Solution-State Studies, X-ray Structure Determination and Luminescence Properties of an Ag(I) Heteroleptic Complex Containing 2,9-Bis(styryl)-1,10-phenanthroline Derivative and Triphenylphosphine
Abstract
:1. Introduction
2. Results and Discussion
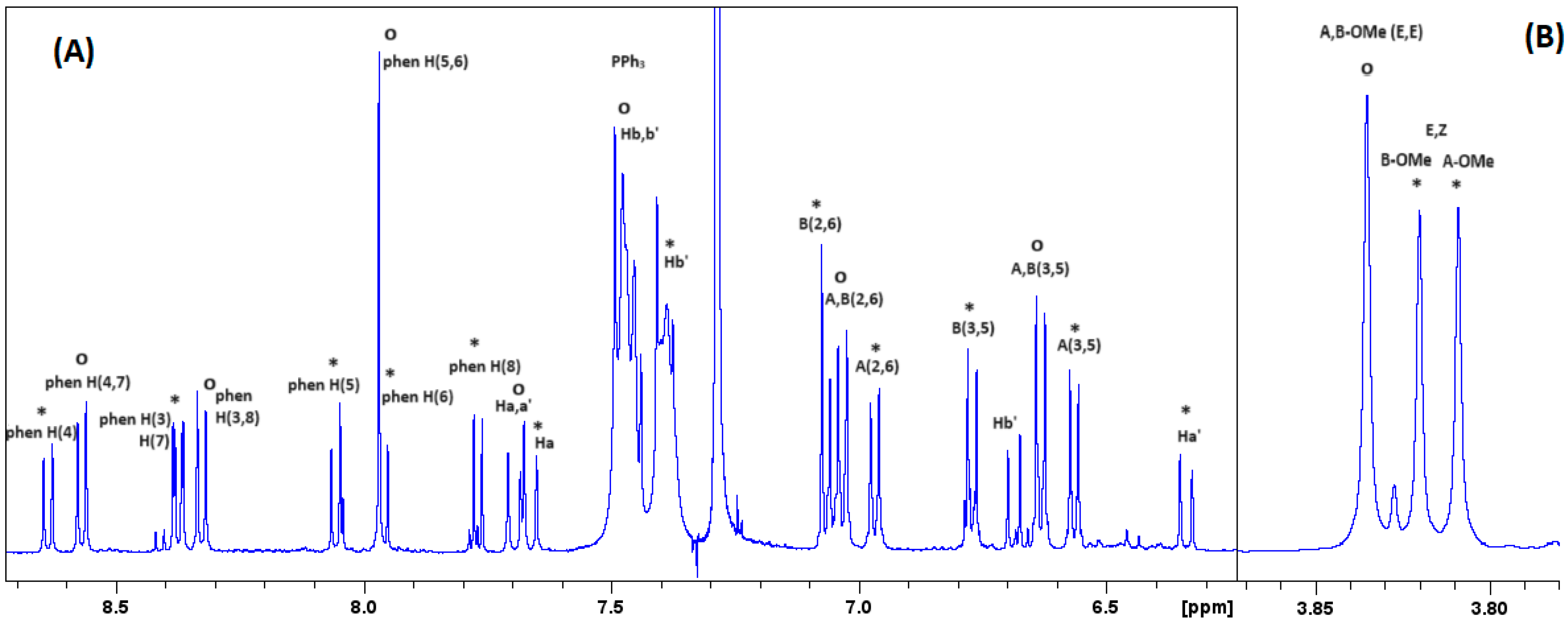
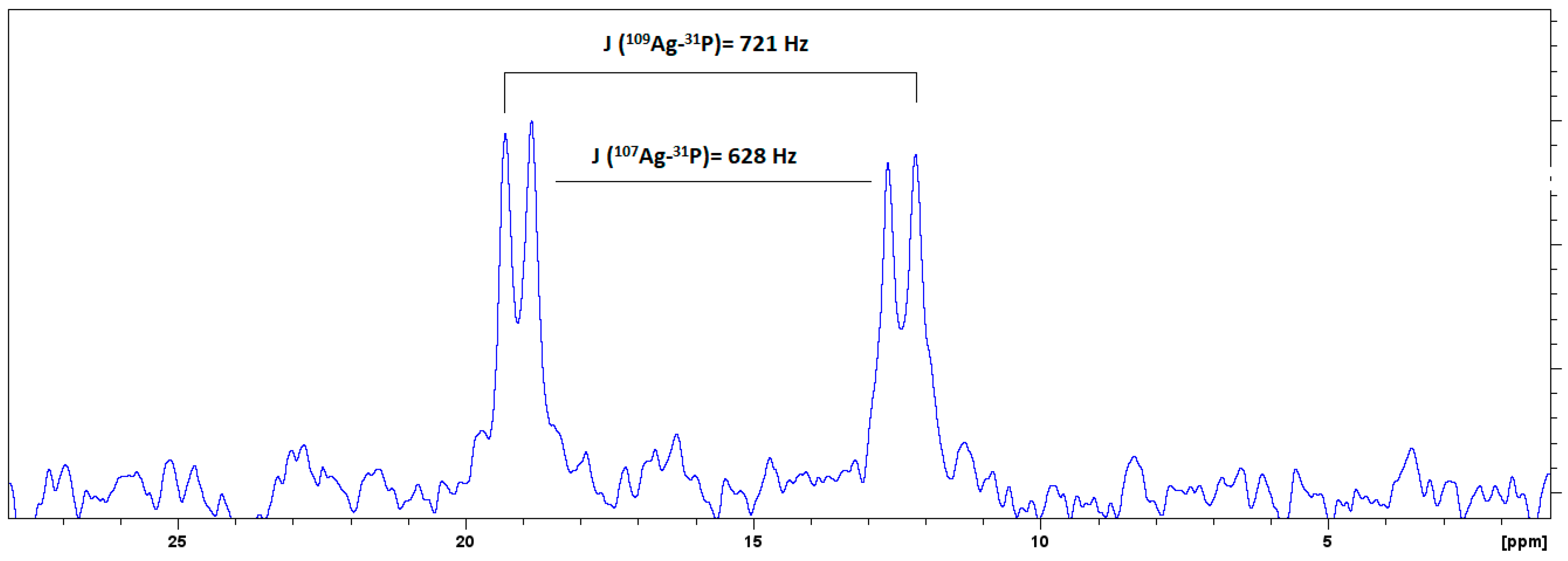
2.1. Characterization with NMR Spectroscopy
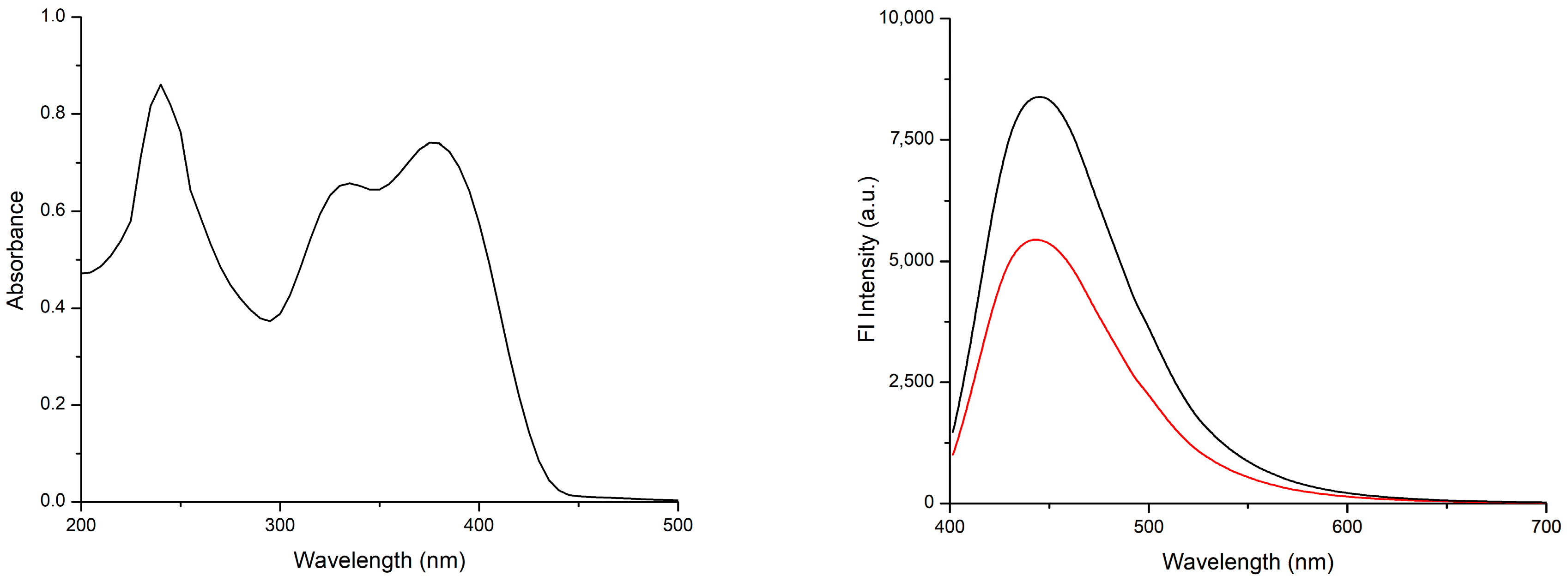
2.2. Optical Properties in Solution
2.3. Absorption Spectrum (DRS)
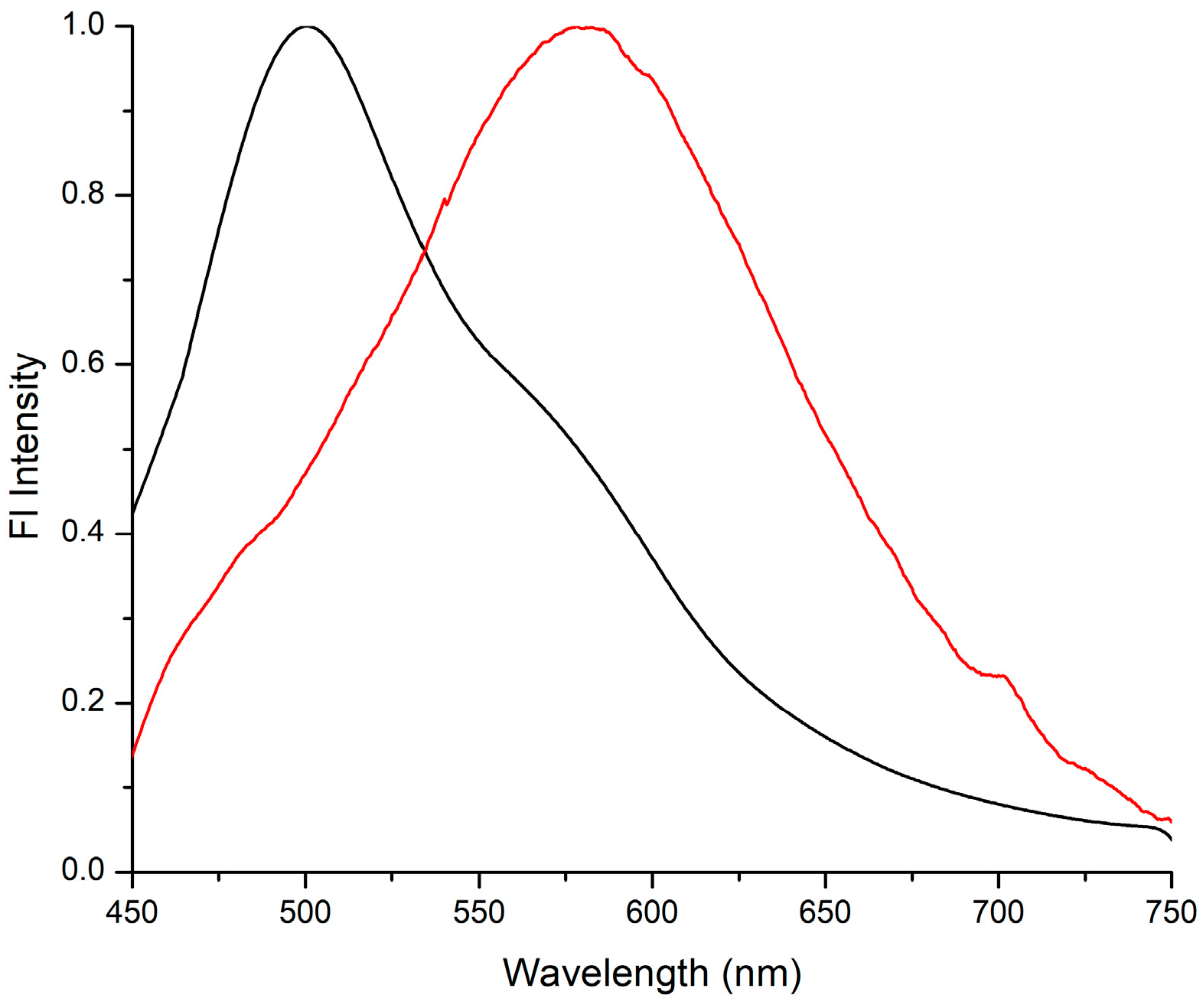
2.4. Luminescent Behavior in the Solid State
2.5. IR Spectroscopy
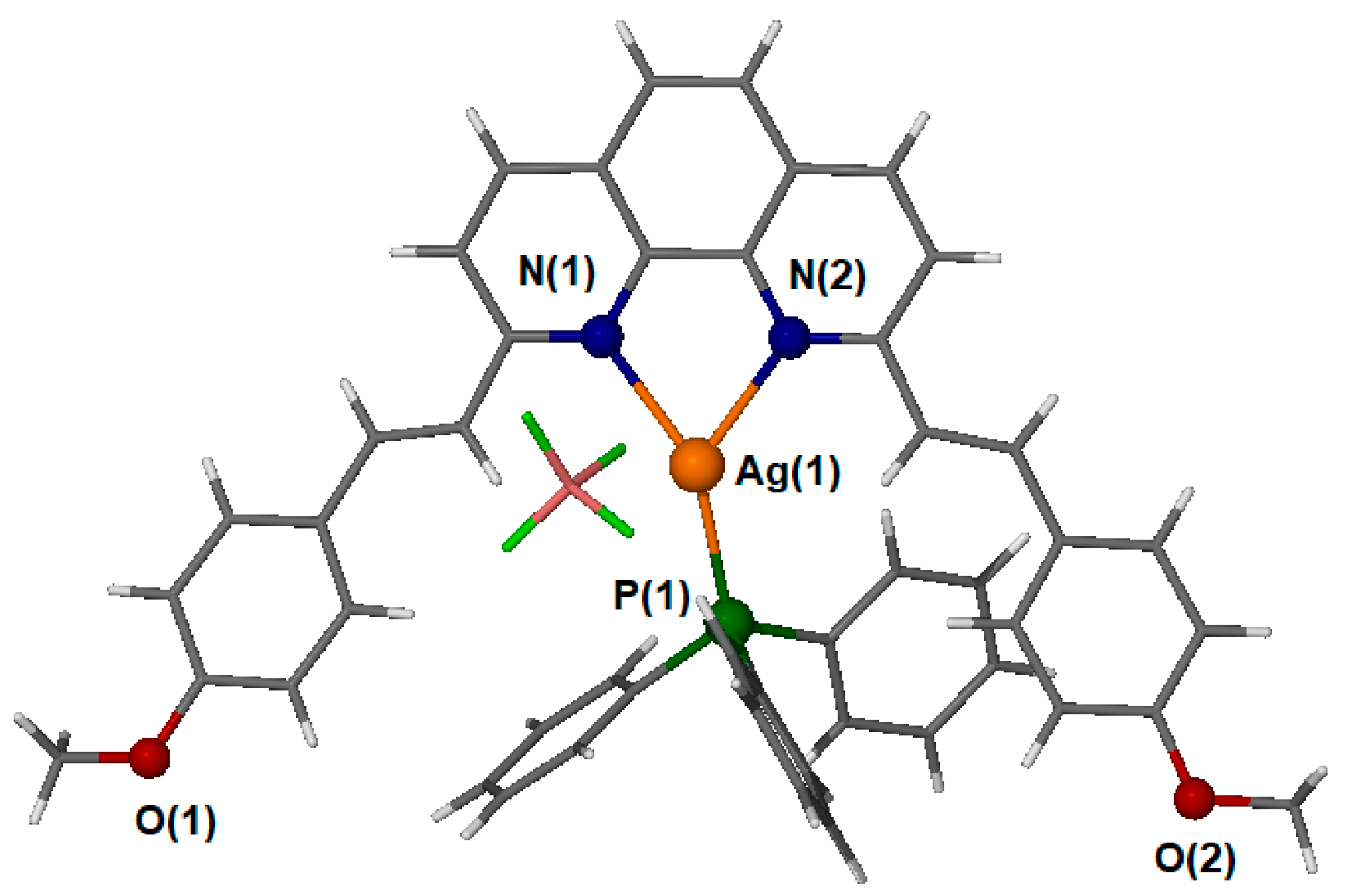
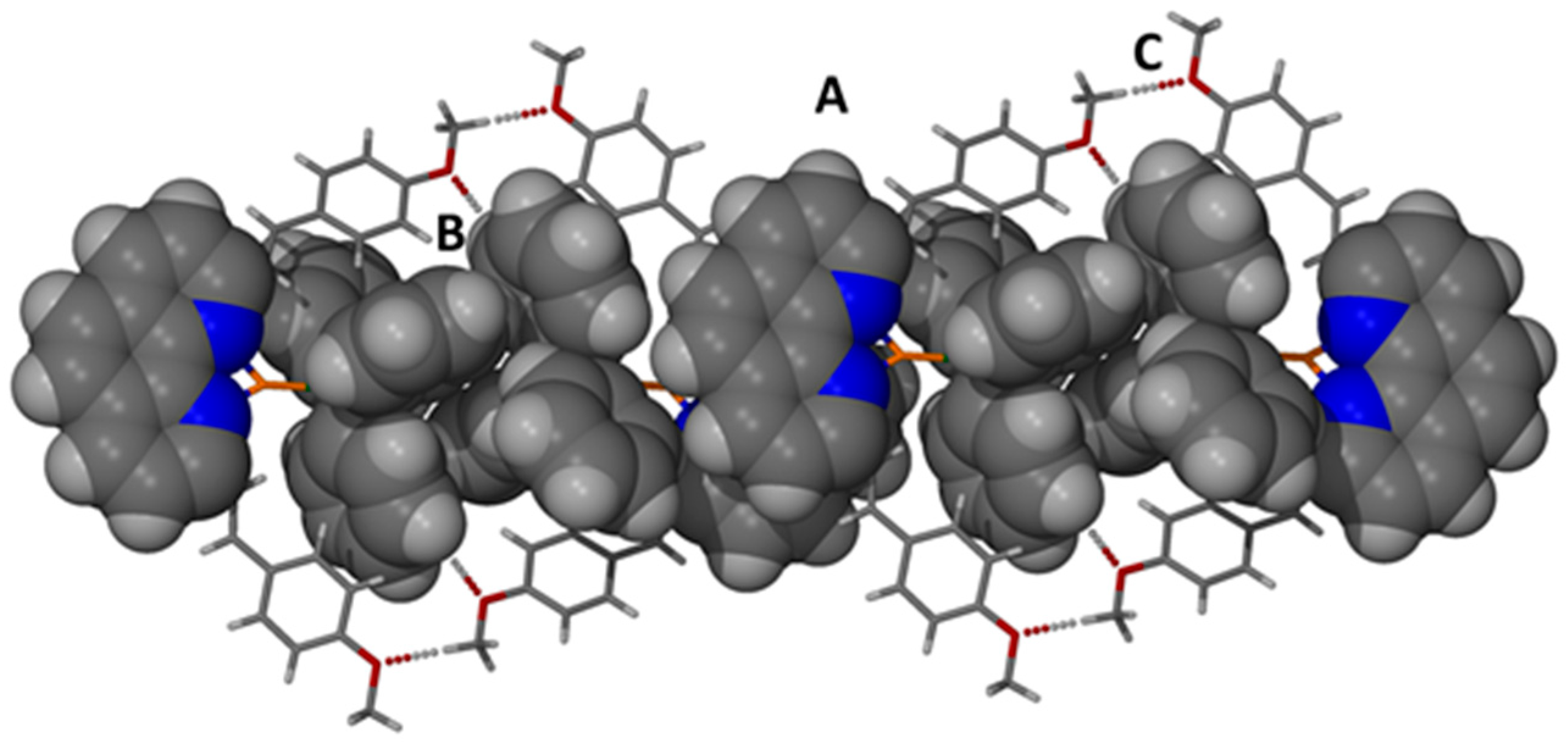
2.6. Description of the Structure
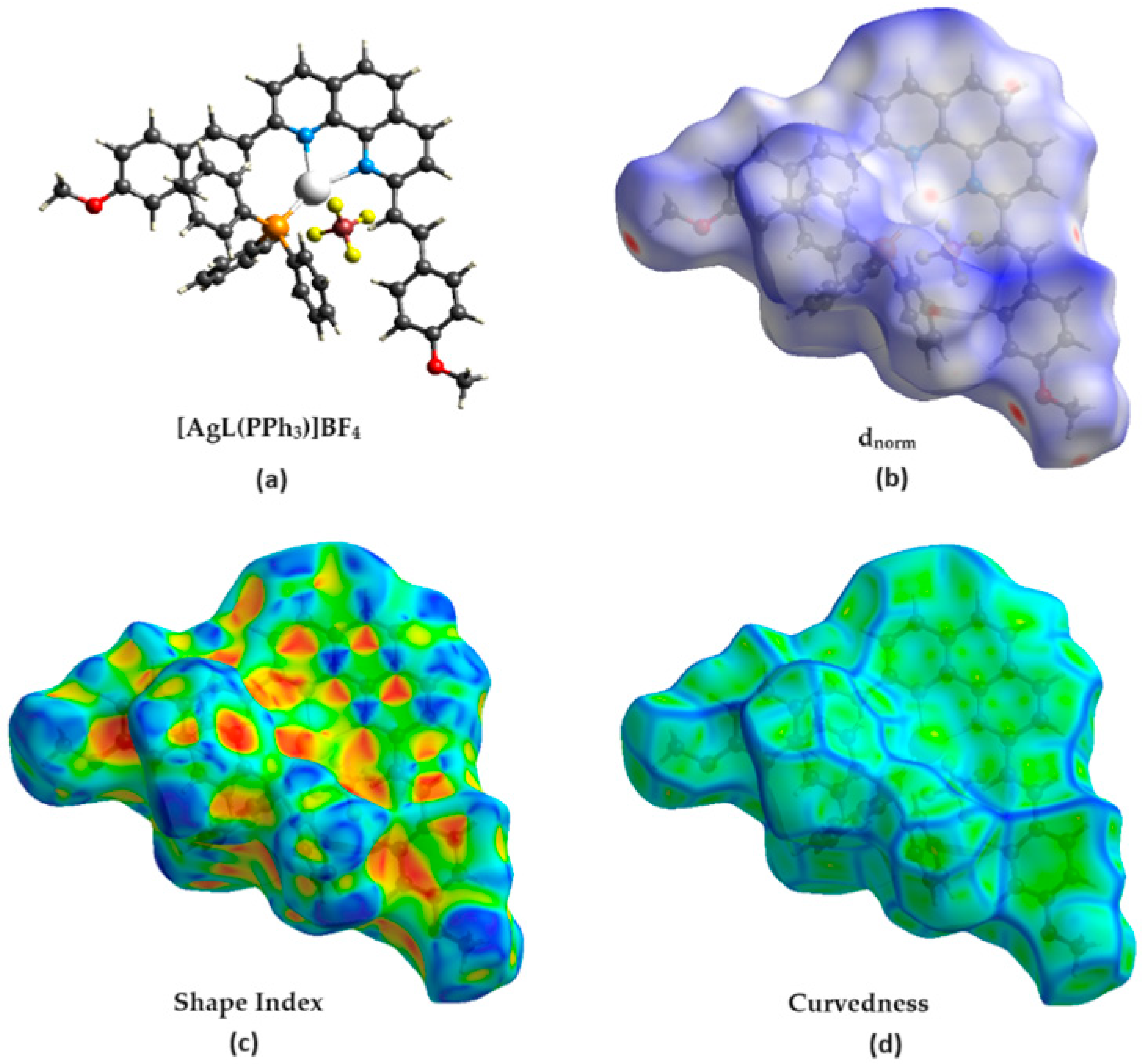
2.7. Hirshfeld Surface Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Crystal Structure Determination
3.4. Synthesis
3.4.1. Synthesis of Ligand L
3.4.2. Synthesis of [AgL(PPh3)]BF4 Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent Heavy-Metal Complexes for Bioimaging. Chem. Soc. Rev. 2011, 40, 2508. [Google Scholar] [CrossRef]
- Abbas, S.; Din, I.; Raheel, A.; Tameez ud Din, A. Cyclometalated Iridium (III) Complexes: Recent Advances in Phosphorescence Bioimaging and Sensing Applications. Appl. Organomet. Chem. 2020, 34, e5413. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated Iridium(III) Complexes for Life Science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef]
- Shon, J.-H.; Kim, D.; Rathnayake, M.D.; Sittel, S.; Weaver, J.; Teets, T.S. Photoredox Catalysis on Unactivated Substrates with Strongly Reducing Iridium Photosensitizers. Chem. Sci. 2021, 12, 4069–4078. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Q.; Sun, W.; Cheng, Y.; Wang, L. Neutral Copper(i) Phosphorescent Complexes from Their Ionic Counterparts with 2-(2′-Quinolyl)Benzimidazole and Phosphine Mixed Ligands. Dalton Trans. 2011, 40, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ding, J.; Cheng, Y.; Wang, L.; Xie, Z.; Jing, X.; Wang, F. Novel Heteroleptic CuI Complexes with Tunable Emission Color for Efficient Phosphorescent Light-Emitting Diodes. Adv. Funct. Mater. 