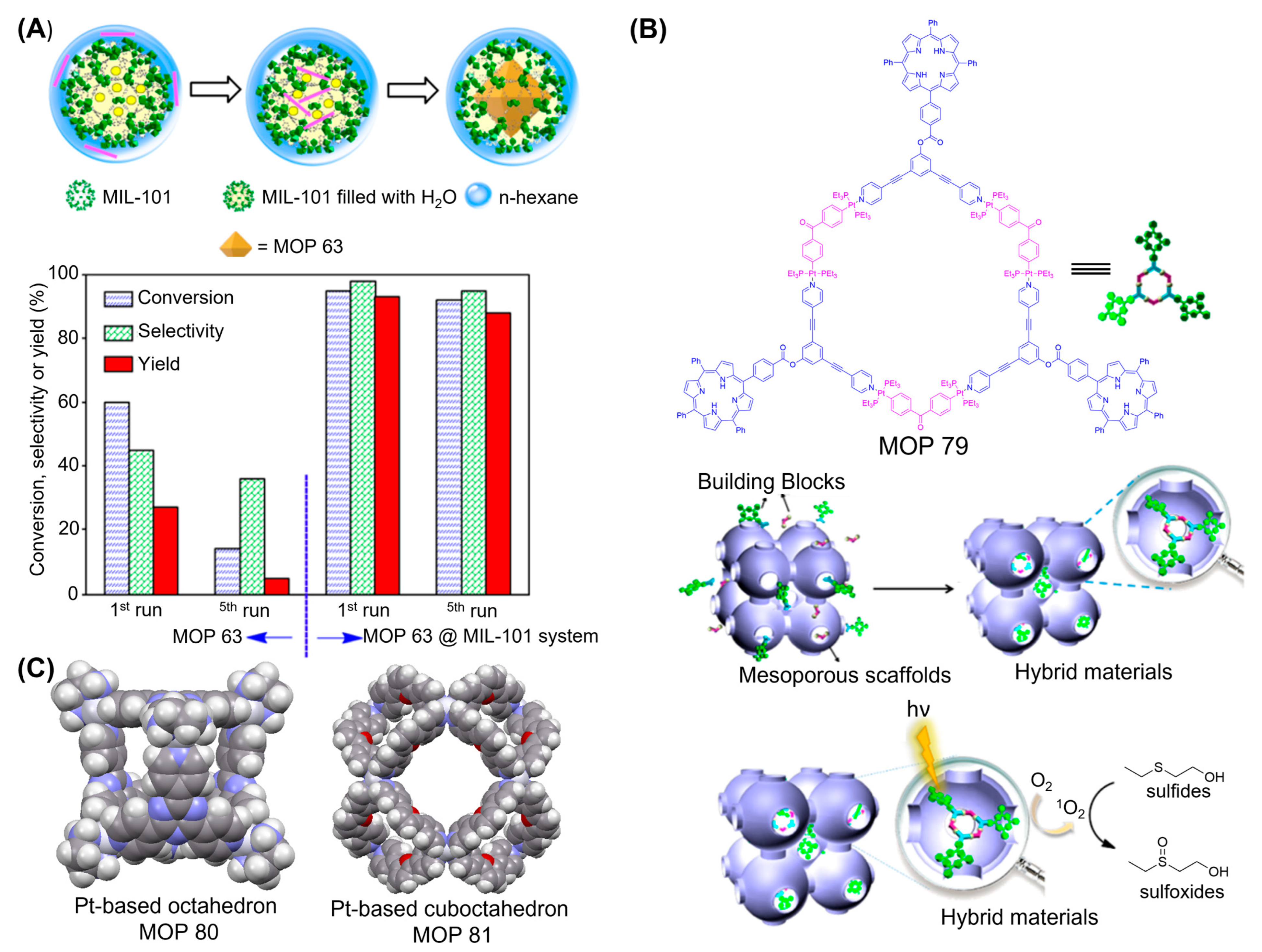
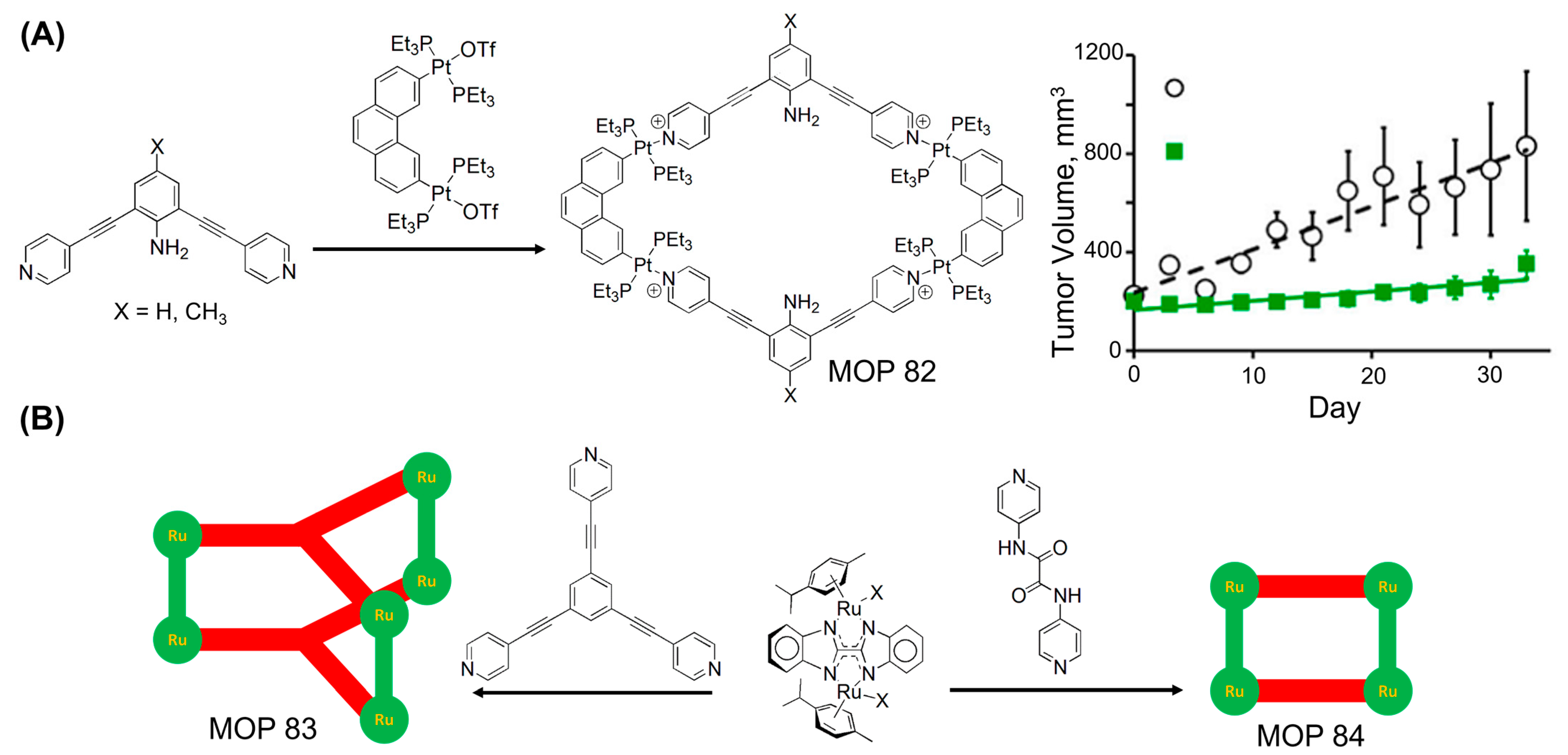
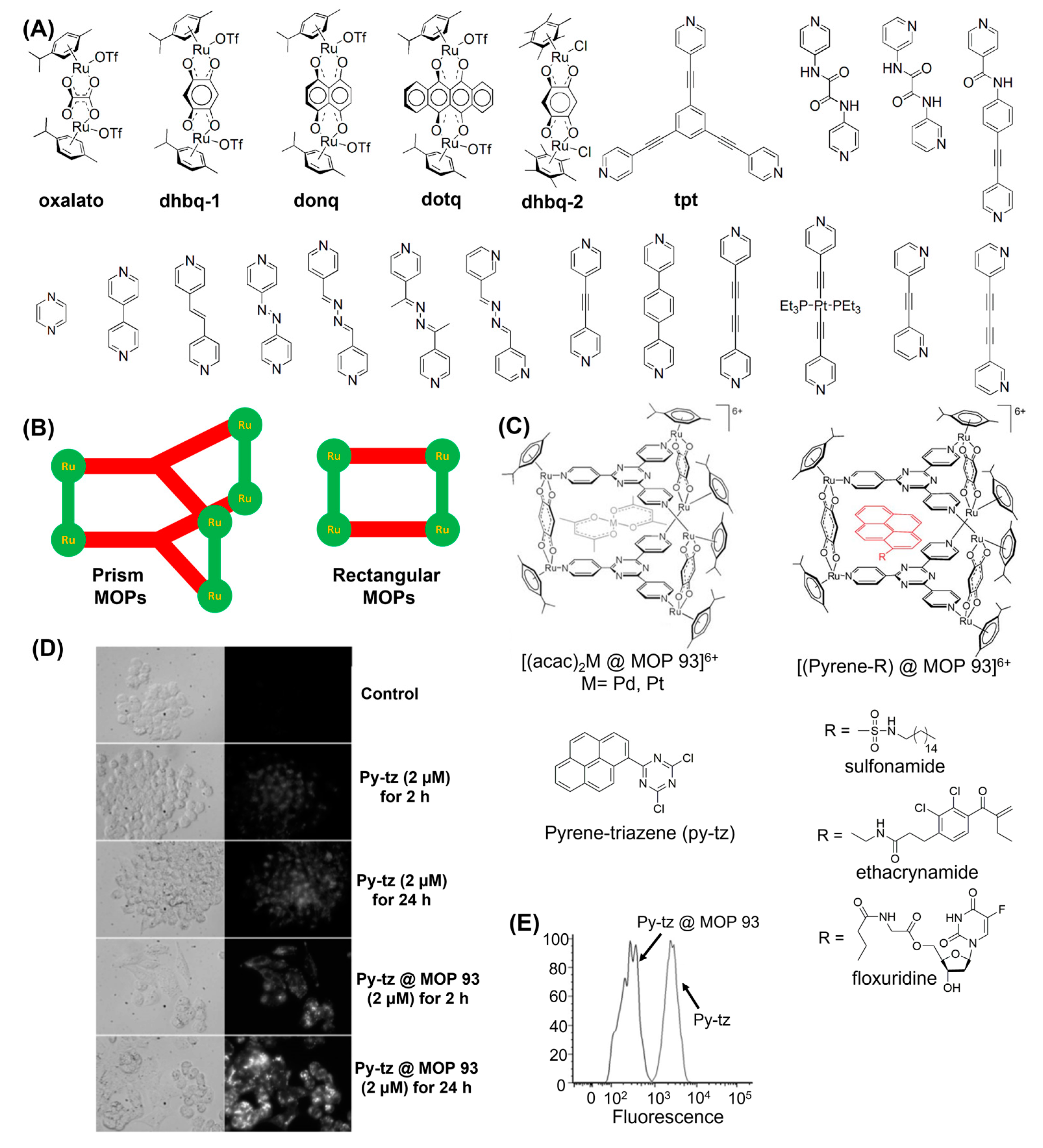
3. MOPs Containing O-Donor Ligands
Strong directional metal-ligand interactions enable rational designing of discrete structures which become less obvious when other noncovalent interactions like H-bonds, π-π, dipole-dipole and Van der Waals interactions [
78,
79] are at play. MOPs with varied shapes and topologies were prepared by choosing appropriate metal ions and rigid organic linkers. Variety of organic ligands in combination with wide range of metal ions led to the synthesis of novel metal organic materials with emerging functionalities. Well-defined coordination geometries of transition metal ions make them suitable candidates for the preparation of MOPs with desired shapes and topologies. Extensive works on coordination polymers establishes the carboxylate-based dinuclear paddlewheel complexes, M
2(COO)
4 as novel secondary building units (SBUs) which were then used in combination with various carboxylate-based multitopic rigid or flexible linkers in order to prepare coordination polymers and porous coordination polymers. Extensive works on coordination polymers eventually led to the evolution of giant field of crystalline porous solid materials [
13,
14,
15]. Dinuclear paddlewheel complex, M
2(COO)
4 uses two metal ions which are interconnected by four carboxylate groups at the equatorial position of each metal center. Some dinuclear metal complex like Mo
2(COO)
4 exhibits metal-metal quadruple bond between metal centers [
80]. In last two decades, dinuclear paddlewheel complexes M
2(COO)
4 were emerged as prime candidates for the preparation of carboxylate-based MOPs. Designing of such MOPs are significantly different from the MOPs prepared from other metal ions. Initial reports on the preparation of self-assembled carboxylate-based MOPs were independently documented by the groups of Yaghi [
22], Zaworotko [
23] and Zhao [
30]. Yaghi and coworkers had demonstrated the synthesis of porous MOPs constructed from copper-based SBU and ditopic carboxylate linkers (
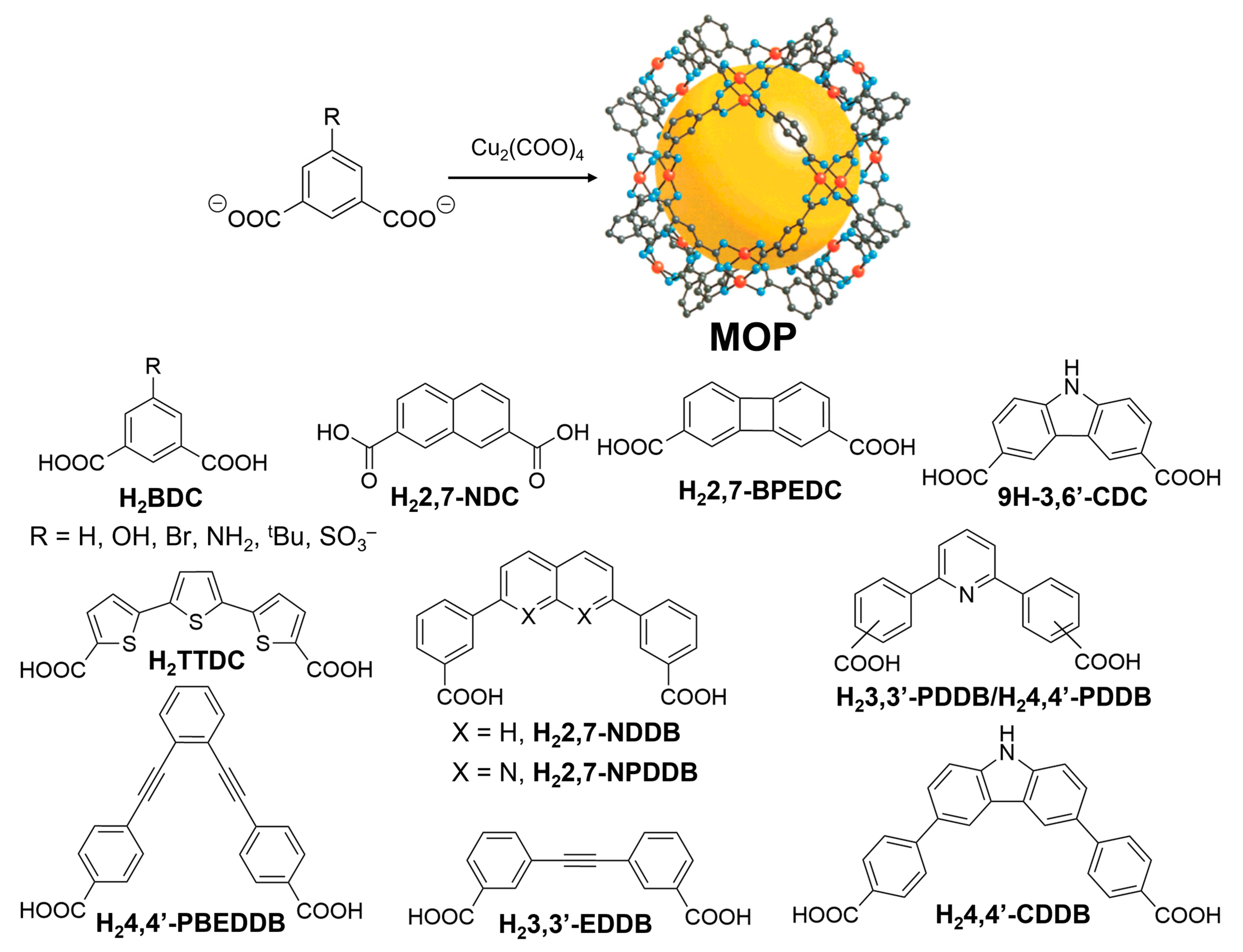
Figure 5). Use of copper(II) acetate, Cu
2(COO)
4 paddlewheel cluster as rigid SBU with linear linker 1,4-benzene dicarboxylate (BDC)—a source of O-donor ligand produces metal organic frameworks with permanent porosity [
81]. Analogous benzene-1,3-dicarboxylate (
m-BDC) linker with 120° bite angle and dicopper paddlewheel SBU produces truncated-cuboctahedron containing 12-paddlewheels linked with 24
m-BDC linkers. The polyhedron (MOP 1) was characterized as Cu
24(
m-BDC)
24(DMF)
14(H
2O)
10 with open metal sites axially coordinated by solvent molecules suitable for post-functionalization both at the inner cavity and outer surface of MOP using external ligands. Dicopper secondary building units are connected to four identical linkers in identical fashion to form a planar vertex arrangement, often called as augmentation giving rise to augmented-cuboctahedron with an average diameter of 15 Å and a volume of 1766 Å
3 [
22]. Parallel to this report, Zaworotko and coworkers demonstrated that upon addition of 2,6-dimethylpyridine to the mixture of a methanolic solution of 5-OH-BDC and Cu(NO
3)
2 spontaneously produces Cu
24(
m-BDC)
24(DMSO)
12(MeOH)
12 (MOP 2) as rhombihexahedron. Polar hydroxy groups located at the outer surface make MOP 2 highly soluble and stable in polar solvents compared to the pristine MOP 1 [
58]. Subsequently, great varieties of MOPs were synthesized by putting various substituents at the 5-position of 1,3-benzene dicarboxylic acid. Bromo and amine-functionalized BDCs gave large-sized rhombicuboctahedron MOPs that are isoreticular to MOP 1 with lower tetragonal symmetry for amine-functionalized MOP compared to bromo-functionalized MOP [
82].
Independently, Zhou and coworkers had demonstrated the synthesis of carboxylate-based cuboctahedron MOPs by reacting dimolybdenum and dicopper paddlewheel clusters with rigid organic linkers 5-
tBu-BDC (BBDC) and
m-BDC with 120° bent angle [
80]. The coordination assemblies, Mo
24(BDC)
24 and [Mo
24(BBDC)
24](py)
12 afforded anticuboctahedron and cuboctahedron geometries, respectively depending on the manner two halves of the paddlewheel clusters are aligned. Each pyridine molecule is axially coordinated to the outer metal center of each Mo-Mo paddlewheel pair. Interestingly coordination assemblies of Cu
24(BBDC)
24(S)
24 and Cu
24(BDC)
24(S)
24 afforded only cuboctahedron clusters with enclosed voids of ~15 Å diameter. Similarly, other variants of MOP 1 were synthesized by various groups including Yaghi, Tonigold, Larsen, Zhou and others [
82,
83,
84,
85,
86,
87]. For example, sulfonated nanoball was synthesized by Zaworotko and coworkers with molecular formula [Cu
2(5-SO
3-BDC)
2(4-methoxypyridine)
0.5(CH
3OH)(H
2O)
1.5•x]
12 [
88]. Other variants of MOP 1 include the synthesis of aliphatic chain, alkene and alkyne-functionalized MOPs using dicopper paddlewheel as SBUs with isoreticular structural features compared with pristine MOP 1 [
74,
82,
85,
86]. Attaching hydrophobic units imparted higher solubility as observed for MOPs functionalized with long aliphatic and 2-(2-hydroxyethoxy)ethoxy chains [
84].
