Abstract
The structure and bonding of two novel tungstaboranes which were synthesized using diverse synthetic methods are described. (i) The room-temperature photolysis of [Cp*W(CO)3Me] with [BH3·SMe2] led to the isolation of the hydrogen-rich tungstaborane [Cp*W(CO)2B3H8] (1). Its geometry consists of an arachno butterfly core similar to tetraborane(10) and obeys the Wade-Mingos electron counting rules (n vertices, n + 3 skeletal electron pairs (seps)). (ii) Further, the tungstaborane [(Cp*W)3(μ-H)2(μ3-H)(μ-CO)2B4H4] (4) was isolated by thermolysis reaction of a tungsten intermediate, obtained by low temperature reaction of [Cp*WCl4] and [LiBH4·THF] with [Cr(CO)5·THF]. Compound 4 which seems to have formed by replacement of a BH unit in [(Cp*W)2B5H9] by the isoelectronic fragment {Cp*W(CO)2}, adopts an oblato-nido hexagonal-bipyramidal core (n vertices, n–1 seps). Both compounds were characterized using multinuclear NMR, IR spectroscopy, mass spectrometry as well as single crystal X-ray diffraction analysis. In addition, density functional theory (DFT) calculations were performed in order to elucidate their bonding and electronic structures.
1. Introduction
After Lipscomb’s groundbreaking research on three-center bonds in boron hydrides [1], numerous pioneers in the field of main group chemistry had thoroughly investigated various bonding modes exhibited by boron atoms in three-dimensional structures [2,3,4,5] and successfully constructed clusters starting from small to 16-vertex boranes [6,7,8,9,10,11,12,13]. Hoffmann’s isolobal analogy [14] and electron-counting rules established by Wade [15], Mingos [16], and Jemmis [17] played a powerful role in shaping and understanding the structures and chemistry of these polyhedral boranes and their derivatives. Indeed, incorporation of transition metal fragment(s) into a polyhedral borane skeleton using the isolobal analogy offered distinctive chemistry of the three-dimensional polyhedral metallaborane clusters that enhanced diversity to the clusters in terms of their unique structural features and various degrees of reactivity [18,19,20,21,22]. In this connection, there has been significant progress over the years in boron-cluster chemistry, specifically in the field of polyhedral metallaborane [22,23,24,25,26] and metallaheteroborane [27,28] chemistry.
Metallaboranes were initially synthesized either through thermolysis or basic metathesis reactions involving pre-existing polyborane anions and transition-metal halides. Subsequently, it was shown that inclusion of monoboranes (such as [LiBH4·THF], [BH3·THF], or [BH3·SMe2]) in mono-cyclopentadienyl metal chlorides ([Cp*MCl]n) represents a highly effective approach for producing metallaboranes that incorporate transition metals spanning from groups 5 to 9. Both of these methodologies have been employed to synthesize several group 6 metallaboranes. For example, Pett et al. isolated the first tungstaborane [(PMe2Ph)3H2WB9H13] by the direct reaction of [WH6(PMe2Ph)3] with the arachno-[B9HI4]− anion [29]. Similarly, Girolami and co-workers synthesized the homoleptic transition metal complex Cr(B3H8)2, from the grinding of CrCl3 with NaB3H8, which turned out to be an excellent precursor for chemical vapor deposition to metal diboride [30]. Fehlner et al. synthesized [(Cp*Cr)2B4H8] using [Cp*CrCl]2 with [BH3·THF] which is a noteworthy example using the later approach [31]. Although metallaboranes have been synthesized from various transition metals across the periodic table, utilizing various synthetic strategies and conditions, synthesis of tungstaboranes is relatively less encountered. With regards to tungstaboranes, the clusters with smaller number of boron atoms are interesting both in terms of geometry as well as cluster build up chemistry. After it was discovered that the reaction of the hydrogen-rich [(Cp*WH3)B4H8] with [BH3·THF] led to the isolation of [(Cp*W)2B5H9], [(Cp*W)2B6H10], [(Cp*W)2B7H9], and [(Cp*W)3(μ-H)B8H8], the search of higher and lower-vertex tungstaborane became a topic of interest [32,33]. Subsequently, our group has synthesized a few tungstaboranes such as [{Cp*W(CO)2}2B2H2W(CO)4] [34], [{Cp*W(CO)}2B4H6] [35] and [(Cp*W)2B4H8W(CO)4] [36]. Therefore, in an attempt to synthesize some higher-vertex tungstaborane clusters, we have explored the reactivity of Cp*WLn (Ln = (CO)3Me or Cl4) in different conditions that led to the isolation of two different tungstaboranes with one and three W metal atoms, respectively. In this article, we report the structures and bonding of these two metallaboranes with the heaviest metal of group 6 triad. Furthermore, we conducted density functional theory (DFT) calculations to understand their bonding and electronic structures.
2. Results and Discussion
2.1. Photolysis of [Cp*M(CO)3Me] (M = W, Mo) with [BH3·SMe2]
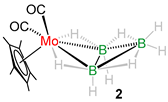
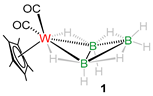
With the purpose of making higher nuclearity group 6 clusters, we performed the reaction of [Cp*M(CO)3Me] (M = W, Mo) with [BH3·SMe2] in photolytic conditions that led to the formation of air and moisture sensitive yellow solid products 1 and 2 in 27% and 31% yield, respectively (Scheme 1). Note that some low-yielded products were also formed in these reactions which could not be isolated. Compounds 1 and 2 were characterized using different spectroscopic techniques. Compound 2 was characterized as [Cp*Mo(CO)2B3H8] [37] which was reported earlier from the reaction of [Cp*Mo(CO)3Me] with [BH3·THF] in similar conditions. Detailed spectroscopic and structural characterization of compound 1 is given below.
