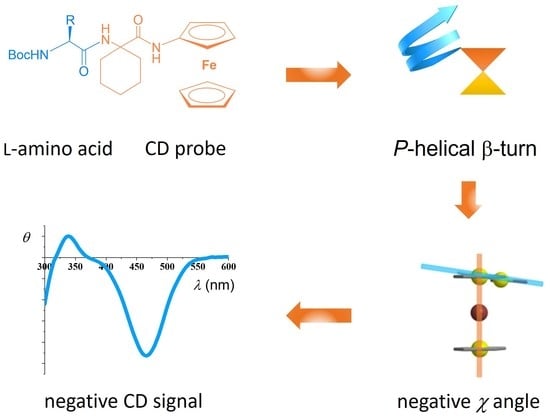
Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore
Abstract
1. Introduction
2. Results and Discussion
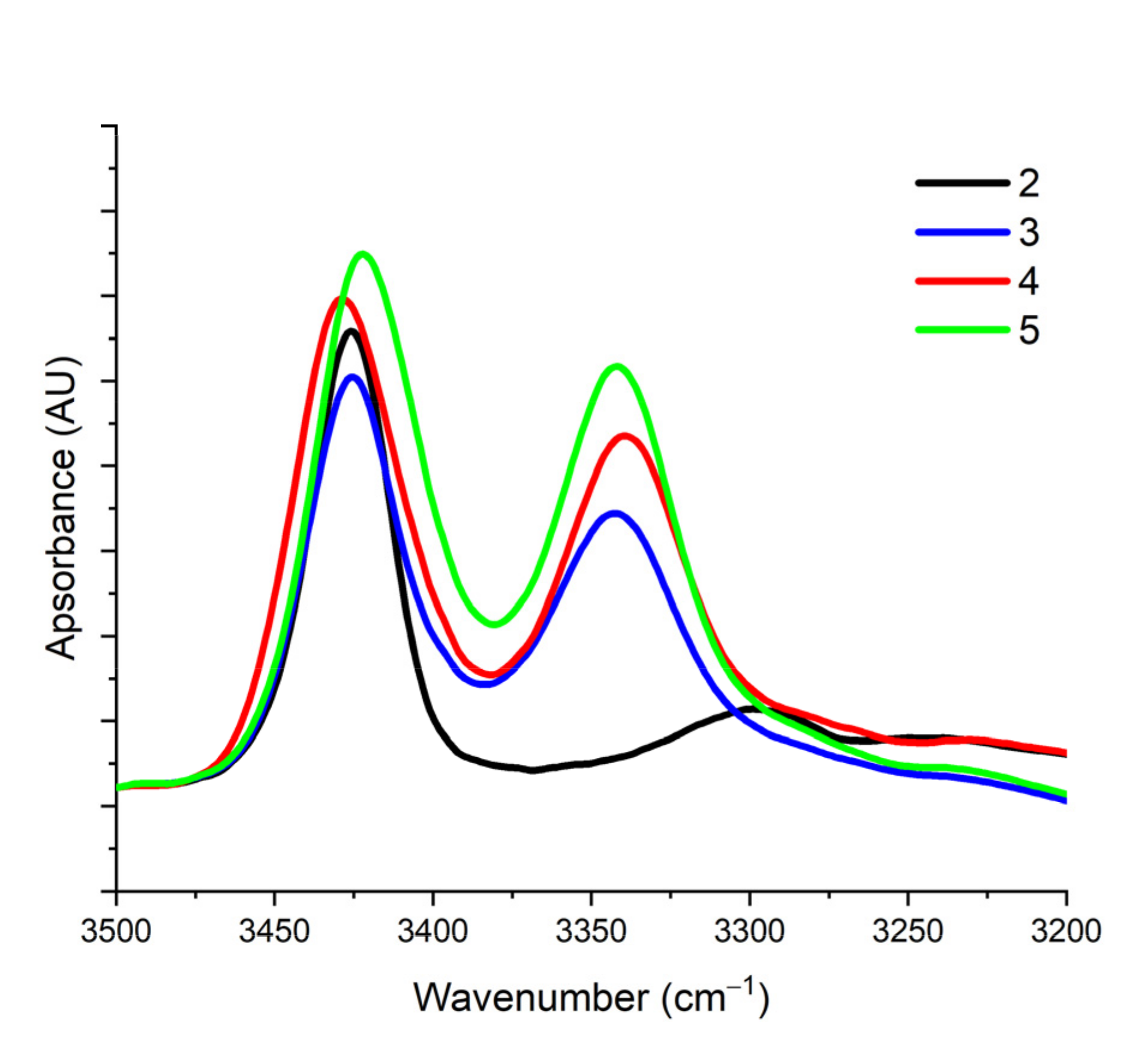
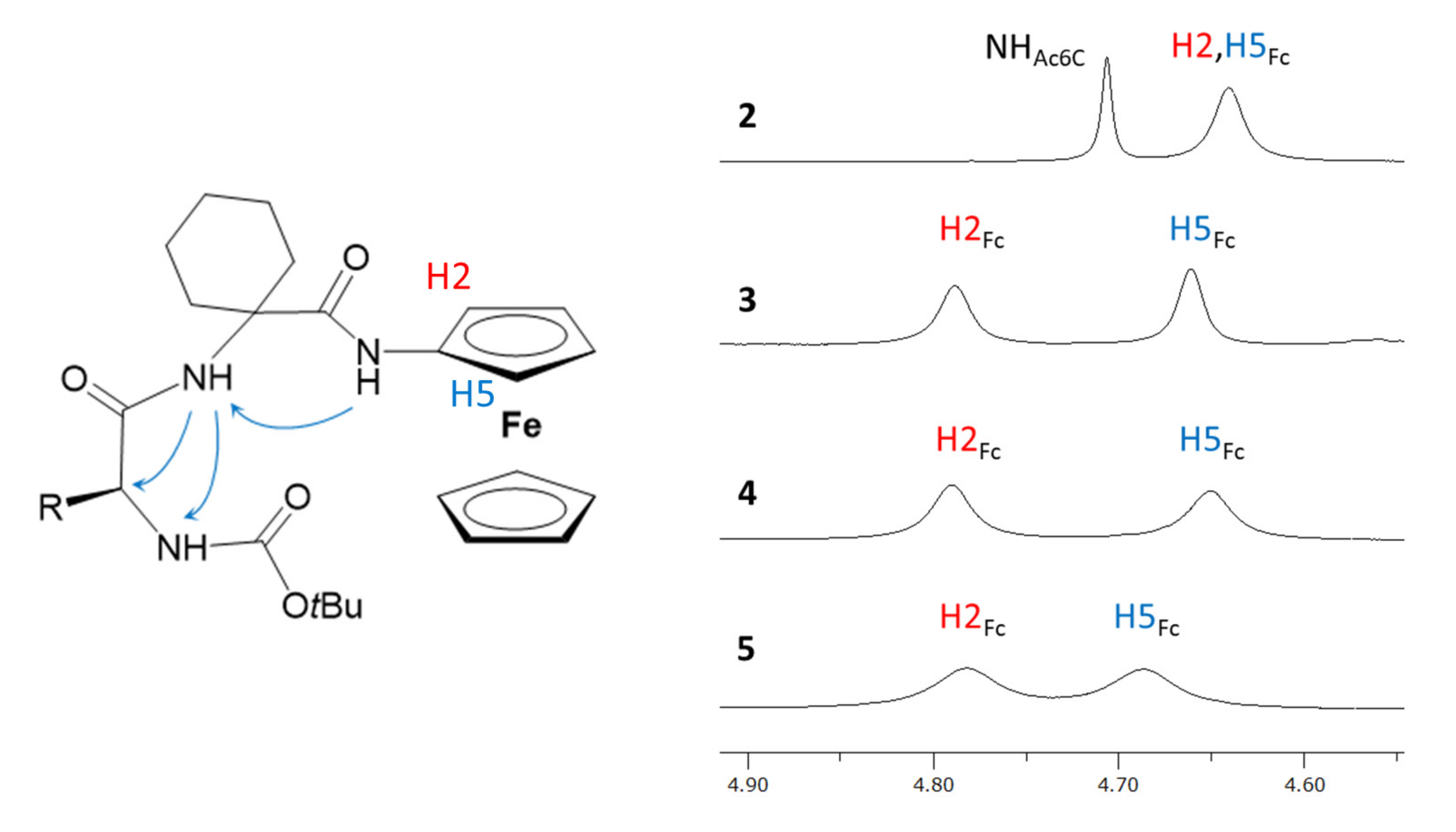
2.1. Infrared (IR) and NMR Studies
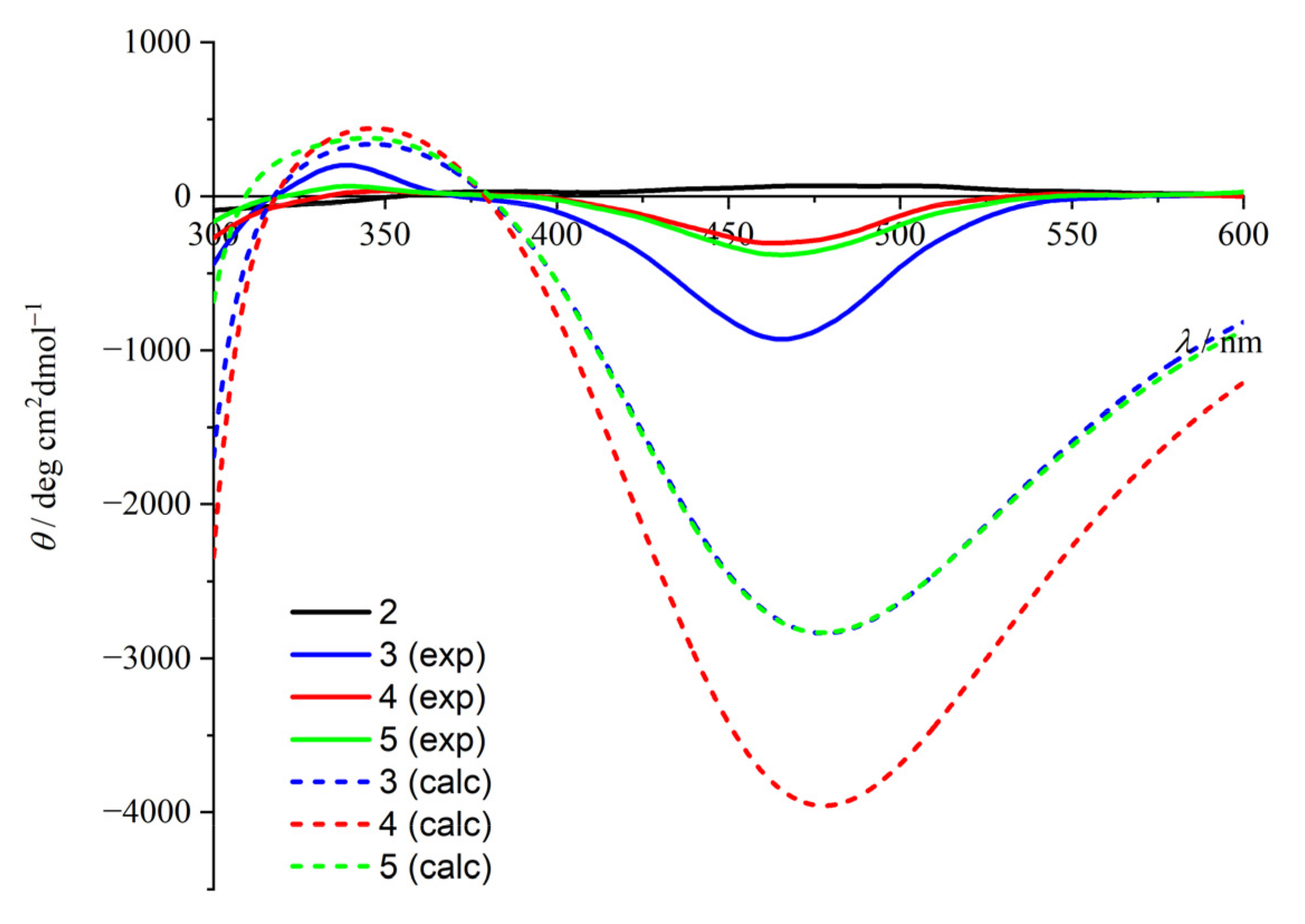
2.2. CD Studies
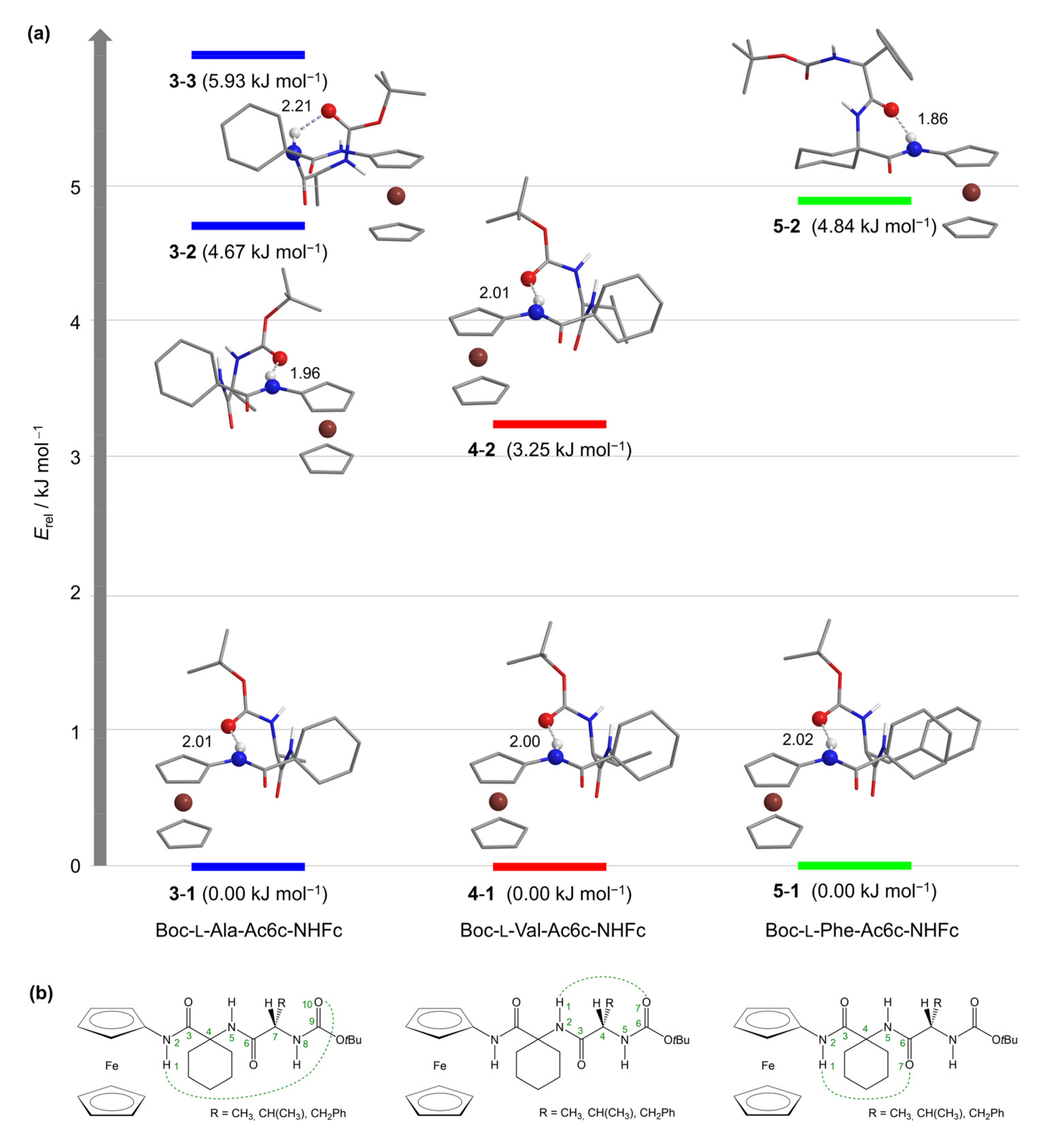
2.3. Computational Studies
3. Materials and Methods
3.1. General
3.2. Synthesis of Boc−Ac6c−NH−Fc (2) and Boc−AA−Ac6c−NH−Fc (3–5)
3.2.1. Boc–Ac6c–NH–Fc (2)
3.2.2. Boc–L–Ala–Ac6c–NH–Fc (3)
3.2.3. Boc–L–Val–Ac6c–NH–Fc (4)
3.2.4. Boc–L–Phe–Ac6c–NH–Fc (5)
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozcelik, A.; Pereira-Cameselle, R.; Poklar Ulrih, N.; Petrovic, A.G.; Alonso-Gómez, J.L. Chiroptical Sensing: A Conceptual Introduction. Sensors 2020, 20, 974. [Google Scholar] [CrossRef]
- Saito, F.; Schreiner, P.R. Determination of the Absolute Configurations of Chiral Alkanes—An Analysis of the Available Tools. Eur. J. Org. Chem. 2020, 2020, 6328–6339. [Google Scholar] [CrossRef]
- Herrera, B.T.; Pilicer, S.L.; Anslyn, E.V.; Joyce, L.A.; Wolf, C. Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc. 2018, 140, 10385–10401. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luoa, L.; Wei, W. Simultaneous determination of the concentration and enantiomeric excess of amino acids with a coumarin-derived achiral probe. Anal. Methods 2021, 13, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zha, D.; Ye, H.; Hai, Y.; Zhou, Y.; Anslyn, E.V.; You, L. Dynamic covalent chemistry within biphenyl scaffolds: Reversible covalent bonding, control of selectivity, and chirality sensing with a single system. Angew. Chem. Int. Ed. 2018, 57, 1300–1305. [Google Scholar] [CrossRef]