Use of angular ditopic ligands other than prototypical benzene dicarboxylic acid (BDC) allows the expansion of the library of carboxylate-based MOPs. For example, 2,7-naphthalenedicarboxylic acid (2,7-NDC) and 2,7-biphenylenedicarboxylic acid (2,7-BPEDC) were successfully utilized to prepare copper-based MOPs [Cu
24(2,7-NDC)
24(DEF)
8(H
2O)
16] and [Cu
24(2,7-BPDC)
24(NMP)
12(H
2O)
12], respectively with same topology as MOP 1 [
82,
89,
90]. Both structures are isoreticular in nature. Angular ditopic linkers containing heteroatoms were also used to prepare diverse and novel class of MOPs. 2,2′:5,2″-terthiophene-5,5″-dicarboxylic acid (H
2TTDC) and 9H-carbazole-3,6-dicarboxylic acid (H
29H-3,6-CDC) linkers with bite angle very close to 90° gave copper-based MOPs [Cu
12(TTDC)
12(NMP)
6(H
2O)
6] and [Cu
12(9H-3,6-CDC)
12(DMA)
6(H
2O)
6], although the general formulas are different from the conventional copper-based MOPs constructed from 120° angular linkers [
91,
92]. Interestingly, methodical probing allows the angle-driven self-assembly approaches to prepare MOP architectures with varied topologies. Tuning the bite angles (0° ≤ θ < 180°) between two carboxylic acid reaction centers and the size of the linkers facilitated the designing of specific polyhedra structures. Most commonly observed bite angles are 0°, 60°, 90° and 120°. Dicopper and dimolybdenum (containing quardruply bonded Mo-Mo) paddlewheel metal clusters in combination with dicarboxylate linkers with variable lengths and varied bridging angles (between 0° and 120°) gave diverse class of carboxylate-based MOPs.
Figure 5.
(
Top) General designing of metal-carboxylate self-assembled MOP prepared from 5-substituted benzene 1,3-dicarboxylate and dimetallic paddlewheel cluster. (
Bottom) Several ditopic carboxylic acid linkers used to prepare diverse class of metal-organic polyhedra (MOPs). Reprinted by permission from Ref. [
22]. Copyright 2001 from American Chemical Society.
Figure 5.
(
Top) General designing of metal-carboxylate self-assembled MOP prepared from 5-substituted benzene 1,3-dicarboxylate and dimetallic paddlewheel cluster. (
Bottom) Several ditopic carboxylic acid linkers used to prepare diverse class of metal-organic polyhedra (MOPs). Reprinted by permission from Ref. [
22]. Copyright 2001 from American Chemical Society.
Parallel arrangements of carboxylic acid groups in H
2(2,7-NDDB) and H
2(2,7-NPDDB) with the bridging angle of 0° gave isostructural large tetragonal MOPs [Cu
2(2,7-NDDB)
2(DMA)
2]
2, [Cu
2(2,7-NPDDB)
2(DMA)
2]
2 and [Mo
2(2,7-NPDDB)
2(DMA)
2]
2 with four bridging ligands surrounding two paddlewheel dicopper/dimolybdenum metal clusters [
93]. Similarly, ligands 3,3′-PDDB and 3,3′-PBEDDB gave lantern-type isostructural MOPs with molecular formulas [Mo
2(3,3′-PDDB)
2(S)
2]
2 and [Mo
2(3,3′-PBEDDB)
2(NMP)
2]
2 [
94]. Octahedron structures were derived when dicarboxylic acid linkers of 90° bend angles are used. Examples of Mo-Mo paddlewheel cluster-based octahedron structures include [Mo
2(9H-3,6-CDC)
2(DMPU)]
6 and [Mo
2(4,4-CDDB)
2(S)
7/6]
6, where DMPU represents 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone [
94]. Organic linkers with 120° bridging angles gave cuboctahedron and anticuboctahedron MOP as discussed earlier when BDC (vide supra) and their derivatives linkers were used [
80]. Linkers with 60° bridging angles produce geometrical structures similar to trigonal cages which include the example of [Mo
2(3,3′-EDDB)
2(S)
2]
3, [Mo
2(4,4′-PBEDDB)
2(S)]
3 and [Mo
2(4,5-(MeO)
2-4,4′-PBEDDB)
2(S)
2]
3 (S represents solvent molecules) [
94].
Till date numerous reports have been documented on the preparation of diverse class of MOPs using O-donor ligands. However, their aqueous instabilities have significantly eluded their utilities for the real-world applications as the stability of weak metal-ligand interactions becoming the crucial factor for determining the hydrolytic stability of coordination complexes and MOPs. Amongst several available dimetallic paddlewheel clusters, copper-based carboxylate MOPs were abundantly synthesized and studied. Labile metal coordination sites of copper-based carboxylate MOPs enables the metal centers to interact post-synthetically with smaller guest molecules or co-ligands in order to solubilize the molecular architectures. However, MOPs constructed from dicopper paddlewheel clusters and carboxylate linkers have several pitfalls—they often fall apart in aqueous media and transform into 3D network in presence of excess ligands, however mostly they remain stable in organic solvents. Tonigold and Volkmer had studied the relative stabilities of MOPs constructed from 5-(2-(2-hydroxyethoxy)ethoxy)benzene-1,3-dicarboxylic acid and Cu(II) ions in comparison with dicopper paddlewheel complex, Cu
2(COO)
4 which undergoes easy decomposition in aqueous solution and other polar solvents [
84]. Study suggested the relative thermodynamic stability of Cu-based MOP compared with Cu
2(COO)
4 paddle-wheel complex due to combined effect of chelate and positive cooperativity arising from the interplay between multiple metal ions and organic linkers. Therefore, enhancing the (aqueous)-stability of Cu-based MOPs is crucial for further extension of the technology to be applicable for real-world benefits. Accordingly, several methodologies adopted to stabilize MOPs include post-functionalization method, hydrophobic shielding of metal ions, immobilization of MOPs on other surfaces, building extended frameworks from MOPs etc. Post-synthetic modification (PSM) strategy is found to be a legitimate route for the construction of hydrolytically stable metal-coordination structures including MOPs and MOFs [
95]. Extensive investigations have been carried out on PSM amendment of MOFs [
95,
96,
97,
98] and very few reports are known hitherto on PSM of MOPs as they seldom retain their structural integrities after post-functionalization. Zhou and coworkers reported the synthesis of alkyne-functionalized MOP 3 constructed from dicopper paddlewheel complex and 5-substitutted-1,3-benzene dicarboxylic acid [
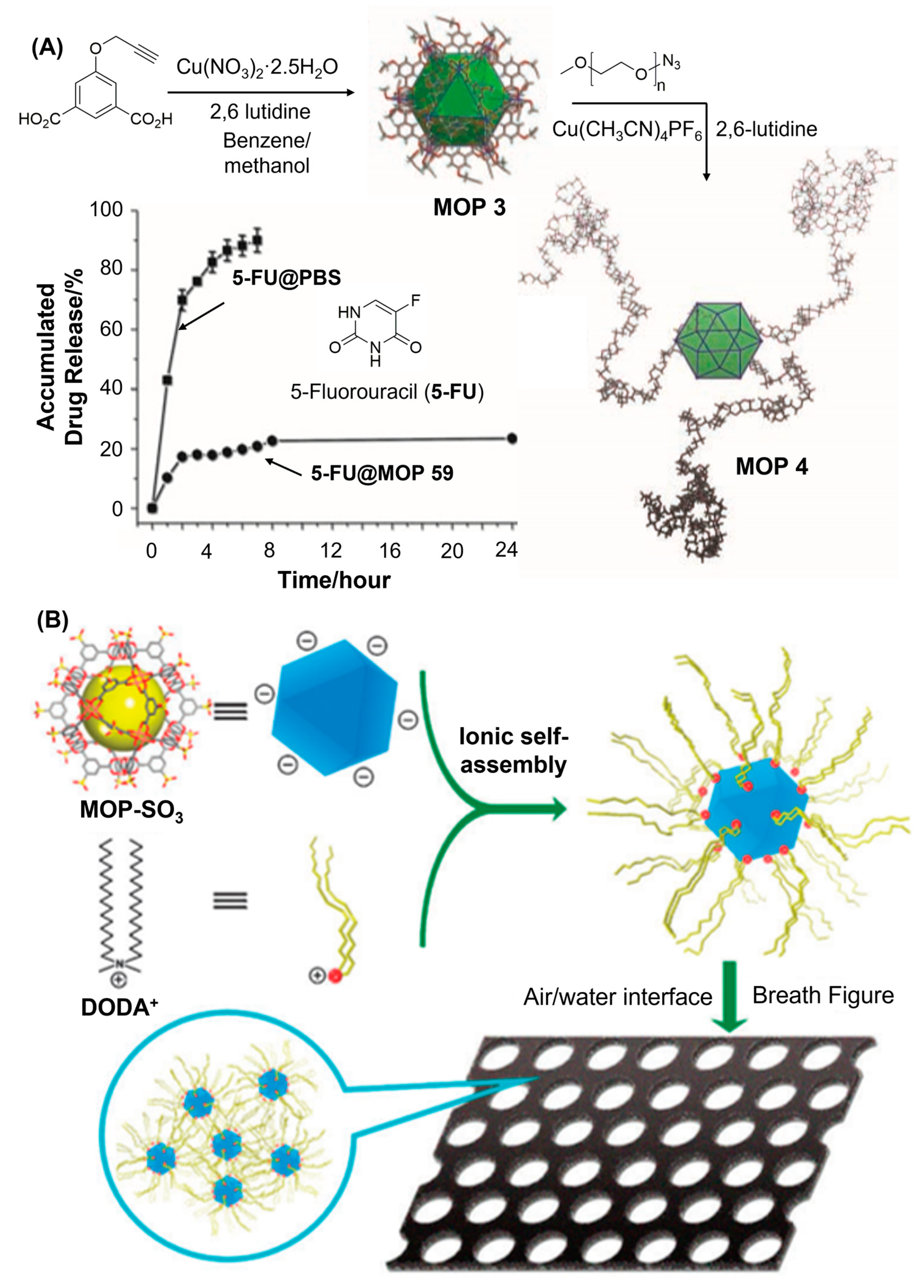
74]. Given the liability of dicopper paddlewheel complex in aqueous environment, the hydrolytic stability of alkyne-functionalized MOP 3 was tested in water following PSM amendments (
Figure 6A). Alkyne-substituted MOP was reacted with azide-terminated polyethylene glycols (PEG) using click chemistry to create MOP 4. Following PSM amendment, MOP 4 was found to be remain water soluble and stable. Presence of long polymeric shells at the outer surface of MOP and presumably the intermolecular aggregation of functionalized MOPs prevented water molecules from attacking the dicopper paddlewheel clusters rendering them water stabile in order to be useful for drug transport. Controlled release of 5-fluorouracil, an anticancer drug which was loaded inside the cavity of MOP 4, was observed over a period of 24 h demonstrating the ability of Cu-based MOPs to be applicable for drug delivery applications. Paramagnetic behaviours, aqueous availabilities and stabilities of PEG-functionalized copper(II)-based MOPs make them suitable candidate to be useful as contrast agents. Akin to covalent modifications, noncovalent post-synthetic modification was also carried out on dicopper paddlewheel cluster-based MOPs and the amendment was done successfully leading to the development of new emergent materials. Huo and coworkers demonstrated that noncovalent post-synthetic amendment can be successfully done on prototypical MOP-SO
3 structure which is successfully constructed from 5-sulfonate-1,3-benzenedicarboxylic acid and dicopper paddlewheel cluster [
99]. By virtue of 24 negative charges sticking out of MOP surface, sulfonated MOP becomes better water soluble and can be surface-functionalized with surfactants like dimethyldistearyl ammonium chloride via ionic self-assembly (
Figure 6B). Structural integrity of MOP was fully preserved following the electrostatic interaction-based surfactant functionalization, which therefore excludes all the possibilities of disassembly during synthetic amendments enabling them to take part in the preparation of ordered honeycomb architecture at the air/water interface. Two dimensional hexagonally ordered pore array with average pore size of 3.5 μm was realized from scanning electron microscopy (SEM) images. The aggregates of surfactant encapsulated MOP nanocages are ~20–30 nm in diameter and the discrete particles are about 3 nm in diameter. Hydrophobic surfactants decorated on the MOP surface significantly reduces the chances of MOP to be exposed to water/air environment, thereby greatly enhances humidity-resistance. In a recent report, Sun and coworkers demonstrated that photopolymerization can significantly enhance the hydro-stability, dispersity and processability of a prototypical copper-based MOP 5 [
100]. MOP 5 constructed from dicopper paddlewheel and amino-isophthalic acid linker was used as a precursor in a reaction with methacrylic anhydride to produce methacrylic-functionalized MOP 6. UV irradiation of methacrylic-functionalized MOP 6 in presence of butyl methacrylate as crosslinking agent afforded photopolymerized MOP-BMA (MOP 7). The hydrophobic polymer segment renders humidity resistance to cross-linked MOP-BMA. In comparison with pristine MOP which degraded upon exposure to the humid condition over a period of 6 h, MOP-BMA remains stable over a period of 24 h. While MOPs in the polymer matrix are observed with high dispersity and thermal stability compared with the pristine MOPs, processability of the material is of particular importance from the perspective of practical applications. Crosslinked MOP exhibits better reusability in comparison with precursor crystalline MOPs demonstrating the viability of post-synthetic modification strategy for better usage of Cu-based MOPs.
A more generalized way to prepare stable MOPs is to shield the labile metal centers from water/chemical-attack. Anchoring hydrophobic functional groups on the outer surface of MOPs would turn the MOPs more water-resistant [
101] and thus should be considered as more generalized and straightforward approach to prepare stable MOPs irrespective of the metal ions and linkers used during the synthesis. Yaghi and coworkers had synthesized dicopper paddlewheel-based carboxylate MOPs functionalized with dodecoxyalkyl chains [
85]. Kim and coworkers demonstrated the ability of dodecoxyalkyl-functionalized MOPs to act as synthetic ion channel for transporting proton and alkali-metal ions across lipid membranes [
102]. The prospect of potential applications of highly stabilized MOPs makes them more appealing for studying their systematic preparation procedures. In a recent report, Ghosh and coworkers had studied the inertness of copper-based MOPs toward water by systematically putting hydrophobic moieties around the metal centers [
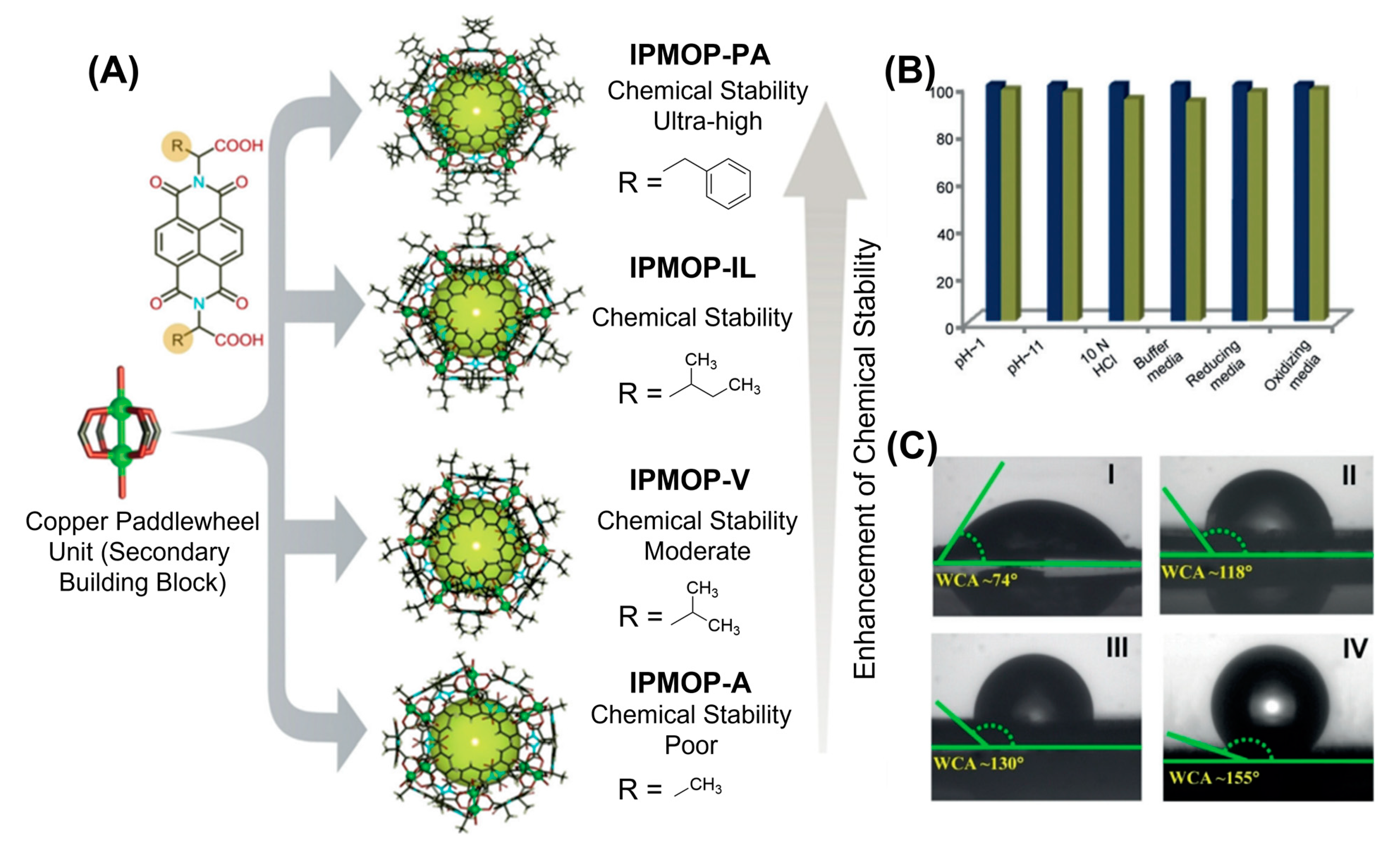
75] and had synthesized series of chemically ultrastable copper-based carboxylate MOPs (
Figure 7). Four neutral spherical MOPs with general formula [Cu
12(L
x)
12(H
2O)
12] (L
x: NDI core-based amino acid variant) were synthesized on a large scale by solvothermal method from dicopper paddlewheel cluster and naphthalene diimide linkers appended with amino acid variants—alanine, valine, isoleucine and phenyl alanine. Eight triangular windows and 24-alkyl groups are appended to each cage structures. Chemical stabilities of those MOPs were investigated under both acidic and basic conditions as well as under several oxidizing and reducing conditions. Smaller alkyl chains on the outer surface render MOPs unstable in water when compared with bulkier alkyl groups-functionalized MOPs. MOPs substituted with isoleucine and valine variants show moderate aqueous stability in comparison with phenyl alanine modified MOP which exhibits ultrastability under a wide range of pH values, in strong oxidizing and reducing media over a period of several days. Such exceptional stability can be attributed to the dense hydrophobic shielding of dicopper paddlewheel metal clusters preventing water molecules and other chemicals to undergo chemical ligation at the labile metal centers. Contact angle measurement revealed that hydrophobic (WCA ≈ 74°) to superhydrophobic surface (WCA ≈ 155°) properties can be achieved for copper-based carboxylate MOPs by simply fine-tuning the alkyl-side chains on the outer surface of MOPs.