2007, 17, 2983–2990. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Cheng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly Efficient Electroluminescence from Green-Light-Emitting Electrochemical Cells Based on CuI Complexes. Adv. Funct. Mater. 2006, 16, 1203–1208. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Holler, M.; Moudam, O.; Nierengarten, J.-F.; Zhou, Z.; Wegh, R.T.; Welter, R. Highly Luminescent Cu I Complexes for Light-Emitting Electrochemical Cells. Adv. Mater. 2006, 18, 1313–1316. [Google Scholar] [CrossRef]
- Safin, D.A.; Mitoraj, M.P.; Robeyns, K.; Filinchuk, Y.; Vande Velde, C.M.L. Luminescent Mononuclear Mixed Ligand Complexes of Copper(i) with 5-Phenyl-2,2′-Bipyridine and Triphenylphosphine. Dalton Trans. 2015, 44, 16824–16832. [Google Scholar] [CrossRef]
- Li, G.F.; Zhang, X.Y.; Li, R.F.; Liu, X.F. Synthesis and Properties of Two New Cu(I) Complexes Based on 5,6-Substituted Imidazole-2,9-Dimethyl-1,10-Phenanthroline and Triphenylphosphine. Russ. J. Gen. Chem. 2016, 86, 387–390. [Google Scholar] [CrossRef]
- Takeda, H.; Kobayashi, A.; Tsuge, K. Recent Developments of Photoactive Cu(I) and Ag(I) Complexes with Diphosphine and Related Ligands. Coord. Chem. Rev. 2022, 470, 214700. [Google Scholar] [CrossRef]
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on Cu I and Ag I Complexes. ChemPhysChem 2017, 18, 3508–3535. [Google Scholar] [CrossRef] [PubMed]
- Shafikov, M.Z.; Czerwieniec, R.; Yersin, H. Ag(i) Complex Design Affording Intense Phosphorescence with a Landmark Lifetime of over 100 Milliseconds. Dalton Trans. 2019, 48, 2802–2806. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Suleymanova, A.F.; Czerwieniec, R.; Yersin, H. Thermally Activated Delayed Fluorescence from Ag(I) Complexes: A Route to 100% Quantum Yield at Unprecedentedly Short Decay Time. Inorg. Chem. 2017, 56, 13274–13285. [Google Scholar] [CrossRef] [PubMed]
