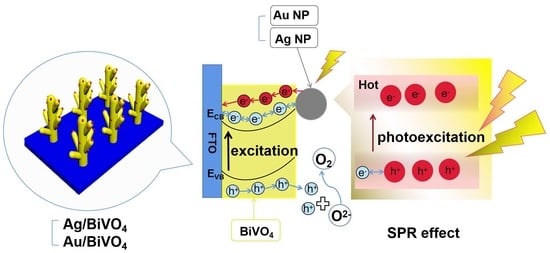
Surface Plasmon Resonance Effect of Noble Metal (Ag and Au) Nanoparticles on BiVO4 for Photoelectrochemical Water Splitting
Abstract
1. Introduction
Characterization
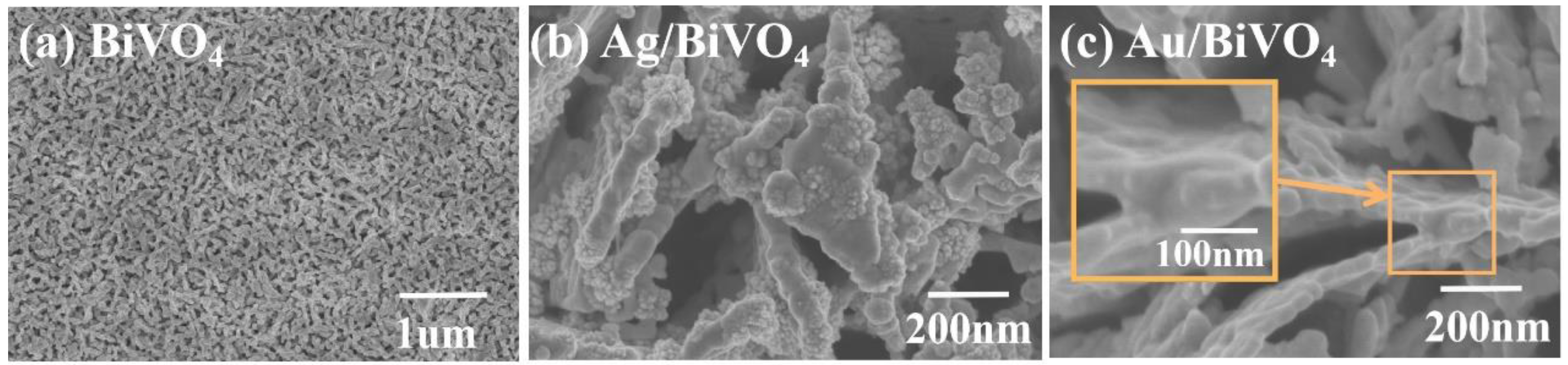
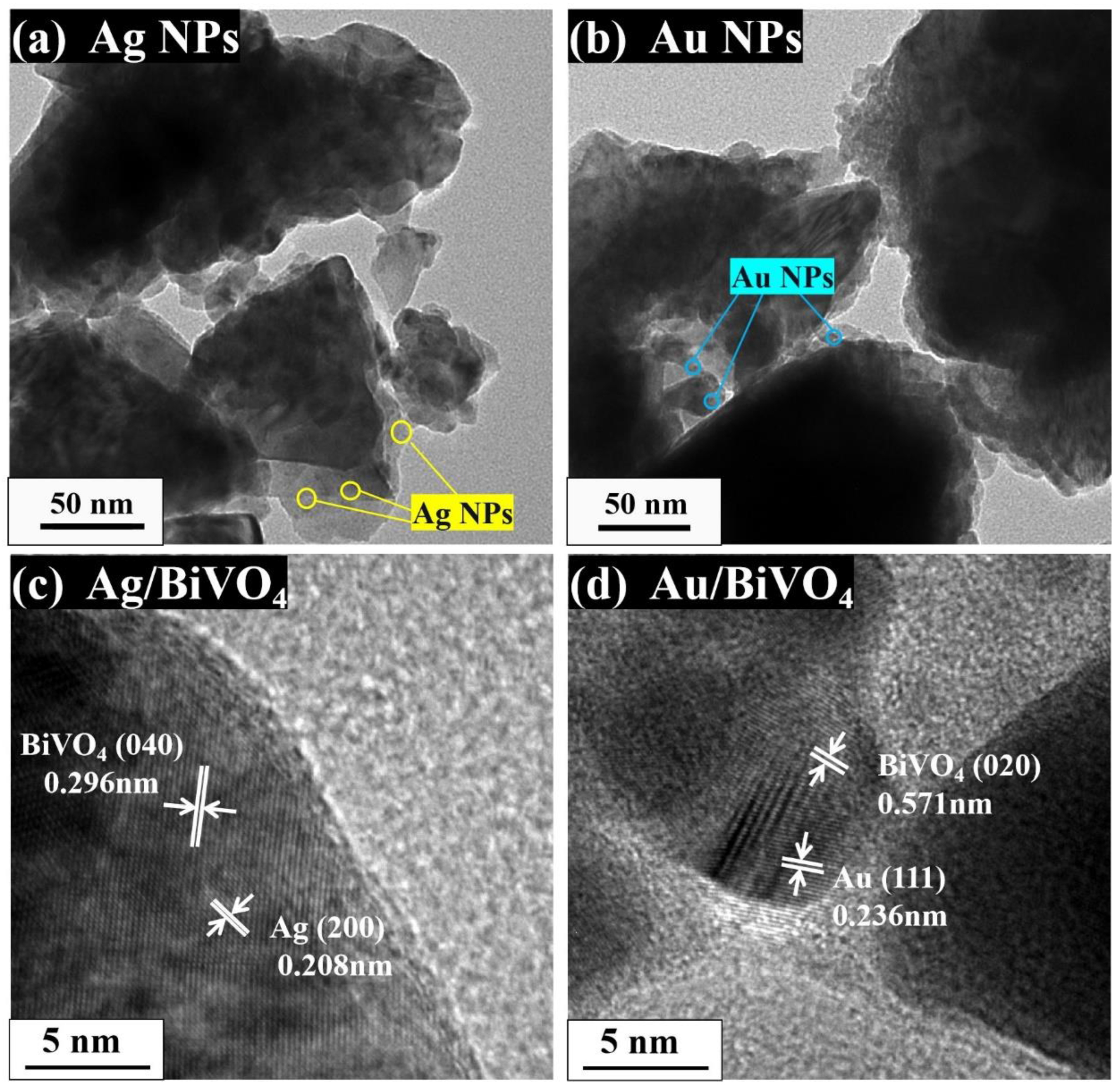
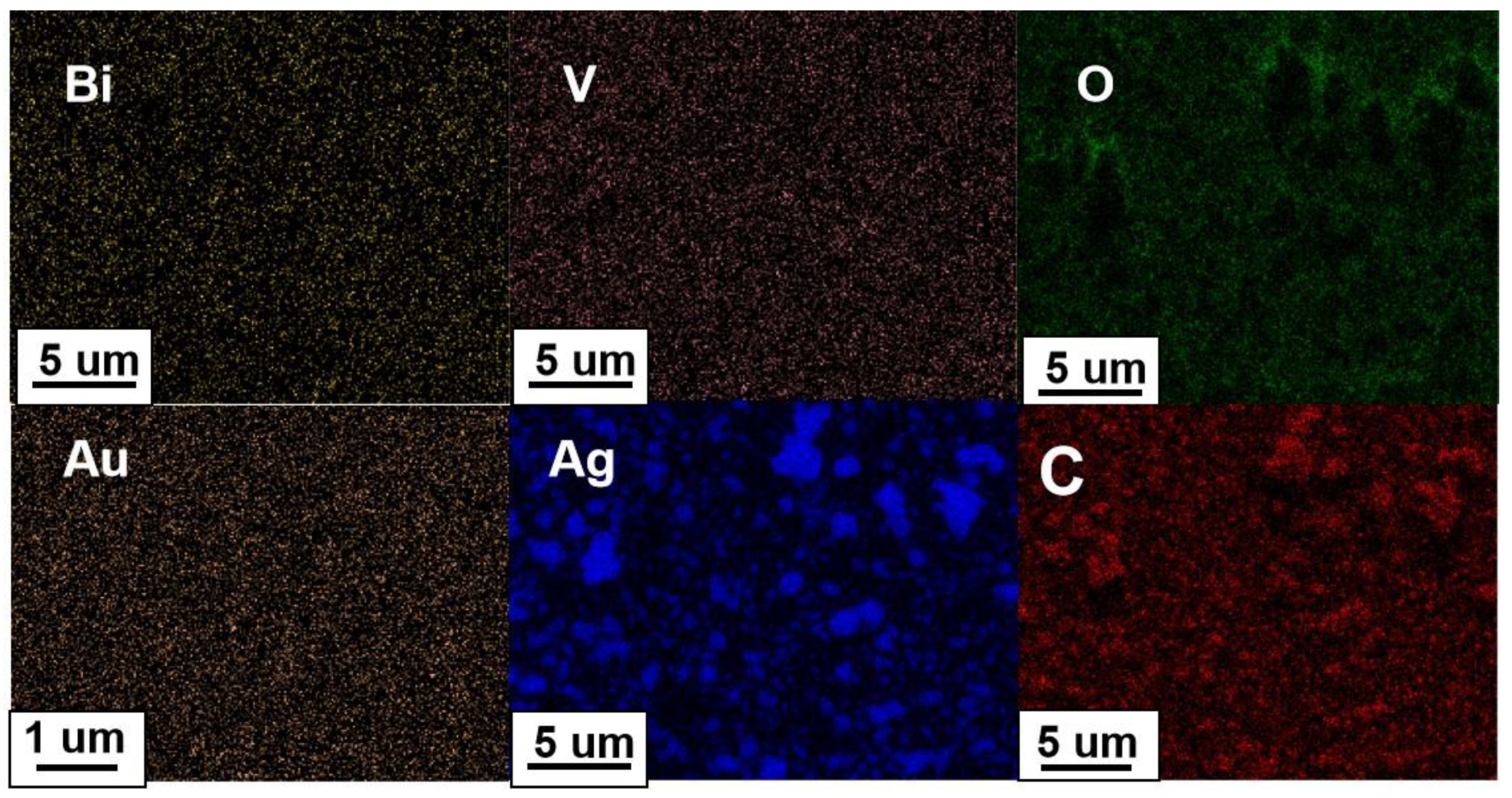
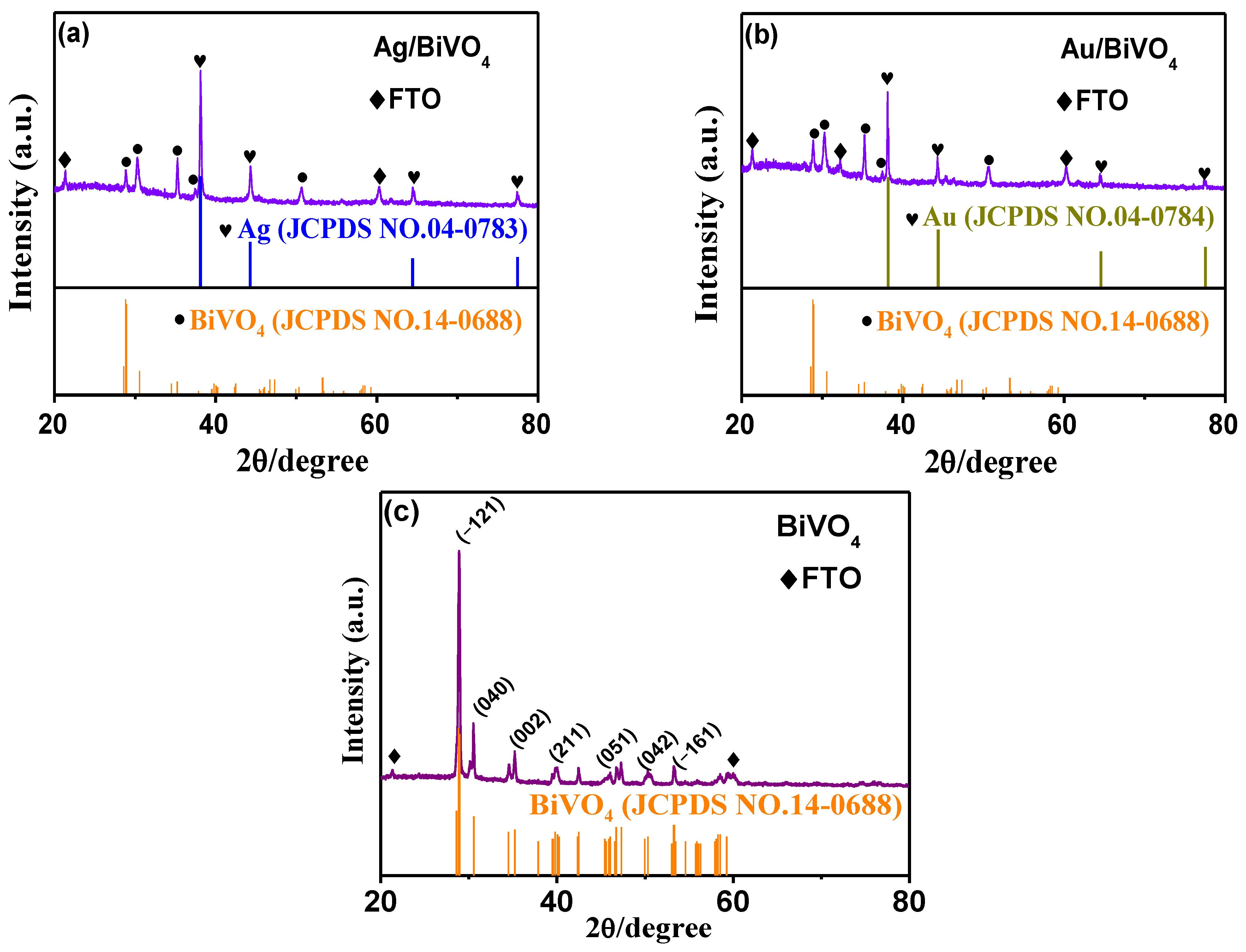
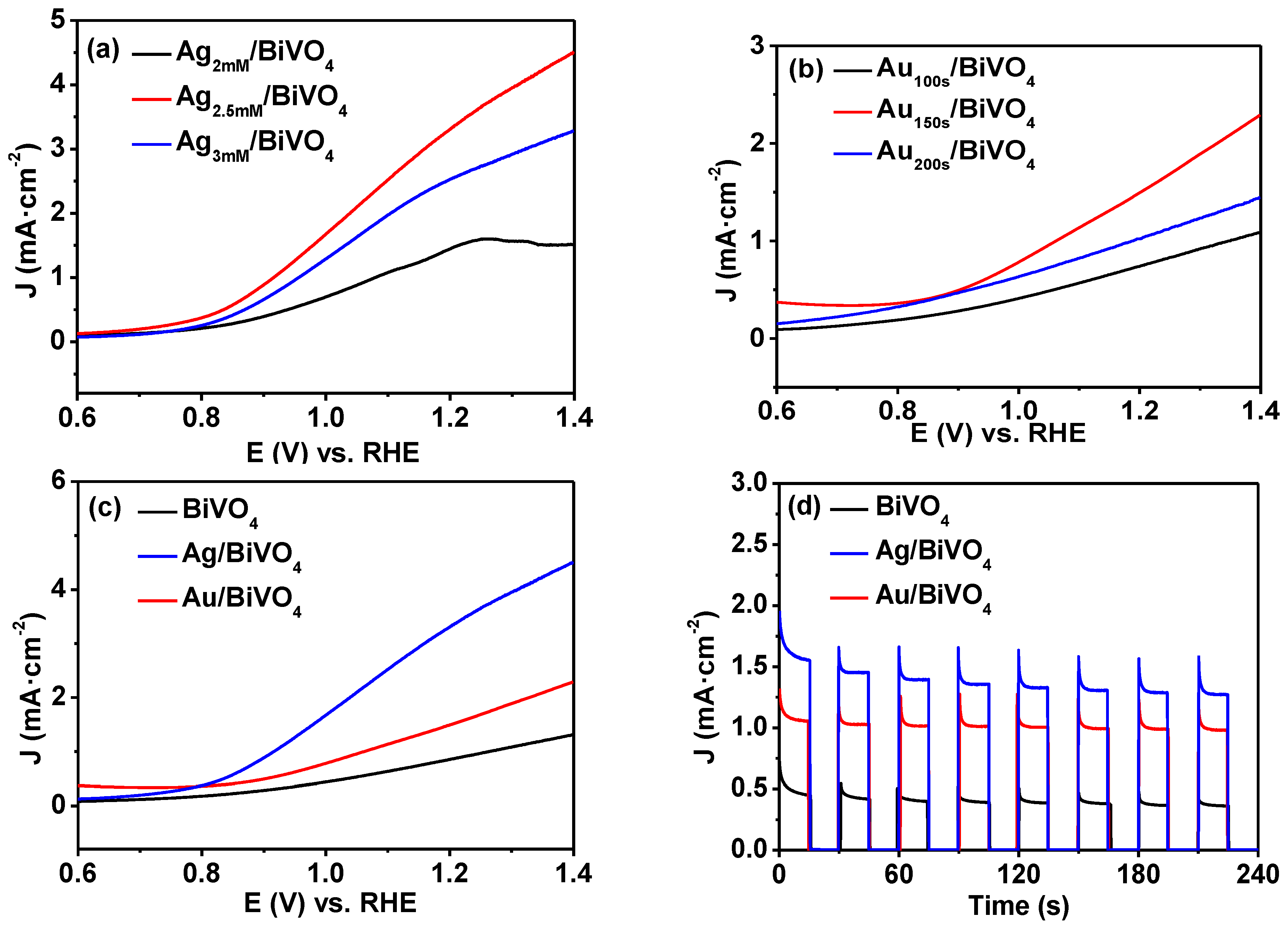
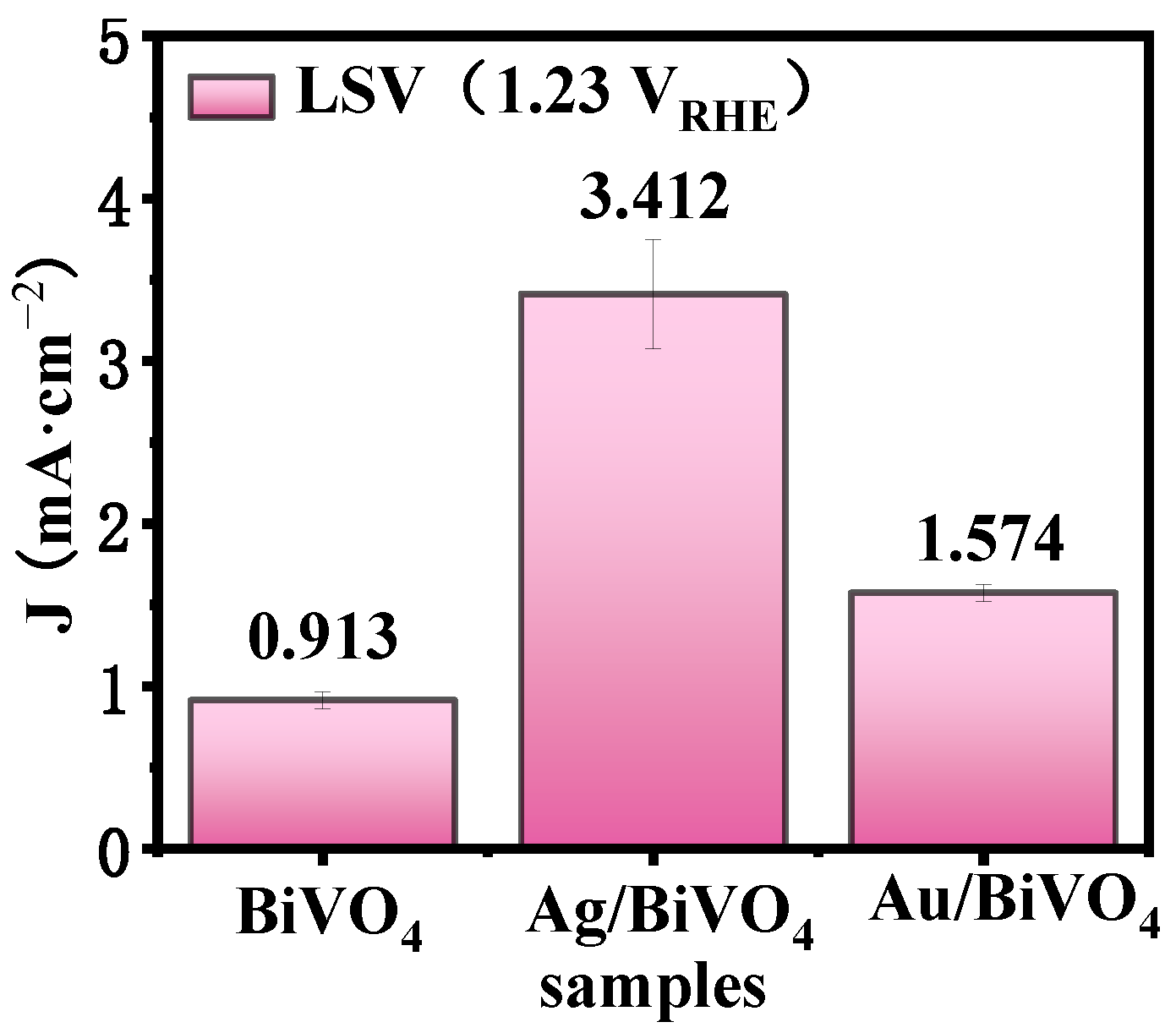
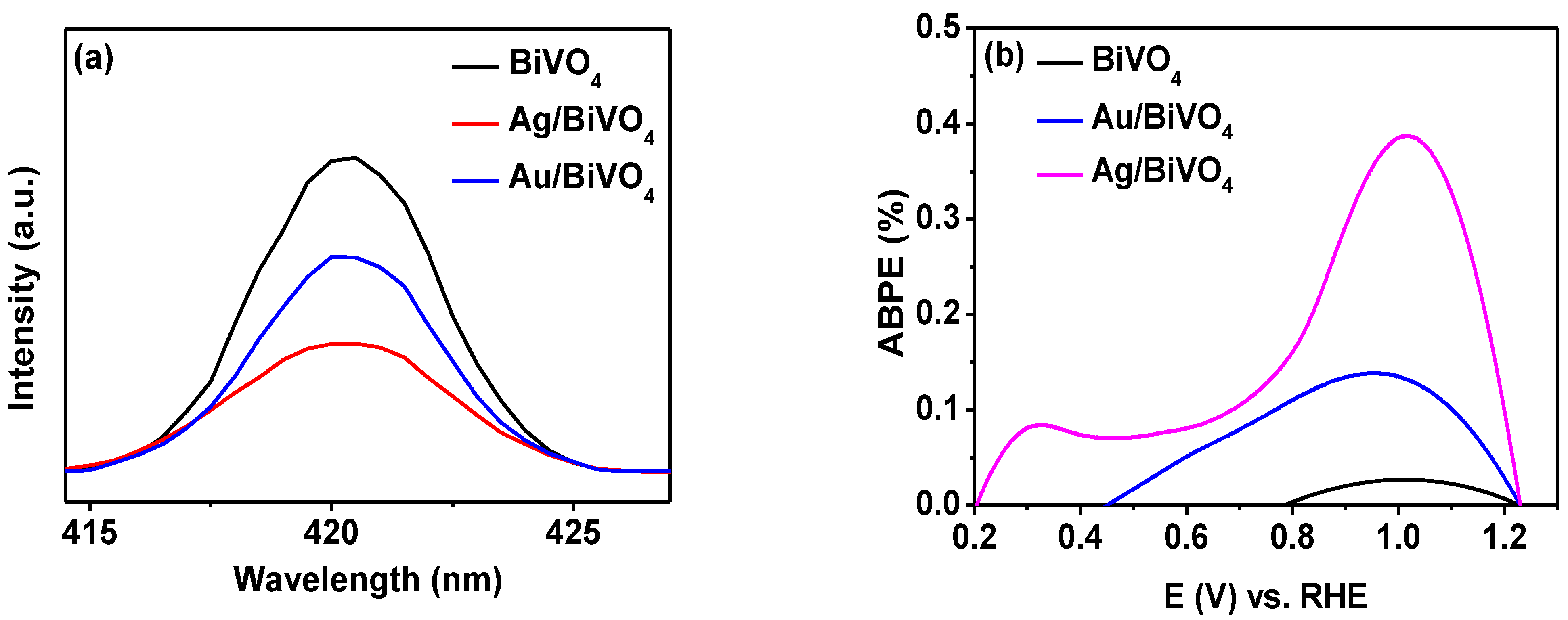
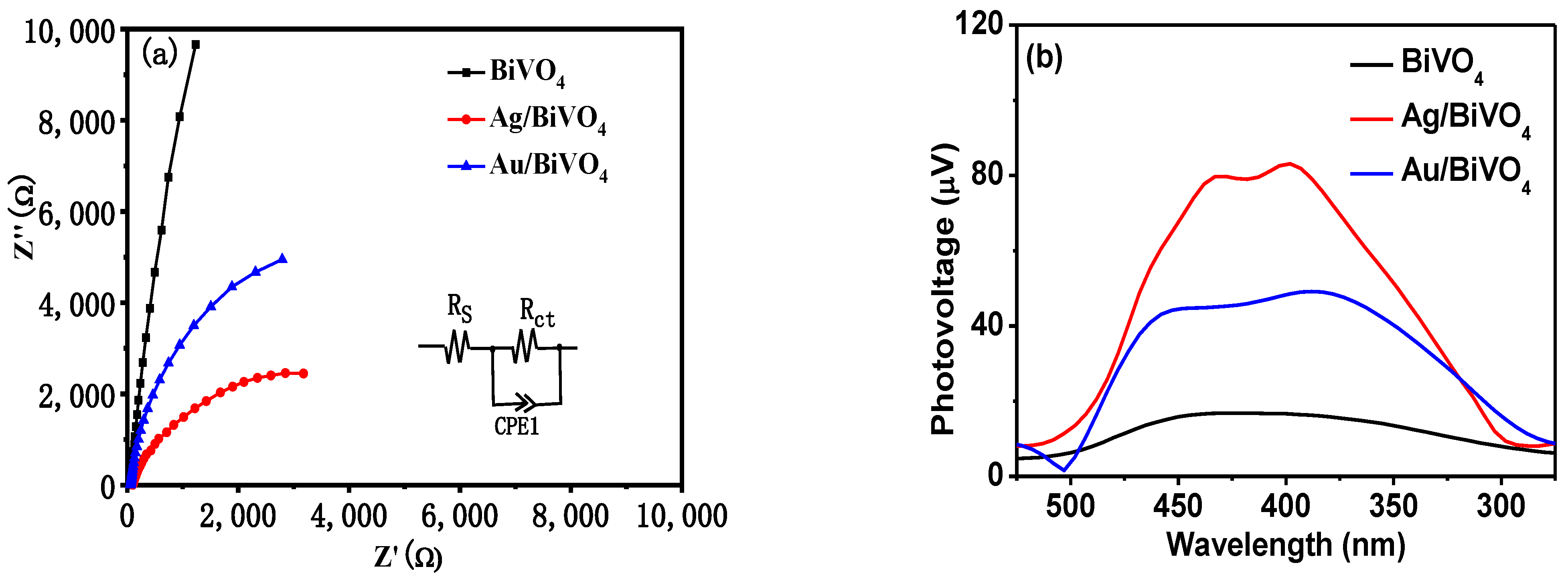
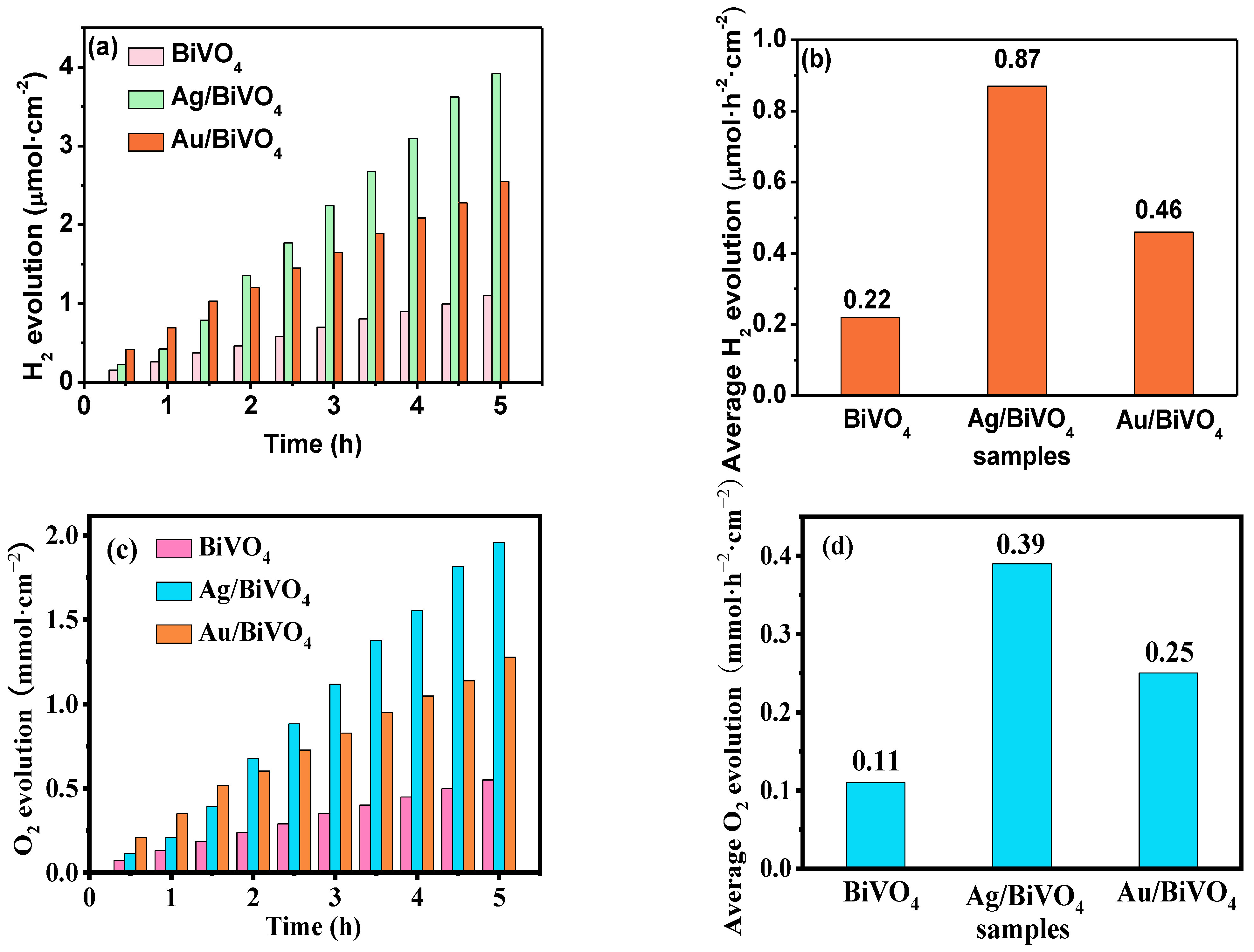
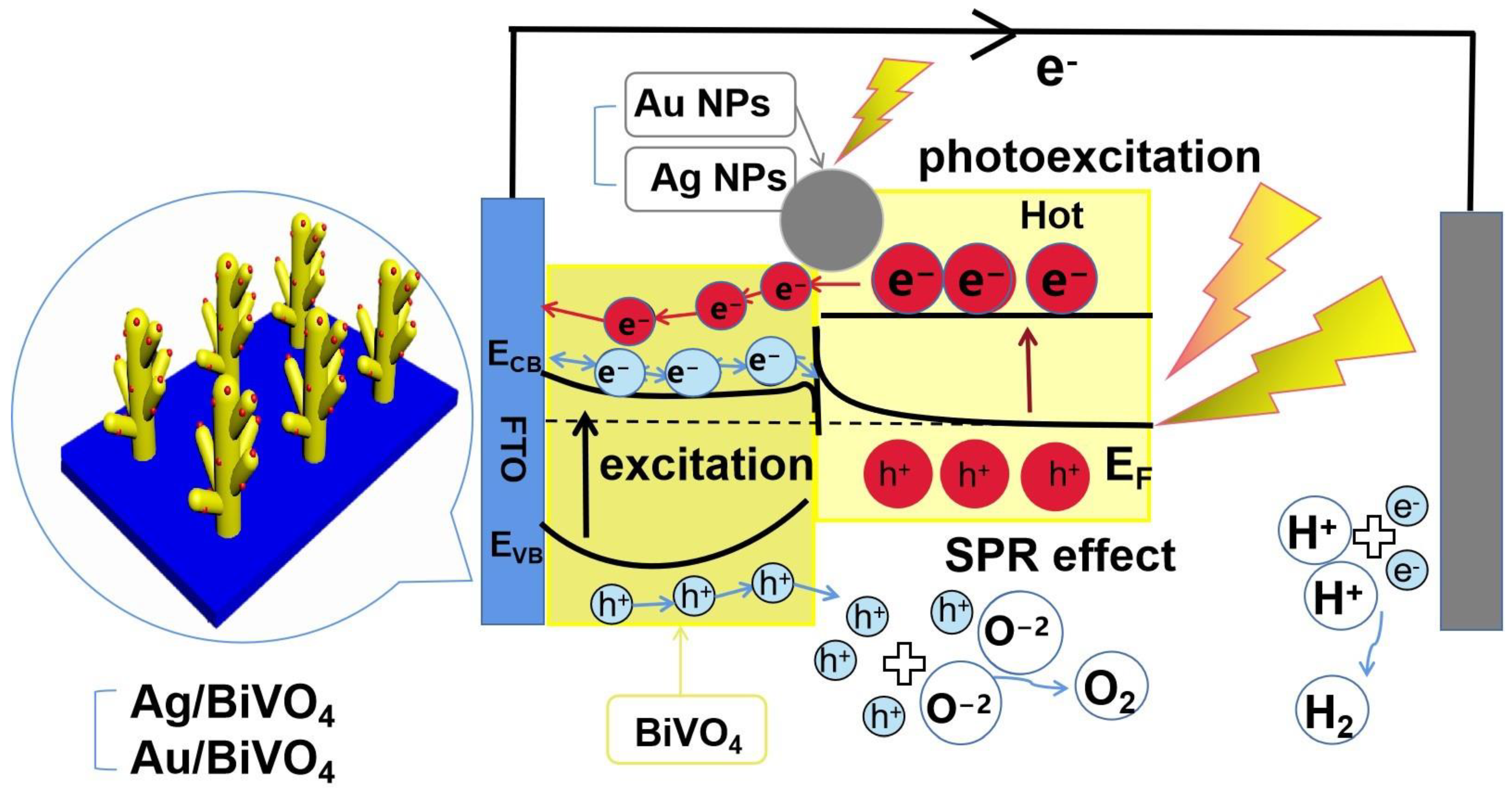
2. Results and Discussion
3. Conclusions
4. Experimental Section
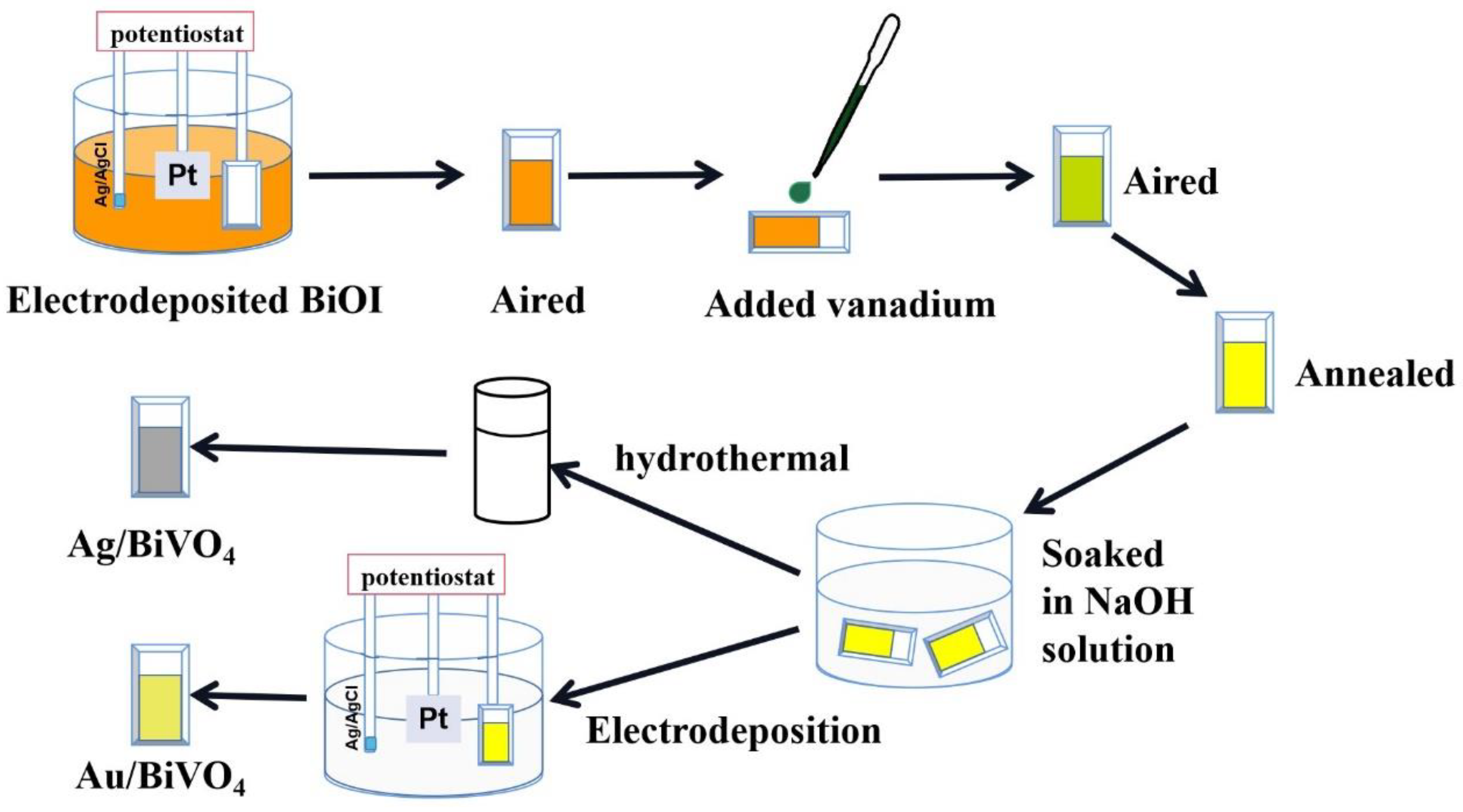
4.1. Synthesis of BiVO4 Films
4.2. Fabrication of Ag/BiVO4 and Au/BiVO4 Photoanodes
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Liu, Z.F.; Guo, Z.G.; Yan, W.G.; Xin, Y. Enhancing light harvesting and charge separation of Cu2O photocathodes with spatially separated noble-metal cocatalysts towards highly efficient water splitting. J. Mater. Chem. A 2018, 6, 20393–20401. