Pt(II) Complexes with a Novel Pincer N^C^N Ligand: Synthesis, Characterization, and Photophysics
Abstract
1. Introduction
2. Results and Discussion
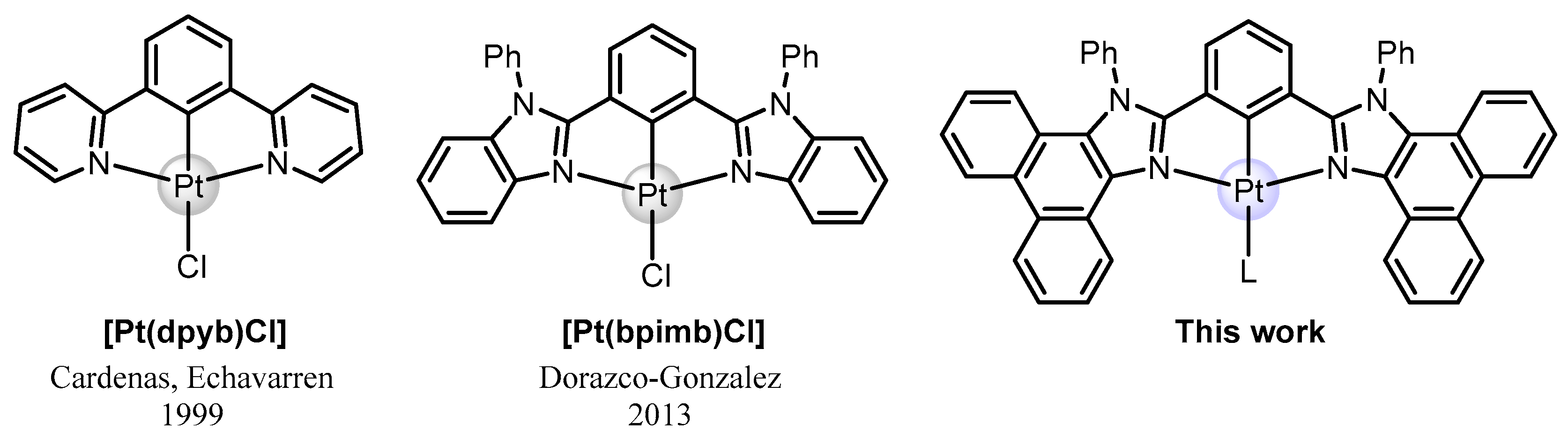
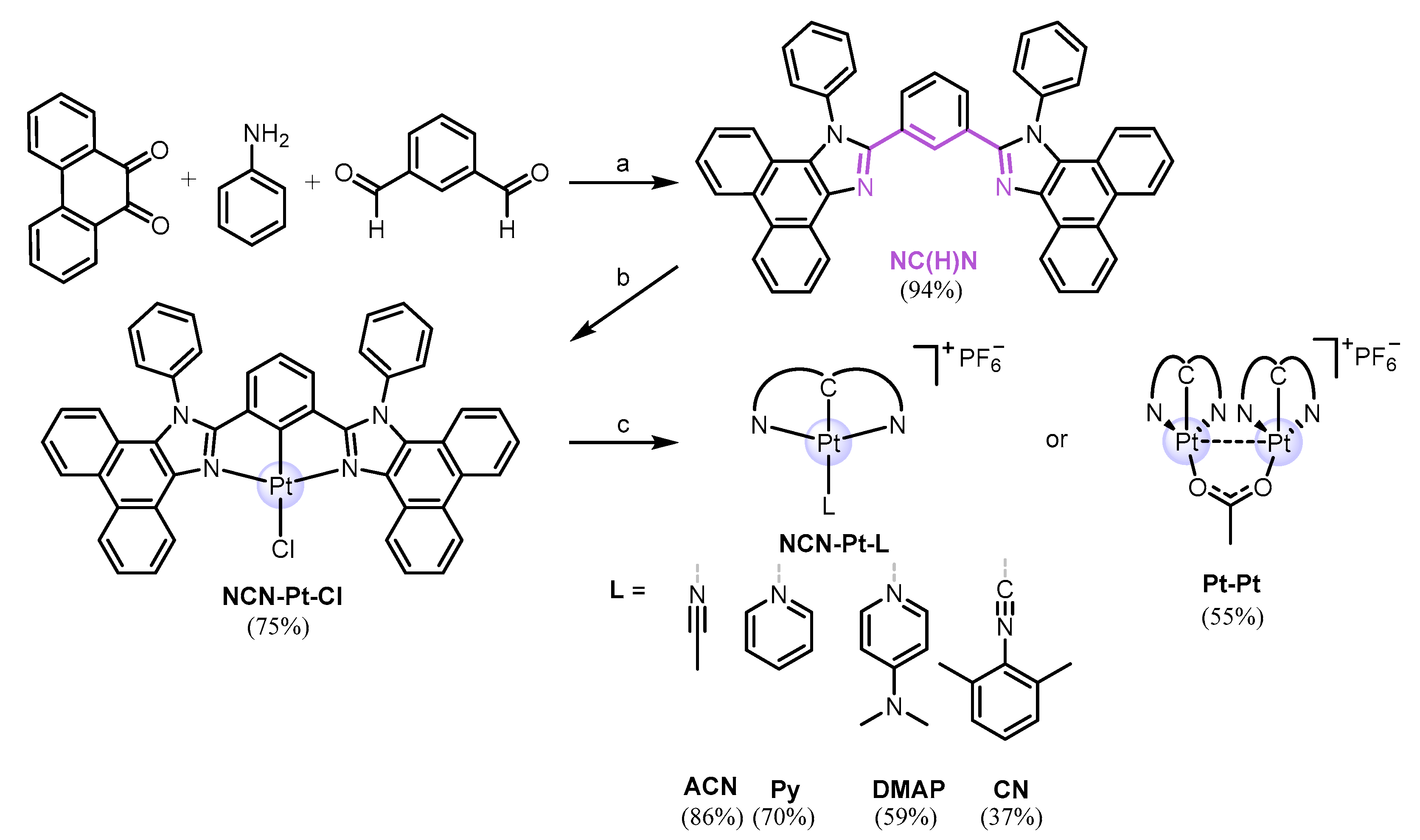
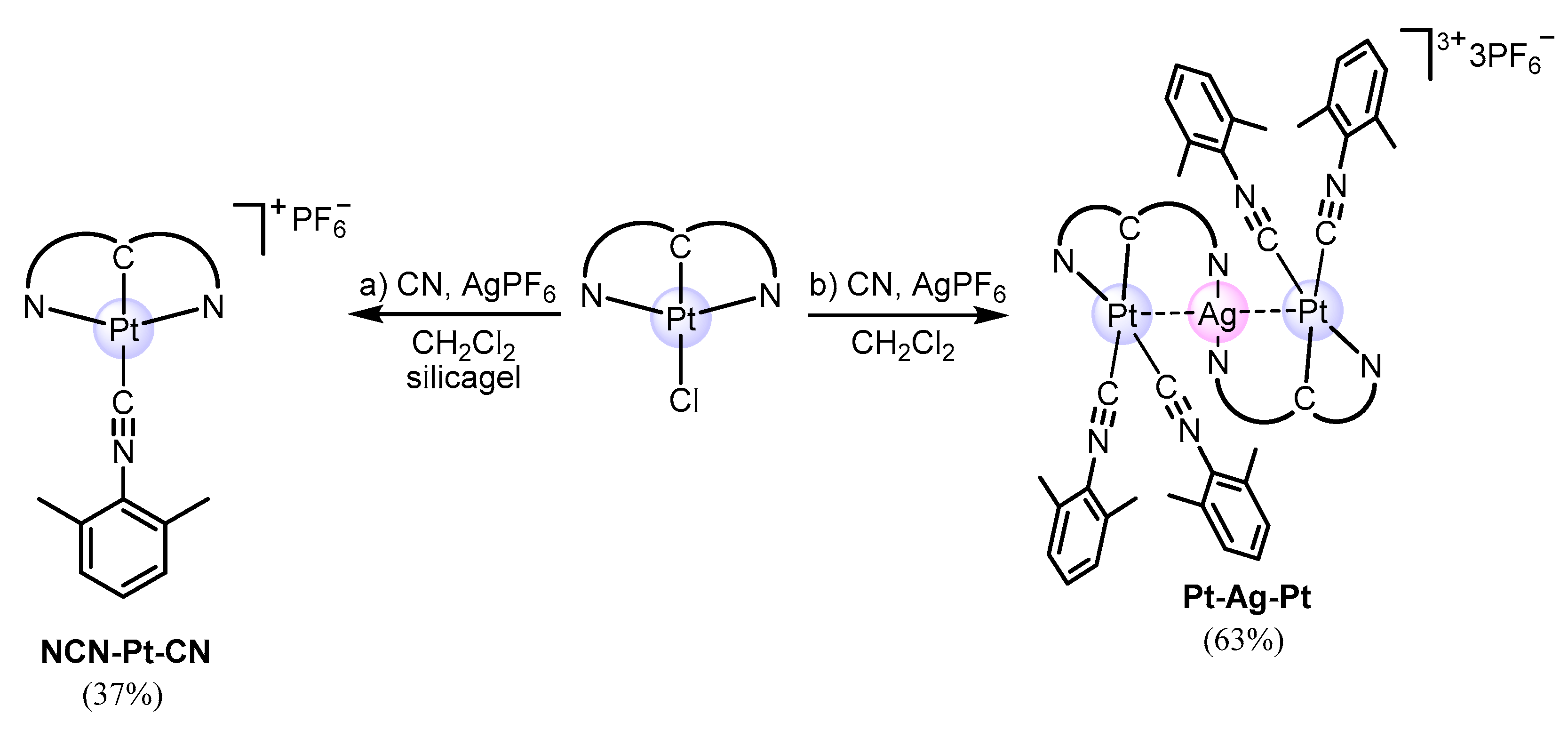
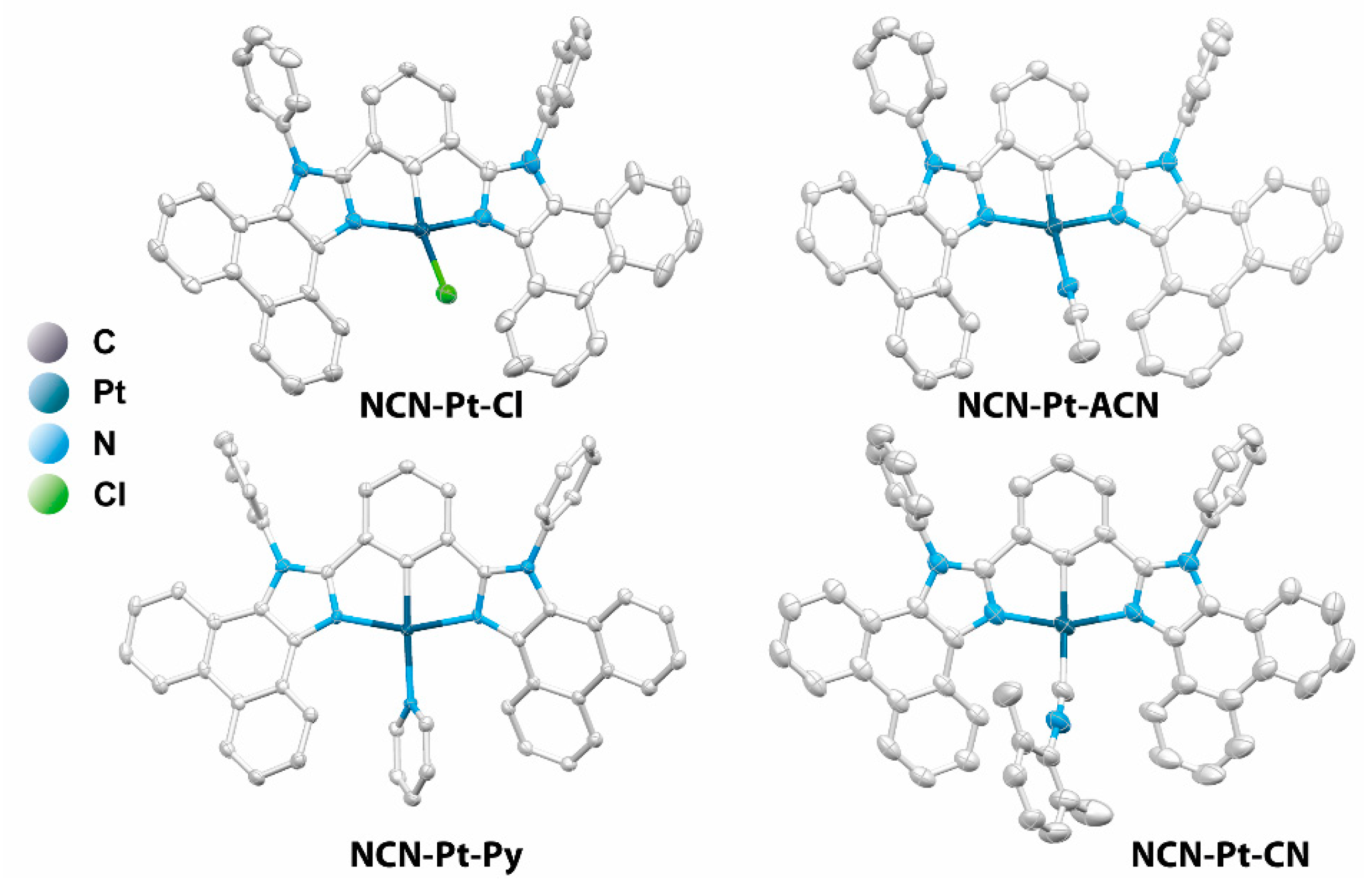
2.1. Design, Synthesis, and Characterization of Mononuclear and Polynuclear Complexes
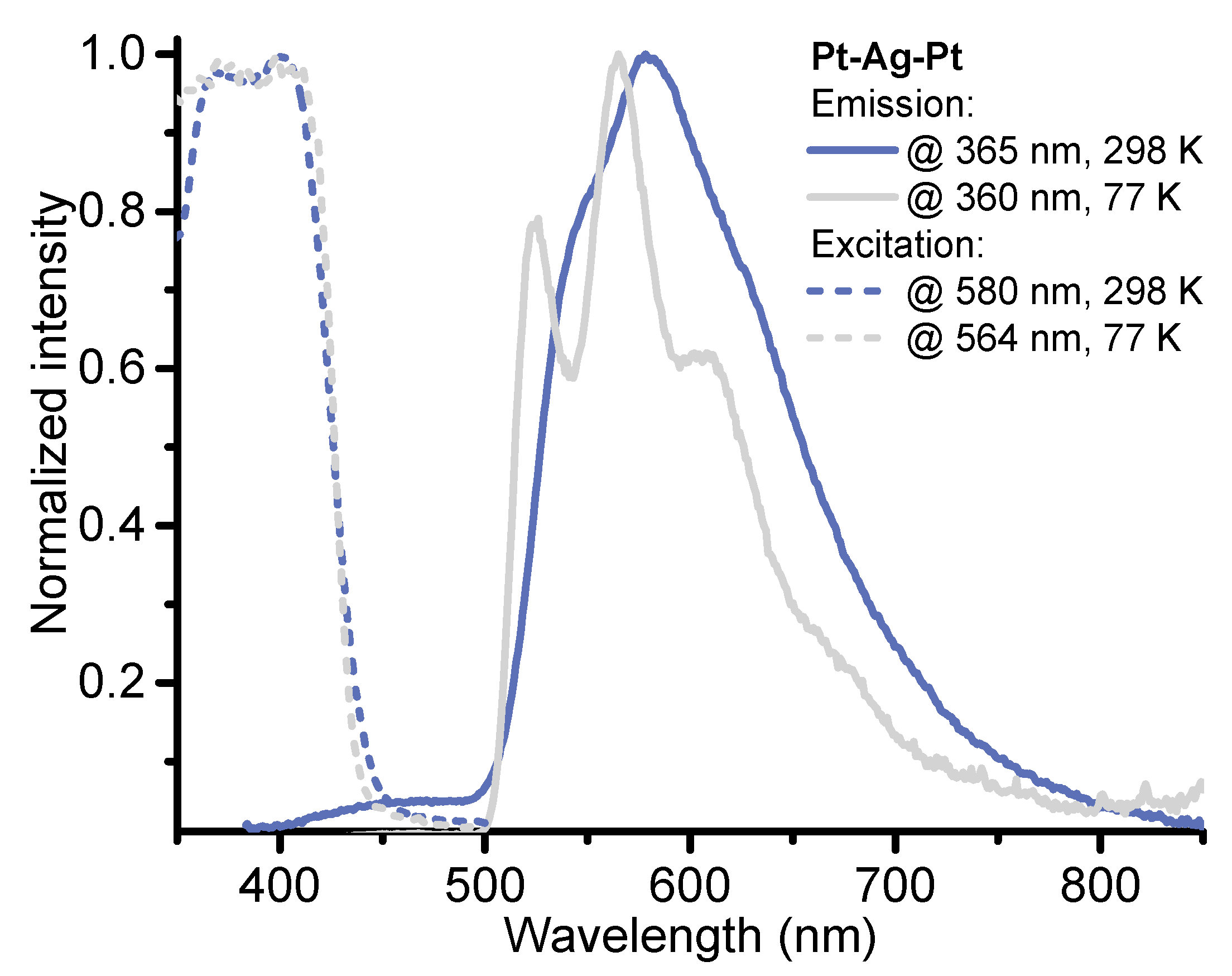
2.2. Photophysical Properties and Computational Studies
3. Materials and Methods
3.1. Synthesis of the Ligand and Complexes
3.2. X-ray Diffraction Analysis
3.3. Photophysical Measurements
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Ming Tong, G.S.; Wan, Q.; Cheng, G.; Tong, W.-Y.Y.; Ang, W.-H.H.; Kwong, W.-L.L.; Che, C.-M.M. Highly phosphorescent platinum(II) emitters: Photophysics, materials and biological applications. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef]
- Huo, S.; Carroll, J.; Vezzu, D.A.K. Design, Synthesis, and Applications of Highly Phosphorescent Cyclometalated Platinum Complexes. Asian J. Org. Chem. 2015, 4, 1210–1245. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Dorazco-González, A. Use of Pincer Compounds as Metal-Based Receptors for Chemosensing of Relevant Analytes. In Pincer Compounds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 587–597. [Google Scholar]
- Yeung, M.C.-L.; Yam, V.W.-W. Luminescent cation sensors: From host–guest chemistry, supramolecular chemistry to reaction-based mechanisms. Chem. Soc. Rev. 2015, 44, 4192–4202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, N.; Zhao, D.; Ma, Y. New cyclometalated transition-metal based photosensitizers for singlet oxygen generation and photodynamic therapy. Sci. China Chem. 2016, 59, 40–52. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Choi, A.W.-T.; Law, W.H.-T. Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes. Dalt. Trans. 2012, 41, 6021. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Aliprandi, A.; Septiadi, D.; Kehr, N.S.; De Cola, L. When self-assembly meets biology: Luminescent platinum complexes for imaging applications. Chem. Soc. Rev. 2014, 43, 4144–4166. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Chen, Y.; Rees, T.W.; Ji, L.; Chao, H. Organelle-targeting metal complexes: From molecular design to bio-applications. Coord. Chem. Rev. 2019, 378, 66–86. [Google Scholar] [CrossRef]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Arrowsmith, R.L.; Pascu, S.I.; Smugowski, H. New developments in the biomedical chemistry of metal complexes: From small molecules to nanotheranostic design. In Organometallic Chemistry; RSC Publishing: Cambridge, UK, 2012; Volume 38, pp. 1–35. ISBN 9781849733762. [Google Scholar]
- Tunik, S.P.; Chelushkin, P.S.; Shakirova, J.R.; Kritchenkov, I.; Baigildin, V.A. Phosphorescent NIR emitters for biomedicine: Applications, advances and challenges. Dalt. Trans. 2021. [Google Scholar] [CrossRef]