- Mahoro, G.U.; Fernandez-Cestau, J.; Renaud, J.; Coto, P.B.; Costa, R.D.; Gaillard, S. Recent Advances in Solid-State Lighting Devices Using Transition Metal Complexes Exhibiting Thermally Activated Delayed Fluorescent Emission Mechanism. Adv. Opt. Mater. 2020, 8, 2000260. [Google Scholar] [CrossRef]
- Sun, L.-Z.; Kuang, X.-N.; Lin, S.; Zhao, L.; Yu, X.; Li, Z.-F.; Liu, M.; Xin, X.-L.; Yang, Y.-P.; Jin, Q.-H. Nine Heteroleptic Copper(I)/Silver(I) Complexes Prepared from Phosphine and Diimine Ligands: Syntheses, Structures and Terahertz Spectra. Polyhedron 2020, 175, 114177. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, X.-N.; Cui, Y.-Z.; Xin, X.-L.; Han, H.-L.; Liu, M.; Yang, Y.-P.; Jin, Q.-H. Synthesis, Structure, Luminescent Properties, and Photocatalytic Behavior of 0D–3D Silver(I) Complexes Bearing Both Diphosphine Ligands and 1,10-Phenanthroline Derivatives. Polyhedron 2018, 155, 135–143. [Google Scholar] [CrossRef]
- Cui, Y.; Yuan, Y.; Han, H.; Li, Z.; Liu, M.; Jin, Q.; Yang, Y.; Zhang, Z. Synthesis, Characterization, and Luminescent Properties of Silver(I) Complexes Based on Diphosphine Ligands and 2,9-Dimethyl-1,10-phenanthroline. Z. Anorg. Allg. Chem. 2016, 642, 953–959. [Google Scholar] [CrossRef]
- Brunner, F.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Positional Isomerism in the N^N Ligand: How Much Difference Does a Methyl Group Make in [Cu(P^P)(N^N)]+ Complexes? Molecules 2020, 25, 2760. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Alkan-Zambada, M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-Yl)Quinoline. Crystals 2020, 10, 255. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Zhang, Q.; Zhang, H.; Kang, B. μ-1,4-Bis(Diphenylphosphino)Butane-κ2P:P′-Bis[(2,2′-Bipyridyl)Silver(I)] Ditetrafluoroborate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m536–m537. [Google Scholar] [CrossRef]
- Wang, X.-J.; Gui, L.-C.; Ni, Q.-L.; Liao, Y.-F.; Jiang, X.-F.; Tang, L.-H.; Zhang, Z.; Wu, Q. π-Stacking Induced Complexes with Z-Shape Motifs Featuring a Complementary Approach between Electron-Rich Arene Diamines and Electron-Deficient Aromatic N-Heterocycles. CrystEngComm 2008, 10, 1003. [Google Scholar] [CrossRef]