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Qi, L.M. Light Management with patterned micro- and nanostructure arrays for photocatalysis, photovoltaics, and optoelectronic and optical devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Tilley, S.D. Recent advances and emerging trends in photoelectrochemical solar energy conversion. Adv. Energy Mater. 2019, 9, 1802877. [Google Scholar] [CrossRef]
- Feng, J.N.; Bian, J.; Bai, L.L.; Xi, S.B.; Wang, Y.; Chen, C.L.; Jing, L.Q. Efficient wide-spectrum photocatalytic overall water splitting over ultrathin molecular nickel phthalocyanine/BiVO4 Z-scheme heterojunctions without noble metals. Appl. Catal. B Environ. 2021, 295, 120260. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Yin, M.M.; Ge, R.; Wei, J.; Su, B.Y.; Chen, X.; Chen, X.M. Competitive near-infrared PEC immunosorbent assay for monitoring okadaic acid based on a disposable flower-like WO3-Modified screen-printed electrode. Biosens. Bioelectron. 2021, 185, 113278. [Google Scholar] [CrossRef]
- Dou, Y.B.; Zhou, J.; Zhou, A.; Li, J.R.; Nie, Z.R. Visible-light responsive MOF encapsulation of noble-metal-sensitized semiconductors for high-performance photoelectrochemical water splitting. J. Mater. Chem. 2017, 5, 19491. [Google Scholar] [CrossRef]
