The Mechanical Properties of Geopolymers from Different Raw Materials and the Effect of Recycled Gypsum
Abstract
1. Introduction
2. Results and Discussion
2.1. Setting Time
2.2. Shrinkage
2.2.1. Plastic Shrinkage
2.2.2. Drying Shrinkage
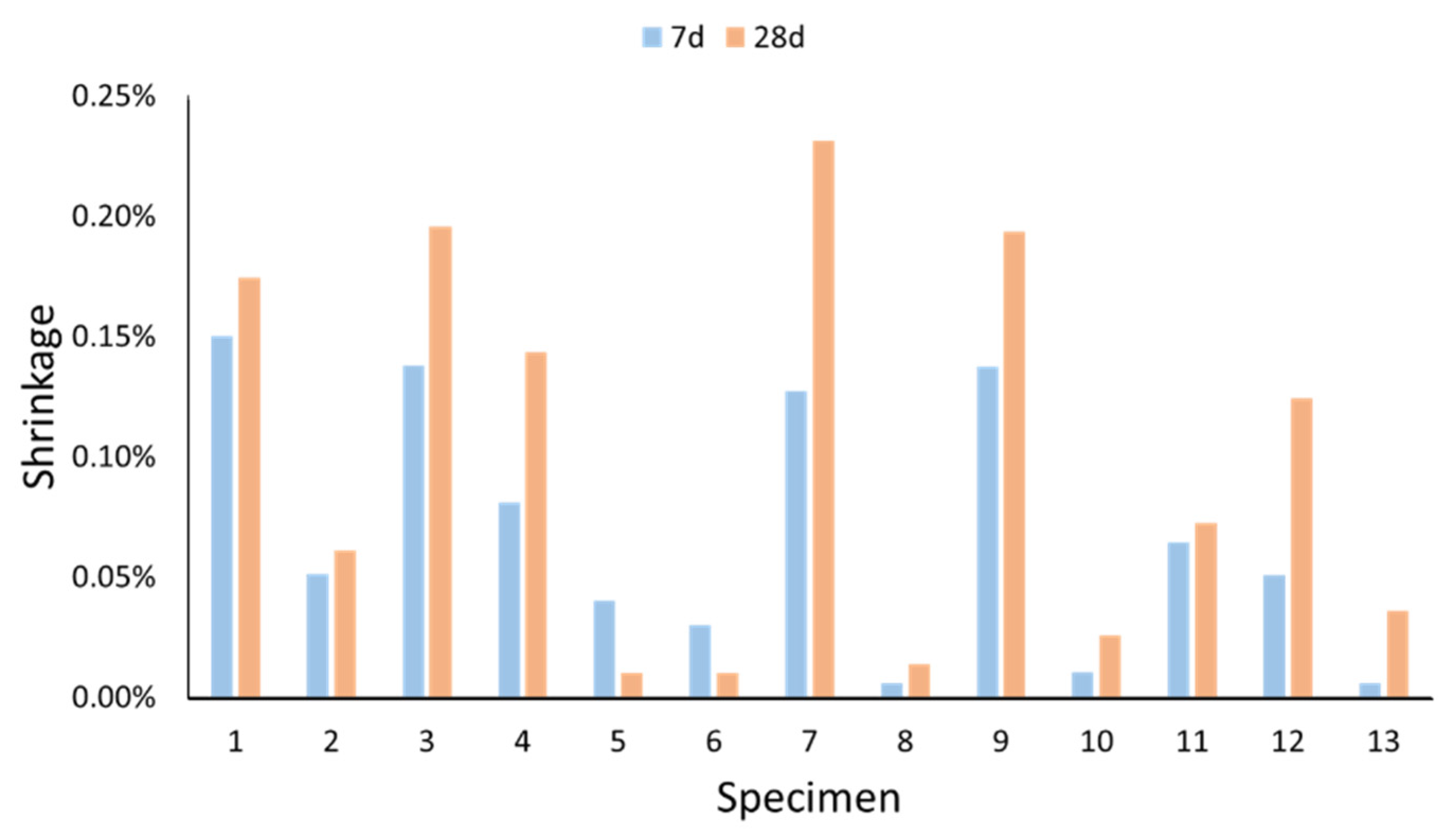
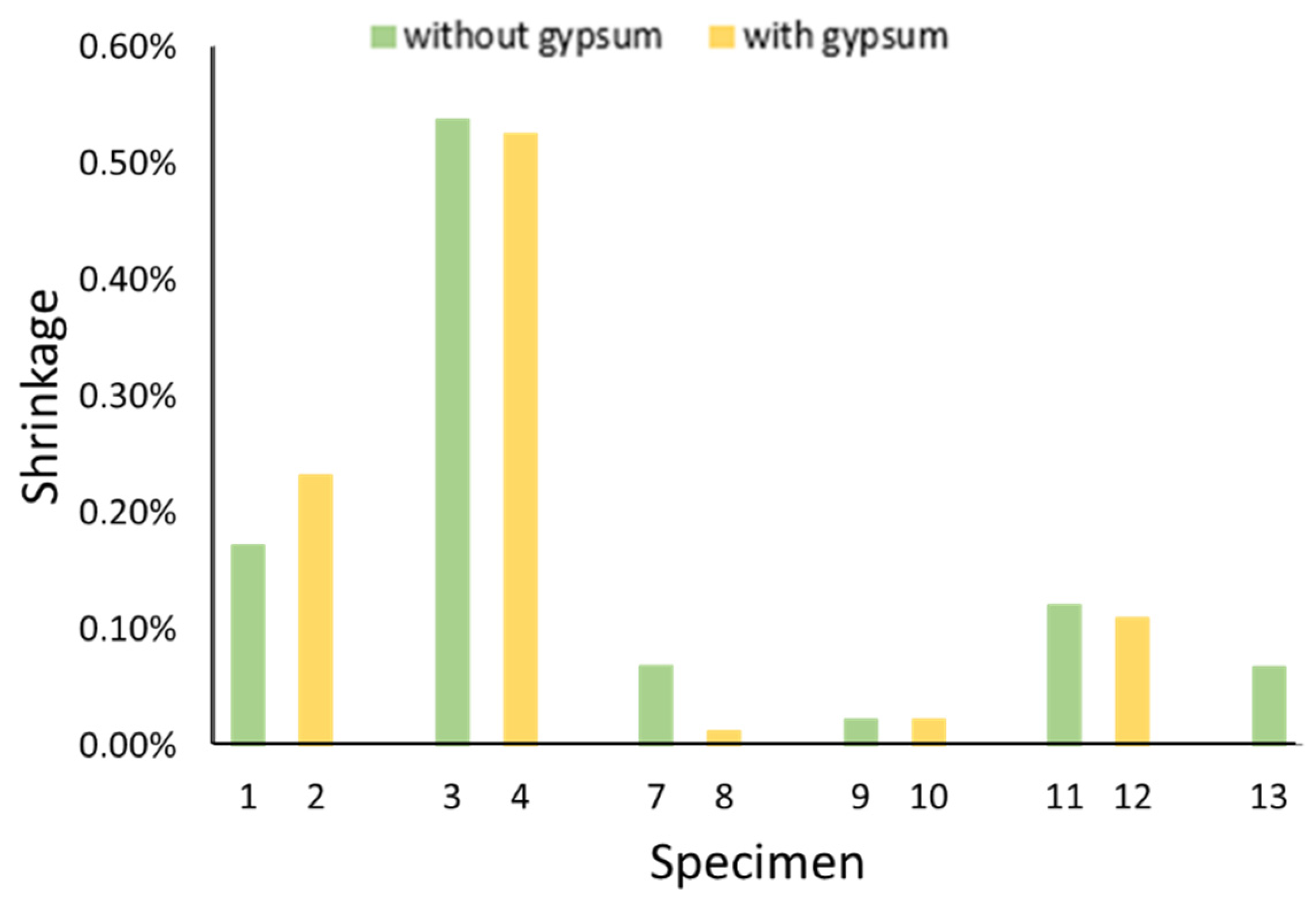
2.2.3. Total Shrinkage
2.3. Strength
2.3.1. Flexural Strength
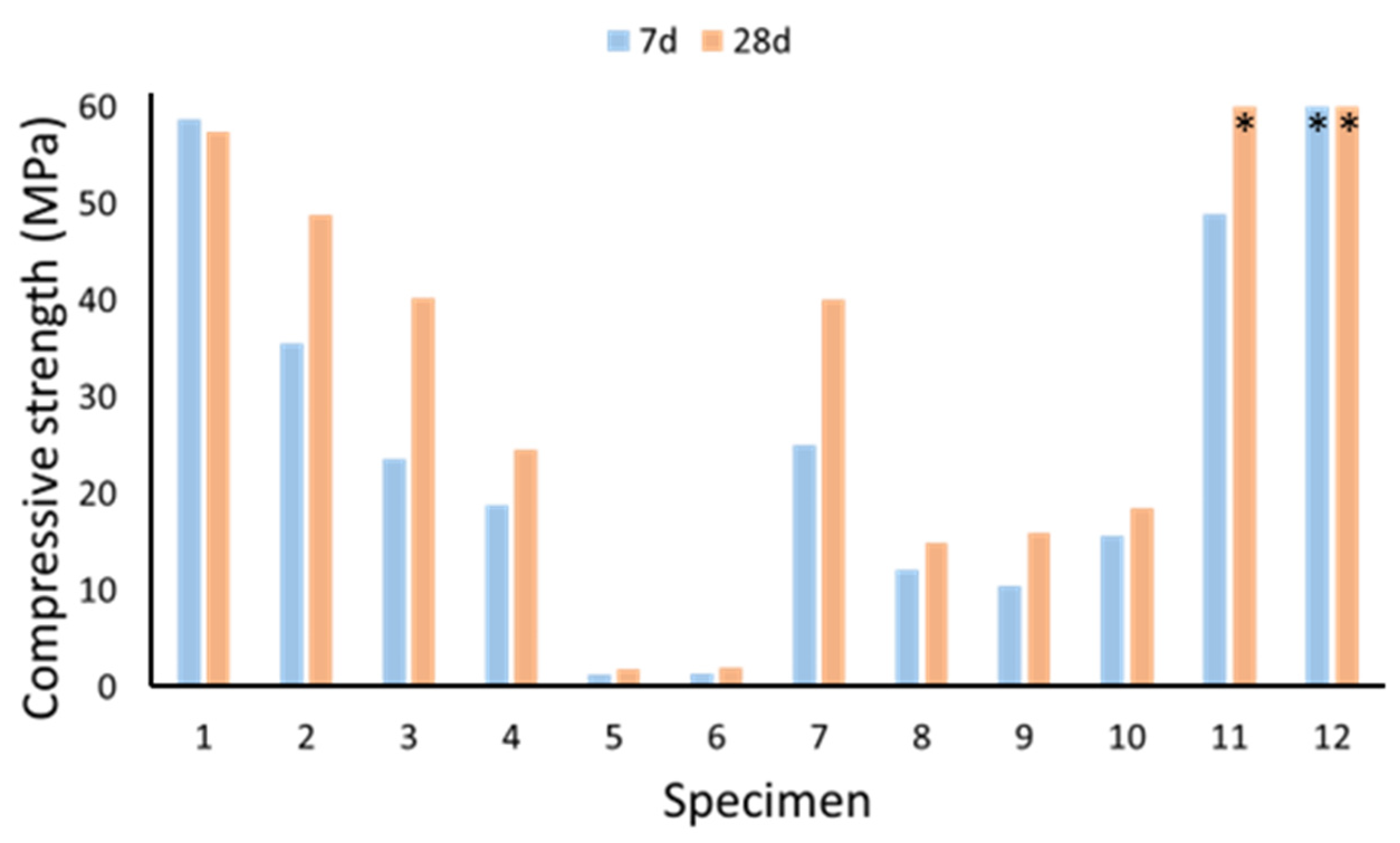
2.3.2. Compressive Strength
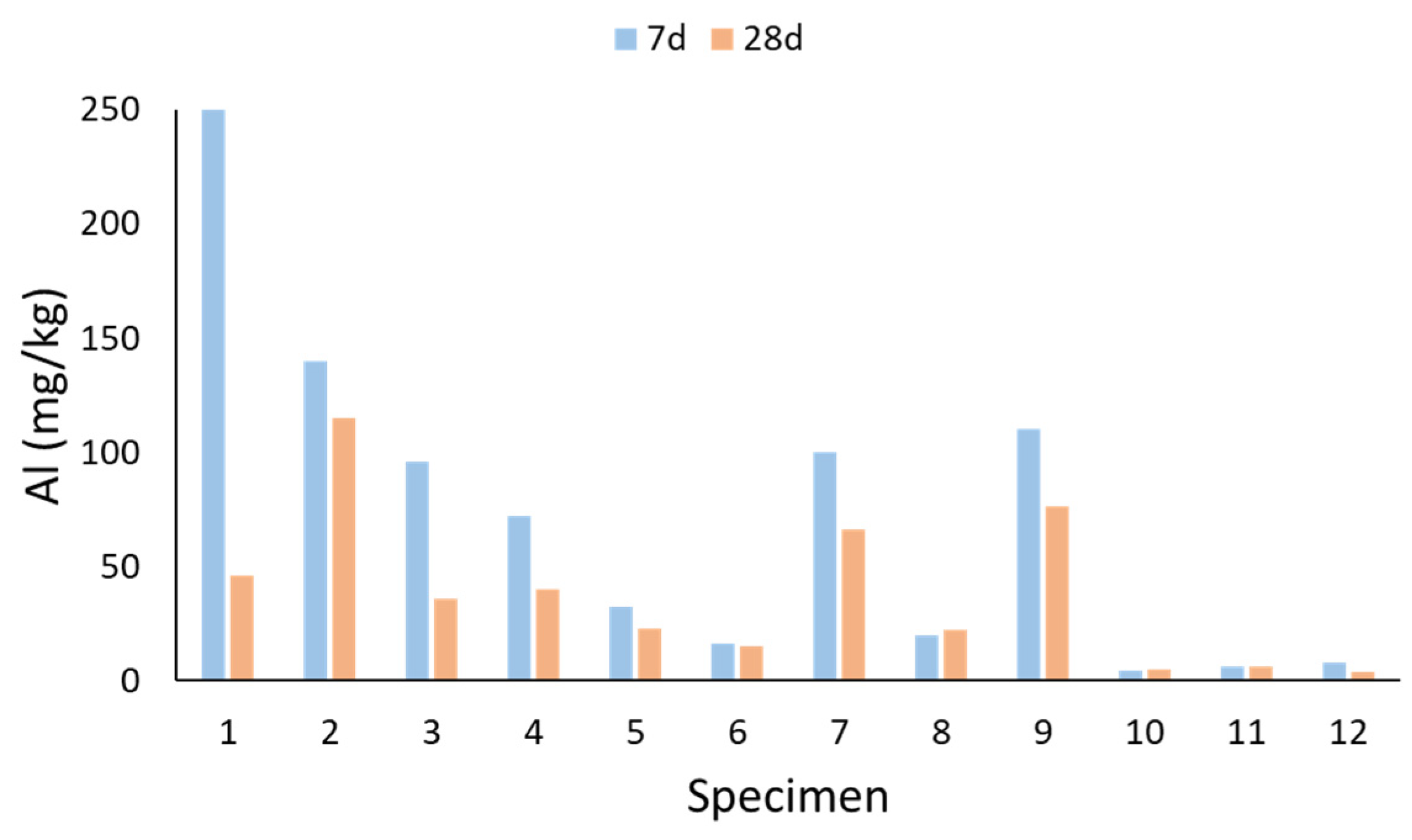
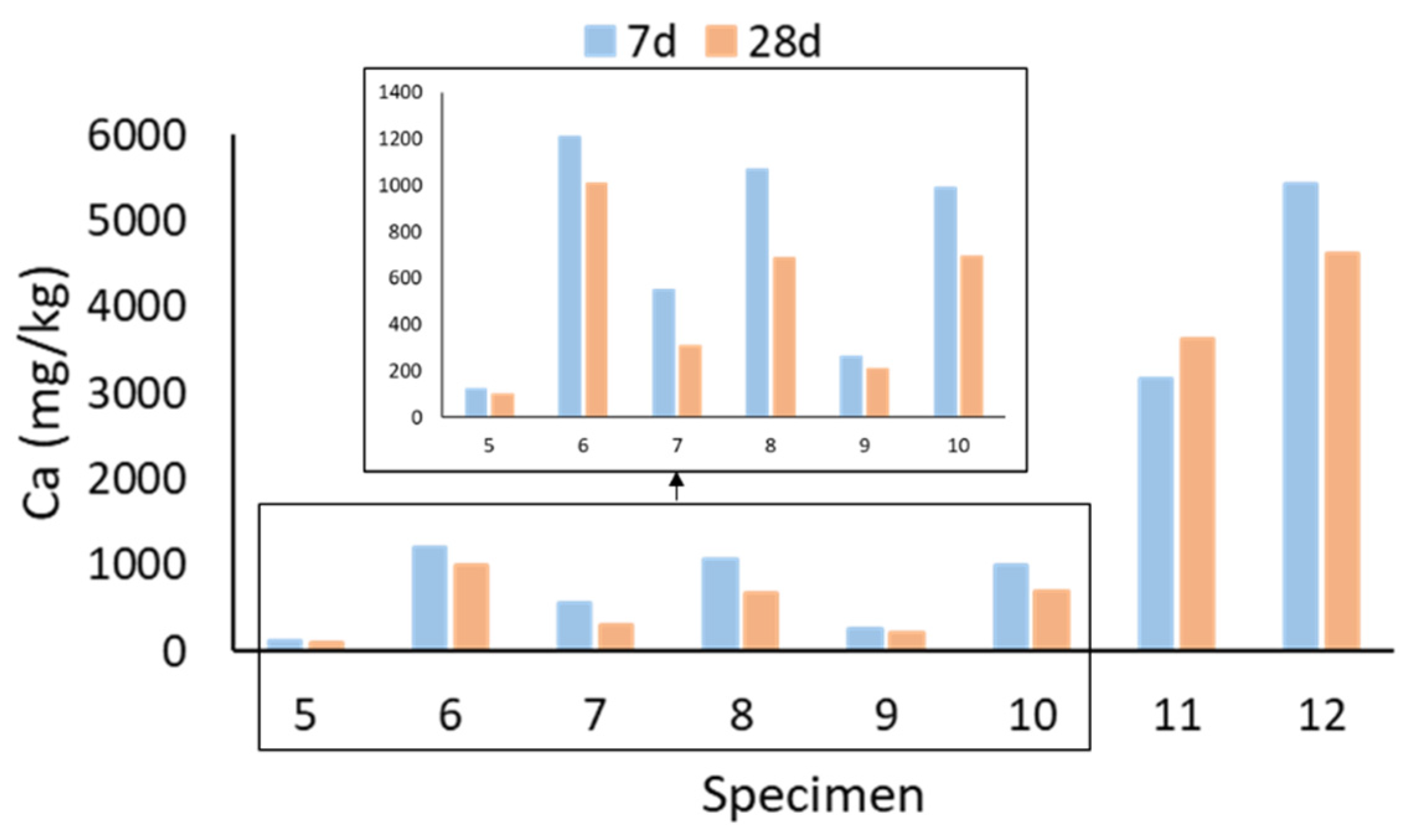
2.4. Leaching of Si, Al, and Ca
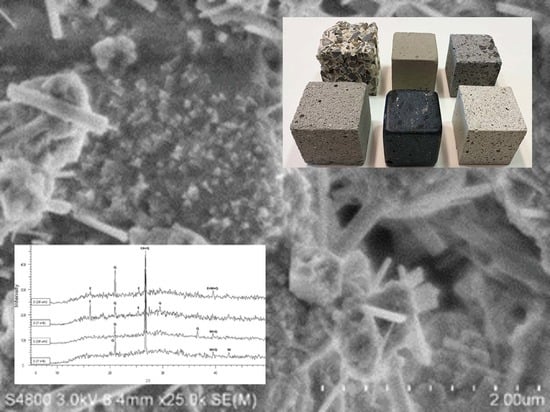
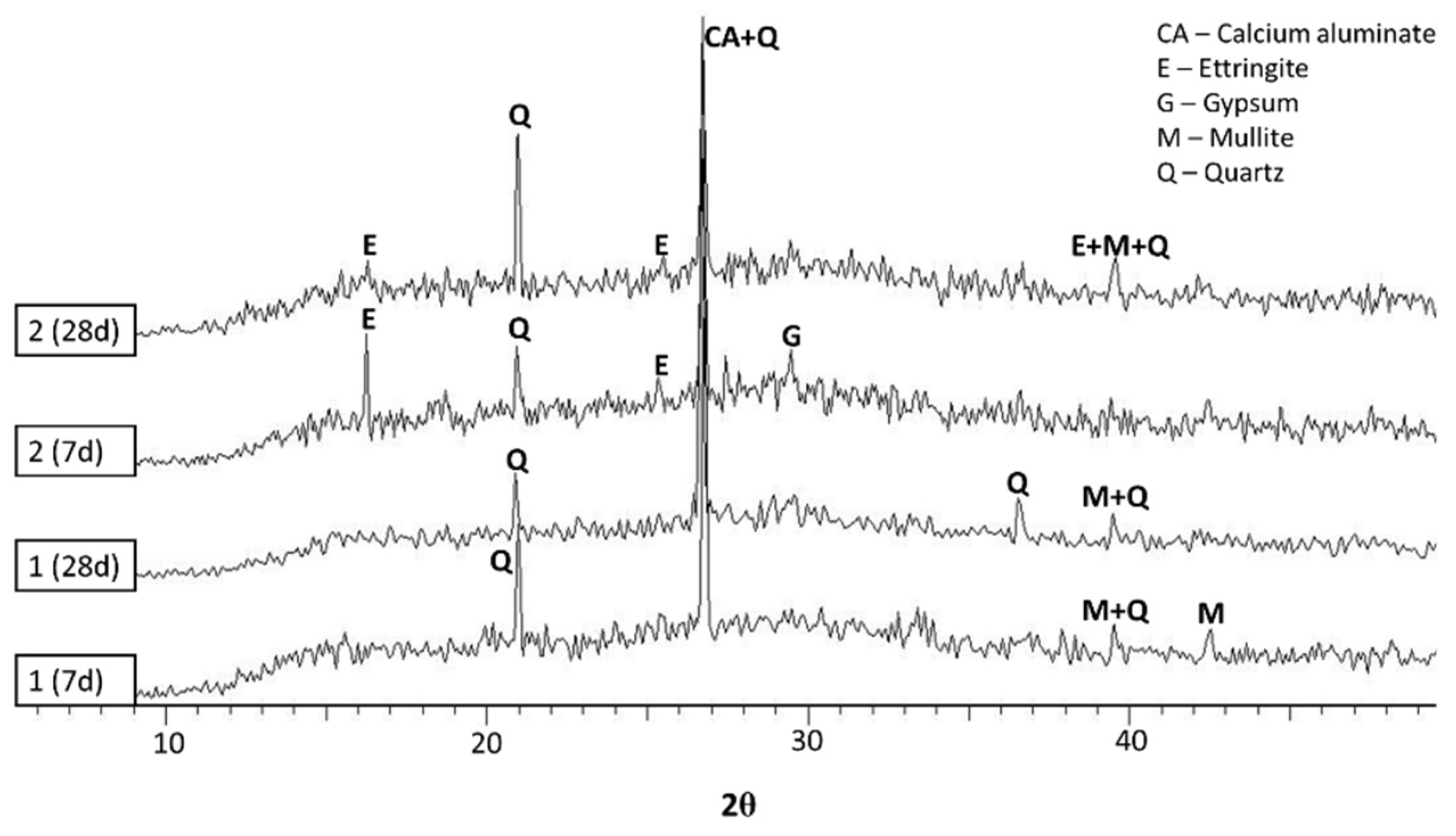
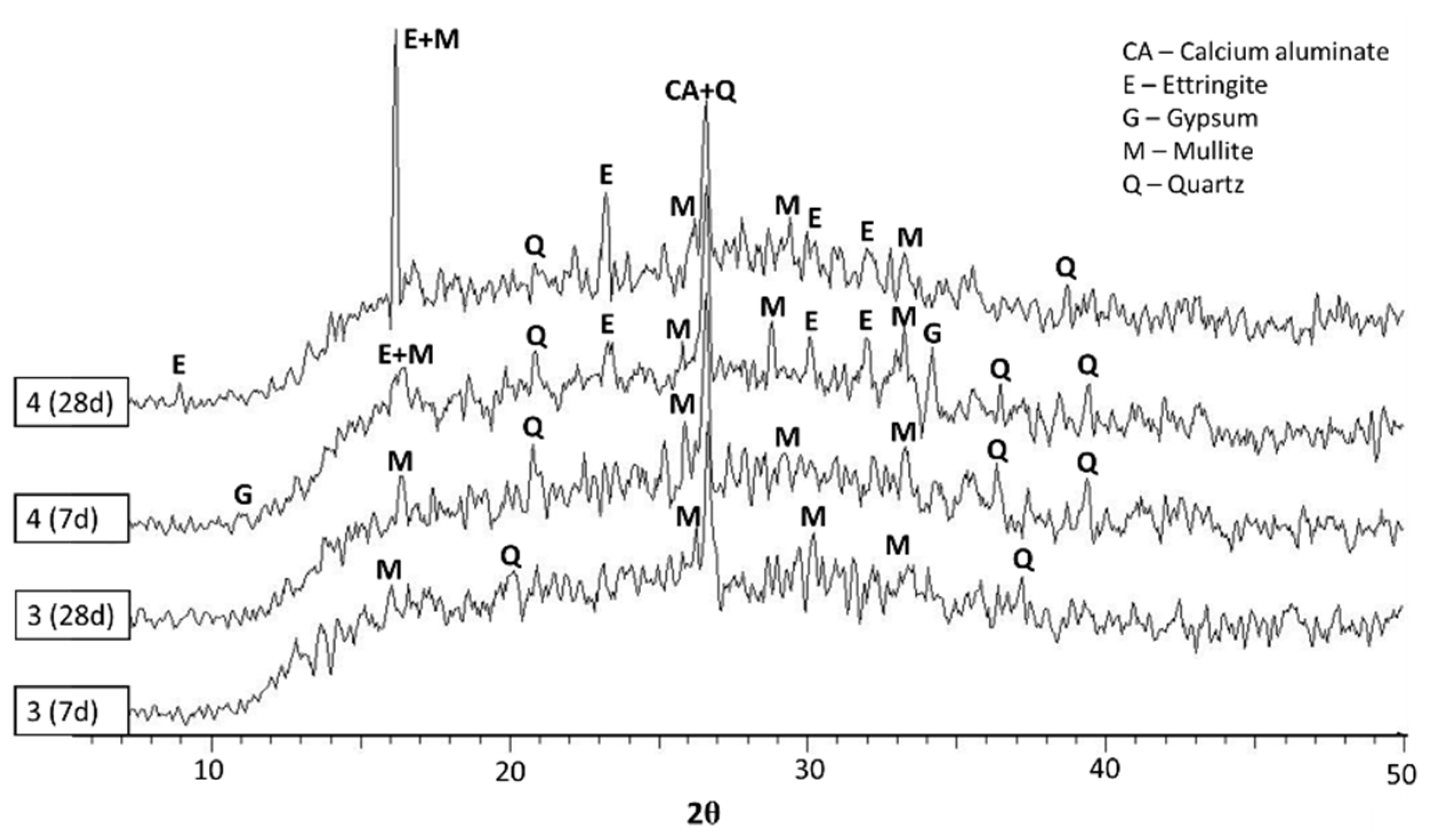
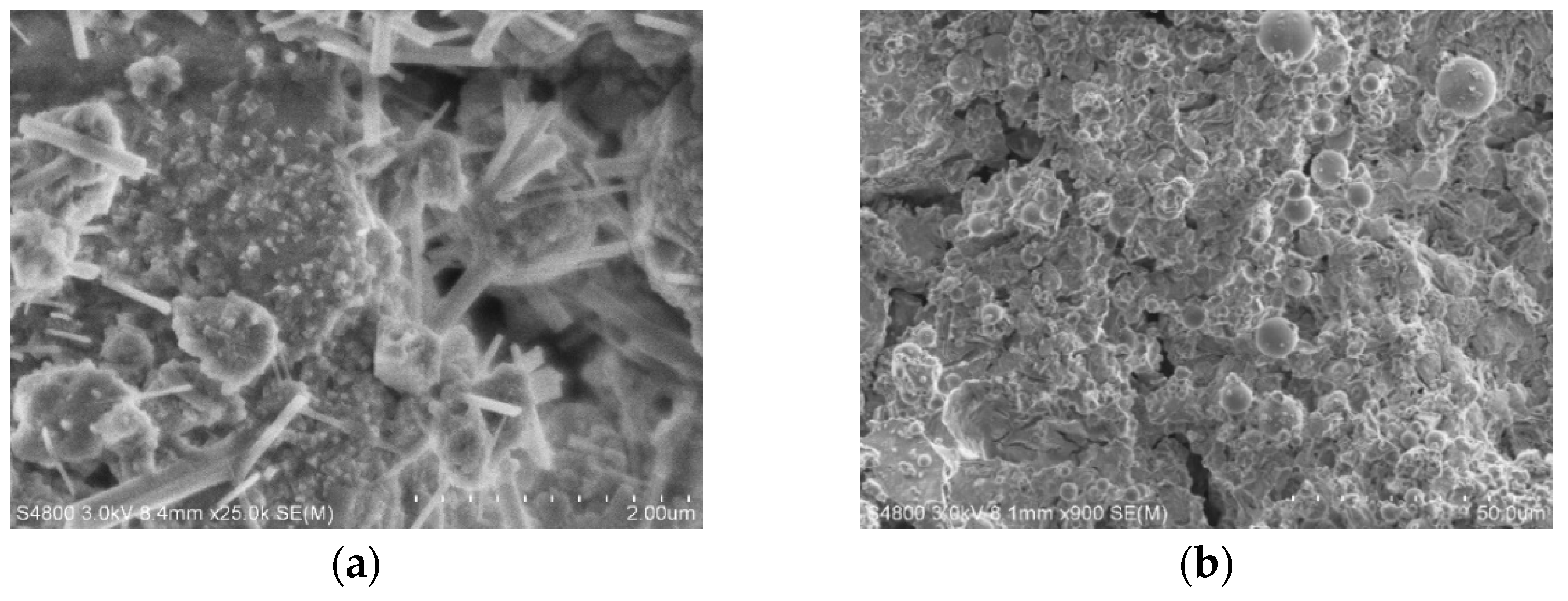
2.5. Crystalline Phase and SEM Measurements
3. Materials and Methods
3.1. Materials
| Raw Material | Na + K | Ca | Si | Al |
|---|---|---|---|---|
| Metakaolin | 1 | - | 25.7 | 21.2 |
| CAC | - | 27.2 | 1.7 | 22.0 |
| Fly ash | 1.7 | 14.3 | 21.0 | 10.0 |
| Biomass ash | 5.3 | 31.0 | 6.1 | 6.5 |
| GGBS | 0.9 | 27.9 | 17.8 | 4.8 |
| OPC | 0.4 | 36.3 | 7.2 | 7.3 |
| Specimen | Solid Raw Materials (Mass% of Total) | WC (%) | Specimen | Solid Raw Materials (Mass% of Total) | WC (%) |
|---|---|---|---|---|---|
| 1 | Metakaolin (34) CAC (12) | 32.7 | 2 | Metakaolin (32) CAC (11.4) CaSO4·2H2O (5) | 31.0 |
| 3 | Fly ash (54) CAC (9.1) | 22.3 | 4 | Fly ash (51) CAC (8.7) CaSO4·2H2O (5) | 21.3 |
| 5 | Fly ash (52) Biomass ash (24) | 27.9 | 6 | Fly ash (49) Biomass ash (21) CaSO4·2H2O (5) | 26.8 |
| 7 | GGBS (74) | 25.1 | 8 | GGBS (70) CaSO4·2H2O (5) | 23.9 |
| 9 | GGBS (48) Biomass ash (21) | 25.8 | 10 | GGBS (45.7) Biomass ash (20) CaSO4·2H2O (5) | 25.3 |
| 11 | OPC (75.8) | 24.2 | 12 | OPC (72.8) CaSO4·2H2O (5) | 22.2 |
| 13 | Metakaolin (10.3) CAC (3.6) Aggregates | 16.2 |
3.2. Preparation Process
3.3. Testing Methods
3.3.1. Setting Time
3.3.2. Shrinkage
3.3.3. Strength
3.3.4. Leaching of Si, Al, and Ca
3.3.5. XRD
3.3.6. SEM
4. Conclusions
- The metakaolin-based specimen (1) had the same setting time as OPC and its compressive strength was the closest to OPC, but the shrinkage was significantly greater, and the specimen had cracks caused by shrinkage. The GBSS and biomass ash-based specimen (9) was the closest to OPC in shrinkage without cracking, but had a much lower compressive strength and a much longer setting time.
