Ferrocene-Based Electrochemical Sensors for Cations
Abstract
1. Introduction
2. Why Ferrocene?
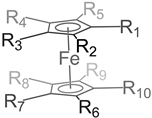 | Solvent | Electrolyte | Em vs. Fc0/+ (V) | Ref |
|---|---|---|---|---|
| R1–10: H | DCM | 0.1 M [Bu4N][ClO4] | 0.000 | [2] |
| R1–10: CH3 | DCM | 0.1 M [Bu4N][ClO4] | −0.570 | [2] |
| R1–5: CH3; R6–10: H | DCM | 0.1 M [Bu4N][ClO4] | −0.270 | [2] |
| R1,10: CH3; R2–9: H | CH3CN | 0.1 M [Bu4N][ClO4] | −0.113 | [39] |
| CH3CN | 0.1 M [Bu4N][PF6] | −0.096 | [40] | |
| MeOH | 0.1 M [Bu4N][ClO4] | −0.104 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | −0.075 | [39] | |
| R2–9: CH3; R1,10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.406 | [40] |
| R1,3,7,10: t-Bu; R2,4,5,6,8,9: H | CH3CN | 0.1 M [Bu4N][ClO4] | −0.238 | [39] |
| CH3CN | 0.1 M [Bu4N][PF6] | −0.233 | [40] | |
| MeOH | 0.1 M [Bu4N][ClO4] | −0.229 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | −0.097 | [39] | |
| R1: n-Bu; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | −0.062 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | −0.055 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | −0.073 | [39] | |
| R1–10: CH2Ph | DCM | 0.1 M [Bu4N][ClO4] | −0.070 | [2] |
| R1,10: CF3; R2–9: H | DCM | 0.1 M [Bu4N][ClO4] | 0.640 | [2] |
| R1: CH=CH2; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.022 | [41] |
| R1: CH2OH; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.029 | [41] |
| CH3CN | 0.1 M [Bu4N][ClO4] | −0.012 | [39] | |
| CH3CN | 0.1 M [Bu4N][PF6] | 0.016 | [38] | |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.005 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | −0.044 | [39] | |
| R1: (CH2)2OH; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.046 | [38] |
| R1: (CH2)3OH; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.052 | [38] |
| R1: (CH2)4OH; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.054 | [38] |
| R1: CH(CH3)OH; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | −0.008 | [41] |
| R1,10: CH(CH3)OH; R2–9: H | CH3CN | 0.1 M [Et4N][ClO4] | −0.013 | [41] |
| R1: CH2CONH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.003 | [38] |
| R1: (CH2)2CONH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.027 | [38] |
| R1: (CH2)3CONH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.049 | [38] |
| R1: COOH; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.239 | [41] |
| CH3CN | 0.1 M [Bu4N][ClO4] | 0.234 | [39] | |
| CH3CN | 0.1 M [Li][ClO4] | 0.239 | [37] | |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.233 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.157 | [39] | |
| R1: CH2COOH; R2–10: H | CH3CN | 0.1 M [Li][ClO4] | −0.006 | [37] |
| R1: (CH2)2COOH; R2–10: H | CH3CN | 0.1 M [Li][ClO4] | −0.022 | [37] |
| R1: (CH2)3COOH; R2–10: H | CH3CN | 0.1 M [Li][ClO4] | −0.047 | [37] |
| R1: COOCH3; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.243 | [41] |
| CH3CN | 0.1 M [Bu4N][ClO4] | 0.237 | [39] | |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.263 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.214 | [39] | |
| R1,10: COOCH3; R2–9: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.470 | [41] |
| R1: COCH3; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.244 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.271 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.191 | [39] | |
| R1,10: COCH3; R2–9: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.482 | [41] |
| R1: COPh; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.250 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.272 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.214 | [39] | |
| R1: CONH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | 0.183 | [38] |
| R1: CHO; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.285 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.304 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.259 | [39] | |
| R1: CH2NH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.014 | [38] |
| R1: (CH2)2NH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.037 | [38] |
| R1: (CH2)3NH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.046 | [38] |
| R1: (CH2)4NH2; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.060 | [38] |
| R1: CH2N(CH3)2; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | −0.004 | [39] |
| MeOH | 0.1 M [Bu4N][ClO4] | 0.046 | [39] | |
| Toluene | 0.5 M [Hex4N][ClO4] | 0.009 | [39] | |
| CH3CN | 0.1 M [Bu4N][PF6] | −0.023 | [42] | |
| DCM:CH3CN 1:4 | 0.1 M [Bu4N][PF6] | 0.003 | [7] | |