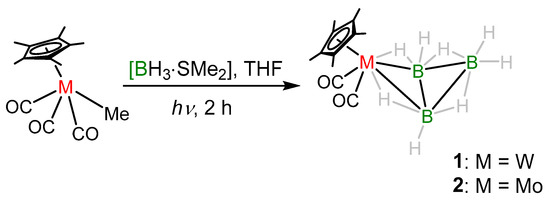
Scheme 1.
Synthesis of clusters 1 and 2.
The mass spectrum for compound 1 shows isotopic distribution pattern at m/z 413 which corresponds to [C12H22B3O2W]+. Besides the presence of hydrogen atoms of the Cp* ligand, the 1H NMR spectrum indicates the existence of B−H−B bridging protons at δ = −0.43 ppm and W−H−B protons at δ = −11.33 ppm. The 11B{1H} NMR shows two peaks at δ = 1.8 and −41.8 ppm which resemble the characteristic peaks of metal-B3H8 complexes. The 1H{11B} NMR experiments further allowed us to assign the terminal hydrogen peaks for 1 at δ = 2.40 and 4.48 ppm. Moreover, the 13C NMR spectrum confirmed the presence of Cp* and CO ligands. The IR spectrum shows peaks that supported the existence of terminal carbonyl ligands and terminal B-H groups. However, a clear explanation eluded us until the single-crystal X-ray diffraction analysis of 1 was performed.
The molecular structure of 1 was determined using single-crystal X-ray diffraction analysis, which confirms the structural inferences made on the basis of spectroscopic data. Figure 1 depicts the molecular structure of 1, which exhibits a WB3 butterfly core geometry that closely resembles that of [B4H10] where one of the wing-tip is replaced by a {Cp*W(CO)2} fragment. The dihedral angle between W1−B1−B2 and B1−B2−B3 plane is 121.54° and is comparable with that of related arachno-octahedral metallaboranes, shown in Table 1 [30,37,38,39,40,41,42]. The average W−B and B−B bond lengths of 2.511(5) Å and 1.767 Å, respectively are in the expected region for such molecules [30,37,38,39,40,41,42]. From the 11B{1H} NMR spectrum, the chemical shift at δ = 1.8 ppm corresponds to atom B3 and that at δ = −41.9 ppm corresponds to atoms B1 and B2. Further, a 1H-11B{1H} HSQC (Heteronuclear Single Quantum Coherence) experiment was performed that confirmed a correlation between the bridging W−H−B hydrogen atoms and the boron atoms B1 and B2.

Figure 1.
Molecular structure and labelling diagram of 1. Selected bond lengths (Å) and angles (deg): W1−B1 2.511(5), W1−B2 2.511(6), B1−B2 1.705(8), B1−B3 1.802(8), B2−B3 1.794(9); W1−B1−B3 105.7(3), W1−B1−B2 70.2(3), B2−B1−B3 61.5(4).

Table 1.
Selected structural parameters and NMR chemical shifts of selected metal-octahydrotetraborane complexes.
Based on Wade’s rules [15] and the molecular formula, it can be concluded that [Cp*W(CO)2B3H8], 1, adopts an arachno-octahedral butterfly geometry (n = 4 vertices) with seven (n + 3 = 7) skeletal electron pairs (seps) [{Cp*W(CO)2} 3 × 1 + (BH) 2 × 2 + (BH2) 3 × 1 + (μ-H) 1 × 4 = 14 electrons, i.e., seven seps]. It is structurally and electronically analogous to the molybdaborane congener (2) reported earlier by us [37] and its geometry matches with other arachno-type butterfly clusters reported earlier (Table 1). Note that the Cp analogue of 1 was first reported by Gaines in 1978, generated from the photolysis reaction of [CpW(CO)3Cl] in presence of [(CH3)4NB3H8] [43]. However, its structure was established only based on preliminary spectroscopic studies.
To gain further insight into the bonding and electronic properties, computational density functional theory (DFT) studies were undertaken on model 1′ (Cp analogue used to mimic 1) at the B3LYP/def2-TZVP level of theory (see Section 3.3 for computational details). The optimized core structure of 1′ is in a good agreement with the solid-state X-ray structure determination (see Table S1). The calculated 11B NMR chemical shifts are also in well accordance with the values experimentally measured for cluster 1 (see Table S2). Subsequently, DFT studies were also carried out for the Mo and Cr analogues 2′ (Cp analogue of 2) and 3′ (Cp analogue of Cp*Cr(CO)2B3H8), respectively, for a comparative bonding analysis. A molecular orbital (MO) analysis suggests that both the HOMO and LUMO predominantly show a bonding character between tungsten and carbonyl ligands. As expected, a similar kind of interaction occurs for 2′ and 3′. Interestingly, the energy of the HOMOs decreases as we move from W to Mo to Cr, whereas, that of the LUMOs get more stabilized in the case of 2′ than that of 3′ when compared with 1′. The result is that the HOMO-LUMO gap of these group 6 metal-B3H8 clusters decrease while moving down the triad (Figure 2a). In this connection, a natural bond order (NBO) analysis suggested a decreasing trend of negative natural charge on the metal center, while moving from 3′ to 1′. Furthermore, an important σ-bonding interaction was observed in HOMO−8 that involves d-orbitals of the metal and p-orbitals of the boron atoms (Figure 2b). Likewise, as shown in Figure 2c, π-type overlap of boron p orbitals generate B−B π-bonding interaction delocalized throughout the triangular B3 framework. All the above findings seemingly justify the stability of the butterfly core in these species.
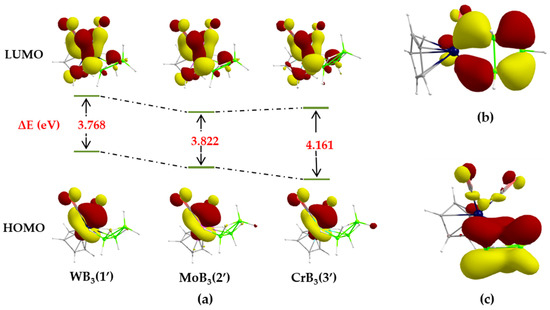
Figure 2.