Protecting labile metal ions from water-attack via hydrophobic shielding and/or post-synthetic amendment are definitely the viable strategies to prepare stable MOPs. However, ideal strategy would be to enhance the inertness of metal-ligand interactions by increasing metal-ligand bond strengths. For example, high-valent metal ions with high charge density and small ionic radii form stronger bonds with carboxylate linkers rendering the metal-ligand bonds less susceptible (
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13) to aqueous instabilities. In general, Ti
4+, Zr
4+, Hf
4+, Cr
3+, Ga
3+, Al
3+ and other metal ions with high charge density and carboxylate ligands are considered to be hard acid and hard base, respectively and they form metal-ligand bonds stronger than Cu-O bond [
103]. Wide varieties of metal organic frameworks were reported using first row transition metal ions starting from Cr
2+ to Zn
2+. Interestingly great varieties of MOPs which are amenable for the incorporation of M
2 paddlewheel metal clusters other than dicopper paddlewheel as secondary building units are created in order to tune their porosities and stabilities. In the first-row transition metal ions of the periodic table, Cr
2+ metal complexes experience lesser Jahn-Teller distortion effect compared with Cu
2+ metal complexes, therefore higher kinetic stability of Cr complexes is anticipated. Among M
2 paddlewheel units, dichromium paddlewheel cluster, Cr
2(COO)
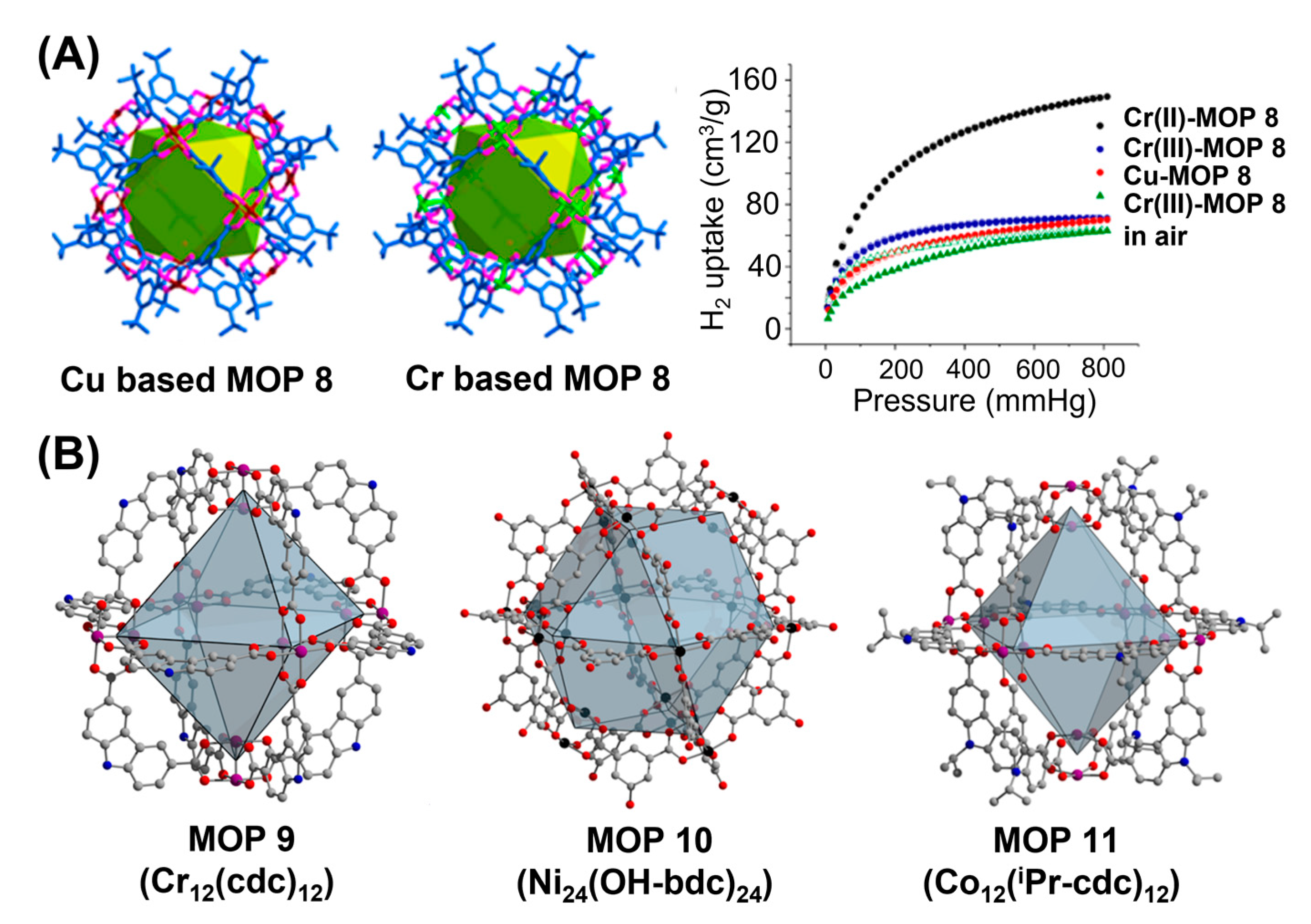
4 forms well-known coordination polymers with carboxylate linkers. Zhou and coworkers had first reported the synthesis of Cr(II)-based MOP (MOP-8) by reacting Cr
2(OAc)
4 with several isophthalate ligands and the BET surface area was measured to be 1000 m
2/g, one of the highest reported surface area among MOP-based systems [
104]. Cr-based MOP 8 was found to be isostructural with the corresponding Cu/Mo-based MOP 8 with each dichromium unit, Cr
2 assembled with four carboxylate linkers originating from four different isophthalate linkers and 24 ligands coordinated to 12 units of Cr
2 units serving as vertices to form cuboctahedron cage. Gas adsorption study revealed better N
2 uptake behaviour of Cr(II)-MOPs at higher temperature compared with analogous Cu(II)-MOPs. High dissociation energy (513 kJ/mol) of Cr-O bond makes Cr(II)-MOPs thermally more stable as shown by their thermogravimetric analysis. Cu(II)-MOPs showed step-by-step increase in N
2 uptake upto 200 °C and lower uptake was observed beyond 200 °C. Interestingly, Cr(II)-MOPs showed negligible uptake until the activation temperature of 160 °C is achieved and finally N
2 uptake reached to 387 cm
3/g upon activation at 200 °C demonstrating the good maintenance of porosities of chromium-based materials following activation. Higher coordination bond strength between the coordinating solvent molecules and chromium metal centers makes the activation temperature higher for gas uptake. Cr(II)-MOPs are susceptible to arial oxidations and produces Cr(III)-MOPs following the oxidation of Cr
2+ metal ions to high valence Cr
3+ metal ions (
Figure 8A). Unlike Cu(II)-MOPs which are unstable in air, strong Cr
3+-O coordination bond makes oxidized chromium-MOPs exceptionally stable to preserve their porous structures in the solid state to exhibit good H
2 uptake properties even after leaving the materials for one month in air. Bloch and coworkers had demonstrated that carbazole containing MOPs which are part of metal organic frameworks like DUT-49 [
105] and PCN-82 [
106] showed excellent methane and hydrogen storage capacities, can be synthesized independently to study their gas adsorption properties (
Figure 8B). Accordingly, chromium(II)-based octahedron cage, Cr
12(cdc)
12•nDMF (MOP-9) was synthesized by reacting anhydrous Cr
2(OAc)
4 with carbazole dicarboxylic acid (H
2cdc) in dimethylformamide-methanol mixture under air-free condition [
107]. TGA analysis revealed the excess of 60% solvent accessible void in Cr
12(cdc)
12 crystals and initial solvent exchange triggered structural rearrangement. Cr
12(cdc)
12 exhibits a BET surface area of 1235 m
2/g upon activation at 70 °C, one of the highest surface area reported for molecular metal organic materials. Analogous isostructural MOPs, Mo
12(cdc)
12 and Cu
12(cdc)
12 exhibited BET surface areas of 1108 m
2/g and 657 m
2/g, respectively which are much lower compared with chromium-based MOPs. Similar BET surface area trends were observed from gravimetric uptake analysis for methane storage with capacities of 148, 135, 81 cm
3/g for Cr
12(cdc)
12, Cu
12(cdc)
12 and Mo
12(cdc)
12, respectively (
Table 1). Nevertheless, surface areas shown by these molecular materials fall short of the record values displayed by porous metal organic frameworks like DUT-49 [
108] and PCN-81 [
106] in order to be useful for high-pressure gas storage application. Achieving permanent porosity requires activation of MOPs which remains challenging mostly due to collapse of the extended structures and thus very much dependent on activation conditions [
109]. Bloch and coworkers had reported the synthesis of chromium(II)-based MOPs using benezene-1,3-dicarboxylic acid ligand which is also known to produce MOPs in combination with other metal ions like copper (II) and molybdenum (II) [
110]. Interestingly,
tBu-substituted chromium(II)-MOP (Cr-
tBu-bdc) was found to be significantly thermally stable with optimal activation temperature of 75 °C in comparsion with Cr-OH-bdc MOP with having the Langmuir surface area of 187 m
2/g. Under this condition, Cr-
tBu-bdc MOP showed BET surface area of 1796 m
2/g which is the highest surface area reported for metal organic polyhedra. Importantly, the materials retained significant surface area of 1588 m
2/g even after heating upto 175 °C.
Other first row transition metal ions like nickel, cobalt, iron, zinc were also successfully utilized to prepare porous coordination cages in order to tune their porosities and stabilities. Introduction of paddlewheel clusters from variable metal ions led to the synthesis of diverse class of coordination cages. Recently, Bloch et al. had reported the preparation of coordination cages using cobalt(II) and nickel(II) metal ions [
111]. Zinc(II)-containing paddlewheel units lack metal-metal bonding. Instability of square-planar Zn
2+ complexes make them particularly challenging to retain the porosities of Zn-based MOPs and MOFs after removal of coordinated solvent molecules which results in degradation of porous materials. Reaction between 5-hydroxy-isophthalic acid and nickel(II) acetate yielded Ni
24(OH-bdc)
24 cuboctahedron cage in high yield with average Ni-Ni distance in the paddlewheel of 2.65 Å comparable to Cu-Cu distance in Cu
24(OH-bdc)
24 (
Figure 8B) but significantly longer than quadruply bonded Mo-Mo distance in Mo
24(OH-bdc)
24. Moreover, topological variants of MOPs can be synthesized by tuning the bridging angles between carboxylic acid groups. Reaction of 9-isopropylcarbazoledicarboxylic (
iPr-cdc) acid with NiOAc
2•4H
2O or CoCl
2•6H
2O afforded octahedron coordination cages Ni
12(
iPr-cdc)
12 and Co
12(
iPr-cdc)
12, respectively with having six bimetallic paddlewheel units in each coordination cage. All three MOPs maintain their crystallinities after solvent exchanges and their three-dimensional structural packing result in potentially accessible channels within their structures following activation. Structural rearrangements due to lack of three-dimensional inter-cage interactions result in significant loss in porosity in Ni
24(OH-bdc)
24 MOP upon activation. Interestingly, carbazole-based materials exhibited excellent thermal stability under evacuation to retain their porosities upto activation temperature of 200 °C. Therefore, precise ligand functionalization with judiciously chosen metal ions can potentially be the best combination to optimize the porosities and stabilities of metal organic polyhedra (MOPs).
Analogous to other first row transition metal ions, titanium metal particularly high valent Ti
4+ metal ion can be used to prepare stable metal organic polyhedra. Compared with other metal ions, Ti-based metal organic polyhedra are very rare in literature. Zhang and coworkers had demonstrated the synthesis of water soluble and ultrastable Ti-based tetrahedron [
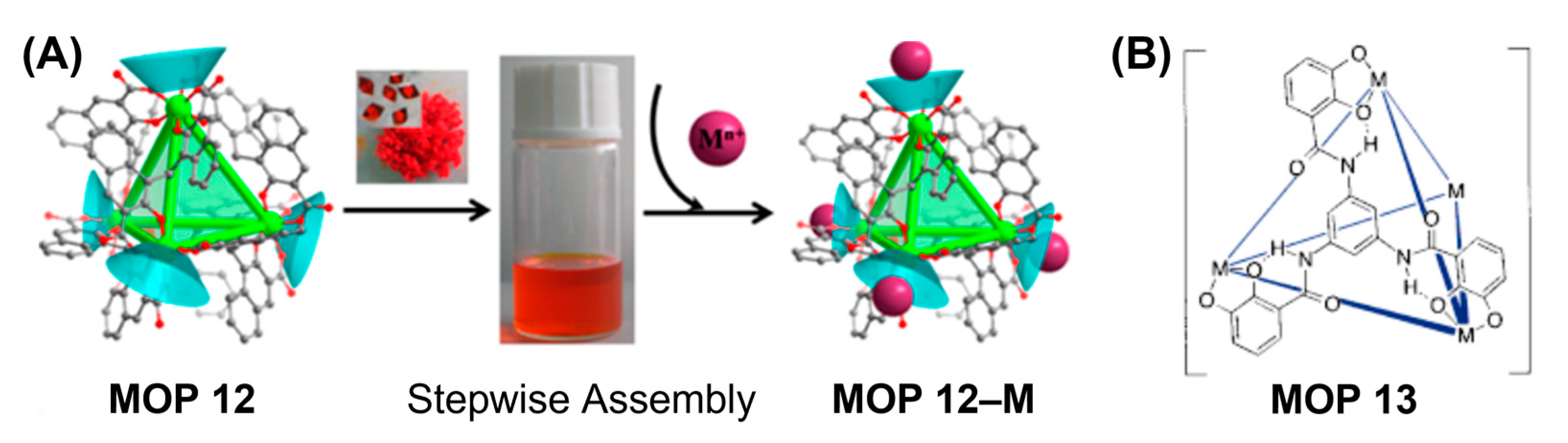
112]. Solvothermal assembly of Ti(
iOPr)
4 or TiCl
4 with embonic acid gave rise to anionic tetrahedral Ti
4L
6 cage (MOP 12) in orthorhombic (PTC-101) or cubic (PTC-102) arrangements (
Figure 9A). Each Ti-center is adopted to distorted octahedron coordination geometry chelated by three carboxyl-phenol groups with uncoordinated oxygen atoms pointing outwards. Solubility and ultra-stability of Ti
4L
6 cage in water is confirmed by electrospray ionization mass spectrometry affording stepwise assembly function with other metal ions. The crystals of PTC-101 and PTC-102 can be easily dissolved in water. Unusual mixed valency of Ti
3+/4+ was observed in the Ti
4L
6 cage as identified by electron spin resonance and X-ray photoelectron spectroscopy analysis. Visible-light induced homogeneous photo-decomposition of acid blue 93 and alkali blue 4B were successfully achieved by these cages. Raymond and coworkers had expanded the synthesis of Ti-based metal coordination cage using catecholate-based organic linkers which satisfy the coordination sphere of tetravalent titanium (Ti
IV) metal ions by having three coordinating chelating groups (
Figure 9B). Reaction of three-fold symmetric tris-catecholate based ligand with the methanolic solution of [Ti(
OnBu)
4] afforded the formation of tetrahedral cluster of stoichiometry (MOP 13) Raymond and coworkers had further demonstrated that other hard trivalent or tetravalent transition and main group metal ions e.g., Ga
III, Fe
III, Al
III, Ge
IV, Sn
IV can be successfully combined with catecholate ligands to prepare coordination complexes of high thermodynamic stabilities and enough labilities to ensure clean formation of the tetrahedral clusters [
59,
113,
114]. Higher stability for the tetravalent Ti
IV and Ge
IV-based hosts can be anticipated from slower guest exchange rate compared with trivalent Ga
III, Fe
III, Al
III hosts as the crystal field effects remain insignificant for metal ions with similar ionic radii, the lability depends on the charge density of the metal ions with more highly charged metal ions having slower water exchange rate [
115]. In contrast, guest exchange in hosts having more inert metal-ligand bonds is as facile as the guest exchange in hosts with more labile metal-ligand bonds, rather distortion of host structure is sufficient for the passage of guests and certainly does not require metal-ligand bonds rupture within the hosts.
Figure 10.
O-donor based MOPs prepared using second row transition metal ions. Chemical structures of Pd-based MOP 14 and MOP 15. Crystal structures of Rh-based anticubooctahedron MOP 16 and Ru-based two cuboctahedron cages, MOP 19 and MOP 20. Crystal structures of Zr
IV-based tetrahedron MOP 21, MOP 22, MOP 23 and MOP 24 are shown. MOP 25 is the crystal structure of Zr-bpydc-CuCl
2. Reprinted by permission from Refs. [
116,
117,
118,
119,
120,
121]. Copyright 2009 from Royal Society of Chemistry. Copyright 2003, 2013, 2016 from American Chemical Society. Copyright 2016 from Wiley-VCH. Copyright 2015 from Elsevier.
Figure 10.
O-donor based MOPs prepared using second row transition metal ions. Chemical structures of Pd-based MOP 14 and MOP 15. Crystal structures of Rh-based anticubooctahedron MOP 16 and Ru-based two cuboctahedron cages, MOP 19 and MOP 20. Crystal structures of Zr
IV-based tetrahedron MOP 21, MOP 22, MOP 23 and MOP 24 are shown. MOP 25 is the crystal structure of Zr-bpydc-CuCl
2. Reprinted by permission from Refs. [
116,
117,
118,
119,
120,
121]. Copyright 2009 from Royal Society of Chemistry. Copyright 2003, 2013, 2016 from American Chemical Society. Copyright 2016 from Wiley-VCH. Copyright 2015 from Elsevier.
Compared with first row transition metal ions, second row transition metal ions tend to form relatively stable complexes. Examples include dirhodium paddlewheel motifs which are particularly interesting for the preparation of stable carboxylate-based MOPs due to their high chemical stabilities, but the relative inertness of their equatorially-oriented carboxylate groups makes it very challenging to prepare the MOPs via ligand-exchange reactions. Similarly, it was believed that MOPs bearing Pt-O or Pd-O bonds would be unstable due to hard-soft combination. Mukherjee and coworkers had reported the synthesis of neutral molecular rectangular MOP driven by Pd-O interaction [
116]. Dipalladium(II)-based organometallic clip containing anthracene unit in combination with disodium fumarate salt afforded molecular rectangular MOP 14 driven by the electrostatic interaction between the anionic fumarate and Pd(II) metal ion (
Figure 10). Utilizing Pt-O interaction, Stang and coworkers had reported the synthesis of MOP 15 (
Figure 10) from phenanthrene-based diplatinum molecular clip and dicarboxylate linkers [
117,
122]. Linear ditopic carboxylate linker gave triangular MOP whereas angular ditopic carboxylate linker produced rhomboidal MOP. Kitagawa and coworkers had demonstrated the synthesis of rhodium-based metal-organic cuboctahedra MOP 16 (
Figure 10) with molecular formula [Rh
2(bdc)
2(S)
2]
12•(S); S = Solvent molecules [
118]. Relative stability of rhodium-based MOP 16 was assessed in comparison with isostructural Cu(II)-analogue. Interestingly, copper(II)-based MOP showed very limited nitrogen uptake following activation at 323 K indicating the copper(II) analogue becoming nonporous after solvent removal. Dirhodium-based MOP can be successfully activated upon heating at 368 K and showed nitrogen adsorption capacity of 130 cm
3/g at relatively low pressure (P/P
0 = 0.1) confirming the presence of microporous cavities inside dirhodium-based MOP. Rhodium-paddlewheel motif provides rigidity to the molecular backbone and imparts high thermal and chemical stabilities. Maspoch and coworkers had reported the role of strategic use of protecting groups in the synthesis of Rh(II)-based MOPs with previously inaccessible functionalities [
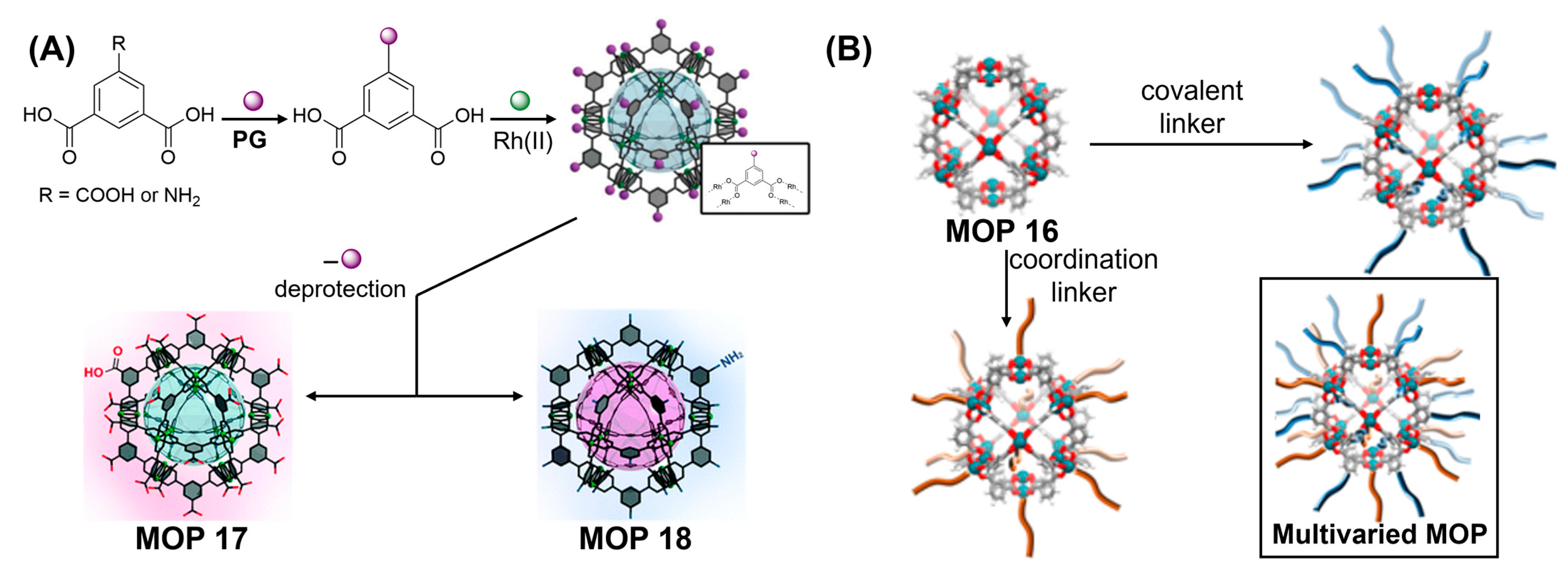
123]. Synthesis of both carboxylic and amine-functionalized MOPs need careful designing of protection and deprotection strategies in order to prevent the formation of undesired coordination polymers facilitated by strong affinities of Rh(II)-paddlewheels towards carboxylic acids and N-donor groups (
Figure 11A). Protection of one of the three carboxylic acid groups of 1,3,5-benzenetricarboxylic acid by 2-(trimethylsilyl)ethanol followed by the treatment with Rh
2(OAc)
2 under solvothermal condition gave COOTSE-RhMOP in cuboctahedron geometry consisting of 12 dirhodium paddlewheel units and 24 protected carboxylic acid linkers. Successful deprotection strategy led to the formation of carboxylic acid functionalized Rh(II)-MOP (MOP 17) demonstrating the ability of Rh(II)-MOP to withstand post-synthetic modification. Interestingly, deprotected Rh(II)-MOP was found to maintain the structural porosity compared with protected MOP as revealed from their increased gas uptake capacities which can be attributed to the removal of bulky protecting groups having beneficial effects on the porous properties of the materials [
124,
125]. COOH-Rh(II)MOP can be dissolved in water at near physiological pH and did not show any signs of degradation even after leaving in aqueous solution for 7 days demonstrating exceptional chemical stability of Rh(II)-based MOP. Similarly, amine-functionalized Rh(II)-MOP (MOP 18) was successfully synthesized by BOC-protecting the amine group of 5-aminoisophthalic acid linker. Owing to the strong processability, deprotection of NBoc-Rh(II)MOP either by thermolysis or by treatment with trifluoroacetic acid (TFA) gave the amine-functionalized Rh(II)-MOP which becomes more porous than the protected starting materials. Interestingly, NH
2-functionalized Rh(II)-MOP exhibits better gas uptake behaviour compared with Cu(II) analogue [
126].