- Zong, Z.; Cao, Z.; Hao, A.; Xing, P. Dynamic axial chirality of ferrocene diamino acids: Hydration effects and chiroptical applications. J. Mater. Chem. C 2021, 9, 12191–12200. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Sripada, A.; Wolf, C. Quantitative Chiroptical Sensing of Free Amino Acids, Biothiols, Amines, and Amino Alcohols with an Aryl Fluoride Probe. J. Am. Chem. Soc. 2019, 141, 16382–16387. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Zandia, L.S.; Wolf, C. Chiroptical sensing of homocysteine. Org. Biomol. Chem. 2020, 18, 8629–8632. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Balaraman, K.; Wolf, C. Quantitative Chirality and Concentration Sensing of Alcohols, Diols, Hydroxy Acids, Amines and Amino Alcohols using Chlorophosphite Sensors in a Relay Assay. Angew. Chem. Int. Ed. 2020, 59, 21382–21386. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Wolf, C. Optical Terpene and Terpenoid Sensing: Chiral Recognition, Determination of Enantiomeric Composition and Total Concentration Analysis with Late Transition Metal Complexes. J. Am. Chem. Soc. 2020, 142, 4121–4125. [Google Scholar] [CrossRef]
- Hassan, D.S.; Thanzeel, F.Y.; Wolf, C. Stereochemical analysis of chiral amines, diamines, and amino alcohols: Practical chiroptical sensing based on dynamic covalent chemistry. Chirality 2020, 32, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.C.; De los Santos, Z.A.; Wolf, C. Chiroptical sensing of unprotected amino acids, hydroxy acids, amino alcohols, amines and carboxylic acids with metal salts. Chem. Commun. 2019, 55, 6297–6300. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Lynch, C.C.; Wolf, C. Dynamic Covalent Optical Chirality Sensing with a Sterically Encumbered Aminoborane. Chem. Eur. J. 2022, 28, e202202028. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.S.; De los Santos, Z.A.; Brady, K.G.; Murkli, S.; Isaacs, L.; Wolf, C. Chiroptical sensing of amino acids, amines, amino alcohols, alcohols and terpenes with π-extended acyclic cucurbiturils. Org. Biomol. Chem. 2021, 19, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Formen, J.S.S.K.; Wolf, C. Chiroptical Switching and Quantitative Chirality Sensing with (Pseudo)halogenated Quinones. Angew. Chem. Int. Ed. 2021, 60, 27031–27038. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Patti, A.; Pedotti, S.; Mazzeo, G.; Longhi, G.; Abbate, S.; Paoloni, L.; Bloino, J.; Rampino, S.; Barone, V. Ferrocenes with simple chiral substituents: An in-depth theoretical and experimental VCD and ECD study. Phys. Chem. Chem. Phys. 2019, 21, 9419–9432. [Google Scholar] [CrossRef]
- Brahma, S.; Ikbal, S.S.; Dhamija, A.; Prasad Rath, S. Highly Enhanced Bisignate Circular Dichroism of Ferrocene-Bridged Zn(II) Bisporphyrin Tweezer with Extended Chiral Substrates due to Well-Matched Host–Guest System. Inorg. Chem. 2014, 53, 2381–2395. [Google Scholar] [CrossRef]