- The leaching of Al, Si, and Ca from the specimens was mostly affected by the age and the raw materials of the specimen, as well as the leaching of other substances. However, the leaching of the measured substances did not seem to be related to the mechanical properties of the specimen.
- Recycled gypsum was found to have a notable effect on the setting time. Depending on the calcium content of the raw materials, it either accelerated or decreased the setting time.
- Gypsum effectively reduced the plastic shrinkage of the geopolymer specimens but had no effect on, or even increased, their drying shrinkage. This indicates that the shrinkage-reducing effect of gypsum is based on ettringite, which is not a stable compound. It decomposes over time and thus its shrinkage-reducing effect is also gradually lost.
- Gypsum had almost no effect on the total shrinkage of the geopolymers, as most of this consisted of drying shrinkage.
- It could be concluded from the diffractograms that the formation and decomposition of ettringite depends on the raw materials of the geopolymer.
- In this study, recycled gypsum reduced the compressive strength of the specimens, but this could perhaps be avoided by using a finer (<1 mm) gypsum powder. On the other hand, ettringite can also cause the material to become brittle.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A Review on Geopolymers as Emerging Materials for the Adsorption of Heavy Metals and Dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Sanjayan, J.G. Synthesis of Geopolymer from Industrial Wastes. J. Clean. Prod. 2015, 99, 297–304. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.-Y. Effect of Na/Al on Formation, Structures and Properties of Metakaolin Based Na-Geopolymer. Constr. Build. Mater. 2019, 226, 250–258. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Y. Preparation of Geopolymer Composites Based on Alkali Excitation. Arab. J. Geosci. 2021, 14, 600. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C.; Torroja, E. Ambient Temperature Drying Shrinkage and Cracking in Metakaolin-Based Geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