| R1,10: CH2N(CH3)2; R2–9: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.017 | [42] |
| R1,10: (CH2)2N(CH3)2; R2–9: H | CH3CN | 0.1 M [Bu4N][PF6] | −0.077 | [42] |
| R1,10: CH2N(CH2Ph)2; R2–9: H | DCM | 0.2 M [Bu4N][PF6] | −0.001 | [9] |
| R1,10: C(CH3)=N(CH2)5CH3; R2–9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.211 | [43] |
R1,10: 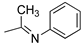 ; R2–9: H ; R2–9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.289 | [43] |
R1,10: 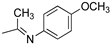 ; R2–9: H ; R2–9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.245 | [43] |
R1,10:  ; R2–9: H ; R2–9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.261 | [43] |
R1,10: 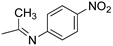 ; R2–9: H ; R2–9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.435 | [43] |
R1,10: 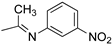 ; R2–9: H ; R2–9: H | DCM:CH3CN 1:1 | 0.1 M [Bu4N][PF6] | 0.390 | [43] |
| R1,10: SH; R2–9: H | DCM | 0.1 M [Bu4N][ClO4] | 0.200 | [2] |
| R1: S(CH2)2OH; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.010 | [41] |
| R1: SCH2CH(CH3)COOH; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.039 | [41] |
| R1: CH(CH3)SPh; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.020 | [41] |
| R1: CH(Ph)SPh; R2–10: H | CH3CN | 0.1 M [Et4N][ClO4] | 0.043 | [41] |
R1: 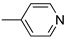 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.180 | [44] |
R1,10: 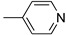 ; R2–9: H ; R2–9: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.350 | [44] |
R1: 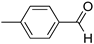 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.170 | [44] |
R1,10: 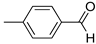 ; R2–9: H ; R2–9: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.180 | [44] |
R1: 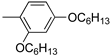 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.020 | [44] |
R1: 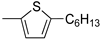 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.090 | [44] |
R1: 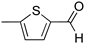 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.130 | [44] |
R1: 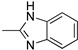 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.075 | [45] |
R1:  ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.085 | [45] |
R1,10: 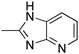 ; R2–9: H ; R2–9: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.205 | [45] |
R1,10: 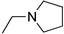 ; R2–9: H ; R2–9: H | DCM | 0.2 M [Bu4N][PF6] | 0.002 | [9] |
R1,10:  ; R2–9: H ; R2–9: H | DCM | 0.2 M [Bu4N][PF6] | −0.001 | [9] |
R1,10: 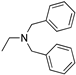 ; R2–9: H ; R2–9: H | DCM | 0.2 M [Bu4N][PF6] | −0.001 | [9] |
R1: 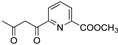 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][ClO4] | 0.366 | [45] |
R1: 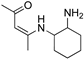 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [Bu4N][PF6] | 0.100 | [46] |
R1: 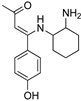 ; R2–10: H ; R2–10: H | CH3CN | 0.1 M [BuN][PF6] | 0.120 | [46] |
3. Ferrocene-Based Electrochemical Sensors Using Oxygen-Containing Host Molecules
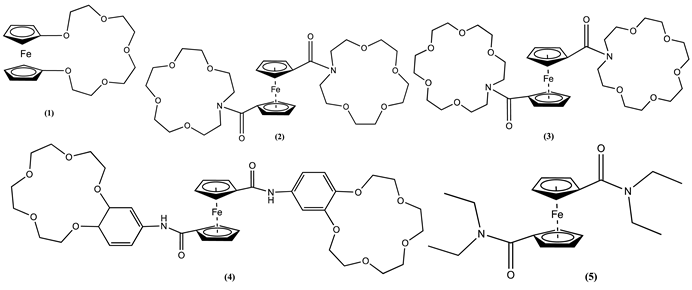
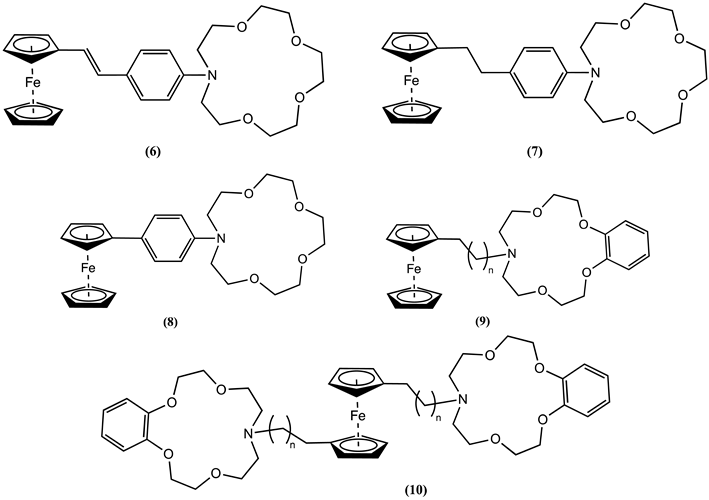

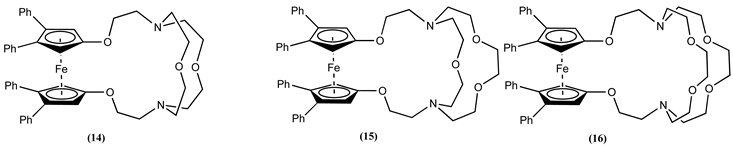
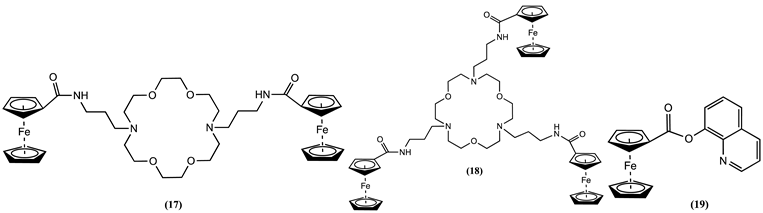
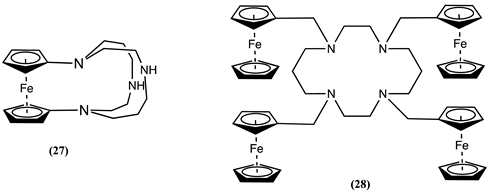
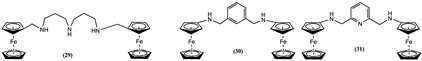
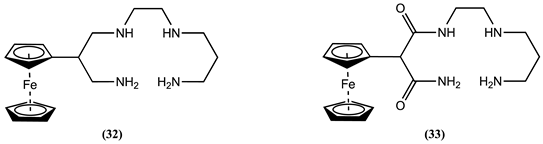
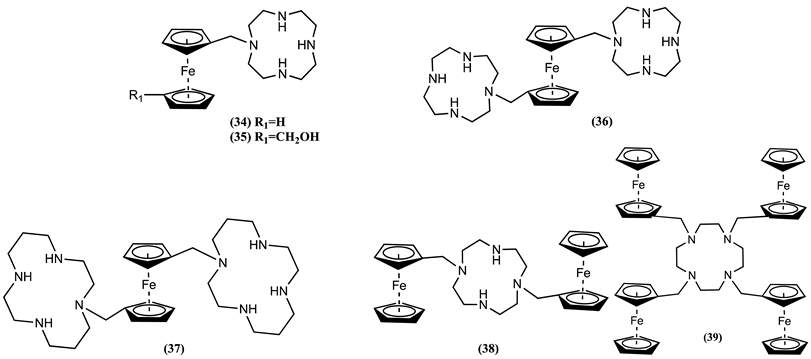
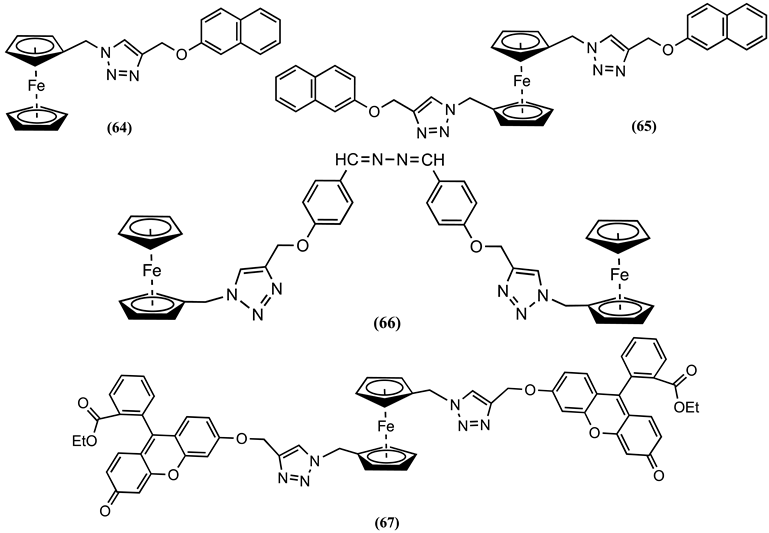
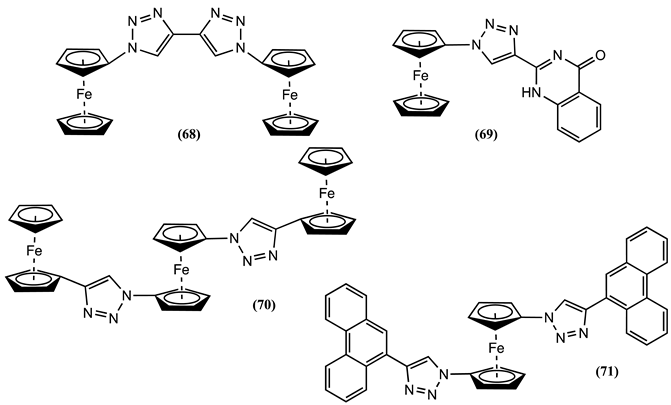
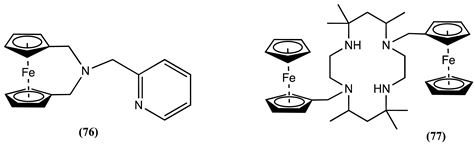
4. Ferrocene-Based Electrochemical Sensors Using Nitrogen-Containing Host Molecules
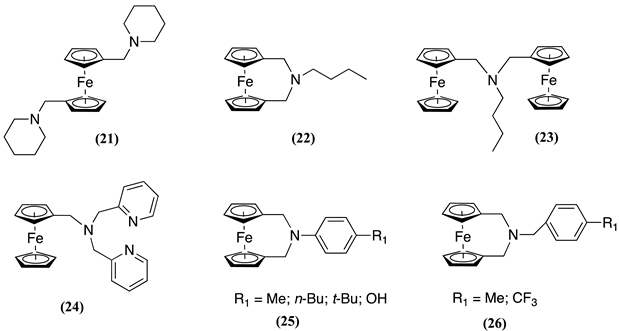




5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.M. Supramolecular Chemistry; Wiley-VCH: New York, NY, USA, 1995; pp. I–X. [Google Scholar]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Molina, P.; Tárraga, A.; Caballero, A. Ferrocene-Based Small Molecules for Multichannel Molecular Recognition of Cations and Anions. Eur. J. Inorg. Chem. 2008, 2008, 3401–3417. [Google Scholar] [CrossRef]
- Togni, A.; Hayashi, T. (Eds.) Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science; VCH: New York, NY, USA, 1995. [Google Scholar]
- Casado, C.M.; Alonso, B.; García-Armada, M.P. 7.02—Ferrocenes and Other Sandwich Complexes of Iron. In Comprehensive Organometallic Chemistry IV; Parkin, G., Meyer, K., O′hare, D., Eds.; Elsevier: Oxford, UK, 2022; pp. 3–45. [Google Scholar]