(a) Comparison of HOMO-LUMO energy gaps in group 6 metal-B3H8 clusters; (b) HOMO−8 of 1′; (c) HOMO−9 of 1′. Isocontour values: ±0.043 [e.bohr−3]1/2.
2.2. Reactivity of [Cp*WCl4] with [Cr(CO)5∙THF]
The reactivity of [Cp*WCl4] with various boranes has been explored over several decades and has led to the formation of different tungstaborane clusters [33,34,44,45]. Thus, with an objective to get higher-vertex hybrid-clusters, we further explored the reactivity of [Cp*WCl4] with an excess of [LiBH4·THF] followed by treating the solution with [Cr(CO)5⋅THF]. The compound [(Cp*W)3(μ-H)2(μ3-H)(μ-CO)2B4H4] (4) was isolated as a green solid in 7% yield along with the already reported compounds [(Cp*W)2B4H8Cr(CO)4] (A) (6% yield) [36] and [(Cp*W)2B5H9] (26% yield) [46] (Scheme 2). Thin-layer chromatography (TLC) was used to separate and purify the products. Detailed spectroscopic and structural characterisation of cluster 4 is described below.
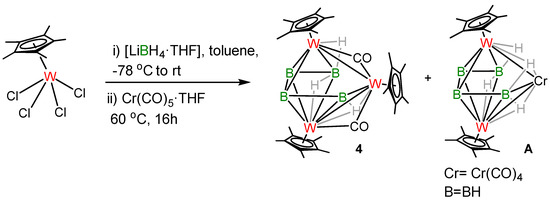
Scheme 2.
Synthesis of cluster 4.
The mass spectrum of 4 showed a molecular ion peak at m/z = 1063 which corresponds to [C32H52B4O2W3]+. The 11B{1H} NMR spectrum shows peaks of equal intensity at δ = 25.7, 36.2, 69.1 and 73.8 ppm which confirm the presence of four boron atoms. In addition, the 1H NMR spectrum of 4 shows three distinct peaks for Cp* protons at δ = 1.95, 2.01 and 2.11 ppm in a 1:1:1 ratio. Further, 13C NMR also confirmed the presence of three types of Cp*. In addition, there are three peaks in the upfield region of 1H NMR at δ = −9.09, −9.36 and −12.86 ppm, indicating three types of bridging hydrogen atoms, for example, W−H−W and W−H−B protons. IR spectroscopy indicated the presence of bridging carbonyl ligands and terminal B−H groups. Although mass spectrometric data along with all the other spectroscopic data provided some guidelines about the structure of 4, the core geometry was truly established when the single crystal X-ray diffraction analysis was carried out (Figure 3). For that, a suitable crystal of 4, grown by the slow evaporation of hexane/dichloromethane solution at −5 °C was used.
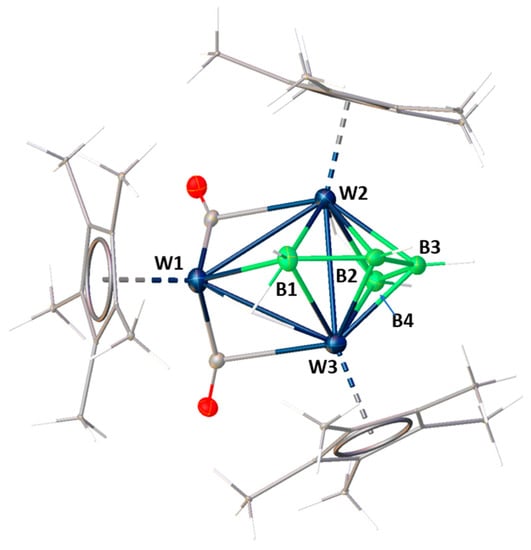
Figure 3.
Molecular structure and labeling diagram of 4. Selected bond lengths (Å) and angles (deg): W1−W2 2.9613(2), W1−W3 2.9482(3), W2−W3 2.8449(2), W1−B1 2.336(5), W2−B2 2.226(6), W2−B3 2.207(6), W3−B2 2.205(5), W3−B3 2.217(5), B1−B2 1.714(8), B2−B3 1.716(8); W3−W1−W2 57.555(6), B1−W1−W2 49.03(15), B3−W2−B2 45.6(2), B2−W2−B1 44.8(2), B3−W2−B4 45.2(2), B1−W2−B4 104.5(2), B1−W2−W3 52.94(15), B3−W2−W1 111.12(14), B1−B2−B3 123.5(4).
Its molecular structure can be seen as [(Cp*W)3(μ-H)2(μ3-H)(μ-CO)2B4H4] and is comparable with its molybdenum analogue previously synthesized by us from the reaction of [(Cp*Mo)2(μ-Cl)2B2H6] with CO [47]. The polyhedral skeletal electron count rules predict that 4 can be viewed as a 7-vertex−6-sep ({Cp*W} (−1) × 3 + (BH) 2 × 4 + (μ-CO) 2 × 2 +(μ-H) 1 × 3 = 12 electrons, i.e., 6 seps) oblato-nido hexagonal-bipyramidal cluster. Consequently, the core structure of 4 exhibits remarkable resemblance to [(Cp*W)2B5H9] [34], wherein the substitution of an isoelectronic unit, Cp*W(CO)2, replaces one of the [BH2] vertices on the open face. The W2−W3 intracluster bond distance in 4 of 2.8449(2) Å is rather short and comparable with that observed in other similar tungstaboranes having 6 seps. The average B−B bond distance (1.721 Å) is in the range of molecules having a [W2B5] framework [36,48].