- Aliprandi, A.; Genovese, D.; Mauro, M.; De Cola, L. Recent Advances in Phosphorescent Pt(II) Complexes Featuring Metallophilic Interactions: Properties and Applications. Chem. Lett. 2015, 44, 1152–1169. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Galenko, E.E.; Kozina, D.O.; Kalinichev, A.A.; Baigildin, V.A.; Prudovskaya, N.A.; Shakirova, J.R.; Khlebnikov, A.F.; Porsev, V.V.; Evarestov, R.A.; et al. Nonsymmetric [Pt(C^N*N′^C′)] Complexes: Aggregation-Induced Emission in the Solid State and in Nanoparticles Tuned by Ligand Structure. Chem. A Eur. J. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.T.; Pigge, F.C. Platinum(II) Complexes with Sterically Expansive Tetraarylethylene Ligands as Probes for Mismatched DNA. Inorg. Chem. 2018, 57, 12641–12649. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Chung, C.Y.-S.; Yam, V.W.-W. Parallel folding topology-selective label-free detection and monitoring of conformational and topological changes of different G-quadruplex DNAs by emission spectral changes via FRET of mPPE-Ala-Pt(II) complex ensemble. Chem. Sci. 2016, 7, 2842–2855. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.G.; Beeby, A.; Davies, E.S.; Weinstein, J.A.; Wilson, C. An Alternative Route to Highly Luminescent Platinum(II) Complexes: Cyclometalation with N∧C∧N-Coordinating Dipyridylbenzene Ligands. Inorg. Chem. 2003, 42, 8609–8611. [Google Scholar] [CrossRef]
- Puttock, E.V.; Walden, M.T.; Williams, J.A.G. The luminescence properties of multinuclear platinum complexes. Coord. Chem. Rev. 2018, 367, 127–162. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Haukka, M.; Levin, O.V.; Frontera, A.; Kukushkin, V.Y. Supramolecular Assembly of Metal Complexes by (Aryl)I⋅⋅⋅d[Pt II ] Halogen Bonds. Chem. A Eur. J. 2020, 26, 7692–7701. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Lalinde, E.; Moreno, M.T. Luminescent cyclometalated-pentafluorophenyl PtII, PtIV and heteropolynuclear complexes. Coord. Chem. Rev. 2018, 366, 69–90. [Google Scholar] [CrossRef]
- Baya, M.; Belío, Ú.; Forniés, J.; Martín, A.; Perálvarez, M.; Sicilia, V. Neutral benzoquinolate cyclometalated platinum(II) complexes as precursors in the preparation of luminescent Pt–Ag complexes. Inorganica Chim. Acta 2015, 424, 136–149. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Chang, V.Y.; Liu, J.; Yang, X.-L.; Huang, W.; Li, Y.; Li, C.-H.; Muller, G.; You, X.-Z. Potential Switchable Circularly Polarized Luminescence from Chiral Cyclometalated Platinum(II) Complexes. Inorg. Chem. 2015, 54, 143–152. [Google Scholar] [CrossRef]
- Berenguer, J.R.; Lalinde, E.; Teresa Moreno, M. An overview of the chemistry of homo and heteropolynuclear platinum complexes containing bridging acetylide (μ-C≡CR) ligands. Coord. Chem. Rev. 2010, 254, 832–875. [Google Scholar] [CrossRef]
- Williams, J.A.G. Photochemistry and Photophysics of Coordination Compounds: Platinum. In Photochemistry and Photophysics of Coordination Compounds II; Balzani, V., Campagna, S., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2007; Volume 281, pp. 205–268. ISBN 978-3-540-73348-5, 978-3-540-73349-2. [Google Scholar]
- Williams, J.A.G. The coordination chemistry of dipyridylbenzene: N-deficient terpyridine or panacea for brightly luminescent metal complexes? Chem. Soc. Rev. 2009, 38, 1783–1801. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.S.-M.; Che, C.-M. Emissive or nonemissive? A theoretical analysis of the phosphorescence efficiencies of cyclometalated platinum(II) complexes. Chem. A Eur. J. 2009, 15, 7225–7237. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wen, Y.; Wang, L.; Li, J.; Zhang, J. Comprehension of the Effect of a Hydroxyl Group in Ancillary Ligand on Phosphorescent Property for Heteroleptic Ir(III) Complexes: A Computational Study Using Quantitative Prediction. Inorg. Chem. 2017, 56, 8986–8995. [Google Scholar] [CrossRef]