- Adhikari, B.; Kraatz, H.-B. Redox-triggered changes in the self-assembly of a ferrocene–peptide conjugate. Chem. Commun. 2014, 50, 5551–5553. [Google Scholar] [CrossRef]
- Moriuchi, T.; Hirao, T. Dipeptide-induced chirality organization. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 23–40. [Google Scholar] [CrossRef]
- Kirin, S.I.; Wissenbach, D.; Metzler-Nolte, N. Unsymmetrical 1,n′-disubstituted ferrocenoyl peptides: Convenient one pot synthesis and solution structures by CD and NMR spectroscopy. New J. Chem. 2005, 29, 1168–1173. [Google Scholar] [CrossRef]
- Kovačević, M.; Kodrin, I.; Cetina, M.; Kmetič, I.; Murati, T.; Čakić Semenčić, M.; Roca, S.; Barišić, L. The conjugates of ferrocene-1,1′-diamine and amino acids. A novel synthetic approach and conformational analysis. Dalton Trans. 2015, 44, 16405–16420. [Google Scholar] [CrossRef] [PubMed]
- Čakić Semenčić, M.; Siebler, D.; Heinze, K.; Rapić, V. Bis- and Trisamides Derived From 1′-Aminoferrocene-1-carboxylic Acid and α-Amino Acids: Synthesis and Conformational Analysis. Organometallics 2009, 28, 2028–2037. [Google Scholar] [CrossRef]
- Herrick, R.S.; Jarret, R.M.; Curran, T.P.; Dragoli, D.R.; Flaherty, M.B.; Lindyberg, S.E.; Slate, R.A.; Thornton, L.C. Ordered conformations in bis(amino acid) derivatives of 1,1′-ferrocenedicarboxylic acid. Tetrahedron Lett. 1996, 37, 5289–5292. [Google Scholar] [CrossRef]
- Kovač, V.; Čakić Semenčić, M.; Kodrin, I.; Roca, S.; Rapić, V. Ferrocene-dipeptide conjugates derived from aminoferrocene and 1-acetyl-1′-aminoferrocene: Synthesis and conformational studies. Tetrahedron 2013, 69, 10497–10506. [Google Scholar] [CrossRef]