In order to investigate the electronic properties of 4, DFT calculations of the Cp analogue of 4 (4′) was carried out at the B3LYP/def2-TZVP level of theory. An MO analysis indicates that both the HOMO and LUMO are mostly non-bonding metal d-type orbitals with almost insignificant bonding interactions between the metal and boron atoms. Indeed, as shown in Figure 4a, the p orbitals of all four boron centers overlap to generate low energy-lying σ-bonding interactions in a delocalized fashion resulting in a region of charge concentration along the B1–B2, B2–B3 and B3−B4 bonds. This also shows high covalent character, which is also reflected by the average electron density [ρr] and energy density [H(r)] values (Table S3). Although any bonding interaction along the cross-cluster W2−W3 bond in the MO analysis is not straightforward, an NBO analysis showed indeed some bonding interaction. The NBO analysis suggested also the existence of bonding interactions along the W1−W2 and W1−W3 bonds. Furthermore, a substantial Wiberg bond indices value of 0.738 suggests the presence of a strong bonding interaction along the W2−W3 bond as compared to the W1−W3 and W1−W2 bonds (0.545 and 0.391 respectively). Therefore, based on these theoretical results, it is reasonable to assume that the cross-cluster W–W bond is even stronger as compared to the other W−W bonds (Table S1).
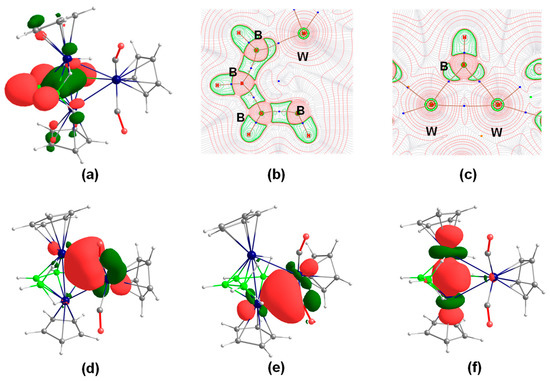
Figure 4.
(a) HOMO–18 of 4′; (b) and (c) contour line diagram along the B1–B2–B3–B4 plane obtained from the Laplacian of electron density of 4′. Charge concentration (∇2ρ(r) < 0) regions are represented by solid red lines, while the areas of charge depletion (∇2ρ(r) > 0) are shown in dashed green lines. Blue dots and bold dark-brown lines depict bond critical points (bcps) and bond paths, respectively; (d), (e) and (f) Selected NBOs of 4′. Isocontour values: ±0.043 [e.bohr−3]1/2.
3. Materials and Methods
3.1. General Methods and Instrumentation
All experiments were carried out utilizing standard Schlenk and glove-box techniques within an argon atmosphere. Prior to usage, solvents were distilled under an argon environment. Other chemicals such as Cp*H, [BH3.SMe2], [Cr(CO)6], and MeI (Aldrich) were used as received. [Cp*W(CO)3Me] [49] and [Cp*WCl4] [50] were prepared according to a literature procedure. Thin-layer chromatography was carried on 250-µm diameter aluminum supported silica gel TLC plates (MERCK TLC Plates). The NMR spectra were recorded on a 400 and 500 MHz Bruker FT-NMR spectrometer. The Infraref spectra (IR) were recorded using a JASCO FT/IR -4100 spectrometer. The mass spectra were recorded on an Agilent Technologies 6545-Q-TOF LC/MS spectrometer. The experimental photoreactions discussed in this study were performed using a Luzchem LZC-4 V photoreactor under irradiation conditions ranging from 254 to 350 nm. Synthesis of 1: A solution of [Cp*W(CO)3Me] (0.05 g, 0.15 mmol) and [BH3·SMe2] (0.7 mL, 7 mmol) in THF (12 mL) was prepared in a flame-dried Schlenk tube. The reaction mixture was then exposed to irradiation at room temperature for a duration of 2 h. Following the reaction, the volatile components were removed under vacuum, and the remaining residue was extracted into hexane and passed through Celite. The solvent was subsequently evaporated, and the resulting residue underwent chromatographic purification using silica gel TLC plates. Elution with a mixture of hexane and CH2Cl2 (95:5 v/v) led to the isolation of arachno-1 as a yellow solid (0.016 g, 27% yield).
1: MS (ESI+): m/z found: 413 [M-H]+; calcd mass for 12C121H2311B3184W16O2 414; 11B NMR (160 MHz, CDCl3, 22⁰ C): δ = 1.8 (s, 1B), −41.8 (d, 2B); 1 H NMR (400 MHz, CDCl3, 22 °C): δ = 4.48 (br, 1B-Ht), 2.40 (br, 2B-Ht), 2.05 (s, 15H, Cp*), −0.43 (br, 2B-H-B), −11.34 (br, 2W-H-B); 13C NMR (100 MHz, CDCl3, 22 °C): δ = 105.3 (s, ⴄ5-C5Me5), 11.2 (s, ⴄ5-C5Me5), 234.3 (s, 2CO); IR (hexane, cm−1): 2445 (B-Ht), 1957, 1882 (CO).
Synthesis of 4: In a flame-dried Schlenk tube [Cp*WCl4], (0.1 g, 0.22 mmol) in 15 mL of toluene was treated with an excess of [LiBH4·THF] (1.1 mL, 2.2 mmol) at a temperature of −78 °C, followed by stirring at room temperature for one hour. After removing the solvent, the resulting residue was extracted into hexane and filtered through a frit containing Celite. The obtained brownish-green hexane extract was then dried under vacuum and dissolved in 15 mL of THF. The solution was heated at 65 °C in the presence of [Cr(CO)5·THF] for a duration of 22 h. Subsequently, the solvent was evaporated under vacuum, and the residue was extracted into hexane and passed through Celite. Upon removal of the solvent from the obtained filtrate, the residue underwent chromatographic purification using silica gel TLC plates. Elution with a mixture of hexane and CH2Cl2 (75:15 v/v) resulted in the isolation of green compound 4 (0.016 g, 7% yield), along with previously known compounds [(Cp*W)2B5H9] (0.040 g, 26% yield) and [(Cp*W)2B4H8Cr(CO)4] (0.011 g, 6% yield).