- Keller, S.; Camenzind, T.N.; Abraham, J.; Prescimone, A.; Häussinger, D.; Constable, E.C.; Housecroft, C.E. Self-Assembly of Heteroleptic Dinuclear Silver(i) Complexes Bridged by Bis(Diphenylphosphino)Ethyne. Dalton Trans. 2018, 47, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.-C.; Ni, Q.-L.; Jiang, X.-F.; Zeng, J.-Q.; Wang, X.-J. [μ- N, N′-Bis(Diphenylphosphinomethyl)Benzene-1,4-Diamine-κ2 P:P′]Bis[(2,2′-Bipyridine-κ2N,N′)Silver(I)] Bis(Perchlorate) Acetone Disolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m589–m590. [Google Scholar] [CrossRef]
- Effendy; Marchetti, F.; Pettinari, C.; Pettinari, R.; Skelton, B.W.; White, A.H. Synthesis and Structural Characterization of Adducts of Silver(I) Oxyanion Salts, AgX (X=ClO4, NO3), with Ph2E(CH2)XEPh2 (‘Dpex’; E=P, As; X=1–4) and Oligodentate Aromatic N-Bases Derivative of 2,2′-Bipyridyl, ‘L’, AgX:Dpex:L (2:1:2). Inorganica Chim. Acta 2007, 360, 1388–1413. [Google Scholar] [CrossRef]
- Sun, Z.-Z.; Hu, F.-Z.; Gao, C.-J.; Mou, W.-L.; Wang, G.; Zhu, N.; Pan, X.; Li, Z.-F.; Han, H.-L.; Fu, H.; et al. One-Dimensional Infinite Chain Ag(i) Complex with High Quantum Yield and TADF Property: Prepared by Metal Ion Adjustment. CrystEngComm 2023, 25, 5405–5412. [Google Scholar] [CrossRef]
- Kaeser, A.; Delavaux-Nicot, B.; Duhayon, C.; Coppel, Y.; Nierengarten, J.-F. Heteroleptic Silver(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands. Inorg. Chem. 2013, 52, 14343–14354. [Google Scholar] [CrossRef]
- Burgos, M.; Crespo, O.; Gimeno, M.C.; Jones, P.G.; Laguna, A. Gold, Silver and Palladium Complexes with the 2,2′-Dipyridylamine Ligand. Eur. J. Inorg. Chem. 2003, 2003, 2170–2174. [Google Scholar] [CrossRef]
- Cui, L.-N.; Hu, K.-Y.; Jin, Q.-H.; Li, Z.-F.; Wu, J.-Q.; Zhang, C.-L. Polynuclear Silver(I) Complexes of Triphenylphosphine and 2-Aminopyrimidine: Synthesis, Structural Characterization and Spectroscopic Properties. Polyhedron 2011, 30, 2253–2259. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Amirnasr, M.; Aoki, K. Crystal Structures of [Ag(Bpy)(PPh3)]X (1: X = PF6, 2: X = ClO4) Complexes. Anal. Sci. X-ray Struct. Anal. Online 2006, 22, X87–X88. [Google Scholar] [CrossRef]
