Synthesis and Molecular Structures of a Redox-Related Pair of Lanthanide Complexes. J. Chem. Soc. Chem. Commun. 1994, 885. [Google Scholar] [CrossRef]
- Momin, A.; Carter, L.; Yang, Y.; McDonald, R.; Essafi (née Labouille), S.; Nief, F.; Del Rosal, I.; Sella, A.; Maron, L.; Takats, J. To Bend or Not To Bend: Experimental and Computational Studies of Structural Preference in Ln(TpiPr2)2 (Ln = Sm, Tm). Inorg. Chem. 2014, 53, 12066–12075. [Google Scholar] [CrossRef]
- Saliu, K.O.; Takats, J.; Ferguson, M.J. Bis[Tris(3-tert-butyl-5-Methylpyrazol-1-Yl)Hydridoborato]Ytterbium(II) Toluene Solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m643–m644. [Google Scholar] [CrossRef]
- Suta, M.; Kühling, M.; Liebing, P.; Edelmann, F.T.; Wickleder, C. Photoluminescence Properties of the “Bent Sandwich-like” Compounds [Eu(TpiPr2)2] and [Yb(Tp iPr2)2]–Intermediates between Nitride-Based Phosphors and Metallocenes. J. Lumin. 2017, 187, 62–68. [Google Scholar] [CrossRef]
- Arikawa, Y.; Inada, K.; Onishi, M. Side-on Coordination Mode of a Pyrazolyl Group in the Structure of a Divalent [Sm{B(3-Mepz)4}2] Complex (3-Mepz Is 3-Methylpyrazol-1-Yl). Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 838–841. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zhai, Y.-Q.; Chen, W.-P.; Ding, Y.-S.; Zheng, Y.-Z. Air-Stable Hexagonal Bipyramidal Dysprosium(III) Single-Ion Magnets with Nearly Perfect D6h Local Symmetry. Chem.-Eur. J. 2019, 25, 16219–16224. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Martí, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D 6 h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef]
- Li, Q.-W.; Wan, R.-C.; Chen, Y.-C.; Liu, J.-L.; Wang, L.-F.; Jia, J.-H.; Chilton, N.F.; Tong, M.-L. Unprecedented Hexagonal Bipyramidal Single-Ion Magnets Based on Metallacrowns. Chem. Commun. 2016, 52, 13365–13368. [Google Scholar] [CrossRef]
- Gil, Y.; Castro-Alvarez, A.; Fuentealba, P.; Spodine, E.; Aravena, D. Lanthanide SMMs Based on Belt Macrocycles: Recent Advances and General Trends. Chem.-Eur. J. 2022, 28, e202200336. [Google Scholar] [CrossRef]
- Meyer, G. All the Lanthanides Do It and Even Uranium Does Oxidation State +2. Angew. Chem. Int. Ed. 2014, 53, 3550–3551. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barcelona, Spain, 2013; Volume 2103. [Google Scholar]
- Li, T.; Zhang, G.; Guo, J.; Wang, S.; Leng, X.; Chen, Y. Tris(Pyrazolyl)Methanide Complexes of Trivalent Rare-Earth Metals. Organometallics 2016, 35, 1565–1572. [Google Scholar] [CrossRef]
- Keene, F.R.; Snow, M.R.; Stephenson, P.J.; Tiekink, E.R.T. Ruthenium(II) Complexes of the C3v Ligands Tris(2-Pyridyl)Amine, Tris(2-Pyridyl)Methane, and Tris(2-Pyridyl)Phosphine. 1. Synthesis and x-Ray Structural Studies of the Bis(Ligand) Complexes. Inorg. Chem. 1988, 27, 2040–2045. [Google Scholar] [CrossRef]
- García, F.; Hopkins, A.D.; Humphrey, S.M.; McPartlin, M.; Rogers, M.C.; Wright, D.S. The First Example of a Si-Bridged Tris(Pyridyl) Ligand; Synthesis and Structure of [MeSi(2-C5H4N)3LiX] (X = 0.2Br, 0.8Cl). Dalton Trans. 2004, 361–362. [Google Scholar] [CrossRef]
