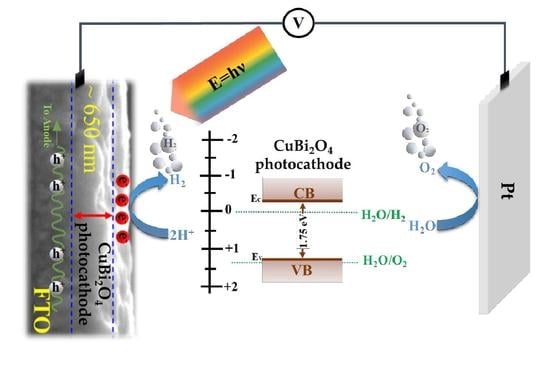
Photoelectrochemical Performance of a CuBi2O4 Photocathode with H2O2 as a Scavenger
Abstract
1. Introduction
2. Experimental Section
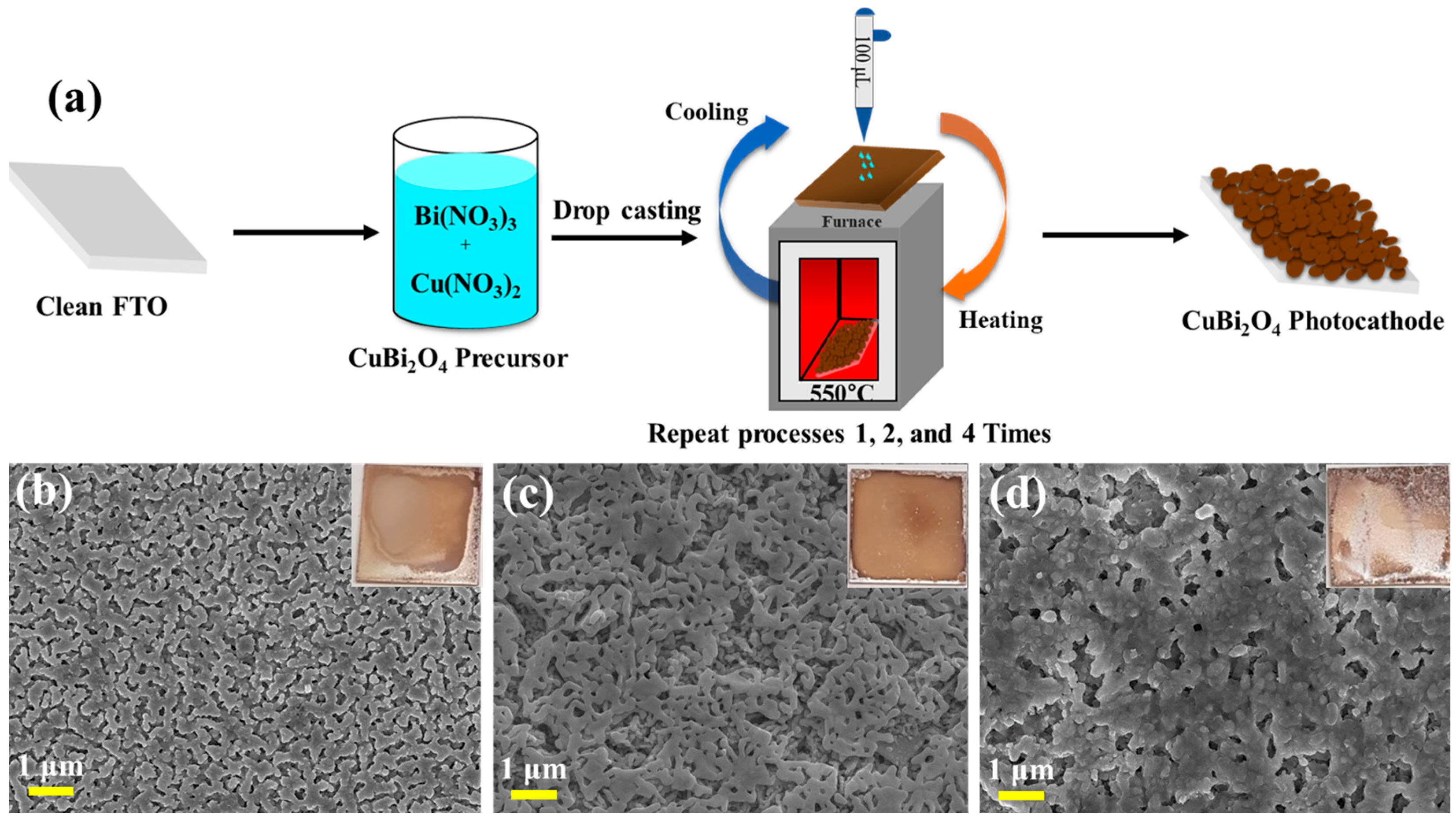
2.1. Fabrication of CuBi2O4 Photocathodes’ Thin Films
2.2. Photoelectrochemical (PEC) Measurements
3. Results and discussion
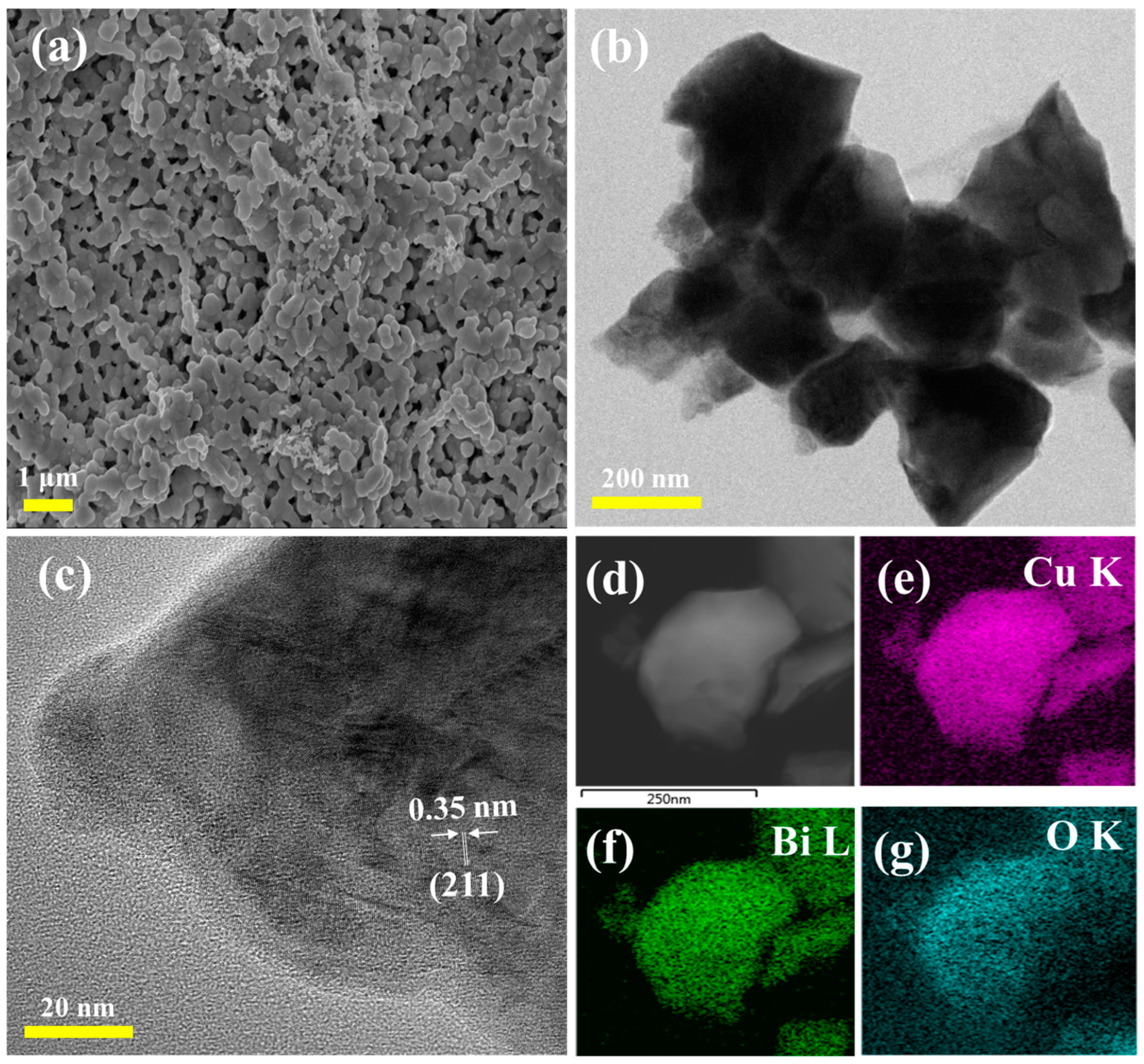
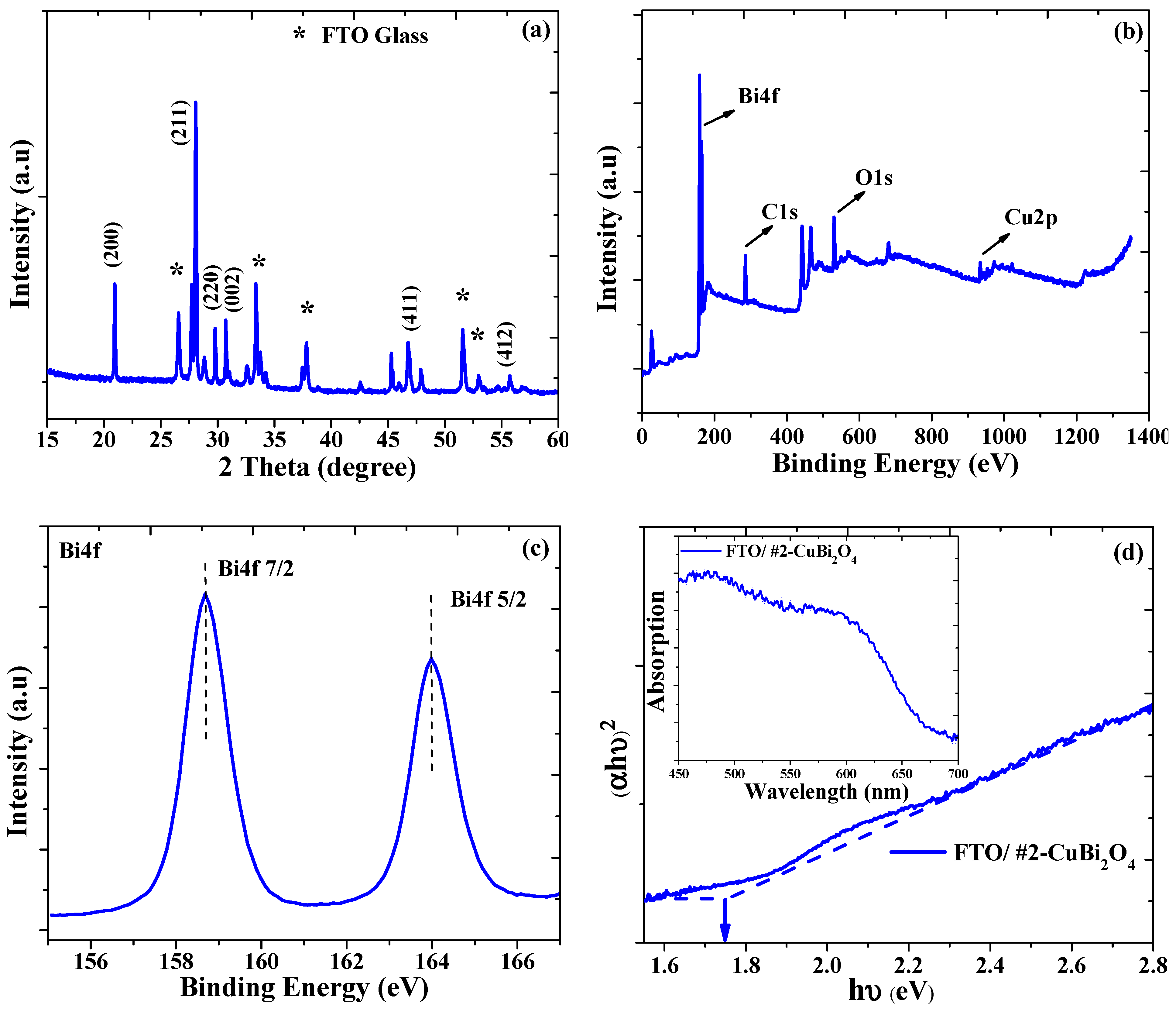
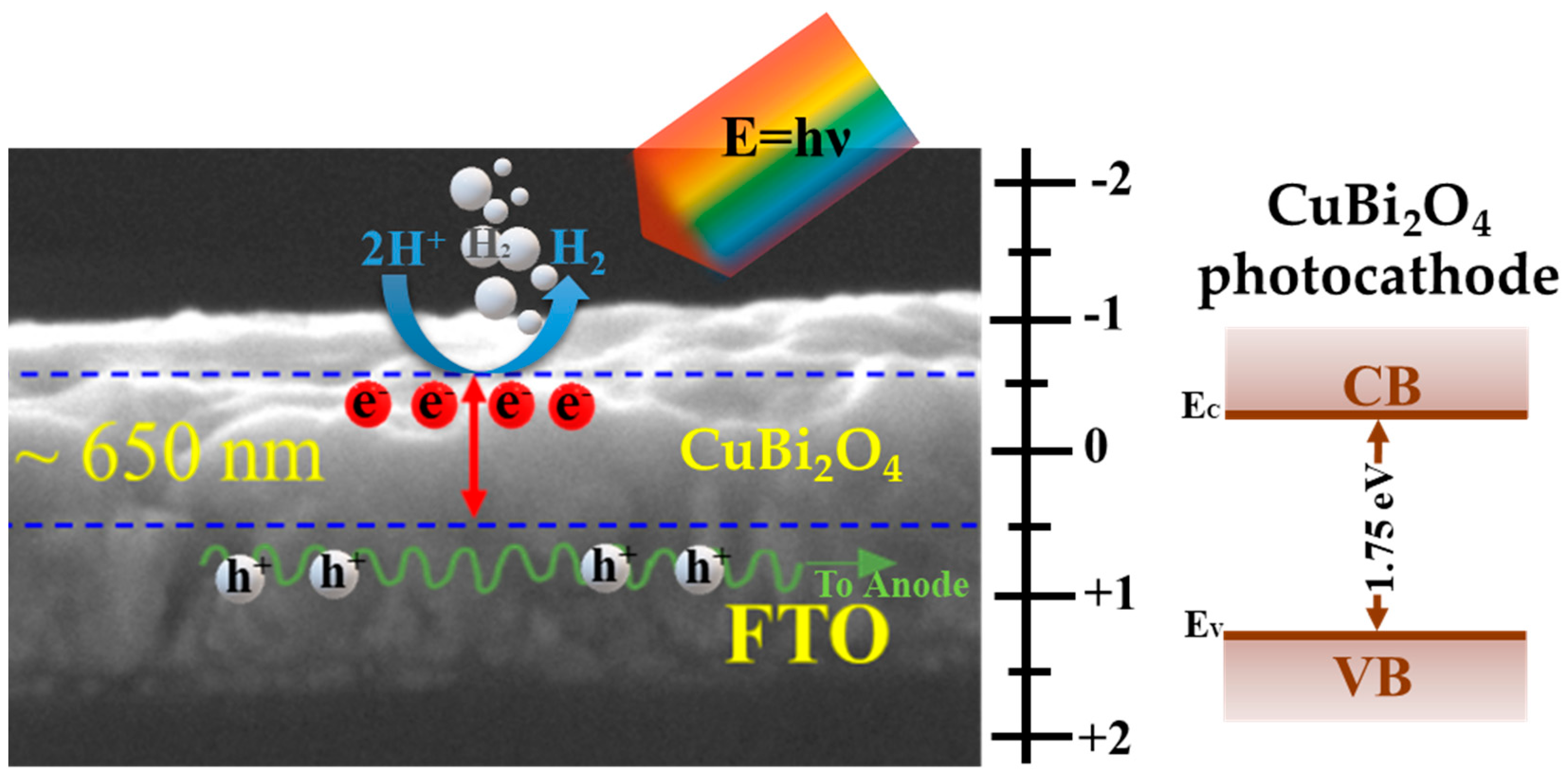
3.1. Physiochemical and Morphological Properties
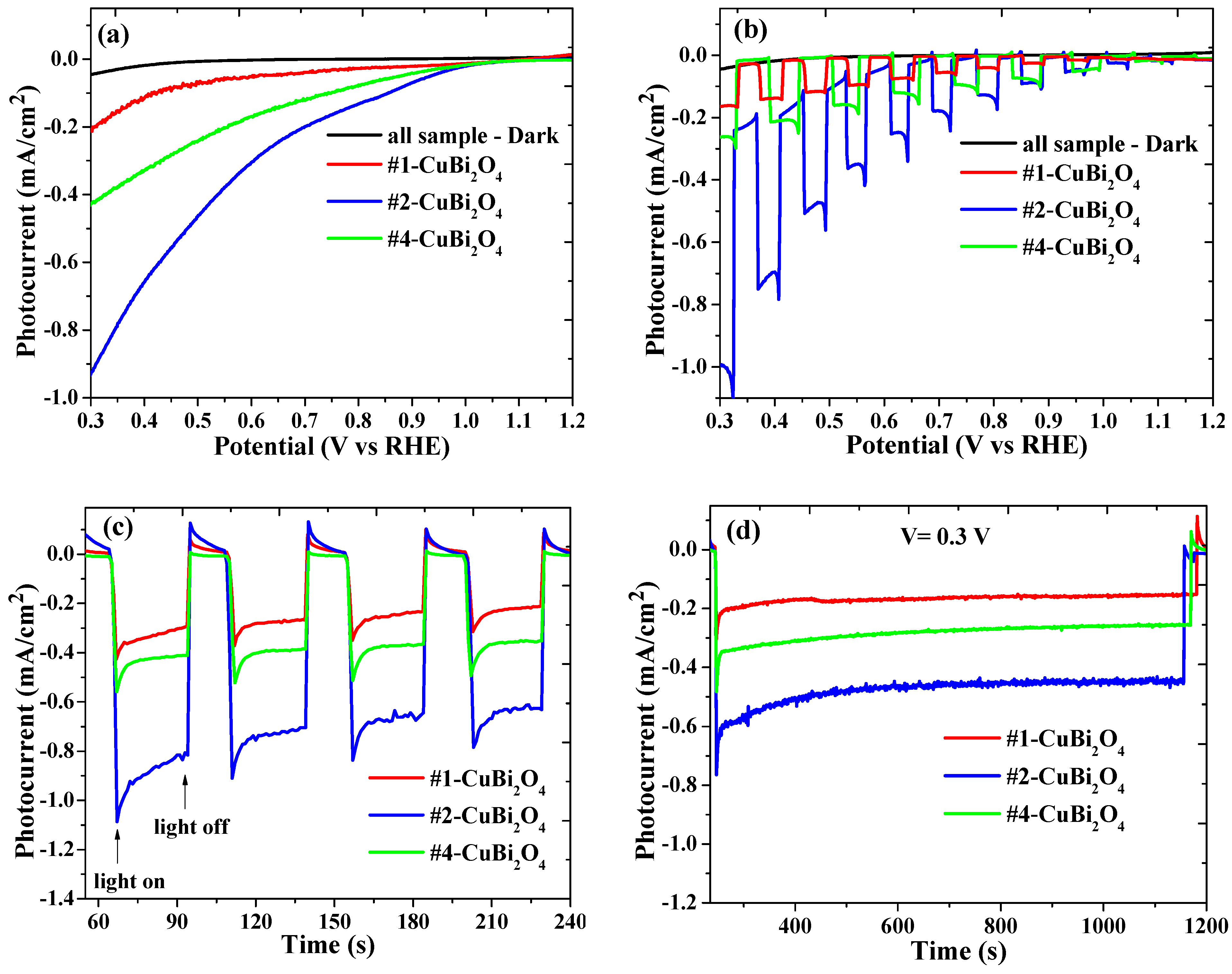
3.2. PEC Water Splitting Properties
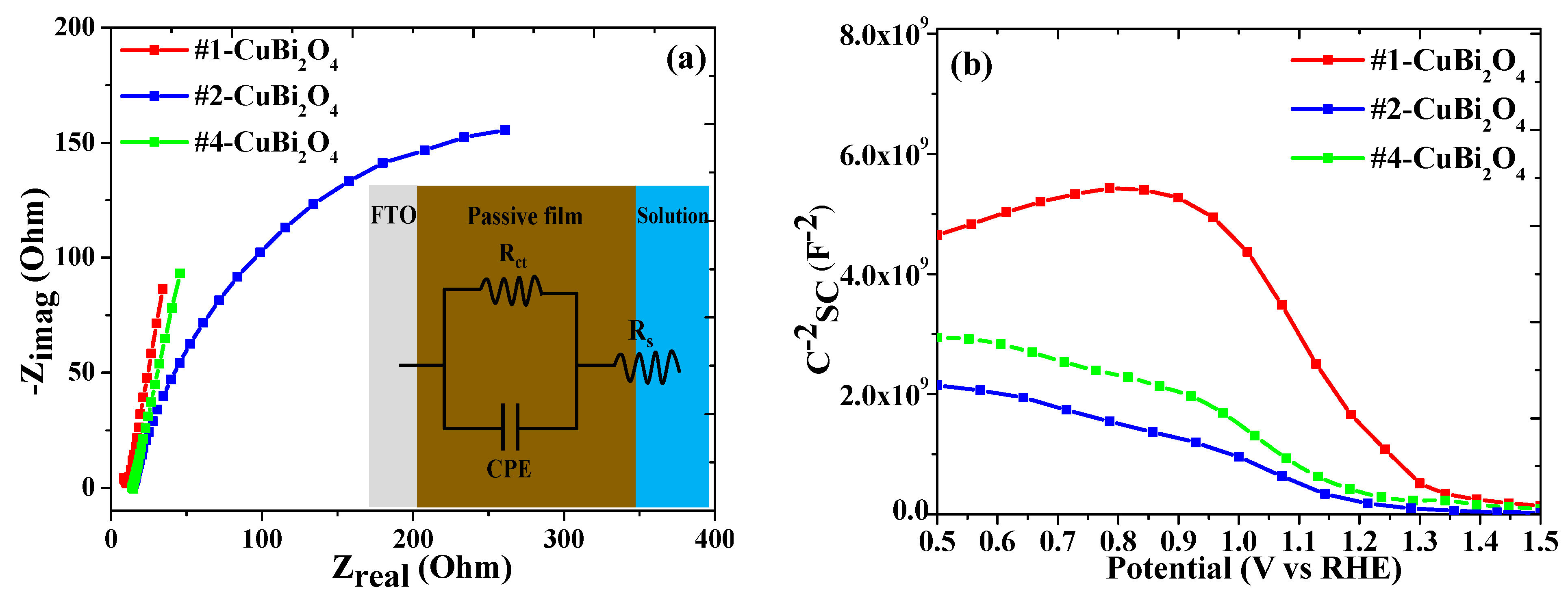
3.3. Nyquist (Impedance Analysis) and Mott–Schottky Plots
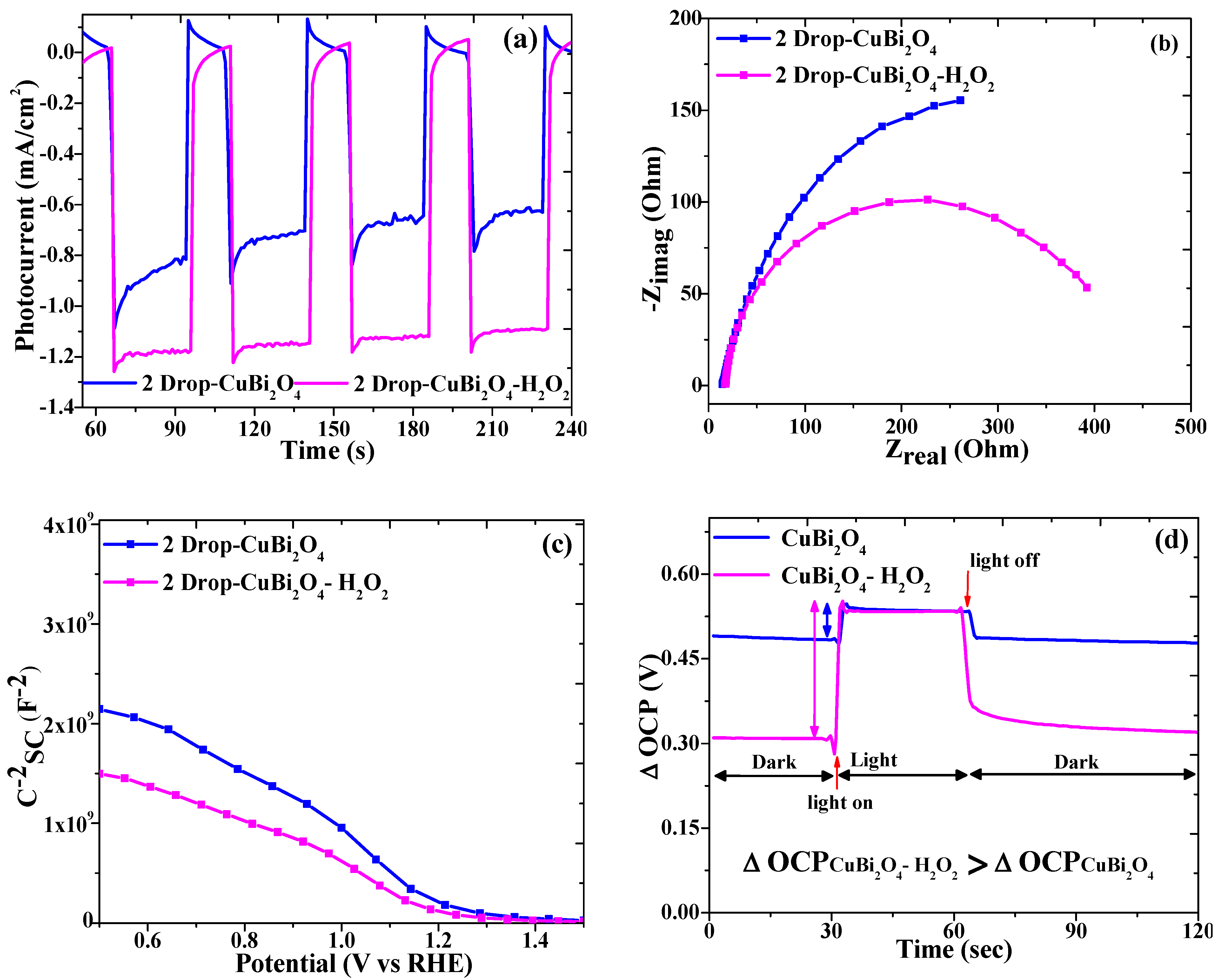
3.4. Effect of H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tayebi, M.; Lee, B.-K. Recent advances in BiVO4 semiconductor materials for hydrogen production using photoelectrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 111, 332–343. [Google Scholar] [CrossRef]
- Dashtian, K.; Shahbazi, S.; Tayebi, M.; Masoumi, Z. A review on metal-organic frameworks photoelectrochemistry: A headlight for future applications. Coord. Chem. Rev. 2021, 445, 214097. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Andrews, N.G.; Sellappan, R. Carbon-Protected BiVO4—Cu2O Thin Film Tandem Cell for Solar Water Splitting Applications. Catalysts 2023, 13, 144. [Google Scholar] [CrossRef]
- Lee, D.J.; Kumar, G.M.; Ganesh, V.; Jeon, H.C.; Kim, D.Y.; Kang, T.W.; Ilanchezhiyan, P. Novel Nanoarchitectured Cu2Te as a Photocathodes for Photoelectrochemical Water Splitting Applications. Nanomaterials 2022, 12, 3192. [Google Scholar] [CrossRef]