- Stepnicka, P. (Ed.) Ferrocenes: Ligands, Materials and Biomolecules; John Wiley & Sons, Ltd.: Chichester, UK, 2008. [Google Scholar]
- Torriero, A.A.J.; Zeng, Z.; Mruthunjaya, A.K.V.; Bond, A.M. Electrochemical Properties of Cyclen and Cyclam Macrocycles Bearing Ferrocenyl Pendants and Their Transition Metal Complexes. J. Electroanal. Chem. 2023, 945, 117687. [Google Scholar] [CrossRef]
- Milaeva, E.R.; Tyurin, V.Y.; Shpakovsky, D.B.; Moiseeva, A.A.; Gracheva, Y.A.; Antonenko, T.A.; Maduar, V.V.; Osolodkin, D.I.; Palyulin, V.A.; Shevtsova, E.F. Redox-active metal complexes with 2,2′-dipicolylamine containing ferrocenyl moiety: Synthesis, electrochemical behavior and biological activity. J. Organomet. Chem. 2017, 839, 60–70. [Google Scholar] [CrossRef]
- Dwadnia, N.; Allouch, F.; Pirio, N.; Roger, J.; Cattey, H.; Fournier, S.; Penouilh, M.-J.; Devillers, C.H.; Lucas, D.; Naoufal, D.; et al. Aminomethyl-Substituted Ferrocenes and Derivatives: Straightforward Synthetic Routes, Structural Characterization, and Electrochemical Analysis. Organometallics 2013, 32, 5784–5797. [Google Scholar] [CrossRef]
- Osakada, K.; Sakano, T.; Horie, M.; Suzaki, Y. Functionalized ferrocenes: Unique properties based on electronic communication between amino group of the ligand and Fe center. Coord. Chem. Rev. 2006, 250, 1012–1022. [Google Scholar] [CrossRef]
- Fabbrizzi, L. The ferrocenium/ferrocene couple: A versatile redox switch. ChemTexts 2020, 6, 22. [Google Scholar] [CrossRef]
- Bayly, S.R.; Beer, P.D.; Chen, G.Z. Ferrocene Sensors. In Ferrocenes: Ligands, Materials and Biomolecules; Stepnicka, P., Ed.; John Wiley & Sons: Chichester, UK, 2008; pp. 281–318. [Google Scholar]
- Noviandri, I.; Brown, K.N.; Fleming, D.S.; Gulyas, P.T.; Lay, P.A.; Masters, A.F.; Phillips, L. The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer. J. Phys. Chem. B 1999, 103, 6713–6722. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L. Theoretical investigations of ferrocene/ferrocenium solvation in imidazolium-based room-temperature ionic liquids. PCCP Phys. Chem. Chem. Phys. 2013, 15, 2669–2683. [Google Scholar] [CrossRef]
- Torriero, A.A.J. Characterization of decamethylferrocene and ferrocene in ionic liquids: Argon and vacuum effect on their electrochemical properties. Electrochim. Acta 2014, 137, 235–244. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Sunarso, J.; Forsyth, M.; Pozo-Gonzalo, C. Assessment of permethylated transition-metal sandwich complexes as internal reference redox systems in ionic liquids. PCCP Phys. Chem. Chem. Phys. 2013, 15, 2547–2553. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Howlett, P.C. Ionic liquid effects on the redox potential of ferrocene. Electrochem. Commun. 2012, 16, 84–87. [Google Scholar] [CrossRef]
- Torriero, A.A.; Sunarso, J.; Howlett, P.C. Critical evaluation of reference systems for voltammetric measurements in ionic liquids. Electrochim. Acta 2012, 82, 60–68. [Google Scholar] [CrossRef]
- Torriero, A.A.J. Reference systems for voltammetric measurements in ionic liquids. In Electrochemistry in Ionic Liquids. Volume 1: Fundamentals; Torriero, A.A.J., Ed.; Springer: Cham, Switzerland, 2015; Volume 1. [Google Scholar]
- Torriero, A.A.J. On Choosing Ferrocene as an Internal Reference Redox Scale for Voltammetric Measurements: A Cautionary Tale. Med. Anal. Chem. Int. J. 2019, 3, 000151. [Google Scholar] [CrossRef]
- Shvartsev, B.; Cohn, G.; Shasha, H.; Eichel, R.-A.; Ein-Eli, Y. Reference electrode assembly and its use in the study of fluorohydrogenate ionic liquid silicon electrochemistry. PCCP Phys. Chem. Chem. Phys. 2013, 15, 17837–17845. [Google Scholar] [CrossRef] [PubMed]
- Inzelt, G.; Lewenstam, A.; Scholz, F. Handbook of Reference Electrodes; Springer: Berlin, Germany, 2013. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Izutzu, K. Electrochemistry in Nonaqueous Solutions; Wiley-VCH: New York, NY, USA, 2002. [Google Scholar]
- Smith, T.J.; Stevenson, K.J. Reference electrodes. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Natherlands, 2007; pp. 73–110. [Google Scholar]
- Ives, D.J.G.; Janz, G.J.; Ives, D.J.D. Reference Electrodes, Theory and Practice; Academic Press: London, UK, 1961. [Google Scholar]
- Torriero, A.A.J. Understanding the Differences between a Quasi-Reference Electrode and a Reference Electrode. Med. Anal. Chem. Int. J. 2019, 3, 000144. [Google Scholar] [CrossRef]
