Import and Implications of Vanadium in Live Aspects
Abstract
1. Introduction
2. Biogeochemical Cycling
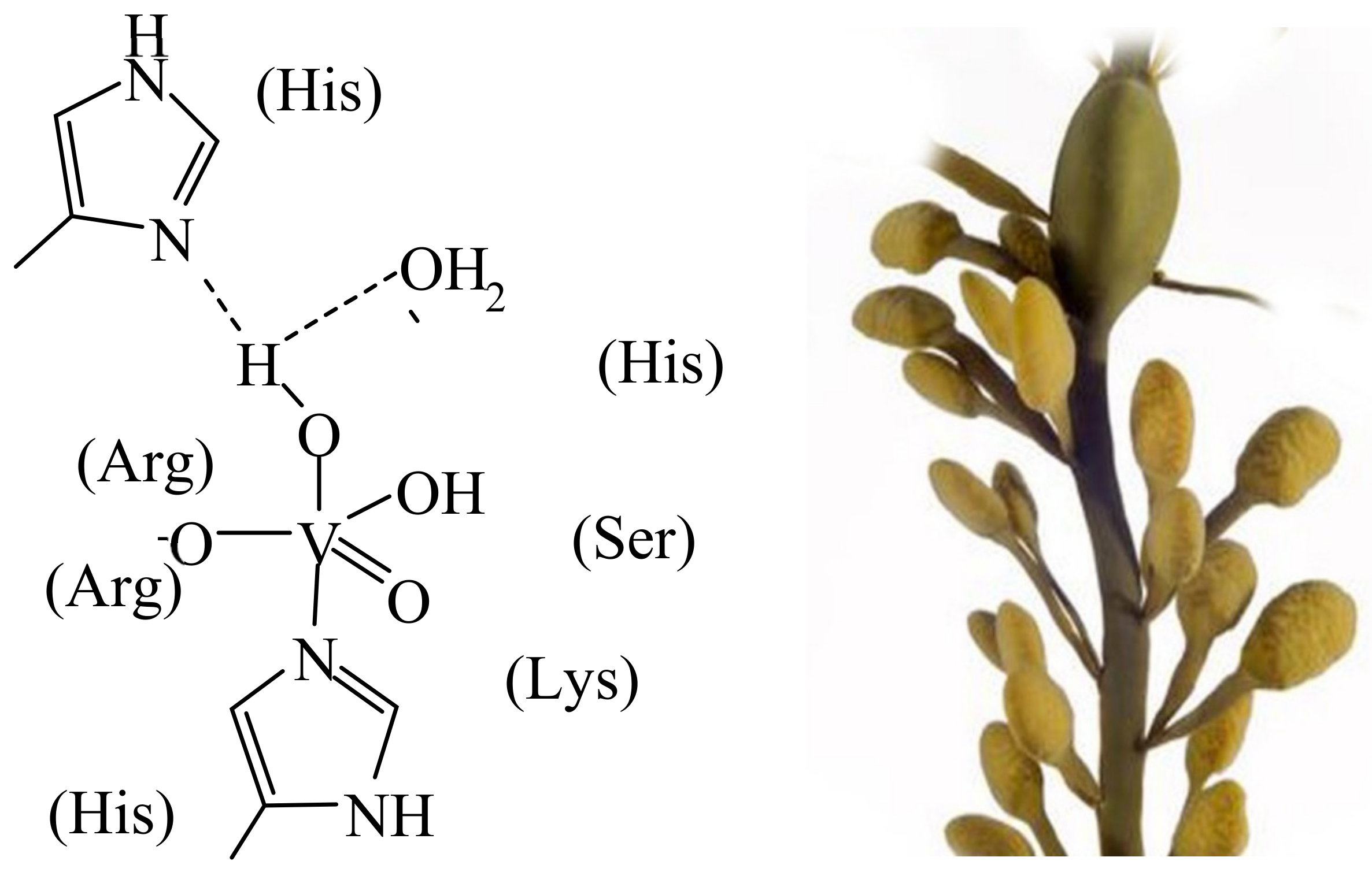
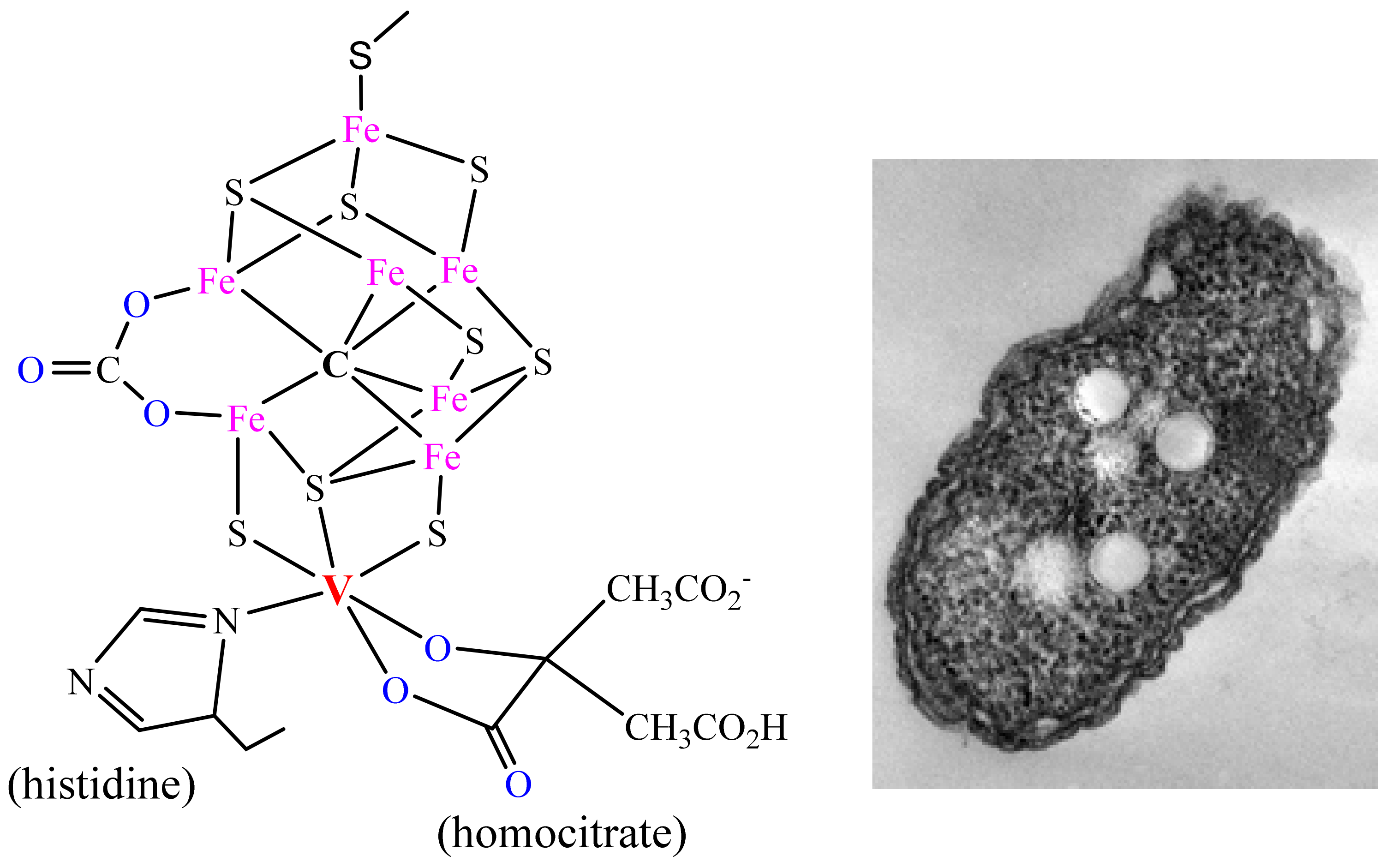
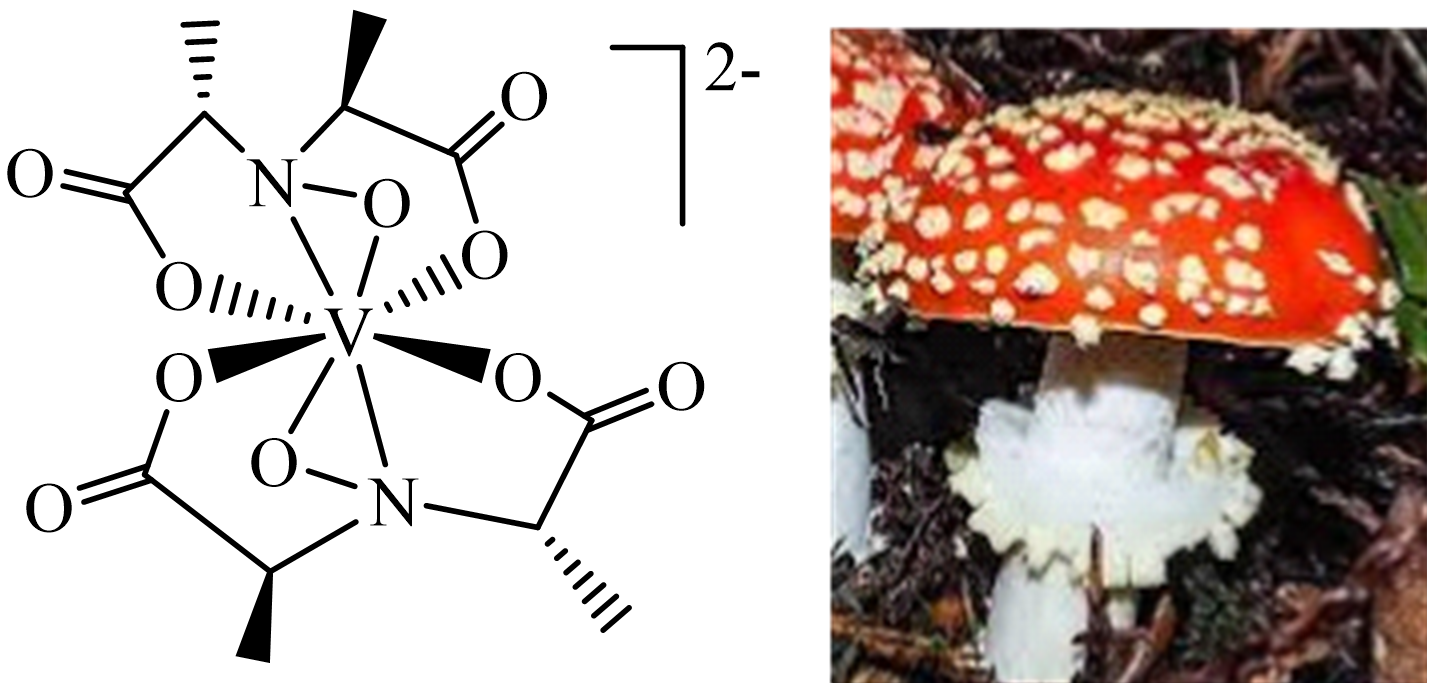
3. Vanadium in Haloperoxidases, Nitrogenases, Amanita Mushrooms, Ascidians and Fan Worms
4. Bacterial Issues
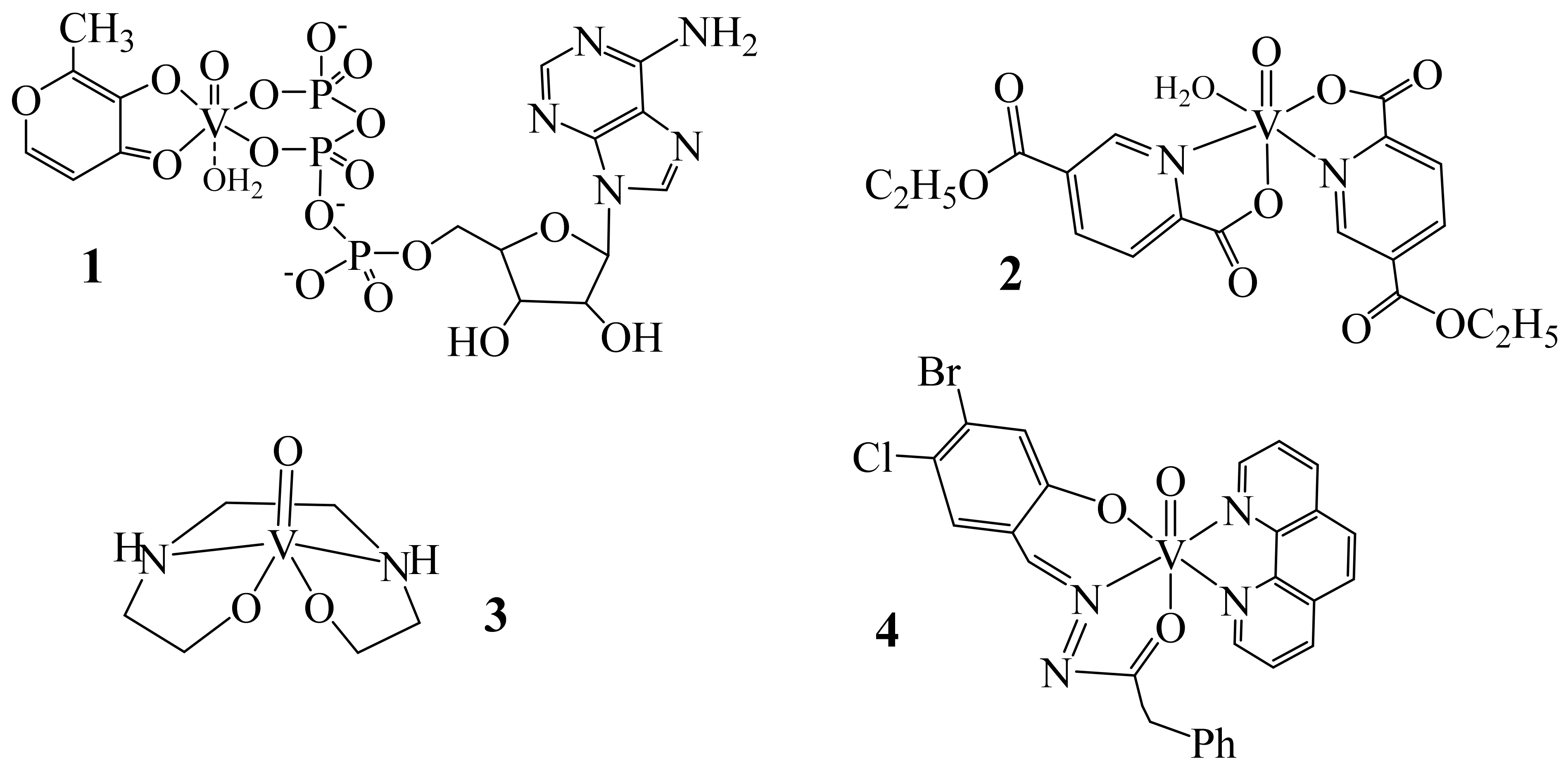
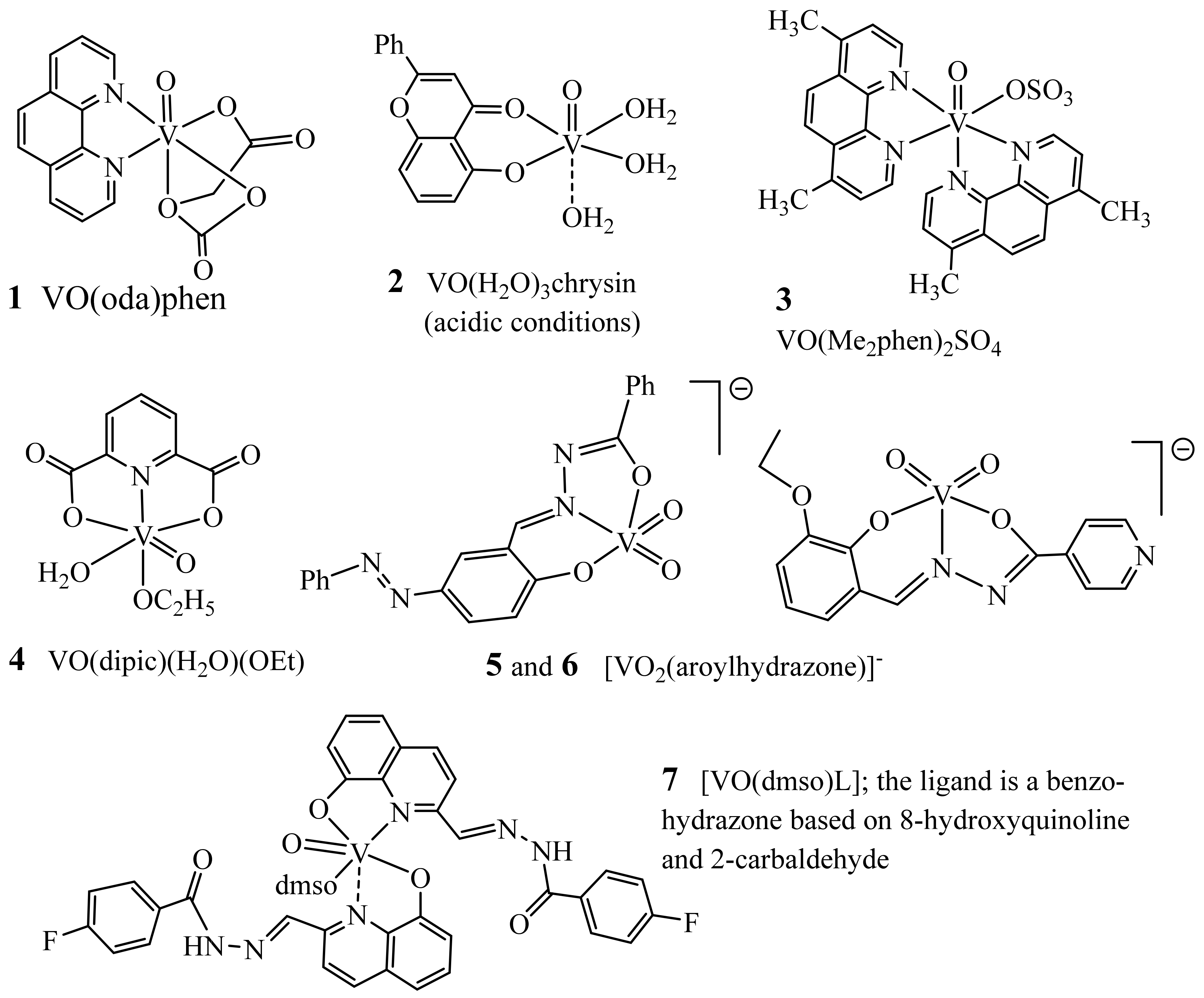
5. Medicinal Applications
6. Conclusions
7. Outlook and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, J.-H.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio)geochemistry. Chem. Geol. 2015, 417, 68–89. [Google Scholar] [CrossRef]
- Awan, R.S.; Liu, C.; Yang, S.; Wu, Y.; Zang, Q.; Khan, A.; Li, G. The occurrence of vanadium in nature: Its biogeochemical cycling and relationship with organic matter—A case study of the early Cambrian black rocks of the niutitang formation, western Hunan, China. Acta Geochim. 2021, 40, 973–997. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wiszniewska, A.; Kaminska, I. A dual role of vanadium in environmental systems—Beneficial and detrimental effects on terrestrial plants and humans. Plants 2021, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Garribba, E.; Sanna, D.; Dinda, R.; Costa Pessoa, J.; Crans, D.C. Hydrolysis, ligand exchange and redox properties of vanadium compounds. Implications of solution transformation and environment, biology and therapeutic applications. Chem. Rev. 2023, in press. [Google Scholar]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere—Speciation, soli-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic applications of vanadium: A mechanistic perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Fournier, J.B.; Rebuffet, E.; Delage, L.; Grijol, R.; Meslet-Gladièr, L.; Rzonca, J.; Potin, P.; Michel, G.; Czjzek, M.; Leblanc, C. The vanadium iodoperoxidases from the marine Flavobacteriaceae Species Zobiella galactanivorans reveals novel molecular and evolutionary features of halide specifity in the vanadium haloperoxidase enzyme family. Appl. Environ. Microbiol. 2014, 80, 7561–7573. [Google Scholar] [CrossRef]
- Xu, G.; Wang, B.-G. Independent evolution of six families of halogenating enzymes. PLoS ONE 2016, 11, e0154619. [Google Scholar] [CrossRef]
- Vilter, H.; Glombitza, K.-W.; Grave, A. A role for vanadium in ascidians and marine algae. Bot. Mar. 1983, 26, 331–340. [Google Scholar]
- Butler, A. Vanadium peroxidases. Curr. Opin. Chem. Biol. 1998, 2, 279–285. [Google Scholar] [CrossRef]
- Baumgartner, J.T.; McKinnie, S.M.K. Investigating the role of vanadium-dependent haloperoxidase enzymology in microbial secondary metabolism and chemical ecology. mSystems 2021, 6, e00780-21. [Google Scholar] [CrossRef]
- Harwood, C.S. Iron-only and vanadium nitrogenase: Fail-safe enzymes or something more? Ann. Rev. 2020, 74, 247–266. [Google Scholar] [CrossRef]
- Darnajoux, R.; Bradley, R.; Bellenger, J.-P. In Vivo temperature dependence of molybdenum and vanadium nitrogenase activity in the heterocystous cyanobacteria Anabaena variabilis. Environ. Sci. Technol. 2022, 56, 2760–2769. [Google Scholar] [CrossRef]
- Hodkinson, B.P.; Allen, J.L.; Forrest, L.L.; Goffinet, B.; Sérusiaux, E.; Andrésson, Ó.S.; Miao, V.; Bellenger, J.P.; Lutzoni, F. Lichen-symbiontic cyanobacteria associated with Peltigera have an alternative vanadium-dependent nitrogen fixing system. Eur. J. Phycol. 2014, 49, 11–19. [Google Scholar] [CrossRef]