4: MS (ESI+): m/z found: 1063 [M]+; calcd. mass for 12C321H5211B4184W316O2 1063; 11B NMR (160 MHz, CDCl3, 22 °C): δ = 73.8 (s, 1B), 69.1 (s, 1B), 36.2 (s, 1B), 25.7 (s, 1B); 1 H NMR (400 MHz, CDCl3, 22 °C): δ = 4.14 (br, 4B-Ht), 2.18 (s, 15H, Cp*), 2.08 (s, 15H, Cp*), 2.02 (s, 15H, Cp*), −9.06 (br, 1W-H-B), −9.31 (br, 1W-H-B), −12.72 (br, 1W-H-B); 13C NMR (100 MHz, CDCl3, 220 C): δ = 107.8 (s, ⴄ5 -C5Me5), 106.3 (s, ⴄ5 -C5Me5), 98.9 (s, ⴄ5-C5Me5), 13.6 (s, ⴄ5-C5Me5), 13.3 (s, ⴄ5-C5Me5), 11.4 (s, ⴄ5-C5Me5); IR (hexane, cm−1): 2491 (B-Ht), 1755 (CO).
3.2. Single Crystal X-ray Diffraction Analysis
Slow evaporation of a hexane−CH2Cl2 solution of compound 1 and 4 allowed us to grow crystals which are suitable for XRD analysis. Table 2 provides crystallographic and structural refinement data for 1 and 4. A Bruker D8 VENTURE diffractometer with PHOTON II detector at 296(2) K was used to collect and integrate crystal diffraction data of 1. Further, a Bruker APEX-II CCD diffractometer was used to collect as well as integrate the crystal data of 4. A dual-space algorithm using SHELXT program [51] was used to solve the structures and refinement was achieved utilizing full-matrix least-squares methods based on F2 using SHELXL-97 software [52]. Olex2-1.3 [53] was used to draw molecular structures. The refinement of non-hydrogen atoms was aided by anisotropic displacement parameters (ADPs), while the introduction of hydrogen atoms, except for bridging hydrogens, was achieved through analysis of Fourier difference maps. H atoms were finally included in their calculated positions and treated as ‘riding’ on their parent atom with constrained thermal parameters. Crystallographic data can be acquired for free from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif, accessed on 31 May 2023.

Table 2.
Crystallographic and structural refinement data for compounds 1 and 4.
3.3. Computational Details
Geometry optimizations of all model clusters were carried out using the Gaussian 09 software package [54]. In order to reduce the computational efforts, the computations were carried out with the Cp ligands instead of Cp* (for optimized model clusters, see Figures S14–S17). The Becke-Lee-Yang-Parr (B3LYP) hybrid functional [55], along with the def2-TZVP basis set [56] from the EMSL (Environmental Molecular Sciences Laboratory) Basis Set Exchange Library [57] for all atoms. Optimizations were carried out without any solvent effects or symmetry constraints. To ensure that the optimized geometries represented genuine minima on the potential energy hypersurface, frequency calculations were conducted for all structures. These calculations were used to examine the nature of the stationary states and verify that there were no imaginary frequencies present. Using the optimized geometries at the B3LYP/def2-TZVP level, NMR chemical shifts were calculated using the gauge-including atomic orbitals (GIAOs) method [58,59,60]. The 11B NMR chemical shifts were calculated relative to B2H6, with a B3LYP shielding constant of 83.6 ppm. To convert these values to the standard [BF3·OEt2] scale, the computed values were adjusted by adding 16.6 ppm (the experimental δ (11B) value of B2H6). The internal standard used for calculating 1H NMR chemical shifts was TMS (SiMe4). Orbital graphics and optimized structure plots were generated using Chemcraft [61]. To obtain the Wiberg bond indices (WBI) [62], a natural bond orbital (NBO) analysis [62] was conducted.
4. Conclusions
The present work describes the isolation and structural characterization of a mono- and a tri-metallic tungstaborane clusters generated from two different metal complex precursors, namely [Cp*W(CO)3Me] and [Cp*WCl4]. First, an efficient route to the synthesis of arachno-[Cp*W(CO)2B3H8] (1) has been described using [Cp*W(CO)3Me] and borane reagent [BH3⋅SMe2]. The geometry of this open butterfly cluster is very interesting as it can be utilized for further cluster build up chemistry. The second route allowed us to demonstrate the synthesis of the tungstaborane [(Cp*W)3(μ-H)2(μ3-H)(μ-CO)2B4H4] (4) using [Cp*WCl4], [LiBH4] and [Cr(CO)5·THF]. Derivatives containing a W3B4 core present in 4 are rather rare. With a butterfly [WB3] core, cluster 1 obeys the Wade-Mingos electron counting rules (n vertices, n + 3 seps), whereas species 4 is an oblato-nido hexagonal-bipyramidal cluster (n vertices, n − 1 seps).
Supplementary Materials
Some supplementary information is available online at https://www.mdpi.com/article/10.3390/inorganics11060248/s1. It contains 1H, 11B{1H}, 13C{1H} NMR, IR, and mass spectra (Figures S1–S12); X-ray analysis details and CIF and checkCIF files as well as the xyz coordinates of the DFT-optimized model clusters, calculated HOMO and LUMO energy levels and HOMO-LUMO gaps, selected bond parameters and their Wiberg bond indices (WBI) for clusters 1’–4’ (Figures S13–S18). Table S1. Selected geometrical parameters and Wiberg bond indices (WBI) of 1’–4’. Table S2. Experimentally observed and calculated 11B chemical shifts of 1’–4’. Table S3. Calculated natural charges (q), natural valence population (Pop) and HOMO–LUMO gaps of 1’–3’.