- Gritzner, G.; Kuta, J. Recommendations on reporting electrode potentials in nonaqueous solvents. Pure Appl. Chem. 1983, 56, 461–466. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Feldberg, S.; Zhang, J.; Simonov, A.; Bond, A. On choosing a reference redox system for electrochemical measurements: A cautionary tale. J. Solid State Electrochem. 2013, 17, 3021–3026. [Google Scholar] [CrossRef]
- Aranzaes, J.R.; Daniel, M.-C.; Astruc, D. Metallocenes as references for the determination of redox potentials by cyclic voltammetry—Permethylated iron and cobalt sandwich complexes, inhibition by polyamine dendrimers, and the role of hydroxy-containing ferrocenes. Can. J. Chem. 2006, 84, 288–299. [Google Scholar] [CrossRef]
- Ruiz, J.; Astruc, D. Permethylated electron-reservoir sandwich complexes as references for the determination of redox potentials. Suggestion of a new redox scale. Comptes Rendus De L′academie Des Sci. Ser. IIc Chim. 1998, 1, 21–27. [Google Scholar] [CrossRef]
- Matsumoto, M.; Swaddle, T.W. The Decamethylferrocene(+/0) Electrode Reaction in Organic Solvents at Variable Pressure and Temperature. Inorg. Chem. 2004, 43, 2724–2735. [Google Scholar] [CrossRef]
- Freyberg, D.P.; Robbins, J.L.; Raymond, K.N.; Smart, J.C. Crystal and molecular structures of decamethylmanganocene and decamethylferrocene. Static Jahn-Teller distortion in a metallocene. J. Am. Chem. Soc. 1979, 101, 892–897. [Google Scholar] [CrossRef]
- Barriere, F.; Geiger, W.E. Use of Weakly Coordinating Anions to Develop an Integrated Approach to the Tuning of ∆E1/2 Values by Medium Effects. J. Am. Chem. Soc. 2006, 128, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.A.; Bhargava, S.K.; Bond, A.M.; Burgar, I.M.; Guo, S.X.; Kar, G.; Priver, S.H.; Wagler, J.; Willis, A.C.; Torriero, A.A.J. Synthesis, X-ray structure and electrochemical oxidation of palladium(II) complexes of ferrocenyldiphenylphosphine. Dalton Trans. 2010, 39, 9079–9090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, Z.; Torriero, A.A.J.; Belousoff, M.J.; Bond, A.M.; Spiccia, L. Synthesis, X-ray Structure of Ferrocene Bearing bis(Zn-cyclen) Complexes and the Selective Electrochemical Sensing of TpT. Chemistry 2009, 15, 10988–10996. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.F.; Neuse, E.W.; Thomas, H.G. Electrochemical characterization of some ferrocenylcarboxylic acids. Transit. Met. Chem. 1987, 12, 301–306. [Google Scholar] [CrossRef]
- Nonjola, P.T.N.; Siegert, U.; Swarts, J.C. Synthesis, Electrochemistry and Cytotoxicity of Ferrocene-Containing Amides, Amines and Amino-Hydrochlorides. J. Inorg. Organomet. Polym. Mater. 2015, 25, 376–385. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Matsumura-Inoue, T.; Ichimura, A. Solvent effect on the redox potential of ferrocene derivatives using an ultramicroelectrode. Anal. Sci. 1992, 8, 877–879. [Google Scholar] [CrossRef]
- Paul, A.; Borrelli, R.; Bouyanfif, H.; Gottis, S.; Sauvage, F. Tunable Redox Potential, Optical Properties, and Enhanced Stability of Modified Ferrocene-Based Complexes. ACS Omega 2019, 4, 14780–14789. [Google Scholar] [CrossRef]
- Scholl, H.; Sochaj, K. Cyclic voltammetry of some ferrocenophanes in acetonitrile. Electrochim. Acta 1991, 36, 689–694. [Google Scholar] [CrossRef]
- Plenio, H.; Yang, J.; Diodone, R.; Heinze, J. Redox-Switched Bonding of Protons to Ferrocenophanes, Ferrocene Cryptands, and Simple Ferrocene Amines. Correlation of X-ray Structural Data and Cyclic Voltammetry Derived Redox Potentials. Inorg. Chem. 1994, 33, 4098–4104. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Chiang, P.-R.; Tsai, M.-C.; Lin, C.-Y.; Huang, J.-H. From diacetylferrocene to 1,1′-ferrocenyldiimines: Substituent effects on synthesis, molecular structure, electrochemical behavior and optical absorption property. J. Mol. Struct. 2009, 935, 102–109. [Google Scholar] [CrossRef]
- Manfredi, N.; Decavoli, C.; Boldrini, C.L.; Coluccini, C.; Abbotto, A. Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties. Energies 2020, 13, 3937. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; Tárraga, A.; Molina, P. Imidazole-Annelated Ferrocene Derivatives as Highly Selective and Sensitive Multichannel Chemical Probes for Pb(II) Cations. J. Org. Chem. 2009, 74, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Celedón, S.; Hamon, P.; Artigas, V.; Fuentealba, M.; Kahlal, S.; Carrillo, D.; Saillard, J.-Y.; Hamon, J.-R.; Manzur, C. Ferrocene functionalized enantiomerically pure Schiff bases and their Zn(II) and Pd(II) complexes: A spectroscopic, crystallographic, electrochemical and computational investigation. New J. Chem. 2022, 46, 3948–3960. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A.; Chen, G.Z. Electrochemical molecular recognition: Pathways between complexation and signalling. J. Chem. Soc. Dalton Trans. 1999, 1897–1910. [Google Scholar] [CrossRef]