- Rohde, M.; Laun, K.; Einsle, O. Two ligands binding sites in CO-reducing V nitrogenase reveal a general mechanistic principle. Sci. Adv. 2021, 7, eabg4474. [Google Scholar] [CrossRef]
- Zheng, Y.; Harrsi, D.F.; Yu, Z.; Fu, Y.; Poudel, S.; Ledbetter, R.N.; Fixen, K.R.; Yang, Z.-Y.; Boyd, E.S.; Lidstrom, M.E.; et al. A pathway for biological methane production using bacterial iron-only nitrogenase. Nat. Microbiol. 2018, 3, 281–286. [Google Scholar] [CrossRef]
- Braeuer, S.; Walenta, M.; Steiner, L.; Goessler, W. Determination of the naturally occurring vanadium complex amavadin in Amanita muscaria with HPLC-ICPMS. J. Anal. At. Spectrom. 2021, 36, 954–967. [Google Scholar] [CrossRef]
- Dias, L.; Bekhti, N.; Kuznetsov, M.L.; Ferreira, J.A.B.; Bacariza, M.C.; da Silva, J.A.L. Nitrite reduction in aqueous solution mediated by amavadin homologues: N2O formation and water oxidation. Chem. Eur. J. 2017, 24, 2474–2482. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Yoshinaga, M.; Kamino, K.; Ueki, T. Vanadium-binding ability of nucleoside diphosphate kinase from the vanadium-rich fan worm, Pseudopotomilla occelata. Zool. Sci. 2016, 33, 266–271. [Google Scholar] [CrossRef]
- Ueki, T.; Yamaguchi, N.; Romaidi; Isago, Y.; Tanahashi, H. Vanadium accumulation in ascidians: A system overview. Coord. Chem. Rev. 2015, 301–302, 300–308. [Google Scholar] [CrossRef]
- Romaidi; Ueki, T. Bioaccumulation of Vanadium by Vanadium-Resistant Bacteria Isolated from the Intestine of Ascidia sydneiensis samea. Mar. Biotechnol. 2016, 18, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Fujie, M.; Romaidi; Satoh, N. Symbiotic bacteria associated with ascidian vanadium accumulation identified by 16S rRNA amplicon sequencing. Mar. Genom. 