- Kolezynski, A.; Król, M.; Zychowicz, M. The Structure of Geopolymers—Theoretical Studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential Applications of Geopolymer Concrete in Construction: A Review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead: Boca Raton, FL, USA; CRC Press: Oxford, UK, 2009; pp. 1–11. [Google Scholar]
- van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and Commercial Progress in the Adoption of Geopolymer Cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Kumar Mehta, P. Reducing the Environmental Impact of Concrete. Concr. Int. 2001, 23, 61–66. [Google Scholar]
- Mclellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and Carbon Emissions for Geopolymer Pastes in Comparison to Ordinary Portland Cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Ridtirud, C.; Chindaprasirt, P.; Pimraksa, K. Factors Affecting the Shrinkage of Fly Ash Geopolymers. Int. J. Miner. Metall. Mater. 2011, 18, 100–104. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Davidovits, J., Ed.; Institut Géopolymère: Saint-Quentin, France, 2015. [Google Scholar]
- Wang, R.; Wang, J.; Dong, T.; Ouyang, G. Structural and Mechanical Properties of Geopolymers Made of Aluminosilicate Powder with Different SiO2/Al2O3 Ratio: Molecular Dynamics Simulation and Microstructural Experimental Study. Constr. Build. Mater. 2020, 240, 117935. [Google Scholar] [CrossRef]
- Kenne Diffo, B.B.; Elimbi, A.; Cyr, M.; Dika Manga, J.; Tchakoute Kouamo, H. Effect of the Rate of Calcination of Kaolin on the Properties of Metakaolin-Based Geopolymers. J. Asian Ceram. Soc. 2015, 3, 130–138. [Google Scholar] [CrossRef]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of Activator Concentration on the Strength, ITZ and Drying Shrinkage of Fly Ash/Slag Geopolymer Concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal Evolution of Metakaolin Geopolymers: Part 1-Physical Evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Synthesis and Thermal Behaviour of Potassium Sialate Geopolymers. Mater. Lett. 2003, 57, 1477–1482. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-Friendly Geopolymer Materials: A Review of Performance Improvement, Potential Application and Sustainability Assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. New Insights into the Early Reaction of NaOH-Activated Slag in the Presence of CaSO4. Compos. B Eng. 2020, 198, 108207. [Google Scholar] [CrossRef]
- El Alouani, M.; Saufi, H.; Moutaoukil, G.; Alehyen, S.; Nematollahi, B.; Belmaghraoui, W.; Taibi, M. Application of Geopolymers for Treatment of Water Contaminated with Organic and Inorganic Pollutants: State-of-the-Art Review. J. Environ. Chem. Eng. 2021, 9, 105095. [Google Scholar] [CrossRef]
- Pol Segura, I.; Jensen, P.A.; Damø, A.J.; Ranjbar, N.; Jensen, L.S.; Canut, M. Influence of Sodium-Based Activators and Water Content on the Fresh and Hardened Properties of Metakaolin Geopolymers. SN Appl. Sci. 2022, 4, 283. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Effect of Curing Condition on the Mechanical Properties of Fly Ash-Based Geopolymer Concrete. SN Appl. Sci. 2019, 1, 1694. [Google Scholar] [CrossRef]
- Fang, G.; Bahrami, H.; Zhang, M. Mechanisms of Autogenous Shrinkage of Alkali-Activated Fly Ash-Slag Pastes Cured at Ambient Temperature within 24 h. Constr. Build. Mater. 2018, 171, 377–387. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Mohammadi Firouz, R.; Illikainen, M. Drying Shrinkage in Alkali-Activated Binders—A Critical Review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Ranjbar, N.; Zhang, M. Fiber-Reinforced Geopolymer Composites: A Review. Cem. Concr. Compos. 2020, 107, 103498. [Google Scholar] [CrossRef]
- Kotrla, J.; Soukal, F.; Bilek, V.; Alexa, M. Effects of Shrinkage-Reducing Admixtures on Autogenous Shrinkage in Alkali-Activated Materials. IOP Conf. Ser. Mater. Sci. Eng. 2019, 583, 012023. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Effect of Admixtures on Properties of Alkali-Activated Slag Concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Tao, S.; Yumei, Y. Quantitative Analysis of Ettringite Formed in the Hydration Products of High-Alite Cements. Adv. Cem. Res. 2015, 27, 497–505. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Zhang, E.Q.; Ren, J. Shrinkage Behaviour, Early Hydration and Hardened Properties of Sodium Silicate Activated Slag Incorporated with Gypsum and Cement. Constr. Build. Mater. 2020, 248, 118687. [Google Scholar] [CrossRef]