- Mann, F.G.; Watson, J. Conditions of Salt Formation in Polyamines and Kindred Compounds. Salt Formation in the Tertiary 2-Pyridylamines, Phosphines and Arsines. J. Org. Chem. 1948, 13, 502–531. [Google Scholar] [CrossRef]
- García, F.; Hopkins, A.D.; Kowenicki, R.A.; McPartlin, M.; Rogers, M.C.; Silvia, J.S.; Wright, D.S. Syntheses and Structure of Heterometallic Complexes Containing Tripodal Group 13 Ligands [RE(2-Py)3]− (E = Al, In). Organometallics 2006, 25, 2561–2568. [Google Scholar] [CrossRef]
- Beswick, M.A.; Belle, C.J.; Davies, M.K.; Halcrow, M.A.; Raithby, P.R.; Steiner, A.; Wright, D.S. One-Pot Synthesis of a Novel Tridentate Tin(IV) Ligand; Syntheses and Structures of [BunSn(NC5H4-C,N)3MBr](M = Li, Cu). Chem. Commun. 1996, 2619. [Google Scholar] [CrossRef]
- Zeckert, K.; Zahn, S.; Kirchner, B. Tin–Lanthanoid Donor–Acceptor Bonds. Chem. Commun. 2010, 46, 2638. [Google Scholar] [CrossRef] [PubMed]
- Beswick, M.A.; Davies, M.K.; Raithby, P.R.; Steiner, A.; Wright, D.S. Synthesis and Structure of [Pb(2-Py)3Li·THF], Containing a Low-Valent Group 14 Tris(Pyridyl) Ligand (2-Py = 2-Pyridyl). Organometallics 1997, 16, 1109–1110. [Google Scholar] [CrossRef]
- Goura, J.; McQuade, J.; Shimoyama, D.; Lalancette, R.A.; Sheridan, J.B.; Jäkle, F. Electrophilic and Nucleophilic Displacement Reactions at the Bridgehead Borons of Tris(Pyridyl)Borate Scorpionate Complexes. Chem. Commun. 2022, 58, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Reichart, F.; Kischel, M.; Zeckert, K. Lanthanide(II) Complexes of a Dual Functional Tris(2-Pyridyl)Stannate Derivative. Chem.-Eur. J. 2009, 15, 10018–10020. [Google Scholar] [CrossRef] [PubMed]
- Zeckert, K. Syntheses and Structures of Lanthanoid(II) Complexes Featuring Sn–M (M = Al, Ga, In) Bonds. Dalton Trans. 2012, 41, 14101–14106. [Google Scholar] [CrossRef]
- Zeckert, K. Pyridyl Compounds of Heavier Group 13 and 14 Elements as Ligands for Lanthanide Metals. Organometallics 2013, 32, 1387–1393. [Google Scholar] [CrossRef]
- Zeckert, K.; Griebel, J.; Kirmse, R.; Weiß, M.; Denecke, R. Versatile Reactivity of a Lithium Tris(Aryl)Plumbate(II) Towards Organolanthanoid Compounds: Stable Lead-Lanthanoid-Metal Bonds or Redox Processes. Chem.-Eur. J. 2013, 19, 7718–7722. [Google Scholar] [CrossRef]
- Hellmann, K.W.; Gade, L.H.; Gevert, O.; Steinert, P.; Lauher, J.W. Tripodal Triamidostannates and -Plumbates. Inorg. Chem. 1995, 34, 4069–4078. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Simmonds, H.R.; Wright, D.S. Formation of a Heterometallic AlIII/SmIII Complex Involving a Novel [EtAl(2-Py)2O]2– Ligand (2-Py = 2-Pyridyl). Organometallics 2014, 33, 7113–7117. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Kopf, S.; Wright, D.S. Modifying the Donor Properties of Tris(Pyridyl)Aluminates in Lanthanide(Ii) Sandwich Compounds. Dalton Trans. 2018, 47, 2232–2239. [Google Scholar] [CrossRef]