2019, 43, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, D.-J.; Pan, H.R.; Wu, J.; Liu, H.-K.; Hsu, H.-F. Reversible conversion of disulfide/dithiolate occurring at a vanadium(IV) centre: A biomimetic system for redox exchange in vanabin. Inorg. Chem. 2022, 61, 19882–19889. [Google Scholar] [CrossRef]
- Boison, G.; Steingen, C.; Stal, L.J.; Bothe, H. The rice field cyanobacteria Anabaena azotica and Anabaena sp. CH1 express vanadium-dependent nitrogenase. Arch. Microbiol. 2006, 186, 367–376. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, H.; Zhao, L.; McCarrick, R.; Agrawal, A. Microbial reduction and precipitation of vanadium by mesophilic and thermophilic methanogens. Chem. Geol. 2014, 370, 29–39. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Li, T.; Li, Z.; Fu, J. Soil vanadium(V) reducing related bacteria drive community response to vanadium pollution from a smelting plant over multiple gradients. Environ. Int. 2020, 138, 105630. [Google Scholar] [CrossRef]
- Semiz, S. Vanadium as potential therapeutic agent for COVID-19: A focus on its antiviral, anti-inflammatory, and antihyperglycemic effects. J. Trace Elem. Med. Biol. 2022, 69, 126887. [Google Scholar] [CrossRef]
- Lyonnet, B.; Martz, X.; Martin, E. L’emploi thérapeutique des dérivés du vanadium. La Presse Méd. 1899, 32, 191–192. [Google Scholar]
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Anwar, M.J.; Quershi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic properties of vanadium complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Wang, J.P.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Xuan, Y.; Su, Z.W. High-fat diet increased renal and hepatic oxidative stress induced by vanadium Wistar rat. Biol. Trace Elem. Res. 2016, 170, 415–423. [Google Scholar] [CrossRef]