- Zhou, T.S.; Chen, S.; Wang, J.C.; Zhang, Y.; Li, J.H.; Bai, J.; Zhou, B.X. Dramatically enhanced solar-driven water splitting of BiVO4 photoanode via strengthening hole transfer and light harvesting by co-modification of CQDs and ultrathin beta-FeOOH layers. Chem. Eng. J. 2021, 403, 126350. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Son, M.K.; Pan, L.F.; Mayer, M.T.; Hagfeldt, A.; Gratzel, M.; Luo, J.S. Structural and compositional investigations on the stability of cuprous oxide nanowire photocathodes for photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2021, 13, 55080–55091. [Google Scholar] [CrossRef]
- Luo, W.J.; Yang, Z.S.; Li, Z.S.; Zhang, J.Y.; Liu, J.G.; Zhao, Z.Y.; Wang, Z.Q.; Yan, S.C.; Yu, T.; Zou, Z.G. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, Y.; Pan, L.; Wang, P.H.; Cui, Y.; Kong, D.C.; Wang, L.; Zhang, X.W.; Zou, J.J. Multi-layer monoclinic BiVO4 with oxygen vacancies and V4+ species for highly efficient visible-light photoelectrochemical applications. Appl. Catal. B Environ. 2018, 221, 187–195. [Google Scholar] [CrossRef]
- Ye, K.Y.; Chai, Z.S.; Gu, J.W.; Yu, X.; Zhao, C.X.; Zhang, Y.M.; Mai, W.J. BiOI-BiVO4 photoanodes with significantly improved solar water splitting capability: P-n junction to expand solar adsorption range and facilitate charge carrier dynamics. Nano Energy 2015, 18, 222–231. [Google Scholar] [CrossRef]