Figure 11.
(
A) Scheme of protecting group strategy to synthesize COOH-tagged cuboctahedron Rh(II)-based MOP 17 and NH
2-tagged Rh(II)-based MOP 18. (
B) Post-synthetic covalent and coordination modification of cuboctahedron MOP 16. (Inset) Illustration of a cuboctahedron Rh-MOP functionalized on the 12 dirhodium paddlewheels through coordination chemistry (in orange) and on the 24 organic linkers through covalent chemistry (in blue). Reprinted by permission from Refs. [
123,
127]. Copyright 2019 from Royal Society of Chemistry and copyright 2019 from American Chemical Society.
Figure 11.
(
A) Scheme of protecting group strategy to synthesize COOH-tagged cuboctahedron Rh(II)-based MOP 17 and NH
2-tagged Rh(II)-based MOP 18. (
B) Post-synthetic covalent and coordination modification of cuboctahedron MOP 16. (Inset) Illustration of a cuboctahedron Rh-MOP functionalized on the 12 dirhodium paddlewheels through coordination chemistry (in orange) and on the 24 organic linkers through covalent chemistry (in blue). Reprinted by permission from Refs. [
123,
127]. Copyright 2019 from Royal Society of Chemistry and copyright 2019 from American Chemical Society.
It has been challenging to implement post-synthetic functionalization on MOPs due to their chemical instabilities. However, stable MOPs synthesized using inert metal-carboxylate bonds like Rh-O however remain amenable for post-functionalization. Maspoch and coworkers had demonstrated the post-synthetic covalent and noncovalent functionalization methods on Rh(II)-based MOPs [
127]. Two robust octahedral Rh(II)-based MOP 16 with varied solvents, [Rh
2(bdc)
2(H
2O)
2]
12 and [Rh
2(OH-bdc)
2(H
2O)(DMA)]
12 were selected for post-functionalization mainly due to their chemical robustness and presence of intrinsic micropores. Carboxylate-based Rh-Rh paddlewheel clusters are mostly inert at their equatorial sites however they remain highly reactive at their axial sites. Given these affinities, ligand-exchange reaction at the axial sites of metal centers would enable surface functionalization of Rh(II)-MOPs using N-donor ligands like 4-tertiarybutyl pyridine (
tertPy) which can axially coordinate to the outer metal centers of all Rh
2 paddlewheel units giving rise to surface-functionalized Rh-MOP with chemical formula of [Rh
2(bdc)
2(
tertPy)(H
2O)]
12. Covalent functionalization was carried out by activating the hydroxyl groups of organic linkers pointing outwards of the Rh(II)-MOP. Sodium hydroxide was reacted with acyl chloride or acid anhydrides. Orthogonal reactivities of Rh-Rh paddlewheels and the organic linkers therefore yielded doubly-functionalized Rh-MOPs featuring 36 new functional molecules. Although, post-functionalization at the outer surface induces surface area reduction compared with pristine materials, post-functionalized Rh(II)-MOPs importantly maintain their intrinsic microporosities in the solid state.
Designing of supramolecular polymers from metal organic polyhedra (MOPs) with permanent microporosities is very challenging as the requirement of crystallinity impedes the materials processing and often amorphous materials end up being nonporous. Furukawa and coworkers had reported novel strategy to prepare amorphous supramolecular polymeric materials with controlled microporosities [
128]. Porous Rh-based carboxylate MOP 16 ([Rh
2(bdc)
2]
12, bdc = benzene-1,3-dicarboxylic acid) was allowed to react with ditopic imidazole linker 1,4-bis(imidazole-1-ylmethyl)benzene (bix) at the labile axial position of each Rh metal centers to yield cross-linked supramolecular polymers. By controlling the self-assembly pathways, macroscopic morphologies of supramolecular polymers can be successfully fine-tuned from spherical colloidal particles to colloidal gels with increasing hierarchical porosities. Gas adsorption experiments which were performed on colloidal polymeric particles and aerogels demonstrated that the intrinsic porosities of the monomers were not only preserved during the supramolecular polymerization, but also supramolecular polymeric materials outperformed the CO
2 uptake of the porous monomers. This can be attributed to the presence of micro and macropores in supramolecular polymers compared with monomeric Rh(II)-MOP 16 which undergoes aggregation in the solid state. Better gas uptake behaviour of aerogels over polymeric particles further emphasizes the influences of macroscopic morphologies on the gas adsorption properties.
Other second row transition metal ions like ruthenium also forms inert metal-ligand complexes and therefore are expected to produce robust and stable MOPs. Dinuclear Ru
2n+ paddlewheel clusters with oxidation states ranging from +4 to +6 were reacted with various carboxylate-based organic linkers to produce metal organic composites. Zhou and coworkers first reported the synthesis of metal organic polyhedra constructed (
Figure 10) from dinuclear ruthenium paddlewheels [
119]. Reaction of H
2CDC (9H-3,6-carbazoledicarboxylic acid), H
2BBDC (5-tert-butyl-1,3-benzenedicarboxylic acid) and H
2BDC (1,3-benzenedicarboxylic acid) with Ru
2n+ [n = 4 for Ru
2(OAc)
4 and n = 5 for Ru
2(OAc)
4Cl] under solvothermal condition gave two octahedron cages [Ru
2(CDC)
2Cl]
6 and [Ru
2(CDC)
2]
6 and two cuboctahedron cages [Ru
2(BBDC)
2Cl]
12 (MOP 19) and [Ru
2(BDC)
2]
12 (MOP 20).
Among other metal-ligand bonds, the Zr-O coordination bond is exceptionally strong with the bond dissociation energy upto 766 kJ/mol. Zr
4+ and carboxylate linkers are hard acid and hard base, respectively; and MOPs constructed from zirconium metal ion and carboxylate linkers are thus expected to be robust and stable. Liu and coworkers had first reported the synthesis of four Zr
IV-based carboxylate MOPs—MOP 21, MOP 22, MOP 23 and MOP 24 with molecular formulas {[Cp
3Zr
3μ
3-O(μ
2-OH)
3]
4(BDC)
6}Cl
4•nS, {[Cp
3Zr
3μ
3-O(μ
2-OH)
3]
4(BTC)
4}Cl
4•4DMF•nS, {[Cp
3Zr
3μ
3-O(μ
2-OH)
3]
4(BPDC)
6}•Cl
4•nS, and {[Cp
3Zr
3μ
3-OH)
3]
4(BTB)
4}•Cl
4•nS (Cp = cyclopentadienyl, bdc = benzene-1,4-dicarboxylate, btc = benezene-1,3,5-tricarboxylate, bpdc = 4,4′-biphenyldicarboxylate, H
3btb = 4,4′,4″-benzene-1,3,5-triyltris (benzoic acid) and S represents free solvent molecules), respectively [
120]. All four cages have tetrahedral geometries and they are isostructural in nature. Trinuclear zirconocene cluster appears to be a 3-connected secondary building unit having pyramidal geometry with carboxylate ligands oriented to one face and the μ-OH groups oriented to the other, in which the C
2 axes in carboxylate ligands forms an angle of about 60°. Hong and coworkers had reported the synthesis of hetero-metal decorated Zr
IV-based carboxylate tetrahedral MOP 25, Zr-bpydc-MCl
2 (M = Pd
2+ or Cu
2+, H
2bpydc = 2,2-bipyridine-5,5-dicarboxylic acid) [
121]. Effective use of hard/soft—acid/base principle allows the orthogonal self-assembly of Zr-bpydc-CuCl
2 cage via three different strategies—postsynthetic metallation, a stepwise metallo-ligand approach and one-pot reaction. Preparation of stable porous materials with permanent porosities and good solution processabilities is exceedingly challenging. Chen and coworkers had reported the preparation of Zr-based MOP 26, [Cp
3Zr
3(μ
3-O)(μ
2-OH)
3]
8(D-cam)
12·Cl
8·nS (D-cam = D-camphoric acid, S = free solvent molecule) which possesses both intrinsic and extrinsic porosities (
Figure 12A) [
129]. MOP-based supramolecular framework maintains their original structure after activation and exhibits permanent porosity with moderately high surface area of 1201 m
2/g as demonstrated by gas adsorption isotherm. Each single vertex of the cage was held together with other three vertices from three different cages using O-H•••Cl•••H-O hydrogen bonds with 24 of those H-bonds of one cage linked with the surrounding cages. Charge-assisted H-bonding interaction enables cubic cages to form porous hydrogen-bonded metal organic framework. Yuan and coworkers had demonstrated that the porosity and stability of Zr-MOP based hydrogen-bonded framework depends on the degree and strength of the charge-assisted H-bonding interactions [
130]. Two previously reported zirconium MOPs with molecular formula {[Cp
3Zr
3(μ
3-O)(μ
2-OH)
3(bdc)
1.
5]
+•4(DMF)
4Cl
−}
4•31DMF•90H
2O (bdc = 1,4-benzenedicarboxylic acid) and {[Cp
3Zr
3(μ
3-O)(μ
2-OH)
3(btc)]
+•4(DMF)
2Cl
−}
2•26DMF•35H
2O (btc = 1,3,5-benzenetricarboxylic acid) when treated with different solvent like dimethyl amine, two new hydrogen-bonded frameworks {[Cp
3Zr
3(μ
3-O)(μ
2-OH)
3(bdc)
1.
5]
+ 4(Me
2NH
2)
+ 2Cl
−6}•35H
2O and {[Cp
3Zr
3(μ
3-O)(μ
2-OH)
3(btc)]
+•4(Me
2NH
2)
2+Cl
−6}•24H
2O are formed. Compared with two pristine MOPs, newly formed MOPs not only exhibit higher ratio of O-H•••Cl
− bond, but also contain stronger bridging H-bonding interactions which results in enhanced porosity and robustness of the framework in guest-free state. PXRD analysis after gas adsorption reveals that crystallinity of newly formed MOPs are well maintained while the pristine MOPs collapse to amorphous materials indicating lower N
2 uptake for pristine MOPs owing to the existence of the intrinsic pores with collapsed interstitial cavities. Higher N
2 uptake was observed for newly formed MOPs which can be attributed to the existence of well-maintained intrinsic and extrinsic pores following activation.
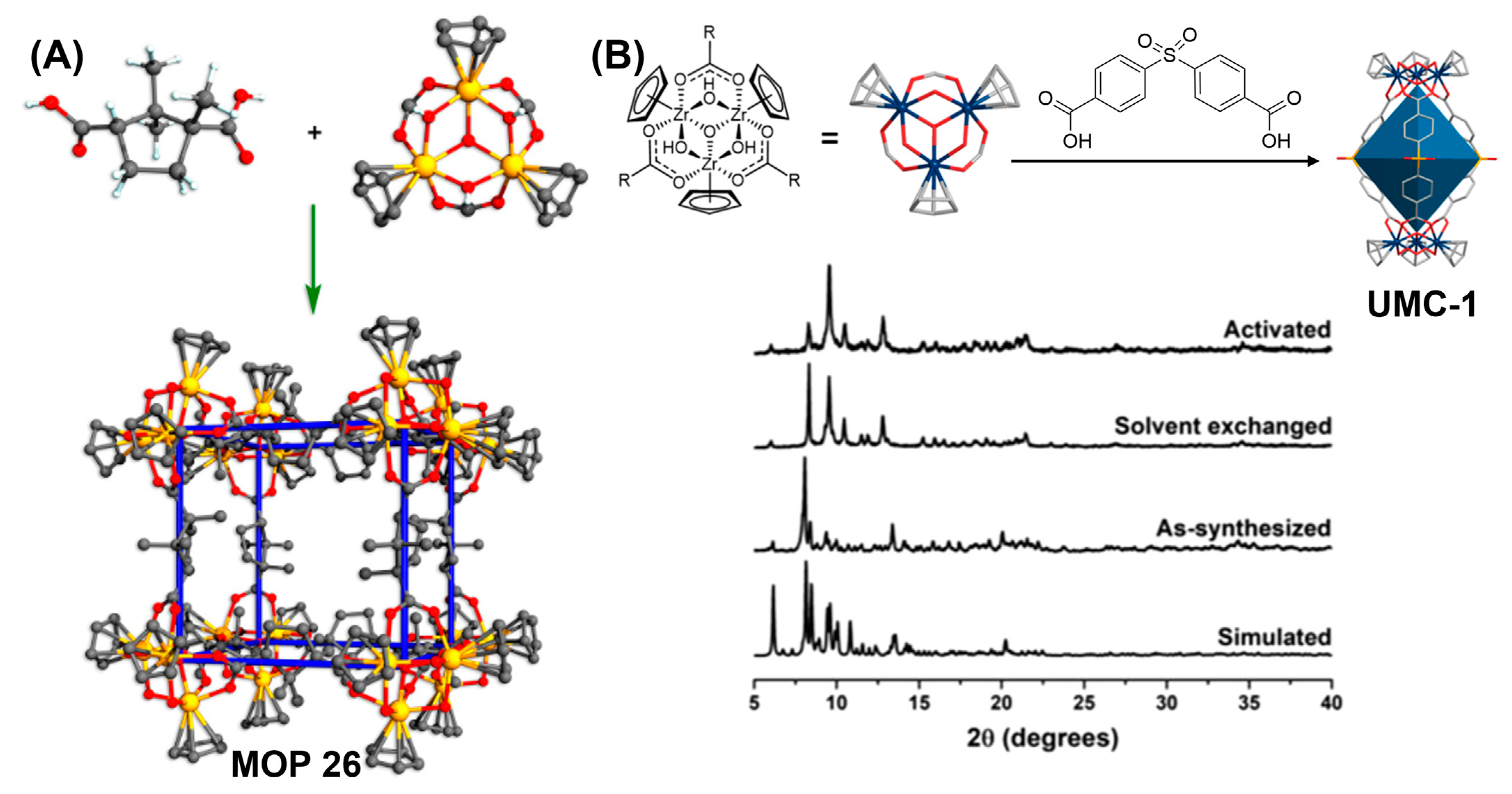
Figure 12.
(
A) Self-assembly of cubic cage [Cp
3Zr
3μ
3-O(μ
2-OH)
3]
8(D-cam)
12 from D-camphoric acid and Cp
3Zr
3O(OH)
3. (
B) Synthesis of UMC-1. Simulated and experimental powder X-ray diffraction patterns of UMC-1 when activated. Afterward, the crystallinity of UMC-1 was preserved. Reprinted by permission from Refs. [
129,
131]. Copyright 2017 from Wiley-VCH and copyright 2018 from American Chemical Society.
Figure 12.
(
A) Self-assembly of cubic cage [Cp
3Zr
3μ
3-O(μ
2-OH)
3]
8(D-cam)
12 from D-camphoric acid and Cp
3Zr
3O(OH)
3. (
B) Synthesis of UMC-1. Simulated and experimental powder X-ray diffraction patterns of UMC-1 when activated. Afterward, the crystallinity of UMC-1 was preserved. Reprinted by permission from Refs. [
129,
131]. Copyright 2017 from Wiley-VCH and copyright 2018 from American Chemical Society.
Choe and coworkers had reported the synthesis of porous Zr-based coordination cages with synergistic binding centers for CO
2 molecules [
131]. Major challenges to enhance gas adsorption behaviour include: (1) strengthening intercage interactions for enhanced permanent porosity and (2) decorating MOPs with strong ligand binding centers for CO
2 adsorption. Zr(IV)-based M
6L
3 cage UMC-1: {[Cp
3Zr
3O(μ
2-OH)
3]
2(SDB)
3}·Cl
2 (H
2SDB: 4,4′-sulfonyldibenzoic acid; Cp: cyclopentadienyl) was accordingly prepared from trinuclear Zr cluster and banana-shaped organic linker. The SO
2 group in the organic linker facilitates the intercage interactions providing strong binding centers for CO
2. Powder X-ray diffraction pattern reveals that UMC-1 retains both intrinsic and extrinsic porosities which can be attributed to proper maintaining of the intercage interactions even after activation (
Figure 12B). Interestingly, UMC-1 remains stable under aqueous acidic solution (pH = 1) to neutral pH = 7 after prolonged exposure for 24 h. Exceptional hydrolytic stability of UMC-1 can be attributed to the strong Zr-O bond strength. Interestingly, another variant of Zr-based MOP, UMC-2 which features the absence of SO
2 group has the same molecular geometry as UMC-1 but different packing pattern. UMC-2 becomes amorphous following activation. Unsurprisingly, UMC-2 with no porosity has significantly lower CO
2 adsorption capacity compared with UMC-1 but has better CO
2 uptake selectivity over N
2 adsorption due to the availability of synergistic carbon dioxide binding centers at the organic linkers. Given the solution processability and aqueous stability, UMC-1 represents a model material for carbon separation application based on its high adsorption selectivity. Zhang and coworkers had reported the synthesis of Zr-based carboxylate MOP with molecular formula of [Cp
3Zr
3(μ
3-O)(μ
2-OH)
3]
2L
3•4Na•4H
2O constructed from cationic SBUs of Cp
3Zr
3(μ
3-O)(μ
2-OH)
3 and broken linear organic ligand Na
2H
2L containing two sulfonate groups [
132]. Multiple charge-assisted H-bonding interactions between μ
2-OH and SO
3− groups decorated on the MOP surface and electrostatic interactions between cationic vertexes and anionic SO
3− groups significantly consolidated the porous frameworks in order to maintain their porosity after desolvation, heating to 150 °C and immersion in water, strong acid or weak base solution as revealed by unaltered PXRD profiles after each treatment. Superior stability of Zr-based MOP further prompted its potential applicability towards gas adsorption which reveals the CO
2-selective capture profile over N
2 making them suitable for CO
2 separation from flue gas mixtures. Sulfonate functionalities render MOP highly proton conductive (1.14 × 10
−3 Scm
−1) and humid (98% relative humidity).