- Čakić Semenčić, M.; Kodrin, I.; Barišić, L.; Nuskol, M.; Meden, A. Synthesis and Conformational Study of Monosubstituted Aminoferrocene- Based Peptides Bearing Homo-and Heterochiral Pro-Ala Sequences. Eur. J. Inorg. Chem. 2017, 2017, 306–317. [Google Scholar] [CrossRef]
- Nuskol, M.; Studen, B.; Meden, A.; Kodrin, I.; Čakić Semenčić, M. Tight Turn in Dipeptide Bridged Ferrocenes: Synthesis, X-Ray Structural, Theoretical and Spectroscopic Studies. Polyhedron 2019, 161, 137–144. [Google Scholar] [CrossRef]
- Nuskol, M.; Šutalo, P.; Đaković, M.; Kovačević, M.; Kodrin, I.; Čakić Semenčić, M. Testing the Potential of the Ferrocene Chromophore as a Circular Dichroism Probe for the Assignment of the Screw-Sense Preference of Tripeptides. Organometallics 2021, 40, 1351–1362. [Google Scholar] [CrossRef]
- Nuskol, M.; Šutalo, P.; Kodrin, I.; Čakić Semenčić, M. Sensing of the Induced Helical Chirality by the Chiroptical Response of the Ferrocene Chromophore. Eur. J. Inorg. Chem. 2022, 2022, e202100880. [Google Scholar] [CrossRef]
- Venkatraman, J.; Shankaramma, S.C.; Balaram, P. Design of folded peptides. Chem. Rev. 2001, 101, 3131–3152. [Google Scholar] [CrossRef]
- Clayden, J.; Castellanos, A.; Solà, J.; Morris, G.A. Quantifying End-to-End Conformational Communication of Chirality through an Achiral Peptide Chain. Angew. Chem. Int. Ed. 2009, 48, 5962–5965. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, C.; Crisma, M.; Formaggio, F.; Peggion, C. Control of peptide conformation by the Thorpe-Ingold effect (C alpha-tetrasubstitution). Biopolymers 2001, 60, 396–419. [Google Scholar] [CrossRef] [PubMed]