- Penney, M.K.; Potter, C.; Burroughs, M.A.; Klausmeyer, K.K. Silver Coordination Complexes of 2-(Diphenylphosphinomethyl)Aminopyridine with Weakly Interacting Counterions. Polyhedron 2015, 102, 207–215. [Google Scholar] [CrossRef]
- Durini, S.; Ardizzoia, G.A.; Therrien, B.; Brenna, S. Tuning the Fluorescence Emission in Mononuclear Heteroleptic Trigonal Silver(i) Complexes. New J. Chem. 2017, 41, 3006–3014. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. 1,10-Phenanthroline: A Versatile Building Block for the Construction of Ligands for Various Purposes. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile Building Blocks for Luminescent Molecules, Materials and Metal Complexes. Chem. Soc. Rev. 2009, 38, 1690. [Google Scholar] [CrossRef]
- Alreja, P.; Kaur, N. Recent Advances in 1,10-Phenanthroline Ligands for Chemosensing of Cations and Anions. RSC Adv. 2016, 6, 23169–23217. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Cesaretti, A.; Elisei, F.; Mencaroni, L.; Spalletti, A.; Fortuna, C.G. New Styryl Phenanthroline Derivatives as Model D−π−A−π−D Materials for Non-Linear Optics. ChemPhysChem 2018, 19, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewska, B.; Gordel, M.; Szeremeta, J.; Kaczorowska, M.A.; Józefowicz, M.; Samoć, M. One- and Two-Photon-Induced Isomerization of Styryl Compounds Possessing A-π-A′ Structure. Dye. Pigment. 2016, 132, 237–247. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Ośmiałowski, B.; Zaleśny, R. Application of Spectroscopic and Theoretical Methods in the Studies of Photoisomerization and Photophysical Properties of the Push—Pull Styryl-Benzimidazole Dyes. Photochem. Photobiol. Sci. 2016, 15, 117–128. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Gordel, M.; Szeremeta, J.; Grela, I.; Samoć, M. Photostability of Push-Pull Phenanthroimidazole Derivative upon One- and Two-Photon Excitation. Dye. Pigment. 