Furthermore, exceptional aqueous and thermal stabilities of Zr-based carboxylate MOPs make them amenable for post-synthetic modifications [
133]. For post-synthetic modification, Choe and coworkers had chosen amine-functionalized Zr-based tetrahedron MOP 27 as prototypical MOP with molecular formula [Cp
3Zr
3(μ
2-O)(μ
3-OH)
3]
4[BDC-NH
2]
6[(C
2H
5)
2NH
2]
2Cl
6 constructed from trinuclear zirconium cluster and 2-amine-1,4-dicarboxylic acid linker [
134]. Good solution processibility of Zr-based MOP was also demonstrated by exceptional aqueous stability. Retention and/or enhancement of permanent porosity of carboxylate-based MOPs following post-synthetic polymerization is particularly challenging task. NH
2-functionalized Zr-based MOP 27 was covalently crosslinked using flexible acyl chloride linkers by utilizing the condensation reaction to form cross-linked MOPs without significant structural changes of Zr-based MOP. Microporosities of tetrahedron MOPs in cross-linked material are well maintained based on gas adsorption study. This crosslinking strategy can be used for advanced applications like synthesis of polymeric membranes and molecular separations. Guo and coworkers had demonstrated successful utilization of covalently cross-linked MOPs for the preparation of interfacial microstructure with enhanced water permeance and attractive separation performance [
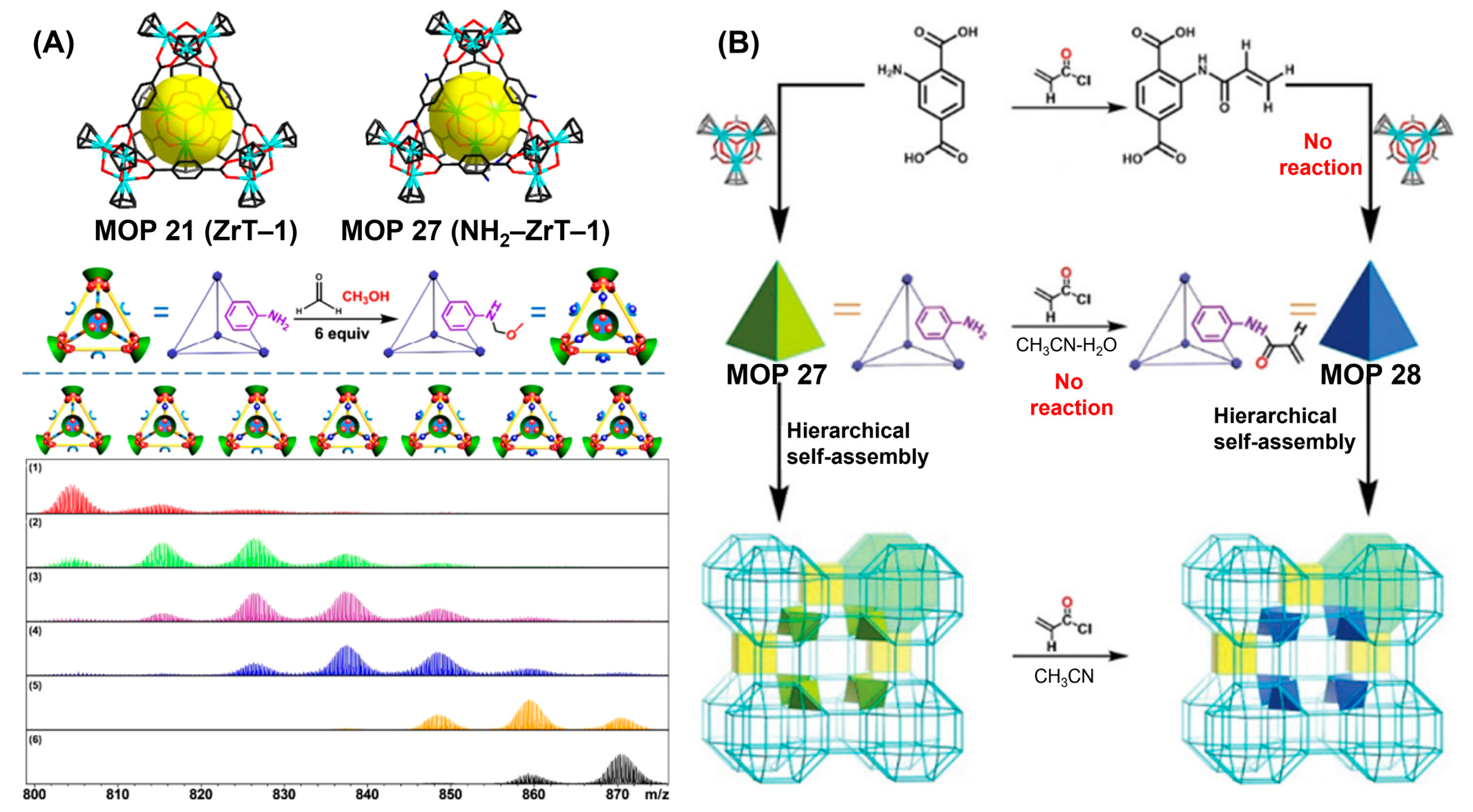
135]. Permanent pores inherited from amine-functionalized Zr-based MOP 27 and network pore originated from rigid cross linkers (e.g., trimesoyl chloride) enable cross-linked MOPs to exhibit better separation efficiency by letting through monovalent and divalent salt ions and rejecting larger dye molecules. Therefore, strong Zr-O metal-ligand interaction enables scientists to prepare aqueous and thermostable MOPs useful for performing various applications like gas storage and separation. In a recent report, Zhao and coworkers systematically studied the aqueous stabilities of several Zr-based MOP 27 (
Figure 13A) over wide range of pH [
136]. Amine-functionalized Zr-MOP 27 (NH
2-ZrT-1) and three others Zr-MOPs (ZrT-1, ZrT-3 and ZrT-4) with no amine groups but with different organic linkers (1,4-benzenedicarboxylic acid, 1,1′-dicarboxylic acid-4,4′-biphenyl and 1,3,5-tris(4-phenylcarboxylic acid)benzene) were chosen for the study. All four Zr-MOPs exhibited exceptional stabilities under neutral, acidic and weakly basic aqueous environments (pH = 2.2 to pH = 9.8). Exceptional stability also allows NH
2ZrT-1 MOP 27 to undergo covalent post-assembly modification (PAM) without any sign of decomposition of the MOP. Mannich reaction was carried out to study the process-tracing and was conducted using formaldehyde in methanol/water solvent mixtures at 25 °C.
1H NMR and ESI-TOF-MS were successfully utilized to monitor the sequential nature of PAM processes by recording the reaction course at various time intervals.
In an another report, Zhao, Yuan and coworkers demonstrated the heterogeneous post-assembly modification strategy (
Figure 13B) in order to prepare supramolecular frameworks [
137]. Exceptionally stable zirconium-based MOP was used to prepare metal organic frameworks (MOFs) amenable for further post-functionalization, which is otherwise impossible to achieve using other synthetic approaches starting from post-functionalized organic ligands mainly due to the reactivities and instabilities of the linkers during the coordination-driven self-assembly process. It was envisaged that 2-acrylamidoterephthalic acid and trinuclear zirconium cluster would give rise to tetrahedron ZrT-1-AA MOP 28 bearing acrylamido group. Although 2-aminoterephthalate was successfully post-functionalized with acryolyl chloride to prepare 2-acrylamidoterephthalic acid, subsequent self-assembly reaction remained unsuccessful. Under aqueous hydrochloric acid condition 2-acrylamidoterephthalic acid undergoes partial hydrolysis to release 2-aminoterephthalic acid, certain portion of the post-functionalized ligand also transforms into 2-(bicyclo[2.2.1]hept-5-ene-2-carboxamido)terephthalic acid via the Diels–Alder (D–A) reaction by reacting with the in situ formed cyclopentadiene from zirconium metal clusters. Also, the synthesis of ZrT-1-AA MOP 28 was unsuccessful via homogeneous post-synthetic modification of ZrT-1-NH
2 MOP 27 which was prepared via self-assembly from 2-amino-1,4-benzenedicarboxylic acid and trinuclear zirconium metal cluster. Homogeneous postsynthetic modification proceeded with low efficiency in methanol partly due to the alcoholysis of acrolyl chloride with methanol. Accordingly, ZrT-1-AA MOP 28 was synthesized via heterogeneous PAM from the bulk ZrT-1-NH
2 MOP 27 crystals. The amino motifs in the ZrT-1-NH
2-based crystalline supramolecular frameworks remain accessible due to framework’s interpenetrated porosity. Cubic ZrT-1-NH
2 crystals following solvent exchange were treated with acryloyl chloride in hexane for one day to give ZrT-1-AA. PAM of MOFs was studied via ESI-TOF-MS. With the increase of reaction time, the peaks gradually shifted toward higher
m/
z ratio as more amino groups of ZrT-1-NH
2 were functionalized. Furthermore, ZrT-1-AA is crosslinked into shaped materials by using poly(ethylene glycol) diacrylate binder. The relative proportion of the binder determines the flexibility, shape, amount of loaded MOPs in the materials and prevents the leakage of MOPs from the composite materials. Shaped materials exhibited moderate CO
2 uptake compared with as-synthesized ZrT-1-AA which is amorphous powder demonstrating the applicability of large-scale fabrication of functional MOPs for practical applications.
Figure 13.
(
A) Crystal structure of Zr-based MOP 21 and MOP 27. Schematic representation of post-assembly modification of MOP 27 upto six available sites in each cage. ESI-TOF MS spectra of MOP 27 in acetonitrile/water over a wide pH range. (
B) Transformation of MOP 27 into MOP 28 via heterogeneous PAM in the supramolecular framework. Reprinted by permission from Refs. [
136,
137]. Copyright from 2018 from American Chemical Society and copyright 2021 from Royal Society of Chemistry.
Figure 13.
(
A) Crystal structure of Zr-based MOP 21 and MOP 27. Schematic representation of post-assembly modification of MOP 27 upto six available sites in each cage. ESI-TOF MS spectra of MOP 27 in acetonitrile/water over a wide pH range. (
B) Transformation of MOP 27 into MOP 28 via heterogeneous PAM in the supramolecular framework. Reprinted by permission from Refs. [
136,
137]. Copyright from 2018 from American Chemical Society and copyright 2021 from Royal Society of Chemistry.
Various other strategies to enhance the stabilities and porosities of MOPs include mixed functionality strategy to give mixed-composition products, but not necessarily mixed-composition starting materials or reaction solutions may yield mixed-composition products. Accordingly, mixed-metal, mixed-ligand and mixed-metal counter anion strategies have been reported for the synthesis of mixed functionality MOPs [
138,
139,
140]. Hong and coworkers had first demonstrated the synthesis of high nuclearity heterometallic calixarene-based carboxylate MOP 29 with chemical composition of Na
4Ni
24Ln
4L
3 (L = dicarboxylate linkers) constructed from large mixed-metal clusters Na
2Ni
12Ln
2 (Ln = Dy and Tb) and linear dicarboxylate linkers [
141]. Based on gas adsorption studies, good permanent porosity was shown by all heterometallic metal organic polyhedra (HMOP). Doonan and coworkers had reported the synthesis of heterobimetallic metal-organic polyhedra MOP 31 constructed from heterometallic paddlewheel nodes using Pd(II) metal ion along with other transition metal ions like Cu(II), Ni(II), Zn(II) and 5-tert-butyl-1,3-benzenedicarboxylate organic linker [
140]. Interestingly, the Pd(II) metal ions are preferentially aligned toward the inner surface of the cuboctahedron MOP and first row metal ions are predominantly positioned on the cage periphery. Activation produces MOPs with coordinatively unsaturated metal sites decorated on the external surface for proposed interaction with gas molecules in the solid state, with H
2-adsorption enthalpies in excess of −12 kJ mol
−1 for NiPd-bimetallic MOP.
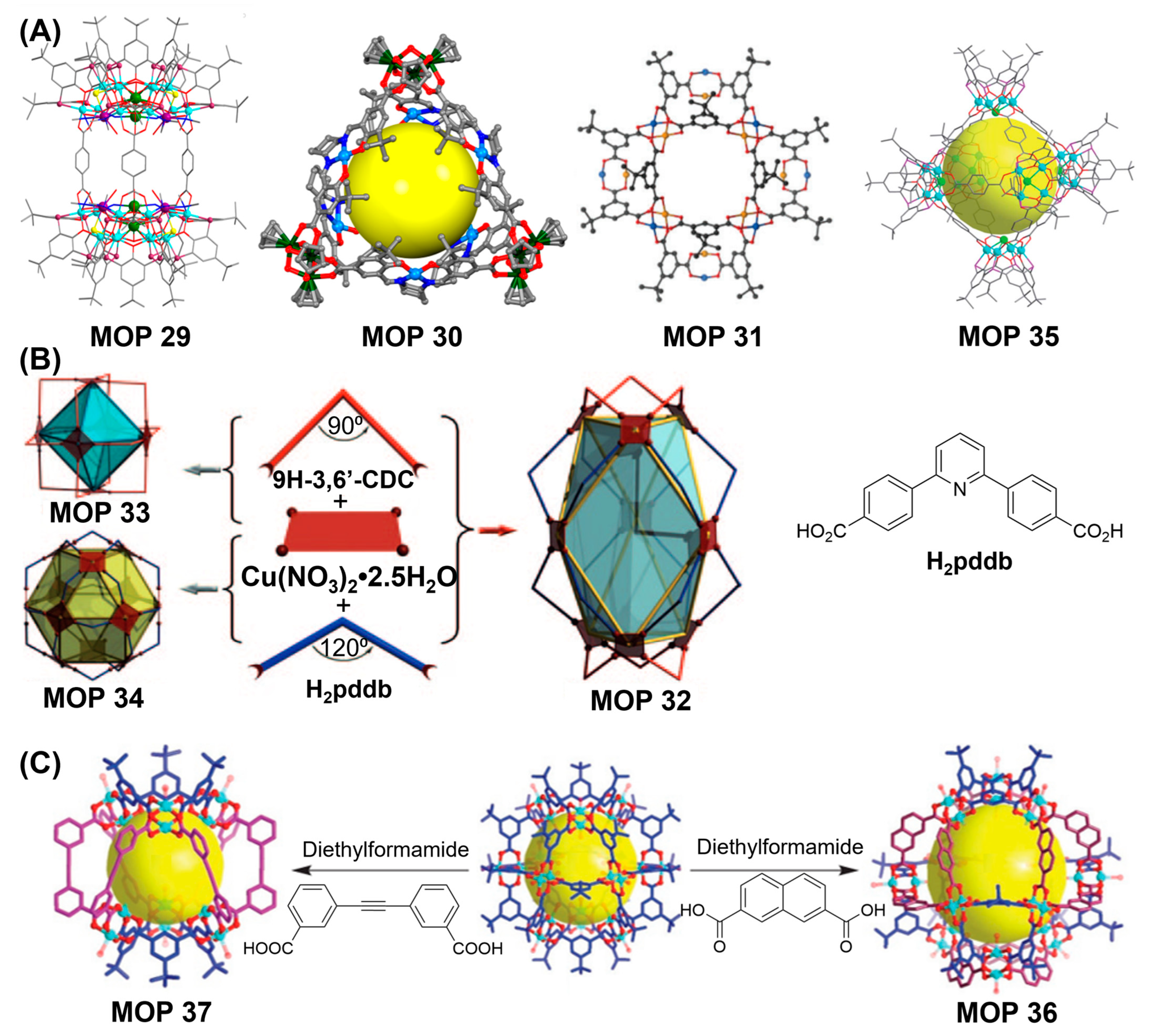
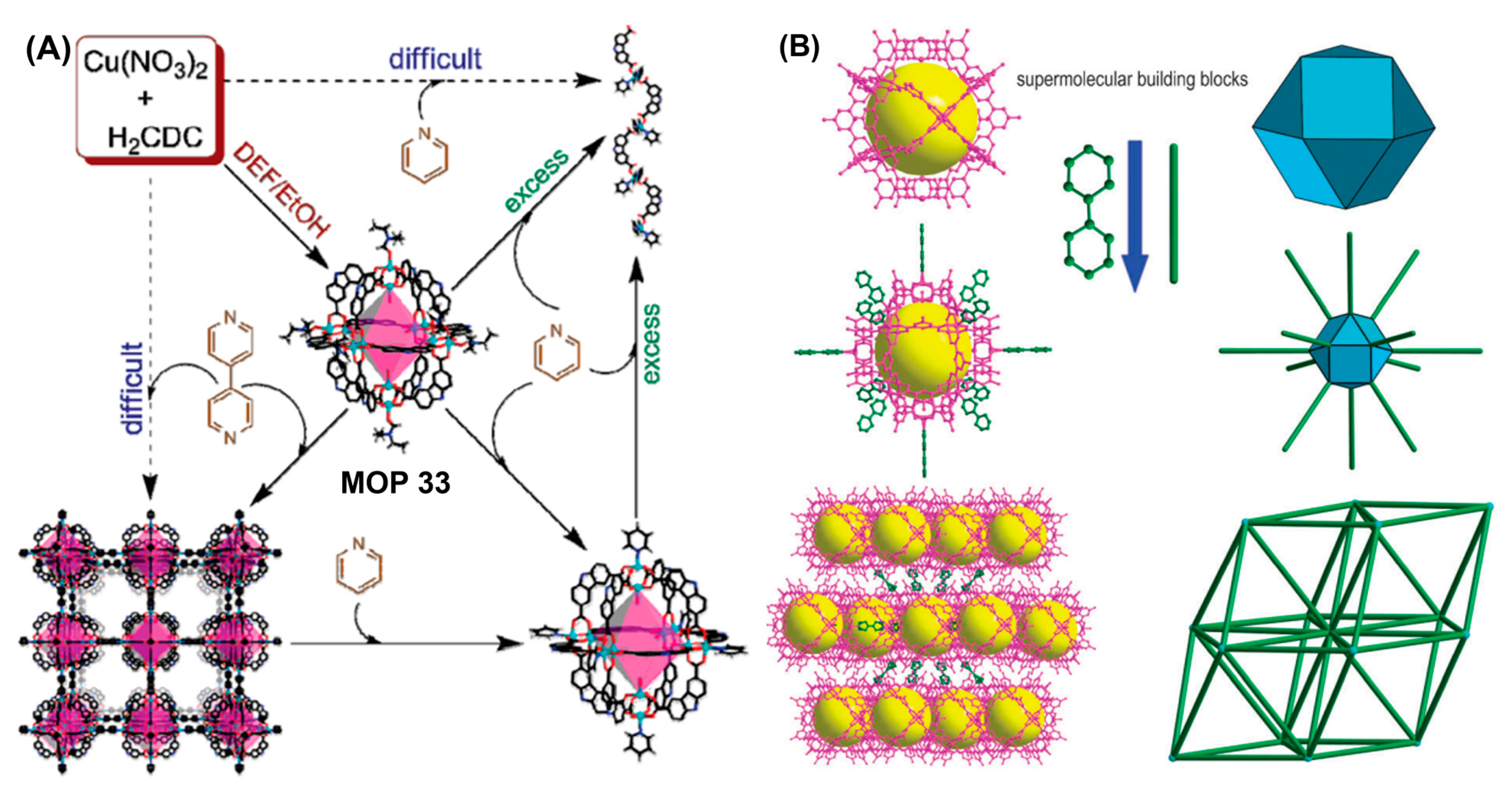
Mixed-ligand strategies worked out beautifully to prepare carboxylate-based MOPs. MOPs prepared from different types of linkers can assume different topologies and feature multiple functionalities as well. Combination of angular ditopic ligands with dissimilar bend angles are particularly intriguing. Following this approach, Zhou and coworkers had demonstrated the synthesis of metal organic polyhedra featuring mixed functionalities [
138]. Combination of ditopic carboxylic acid linkers, 9H-carbazole-3,6-dicarboxylate (9H-3,6′-CDC) with 90° bend angle and 4,4′-pyridine-2,6-diyldibenzoate (H
2pddb) with 120° bend angle with dicopper paddlewheel metal cluster generates hendecahedra MOP 32 with odd numbers of faces (eleven) and odd numbers of vertices (nine). In contrast, ditopic linker with 90° bend angle and dicopper paddlewheel form an octahedron MOP 33 and linker with 120° bend angle yields a cuboctahedron MOP 34 (
Figure 14B). Preliminary gas adsorption studies revealed type I sorption behaviour indicative of a microporous material with Langmuir surface area of 372 m
2/g which is relatively low compared with calculated accessible surface area. Sample became almost amorphous following activation as revealed by powder X-ray diffraction (PXRD) which is attributed to the blockage of the cavity due to the positional rearrangement of molecular structure. Hong and coworkers had demonstrated the synthesis of mixed-ligand carboxylate MOP 35 with molecular formula [Co
24(BTC4A)
6(μ
4-Cl)
6(1,4-BDC)
12]•6Hdma (Hdma = 1,4-dimethylamine cation) via [6 + 12] condensation of cationic tetranuclear-metal building blocks generated in situ from p-
tert-butylthiacalix[4]arene and 1,4-benzenedicarboxylic acid organic linker [
142]. Permanent porosity was determined by the gas adsorption isotherm of activated sample showing pseudo-type I isotherm typical of materials with permanent microporosity. In another report, Zhou and coworkers had reported the bridging-ligand-substitution strategy to successfully prepare mixed-ligand MOPs via the interconversion of MOPs prepared from various angular ditopic carboxylic linkers [
89]. Mixed-ligand quasi-spherical structure MOP 36 [Cu
24(5-
tBu-BDC)
12(2,7-NDC)
12(S)
24] and distorted octahedron MOP 37 [Cu
12(5-
tBu-BDC)
6(3,3′-EDDB)
6(S)
12] were synthesized by ligand substitution reaction of octahedron MOP (prepared from 5-
tBu-BDC and dicopper paddlewheel) using H
2(2,7-NDC) and H
2(3,3′-EDDB) ligands with bend angles of 120° and 60°, respectively (
Figure 14C). Synthesis of mixed-ligand cages were favoured either thermodynamically or kinetically in bridging-ligand-substitution reactions. Solubility of MOPs and substituting ligands in the appropriate solvent mixtures are pre-requisite for bridging-ligand-substitution strategies. Sulfonate and hydroxyl-functionalized dicopper paddlewheel-based MOPs were successfully converted into carbazole-based MOPs using carbazole dicarboxylic acid ligands before finally converting them into 1,4-benzenedicarboxylic acid-based MOP using 1,4-benzenedicarboxylic acid as substituting ligand. Bridging linkers with different properties enable the synthesis of new metal organic polyhedra with distinct topologies (like size, shape) and functionalities.