- Sajoto, T.; Djurovich, P.I.; Tamayo, A.B.; Oxgaard, J.; Goddard, W.A.; Thompson, M.E. Temperature Dependence of Blue Phosphorescent Cyclometalated Ir(III) Complexes. J. Am. Chem. Soc. 2009, 131, 9813–9822. [Google Scholar] [CrossRef]
- Wang, Z.; Turner, E.; Mahoney, V.; Madakuni, S.; Groy, T.; Li, J. Facile Synthesis and Characterization of Phosphorescent Pt(N∧C∧N)X Complexes. Inorg. Chem. 2010, 49, 11276–11286. [Google Scholar] [CrossRef]
- Cárdenas, D.J.; Echavarren, A.M.; Ramírez de Arellano, M.C. Divergent Behavior of Palladium(II) and Platinum(II) in the Metalation of 1,3-Di(2-pyridyl)benzene. Organometallics 1999, 18, 3337–3341. [Google Scholar] [CrossRef]
- Farley, S.J.; Rochester, D.L.; Thompson, A.L.; Howard, J.A.K.; Williams, J.A.G. Controlling Emission Energy, Self-Quenching, and Excimer Formation in Highly Luminescent N∧C∧N-Coordinated Platinum(II) Complexes. Inorg. Chem. 2005, 44, 9690–9703. [Google Scholar] [CrossRef]
- Tarran, W.A.; Freeman, G.R.; Murphy, L.; Benham, A.M.; Kataky, R.; Williams, J.A.G. Platinum(II) complexes of N^C^N-coordinating 1,3-bis(2-pyridyl)benzene ligands: Thiolate coligands lead to strong red luminescence from charge-transfer states. Inorg. Chem. 2014, 53, 5738–5749. [Google Scholar] [CrossRef]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef]
- Dorazco-Gonzalez, A. Chemosensing of Chloride Based on a Luminescent Platinum(II) NCN Pincer Complex in Aqueous Media. Organometallics 2014, 33, 868–875. [Google Scholar] [CrossRef]
- Tam, A.Y.-Y.; Tsang, D.P.-K.; Chan, M.-Y.; Zhu, N.; Yam, V.W.-W. A luminescent cyclometalated platinum(II) complex and its green organic light emitting device with high device performance. Chem. Commun. 2011, 47, 3383. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.S.-H.; Tsang, D.P.-K.; Lam, W.H.; Tam, A.Y.-Y.; Chan, M.-Y.; Wong, W.-T.; Yam, V.W.-W. Luminescent Platinum(II) Complexes of 1,3-Bis( N -alkylbenzimidazol- 2′-yl)benzene-Type Ligands with Potential Applications in Efficient Organic Light-Emitting Diodes. Chem. A Eur. J. 2013, 19, 6385–6397. [Google Scholar] [CrossRef]
- Lam, E.S.-H.; Tam, A.Y.-Y.; Chan, M.-Y.; Yam, V.W.-W. A New Class of Luminescent Platinum(II) Complexes of 1,3-Bis( N -alkylbenzimidazol-2′-yl)benzene-Type Ligands and Their Application Studies in the Fabrication of Solution-Processable Organic Light-Emitting Devices. Isr. J. Chem. 2014, 54, 986–992. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Wang, J.; Wu, C.; Zhang, X.; Yin, X. Amplification of circularly polarized luminescence from chiral cyclometalated platinum(II) complexes by the formation of excimer. J. Organomet. Chem. 2022, 973–974, 122394. [Google Scholar] [CrossRef]
- Chan, M.H.-Y.; Wong, H.-L.; Yam, V.W.-W. Synthesis and Photochromic Studies of Dithienylethene-Containing Cyclometalated Alkynylplatinum(II) 1,3-Bis( N -alkylbenzimidazol-2′-yl)benzene Complexes. Inorg. Chem. 2016, 55, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yuan, B.; Hou, X.; Yan, C.; Sun, X.; Xie, Z.; Shao, X.; Zhou, S. Broadband optical limiting of a novel twisted tetrathiafulvalene incorporated donor–acceptor material and its Ormosil gel glasses. J. Mater. Chem. C 2018, 6, 8495–8501. [Google Scholar] [CrossRef]