- Shechter, Y.; Karlish, S.J.D. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl(IV) ions. Nature 1980, 284, 556–558. [Google Scholar] [CrossRef]
- Elvingson, K.; Baró, A.G.; Pettersson, L. Speciation in vanadium bioinorganic systems. 2. An NMR, ESR, and Potentiometric Study of the Aqueous H+-Vanadate-Maltol System. Inorg. Chem. 1996, 33, 3388–3393. [Google Scholar] [CrossRef]
- Thomsen, K.H.; Orvig, C.J. Vanadium in diabetes: 100 years from phase 0 to phase I. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar]
- Sanna, D.; Serra, M.; Micera, G.; Garribba, E. Interaction of antidiabetic vanadium compounds with hemoglobin and red blood cells and their distribution between plasma and erythrocytes. Inorg. Chem. 2014, 53, 1449–1464. [Google Scholar] [CrossRef]
- Dörnyei, Á.; Marcão, S.; Costa Pessoa, J.; Jakusch, T.; Kiss, T. Interactions of Insulin-Mimetic Vanadium Complexes with the Cell Constituents ATP and Glutathione. Eur. J. Inorg. Chem. 2006, 18, 3614–3621. [Google Scholar] [CrossRef]
- Gätjens, J.; Meier, B.; Kiss, T.; Nagy, E.M.; Buglyó, P.; Sakurai, H.; Kawabe, K.; Rehder, D. A new family of insulin-mimetic vanadium complexes derived from 5-carboxyalkoxypicolinates. Chem. Eur. J. 2003, 9, 4924–4935. [Google Scholar] [CrossRef]
- Levina, A.; McLeod, A.I.; Kremer, L.E.; Aitken, J.B.; Glover, C.J.; Johannessen, B.; Lay, P.A. Reactivity-activity relationship of oral antidiabetic vanadium complexes in gastrointestinal media: An X-ray absorption spectroscopic study. Metallomics 2014, 6, 180–1888. [Google Scholar] [CrossRef]