2017, 136, 150–160. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pal, P.; Maity, D.; Baitalik, S. Photophysics and Luminescence Switching Properties of a Series of Photochromic Styrylbenzene-Terpyridine Conjugate: Experimental and DFT/TD-DFT Investigation. J. Photochem. Photobiol. A Chem. 2019, 378, 94–104. [Google Scholar] [CrossRef]
- Gordillo, M.A.; Soto-Monsalve, M.; Carmona-Vargas, C.C.; Gutiérrez, G.; D’vries, R.F.; Lehn, J.; Chaur, M.N. Photochemical and Electrochemical Triggered Bis(Hydrazone) Switch. Chem. A Eur. J. 2017, 23, 14872–14882. [Google Scholar] [CrossRef]
- Gupta, D.; Gaur, A.K.; Kumar, H.; Singh, S.; Venkataramani, S. Light-Switchable Metal Complexes: Introducing Photoresponsive Behaviour through Azoheteroarenes. ChemPhotoChem 2023, 7, e202300068. [Google Scholar] [CrossRef]
- Valora, G.; Bonaccorso, C.; Cesaretti, A.; Fortuna, C.G.; Spalletti, A.; Elisei, F. Metal Complexes with Sterically Demanding Phenanthroline Ligands: A Combined Spectroscopic Study. Dye. Pigment. 2021, 187, 109150. [Google Scholar] [CrossRef]
- Glykos, D.; Plakatouras, J.C.; Malandrinos, G. [4,4′-Bis(4-Fluorophenyl)-6,6′-Dimethyl-2,2′-Bipyridine] [Bis (2-(Diphenylphosphino) Phenyl) Ether] Silver(I) Hexafluorophosphate. Molbank 2023, 2023, M1675. [Google Scholar] [CrossRef]
- Moudam, O.; Tsipis, A.C.; Kommanaboyina, S.; Horton, P.N.; Coles, S.J. First Light-Emitting Electrochemical Cell with [Ag(i)(N^N)(P^P)] Type Complex. RSC Adv. 2015, 5, 95047–95053. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Patroniak, V.; Kubicki, M.; Kądziołka, G.; Przepiórski, J.; Michalkiewicz, B. Synthesis, Structure, and Photocatalytic Properties of New Dinuclear Helical Complex of Silver(I) Ions. J. Catal. 2012, 291, 1–8. [Google Scholar] [CrossRef]