- Hajiashrafi, T.; Nemati Kharat, A.; Love, J.A.; Patrick, B.O. Synthesis, Characterization and Crystal Structure of Three New Lanthanide (III) Complexes with the [(6-Methyl-2-Pyridyl)Methyl]Bis(2-Pyridylmethyl)Amine (MeTPA) Ligand; New Precursors for Lanthanide (III) Oxide Nano-Particles. Polyhedron 2013, 60, 30–38. [Google Scholar] [CrossRef]
- Hay, M.A.; Gable, R.W.; Boskovic, C. Modulating the Electronic Properties of Divalent Lanthanoid Complexes with Subtle Ligand Tuning. Dalton Trans. 2023, 52, 3315–3324. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.; Tan, P.; Lv, W.; Cui, H.; Chen, L.; Cai, X.; Zhao, Y.; Yuan, A. Tuning the Ligand Field in Seven-Coordinate Dy(III) Complexes to Perturb Single-Ion Magnet Behavior. New J. Chem. 2021, 45, 8591–8596. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Tanaka, N.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Upward Temperature Shift of the Intrinsic Phase Lag of the Magnetization of Bis(Phthalocyaninato)Terbium by Ligand Oxidation Creating an S = 1/2 Spin. Inorg. Chem. 2004, 43, 5498–5500. [Google Scholar] [CrossRef]
- Pederson, R.; Wysocki, A.L.; Mayhall, N.; Park, K. Multireference Ab Initio Studies of Magnetic Properties of Terbium-Based Single-Molecule Magnets. J. Phys. Chem. A 2019, 123, 6996–7006. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong Exchange and Magnetic Blocking in N23−-Radical-Bridged Lanthanide Complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef]
- Vieru, V.; Iwahara, N.; Ungur, L.; Chibotaru, L.F. Giant Exchange Interaction in Mixed Lanthanides. Sci. Rep. 2016, 6, 24046. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Ovcharenko, V.I. The Chemistry of Nitroxide Radicals in the Molecular Design of Magnets. Russ. Chem. Rev. 2009, 78, 971. [Google Scholar] [CrossRef]
- Vostrikova, K.E. High-Spin Molecules Based on Metal Complexes of Organic Free Radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Vostrikova, K.E.; Romanenko, G.V.; Ikorski, V.N.; Podberezskaya, N.V.; Larionov, S.V. Synthesis, Crystal Structure and Magnetic Properties of Di(Methanol) and Di(Ethanol)-Bis 2,2,5,5-Tetramethyl-1-Oxyl-3-Imidazoline-4-(3′,3′,3′-Trifluoromethyl-1-Propenyl-2′-Oxyato Nickel(II)—A New Type of Low Temperature Ferromagnetics. Dokl. Akad. Nauk SSSR 1989, 306, 115–118. [Google Scholar]
- Meng, X.; Shi, W.; Cheng, P. Magnetism in One-Dimensional Metal–Nitronyl Nitroxide Radical System. Coord. Chem. Rev. 2019, 378, 134–150. [Google Scholar] [CrossRef]
- Fegy, K.; Vostrikova, K.E.; Luneau, D.; Rey, P. New Nitroxide Based Molecular Magnetic Materials. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1997, 305, 69–80. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Vostrikova, K.E.; Ikorskii, V.N.; Larionov, S.V.; Sagdeev, R.Z. The Low Temperature Ferromagnet Dimethanol-Bis-[2,2,5,5-Tetramethyl-1-Oxyl-3-Imidazoline-4-(3′,3′,3′-TriFluoromethyl-1′-Propenyl-2′-Oxyato)] Cobalt(II), CoL2(CH3OH)2. Dokl. Akad. Nauk SSSR 1989, 306, 660–662. [Google Scholar]
- Hintermaier, F.; Volodarsky, L.B.; Polborn, K.; Beck, W. New 2,5-Dihydroimidazole-1-Oxyls with Functional Side Groups (N, O, S Donors). Liebigs Ann. 1995, 1995, 2189–2194. [Google Scholar] [CrossRef]