- Divvala, S.; Rani, M.S. Early Strength Properties of Geopolymer Concrete Composites: An Experimental Study. Mater. Today Proc. 2021, 47, 3770–3777. [Google Scholar] [CrossRef]
- Zaheer, M.; Khan, N.; Uddin, F.; Shaikh, A.; Hao, Y.; Hao, H. Synthesis of High Strength Ambient Cured Geopolymer Composite by Using Low Calcium Fly Ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH Solution on the Synthesis of Fly Ash Geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Pane, I.; Imran, I.; Budiono, B. Compressive Strength of Fly Ash-Based Geopolymer Concrete with a Variable of Sodium Hydroxide (NaOH) Solution Molarity. MATEC Web Conf. 2018, 147, 01004. [Google Scholar] [CrossRef]
- Hardjasaputra, H.; Cornelia, M.; Gunawan, Y.; Surjaputra, I.V.; Lie, H.A.; Pranata Ng, G. Study of Mechanical Properties of Fly Ash-Based Geopolymer Concrete. IOP Conf. Ser. Mater. Sci. Eng. 2019, 615, 012009. [Google Scholar] [CrossRef]
- Talha Ghafoor, M.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of Alkaline Activators on the Mechanical Properties of Fly Ash Based Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Gómez-Casero, M.A.; de Dios-Arana, C.; Bueno-Rodríguez, J.S.; Pérez-Villarejo, L.; Eliche-Quesada, D. Physical, Mechanical and Thermal Properties of Metakaolin-Fly Ash Geopolymers. Sustain. Chem. Pharm. 2022, 26, 100620. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Cao, L.; Qi, S.L. Fiber Reinforced Geopolymers for Fire Resistance Applications. Procedia Eng. 2014, 71, 153–158. [Google Scholar] [CrossRef]
- Cao, Y.F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of Calcium Aluminate Cement on Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Cheng-Yong, H.; Al Bakri, M.; Hussin, K. Structure and Properties of Clay-Based Geopolymer Cements: A Review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Juengsuwattananona, K.; Winnefeldb, F.; Chindaprasirtc, P.; Pimraksa, K. Correlation between Initial SiO2/Al2O3, Na2O/Al2O3, Na2O/SiO2 and H2O/Na2O Ratios on Phase and Microstructure of Reaction Products of Metakaolin-Rice Husk Ash Geopolymer. Constr. Build. Mater. 