- Qiu, Y.; Feng, Y.; Zhao, Q.; Wang, H.; Guo, Y.; Qiu, D. White light emission from a green cyclometalated platinum(II) terpyridylphenylacetylide upon titration with Zn(II) and Eu(III). Dalt. Trans. 2020, 49, 11163–11169. [Google Scholar] [CrossRef]
- Kong, F.K.-W.; Tang, M.-C.; Wong, Y.-C.; Chan, M.-Y.; Yam, V.W.-W. Design Strategy for High-Performance Dendritic Carbazole-Containing Alkynylplatinum(II) Complexes and Their Application in Solution-Processable Organic Light-Emitting Devices. J. Am. Chem. Soc. 2016, 138, 6281–6291. [Google Scholar] [CrossRef]
- Marqués-López, E.; Herrera, R.P. Essential Multicomponent Reactions I. In Multicomponent Reactions; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 382–415. [Google Scholar]
- Dias, G.G.; Paz, E.R.S.; Nunes, M.P.; Carvalho, R.L.; Rodrigues, M.O.; Rodembusch, F.S.; Silva Júnior, E.N. Imidazoles and Oxazoles from Lapachones and Phenanthrene-9,10-dione: A Journey through their Synthesis, Biological Studies, and Optical Applications. Chem. Rec. 2021, 21, 2702–2738. [Google Scholar] [CrossRef]
- Bagheri, M.; Mirzaee, M.; Hosseini, S.; Gholamzadeh, P. The photochromic switchable imidazoles: Their genesis, development, synthesis, and characterization. Dye. Pigment. 2022, 203, 110322. [Google Scholar] [CrossRef]
- Ye, S.; Zhuang, S.; Pan, B.; Guo, R.; Wang, L. Imidazole derivatives for efficient organic light-emitting diodes. J. Inf. Disp. 2020, 21, 173–196. [Google Scholar] [CrossRef]
- Tu, L.; Xie, Y.; Li, Z.; Tang, B. Aggregation-induced emission: Red and near-infrared organic light-emitting diodes. SmartMat 2021, 2, 326–346. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Wei, J.; Miao, Y.; Zhang, Z.; Zhang, Z.; Liu, Y.; Wang, Y. Structurally simple phenanthroimidazole-based bipolar hosts for high-performance green and red electroluminescent devices. RSC Adv. 2015, 5, 73926–73934. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Kuznetsov, K.M.; Gurzhiy, V.V.; Pavlovskiy, V.V.; Porsev, V.V.; Evarestov, R.A.; Tunik, S.P.; Solomatina, A.I.; Kuznetsov, K.M.; Gurzhiy, V.V.; et al. Luminescent organic dyes containing a phenanthro[9,10- D ]imidazole core and [Ir(N^C)(N^N)] + complexes based on the cyclometalating and diimine ligands of this type. Dalt. Trans. 2020, 49, 6751–6763. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. Cyclometalation using d-block transition metals: Fundamental aspects and recent trends. Chem. Rev. 2010, 110, 576–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Z.; Hao, X.-Q.; Niu, J.-L.; Wei, D.; Tu, T.; Gong, J.-F.; Song, M.-P. Neutral and Cationic NCN Pincer Platinum(II) Complexes with 1,3-Bis(benzimidazol-2′-yl)benzene Ligands: Synthesis, Structures, and Their Photophysical Properties. Organometallics 2014, 33, 1563–1573. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, M. Regulation of metal–metal interactions and chromic phenomena of multi-decker platinum complexes having π-systems. Coord. Chem. Rev. 2018, 355, 101–115. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef]
- Fernández-Moreira, V.; Thorp-Greenwood, F.L.; Coogan, M.P. Application of d6 transition metal complexes in fluorescence cell imaging. Chem. Commun. 2010, 46, 186–202. [Google Scholar] [CrossRef]