- Beer, P.D.; Danks, J.P.; Hesek, D.; McAleer, J.F. A potassium-selective sulfide-linked redox-active ferrocene ionophore that exhibits extraordinary electrochemical recognition behaviour. J. Chem. Soc. Chem. Commun. 1993, 1735–1737. [Google Scholar] [CrossRef]
- Beer, P.D. Transition Metal and Organic Redox-Active Macrocycles Designed to Electrochemically Recognize Charged and Neutral Guest Species. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1992; Volume 39, pp. 79–157. [Google Scholar]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Lindoy, L.F. The Chemistry of Macrocyclic Ligand Complexes; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Dobler, M. Macrocyclic chemistry: Aspects of organic and inorganic supramolecular chemistry by B. Dietrich, P. Viout and J.-M. Lehn. Acta Crystallogr. Sect. B 1993, 49, 1074. [Google Scholar] [CrossRef]
- Beer, P.D.; Keefe, A.D.; Sikanyika, H.; Blackburn, C.; McAleer, J.F. Metallocene bis(aza-crown ether) ligands and related compounds. Their syntheses, coordination chemistry, and electrochemical properties. J. Chem. Soc. Dalton Trans. 1990, 3289–3294. [Google Scholar] [CrossRef]
- Saji, T.; Kinoshita, I. Electrochemical ion transport with ferrocene functionalized crown ether. J. Chem. Soc. Chem. Commun. 1986, 716–717. [Google Scholar] [CrossRef]
- Beer, P.D.; Sikanyika, H.; Slawin, A.M.Z.; Williams, D.J. The synthesis, coordination and electrochemical studies of metallocene bis(crown ether) receptor molecules. Single-crystal x-ray structure of a ferrocene bis(crown ether) potassium complex. Polyhedron 1989, 8, 879–886. [Google Scholar] [CrossRef]
- Saji, T. Electrochemically switched cation binding in pentaoxa [13] ferrocenophane. Chem. Lett. 1986, 15, 275–276. [Google Scholar] [CrossRef]
- Beer, P.D.; Sikanyika, H.; Blackburn, C.; McAleer, J.F.; Drew, M.G.B. Redox responsive crown ethers containing a direct link between the ferrocene redox-active centre and benzo crown ether. Crystal structure of a ferrocene benzo-15-crown-5 sodium complex. J. Organomet. Chem. 1988, 356, C19–C22. [Google Scholar] [CrossRef]
- Beer, P.D.; Blackburn, C.; McAleer, J.F.; Sikanyika, H. Redox-responsive crown ethers containing a conjugated link between the ferrocene moiety and a benzo crown ether. Inorg. Chem. 1990, 29, 378–381. [Google Scholar] [CrossRef]
- Jin, S.; Wang, D.; Jin, X.; Chen, G.Z. Intramolecular Electrostatics: Coulomb’s Law at Sub-Nanometers. ChemPhysChem 2004, 5, 1623–1629. [Google Scholar] [CrossRef]
- Hall, C.D. Macrocycles and Cryptands Containing the Ferrocene Unit. In Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Materials Science; Togni, A., Hayashi, T., Eds.; VCH: New York, NY, USA, 1995; pp. 279–316. [Google Scholar]
- Hall, C.D.; Sharpe, N.W.; Danks, I.P.; Sang, Y.P. Cyclic voltammetry studies on the complexation of metal cations by cryptands containing the ferrocene unit. J. Chem. Soc. Chem. Commun. 1989, 419–421. [Google Scholar] [CrossRef]
- Dennis Hall, C.; Chu, S.Y.F. Cyclic voltammetry of cryptands and cryptates containing the ferrocene unit. J. Organomet. Chem. 1995, 498, 221–228. [Google Scholar] [CrossRef]
- Medina, J.C.; Goodnow, T.T.; Rojas, M.T.; Atwood, J.L.; Lynn, B.C.; Kaifer, A.E.; Gokel, G.W. Ferrocenyl iron as a donor group for complexed silver in ferrocenyldimethyl[2.2]cryptand: A redox-switched receptor effective in water. J. Am. Chem. Soc. 1992, 114, 10583–10595. [Google Scholar] [CrossRef]
- Plenio, H.; Aberle, C. Oxaferrocene Cryptands as Efficient Molecular Switches for Alkali and Alkaline Earth Metal Ions. Organometallics 1997, 16, 5950–5957. [Google Scholar] [CrossRef]
- Beer, P.D.; Crowe, D.B.; Ogden, M.I.; Drew, M.G.B.; Main, B. Ammonium redox-responsive receptors containing multiple ferrocene and quinone redox-active centres attached to di- and tri-aza crown ether macrocycles. J. Chem. Soc. Dalton Trans. 1993, 2107–2116. [Google Scholar] [CrossRef]
- Shi, L.; Song, W.; Li, Y.; Li, D.-W.; Swanick, K.N.; Ding, Z.; Long, Y.-T. A multi-channel sensor based on 8-hydroxyquinoline ferrocenoate for probing Hg(II) ion. Talanta 2011, 84, 900–904. [Google Scholar] [CrossRef]
- Li, S.-H.; Chen, F.-R.; Zhou, Y.-F.; Wang, J.-N.; Zhang, H.; Xu, J.-G. Enhanced fluorescence sensing of hydroxylated organotins by a boronic acid-linked Schiff base. Chem. Commun. 2009, 4179–4181. [Google Scholar] [CrossRef]