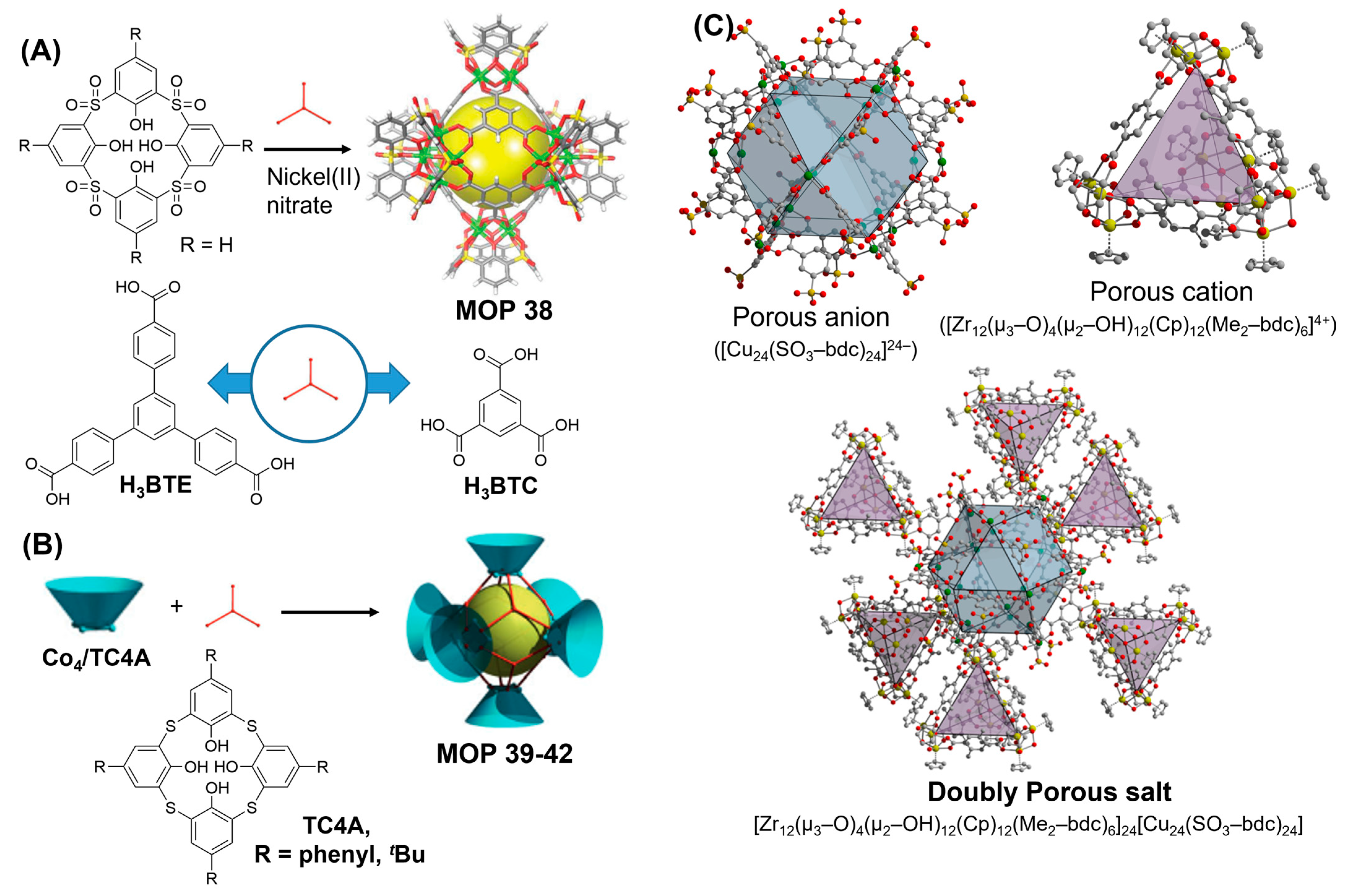
Calix[4]arenes are versatile macrocyclic synthetic container compounds which have been highlighted for use as building blocks for the preparation of cage compounds with various metal ions in combination with other carboxylic acid linkers [
144]. Self-assembled calixarene-based tetranuclear cluster complexes are prepared from quadruply deprotonated
p-tert-butylsulfonylcalix[4]arene, transition metal ions like Mn(II), Co(II), Ni(II) etc. and acetate anions where four sulfonyl oxygen atoms and four phenoxo atoms are coordinated to four metal ions that are further attached to four acetate groups and one μ
4-hydroxo oxygen atom (
Figure 15A). Subsequently, calixarene-based tetranuclear metal cluster and tricarboxylate ligand gave Co(II) and Ni(II) polyhedra with molecular formula as [M
4(μ
4-H
2O)(BTC4A)]
6(1,3,5-BTC)
8; M = Co(II) and Ni(II). Interestingly, Ni(II)-based MOP 38 was found to be remarkably robust and chemically stable upto 400 °C as revealed by thermogravimetric analysis (TGA) and has permanent porosity as shown by gas adsorption studies [
145]. Zhang and coworkers had reported the synthesis of Co(II)-based four octahedron MOPs by allowing ternary acids 1,3,5-benzenetricarboxylic acid (H
3BTC) or 4,4′,4″-benzene-1,3,5-triyl-tribenzoic acid (H
3BTB) to react with
p-tert-butylthiacalix[4]arene (H
4TC4A) or p-phenylthiacalix[4]arene (H
4PTC4A) in [6+8] solvothermal condensation reactions [
146]. Six cobalt-calix[4]arene clusters with octahedron arrangement are bridged together by eight BTC or BTB linker [
147] giving rise to MOP 39-42 {[Co
4(TC4A)Cl]
6(BTB)
8}
6−, {[Co
4(TC4A)Cl]
6(BTC)
8}
6−, {[Co
4(PTC4A)Cl]
6(BTB)
8}
6−, and {[Co
4(PTC4A)Cl]
6(BTC)
8}
6− (
Figure 15B) with periphery diameters of up to 4.7 nm, internal voids of up to 1.7 nm and with exceptionally high thermal stabilities. Wang and coworkers had demonstrated the synthesis of type II calix[4]arene-based metal organic polyhedra assembled from divalent metal ions Ni(II) or Co(II), sulfonylcalix[4]arenes and linear 1,4-benzenedicarboxylic acid linker [
148]. The octahedron geometry arranges six calix[4]arene-based metal clusters at each vertices bridged together by twelve 1,4-benzenedicarboxylic acid linkers spanning across the edges of the octahedron. While guest-binding behaviour is determined by individual cages, the solid-state binding phenomena is dependent on the spatial organization of MOP. At solid-liquid interface, Co(II) MOP adsorbs 5 equiv. of methylene blue (MB) from the aqueous solution at much faster rate compared with Ni(II) MOP demonstrating their hydrolytic stabilities. At solid-gas interface, Ni(II) MOP shows better performance towards gas adsorption studies compared with Co(II) MOP, however the gas adsorption capacities of both MOPs are significantly lower which can be attributed to the partial collapse of the solid state packing of the MOPs after solvent evacuation.
Bloch and coworkers had demonstrated a novel strategy to prepare porous ionic cages with mixed functionalities and tunable properties where both counterions (cation and anion) are intrinsically porous in nature [
139]. Prerequisite conditions suitable for the preparation of doubly porous cages include having the requisite charges, permanent porosities, high stabilities and solubilities in various solvents or solvent mixtures in order to be compatible with solvent metathesis. Prototypical cuboctahedron sulfonate-functionalized copper paddlewheel-based MOP [Cu
24(SO
3-bdc)
24]
24− was selected as anionic MOP and highly stable zirconium-based MOP [Zr(μ
3-O)
4(μ
2-OH)
12(Cp)
12(Me
2-bdc)
6]Cl
4 was targeted as cationic MOP particularly for their permanent porosities and their abilities to withstand post-synthetic modifications. Combination of methanolic solution of copper-based MOP and zirconium MOP yielded nearly instantaneous precipitation of light-blue insoluble porous salt (
Figure 15C) with proposed molecular formula of [Zr
12(μ
3-O)
4(μ
2-OH)
12(Cp)
12(Me
2-bdc)
6]
6[Cu
24(SO
3-bdc)
24]. Comparatively, porous salt has CO
2 and N
2 accessible BET surface area of 252 m
2/g and 496 m
2/g, respectively much higher than those of constituent MOPs. The resulting doubly porous salt displays the spectroscopic signature of the parent cages representing a new and powerful method for the synthesis of porous solid with tailored functionality.
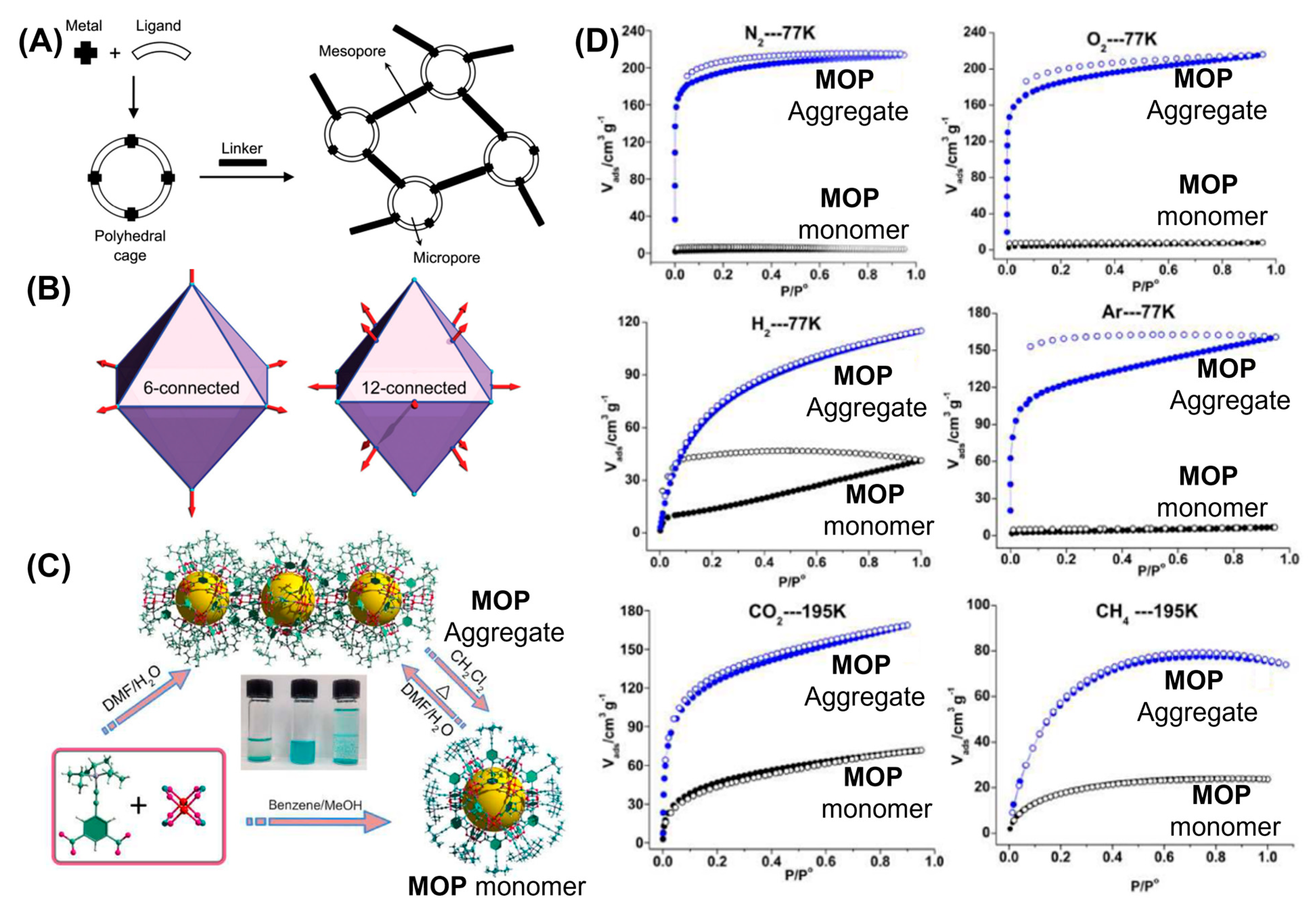
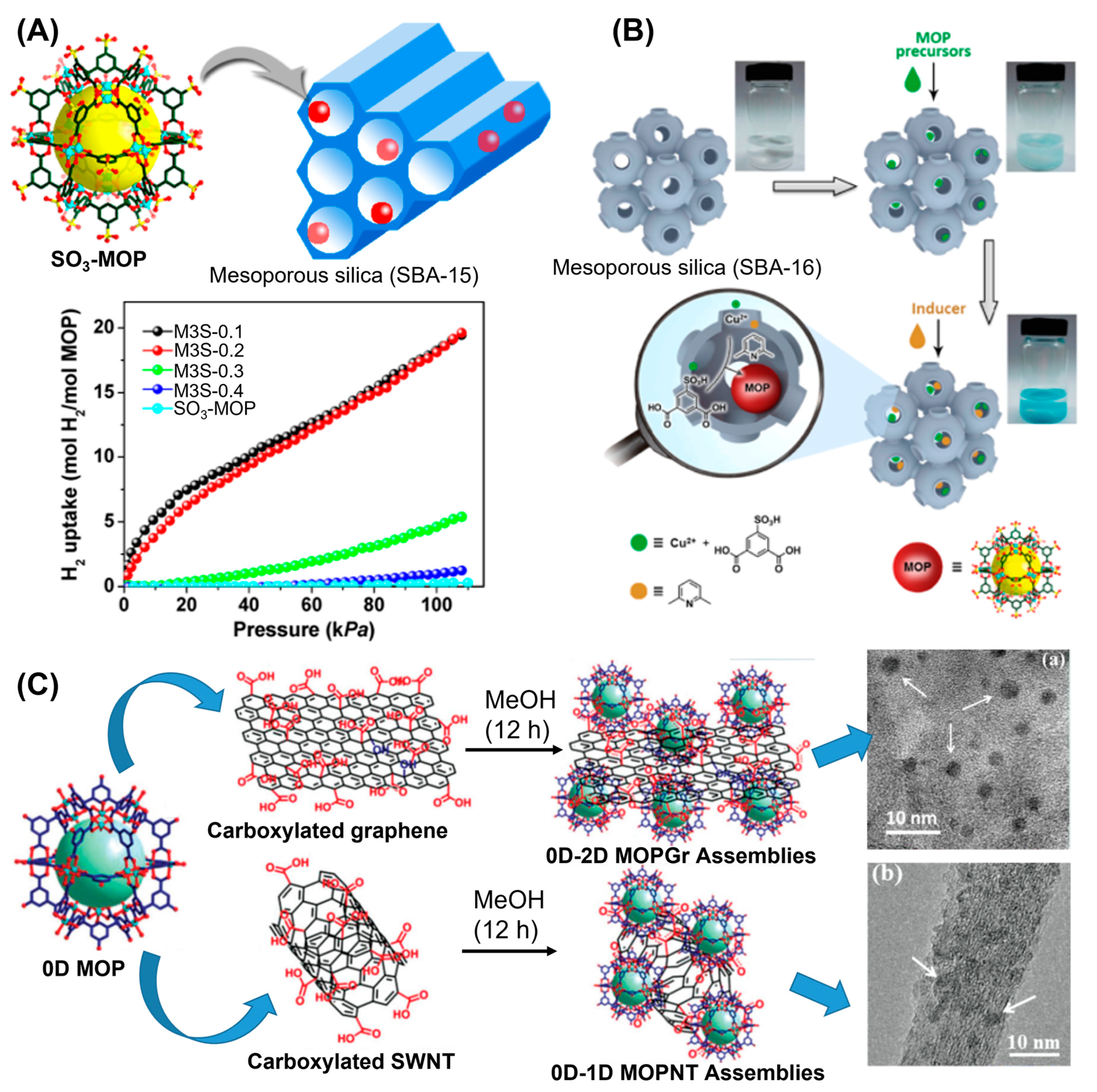
In addition to the chemical and hydrolytic stabilities, maintaining porosities of MOPs in the solid state is crucial for their usages towards various potential applications. Carboxylate-based MOPs are particularly attractive for maintaining their micro- and meso-porosities in their crystalline states. Nevertheless, lack of intercage stabilization interaction increases the chances of random rearrangements of MOPs in the solid state and thus they tend to aggregate upon activation. Aggregation causes the blockage of active sites and pore apertures of individual MOPs by the adjacent cages which results in compromising their guest uptake performances severely. Various strategies to circumvent the undesired aggregation of MOPs include the dispersion of MOPs in a porous matrix and the preparation of metal organic frameworks (MOFs) using MOPs as building units to retain their uniformities and porosities. Porous materials with large cavities can successfully accommodate MOPs and their small apertures can prevent leaching or aggregation. Zhou and coworkers had reported that MOPs performance can significantly be enhanced following their dispersion inside mesoporous silica nanopores of SBS–15 [
77]. Three dicopper paddlewheel-based carboxylate MOPs with general formula of [Cu
24(L
x)
24S
24] (S = solvent molecules) were prepared with varied substituents (
tbutyl, hydroxy and sulfonate) at the 5-position of the 1,3-benzenedicarboxylic acid linker (
Figure 16A). Compared with other two MOPs, sulfonated-functionalized MOPs are well dispersed inside the porous matrix of SBA-15. Sulfonated-MOP loaded SBA-15 samples (M3S-0.2, M3S-0.4 and M3S-0.6) were prepared with varying mass fraction (0.2, 0.4 and 0.6) of MOPs in the samples. N
2 absorption-desorption isotherms showed the hysteresis loop, characteristics feature of SBA-15 porous matrix, which closes at relative pressure of 0.7. For MOP-loaded silica, the hysteresis loop can remain open upto relative pressure of 0.5 and the hysteresis performance significantly enhances with increasing MOPs content in mesoporous silica. TGA analysis revealed that thermal stability of MOPs can be significantly enhanced due to their confinement within silica nanopores compared with pristine MOPs. H
2 uptake ability of MOP-loaded silica was significantly enhanced compared with bulk MOPs. Lower MOPs content-loaded silica renders MOPs more well-dispersed within mesoporous silica compared with higher MOPs content-loaded silica. Superior H
2-sorption behaviour was observed for lower MOPs content-loaded silica nanopore due to lack of MOP aggregation within silica nanopores enabling the MOPs maintaining their porosities and open apertures for guest uptake. In an interesting report, Li and coworkers had demonstrated the synthesis of sulfonated-functionalized dicopper paddlewheel-based carboxylate MOP within the mesoporous silica SBA-16 by utilizing double-solvent strategy [
149]. Mesoporous silica with inner cavity size of ~6 nm and pore entrance of ~2 nm was employed to host MOP molecules with average diameter of 3 nm (
Figure 16B). Interfacial tension drives the precursors containing hydrophilic solution into the hydrophilic cavity of silica nanopore avoiding unnecessary aggregation of MOPs onto the outer surface of pores. Addition of methanolic solution containing precursors and inducer 2,6-lutidine to hexane solution of silica allows methanolic solution to enter the pore via capillary action to prepare isolated MOP molecules within SBA-16 pore. MOP encapsulated SBA-16 showed better sorption behaviour for CO
2 and C
3H
6 compared to bulk MOPs confirming the presence of accessible MOP cavity sites in the mesoporous matrix due to higher dispersities. Not only higher catalytic efficiency was shown by MOPs confined within silica nanopores towards ring-opening reaction of styrene oxide compared with bulk MOPs, also better reusability upto four cycles were demonstrated for the catalytic reaction. Silica-confined MOPs have maintained their structural integrities even after exposing to the humid atmosphere for 7 days, bare are MOPs degraded severely within 24 h demonstrating the ability of silica surfaces to stabilize MOPs. Rao and coworkers had demonstrated a novel strategy to stabilize dicopper paddlewheel-based carboxylate MOPs on the graphene and single-walled carbon nanotube surfaces [
150]. Discrete MOPs are covalently attached to the surfaces by reacting carboxylic acid-functionalized graphene/SWNT with the hydroxy groups decorated on the MOP surfaces through ester bonds (
Figure 16C). Noncovalent interactions between the Cu units of MOPs and carboxylates on graphene/SWNT, and additional π-π interactions contributed to MOP stabilization. Interestingly, higher catalytic efficiencies were shown by composites MOPGr-2 and MOPNT-2 toward conversion of propylene oxide to propylene carbonate compared to bulk MOPs demonstrating the better preservation of MOPs upon depositing them on graphene/SWNT surfaces. Kitagawa, Choe and coworkers had reported the synthesis of sulfonate-functionalized dicopper paddlewheel-based carboxylate MOP inside a porous metal organic framework host (PCN-77) [
151]. Higher proton conductivity and water-vapour capture performance were shown by encapsulated MOPs. Authors claimed that amounts of MOPs inside metal organic frameworks control the volumes of meso- and micropores of the composite materials, opening up the possibilities of hybrid materials for other potential applications like solid-state electrolysis and heterogeneous catalysis.