- Li, J.Q.; Guo, Z.Y.; Liu, H.; Du, J.; Zhu, A.F. Two-step hydrothermal process for synthesis of F-doped BiVO4 spheres with enhanced photocatalytic activity. J. Alloys Compd. 2013, 581, 40–45. [Google Scholar] [CrossRef]
- Wang, S.G.; Chen, P.; Bai, Y.; Yun, J.H.; Liu, G.; Wang, L.Z. New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting. Adv. Mater. 2018, 30, 1800486. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Green-synthesized W and Mo-doped BiVO4 oriented along the 040 facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B Environ. 2016, 180, 630–636. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D.; Han, C.C.; Wang, P.; Tong, Z.F.; Tan, B.H.; Liu, Z.F. The synergistic effect of CuBi2O4 and Co-Pi: Improving the PEC activity of BiVO4-based composite materials. New J. Chem. 2022, 46, 2971–2979. [Google Scholar] [CrossRef]
- Fang, G.Z.; Liu, Z.F.; Han, C.C. Enhancing the PEC water splitting performance of BiVO4 co-modifying with NiFeOOH and Co-Pi double layer cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar] [CrossRef]
- Geng, H.M.; Ying, P.Z.; Zhao, Y.L.; Gu, X.Q. Cactus shaped FeOOH/Au/BiVO4 photoanodes for efficient photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 35280–35289. [Google Scholar] [CrossRef]
- Su, F.L.; Wang, T.; Lv, R.; Zhang, J.J.; Zhang, P.; Lu, J.W.; Gong, J.L. Dendritic Au/TiO2 nanorod arrays for visible-light driven photoelectrochemical water splitting. Nanoscales 2013, 5, 9001–9009. [Google Scholar] [CrossRef]
- Nga, T.T.T.; Huang, Y.C.; Chen, J.L.; Chen, C.L.; Lin, B.H.; Yeh, P.H.; Du, C.H.; Chiou, J.W.; Pong, W.F.; Arul, K.T.; et al. Effect of Ag-decorated BiVO4 on photoelectrochemical water splitting: An X-ray absorption spectroscopic investigation. Nanomaterials 2022, 12, 3659. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tu, X.L.; Su, Y.J.; Lu, J.; Hu, J.; Cai, B.F.; Zhou, Z.H.; Yang, Z.; Zhang, Y.F. Controlled growth of vertically aligned ultrathin In2S3 nanosheet arrays for photoelectrochemical water splitting. Nanoscale 2018, 10, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jo, Y.H.; Kim, J.H.; Lee, J.S. Ultrafast fabrication of highly active BiVO4 photoanodes by hybrid microwave annealing for unbiased solar water splitting. Nanoscale 2016, 8, 17623–17631. [Google Scholar] [CrossRef] [PubMed]