- Hintermaier, F.; Sünkel, K.; Volodarsky, L.B.; Beck, W. Synthesis, Structure, and Magnetic Properties of Transition Metal Complexes of the Nitroxide 2,5-Dihydro-4,5,5-Trimethyl-2,2-Bis(2-Pyridyl)Imidazole-1-Oxyl. Inorg. Chem. 1996, 35, 5500–5503. [Google Scholar] [CrossRef]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [Google Scholar] [CrossRef]
- Rey, P.; Smolentsev, A.I.; Vostrikova, K.E. Oxazolidine Nitroxide Transformation in a Coordination Sphere of the Ln3+ Ions. Molecules 2022, 27, 1626. [Google Scholar] [CrossRef]
- Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. [Google Scholar] [CrossRef]
- Köhn, R.D.; Pan, Z.; Kociok-Köhn, G.; Mahon, M.F. New Sandwich Complexes of Praseodymium(Iii) Containing Triazacyclohexane Ligands. J. Chem. Soc. Dalton Trans. 2002, 2344–2347. [Google Scholar] [CrossRef]
- Wedal, J.C.; Ziller, J.W.; Evans, W.J. Trimethyltriazacyclohexane Coordination Chemistry of Simple Rare-Earth Metal Salts. Dalton Trans. 2023, 52, 4787–4795. [Google Scholar] [CrossRef]
- Hazama, R.; Umakoshi, K.; Kabuto, C.; Kabuto, K.; Sasaki, Y. A Europium(III)-N,N,N′,N′-Tetrakis(2-Pyridylmethyl)-(R)-Propylenediamine Complex as a New Chiral Lanthanide NMR Shift Reagent for Aqueous Neutral Solution. Chem. Commun. 1996, 15–16. [Google Scholar] [CrossRef]
- Vostrikova, K.E.; Samsonenko, D.G. CCDC 2268033: Experimental Crystal Structure Determination. CSD Commun. 2023. [Google Scholar] [CrossRef]
- Ishida, T.; Murakami, R.; Kanetomo, T.; Nojiri, H. Magnetic Study on Radical-Gadolinium(III) Complexes. Relationship between the Exchange Coupling and Coordination Structure. Polyhedron 2013, 66, 183–187. [Google Scholar] [CrossRef]
- Kanetomo, T.; Ishida, T. Strongest Exchange Coupling in Gadolinium(III) and Nitroxide Coordination Compounds. Inorg. Chem. 2014, 53, 10794–10796. [Google Scholar] [CrossRef]
- Kanetomo, T.; Yoshitake, T.; Ishida, T. Strongest Ferromagnetic Coupling in Designed Gadolinium(III)–Nitroxide Coordination Compounds. Inorg. Chem. 2016, 55, 8140–8146. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, M.; Mei, X.; Tian, H.; Ma, Y.; Li, L.; Liao, D. Single-Molecule Magnets Based on Rare Earth Complexes with Chelating Benzimidazole-Substituted Nitronyl Nitroxide Radicals. Dalton Trans. 2012, 41, 14651. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Wang, J.; Li, L. Unusual Gd–Nitronyl Nitroxide Antiferromagnetic Coupling and Slow Magnetic Relaxation in the Corresponding Tb Analogue. Dalton Trans. 2015, 44, 13890–13896. [Google Scholar] [CrossRef]
- Wang, J.; Miao, H.; Xiao, Z.-X.; Zhou, Y.; Deng, L.-D.; Zhang, Y.-Q.; Wang, X.-Y. Syntheses, Structures and Magnetic Properties of the Lanthanide Complexes of the Pyrimidyl-Substituted Nitronyl Nitroxide Radical. Dalton Trans. 2017, 46, 10452–10461. [Google Scholar] [CrossRef]
- Benelli, C.; Caneschi, A.; Gatteschi, D.; Pardi, L. Gadolinium(III) Complexes with Pyridine-Substituted Nitronyl Nitroxide Radicals. Inorg. Chem. 1992, 31, 741–746. [Google Scholar] [CrossRef]