- Lima, L.M.A.; Belia, M.F.; Silva, W.A.; Postal, K.; Kostenkova, K.; Crans, D.C.; Rossiter, A.K.F.F.; da Silva, V.A. Vanadium(IV)-diamin complex with hypoglycemic activity and a reduction of testicular atrophy. J. Inorg. Biochem. 2021, 216, 111312. [Google Scholar] [CrossRef]
- Jasinska, A.; Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Mordyl, B.; Gluch-Lutwin, M. V(III) and V(IV) Schiff base complexes as potential insulin-mimetic compounds—Comparison, characterization and biological activity. Polyhedron 2022, 215, 115682. [Google Scholar] [CrossRef]
- Ferraro, G.; Paolillo, M.; Sciortino, G.; Garribba, E.; Merlino, A. Multiple and Variable Binding of Pharmacologically Active Bis(maltolato)oxidovanadium(IV) to Lysozyme. Inorg. Chem. 2022, 61, 16458–16467. [Google Scholar] [CrossRef] [PubMed]
- León, J.E.; Butenko, N.; Di Virgilio, A.L.; Muglia, C.I.; Cavaco, I.; Etcheverry, S.B. Vanadium and cancer Treatment: Antitumoral mechanisms of three oxidovanadium(IV) complexes on a human osteosarcoma cell line. J. Inorg. Biochem. 2014, 134, 106–117. [Google Scholar] [CrossRef] [PubMed]
- León, I.E.; Díez, P.; Etcheverry, S.B.; Fuentes, M. Deciphering the effect of an oxovanadium(IV) complex with flavonoid chrysin (VOChrys) on intracellular cell signalling pathways in an osteosarcoma cell line. Metallomics 2016, 8, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Ugone, V.; Micera, G.; Buglyó, P.; Bíró, L.; Garribba, E. Speciation in human blood of Metvan, a vanadium based potential anti-tumor drug. Dalton Trans. 2017, 46, 8950–8967. [Google Scholar] [CrossRef] [PubMed]
- Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Drew, M.G.B.; Acharya, K.; Chandra, S. Synthesis, crystal structure, DFT calculations, protein interaction and anticancer activities of water soluble dipicolinic acid-imidazole based oxidovanadium(IV) complexes. Dalton Trans. 2017, 46, 16682–16702. [Google Scholar] [CrossRef]
- Mohanty, M.; Maurya, S.K.; Banerjee, A.; Patra, S.A.; Maurya, M.R.; Crochet, A.; Brzezinski, K.; Dinda, R. In vitro cytotoxicity and catalytic evaluation of dioxidovanadium(V) complexes in an azohydrazone ligand environment. New J. Chem. 2019, 43, 17680–17695. [Google Scholar] [CrossRef]
- Sahu, G.; Banerjee, A.; Samanta, R.; Mohanty, M.; Lima, S.; Tiekink, E.R.T.; Dinda, R. Water-Soluble Dioxidovanadium(V) Complexes of Aroylhydrazones: DNA/BSA Interactions, Hydrophobicity, and cell-Selective Anticancer Potential. Inorg. Chem. 2021, 60, 15291–15309. [Google Scholar] [CrossRef]
- Ribeiro, N.; Bulut, I.; Oósa, V.; Sewrgi, B. Solution chemical properties and anticancer potential of 8-hydroxyquinoline hydrazones and their oxidovanadium(IV) complexes. J. Inorg. Biochem. 2022, 235, 111932. [Google Scholar] [CrossRef]
- Sahu, G.; Patra, S.A.; Pattanayak, P.D.; Kaminsky, W.; Dinda, R. LVVO-Ethyl Maltol-Based Metallodrugs (L2− = Tridentate ONO Ligands): Hydrophobicity, Hydrolytic Stability, and Cytotoxicity via ROS-Mediated Apoptosis. Inorg. Chem. 2023, 62, 6722–6739. [Google Scholar] [CrossRef]
- Nunes, P.; Yildizhan, Y.; Adiguzel, Z.; Marques, F.; Costa Pessoa, J.; Acilan, C. Copper(II) and oxidovanadium(IV) complexes of chromone Schiff bases as potential anticancer agents. J. Biol. Inorg. Chem. 2022, 27, 89–109. [Google Scholar] [CrossRef]