- Zanello, P.; Cinquantini, A.; Fontani, M.; Giardiello, M.; Giorgi, G.; Landis, C.R.; Kimmich, B.F.M. Redox behavior of boronato-functionalized 1,1′-bis(diphenylphosphino)ferrocenes. J. Organomet. Chem. 2001, 637–639, 800–804. [Google Scholar] [CrossRef]
- El Ghachtouli, S.; Cadiou, C.; Déchamps-Olivier, I.; Chuburu, F.; Aplincourt, M.; Turcry, V.; Le Baccon, M.; Handel, H. Spectroscopy and Redox Behaviour of Dicopper(II) and Dinickel(II) Complexes of Bis(cyclen) and Bis(cyclam) Ligands. Eur. J. Inorg. Chem. 2005, 2005, 2658–2668. [Google Scholar] [CrossRef]
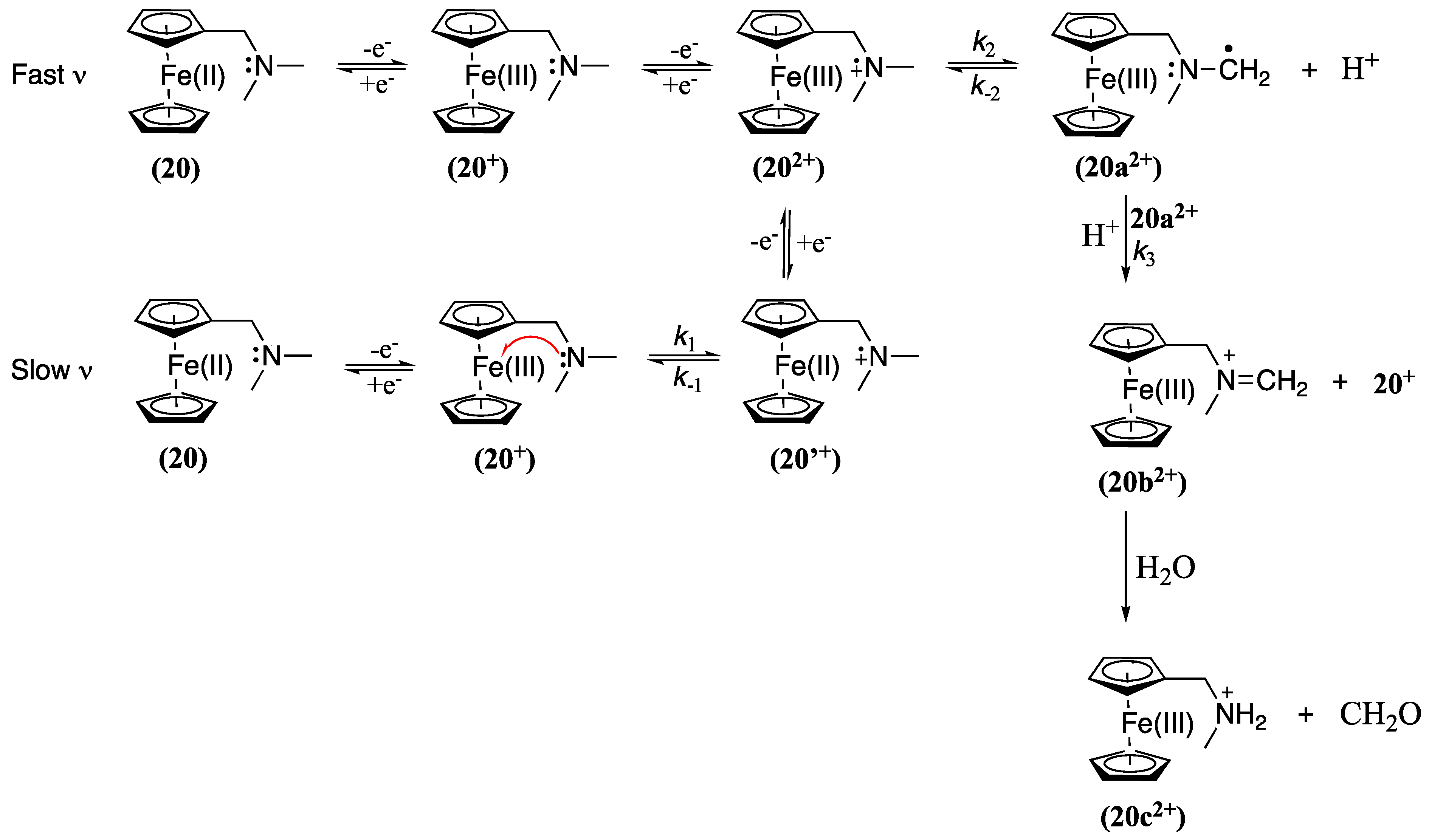
- Mruthunjaya, A.K.V.; Torriero, A.A.J. Mechanistic Aspects of the Electrochemical Oxidation of Aliphatic Amines and Aniline Derivatives. Molecules 2023, 28, 471. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Belousoff, M.J.; Spiccia, L.; Bond, A.M.; Torriero, A.A.J. Macrocycles Bearing Ferrocenyl Pendants and their Electrochemical Properties upon Binding to Divalent Transition Metal Cations. ChemPlusChem 2018, 83, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Torriero, A.A.J.; Bond, A.M.; Spiccia, L. Fluorescent and Electrochemical Sensing of Polyphosphate Nucleotides by Ferrocene Functionalised with Two Zn(II)-(TACN)(pyrene) Complexes. Chemistry 2010, 16, 9154–9163. [Google Scholar] [CrossRef]
- Plenio, H.; Aberle, C. Synthesis of a ferrocene bridged cyclam: A new redox-active macrocycle and the structure of a nickel(II) complex with strongly coupled metal centers. Chem. Commun. 1998, 2697–2698. [Google Scholar] [CrossRef]
- Plenio, H.; Aberle, C.; Al Shihadeh, Y.; Lloris, J.M.; Martínez-Máñez, R.; Pardo, T.; Soto, J. Ferrocene–Cyclam: A Redox-Active Macrocycle for the Complexation of Transition Metal Ions and a Study on the Influence of the Relative Permittivity on the Coulombic Interaction between Metal Cations. Chemistry 2001, 7, 2848–2861. [Google Scholar] [CrossRef]
- Tendero, M.J.L.; Benito, A.; Cano, J.; Lloris, J.M.; Martínez-Máñez, R.; Soto, J.; Edwards, A.J.; Raithby, P.R.; Rennie, M.A. Host molecules containing electroactive cavities obtained by the molecular assembly of redox-active ligands and metal ions. J. Chem. Soc. Chem. Commun. 1995, 1643–1644. [Google Scholar] [CrossRef]
- Beer, P.D.; Smith, D.K. Tunable bis(ferrocenyl) receptors for the solution-phase electrochemical sensing of transition-metal cations. J. Chem. Soc. Dalton Trans. 1998, 417–424. [Google Scholar] [CrossRef]
- De Santis, G.; Fabbrizzi, L.; Licchelli, M.; Pallavicini, P.; Perotti, A. A redox-switchable ligand for which the binding ability is enhanced by oxidation of its ferrocene unit. J. Chem. Soc. Dalton Trans. 1992, 3283–3284. [Google Scholar] [CrossRef]
- Zapata, F.; Caballero, A.; Espinosa, A.; Tárraga, A.; Molina, P. A Simple but Effective Ferrocene Derivative as a Redox, Colorimetric, and Fluorescent Receptor for Highly Selective Recognition of Zn2+ Ions. Org. Lett. 2007, 9, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
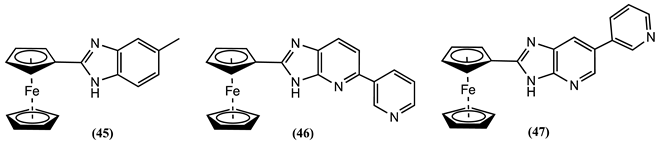
- Nedunchezhian, K.; Nallathambi, S. Mono- and di-ferrocene conjugated 5-methyl benzimidazole based multi-channel receptors for cations/anions with their antimicrobial and anticancer studies. New J. Chem. 2023, 47, 4656–4666. [Google Scholar] [CrossRef]
- Zhang, B.; Suo, Q.; Li, Q.; Hu, J.; Zhu, Y.; Gao, Y.; Wang, Y. Multiresponsive chemosensors based on ferrocenylimidazo[4,5-b]pyridines: Solvent-dependent selective dual sensing of Hg2+ and Pb2+. Tetrahedron 2022, 120, 132878. [Google Scholar] [CrossRef]
- Tian, H.-j.; Tang, R.-r.; Li, S.-f.; Luo, Y.-m. Synthesis, characterization and electrochemical recognition of metal ions of three new ferrocenyl derivatives containing pyridyl moiety. J. Cent. South Univ. 2013, 20, 3379–3384. [Google Scholar] [CrossRef]
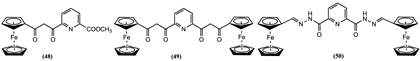
- Kaur, S.; Shalini, B.A.; Ahmad Shiekh, B.; Kumar, V.; Kaur, I. Triazole-tethered naphthalimide-ferrocenyl-chalcone based voltammetric and potentiometric sensors for selective electrochemical quantification of Copper(II) ions. J. Electroanal. Chem. 2022, 905, 115966. [Google Scholar] [CrossRef]
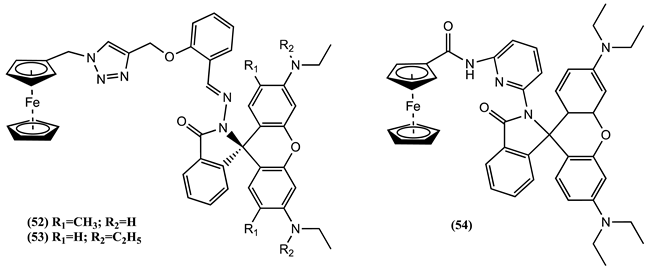
- Arivazhagan, C.; Borthakur, R.; Ghosh, S. Ferrocene and Triazole-Appended Rhodamine Based Multisignaling Sensors for Hg2+ and Their Application in Live Cell Imaging. Organometallics 2015, 34, 1147–1155. [Google Scholar] [CrossRef]
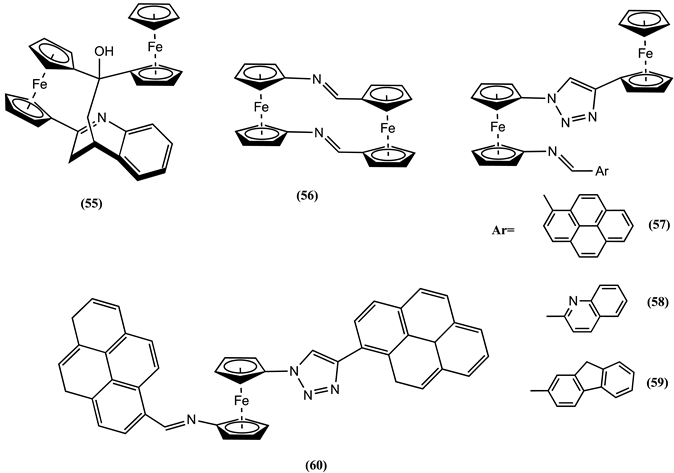
