Hydrothermal Synthesis of Boron-Doped Graphene for High-Performance Zinc-Ion Hybrid Capacitor Using Aloe Vera Gel Electrolyte
Abstract
1. Introduction
2. Experimental
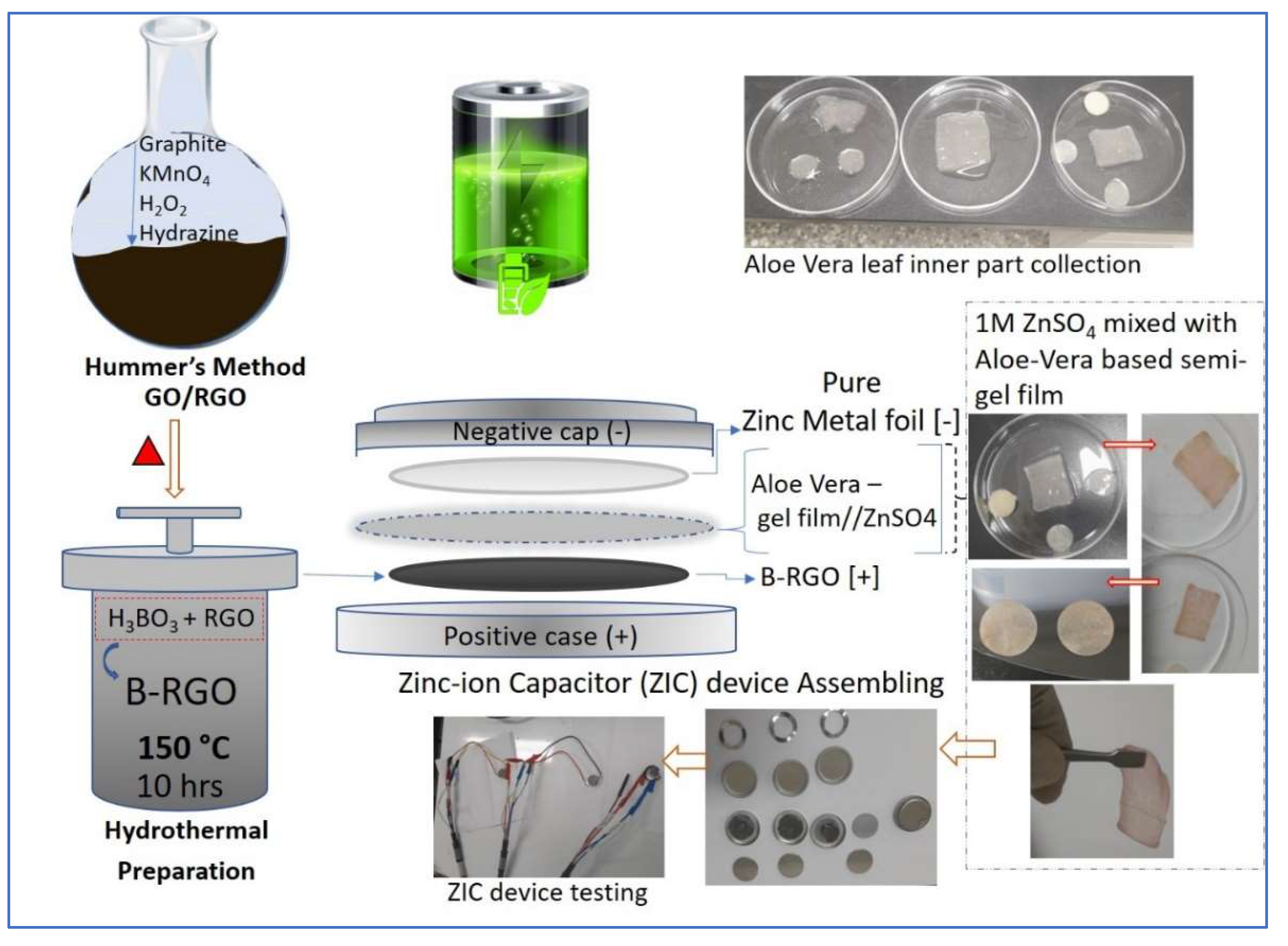
2.1. Materials and Methods
2.2. Experimental Procedure of B-RGO
2.3. Material Characterization
2.4. Electrochemical Characterization and Electrode Preparations
2.5. Semi-Hydrogel Film Preparations
3. Results and Discussion
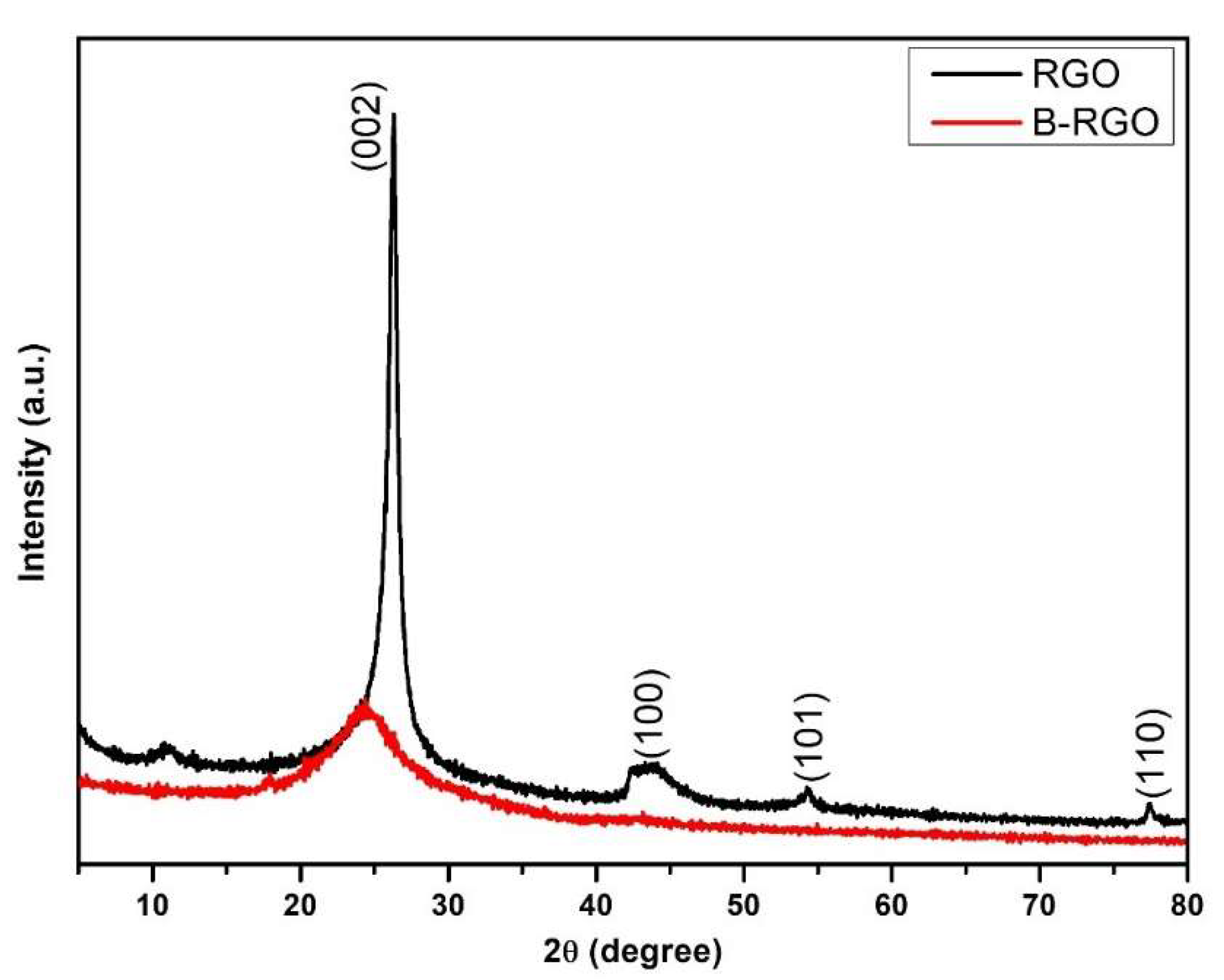
3.1. X-ray Diffraction Analysis
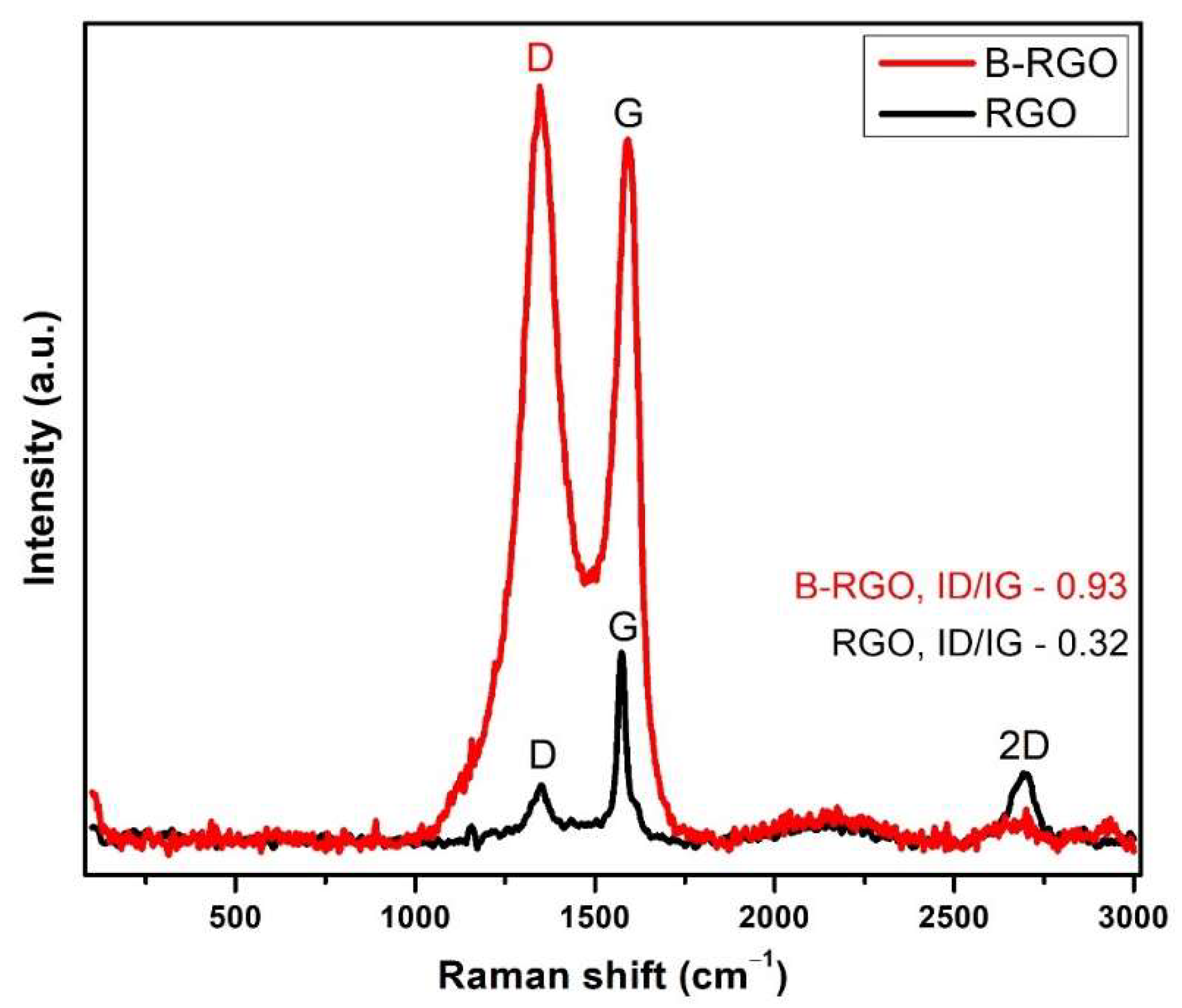
3.2. Micro-Raman Analysis
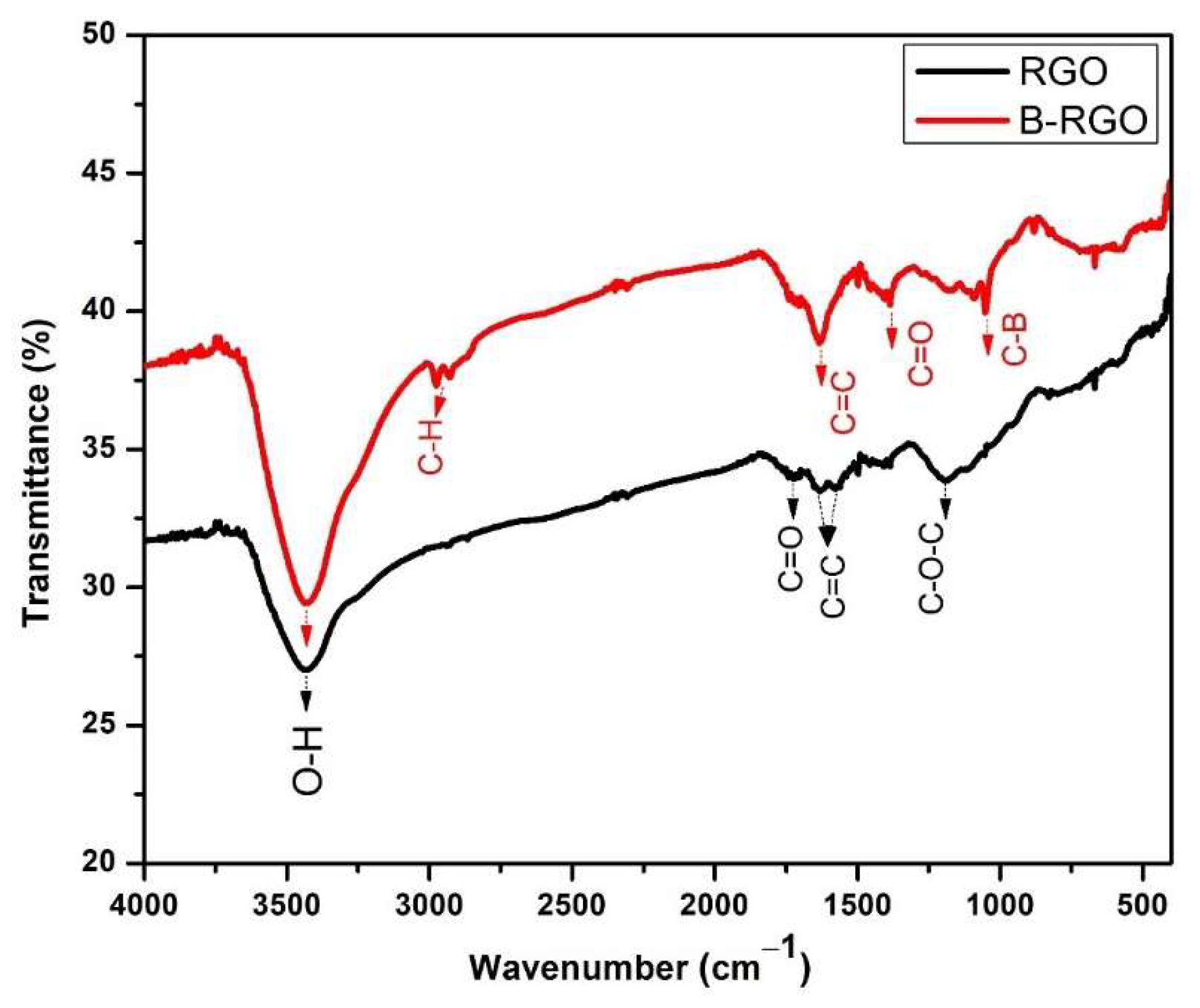
3.3. FT-IR Analysis
3.4. FE-SEM Morphological Analysis
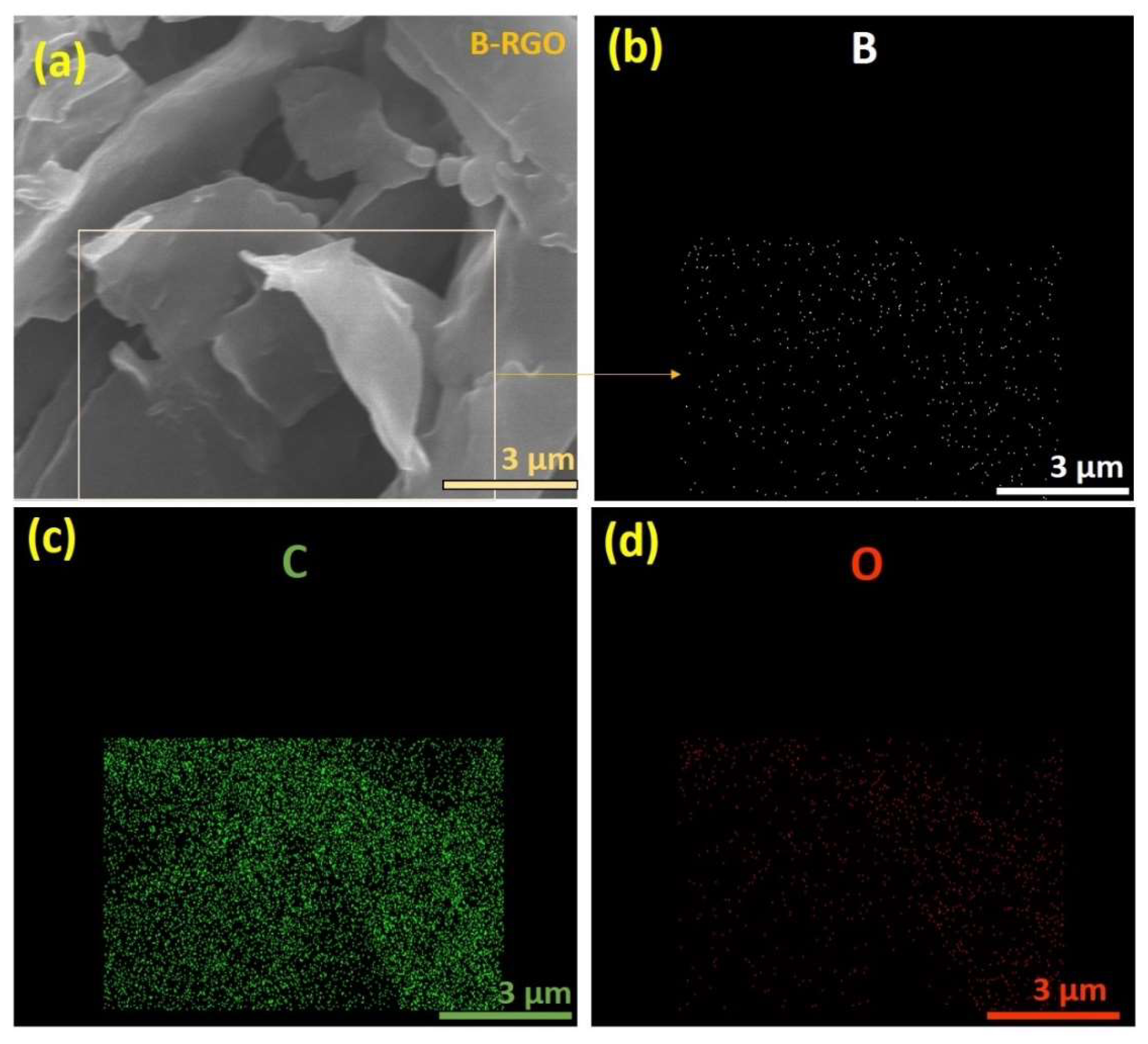
3.5. Energy-Dispersive X-ray (EDX) and Element Mapping Spectroscopy Analysis
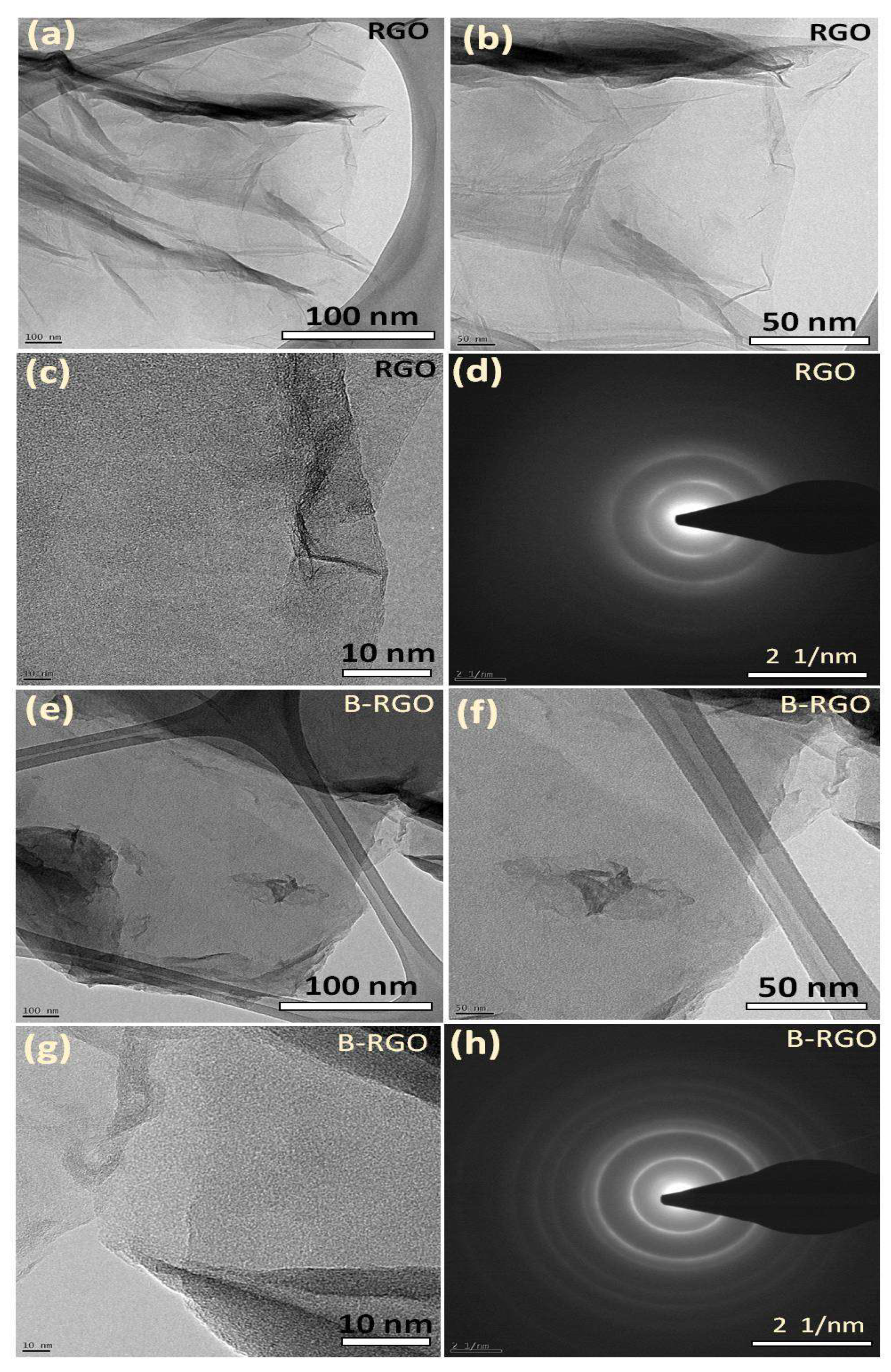
3.6. FE-TEM Analysis
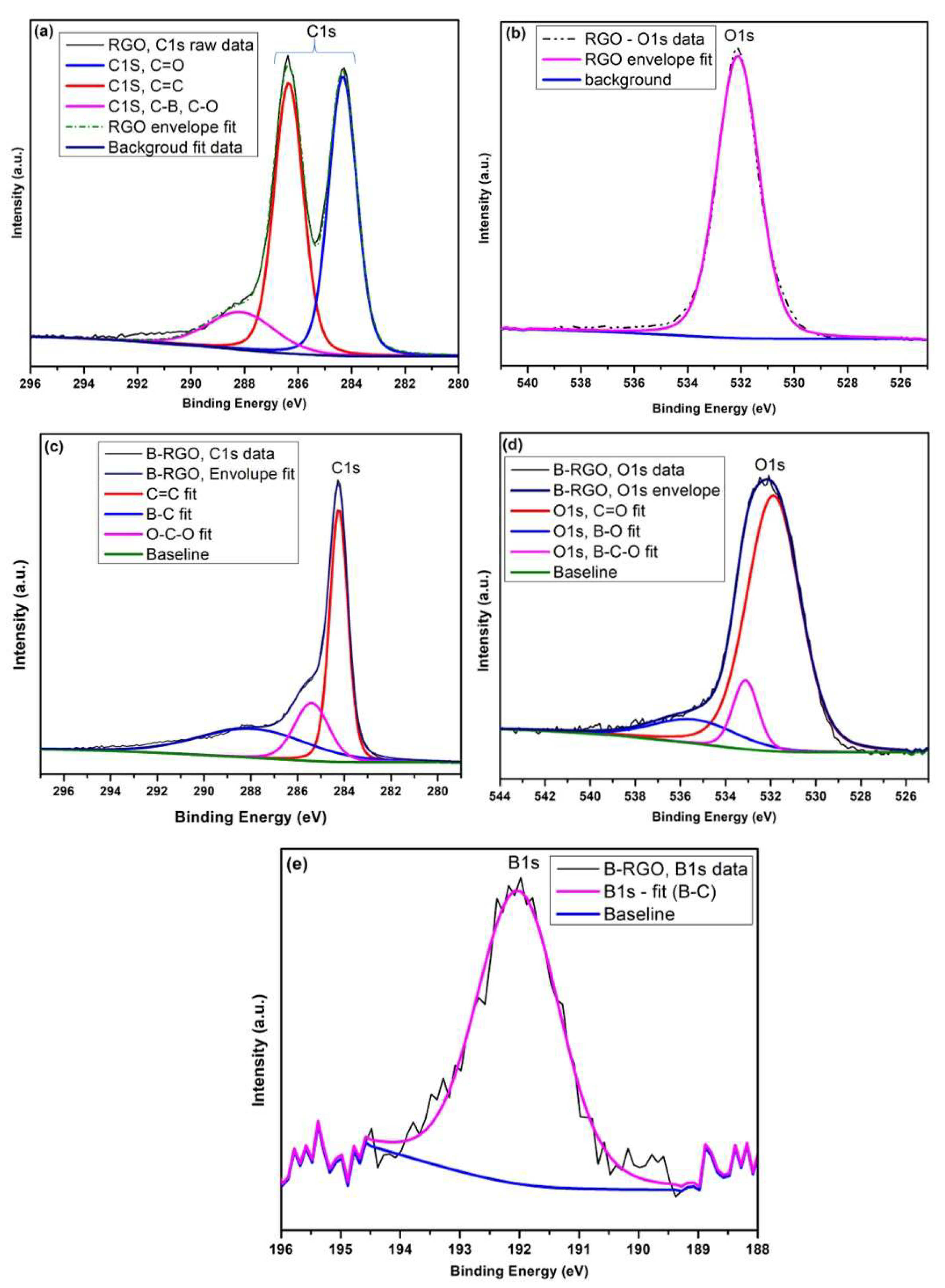
3.7. XPS Analysis
3.8. Electrochemical Measurements ZIC
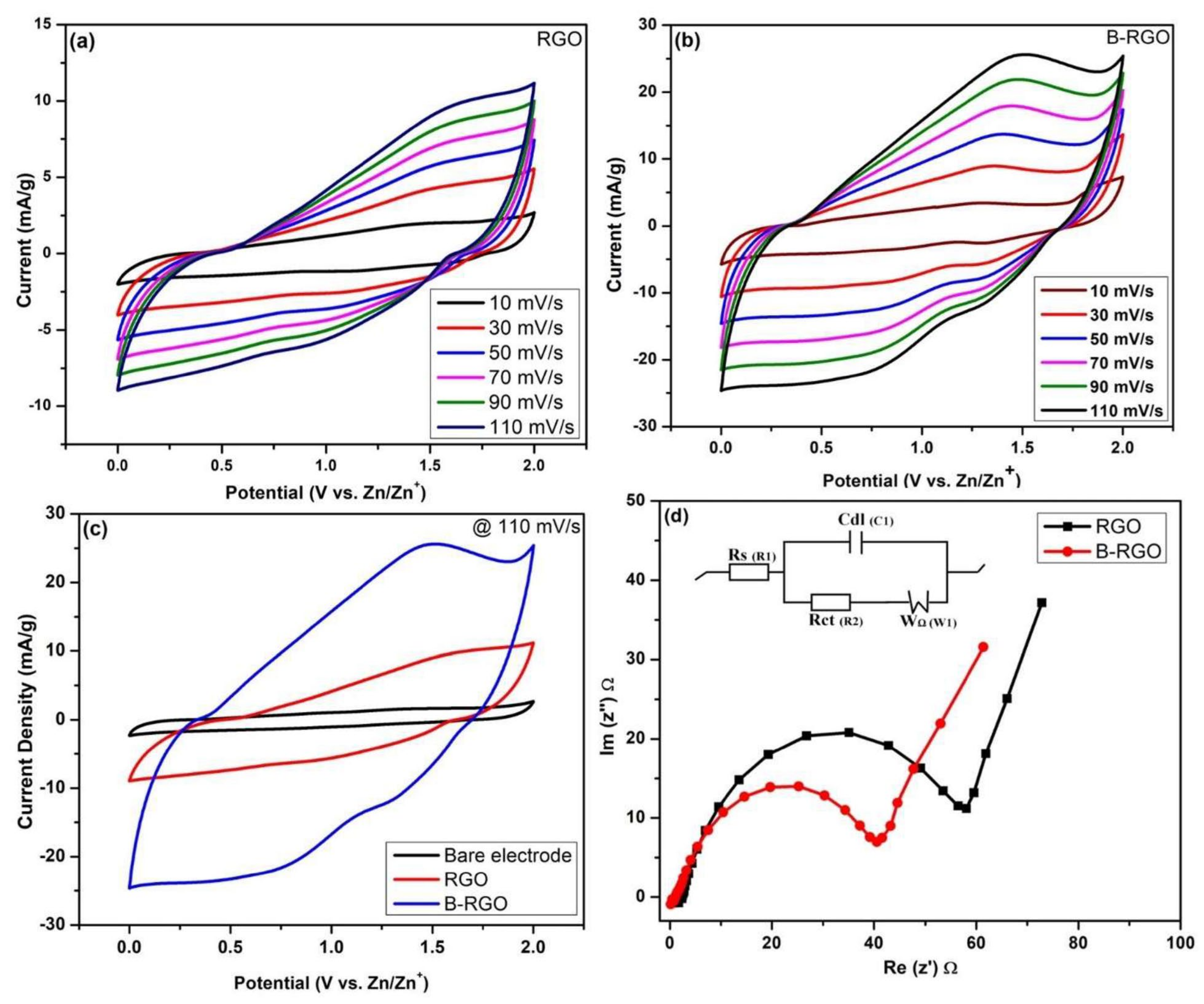
3.8.1. Cyclic Voltammetry and Electrochemical Impedance Spectroscopy (EIS)
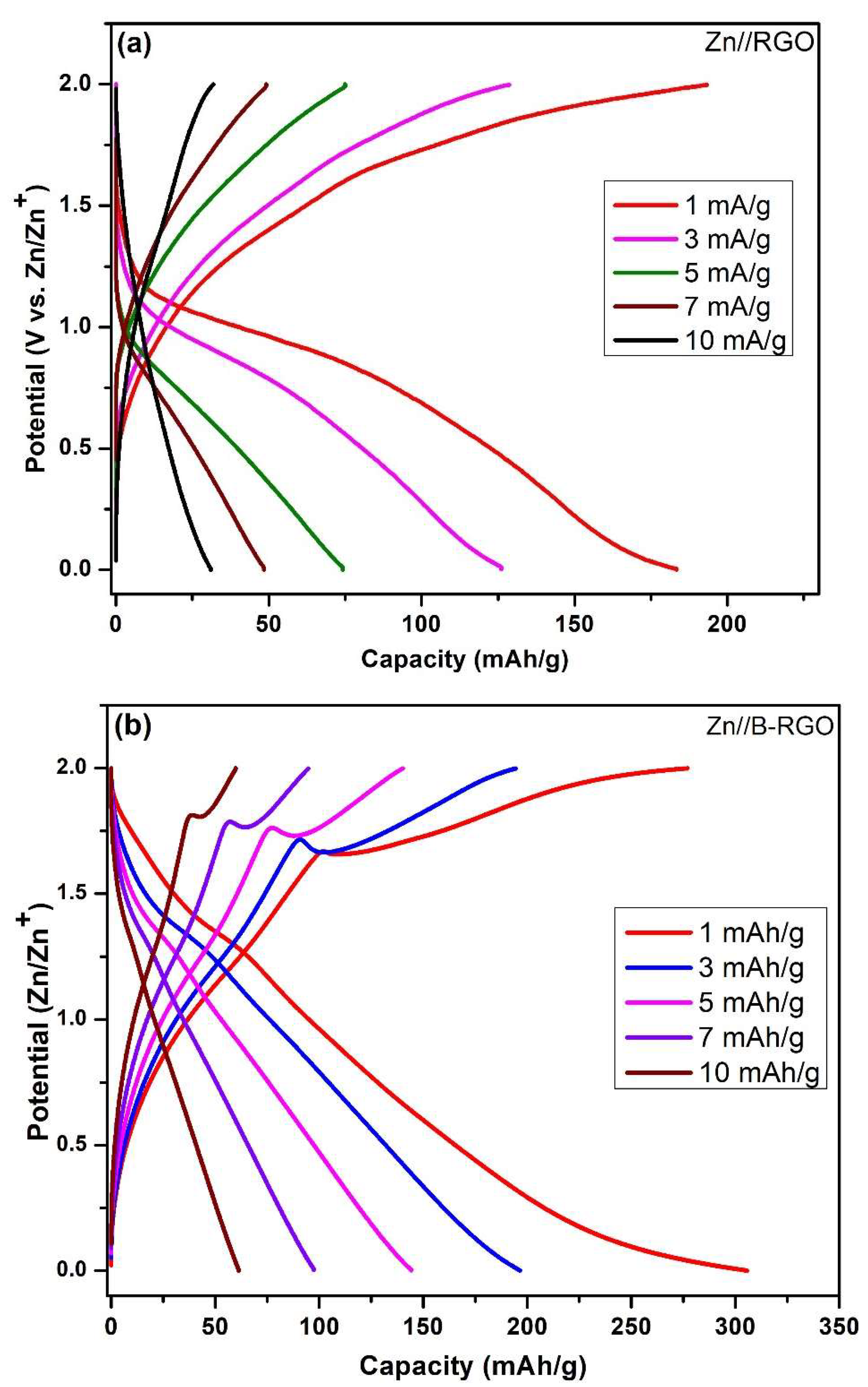
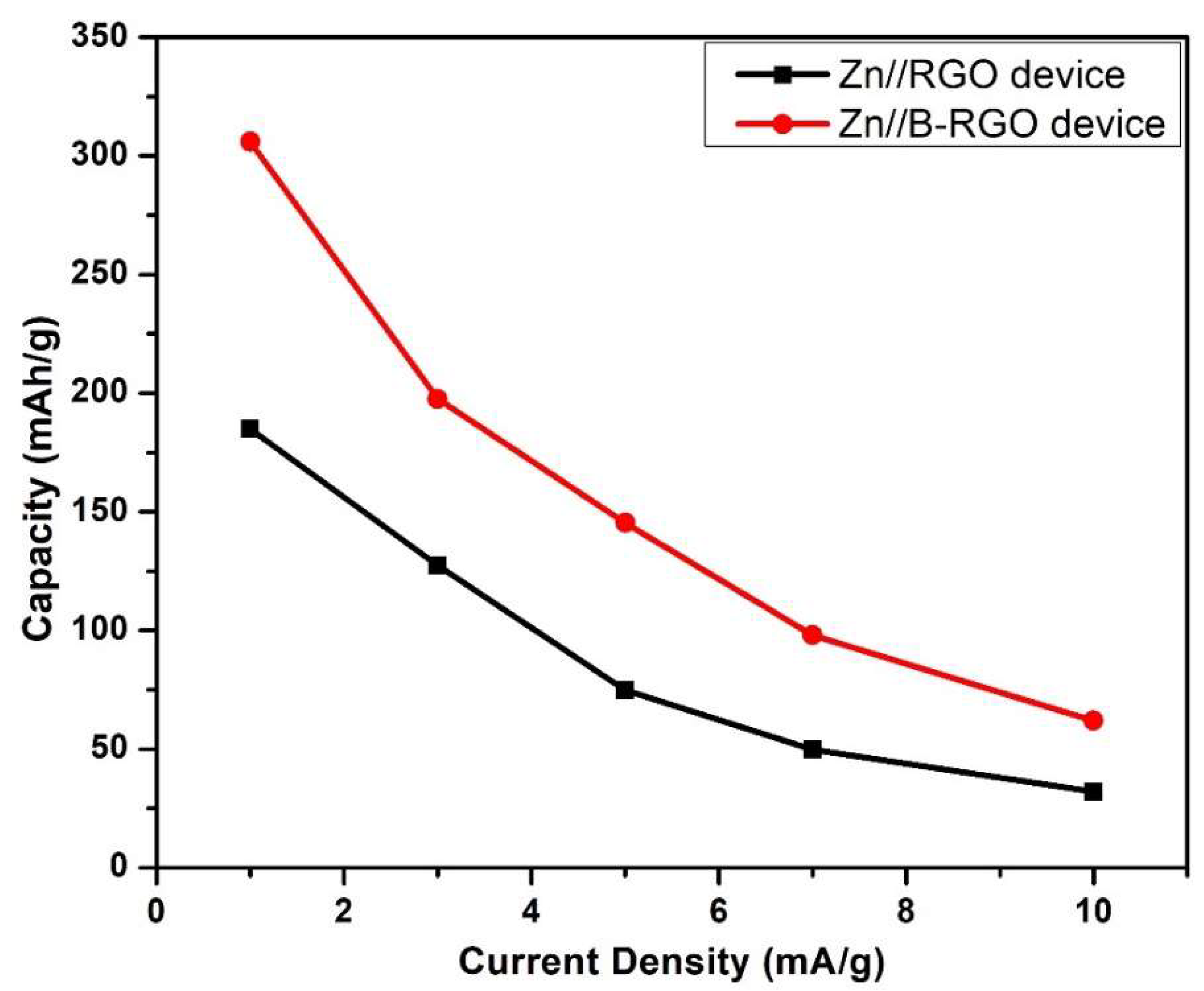
3.8.2. Galvanostatic Charging/Discharging (GCD) Measurements
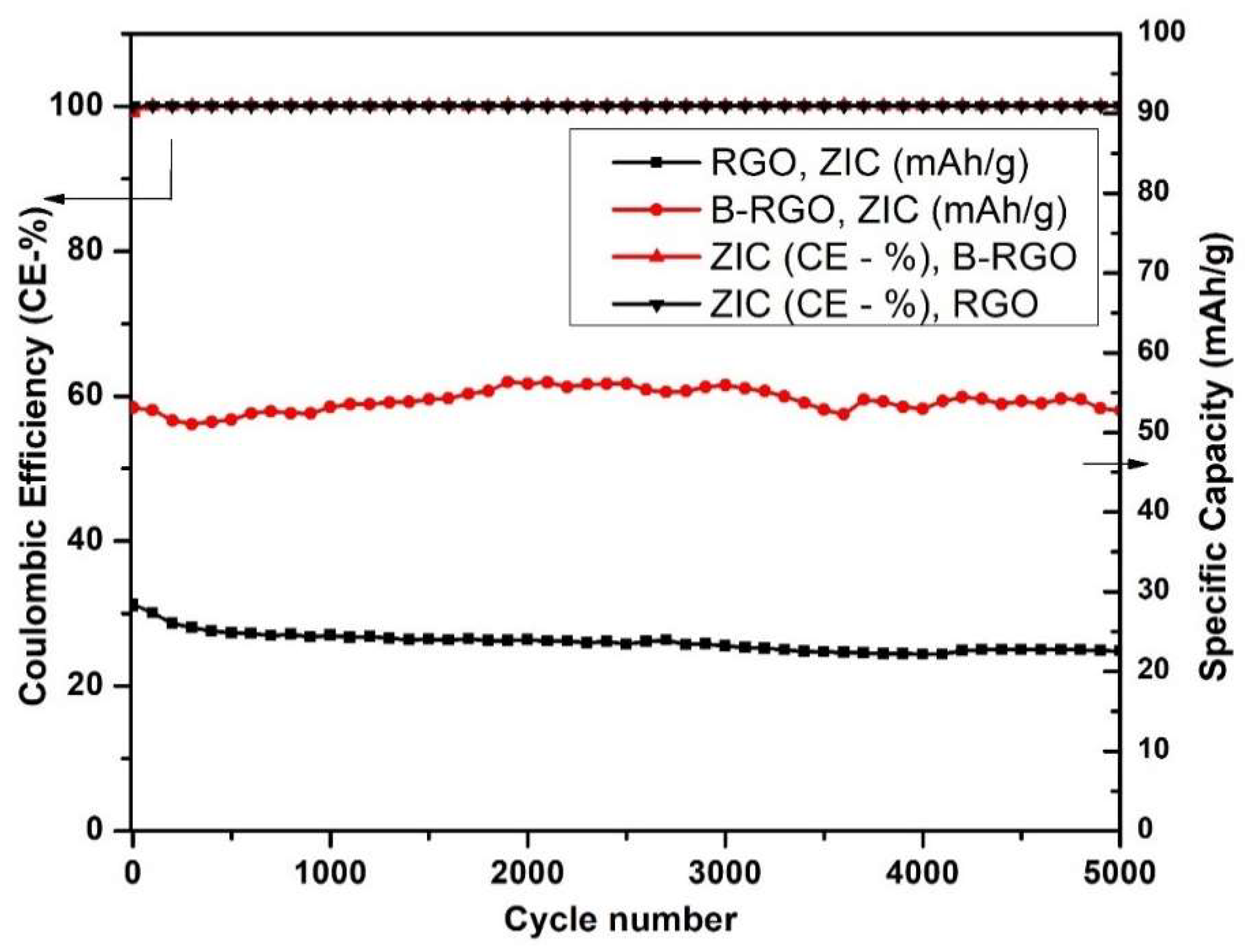
3.8.3. Cyclic stability of Zn//RGO and Zn//B-RGO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Conway, B.E. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, X.; Cheng, Y.; Shi, Z.; Huang, T.; Yang, S.; Zheng, H.; Bi, Y.; Zhang, J.; Shi, Q.; et al. A flexible zinc ion hybrid capacitor integrated system with layers-dependent V2CTx MXene. Chem. Eng. J. 2023, 454, 140360. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.-G.; Zheng, J.P. Progress and perspectives on pre-lithiation technologies for lithium ion capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Dewar, D.; Glushenkov, A.M. Optimisation of sodium-based energy storage cells using pre-sodiation: A perspective on the emerging field. Energy Environ. Sci. 2021, 14, 1380–1401. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, J.; Kou, Z.; Liao, C.; Liu, Z.; Zhou, L.; Wang, J.; Mai, L. Zn2+ pre-intercalation stabilizes the tunnel structure of MnO2 nanowires and enables zinc-ion hybrid supercapacitor of battery-level energy density. Small 2020, 16, e2000091. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, G.; Wang, F.; Yang, S.; Zhu, F.; Zhuang, X.; Schmidt, O.G.; Feng, X. Zn-ion hybrid micro-supercapacitors with ultrahigh areal energy density and long-term durability. Adv. Mater. 2019, 31, 1806005. [Google Scholar] [CrossRef]
- Mo, F.; Chen, Z.; Liang, G.; Wang, D.; Zhao, Y.; Li, H.; Dong, B.; Zhi, C. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-MnO2 Batteries with Superior Rate Capabilities. Adv. Energy Mater. 2020, 10, 2000035. [Google Scholar] [CrossRef]
- Yan, H.; Li, S.; Nan, Y.; Yang, S.; Li, B. Ultrafast Zinc–Ion–Conductor Interface toward High-Rate and Stable Zinc Metal Batteries. Adv. Energy Mater. 2021, 11, 2100186. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Li, C.; Yang, D.; Wang, W.; Yan, W.; Zhou, W.; Wu, Z.; Wang, L.; Huang, Q.; et al. A Low-Cost Zn-Based Aqueous Supercapacitor with High Energy Density. ACS Appl. Energy Mater. 2019, 2, 5835–5842. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent Progress and Perspectives on Aqueous Zn-Based Rechargeable Batteries with Mild Aqueous Electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Kundu, D.; Vajargah, S.H.; Wan, L.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous vs. Nonaqueous Zn-Ion Batteries: Consequences of the Desolvation Penalty at the Interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, T.; Xu, N.; Huang, K. A Semisolid Electrolyte for Flexible Zn-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6904–6910. [Google Scholar] [CrossRef]
- Han, Q.; Chi, X.; Zhang, S.; Liu, Y.; Zhou, B.; Yang, J.; Liu, Y. Durable, Flexible Self-Standing Hydrogel Electrolytes Enabling High-Safety Rechargeable Solid-State Zinc Metal Batteries. J. Mater. Chem. A 2018, 6, 23046–23054. [Google Scholar] [CrossRef]
- Yi, J.; Choe, G.; Park, J.; Lee, J.Y. Graphene oxide-incorporated hydrogels for biomedical applications. Polym. J. 2020, 52, 823–837. [Google Scholar] [CrossRef]
- Hanif, W.; Hardiansyah, A.; Randy, A.; Asri, L.A.T.W. Physically crosslinked PVA/graphene-based materials/aloe vera hydrogel with antibacterial activity. RSC Adv. 2021, 11, 29029–29041. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, R.; Xu, L.; Liu, Y.; Dai, Y.; Huang, M.; Mai, L. Steric Molecular Combing Effect Enables Ultrafast Self-Healing Electrolyte in Quasi-Solid-State Zinc-Ion Batteries. ACS Energy Lett. 2022, 7, 2825–2832. [Google Scholar] [CrossRef]
- Eskusson, J.; Thomberg, T.; Lust, E.; Jänes, A. Electrochemical Characteristics of Zn-Ion Hybrid Supercapacitors Based on Aqueous Solution of Different Electrolytes. J. Electrochem. Soc. 2022, 169, 020512. [Google Scholar] [CrossRef]