- Thorp-Greenwood, F.L. An Introduction to Organometallic Complexes in Fluorescence Cell Imaging: Current Applications and Future Prospects. Organometallics 2012. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Chelushkin, P.S.; Krupenya, D.V.; Podkorytov, I.S.; Artamonova, T.O.; Sizov, V.V.; Melnikov, A.S.; Gurzhiy, V.V.; Koshel, E.I.; Shcheslavskiy, V.I.; et al. Coordination to Imidazole Ring Switches on Phosphorescence of Platinum Cyclometalated Complexes: The Route to Selective Labeling of Peptides and Proteins via Histidine Residues. Bioconjug. Chem. 2017, 28, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.P.; Fernández-Moreira, V. Progress with, and prospects for, metal complexes in cell imaging. Chem. Commun. 2014, 50, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Stacey, O.J.; Ward, B.D.; Coles, S.J.; Horton, P.N.; Pope, S.J.A. Chromophore-labelled, luminescent platinum complexes: Syntheses, structures, and spectroscopic properties. Dalt. Trans. 2016, 45, 10297–10307. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, P.; Journaud, O.; Vanthuyne, N.; Jacquemin, D.; Favereau, L.; Crassous, J.; Ros, A. Helical donor–acceptor platinum complexes displaying dual luminescence and near-infrared circularly polarized luminescence. Dalt. Trans. 2021, 50, 13220–13226. [Google Scholar] [CrossRef]
- Irmler, P.; Winter, R.F. σ-Pt-BODIPY Complexes with Platinum Attachment to Carbon Atoms C2 or C3: Spectroscopic, Structural, and (Spectro)Electrochemical Studies and Photocatalysis. Organometallics 2018, 37, 235–253. [Google Scholar] [CrossRef]
- Geist, F.; Jackel, A.; Irmler, P.; Linseis, M.; Malzkuhn, S.; Kuss-Petermann, M.; Wenger, O.S.; Winter, R.F. Directing Energy Transfer in Panchromatic Platinum Complexes for Dual Vis–Near-IR or Dual Visible Emission from σ-Bonded BODIPY Dyes. Inorg. Chem. 2017, 56, 914–930. [Google Scholar] [CrossRef]
- Horiuchi, S.; Moon, S.; Ito, A.; Tessarolo, J.; Sakuda, E.; Arikawa, Y.; Clever, G.H.; Umakoshi, K. Multinuclear Ag Clusters Sandwiched by Pt Complex Units: Fluxional Behavior and Chiral-at-Cluster Photoluminescence. Angew. Chemie Int. Ed. 2021, 60, 10654–10660. [Google Scholar] [CrossRef]
- Shakirova, J.R.; Hendi, Z.; Zhukovsky, D.D.; Sokolov, V.V.; Jamali, S.; Pavlovskiy, V.V.; Porsev, V.V.; Evarestov, R.A.; Tunik, S.P. NIR emitting platinum pincer complexes based on the N^N^C ligand containing {benz[4,5]imidazo[1,2-a]pyrazin} aromatic system; synthesis, characterization and photophysical study. Inorganica Chim. Acta 2020, 511, 119776. [Google Scholar] [CrossRef]
- Fleetham, T.; Golden, J.H.; Idris, M.; Hau, H.-M.; Muthiah Ravinson, D.S.; Djurovich, P.I.; Thompson, M.E. Tuning State Energies for Narrow Blue Emission in Tetradentate Pyridyl-Carbazole Platinum Complexes. Inorg. Chem. 2019, 58, 12348–12357. [Google Scholar] [CrossRef]
- You, Y.; Kim, K.S.; Ahn, T.K.; Kim, D.; Park, S.Y. Direct Spectroscopic Observation of Interligand Energy Transfer in Cyclometalated Heteroleptic Iridium(III) Complexes: A Strategy for Phosphorescence Color Tuning and White Light Generation. J. Phys. Chem. C 2007, 111, 4052–4060. [Google Scholar] [CrossRef]
- Na, H.; Lai, P.; Cañada, L.M.; Teets, T.S. Photoluminescence of Cyclometalated Iridium Complexes in Poly(methyl methacrylate) Films. Organometallics 2018, 37, 3269–3277. [Google Scholar] [CrossRef]
- CrysAlisPro, Rigaku Oxford Diffraction, Version: 1.171.39.35a 2017.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 2016. Available online: https://gaussian.com (accessed on 28 April 2023).