- Fominski, V.; Demin, M.; Fominski, D.; Romanov, R.; Rubinkovskaya, O.; Shvets, P.; Goikhman, A. Pulsed Laser Phosphorus Doping and Nanocomposite Catalysts Deposition in Forming a-MoSx/NP-Mo//n+p-Si Photocathodes for Efficient Solar Hydrogen Production. Nanomaterials 2022, 12, 2080. [Google Scholar] [CrossRef]
- Tayebi, M.; Masoumi, Z.; Seo, B.; Li, C.-S.; Kim, H.-G.; Lee, B.-K. Efficient and Stable MoOX@Mo-BiVO4 Photoanodes for Photoelectrochemical Water Oxidation: Optimization and Understanding. ACS Appl. Energy Mater. 2022, 5, 11568–11580. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Wu, S.; Wei, X. Field enhanced GaN photocathode and a proposed implementation method. Optik 2017, 144, 281–288. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chen, W.-T.; Chen, Y.-C.; Wei, H.-Y.; Yen, Y.-S.; Huang, K.-C.; Ho, K.-C.; Chu, C.-W.; Lin, J.T. Charge transporting enhancement of NiO photocathodes for p-type dye-sensitized solar cells. Electrochim. Acta 2012, 66, 210–215. [Google Scholar] [CrossRef]
- Ida, S.; Yamada, K.; Matsunaga, T.; Hagiwara, H.; Matsumoto, Y.; Ishihara, T. Preparation of p-Type CaFe2O4 Photocathodes for Producing Hydrogen from Water. J. Am. Chem. Soc. 2010, 132, 17343–17345. [Google Scholar] [CrossRef]
- Joshi, U.A.; Maggard, P.A. CuNb3O8: A p-Type Semiconducting Metal Oxide Photoelectrode. J. Phys. Chem. Lett. 2012, 3, 1577–1581. [Google Scholar] [CrossRef]