- Loizou, M.; Papaphilippou, P.; Vlasiou, M.; Spilia, M.; Peschos, D.; Simos, Y.V.; Keramidas, A.D.; Drouza, C. Binuclear VIV/V, MoVI and ZnII—Hydroquinonate complexes: Synthesis, stability, oxidative activity and anticancer properties. J. Inorg. Biochem. 2022, 235, 111911. [Google Scholar] [CrossRef]
- Talebi, A.; Salehi, M.; Khaleghian, A.; Kubicki, M. Evaluation of anticancer activities, molecular docking, and antioxidant studies of new Ni(II), VO(IV), Cu(II) and Co(III) Schiff base complexes. Inorg. Chim. Acta 2023, 546, 121296. [Google Scholar] [CrossRef]
- Scibior, A. Vanadium (V) and manganese (Mg)—In vivo interaction: A review. Chem.-Biol. Interact. 2016, 258, 214–233. [Google Scholar] [CrossRef]
- Hu, J.; Xia, W.; Pan, X.; Zheng, T.; Zhang, B.; Zhou, A.; Buka, S.L.; Bassig, B.A.; Liu, W.; Wu, C.; et al. Association of adverse birth outcomes with prenatal exposure to vanadium: A population-based cohort study. Lancet Planet. Health 2017, 1, e230–e241. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Vanadium Compounds as Pro-inflammatory Agents: Effects on Cyclooxygenases. Int. J. Mol. Sci. 2015, 16, 12648–12668. [Google Scholar] [CrossRef]
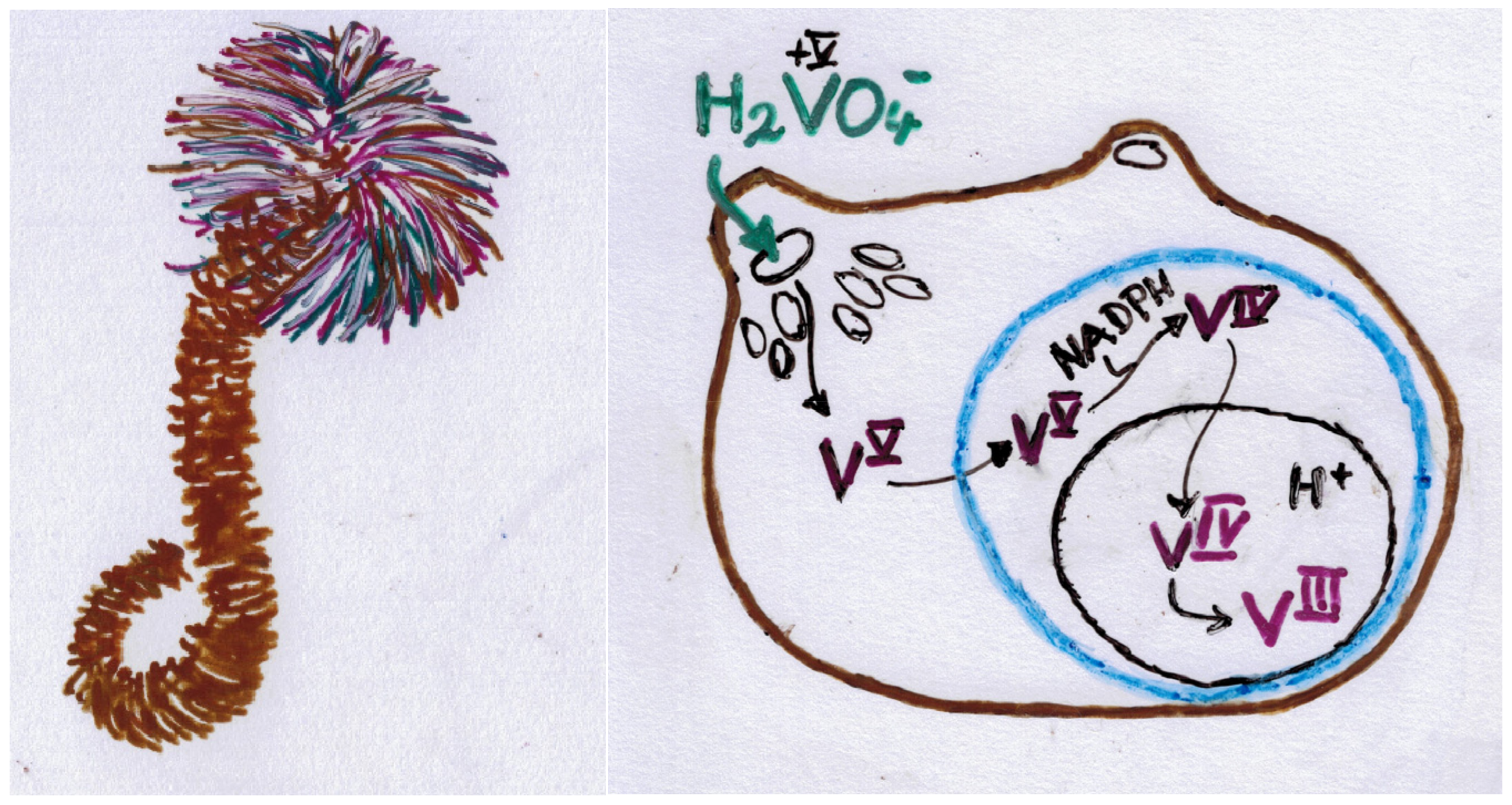
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehder, D. Import and Implications of Vanadium in Live Aspects. Inorganics 2023, 11, 256. https://doi.org/10.3390/inorganics11060256
Rehder D. Import and Implications of Vanadium in Live Aspects. Inorganics. 2023; 11(6):256. https://doi.org/10.3390/inorganics11060256
Chicago/Turabian StyleRehder, Dieter. 2023. "Import and Implications of Vanadium in Live Aspects" Inorganics 11, no. 6: 256. https://doi.org/10.3390/inorganics11060256
APA StyleRehder, D. (2023). Import and Implications of Vanadium in Live Aspects. Inorganics, 11(6), 256. https://doi.org/10.3390/inorganics11060256