- Aloharrif, M.M.; Sandeep, K.V. Aloe vera Their Chemicals Composition and Applications: A Review. Int. J. Biol. Med. Res. 2011, 9, 466–471. [Google Scholar]
- Saberian, H.; Esfahani, Z.H.; Banakar, A. Ohmic heating of aloe vera gel: Electrical conductivity and energy efficiency. Iran. J. Chem. Chem. Eng. 2018, 37, 157–165. [Google Scholar] [CrossRef]
- MElbandy, A.; Abed, S.M.; Gad, S.S.A.; Abdet-Fadeel, M.G. Aloe vera gel as a functional ingredient and natural preservative in mango nectar. World J. Diary Food Sci. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Liu, C.; Tian, Y.; An, Y.; Yang, Q.; Xiong, S.; Feng, J.; Qian, Y. Robust and Flexible Polymer/MXene-Derived Two Dimensional TiO2 Hybrid Gel Electrolyte for Dendrite-Free Solid-State Zinc-Ion Batteries. Chem. Eng. J. 2022, 430, 132748. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Chen, Z.; Jin, Y.; Chen, J. High Voltage and Self-Healing Zwitterionic Double-Network Hydrogels as Electrolyte for Zinc-Ion Hybrid Supercapacitor/Battery. Int. J. Hydrogen Energy 2022, 47, 23909–23918. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S. Graphene-Based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Terrones, H.; Lv, R.; Terrones, M.; Dresselhaus, M.S. The role of defects and doping in 2D graphene sheets and 1D nanoribbons. Rep. Prog. Phys. 2012, 75, 062501. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, B.; Neto, A.H.C. Superconducting states of pure and doped graphene. Phys. Rev. Lett. 2007, 98, 146801. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A 2016, 4, 5002–5025. [Google Scholar] [CrossRef]