Discrete metal-carboxylate polyhedra are used as building blocks to design porous coordination frameworks, which can also be employed to stabilize metal organic polyhedra that are otherwise unstable hydrolytically or chemically, and often undergo spontaneous aggregation in the solid state after guest removal. Higher thermodynamic stabilities of molecular architectures can therefore be envisaged compared with naked metal ions owing to the prevention of potential attack by solvent molecules or other competing ligands to the metal ions by virtue of strong metal-coordination bond. MOPs interconnected by strong coordination bonds using interconnecting ligands render hydrolytically and chemically stabile MOPs, and subsequently prevented MOP reorganization/aggregation upon guest removal. Therefore, MOPs serving as monomers would definitely facilitate to optimize the shapes, sizes and porosities of the 3D networks by introducing strategic tactics to add new linkers to the MOP solutions. Major challenge to prepare MOP-based extended three-dimensional network is the selection of appropriate MOPs with enhanced solubilities, better stabilities and their abilities to grow into three dimensional networks [
152]. Traditional approach is the linking of MOPs by using ditopic and tritopic linkers by replacing axially positioned multiple replaceable solvent molecules attached onto the outer surface of PW metal ions of each MOP. Zhou and coworkers had demonstrated the interconversion between twofold interpenetrated 3D framework (MOF) and molecular octahedron MOP 33 prepared from dicopper-paddlewheel cluster and 9H-carbazole-3,6-dicarboxylate (CDC) with amide (N-H) functional groups (
Figure 17A). Amide groups tethered onto each linker form H-bonds with appropriate solvent molecules and therefore improve the solubility of molecular cage [
92]. Jahn-Teller distorted axially positioned labile coordinated solvent molecules of octahedron MOP undergo ligand exchange reaction with pyridine or 4,4′-bipyridine molecules leading to the conversion into an extended interpenetrated network ([Cu
4(9H-3,6-CDC)
4(4,4′-bpy)(H
2O)
2]
3)
n with six vertices of each octahedron interlinked with bipyridine ligands and about 76% of solvent accessible area. MOF can be transformed reversibly back into molecular octahedron MOP by dissolving in DEF/py (4:1
v/v; py = pyridine) solvent mixture. Furthermore, excess of pyridine rearranges molecular octahedron to a helical 1D chain which is otherwise difficult to achieve. Su and coworkers had demonstrated the synthesis of porous coordination framework prepared from cubohemioctahedron cage [Cu
2(5-NH
2-
mBDC)
2]
12•(H
2O)
24 as precursor which was derived from dicopper paddlewheel SBUs and 5-NH
2-
mBDC ligands [
153]. Solvothermal reaction between cubohemioctahedron cage and 4,4′-bipyridine in N,N′-dimethylacetamide/ethanol (5:1) solvent mixtures afforded 3D framework of molecular formula {{Cu
24(5-NH
2-
mBDC)
24(bpy)
6(H
2O)
12]•72DMA} containing both micropore and mesopore cavities (
Figure 17B). Intrinsic and extrinsic porosities of MOFs allow the encapsulation (23.76 wt% loading) and release of guest like 5-fluorouracil drug. Approximately 80% of the loaded drug was released within few hours followed by a plateau which is presumably due to the initial MOF-structure degradation as confirmed by PXRD pattern. Majority of drugs are loaded inside the mesoporous cavity of MOFs and the rest are encapsulated within the intrinsic pores of MOPs. Su and coworkers had reported the stepwise synthetic strategy for the construction of three 12-connected MOFs with fcu topologies which are the rare examples of MOFs derived from cubohemioctahedron MOP precursor [Cu
24(5-hip)
24] (5-hip = 5-hydroxyisophthalic acid) [
154]. Three different organic linkers pyrazine, 4,4′-bipyridine and 1,2-di(pyridine-4-yl)ethene afforded three different MOFs with molecular formulas {[Cu
24(5-hip)
24(pz)
6(H
2O)
12]•64DMF}, {[Cu
24(5-hip)
24(bpy)
6(H
2O)
12]•119DMF}, and {[Cu
24(5-hip)
24(bpe)
6(H
2O)
12]•138DMF}). Metal organic framework {[Cu
24(5-hip)
24(bpe)
6(H
2O)
12]•138DMF}) was successfully utilized to adsorb and separate methylene blue and methyl orange dyes from mixture of solution containing rhodamine B. Interestingly, MOFs constructed from MOP building units exhibited better performance towards adsorption of dyes compared with pristine MOP precursors. Klosterman and coworkers had reported the synthesis of 1D coordination polymers at room temperature from copper-based M
4L
4 lantern-type MOP which contains inward-directed free amine functional groups and 4,4′-bpy bridging linker. Unsurprisingly the same coordination polymer was not yielded when the M
4L
4-type MOP precursors are mixed together with 4,4′-bpy bridging ligand in solution [
155].
Rigid inter-connected spacers are found to be ideal for maintaining the porosity of metal organic frameworks. Chun, Moon and coworkers had demonstrated the preparation of coordination polymers using flexible linkers as well (
Figure 18A). 2,7-naphthalenedicarboxylate (2,7-ndc) and Cu
2+ afforded cuboctahedron MOP [Cu
24(2,7-ndc)
24(DMF)
10(H
2O)
14] with 12 dicopper paddlewheel units [
90]. Complete amorphization of octahedron MOP occurs after solvent-exchange and evacuation due to full collapsing of molecular packing. Non-framework coordination polymers were prepared by replacing axially coordinated labile solvent molecules on the outer metal ion of paddlewheel complexes with cross-linkers like ethylenediamine, rigid aromatic linker xylenediamine or long flexible linker diaminoheptane. Compared with pristine MOP, distinct surface features for all three coordination polymers were observed from N
2 adsorption isotherms. Unusual isothermal behaviours were observed for all three coordination polymers. When relatively long linkers connect individual MOP molecules, mesopore volume significantly increases to show large hysteresis loop and much broader pore size distribution which can then be optimized systematically by choosing appropriate MOPs and cross linkers.
Common strategy to prepare MOFs from MOP building units is to attach the cross-linkers to the metal centers of paddlewheel metal clusters of each MOP molecules by replacing the axially-coordinated labile solvent molecules. Use of extended organic linkers can be used further to diversify the synthesis of metal organic frameworks derived from MOP precursors. Eddaoudi and coworkers had reported the synthetic strategy to prepare porous metal organic frameworks by using superbuilding blocks (SBB) [
156]. Metal organic truncated cuboctahedron supermolecular building block generated the rhombicuboctahedron tertiary building block in situ was used to prepare a (24-connected)-based MOF. 12 dicopper paddlewheels are joined by 24 molecules of 1,3-BDC linkers with the 5-position of the bend bridging ligand lying exactly on the vertices of the rhombicubooctahedron. Functionalization at the 5-position permits the formation of a rigid triangular molecular building blocks, a 3-connected vertex figure leading to the assembly of a MOF. Solvothermal reaction of 5-tetrazolylisophthalic acid and Cu(NO
3)
2•2.5 H
2O in dimethylformamide/ethanol afforded (3,24)-connected MOF with molecular formula of [Cu
6O(TZI)
3(H
2O)
9(NO
3)]
n(H
2O)
15 consisted of truncated cuboctahedron connected to trigonal Cu
3O(N4CR)
3 trimers through each tetrazolate (N
4CR) moiety. Higher porosity and larger surface area of the MOF can be achieved by expanding the bifunctional organic linker. Large surface area of 2847 m
2/g for the MOF was estimated using BET method with the largest MOP approaches mesoporous ranges, the smallest MOPs are first to be occupied and the largest are subsequently occupied by guest molecules at high pressure. Zhou and coworkers had demonstrated the synthesis of two dicopper(II) paddlewheel-based porous metal-organic frameworks (PCN-81 and PCN-82) that are sustained with 12-connected nanoscopic octahedra [
106]. Solvothermal reaction of tetratopic ligands H
4pbcd and H
4dpbcd with Cu(NO
3)
2•2.5H
2O in DMA and DMSO solvent mixture in the presence of HBF
4 acid afforded MOFs: PCN-81 and PCN-82, respectively with general molecular formula of [Cu
2(ligand)]•2X (X is coordinating solvent). The octahedron building block serves as a 12-connected node to prepare 12-connected network leaving three different types of microporous cages with varied cavity sizes (
Figure 18B). Each octahedron MOPs in PCN-82 with internal diameter of 11 Å are constructed from one end of each twelve 90°-angular carbazole-3,6-dicarboxylate moieties and six metal SBUs. MOPs with medium pore diameter (~12.9 Å) are formed by six organic ligands and 12 dicopper paddlewheel as SBUs. Large MOPs with diameter of 18.1 Å are formed by carbazole moiety on the opposite end of each ligand. Surface area measurement revealed that PCN-82 has higher surface area compared with PCN-81 especially for hydrogen, methane and carbon dioxide due to the availabilities of open metal sites and their microporous nature. Freeze-dried PCN-82 maintains the porosity and high crystallinity during PXRD and multi-cycle gas-adsorption measurements.
Figure 18.
(
A) Stepwise synthesis of non-framework coordination polymers (CPs) with bimodal porosity, where MOP is used with various diamines (ethylenediamine, xylenediamine, or diaminoheptane) as linkers to obtain a series of non-framework CPs. (
B) Structural representations of 6-connected and 12-connected octahedron SBBs. (
C) Interconversion between cuboctahedron MOP and the 1D chain of MOP. (Bottom left) a 5-((triisopropylsilyl)ethynyl)isophthalic acid (TEI) ligand and a dicopper paddlewheel. (Center) MOP monomer synthesized in the mother liquid (left), dissolving chain of 7 in CH
2Cl
2 (middle) leads to the formation of MOP and recrystallization in CH
2Cl
2/DMF (right) yields crystals of MOP. (
D) The sorption isotherms of N
2, O
2, H
2, Ar at 77 K, CO
2 and CH
4 at 195 K for MOP monomer and MOP aggregate (equilibrium time is 5 s for both MOP monomer and MOP aggregate). Reprinted by permission from Refs. [
90,
106,
157]. Copyright 2011 from Korean Chemical Society. Copyright 2013 from Royal Society of Chemistry. Copyright 2012 from American Chemical Society.
Figure 18.
(
A) Stepwise synthesis of non-framework coordination polymers (CPs) with bimodal porosity, where MOP is used with various diamines (ethylenediamine, xylenediamine, or diaminoheptane) as linkers to obtain a series of non-framework CPs. (
B) Structural representations of 6-connected and 12-connected octahedron SBBs. (
C) Interconversion between cuboctahedron MOP and the 1D chain of MOP. (Bottom left) a 5-((triisopropylsilyl)ethynyl)isophthalic acid (TEI) ligand and a dicopper paddlewheel. (Center) MOP monomer synthesized in the mother liquid (left), dissolving chain of 7 in CH
2Cl
2 (middle) leads to the formation of MOP and recrystallization in CH
2Cl
2/DMF (right) yields crystals of MOP. (
D) The sorption isotherms of N
2, O
2, H
2, Ar at 77 K, CO
2 and CH
4 at 195 K for MOP monomer and MOP aggregate (equilibrium time is 5 s for both MOP monomer and MOP aggregate). Reprinted by permission from Refs. [
90,
106,
157]. Copyright 2011 from Korean Chemical Society. Copyright 2013 from Royal Society of Chemistry. Copyright 2012 from American Chemical Society.
![Inorganics 11 00036 g018 Inorganics 11 00036 g018]()
Solvent-induced noncovalent interactions was successfully utilized as a tool to control the interconversion between discrete and porous chain of MOPs [
157]. A “shish-kabob” of MOPs was synthesized by interlinking bulky hydrophobic groups-decorated-MOP either a one-fell-swoop or a step-by-step procedure by varying the dielectric constant of the assembly mixture. Prototypical MOP constructed from 1,3-benzene dicarboxylic acid and dicopper paddlewheel cluster are chosen for the study. A surfactant-like amphiphilic ligand, 5-((triisopropylsilyl)ethnyl) isophthalate ligand along with Cu(II) metal ions in hydrophobic solvent mixture (benzene: methanol = 19:1) afforded a micelle-like cuboctahedron MOP featuring 24 triisopropylsilyl (TIPS) groups attached onto the exterior of MOP (
Figure 18C). Interestingly, the same starting materials in a hydrophilic solvent mixtures (DMF: water = 1:2) afforded the same cuboctahedron MOPs with mutual interdigitation of the TIPS group from adjacent MOPs to give a “shish kabob” of cuboctahedron. Distances between two closest Cu(II) metal atoms from two adjacent cages linked through two carboxylate group in a bidentate-bridging mode with Cu-O bond are 2.272 and 2.426 Å. Hydrophobic effect causes the aggregation which leads to the formation of Cu-O bonds between a pair of adjacent MOPs. Such behaviour enables structural interconversion between discrete MOP and a chain of MOPs by switching the dielectric constants of the solvent mixtures. Suspending the 1D chain of MOPs in CH
2Cl
2 led to the dissociation into discrete MOPs presumably due to solvation effect. Furthermore, the discrete MOPs can be successfully transformed into 1D chain MOP by immersing them in DMF-water solvent mixture, confirming the effect of hydrophobicity in order to drive the formation of the ‘’shish-kabob’’ of MOPs. Unsurprisingly, interdigitation and the formation of Cu-O bonds between neighboring MOPs prevent the aggregation in the solid state after activation and lead to maintain the structural integrity of the ‘’shish-kabob’’ of nanocages rendering them more porous compared with discrete MOPs. This dimension augmentation strategy turns out to be a suitable tool for improving and tuning the stabilities and porosities of MOP-based materials (
Figure 18D).
4. MOPs Containing N-Donor Ligands
Second popular class of metal organic polyhedra are made of N-donor ligands. Compared with MOPs derived from O-donor ligands, MOPs synthesized from N-donor ligands and soft metal ions tend to be hydrolytically more stable. Extensive works were done to prepare large body of metal coordination driven supramolecular polygons and polyhedra (MOPs) using N-donor ligands [
4,
5,
8,
25,
28]. Nevertheless, majority of MOPs have been prepared and studied in organic solvents as the designing of water-soluble and water-stable MOPs has been challenging. It is because not only that water acts as competing solvent but also most of the organic ligands employed in the self-assembly process are poorly water soluble. Additionally the hydrophobic effects engendered by water altered the reaction pathway leading to stacking of organic ligands. Early examples of the synthesis of supramolecular structures using metal-ligand interactions involving N-donor ligands are reported by Lehn et al. Formation of double-helical metal complexes-the helicates results from the self-organization of oligobipyridine strands around tetrahedrally coordinated Cu
+ or Ag
+ metal ions [
158,
159,
160,
161]. Spontaneous self-assembly of hexaazatriphenylene and quaterpyridine with [Cu(MeCN)
4]BF
4 gave cylindrical inorganic complex with internal cavity [
162]. Following this discovery, synthesis of MOP-field experienced a rapid upsurge and the progresses have been documented from various research groups notably Fujita, Stang, Nitschke, Mukherjee, Severin, Schmittel, Shionoya, Ward and many others. Earliest example of N-donor coordinated MOP is reported by Fujita and coworkers followed by the extensive works of Fujita and Stang groups using Pd(II) and Pt(II) metal ions [
4,
60,
61]. Schmittel group have done extensive works on the synthesis of MOPs using transition metal ions like Cu(I), Ag(I) and Zn(II) in order to prepare wide range of N-donor coordinated 2D and 3D metal coordination structures mostly in organic solvents [
8]. Heteroleptic approaches were successfully demonstrated and utilized to prepare Cu and Zn-based MOPs by bringing multiple components together. Shiyonoya and coworkers had reported the synthesis of Cu, Ag and Zn-based MOPs [
8]. Coronado et al. had reported the preparation of metallo-supramolecular hexagon from the mixture of Cu(II) metal ion and a rigid heteroditopic ligand containing phenanthroline and terpyridine binding units [
163]. Zelewsky and co-workers had reported the synthesis of silver metal ion-based interwoven molecular hexagon by employing a pinene-2,2′-bipyridine ligand [
164]. Silver based cationic molecular metallacycles were designed using 4-(2-pyridyl)pyrimidine as the organic linker [
165]. Dunbar et al. have investigated the effect of anions in anion-templated self-assembly reaction between first row transition metal ions and divergent bis-pyridine ligand [
166,
167]. Larger anion like [SbF
6]
— favoured the formation of molecular pentagon [{Ni
5(bptz)
5(CH
3CN)
10}SbF
6]
9+ while relatively smaller counter-anions ClO
4− and BF
4− gave molecular square [{M
4(bptz)
4(CH
3CN)
8}⊂X]
7+ (M = Ni(II), Zn(II); X = ClO
4− and BF
4−). Other transition metal ions like iron, cobalt and nickel were also extensively used to prepare various MOPs with most of them soluble in organic solvents demonstrating their good chemical stabilities [
168]. Water-stable MOPs ideally should be prepared in water but can also be prepared in organic solvents and be brought into aqueous solution by various approaches like redissolving them in water, performing counterions exchange or attaching water solubilizing groups via presynthetic and postsynthetic modifications, are particularly interesting for their abilities to withstand aqueous environments for real-life applications as the water-contact (mostly from atmospheric moisture) for most of the materials is almost unavoidable. The disadvantages that water-soluble MOPs containing N-donor ligands experience are mostly two-folds: (1) instability of metal-ligand bonds and (2) degradation of organic linkers particularly for those MOPs which utilize dynamic combinatorial chemistry (DCC) to stich organic molecules together. Considering their solution processabilities, solid-state recyclabilities and reusabilities; water-soluble and -stable MOPs containing N-donor ligands are rare in literature. Contrast to solid MOFs, solution-based MOPs can easily deform or de-ligate to enable guest molecules larger than the pore sizes to enter within their cavities. Thus, MOPs involving strong metal-ligand interactions and multi-topic chelating ligands are thus expected to render stable MOPs.