- Barišić, L.; Čakić, M.; Mahmoud, K.A.; Liu, Y.-N.; Kraatz, H.-B.; Pritzkow, H.; Kirin, S.I.; Metzler-Nolte, N.; Rapić, V. Helically Chiral Ferrocene Peptides Containing 1′-Aminoferrocene-1-Carboxylic Acid Subunits as Turn Inducers. Chem. Eur. J. 2006, 12, 4965–4980. [Google Scholar] [CrossRef]
- Hirata, T.; Ueda, A.; Oba, M.; Doi, M.; Demizu, Y.; Kurihara, M.; Nagano, M.; Suemune, H.; Tanaka, M. Amino equatorial effect of a six-membered ring amino acid on its peptide 310- and α-helices. Tetrahedron 2015, 71, 2409–2420. [Google Scholar] [CrossRef]
- Doi, M.; Asano, A.; Komura, E.; Ueda, Y. The structure of an endomorphin analogue incorporating 1-aminocyclohexane-1-carboxlylic acid for proline is similar to the β-turn of Leu-enkephalin. Biochemical. Biophysical. Res. Commun. 2002, 297, 138–142. [Google Scholar] [CrossRef]
- Fabiano, N.; Valle, G.; Crisma, M.; Toniolo, C.; Saviano, M.; Lombardi, A.; Isernia, C.; Pavone, V.; Di Blasio, B.; Pedone, C.; et al. Conformational versatility of the Nα-acylated tripeptide amide tail of oxytocin. Int. J. Pept. Protein Res. 1993, 42, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, V.S.; Cameron, T.S. Proline-containing β-turns. Int. J. Pept. Protein Res. 1988, 31, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Jayakumar, R. Role of N-t-Boc group in helix initiation in a novel tetrapeptide. J. Pept. Res. 2002, 59, 249–256. [Google Scholar] [CrossRef]