2019, 226, 406–417. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Wang, K.; Zhang, S.; Sheng, D. Performance Deterioration of Fly Ash/Slag-Based Geopolymer Composites Subjected to Coupled Cyclic Preloading and Sulfuric Acid Attack. J. Clean. Prod. 2021, 321, 128942. [Google Scholar] [CrossRef]
- Kozub, B.; Castro-Gomes, J. An Investigation of the Ground Walnut Shells’ Addition Effect on the Properties of the Fly Ash-Based Geopolymer. Materials 2022, 15, 3936. [Google Scholar] [CrossRef] [PubMed]
- Rojo-López, G.; González-Fonteboa, B.; Martínez-Abella, F.; González-Taboada, I. Rheology, Durability, and Mechanical Performance of Sustainable Self-Compacting Concrete with Metakaolin and Limestone Filler. Case Stud. Constr. Mater. 2022, 17, e01143. [Google Scholar] [CrossRef]
- Weimann, K.; Adam, C.; Buchert, M.; Sutter, J. Environmental Evaluation of Gypsum Plasterboard Recycling. Minerals 2021, 11, 101. [Google Scholar] [CrossRef]
- Ibrahim, Y.E.; Adamu, M.; Marouf, M.L.; Ahmed, O.S.; Drmosh, Q.A.; Malik, M.A. Mechanical Performance of Date-Palm-Fiber-Reinforced Concrete Containing Silica Fume. Buildings 2022, 12, 1642. [Google Scholar] [CrossRef]
- Golewski, G.L. Combined Effect of Coal Fly Ash (CFA) and Nanosilica (NS) on the Strength Parameters and Microstructural Properties of Eco-Friendly Concrete. Energies 2023, 16, 452. [Google Scholar] [CrossRef]
- Alfimova, N.; Pirieva, S.; Levickaya, K.; Kozhukhova, N.; Elistratkin, M. The Production of Gypsum Materials with Recycled Citrogypsum Using Semi-Dry Pressing Technology. Recycling 2023, 8, 34. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Alfimova, N.I.; Kozhukhova, M.I.; Nikulin, I.S.; Glazkov, R.A.; Kolomytceva, A.I. The Effect of Recycled Citrogypsum as a Supplementary Mineral Additive on the Physical and Mechanical Performance of Granulated Blast Furnace Slag-Based Alkali-Activated Binders. Recycling 2023, 8, 22. [Google Scholar] [CrossRef]