- Cheng, X.; Ding, J.; Wu, Y.; Liu, H.; Dawson, G. The photocathodic properties of a Fe2O3 wrapped CuFeO2 layer on ITO glass for water splitting. Chem. Phys. 2018, 513, 241–245. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Liu, G.; Ye, J. A highly durable p-LaFeO3/n-Fe2O3 photocell for effective water splitting under visible light. Chem. Commun. 2015, 51, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.K.; Zhang, Z.; Roychoudhury, S.; Jiang, C.-M.; Gul, S.; Liu, Y.-S.; Dhall, R.; Ceballos, A.; Yano, J.; Prendergast, D.; et al. CuBi2O4: Electronic Structure, Optical Properties, and Photoelectrochemical Performance Limitations of the Photocathode. Chem. Mater. 2021, 33, 934–945. [Google Scholar] [CrossRef]
- Cao, D.; Nasori, N.; Wang, Z.; Mi, Y.; Wen, L.; Yang, Y.; Qu, S.; Wang, Z.; Lei, Y. p-Type CuBi2O4: An easily accessible photocathodic material for high-efficiency water splitting. J. Mater. Chem. A 2016, 4, 8995–9001. [Google Scholar] [CrossRef]
- Berglund, S.P.; Abdi, F.F.; Bogdanoff, P.; Chemseddine, A.; Friedrich, D.; van de Krol, R. Comprehensive Evaluation of CuBi2O4 as a Photocathode Material for Photoelectrochemical Water Splitting. Chem. Mater. 2016, 28, 4231–4242. [Google Scholar] [CrossRef]
- Hahn, N.T.; Holmberg, V.C.; Korgel, B.A.; Mullins, C.B. Electrochemical Synthesis and Characterization of p-CuBi2O4 Thin Film Photocathodes. J. Phys. Chem. C 2012, 116, 6459–6466. [Google Scholar] [CrossRef]
- Sullivan, I.; Zoellner, B.; Maggard, P.A. Copper(I)-Based p-Type Oxides for Photoelectrochemical and Photovoltaic Solar Energy Conversion. Chem. Mater. 2016, 28, 5999–6016. [Google Scholar] [CrossRef]