- Osawa, M.; Kawata, I.; Ishii, R.; Igawa, S.; Hashimoto, M.; Hoshino, M. Application of Neutral D10 Coinage Metal Complexes with an Anionic Bidentate Ligand in Delayed Fluorescence-Type Organic Light-Emitting Diodes. J. Mater. Chem. C 2013, 1, 4375. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Ren, Z.; Lang, J. Silver(I)-Based Complexes Used as High-Performance Photocatalysts for the Degradation of Organic Dyes in Water. Eur. J. Inorg. Chem. 2019, 2019, 1816–1824. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Jin, Q.-H.; Hu, K.-Y.; Zhang, C.-L. (1,10-Phenanthroline-κ2N,N′)(Triphenylphosphine-κP)Silver(I) Trifluoromethanesulfonate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m1096–m1097. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π–π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Ishida, H.; Tobita, S.; Hasegawa, Y.; Katoh, R.; Nozaki, K. Recent Advances in Instrumentation for Absolute Emission Quantum Yield Measurements. Coord. Chem. Rev. 2010, 254, 2449–2458. [Google Scholar] [CrossRef]
- Bruker. APEX 3. In SAINT, SHELXT; Bruker AXS Inc.: Fitchburg, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Barbour, L.J. X-Seed—A Software Tool for Supramolecular Crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]



| H Atoms | L (E,E)/PPh3 | Complex (E, E) | Δδ (Ε,Ε) * | H Atoms | Complex (E,Z) | Δδ (Ε,Ζ) * |
|---|---|---|---|---|---|---|
| H(3) | 7.93 | 8.33 | 0.4 | H(3) | 8.37 | 0.44 |
| H(4) | 8.22 | 8.57 | 0.35 | H(4) | 8.64 | 0.42 |
| H(5) | 7.74 | 7.97 | 0.23 | H(5) | 8.06 | 0.32 |
| H(6) | 7.74 | 7.97 | 0.23 | H(6) | 7.96 | 0.22 |
| H(7) | 8.22 | 8.57 | 0.35 | H(7) | 8.37 | 0.15 |
| H(8) | 7.93 | 8.33 | 0.4 | H(8) | 7.77 | −0.16 |
| H(a,a’) (Ε) | 7.77 | 7.70 | −0.07 | H(a) (E) | 7.67 | −0.1 |
| H(b,b’) (Ε) | 7.62 | 7.43 | −0.19 | H(b) (E) | 7.39 | −0.23 |
| H(a’) (Z) | 6.34 | −1.43 | ||||
| H(b’) (Z) | 6.69 | −0.93 | ||||
| A, H(2,6) | 7.68 | 7.04 | −0.64 | A H(2,6) | 6.97 | −0.71 |
| A, H(3,5) | 6.99 | 6.63 | −0.36 | A H(3,5) | 6.56 | −0.43 |
| Β, H(2,6) | 7.68 | 7.04 | −0.64 | B H(2,6) | 7.07 | −0.61 |
| Β, H(3,5) | 6.99 | 6.63 | −0.36 | B H(3,5) | 6.77 | −0.22 |
| A, (-OMe) | 3.9 | 3.84 | −0.06 | A H(-OMe) | 3.81 | −0.09 |
| Β, (-OΜe) | 3.9 | 3.84 | −0.06 | B H(-OMe) | 3.82 | −0.08 |