- Nawaukkaratharnant, N. Utilization of Gypsum-Bonded Investment Mold Waste from Jewelry and Accessory Industry as Raw Material for Construction Materials Using Geopolymer Technology. Rep. Grant-Support. Res. Asahi Glass Found. 2021, 89, 1–12. [Google Scholar]
- An, Q.; Pan, H.; Zhao, Q.; Du, S.; Wang, D. Strength Development and Microstructure of Recycled Gypsum-Soda Residue-GGBS Based Geopolymer. Constr. Build. Mater. 2022, 331, 127312. [Google Scholar] [CrossRef]
- Abadel, A.A.; Alghamdi, H.; Alharbi, Y.R.; Alamri, M.; Khawaji, M.; Abdulaziz, M.A.M.; Nehdi, M.L. Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud. Materials 2023, 16, 1551. [Google Scholar] [CrossRef]
- Ricciotti, L.; Occhicone, A.; Ferone, C.; Cioffi, R.; Tarallo, O.; Roviello, G. Development of Geopolymer-Based Materials with Ceramic Waste for Artistic and Restoration Applications. Materials 2022, 15, 8600. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Ahmed, H.U.; Mosavi, A. Survey of Mechanical Properties of Geopolymer Concrete: A Comprehensive Review and Data Analysis. Materials 2021, 14, 4690. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. Drying Shrinkage of Slag Blended Fly Ash Geopolymer Concrete Cured at Room Temperature. Procedia Eng. 2015, 125, 594–600. [Google Scholar] [CrossRef]
- Alnkaa, A.; Yaprak, H.; Selcuk, M.; Kaplan, G. Effect of Different Cure Conditions on the Shrinkage of Geopolymer Mortar. Int. J. Eng. Res. Dev. 2018, 14, 51–55. [Google Scholar]
- Lu, T.; Li, Z.; van Breugel, K. Modelling of Autogenous Shrinkage of Hardening Cement Paste. Constr. Build. Mater. 2020, 264, 120708. [Google Scholar] [CrossRef]
- Katare, V.D.; Madurwar, M.V. Experimental Characterization of Sugarcane Biomass Ash—A Review. Constr. Build. Mater. 2017, 152, 1–15. [Google Scholar] [CrossRef]
- EN 12457-2:2002; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges. Comite Europeen de Normalisation: Brussels, Belgium, 2002.
- Levien, L.; Prewitt, C.T.; Weidner, D.J. Structure and Elastic Properties of Quartz at Pressure P = 1 Atm. Am. Mineral. 1980, 65, 920–930. [Google Scholar]
- Balzar, D.; Ledbetter, H. Crystal Structure and Compressibility of 3:2 Mullite. Am. Mineral. 1993, 78, 1192–1196. [Google Scholar]