- Lopez, J.L.; Tárraga, A.; Espinosa, A.; Velasco, M.D.; Molina, P.; Lloveras, V.; Vidal-Gancedo, J.; Rovira, C.; Veciana, J.; Evans, D.J.; et al. A New Multifunctional Ferrocenyl-Substituted Ferrocenophane Derivative: Optical and Electronic Properties and Selective Recognition of Mg2+ Ions. Chemistry 2004, 10, 1815–1826. [Google Scholar] [CrossRef]
- Otón, F.; Ratera, I.; Espinosa, A.; Wurtz, K.; Parella, T.; Tárraga, A.; Veciana, J.; Molina, P. Selective Metal-Cation Recognition by [2.2]Ferrocenophanes: The Cases of Zinc- and Lithium-Sensing. Chemistry 2010, 16, 1532–1542. [Google Scholar] [CrossRef]
- Otón, F.; González, M.d.C.; Espinosa, A.; Tárraga, A.; Molina, P. Synthesis, Structural Characterization, and Sensing Properties of Clickable Unsymmetrical 1,1′-Disubstituted Ferrocene–Triazole Derivatives. Organometallics 2012, 31, 2085–2096. [Google Scholar] [CrossRef]
- González, M.C.; Otón, F.; Orenes, R.A.; Espinosa, A.; Tárraga, A.; Molina, P. Ferrocene–Triazole–Pyrene Triads as Multichannel Heteroditopic Recognition Receptors for Anions, Cations and Ion Pairs. Organometallics 2014, 33, 2837–2852. [Google Scholar] [CrossRef]
- Sola, A.; Otón, F.; Espinosa, A.; Tárraga, A.; Molina, P. Aldimines generated from aza-Wittig reaction between bis(iminophosphoranes) derived from 1,1′-diazidoferrocene and aromatic or heteroaromatic aldehydes: Electrochemical and optical behaviour towards metal cations. Dalton Trans. 2011, 40, 12548–12559. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yan, L.; Xie, R.; Han, L.; Zhu, N. Multi-channel sensing of trivalent metal ions using a simple ferrocenyl Schiff base probe with AIE property. J. Mol. Struct. 2024, 1295, 136629. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Bheemireddy, V.; Vijaykumar, G.; Thakur, A. Triazole appended mono and 1,1′-di-substituted ferrocene-naphthalene conjugates: Highly selective and sensitive multi-responsive probes for Hg(II). Sens. Actuators B Chem. 2017, 240, 640–650. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Mondal, B.; Lima, S.; Thakur, A. Metal-coordination driven intramolecular twisting: A turn-on fluorescent-redox probe for Hg2+ ions through the interaction of ferrocene nonbonding orbitals and dibenzylidenehydrazine. Dalton Trans. 2019, 48, 8209–8220. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, S.R.; Pal, A.; Sarangi, U.K.; Thakur, A. Ferrocene appended fluorescein-based ratiomeric fluorescence and electrochemical chemosensor for Fe3+ and Hg2+ ions in aqueous media: Application in real samples analysis. Inorganica Chim. Acta 2019, 498, 119097. [Google Scholar] [CrossRef]
- Romero, T.; Orenes, R.A.; Tárraga, A.; Molina, P. Preparation, Structural Characterization, Electrochemistry, and Sensing Properties toward Anions and Cations of Ferrocene-Triazole Derivatives. Organometallics 2013, 32, 5740–5753. [Google Scholar] [CrossRef]
- Kamal, A.; Kumar, S.; Kumar, V.; Mahajan, R.K. Selective sensing ability of ferrocene appended quinoline-triazole derivative toward Fe (III) ions. Sens. Actuators B Chem. 2015, 221, 370–378. [Google Scholar] [CrossRef]
- Pandey, R.; Gupta, R.K.; Shahid, M.; Maiti, B.; Misra, A.; Pandey, D.S. Synthesis and Characterization of Electroactive Ferrocene Derivatives: Ferrocenylimidazoquinazoline as a Multichannel Chemosensor Selectively for Hg2+ and Pb2+ Ions in an Aqueous Environment. Inorg. Chem. 2012, 51, 298–311. [Google Scholar] [CrossRef]
- Kiran Kumar, C.; Trivedi, R.; Giribabu, L.; Niveditha, S.; Bhanuprakash, K.; Sridhar, B. Ferrocenyl pyrazoline based multichannel receptors for a simple and highly selective recognition of Hg2+ and Cu2+ ions. J. Organomet. Chem. 2015, 780, 20–29. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Cheng, H.; Chen, X.; Song, M.; Tang, M.; Wei, D.; Wu, Y. Syntheses, structures of N-(substituted)-2-aza-[3]-ferrocenophanes and their application as redox sensor for Cu2+ ion. Appl. Organomet. Chem. 2012, 26, 449–454. [Google Scholar] [CrossRef]
- Krishnapriya, K.R.; Sampath, N.; Ponnuswamy, M.N.; Kandaswamy, M. Synthesis and electrochemical sensing behaviour of a new ferrocene functionalized tet ′a′ macrocyclic receptor towards transition metal ions. Appl. Organomet. Chem. 2007, 21, 311–317. [Google Scholar] [CrossRef]
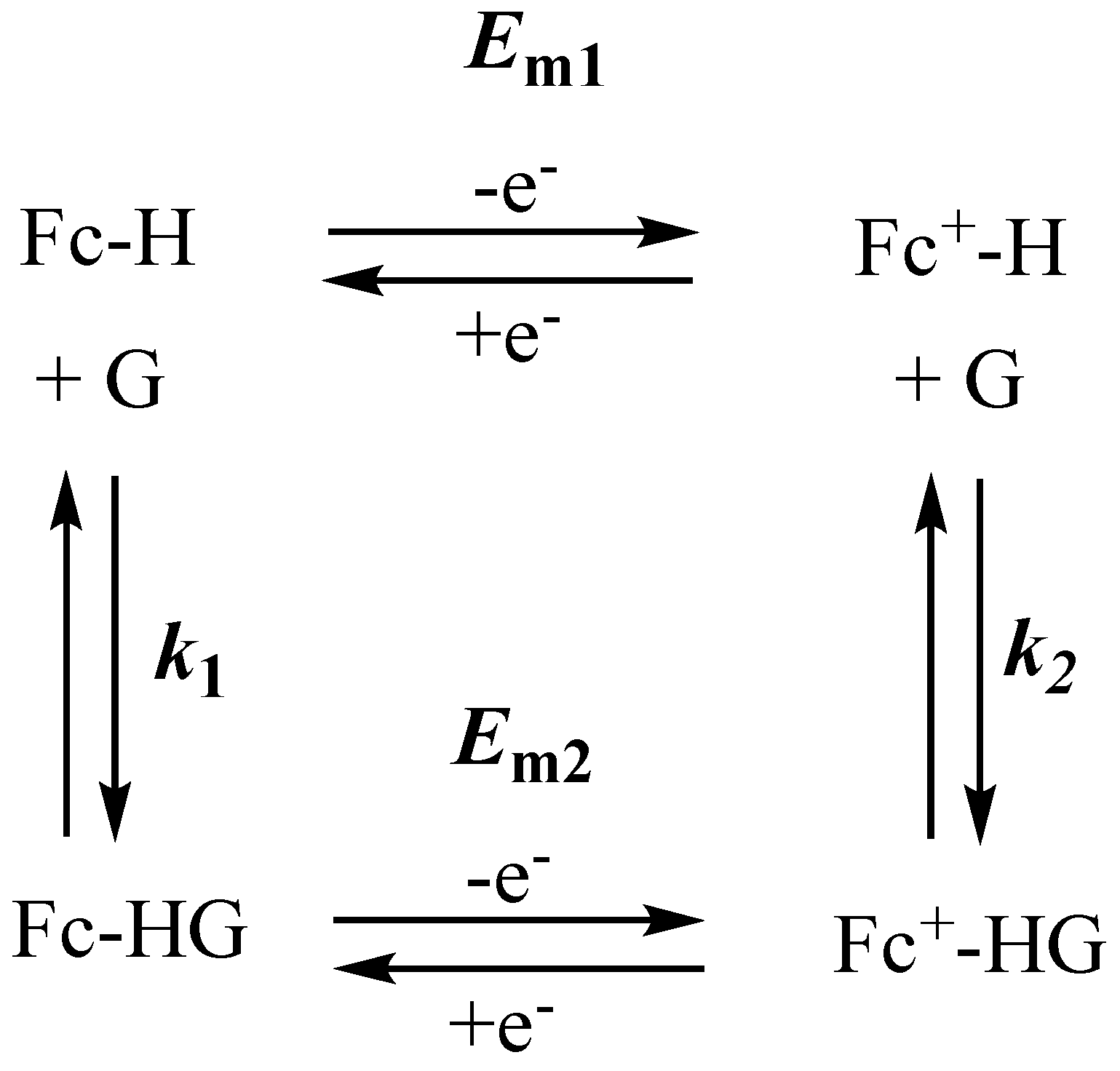
| Solvent | Electrolyte | Em of Fc0/+ vs. DmFc0/+(V) | Ref. |
|---|---|---|---|
| 1,2-dibromoethane | 0.1 M [Bu4N][ClO4] | 0.475 ± 0.007 | [13] |
| 1,2-dichloroethane | 0.1 M [Bu4N][ClO4] | 0.532 ± 0.001 | [13] |
| 1,2-dichlorobenzene | 0.1 M [Bu4N][ClO4] | 0.535 ± 0.001 | [13] |
| 2-propanol | 0.1 M [Bu4N][CF3SO3] | 0.455 ± 0.003 | [13] |
| 2,2,2-trifluoroethanol | 0.1 M [Bu4N][ClO4] | 0.575 ± 0.004 | [13] |