- Niu, L.; Li, Z.; Hong, W.; Sun, J.; Wang, Z.; Ma, L.; Wang, J.; Yang, S. Pyrolytic synthesis of boron-doped graphene and its application as electrode material for supercapacitors. Electrochim. Acta 2013, 108, 666–673. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.L.; Lee, S.; Oh, J.; Lee, K.-S.; Potts, J.R.; Ji, J.; Zhao, X.; Ruoff, R.S.; Park, S. Generation of B-doped graphene nanoplatelets using a solution process and their supercapacitor applications. ACS Nano 2012, 7, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Jiang, Z.; Manthiram, A. Porous B-doped graphene inspired by Fried-Ice for supercapacitors and metal-free catalysts. J. Mater. Chem. A 2013, 1, 13476–13483. [Google Scholar] [CrossRef]
- Marconcini, P.; Cresti, A.; Roche, S. Effect of the channel length on the transport characteristics of transistors based on boron-doped graphene ribbons. Materials 2018, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Sreena, K.; Vinayan, B.; Ramaprabhu, S. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, Z.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Zhu, H. Boron Doping of Graphene for Graphene-Silicon p-n Junction Solar Cells. Adv. Energy Mater. 2012, 2, 425–429. [Google Scholar] [CrossRef]
- Qiao, M.; Tang, C.; He, G.; Qiu, K.; Binions, R.; Parkin, I.P.; Zhang, Q.; Guo, Z.; Titirici, M.M. Graphene/Nitrogen-Doped Porous Carbon Sandwiches for the Metal-Free Oxygen Reduction Reaction: Conductivity versus Active Sites. J. Mater. Chem. A 2016, 4, 12658–12666. [Google Scholar] [CrossRef]