| o,p-PPh3 | 7.32–7.38 | 7.47, 7.48 | - | o,p-PPh3 | 7.47, 7.48 | - |
| m-PPh3 | 7.39 | - | m-PPh3 | 7.39 | - | |
| Absorption | Emission (Solution) | Emission (Solid) | ||||||
|---|---|---|---|---|---|---|---|---|
| λabs (nm) | ε (Μ−1cm−1) | λexc (nm) | λem (nm) | ΦPL (%) | λexc (nm) | λem (nm) | ΦPL (%) | |
| Ligand | 365 | 55,000 | 365 | 433 | 6 | 365 | 570 | 3.8 |
| 1 | 375 | 4008 | 375 | 446 | 8.6 | 375 | 500 | 4.3 |
| Bond Distances | (Å) | Bond Angles | (°) |
|---|---|---|---|
| Ag(1)-N(1) | 2.287(2) | N(1)-Ag(1)-N(2) | 73.26(8) |
| Ag(1)-N(2) | 2.336(2) | N(1)-Ag(1)-P(1) | 151.34(6) |
| Ag(1)-P(1) | 2.3743(7) | N(2)-Ag(1)-P(1) | 134.03(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glykos, D.; Plakatouras, J.C.; Malandrinos, G. Solution-State Studies, X-ray Structure Determination and Luminescence Properties of an Ag(I) Heteroleptic Complex Containing 2,9-Bis(styryl)-1,10-phenanthroline Derivative and Triphenylphosphine. Inorganics 2023, 11, 467. https://doi.org/10.3390/inorganics11120467
Glykos D, Plakatouras JC, Malandrinos G. Solution-State Studies, X-ray Structure Determination and Luminescence Properties of an Ag(I) Heteroleptic Complex Containing 2,9-Bis(styryl)-1,10-phenanthroline Derivative and Triphenylphosphine. Inorganics. 2023; 11(12):467. https://doi.org/10.3390/inorganics11120467
Chicago/Turabian StyleGlykos, Dimitrios, John C. Plakatouras, and Gerasimos Malandrinos. 2023. "Solution-State Studies, X-ray Structure Determination and Luminescence Properties of an Ag(I) Heteroleptic Complex Containing 2,9-Bis(styryl)-1,10-phenanthroline Derivative and Triphenylphosphine" Inorganics 11, no. 12: 467. https://doi.org/10.3390/inorganics11120467
APA StyleGlykos, D., Plakatouras, J. C., & Malandrinos, G. (2023). Solution-State Studies, X-ray Structure Determination and Luminescence Properties of an Ag(I) Heteroleptic Complex Containing 2,9-Bis(styryl)-1,10-phenanthroline Derivative and Triphenylphosphine. Inorganics, 11(12), 467. https://doi.org/10.3390/inorganics11120467