| Acetone | 0.1 M [Bu4N]Cl | 0.451 ± 0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.479 ± 0.004 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.487 ± 0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.504 ± 0.005 | [34] | |
| Acetonitrile (CH3CN) | 0.1 M [Bu4N]Cl | 0.501 ± 0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.505 ± 0.002 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.509 ± 0.003 | [35] | |
| 0.1 M [Bu4N][TFAB] | 0.517 ± 0.005 | [34] | |
| Acetonitrile/dichloromethane (80:20) | 0.1 M [Bu4N][PF6] | 0.512 ± 0.003 | [36] |
| Aniline | 0.1 M [Bu4N][ClO4] | 0.527 ± 0.004 | [13] |
| Anisole | 0.1 M [Bu4N][PF6] | 0.518 ± 0.005 | [34] |
| 0.1 M [Bu4N][TFAB] | 0.607 ± 0.005 | [34] | |
| Benzonitrile | 0.1 M [Bu4N]Cl | 0.524 ± 0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.523 ± 0.001 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.530 ± 0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.543 ± 0.005 | [34] | |
| Benzyl alcohol | 0.1 M [Bu4N][ClO4] | 0.508 ± 0.003 | [13] |
| Bromobenzene | 0.1 M [Bu4N][ClO4] | 0.489 ± 0.005 | [13] |
| Chlorobenzene | 0.1 M [Bu4N][ClO4] | 0.497 ± 0.001 | [13] |
| Chloroform | 0.1 M [Bu4N][ClO4] | 0.483 ± 0.001 | [13] |
| Dichloromethane (DCM) | 0.1 M [Bu4N]Cl | 0.534 ± 0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.532 ± 0.002 | [13] | |
| 0.1 M [Bu4N][ClO4] | 0.570 ± 0.002 | [2] | |
| 0.1 M [Bu4N][PF6] | 0.548 ± 0.003 | [35] | |
| 0.1 M [Et4N][BF4] | 0.541 ± 0.003 | [17] | |
| 0.1 M [Bu4N][TFAB] | 0.614 ± 0.005 | [34] | |
| 0.1 M [C4mPyr][FAP] | 0.589 ± 0.003 | [17] | |
| 0.1 M [C2mim][FAP] | 0.590 ± 0.003 | [17] | |
| 0.1 M [C2mim][B(CN)4] | 0.588 ± 0.003 | [17] | |
| 0.1 M [C4mim][NTf2] | 0.570 ± 0.003 | [17] | |
| 0.1 M [C4mPyr][NTf2] | 0.568 ± 0.003 | [17] | |
| 0.1 M [C2mim][FSI] | 0.569 ± 0.003 | [17] | |
| 0.1 M [C3mim][FSI] | 0.568 ± 0.003 | [17] | |
| 0.1 M [C4mPyr][N(CN)2] | 0.564 ± 0.003 | [17] | |
| 0.1 M [C4mim][PF6] | 0.556 ± 0.003 | [17] | |
| 0.1 M [C4mim][BF4] | 0.557 ± 0.003 | [17] | |
| 0.1 M [C4mim][CF3SO3] | 0.556 ± 0.003 | [17] | |
| Diethyl ether | 0.1 M [Bu4N][BArF24] | 0.550 ± 0.005 | [34] |
| 0.1 M Na[BArF24] | 0.583 ± 0.005 | [34] | |
| Dimethyl sulfoxide | 0.1 M [Bu4N][PF6] | 0.486 ± 0.005 | [34] |
| 0.1 M [Bu4N][TFAB] | 0.493 ± 0.005 | [34] | |
| 0.1 M [Bu4N][ClO4] | 0.468 ± 0.001 | [13] | |
| Ethanol | 0.1 M [Bu4N][ClO4] | 0.473 ± 0.005 | [13] |
| Formamide | 0.1 M [Bu4N][ClO4] | 0.510 ± 0.003 | [13] |
| Methanol (MeOH) | 0.1 M [Bu4N][ClO4] | 0.497 ± 0.002 | [13] |
| Nitrobenzene | 0.1 M [Bu4N][ClO4] | 0.514 ± 0.002 | [13] |
| Nitromethane | 0.1 M [Bu4N]Cl | 0.505 ± 0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.516 ± 0.004 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.510 ± 0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.516 ± 0.005 | [34] | |
| N-methylformamide | 0.1 M [Bu4N][ClO4] | 0.510 ± 0.002 | [13] |
| N,N-dimethylformamide (DMF) | 0.1 M [Bu4N]Cl | 0.475 ± 0.005 | [34] |
| 0.1 M [Bu4N][ClO4] | 0.458 ± 0.003 | [13] | |
| 0.1 M [Bu4N][PF6] | 0.478 ± 0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.493 ± 0.005 | [34] | |
| N,N-dimethylacetamide | 0.1 M [Bu4N][ClO4] | 0.455 ± 0.008 | [13] |
| Propylene carbonate | 0.1 M [Bu4N][ClO4] | 0.495 ± 0.002 | [13] |
| Pyridine | 0.1 M [Bu4N][ClO4] | 0.517 ± 0.004 | [13] |
| Tetrahydrofuran | 0.1 M [Bu4N][BF4] | 0.413 ± 0.005 | [34] |
| 0.1 M [Bu4N][CF3SO3] | 0.438 ± 0.005 | [34] | |
| 0.1 M [Bu4N][ClO4] | 0.423 ± 0.005 | [34] | |
| 0.427 ± 0.002 | [13] | ||
| 0.1 M [Bu4N][PF6] | 0.446 ± 0.005 | [34] | |
| 0.1 M [Bu4N][BPh4] | 0.485 ± 0.005 | [34] | |
| 0.1 M Na[BArF24] | 0.502 ± 0.005 | [34] | |
| 0.1 M [Bu4N][TFAB] | 0.484 ± 0.005 | [34] | |
| 0.1 M [Bu4N][BArF24] | 0.521 ± 0.005 | [34] | |
| Toluene | 0.1 M [Bu4N][BF4] a | 0.430 ± 0.005 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torriero, A.A.J.; Mruthunjaya, A.K.V. Ferrocene-Based Electrochemical Sensors for Cations. Inorganics 2023, 11, 472. https://doi.org/10.3390/inorganics11120472
Torriero AAJ, Mruthunjaya AKV. Ferrocene-Based Electrochemical Sensors for Cations. Inorganics. 2023; 11(12):472. https://doi.org/10.3390/inorganics11120472
Chicago/Turabian StyleTorriero, Angel A. J., and Ashwin K. V. Mruthunjaya. 2023. "Ferrocene-Based Electrochemical Sensors for Cations" Inorganics 11, no. 12: 472. https://doi.org/10.3390/inorganics11120472
APA StyleTorriero, A. A. J., & Mruthunjaya, A. K. V. (2023). Ferrocene-Based Electrochemical Sensors for Cations. Inorganics, 11(12), 472. https://doi.org/10.3390/inorganics11120472