- Strömberg, A.; Yuan, Y.; Li, F.; Manavaimaran, B.; Lourdudoss, S.; Zhang, P.; Sun, Y. Heteroepitaxial Growth of GaP Photocathode by Hydride Vapor Phase Epitaxy for Water Splitting and CO2 Reduction. Catalysts 2022, 12, 1482. [Google Scholar] [CrossRef]
- MKumar; Meena, B.; Subramanyam, P.; Suryakala, D.; Subrahmanyam, C. Emerging Copper-Based Semiconducting Materials for Photocathodic Applications in Solar Driven Water Splitting. Catalysts 2022, 12, 1198. [Google Scholar]
- Yuvaraj, S.; Karthikeyan, K.; Kalpana, D.; Lee, Y.S.; Selvan, R.K. Surfactant-free hydrothermal synthesis of hierarchically structured spherical CuBi2O4 as negative electrodes for Li-ion hybrid capacitors. J. Colloid Interface Sci. 2016, 469, 47–56. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Gao, W.; Hao, H. Synthesis and visible photocatalytic activity of new photocatalyst MBi2O4(M = Cu, Zn). J. Mater. Sci. Mater. Electron. 2015, 26, 1866–1873. [Google Scholar] [CrossRef]
- Elaziouti, A.; Laouedj, N.; Bekka, A. Synergetic effects of Sr-doped CuBi2O4 catalyst with enhanced photoactivity under UVA– light irradiation. Environ. Sci. Pollut. Res. 2016, 23, 15862–15876. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Efficient Complete Oxidation of Acetaldehyde into CO2 over CuBi2O4/WO3 Composite Photocatalyst under Visible and UV Light Irradiation. J. Phys. Chem. C 2007, 111, 7574–7577. [Google Scholar] [CrossRef]