- Luo, X.; Yang, J.; Liu, H.; Wu, X.; Wang, Y.; Ma, Y.; Wei, S.-H.; Gong, X.; Xiang, H. Predicting Two-Dimensional Boron–Carbon Compounds by the Global Optimization Method. J. Am. Chem. Soc. 2011, 133, 16285–16290. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tang, Y.-B.; Yin, L.-C.; Yang, Y.; Bo, X.-H.; Cao, Y.-L.; Wang, H.-E.; Zhang, W.-J.; Bello, I.; Lee, S.-T.; Cheng, H.-M.; et al. Tunable band gaps and p-type transport properties of boron-doped graphenes by controllable ion doping using reactive microwave plasma. ACS Nano 2012, 6, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shen, H.; Sun, L.; Cheng, B.; Liu, B.; Shen, J. Nitrogen and boron doped monolayer graphene by chemical vapor deposition using polystyrene, urea and boric acid. New J. Chem. 2012, 36, 1385–1391. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel di-functional material for enhancing CO2 capture and super-capacitance. J. Mater. Sci. Technol. 2022, 99, 73–81. [Google Scholar] [CrossRef]
- Thirumal, V.; Pandurangan, A.; Jayavel, R.; Ilangovan, R. Synthesis and characterization of boron doped graphene nanosheets for supercapacitor applications. Synth. Met. 2016, 220, 524–532. [Google Scholar] [CrossRef]
- Balaji, S.S.; Karnan, M.; Anandhaganesh, P.; Tauquir, S.M.; Sathish, M. Performance evaluation of B-doped graphene prepared via two different methods in symmetric supercapacitor using various electrolytes. Appl. Surf. Sci. 2019, 491, 560–569. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Tian, X.; Luo, L.; Fang, J.; Gao, H.; Jiang, Z.-J. Hydrothermal Synthesis of Boron and Nitrogen Codoped Hollow Graphene Microspheres with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 19398–19407. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Kang, L.; Tian, Z.; Lai, F.; Brett, D.J.L.; Liu, T.; He, G. Self-assembled carbon nanoribbons with the heteroatom doping used as ultrafast charging cathodes in zinc-ion hybrid supercapacitors. Sci. China Mater. 2022, 65, 1495–1502. [Google Scholar] [CrossRef]
- Lee, Y.-G.; An, G.-H. Synergistic Effects of Phosphorus and Boron Co-Incorporated Activated Carbon for Ultrafast Zinc-Ion Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 41342–41349. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Z.; Wang, Z.; Xi, M.; Xie, X.; Yang, G. Flexible zinc ion hybrid supercapacitors enabled by N/S co-doped porous carbon and bacterial cellulose/ZnSO4 electrolyte. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130424. [Google Scholar] [CrossRef]
- Owusu, K.; Pan, X.; Yu, R.; Qu, L.; Liu, Z.; Wang, Z.; Tahir, M.; Haider, W.; Zhou, L.; Mai, L. Introducing Na2SO4 in aqueous ZnSO4 electrolyte realizes superior electrochemical performance in zinc-ion hybrid capacitor. Mater. Today Energy 2020, 18, 100529. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y. A novel zinc-ion hybrid supercapacitor for long-life and low-cost energy storage applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, Y.; Zhu, X.; Hong, W.; Tong, Y.; Lu, Y.; Pei, G.; Pang, Y.; Shen, Z.; Guan, C. Bamboo-derived porous carbons for Zn-ion hybrid supercapacitors. Mater. Res. Bull. 2021, 139, 111281. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Bai, Z.; Mi, H.; Ji, C.; Pang, H.; Yu, C.; Qiu, J. High energy-power Zn-ion hybrid supercapacitors enabled by layered B/N co-doped carbon cathode. Nano Energy 2019, 66, 104132. [Google Scholar] [CrossRef]