- Wuthrich, K. NMR of Proteins and Nucleic Acids; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Kovačević, M.; Čakić Semenčić, M.; Kodrin, I.; Roca, S.; Perica, J.; Mrvčić, J.; Stanzer, D.; Molčanov, K.; Milašinvić, V.; Brkljačić, L.; et al. Biological Evaluation and Conformational Preferences of Ferrocene Dipeptides with Hydrophobic Amino Acids. Inorganics 2023, 11, 29. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Schrödinger LLC. MacroModel; Schrödinger LLC: New York, NY, USA, 2019. [Google Scholar]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Keith, T.A. AIMAll; Version 19.02.13; TK Gristmill Software: Overland Park, KS, USA, 2017. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuskol, M.; Šutalo, P.; Kovačević, M.; Kodrin, I.; Čakić Semenčić, M. Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore. Inorganics 2023, 11, 225. https://doi.org/10.3390/inorganics11060225
Nuskol M, Šutalo P, Kovačević M, Kodrin I, Čakić Semenčić M. Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore. Inorganics. 2023; 11(6):225. https://doi.org/10.3390/inorganics11060225
Chicago/Turabian StyleNuskol, Marko, Petar Šutalo, Monika Kovačević, Ivan Kodrin, and Mojca Čakić Semenčić. 2023. "Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore" Inorganics 11, no. 6: 225. https://doi.org/10.3390/inorganics11060225
APA StyleNuskol, M., Šutalo, P., Kovačević, M., Kodrin, I., & Čakić Semenčić, M. (2023). Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore. Inorganics, 11(6), 225. https://doi.org/10.3390/inorganics11060225