- Xu, N.; Li, F.; Gao, L.; Hu, H.; Hu, Y.; Long, X.; Ma, J.; Jin, J. Polythiophene coated CuBi2O4 networks: A porous inorganic–organic hybrid heterostructure for enhanced photoelectrochemical hydrogen evolution. Int. J. Hydrogen Energy 2018, 43, 2064–2072. [Google Scholar] [CrossRef]
- Xu, Y.; Jian, J.; Li, F.; Liu, W.; Jia, L.; Wang, H. Porous CuBi2O4 photocathodes with rationally engineered morphology and composition towards high-efficiency photoelectrochemical performance. J. Mater. Chem. A 2019, 7, 21997–22004. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, Z.; Wang, P.; Zhang, Q.; Dai, Y.; Huang, B. Amorphous TiO2-modified CuBi2O4 Photocathode with enhanced photoelectrochemical hydrogen production activity. Chin. J. Catal. 2018, 39, 1704–1710. [Google Scholar] [CrossRef]
- Wang, F.; Septina, W.; Chemseddine, A.; Abdi, F.F.; Friedrich, D.; Bogdanoff, P.; van de Krol, R.; Tilley, S.D.; Berglund, S.P. Gradient Self-Doped CuBi2O4 with Highly Improved Charge Separation Efficiency. J. Am. Chem. Soc. 2017, 139, 15094–15103. [Google Scholar] [CrossRef]
- Shah, A.K.; Sahu, T.K.; Banik, A.; Gogoi, D.; Peela, N.R.; Qureshi, M. Reduced graphene oxide modified CuBi2O4 as an efficient and noble metal free photocathode for superior photoelectrochemical hydrogen production. Sustain. Energy Fuels 2019, 3, 1554–1561. [Google Scholar] [CrossRef]
- Kang, D.; Hill, J.C.; Park, Y.; Choi, K.-S. Photoelectrochemical Properties and Photostabilities of High Surface Area CuBi2O4 and Ag-Doped CuBi2O4 Photocathodes. Chem. Mater. 2016, 28, 4331–4340. [Google Scholar] [CrossRef]