- Peverati, R.; Truhlar, D.G. Screened-exchange density functionals with broad accuracy for chemistry and solid-state physics. Phys. Chem. Chem. Phys. 2012, 14, 16187. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. CCLIB: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
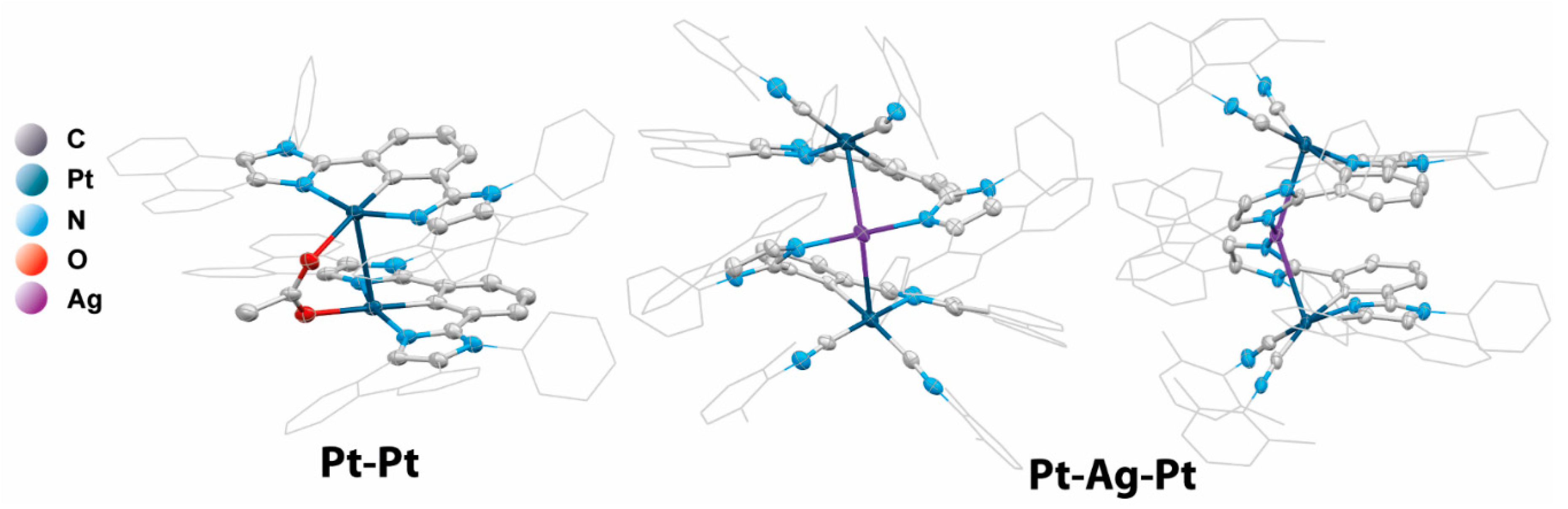
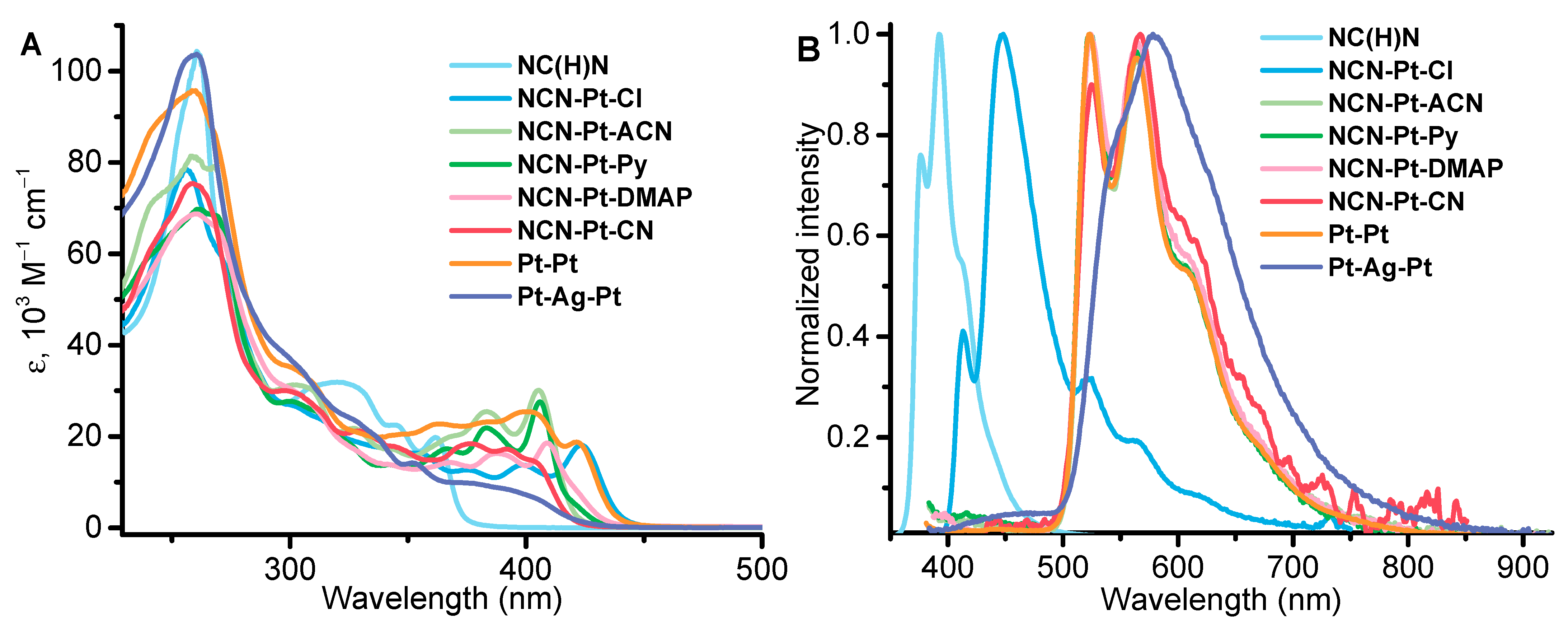
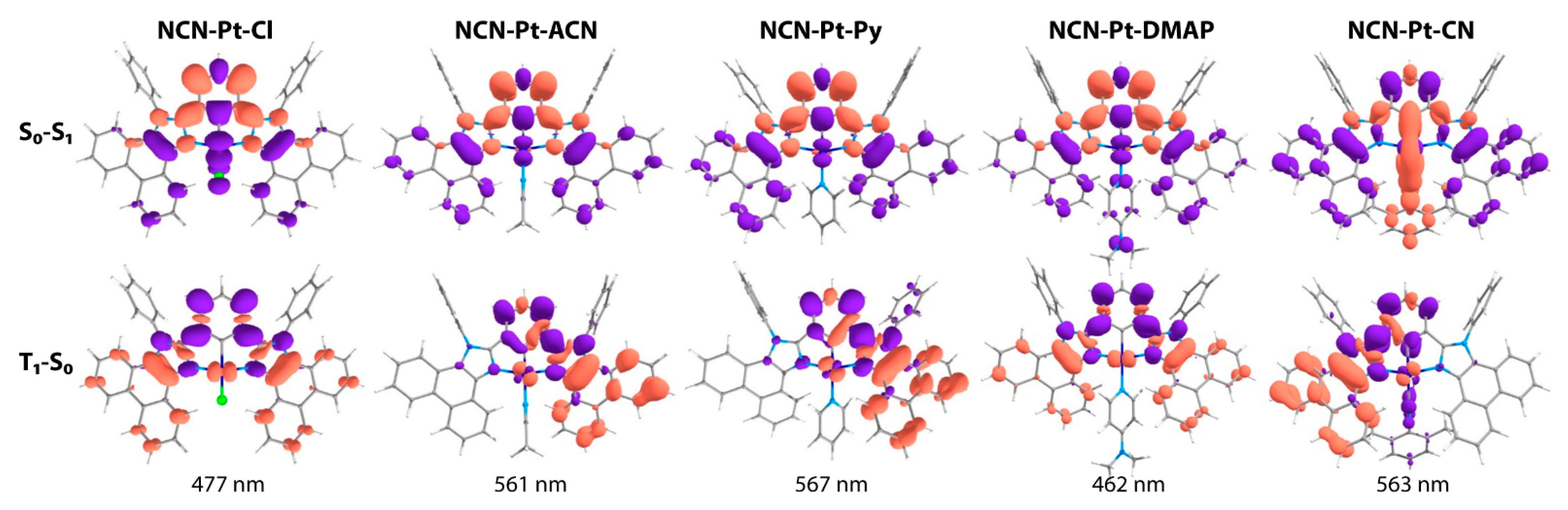
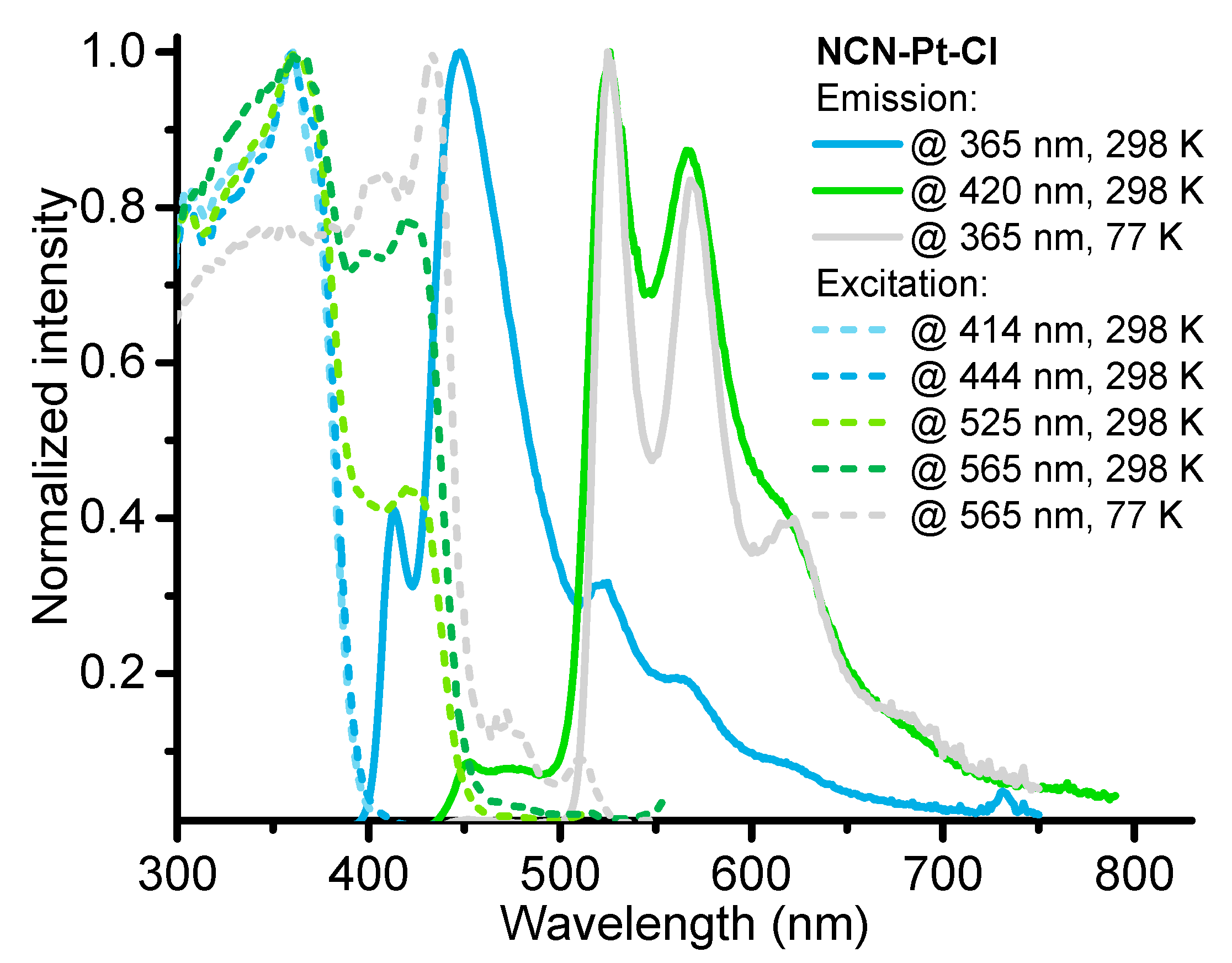

| № | λabs, nm (ε × 10−3, M−1cm−1) | λem, nm 298 K, (77 K) | τobs a (aer/deg) b, μs | Φ (aer/deg) b, % | kr c, s−1 | knr d, s−1 |
|---|---|---|---|---|---|---|
| NC(H)N | 260 (104), 320 (32), 345 (22), 362 (20) | 373, 392, 410sh, 440sh | 1.82 ns @ 450 nm | 30 | 1.65 × 108 | 3.85 × 108 |
| NCN-Pt-Cl | 257 (78), 270sh (61), 300sh (27), 315sh (23), 345sh (18), 356sh (16), 376 (13), 398 (14), 424 (18) | fl: 397, 416, 440, ph: 525, 568, 615sh (526, 570, 622) | fl: 0.0041 @ 450 nm ph: 0.053/0.060 @ 600 nm | fl: 3.03 @ 420 nm ph: 0.56/0.87 @ 600 nm | * | * |
| NCN-Pt-ACN | 247 (67), 270sh (44), 310sh (16), 345sh (18), 358sh (13), 395 (16), 420 (17) | 523, 564, 605sh (522, 564, 613, 670sh) | 0.55/2.27 | 0.75/3.6 | 0.016 × 106 | 0.425 × 106 |
| NCN-Pt-Py | 258 (55), 310sh (18), 364sh (13), 384sh (13), 393sh (13), 405 (13), 420 (9.1) | 523, 564, 605sh | 0.32/0.93 | 0.39/2.3 | 0.025 × 106 | 1.05 × 106 |
| NCN-Pt-DMAP | 261 (61), 270sh (58), 305sh (26), 328sh (15), 340sh (12), 368 (13), 388 (14), 409 (16), 420sh (6.4) | 524, 564, 605sh | 0.36/1.96 | 0.35/1.14 | 0.0058 × 106 | 0.50 × 106 |