- Goetz-Neunhoeffer, F.; Neubauer, J. Refined Ettringite (Ca6Al2(SO4)3(OH)12*26H2O) Structure for Quantitative X-Ray Diffraction Analysis. Powder Diffr. 2006, 21, 4–11. [Google Scholar] [CrossRef]
- Schofield, P.F.; Knight, K.S.; Stretton, I.C. Thermal Expansion of Gypsum Investigated by Neutron Powder Diffraction T = 4.2 K. Am. Mineral. 1996, 81, 847–851. [Google Scholar] [CrossRef]
- Abolhasani, A.; Aslani, F.; Samali, B.; Ghaffar, S.H.; Fallahnejad, H.; Banihashemi, S. Silicate Impurities Incorporation in Calcium Aluminate Cement Concrete: Mechanical and Microstructural Assessment. Adv. Appl. Ceram. 2021, 120, 104–116. [Google Scholar] [CrossRef]
- Pacewska, B.; Nowacka, M.; Aleknevičius, M.; Antonovič, V. Early Hydration of Calcium Aluminate Cement Blended with Spent FCC Catalyst at Two Temperatures. Procedia Eng. 2013, 57, 844–850. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Chen, Q.; Yang, F.; Liu, X.; Wu, J.; Wang, P. Effect of Mineral Composition and w/c Ratios to the Growth of AFt during Cement Hydration by In-Situ Powder X-Ray Diffraction Analysis. Materials 2020, 13, 4963. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korhonen, H.; Timonen, J.; Suvanto, S.; Hirva, P.; Mononen, K.; Jääskeläinen, S. The Mechanical Properties of Geopolymers from Different Raw Materials and the Effect of Recycled Gypsum. Inorganics 2023, 11, 298. https://doi.org/10.3390/inorganics11070298
Korhonen H, Timonen J, Suvanto S, Hirva P, Mononen K, Jääskeläinen S. The Mechanical Properties of Geopolymers from Different Raw Materials and the Effect of Recycled Gypsum. Inorganics. 2023; 11(7):298. https://doi.org/10.3390/inorganics11070298
Chicago/Turabian StyleKorhonen, Henna, Juha Timonen, Sari Suvanto, Pipsa Hirva, Kirsi Mononen, and Sirpa Jääskeläinen. 2023. "The Mechanical Properties of Geopolymers from Different Raw Materials and the Effect of Recycled Gypsum" Inorganics 11, no. 7: 298. https://doi.org/10.3390/inorganics11070298
APA StyleKorhonen, H., Timonen, J., Suvanto, S., Hirva, P., Mononen, K., & Jääskeläinen, S. (2023). The Mechanical Properties of Geopolymers from Different Raw Materials and the Effect of Recycled Gypsum. Inorganics, 11(7), 298. https://doi.org/10.3390/inorganics11070298