- Lee, W.-H.; Kang, J.; Park, H.S.; Nam, K.M.; Cho, S.K. Photoelectrochemical response of Au-decorated CuBi2O4 photocathode in bicarbonate solution. J. Electroanal. Chem. 2019, 838, 172–177. [Google Scholar] [CrossRef]
- Jiang, Z.; Geng, H.; Cai, X.; Mao, L.; Zhao, Y.; Gu, X. Preparation of CuBi2O4 photocathodes for overall water splitting under visible light irradiation. Mater. Sci. Semicond. Process. 2021, 134, 105989. [Google Scholar] [CrossRef]
- Kim, N.-W.; Choi, B.-U.; Yu, H.; Ryu, S.; Oh, J. Formation of high-density CuBi2O4 thin film photocathodes with polyvinylpyrrolidone-metal interaction. Opt. Express 2019, 27, A171–A183. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.A.; Kim, Y.; Varma, P.; Gopannagari, M.; Reddy, K.A.J.; Hong, D.H.; Song, I.; Kumar, D.P.; Kim, T.K. Inverse Opal CuBi2O4 Photocathodes for Robust Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2022, 5, 6050–6058. [Google Scholar] [CrossRef]
- Jin, J.; Hu, J.; Qu, J.; Cao, G.; Lei, Y.; Zheng, Z.; Yang, X.; Li, C.M. Reaction Kinetics of Photoelectrochemical CO2 Reduction on a CuBi2O4-Based Photocathode. ACS Appl. Mater. Interfaces 2022, 14, 17509–17519. [Google Scholar] [CrossRef]
- Lee, S.; Cha, S.; Myung, Y.; Park, K.; Kwak, I.H.; Kwon, I.S.; Seo, J.; Lim, S.A.; Cha, E.H.; Park, J. Orthorhombic NiSe2 Nanocrystals on Si Nanowires for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 33198–33204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Xu, Y.-F.; Chen, B.-X.; Ning, Z.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. 3D Cathodes of Cupric Oxide Nanosheets Coated onto Macroporous Antimony-Doped Tin Oxide for Photoelectrochemical Water Splitting. ChemSusChem 2016, 9, 3012–3018. [Google Scholar] [CrossRef] [PubMed]