- Myung, N.; Ham, S.; Choi, S.; Chae, Y.; Rajeshwar, K. Tailoring interfaces for electrochemical synthesis of semiconductor films: BiVO4, Bi2O3, or composites. J. Phys. Chem. C 2011, 115, 7793–7800. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhang, J.L.; Hou, L.Q.; Fu, X.H.; Yu, X.; Zhu, Y.; Zhang, Y.M. Dual modification with Ag and FeOOH significantly increased the photoelectrochemical water splitting activity of BiVO4 photoanodes. Surf. Interfaces 2021, 25, 101224. [Google Scholar] [CrossRef]
- Gao, X.T.; Bai, Z.Q.; Zhang, S.; Liu, J.C.; Li, Z.H. Highly efficient hamburger-like nanostructure of a triadic Ag/Co3O4/BiVO4 photoanode for enhanced photoelectrochemical water oxidation. RSC Adv. 2020, 10, 45067. [Google Scholar] [CrossRef]
- Zhang, L.; Herrmann, L.O.; Baumberg, J.J. Size dependent plasmonic effect on BiVO4 photoanodes for solar water splitting. Sci. Rep. 2015, 5, 16660. [Google Scholar] [CrossRef]
- Lim, F.S.; Tan, S.T.; Zhu, Y.M.; Chen, J.W.; Wu, B.; Yu, H.; Kim, J.M.; Ginting, R.T.; Lau, K.S.; Chia, C.H.; et al. Tunable plasmon-induced charge transport and photon absorption of bimetallic Au-Ag nanoparticles on ZnO photoanode for photoelectrochemical enhancement under visible light. J. Phys. Chem. 2020, 124, 14105–14117. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; Mckone, J.R.; Boettcher, S.W.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Tian, Y.; Cui, Q.Q.; Xu, L.L.; Jiao, A.X.; Ma, H.; Wang, C.; Zhang, M.Y.; Wang, X.L.; Li, S.; Chen, M. Alloyed AuPt nanoframes loaded on h-BN nanosheets as an ingenious ultrasensitive near-infrared photoelectrochemical biosensor for accurate monitoring glucose in human tears. Biosens. Bioelectron. 2021, 192, 113490. [Google Scholar] [CrossRef] [PubMed]
- Long, X.F.; Gao, L.L.; Li, F.; Hu, Y.P.; Wei, S.Q.; Wang, C.L.; Wang, T.; Jin, J.; Ma, J.T. Bamboo shoots shaped FeVO4 passivated ZnO nanorods photoanode for improved charge separation/transfer process towards efficient solar water splitting. Appl. Catal. B Environ. 2019, 257, 117813. [Google Scholar] [CrossRef]
- Nan, F.; Kang, Z.; Wang, J.; Shen, M.; Fang, L. Carbon quantum dots coated BiVO4 inverse opals for enhanced photoelectrochemical hydrogen generation. Appl. Phys. Lett. 2015, 106, 153901–153905. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, Y.; Ouyang, S.; Yu, H.; Lu, G.; Ye, J.; Bi, Y. BiAg alloy nanospheres: A new photocatalyst for H2 evolution from water splitting. ACS Appl. Mater. Interfaces 2014, 6, 19488–19493. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Zhan, D.; Wang, D.; Han, C.; Fu, Q.; Zhu, H.; Mao, Z.; Liu, Z.-Q. Surface Plasmon Resonance Effect of Noble Metal (Ag and Au) Nanoparticles on BiVO4 for Photoelectrochemical Water Splitting. Inorganics 2023, 11, 206. https://doi.org/10.3390/inorganics11050206
Liu R, Zhan D, Wang D, Han C, Fu Q, Zhu H, Mao Z, Liu Z-Q. Surface Plasmon Resonance Effect of Noble Metal (Ag and Au) Nanoparticles on BiVO4 for Photoelectrochemical Water Splitting. Inorganics. 2023; 11(5):206. https://doi.org/10.3390/inorganics11050206
Chicago/Turabian StyleLiu, Rui, Difu Zhan, Dong Wang, Changcun Han, Qian Fu, Hongxun Zhu, Zhuxiang Mao, and Zhao-Qing Liu. 2023. "Surface Plasmon Resonance Effect of Noble Metal (Ag and Au) Nanoparticles on BiVO4 for Photoelectrochemical Water Splitting" Inorganics 11, no. 5: 206. https://doi.org/10.3390/inorganics11050206
APA StyleLiu, R., Zhan, D., Wang, D., Han, C., Fu, Q., Zhu, H., Mao, Z., & Liu, Z.-Q. (2023). Surface Plasmon Resonance Effect of Noble Metal (Ag and Au) Nanoparticles on BiVO4 for Photoelectrochemical Water Splitting. Inorganics, 11(5), 206. https://doi.org/10.3390/inorganics11050206