Author Contributions
Conceptualization, S.M., S.G. (Sundargopal Ghosh) and J.-F.H.; methodology, S.M. and S.S.; software, S.M. and S.G. (Sourav Gayen); validation, S.G. (Sundargopal Ghosh) and J.-F.H.; formal analysis, S.M., S.G. (Sourav Gayen), S.S.; investigation, S.M., S.G. (Sourav Gayen), S.S.; resources, S.G. (Sundargopal Ghosh); data curation, S.M. and S.G. (Sourav Gayen); writing—original draft preparation, S.M. and S.G (Sourav Gayen); writing—review and editing, S.G. (Sundargopal Ghosh) and J.-F.H.; visualization, S.M., S.G. (Sourav Gayen) and S.G. (Sundargopal Ghosh); supervision, S.G. (Sundargopal Ghosh) and J.-F.H.; project administration, S.G. (Sundargopal Ghosh) and J.-F.H.; funding acquisition, S.G. (Sundargopal Ghosh). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by ERP (Project No.- RF/2223/0528/CY/RFER/008224).
Data Availability Statement
All data are available on request.
Acknowledgments
S.M. thanks IIT Madras for research fellowship. S.G. thanks CSIR, India and S.S. thanks UGC, India for research fellowship. Department of Chemistry, IIT Madras is thankfully acknowledged for X-ray support. The authors express their gratitude to IIT Madras for providing computational facilities. Additionally, they extend their appreciation to P. K. Sudhadevi Antharjanam and Venkatachalam Ramkumar for their valuable contributions in analyzing the single crystal X-ray diffraction data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lipscomb, W.L. Boron Hydrides; Benjamin: New York, NY, USA, 1963. [Google Scholar]
- Longuet-Higgins, H.C. The structures of electron-deficient molecules. Q. Rev. Chem. Soc. 1957, 11, 121–133. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Ward, I.M. Metallaboranes and Metal-Boron Bonding. Chem. Soc. Rev. 1974, 3, 231–271. [Google Scholar] [CrossRef]
- Greenwood, N.N. The concept of boranes as ligands. Coord. Chem. Rev. 2002, 226, 61–69. [Google Scholar] [CrossRef]
- Fehlner, T.P.; Halet, J.-F.; Saillard, J.-Y. Molecular Clusters: A Bridge to Solid-State Chemistry; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kennedy, J.D. The Polyhedral Metallaboranes Part II. Metallaboranes Clusters with Eight Vertices and More. Prog. Inorg. Chem. 1986, 34, 211–434. [Google Scholar]
- Boyd, A.S.F.; Burke, A.; Ellis, D.; Ferrer, D.; Giles, B.T.; Laguna, M.A.; McIntosh, R.; Macgregor, S.A.; Ormsby, D.L.; Rosair, G.M.; et al. Supraicosahedral (metalla)carboranes. J. Pure Appl. Chem. 2003, 75, 1325–1333. [Google Scholar] [CrossRef]
- Bose, S.K.; Geetharani, K.; Varghese, B.; Ghosh, S. Unusual organic chemistry of a metallaborane substrate: Formation of a tantalaborane complex with a bridging Acyl group (μ-n2). Inorg. Chem. 2010, 49, 6375–6377. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Noll, B.C.; Fehlner, T.P. Expansion of iridaborane clusters by addition of monoborane. Novel metallaboranes and mechanistic detail. Dalton Trans. 2008, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Ghosh, S. Borane Polyhedra Beyond Icosahedron. In 50th Anniversary of Electron Counting Paradigms for Polyhedral Molecules; Springer: Cham, Switzerland, 2021; Volume 187, pp. 109–138. [Google Scholar]
- Zhang, A.; Xie, Z. Recent Progress in the Chemistry of Supercarboranes. Chem. Asian J. 2010, 5, 1742–1757. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z. Synthesis, Structure, and Reactivity of 13- and 14-Vertex Carboranes. Acc. Chem. Res. 2014, 47, 1623–1633. [Google Scholar] [CrossRef]
- Dustin, D.F.; Dunks, G.B.; Hawthorne, M.F. Novel 13-Vertex Metallocarborane Complexes Formed by Polyhedral Expansion of 1,2-Dicarba-closo-dodecaborane(12) (1,2-B10C2H12). J. Am. Chem. Soc. 1973, 95, 1109–1115. [Google Scholar] [CrossRef]
- Hoffmann, R. Building Bridges Between Inorganic and Organic Chemistry (Nobel Lecture). Angew. Chem. Int. Ed. 1982, 21, 711–724. [Google Scholar] [CrossRef]
- Wade, K. Skeletal electron counting in cluster species. Some generalisations and predictions. Inorg. Nucl. Chem. Lett. 1972, 8, 559–562. [Google Scholar] [CrossRef]
- Mingos, D.M.P. Polyhedral skeletal electron pair approach. Acc. Chem. Res. 1984, 17, 311–319. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Balakrishnarajan, M.M.; Pancharatna, P.D. A Unifying Electron-Counting Rule for Macropolyhedral Boranes, Metallaboranes, and Metallocenes. J. Am. Chem. Soc. 2001, 123, 4313–4323. [Google Scholar] [CrossRef] [PubMed]
- Bullick, H.J.; Grebenik, P.D.; Green, M.L.H.; Hughes, A.K.; Leach, J.B.; Mountford, P. Reactivity of nido-[2-{Fe(η-C5H5)}B5H10] with some transition-metal hydride complexes. J. Chem. Soc. Dalton Trans. 1994, 3337–3342. [Google Scholar] [CrossRef]