- Hu, P.; Sun, Z.; Wang, X.; Li, L.; Liao, D.; Luneau, D. Magnetic Relaxation in Mononuclear Tb Complex Involving a Nitronyl Nitroxide Ligand. New J. Chem. 2014, 38, 4716–4721. [Google Scholar] [CrossRef]
- Chen, P.Y.; Wu, M.Z.; Shi, X.J.; Tian, L. A Family of Multi-Spin Rare-Earth Complexes Based on a Triazole Nitronyl Nitroxide Radical: Synthesis, Structure and Magnetic Properties. RSC Adv. 2018, 8, 15480–15486. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ishida, T. Magnetic Exchange Interaction in Gadolinium(III) Complex Having Aliphatic Nitroxide Radical TEMPO; AIP Publishing: New York, NY, USA, 2016; p. 020016. [Google Scholar]
- Caneschi, A.; Dei, A.; Gatteschi, D.; Sorace, L.; Vostrikova, K. Antiferromagnetic Coupling in a Gadolinium(III) Semiquinonato Complex. Angew. Chem. Int. Ed. 2000, 39, 246–248. [Google Scholar] [CrossRef]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin Density in a Nitronyl Nitroxide Free Radical. Polarized Neutron Diffraction Investigation and Ab Initio Calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [Google Scholar] [CrossRef]
- Hamada, D.; Fujinami, T.; Yamauchi, S.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Coletti, C.; Re, N. Luminescent DyIII Single Ion Magnets with Same N6O3 Donor Atoms but Different Donor Atom Arrangements, ‘Fac’-[DyIII(HLDL-Ala)3]·8H2O and ‘Mer’-[DyIII(HLDL-Phe)3]·7H2O. Polyhedron 2016, 109, 120–128. [Google Scholar] [CrossRef]
- Murakami, R.; Nakamura, T.; Ishida, T. Doubly TEMPO-Coordinated Gadolinium(III), Lanthanum(III), and Yttrium(III) Complexes. Strong Superexchange Coupling across Rare Earth Ions. Dalton Trans. 2014, 43, 5893–5898. [Google Scholar] [CrossRef]
- Tkachenko, I.A.; Petrochenkova, N.V.; Mirochnik, A.G.; Karasev, V.E.; Kavun, V.Y. Carboxylato-Bis-Dibenzoylmethanates of Europium(III): Luminescence and Magnetic Properties. Russ. J. Phys. Chem. A 2012, 86, 681–684. [Google Scholar] [CrossRef]
- Kahn, M.L.; Sutter, J.-P.; Golhen, S.; Guionneau, P.; Ouahab, L.; Kahn, O.; Chasseau, D. Systematic Investigation of the Nature of the Coupling between a Ln(III) Ion (Ln = Ce(III) to Dy(III)) and Its Aminoxyl Radical Ligands. Structural and Magnetic Characteristics of a Series of {Ln(Radical)2} Compounds and the Related {Ln(Nitrone)2} Derivat. J. Am. Chem. Soc. 2000, 122, 3413–3421. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vostrikova, K.E. The Tripodal Ligand’s 4f Complexes: Use in Molecular Magnetism. Inorganics 2023, 11, 307. https://doi.org/10.3390/inorganics11070307
Vostrikova KE. The Tripodal Ligand’s 4f Complexes: Use in Molecular Magnetism. Inorganics. 2023; 11(7):307. https://doi.org/10.3390/inorganics11070307
Chicago/Turabian StyleVostrikova, Kira E. 2023. "The Tripodal Ligand’s 4f Complexes: Use in Molecular Magnetism" Inorganics 11, no. 7: 307. https://doi.org/10.3390/inorganics11070307
APA StyleVostrikova, K. E. (2023). The Tripodal Ligand’s 4f Complexes: Use in Molecular Magnetism. Inorganics, 11(7), 307. https://doi.org/10.3390/inorganics11070307