- Dong, L.; Ma, X.; Li, Y.; Zhao, L.; Liu, W.; Cheng, J.; Xu, C.; Li, B.; Yang, Q.-H.; Kang, F. Extremely safe, high-rate and ultralong-life zinc-ion hybrid supercapacitors. Energy Storage Mater. 2018, 13, 96–102. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Zhang, K.; Kamruzzaman, M.; Zhi, C.; Zapien, J.A. A flexible solid-state zinc ion hybrid supercapacitor based on co-polymer derived hollow carbon spheres. J. Mater. Chem. A 2019, 7, 7784–7790. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirumal, V.; Rajkumar, P.; Yoo, K.; Kim, J. Hydrothermal Synthesis of Boron-Doped Graphene for High-Performance Zinc-Ion Hybrid Capacitor Using Aloe Vera Gel Electrolyte. Inorganics 2023, 11, 280. https://doi.org/10.3390/inorganics11070280
Thirumal V, Rajkumar P, Yoo K, Kim J. Hydrothermal Synthesis of Boron-Doped Graphene for High-Performance Zinc-Ion Hybrid Capacitor Using Aloe Vera Gel Electrolyte. Inorganics. 2023; 11(7):280. https://doi.org/10.3390/inorganics11070280
Chicago/Turabian StyleThirumal, Vediyappan, Palanisamy Rajkumar, Kisoo Yoo, and Jinho Kim. 2023. "Hydrothermal Synthesis of Boron-Doped Graphene for High-Performance Zinc-Ion Hybrid Capacitor Using Aloe Vera Gel Electrolyte" Inorganics 11, no. 7: 280. https://doi.org/10.3390/inorganics11070280
APA StyleThirumal, V., Rajkumar, P., Yoo, K., & Kim, J. (2023). Hydrothermal Synthesis of Boron-Doped Graphene for High-Performance Zinc-Ion Hybrid Capacitor Using Aloe Vera Gel Electrolyte. Inorganics, 11(7), 280. https://doi.org/10.3390/inorganics11070280