- Varunkumar, K.; Sellappan, R. Photoelectrochemical behaviour of CuBi2O4@MoS2 photocathode for solar water splitting. Mater. Chem. Phys. 2021, 261, 124245. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Yang, K.D.; Kim, D.-H.; Nam, K.T.; Hong, S.-H. p-Type CuBi2O4 thin films prepared by flux-mediated one-pot solution process with improved structural and photoelectrochemical characteristics. Mater. Lett. 2017, 188, 192–196. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, C.-Y.; Reisner, E. Photoelectrochemical reduction of aqueous protons with a CuO|CuBi2O4 heterojunction under visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 22462–22465. [Google Scholar] [CrossRef]
- Song, A.; Plate, P.; Chemseddine, A.; Wang, F.; Abdi, F.F.; Wollgarten, M.; van de Krol, R.; Berglund, S.P. Cu:NiO as a hole-selective back contact to improve the photoelectrochemical performance of CuBi2O4 thin film photocathodes. J. Mater. Chem. A 2019, 7, 9183–9194. [Google Scholar] [CrossRef]
- Monny, S.A.; Zhang, L.; Wang, Z.; Luo, B.; Konarova, M.; Du, A.; Wang, L. Fabricating highly efficient heterostructured CuBi2O4 photocathodes for unbiased water splitting. J. Mater. Chem. A 2020, 8, 2498–2504. [Google Scholar] [CrossRef]