| NCN-Pt-CN | 259 (60), 300sh (24), 330sh (17), 345sh (14), 376 (15), 391 (14), 405sh (11) | 524, 564, 605sh | 0.20/0.96 | 0.10/0.18 | 0.019 × 106 | 1.04 × 106 |
| Pt-Pt | 260 (60), 305sh (20), 325sh (14), 370sh (13), 385 (14), 407sh (15), 420sh (5.6) | 525, 565, 605sh | 0.61/8.54 | 0.67/9.02 | 0.011 × 106 | 0.11 × 106 |
| Pt-Ag-Pt | 260 (132), 300sh (47), 330sh (29), 353 (18), 390sh (11) | 550sh, 578, 630sh (525, 565, 610, 670sh) | 1.57/10.74 | 0.31/1.6 | 0.0015 × 106 | 0.09 × 106 |
| № | Medium | λex, nm | λem, nm | τobs a, μs | Φ, % | kr b, ×104 s−1 | knr c, ×104 s−1 |
|---|---|---|---|---|---|---|---|
| NCN-Pt-Cl | solid state | 435, 477, 510 | 523, 566, 616, 668sh | 0.21 | 2.1 | 10.05 | 468.42 |
| PMMA | 377, 400, 419 | 527, 568, 610sh, 675sh | 5.4 | 12 | 2.24 | 16.40 | |
| NCN-Pt-ACN | solid state | 422, 435, 477, 510 | 524, 566, 611, 668sh | 1.2 | 4.7 | 4.02 | 81.45 |
| PMMA | 368, 386, 407 | 535, 567, 625sh | 10.5 | 11 | 1.05 | 8.49 | |
| NCN-Pt-Py | solid state | 423, 477, 508 | 520, 566, 605sh | 1.9 | 3.8 | 2.05 | 51.80 |
| PMMA | 367, 384, 406 | 525, 560, 610sh | 12.1 | 15 | 1.24 | 7.05 | |
| NCN-Pt-DMAP | solid state | 405, 416, 477, 508 | 524, 566, 608, 668sh | 2.6 | 12 | 4.70 | 34.50 |
| PMMA | 370, 393, 414 | 530, 567, 620sh | 8.5 | 12 | 1.41 | 10.34 | |
| NCN-Pt-CN | solid state | 429, 477, 510 | 525, 567, 608, 670sh | 0.99 | 1.4 | 1.41 | 99.30 |