- Roy, D.K.; Mondal, B.; Anju, R.S.; Ghosh, S. Chemistry of Diruthenium and Dirhodium Analogues of Pentaborane(9): Synthesis and Characterization of Metal N,S-Heterocyclic Carbene and B-Agostic Complexes. Chem. Eur. J. 2015, 21, 3640–3648. [Google Scholar] [CrossRef]
- Ghosh, S.; Lei, X.; Cahill, C.L.; Fehlner, T.P. Symmetrical Scission of the Coordinated Tetraborane in [(Cp*ReH2)2B4H4] on CO Addition and Reassociation of the Coordinated Diboranes on H2 Loss. Angew. Chem. Int. Ed. 2000, 39, 2900–2902. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shang, M.; Fehlner, T.P. Reactions of an Electronically Unsaturated Chromaborane. Coordination of CS2 to (η5-C5Me5)2Cr2B4H8 and Its Hydroboration to a Methanedithiolato Ligand. Organometallics 1996, 15, 1963–1965. [Google Scholar] [CrossRef]
- Green, M.L.H.; Leach, J.B.; Kelland, M.A. Synthesis and Interconversion of Some Small Ruthenaboranes: Reaction of a Ruthenium Borohydride with Pentaborane(9) to Form Larger Ruthenaboranes. Organometallics 2007, 26, 4031–4037. [Google Scholar] [CrossRef]
- Weller, A.S. d- and f-Block Metallaboranes. In Comprehensive Organometallic Chemistry III; Crabtree, R.H., Mingos, D.M.P., Eds.; Elsevier: Oxford, UK, 2006; Volume 3, Chapter 3.04; pp. 133–174. [Google Scholar]
- Fehlner, T.P. Metallaboranes. In Structural and Electronic Paradigms in Cluster Chemistry; Springer: Berlin/Heidelberg, Germany, 1997; Volume 87, pp. 111–135. [Google Scholar]
- Sahoo, S.; Reddy, K.H.K.; Dhayal, R.S.; Mobin, S.M.; Ramkumar, V.; Jemmis, E.D.; Ghosh, S. Chlorinated hypoelectronic dimetallaborane clusters: Synthesis, characterization, and electronic structures of (η-C5Me 5W)2B5HnCIm, (n = 7, m = 2 and n = 8, m = 1). Inorg. Chem. 2009, 48, 6509–6516. [Google Scholar] [CrossRef]
- Ferguson, G.; Jennings, M.C.; Lough, A.J.; Coughlan, S.; Spalding, T.R.; Kennedy, J.D.; Fontaine, X.L.R.; Stibr, B. Novel rhodathiaborane complexes derived from [(PPh3)2RhSB9H10]. J. Chem. Soc. Chem. Commun. 1990, 891–894. [Google Scholar] [CrossRef]
- Bown, M.; Fontaine, X.L.R.; Greenwood, N.N.; Kennedy, J.D. Organoruthenaborane Chemistry. VIII. Reactions of [{(η6-C6Me6)RuCl2}2] and [{(η6-MeC6HPr)RuCl2}2] with Cs[arachno-6-SB9H12]: Isolation of ten-, eleven-, and twelve-vertex ruthenathiaboranes and their characterization by N.M.R. spectroscopy. Z. Anorg. Allg. Chem. 1991, 602, 17–29. [Google Scholar] [CrossRef]
- Mazighi, K.; Carroll, P.J.; Sneddon, L.G. Syntheses and structural characterizations of hypho- and arachno-metalladithiaborane clusters. Inorg. Chem. 1992, 31, 3197–3204. [Google Scholar] [CrossRef]
- Thornton-Pett, M.; Beckett, M.A.; Kennedy, J.D. Polyhedral phosphaborane chemistry: Crystal and molecular structure of the diphenylphosphido-bridged arachno-decaboranyl cluster compound [PMePh3][6,9-µ-(PPh2)B10H12]. J. Chem. Soc. Dalton Trans. 1986, 303–308. [Google Scholar] [CrossRef]
- Goedde, D.M.; Girolami, G.S. A New Class of CVD Precursors to Metal Borides: Cr(B3H8)2 and Related Octahydrotriborate Complexes. J. Am. Chem. Soc. 2004, 126, 12230–12231. [Google Scholar] [CrossRef] [PubMed]
- Deck, K.J.; Nishihara, Y.; Shang, M.; Fehlner, T.P. Preparation and Structure of (Cp*Cr)2B4H8. An Unsaturated Metallaborane Cluster with an Unexpected Structure. J. Am. Chem. Soc. 1994, 116, 8408–8409. [Google Scholar] [CrossRef]
- Weller, A.S.; Shang, M.; Fehlner, T.P. Synthesis and Structure of the Metallaborane Cp*3(μ-H)W3B8H8 from the Thermolysis of Cp*H3WB4H8 (Cp* = η5-C5Me5). A Close-Packed 11-Atom Boron-Rich Cluster. J. Am. Chem. Soc. 1998, 120, 8283–8284. [Google Scholar] [CrossRef]
- Weller, A.S.; Shang, M.; Fehlner, T.P. Synthesis of Mono- and Ditungstaboranes from Reaction of Cp*WCl4 and [Cp*WCl2]2 with BH3·thf or LiBH4 (Cp* = η5-C5Me5). Control of Reaction Pathway by Choice of Monoboron Reagent and Oxidation State of Metal Center. Organometallics 1999, 18, 53–64. [Google Scholar] [CrossRef]
- Mondal, B.; Bag, R.; Ghorai, S.; Bakthavachalam, K.; Jemmis, E.D.; Ghosh, S. Synthesis, Structure, Bonding, and Reactivity of Metal Complexes Comprising Diborane(4) and Diborene(2): [{Cp*Mo(CO)2}2{μ-η2:η2-B2H4}] and [{Cp*M(CO)2}2B2H2M(CO)4], M = Mo,W. Angew. Chem. Int. Ed. 2018, 57, 8079–8083. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Bag, R.; Ghosh, S. Combined Experimental and Theoretical Investigations of Group 6 Dimetallaboranes [(Cp*M)2B4H10] (M = Mo and W). Organometallics 2018, 37, 2419–2428. [Google Scholar] [CrossRef]