- Jeong, H.W.; Zsigmond, T.S.; Samu, G.F.; Janáky, C. Sacrificial Agent Gone Rogue: Electron-Acceptor-Induced Degradation of CsPbBr3 Photocathodes. ACS Energy Lett. 2022, 7, 417–424. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, Z.; Spurgeon, J.; Yang, X. Enhanced photoelectrochemical water-splitting performance of semiconductors by surface passivation layers. Energy Environ. Sci. 2014, 7, 2504–2517. [Google Scholar] [CrossRef]
- Li, J.; Griep, M.; Choi, Y.; Chu, D. Photoelectrochemical overall water splitting with textured CuBi2O4 as a photocathode. Chem. Commun. 2018, 54, 3331–3334. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoumi, Z.; Tayebi, M.; Lari, S.A.M.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Kyung, D.; Tayebi, M. Photoelectrochemical Performance of a CuBi2O4 Photocathode with H2O2 as a Scavenger. Inorganics 2023, 11, 147. https://doi.org/10.3390/inorganics11040147
Masoumi Z, Tayebi M, Lari SAM, Seo B, Lim C-S, Kim H-G, Kyung D, Tayebi M. Photoelectrochemical Performance of a CuBi2O4 Photocathode with H2O2 as a Scavenger. Inorganics. 2023; 11(4):147. https://doi.org/10.3390/inorganics11040147
Chicago/Turabian StyleMasoumi, Zohreh, Mahdi Tayebi, S. Ahmad Masoumi Lari, Bongkuk Seo, Choong-Sun Lim, Hyeon-Gook Kim, Daeseung Kyung, and Meysam Tayebi. 2023. "Photoelectrochemical Performance of a CuBi2O4 Photocathode with H2O2 as a Scavenger" Inorganics 11, no. 4: 147. https://doi.org/10.3390/inorganics11040147
APA StyleMasoumi, Z., Tayebi, M., Lari, S. A. M., Seo, B., Lim, C.-S., Kim, H.-G., Kyung, D., & Tayebi, M. (2023). Photoelectrochemical Performance of a CuBi2O4 Photocathode with H2O2 as a Scavenger. Inorganics, 11(4), 147. https://doi.org/10.3390/inorganics11040147