| PMMA | 370, 395, 405 | 523, 562, 610sh | 15.5 | 13 | 0.84 | 5.62 | |
| Pt-Pt | solid state | 440, 477, 503 | 562, 595, 645sh | 0.71 | 1.8 | 2.53 | 137.92 |
| PMMA | 366, 385, 407 | 520, 560, 610sh | 11.5 | 14 | 1.21 | 7.46 | |
| Pt-Ag-Pt | solid state | 403, 500 | 576 | 8.2 | 9 | 1.10 | 11.08 |
| PMMA | 370, 395sh | 529sh, 562, 610sh | 22.2 | 22 | 0.99 | 3.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luneva, E.E.; Kozina, D.O.; Mozzhukhina, A.V.; Porsev, V.V.; Solomatina, A.I.; Tunik, S.P. Pt(II) Complexes with a Novel Pincer N^C^N Ligand: Synthesis, Characterization, and Photophysics. Inorganics 2023, 11, 198. https://doi.org/10.3390/inorganics11050198
Luneva EE, Kozina DO, Mozzhukhina AV, Porsev VV, Solomatina AI, Tunik SP. Pt(II) Complexes with a Novel Pincer N^C^N Ligand: Synthesis, Characterization, and Photophysics. Inorganics. 2023; 11(5):198. https://doi.org/10.3390/inorganics11050198
Chicago/Turabian StyleLuneva, Evgeniia E., Daria O. Kozina, Anna V. Mozzhukhina, Vitaly V. Porsev, Anastasia I. Solomatina, and Sergey P. Tunik. 2023. "Pt(II) Complexes with a Novel Pincer N^C^N Ligand: Synthesis, Characterization, and Photophysics" Inorganics 11, no. 5: 198. https://doi.org/10.3390/inorganics11050198
APA StyleLuneva, E. E., Kozina, D. O., Mozzhukhina, A. V., Porsev, V. V., Solomatina, A. I., & Tunik, S. P. (2023). Pt(II) Complexes with a Novel Pincer N^C^N Ligand: Synthesis, Characterization, and Photophysics. Inorganics, 11(5), 198. https://doi.org/10.3390/inorganics11050198