- Bag, R.; Saha, S.; Borthakur, R.; Mondal, B.; Roisnel, T.; Dorcet, V.; Halet, J.-F.; Ghosh, S. Synthesis, Structures and Chemistry of the Metallaboranes of Group 4-9 with M2B5 Core having a Cross Cluster M-M Bond. Inorganics 2019, 7, 27. [Google Scholar] [CrossRef]
- Ramalakshmi, R.; Bhattacharyya, M.; Rao, C.E.; Ghosh, S. Synthesis, structure and chemistry of low-boron containing molybdaborane: Arachno-[Cp*Mo(CO)2B3H8]. J. Organomet. Chem. 2015, 792, 31–36. [Google Scholar] [CrossRef]
- Grebenik, P.D.; Leach, J.B.; Green, M.L.H.; Walker, N.M. Transition metal mediated homologation of BH3·THF: Synthesis and crystal structure of [WH3(PMe3)3B3H8]. J. Organomet. Chem. 1988, 345, C31–C34. [Google Scholar] [CrossRef]
- Ghosh, S.; Beatty, A.M.; Fehlner, T.P. The Reaction of Cp*ReH6, Cp* = C5Me5, with Monoborane to Yield a Novel Rhenaborane. Synthesis and Characterization of arachno-Cp*ReH3B3H8. Collect. Czech. Chem. Commun. 2002, 67, 808–812. [Google Scholar] [CrossRef]
- Grebenik, P.D.; Leach, J.B.; Pounds, J.M. Niobium metallaboranes: A novel metallaborane analogue of pentaborane (11). J. Organomet. Chem. 1990, 382, C1–C5. [Google Scholar] [CrossRef]
- Geetharani, K.; Bose, S.K.; Pramanik, G.; Saha, T.K.; Ramkumar, V.; Ghosh, S. An Efficient Route to Group 6 and 8 Metallaborane Compounds: Synthesis of arachno-[Cp*Fe(CO)B3H8] and closo-[(Cp*M)2B5H9] (M = Mo, W). Eur. J. Inorg. Chem. 2009, 2009, 1483–1487. [Google Scholar] [CrossRef]
- Gaines, D.F.; Hildebrandt, S.J. Syntheses and Properties of Some Neutral Octahydrotriborate(l-) Complexes of Chromium-, Manganese-, and Iron-Group Metals. Inorg. Chem. 1978, 17, 794–806. [Google Scholar] [CrossRef]
- Rau, M.S.; Kretz, C.M.; Geoffroy, G.L.; Rheingold, A.L. Reaction of Cp*MoCl4 and Cp*WCl4 with H2O, H2S, amines, and hydrazines. Formation of the trioxo anions [Cp*Mo(O)3]- and [Cp*W(O)3]- and the trisulfido anion [Cp*W(S)3]−. Organometallics 1993, 12, 3447–3460. [Google Scholar] [CrossRef]
- Lupan, A.; King, R.B. Flattened deltahedral structures and bridging hydrogen atoms in hypoelectronic dimolybdaboranes and ditungstaboranes. J. Organomet. Chem. 2014, 754, 94–103. [Google Scholar] [CrossRef]
- Aldridge, S.; Shang, M.; Fehlner, T.P. Directed Synthesis of Chromium and Molybdenum Metallaborane Clusters. Preparation and Characterization of (Cp*Cr)2B5H9, (Cp*Mo)2B5H9 and (Cp*MoCl)2B4H10. J. Am. Chem. Soc. 1997, 119, 2339–2340. [Google Scholar] [CrossRef]
- Mondal, B.; Bag, R.; Roisnel, T.; Ghosh, S. Use of Single-Metal Fragments for Cluster Building: Synthesis, Structure, and Bonding of Heterometallaboranes. Inorg. Chem. 2019, 58, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, S.; Hashimoto, H.; Kawamura, K.; Shang, M.; Fehlner, T.P. Cluster Expansion Reactions of Group 6 Metallaboranes. Syntheses, Crystal Structures, and Spectroscopic Characterizations of (Cp*Cr)2B5H9, (Cp*Cr)2B4H8Fe(CO)3, (Cp*Cr)2B4H7Co(CO)3, and (Cp*Mo)2B5H9Fe(CO)3. Inorg. Chem. 1998, 37, 928–940. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Rest, A.J.; Alt, H.G.; Eichner, E.; Jansen, B.M. Photochemistry of alkyltricarbonyl(η5-cyclopentadienyl)tungsten (alkyl = Et, Prn, Pri, Bun, or CH2Ph), tricarbonyl(η5-cyclopentadienyl) (phenyl)tungsten, tricarbonyl(η5-pentamethylcyclopentadienyl)(n-propyl)tungsten, and tricarbonyl(η5-cyclopentadienyl)(ethyl)-molybdenum in gas matrices at 12 K and in solutions at 243 K. J. Chem. Soc. Dalton Trans. 1984, 175–186. [Google Scholar]
- Murray, R.C.; Blum, L.; Liu, A.H.; Schrock, R.R. Simple Routes to Mono(η5- pentamethylcyclopentadienyl) Complexes of Molybdenum(V) and Tungsten(V). Organometallics 1985, 4, 953–954. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT –Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXS97 and SHELXL97: Program for Crystal Structure Solution and Refinement; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.G.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; Caricato, M.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. A New Basis Set Exchange: An Open, up-to-date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- London, F.J. Quantum theory of interatomic currents in aromatic combinations. J. Phys. Radium 1937, 8, 397–409. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Chemcraft - Graphical Software for Visualization of Quantum Chemistry Computations. Version 1.8, Build 648. Available online: https://www.chemcraftprog.com (accessed on 31 May 2023).
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO Program 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Wiberg, K. Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).