Contrast to MOPs containing O-donor ligands, MOPs containing N-donor ligands have found limited applications in the solid-state, rather they were extensively used to study solution-based properties. Therefore, their aqueous stabilities become indispensable for exploring their properties both in solid and solution state. Majority of MOPs synthesized and studied in organic solvents will not be discussed here as their stabilities have been mostly verified in organic solvents and authors are encouraged to read several excellent review articles available in literature [
4,
5,
8,
25]. First row transition metal ions with well-defined coordination geometries were found to be suitable for the preparation of N-donor ligand-coordinated MOPs. Interestingly, kinetic inertness of the first-row transition metal ions obey the following stability order Ni
II > Fe
II > Co
II > Zn
II > Cd
II. Using first-row transition metal ions, Nitschke and coworkers have done extensive works to prepare N-donor containing MOPs that are water soluble. Nitschke et al. have introduced new type of water-soluble Fe
4L
6 tetrahedron MOP 43 prepared via subcomponent self-assembly approach which involves the simultaneous formation of dynamic coordinative N→M and covalent C→N bonds in turn leading to the in situ formation of pyridyl imine chelate ligands and their organization around metal ion templates [
62]. Subcomponents 2-formylpyridine and 4,4′-diaminobiphenyl-2,2′-disulfonic acid (two sulfonate groups enable water solubility) in combination with Fe(II) metal ion and a base produced water soluble tetrahedron MOP 43 (
Figure 19A). MOP exclusively contains iron (II) metal ions in the low-spin state and was found to be stable in aqueous solution owing to the strong binding interaction and mutual stabilization between iron(II) metal ion and the imine ligands. X-ray crystal structure revealed the formation of an anionic tetrahedron with four Fe
II vertices and six bis-bidenate ligand edges.
Enantiopure water-soluble MOPs were later synthesized by Nitschke and coworkers, suitable for guest encapsulation and catalysis [
169]. Diamino terphenylene subcomponent was functionalized with chiral glycerol groups to prepare enantiopure derivatives, (S,S) and (R,R). Self-assembly reaction of enantiopure derivative either (S,S) or (R,R), 2-formylpyridine and iron(II) sulfate yielded the corresponding chiral [Fe
II4L
6]
8+ MOP ΔΔΔΔ-44 or MOP ΛΛΛΛ-44 in water, respectively (
Figure 19B). Compared with previously synthesized terphenyl-edged tetrahedra, the glycerol groups serve not only as water solubilizing agent to render water-soluble MOP but also helps to dictate the handedness of the iron(II) stereocenters despite having the distance between stereochemical elements. MOP ΔΔΔΔ-44 binds to wide range of chiral organic guests demonstrating the ability of enantiopure MOPs for chiral recognition and to catalyze the hydrolysis of the neurotoxic organophosphate dichlorvos. Ward and coworkers had independently reported the synthetic strategies to prepare N-donor containing water-soluble MOP [
170]. Self-assembly of cobalt(II) ion with organic linkers containing naphthalene-1,5-diyl spacer with two chelating pyrazolyl-pyridine units led to the formation of cubic MOP 45 [Co
II8L
12]
16+ (
Figure 19C). Eight octahedrally organized Co
II corners with twelve bridging ligands spanning across the edges are rearranged to prepare the cubic MOP. The attachment of hydroxymethyl substituents to the pyridyl-C
4 sites of each ligand render the MOP 46 water soluble owing to the presence of outward-facing hydroxyl groups. The internal cavity of MOP 46 remains hydrophobic and binds to wide range of guest molecules by exploiting H-bonding interactions with specific sites on the internal surface of the MOPs, nonpolar interactions such as aromatic, van der Waals interactions and solvophobic interactions. Nitschke and coworkers [
171] had demonstrated that hydroxymethyl functionalized organic linker when reacted with 2-formylpyridine and FeSO
4 in water at 50 °C can render water-soluble Fe
II4L
6 tetrahedron MOP 47 with
fac-stereochemistry at each metal centers (
Figure 19D). Prolonged exposure of iron(II) tetrahedron MOP 47 to the aqueous solution led to the isolation of water-soluble Fe
10L
15 prism MOP 48 which recrystallizes from the aqueous buffer. This observation indicates that the tetrahedron and prism are in equilibrium in solution with prism preferentially crystallizes due to lower solubility. Prism with
mer stereochemistry at each metal center can be separately prepared by mixing the organic subcomponents and FeSO
4 at 20 °C in Water-MeOH (1:9) mixture. Interestingly, complete conversion of prism MOP to the tetrahedron MOP was not achieved in water or methanol/water mixture over a period of two months at room temperature until the solution was heated to 50 °C for one week. This observation led to the conclusion that the prism is kinetic product and tetrahedron is the thermodynamic product which was further consolidated by the stability experiments. Prism falls apart in presence of competitive ligand 4-methoxyaniline, however tetrahedron MOP remain intact even after seven days in presence of 20 equiv. of 4-methoxyaniline.
Figure 19.
(
A) Preparation of tetrahedron MOP 43 by aqueous subcomponent self-assembly. (
B) Enantioselective formation of tetrahedron MOP ΔΔΔΔ-44 and MOP ΛΛΛΛ-44 from enantiopure (S,S/R,R) diamine, 2-formylpyridine and Fe
IISO
4 by subcomponent self-assembly. (
C) Preparation of cubic MOP 45 and MOP 46 from the corresponding ligand and Co(BF
4)
2 salt via self-assembly. (
D) Subcomponent self-assembly of tetrahedron MOP 47 and prism MOP 48, and their interconversion. Reprinted by permission from Refs. [
62,
169,
170,
171]. Copyright 2008 and 2013 from Wiley-VCH. Copyright 2013 from Royal Society of Chemistry.
Figure 19.
(
A) Preparation of tetrahedron MOP 43 by aqueous subcomponent self-assembly. (
B) Enantioselective formation of tetrahedron MOP ΔΔΔΔ-44 and MOP ΛΛΛΛ-44 from enantiopure (S,S/R,R) diamine, 2-formylpyridine and Fe
IISO
4 by subcomponent self-assembly. (
C) Preparation of cubic MOP 45 and MOP 46 from the corresponding ligand and Co(BF
4)
2 salt via self-assembly. (
D) Subcomponent self-assembly of tetrahedron MOP 47 and prism MOP 48, and their interconversion. Reprinted by permission from Refs. [
62,
169,
170,
171]. Copyright 2008 and 2013 from Wiley-VCH. Copyright 2013 from Royal Society of Chemistry.
Attempt to prepare water-soluble MOPs from highly hydrophobic ligands includes the use of different counter-anions during the self-assembly. Attempt to prepare sulfate counter anion-based MOP from FeSO
4 and the corresponding subcomponents in several solvent mixtures remain unsuccessful. Nitschke and coworkers had reported successful anion-exchange strategy to prepare water-soluble MOPs [
76] Reaction of four-fold symmetric tetrakis-amine zinc porphyrin and 2-formylpyridine with Fe(OTf)
2 afforded cubic MOP 49 with eight octahedrally coordinated Fe
II centers located at the corners and six facially-positioned organic ligands (
Figure 20). Triflate salt of the corresponding MOP 49 is soluble in organic solvents but remains water-insoluble. Anion metathesis using potassium sulfate or tetrabutylammonium sulfate precipitated out the corresponding sulfate salt of Fe-MOP 49 that becomes water-soluble. Interestingly, no degradation of the sulfate MOP was observed in the concentration range (0.2–15 mM, aqueous solution) at 25 °C over period of several months. Gu and coworkers had reported the synthesis of four different pairs of enantiomers of water-stable tetrahedron MOP [Ni
4L
6]
8+ via subcomponent self-assembly of 1,8-di(imidazole-2-carboxaldehyde)octane, aryl-substituted ethylamine and NiCl
2•6H
2O mixed together in 3:6:2 molar ratio in acetonitrile/diethyl ether solution [
172]. Four octahedrally arranged Ni
II metal centers are arranged in tetrahedral geometry linked together by six bridging diimidazole ligands spanning across the edges of the tetrahedron MOP. Flexible diimidazole linkers endowed the MOPs with relative water-compatibility. Compared with other transition metal-based tetrahedron MOPs [
173], Ni(II)-based MOPs showed better aqueous stability. Chloride salts of each MOP showed better water solubility and remarkable stability with little decomposition over long periods in acidic solution as well as even at a high temperature of 90 °C. High positive charge and suitable size of the corresponding MOP enable remarkable stabilization of antiparallel G-quadruplex DNA with moderate enantioselectivity.
Nitschke and coworkers had reported the synthetic strategies to prepare sulfate MOPs using different metal ions (M = Co
II, Ni
II, Zn
II and Cd
II) as templates [
174] following the strategies either via direct MOP formation from the corresponding metal(II) sulfate and the organic subcomponents or via anion exchange. Subsequently wide variety of MOPs including M
8L
6 cubes, face-capped M
4L
4 tetrahedra, edge-linked M
4L
6 tetrahedra were prepared. Generally hydrophobic counterparts render MOPs water insoluble, however the use of SO
42− counter anion makes them water-soluble. CoX
2 and NiX
2 metal salts (X = OTf
−, NTf
2−, BF
4−) in combination with di-, tri-, tetratopic organic linkers gave water-insoluble MOPs with various sizes and shapes which become water-soluble following anion metathesis using tetrabutylammonium sulfate salt (
Figure 21). Interestingly, their water solubilities and subsequent stabilities are dictated by the three factors: (1) metal-ligand bond strength, (2) degree of chelation and (3) degree of topicity of organic linker. Interestingly Co(II)-based MOPs prepared from linkers containing higher degree of topicity are endowed with enhanced aqueous stabilities. Co(II)-based MOPs made up of ditopic linkers fall apart in water following anion metathesis compared with MOPs constructed from tritopic and tetratopic linkers which remain intact in water. Interestingly Ni(II)-based MOPs showed enhanced stabilities and the corresponding MOPs prepared from ditopic and tritopic ligands can be further utilized to encapsulate hydrophobic guest molecules within their cavities. Use of labile metal ions like Zn(II) or Cd(II) in combination with tritopic and tetratopic organic linkers render the MOPs only soluble in organic solvents as they are easily disassembled in water following anion metathesis. Their aqueous solubilities and stabilities can be enhanced by tuning the degree of chelation. Various bisaldehyde organic linkers in combination with tritopic amine TREN and TRPN when react with MSO
4 (M = Zn, Cd) gave water soluble helicates and tetrahedra. Similarly, trisaldehyde in combination with tritopic amines produces water soluble tetrahedron MOPs with enhanced stabilities which can be explained by the higher degree of chelation rendering labile metal-N interactions more resistant to water-attack. Comparative study on the relative stability of cubic MOPs prepared from transition metal ions (Fe, Co, Ni, Zn, Cd) revealed that the aqueous stabilities (
Figure 22) obey the metal-ligand bond strengths in the following order Ni
II > Fe
II > Co
II > Zn
II > Cd
II as predicted by the Irving-Williams series (Fe
II < Co
II < Ni
II > Zn
II) [
175]. Ni(II), Co(II) and Fe(II)-based MOPs remain stable in water over several months with Ni(II) MOPs being the most stable owing to the lower ionic radius and higher gain in LFSE which is also reflected from the slower rate of aqua ligand exchange. Fe(II)-based MOPs were found to be more stable than Co(II)-based MOPs due to the formation of low spin Fe
II complexes compared with high-spin Fe
II complex used to prepare the Irving-Williams series. Filled
d-orbital with no gain in LFSE and large ionic radii of render Zn and Cd MOPs less water stable. Cd(II) MOP was found to be stable in water even less than several minutes while Zn(II) MOP was stable only for 30 min which are in good agreements with ~3–4 order faster water exchange rate compared with most stable Ni(II) aqua complex. This study demonstrated that strong metal-ligand interaction in combination with high degree of chelation and enhanced ligand topicity provided the blueprint to prepare water-soluble and water-stable MOPs.
MOPs have found wide applications in separation technology. Parallel to porous solids, porous liquids are beginning to make their footprint in chemical technological applications as well. Porous liquids are described as materials that exist in liquid phase at given temperature range with intrinsic porosity only coming from molecular cavity itself and lack external porosities like conventional liquid. Porous liquid has been classified into three classes [
176,
177]—type I, type II and type III. Type I porous liquid is a class of porous material existed as neat liquid. Type II and type III porous materials are prepared from porous molecular cage and MOFs, respectively in solvents that are remain inaccessible by the cavity of dissolved materials producing the porous liquids with empty intrinsic pores for guest recognition. Relatively better solution processability of MOPs has given them an edge over MOFs in order to be useful for porous liquid. Nitschke and coworkers had reported that porous liquid can be successfully prepared from N-donors based coordination MOPs [
178]. Self-assembly of tritopic pyridine aldehyde linker and para-imidazolyum substituted aniline in the presence with Zn(NTf
2)
2 in acetonitrile gave the corresponding tetrahedron MOP 58 which was then used to prepare the corresponding porous ionic liquid using solvent evaporation (
Figure 23A). Thermogravimetric analysis revealed that neat liquid MOP 58 showed no significant loss of mass between room temperature and 300 °C. Chlorofluoro hydrocarbons (CFCl
3, CF
2Cl
2, CF
3Cl) are long-lived ozone-depleting agents with 1 g of each in the atmosphere being equivalent of several thousand grams of CO
2. Zn(II)-MOP 58 based porous ionic liquid was therefore tested for the encapsulation of chlorofluoro hydrocarbons showing the affinity in the following order CFCl
3 > CF
2Cl
2 > CF
3Cl. Furthermore, empty MOP 58 can also be recovered by releasing the encapsulated guests under a reduced pressure enabling the porous liquid to be recyclable, an important consideration for developing porous industrial materials.
Combining the inert metal ions with organic linkers has been proven to be a viable route for the preparation of water-soluble and water-stable MOPs, however this approach may not be the straight-forward strategy always. Many self-assembly pathways that use inert metal ions often fall into the kinetic traps leading to the formation of oligomeric intermediates. Methods to overcome the kinetic traps include thermal labilization, use of labilizing competitive ligands and post-assembly modification [
181,
182,
183,
184]. In a strategy, labile building blocks are assembled to form supramolecular architectures which are then kinetically locked via subsequent chemical modifications. Taking the benefits of substitutionally non-lability of Co
III metal ion compared with Co
II, Lusby and coworkers [
179] had reported the synthesis of water soluble Co(III)-based tetrahedron MOP 59 from pyridyl-triazole ligand system and Co(ClO
4)
2 followed by oxidation of Co
II to Co
III using ceric ammonium nitrate.
d6 electronic configuration of Co
II renders low spin octahedron coordination complex (
Figure 23B) at each metal center. [Co
II4L
6]
12+ tetrahedron MOP 59 was then reacted with ceric ammonium nitrate to form oxidized Co
III-based MOP 59 with mixed ClO
4−/NO
3− counter-anions. The oxidized MOP 59 becomes more stable and less dynamic due to the enhanced inertness of Co
III-N linkages. Counter-anion exchange using AgNO
3 gave the water-soluble nitrate (NO
3−) salt of the corresponding tetrahedron MOP 59 which was found to be stable for indefinite period. In an interesting example, Nitschke and coworkers had demonstrated the PAM locking strategy in order to stabilize meta-stable states that appears in self-assembly pathways [
180]. Reaction between tris-amine derivative and 2-formylpyridine in presence of Fe(NTf
2)
2 gave the initial metastable kinetic product Fe
II2L
3 triple helicate MOP 60 which subsequently rearranges to form thermodynamically favoured Fe
II4L
4 face-capped tetrahedron MOP 61 after several days (
Figure 23C). Helicate structure MOP 60 only uses two out of three aniline moieties for metal coordination with the third remaining free for subsequent modification. Trapping the third reactive aniline via PAM locking strategy allows the helicate MOP 60 to be trapped at the out-of -equilibrium stage. N-acylation using acid anhydrides or activated esters and subsequent azidation gave PAM-modified kinetically-trapped helicate MOP. Glasson, Lindoy and coworkers had demonstrated that additional covalent buttressing of metallo-supramolecular complexes can render MOPs kinetically more inert and stable [
185]. Linear bis(bipyridine) ligand in presence with Fe(PF
6)
2 afforded either kinetically favoured Fe
II2L
3 or thermodynamically favoured Fe
II4L
6 complex from an equilibrium solution depending on the reaction conditions employed. Subsequently, they can be capped by treating the exposed 5-(tert-butyl)benzaldehyde residues with ammonium acetate followed by reduction with sodium cyanoborohydride. Formation of tertiary amine locked each MOPs in their assembled states.
Second row transition metal ions (Pd, Cd, Ag, Rh, Ru) in combination with N-donor ligands have been extensively used to prepare MOPs that show good water solubility and stability to exhibit their potential usefulness for various applications. Early examples of MOPs documented the use of palladium metal ions. Fujita and coworkers have envisaged that square planar geometry of Pd
II metal complex would be suitable to combine with multitopic organic linkers to give rise diverse class of MOPs. Hydrophobic 4,4′-bipyridine linker self-assembles with [(en)Pd]
2+ capping units (en = ethylenediamine) originating from [(en)Pd](NO
3)
2 salt to render (
Figure 24A) water-soluble MOP 62 [(en)
4Pd
4(bpy)
4]
8+ in square geometry [
60]. Hydrogen-bonding donating ability of the capping unit and the use of hydrophilic nitrate (NO
3−) counter-anions make the MOP water-soluble. Guest encapsulation ability of the MOP was demonstrated by incorporating hydrophobic molecules such as 1,3,5-trimethoxybenzene within its interior. Later, Fujita group had reported that self-assembly of 1,3,5-tris(4-pyridyl)triazene and [(en)Pd](NO
3)
2 salt (in 6:4 ratio) produces the first ever water-soluble octahedron MOP 63, [Pd
6L
4]
12+ [
61]. Four triazine panels are held together by six palladium capping units (
Figure 24B) through metal-ligand interactions to form truncated octahedron with alternate closed and open faces. Large interior cavity volume enables guest molecules to occupy the cavity useful for guest recognitions, chemical transformations and transportation [
186]. Other variants of palladium(II) capping units including [(tmeda)Pd]
2+ (tmeda = N,N,N′,N′-tetramethylethylenediamine) [
187], chiral [(EtCyhex)Pd]
2+ (EtCyhex = cis-(1R, 2R)-N,N′-diethyl-1,2-diaminocyclohexane) and [(bipy)Pd]
2+ (bipy = 2,2′-bipyridine) with two accessible
cis-oriented coordinated sites were also successfully used to make isostructural MOPs analogous to [Pd
6L
4]
12+ octahedron.
General strategy to prepare palladium-based water soluble cages demonstrated by Fujita and coworkers was further expanded by the same group using cis-capped [(en)Pd(NO
3)
2] salt. Pyridine/pyrimidine molecular panels were used to prepare varieties of self-assembled exotic polyhedron architectures [
188,
193]. Despite having used the hydrophobic organic linkers, the self-assembled MOPs remain water soluble due to the cationic charges associated with palladium(II) metal centers and the corresponding nitrate counter-anions further augmenting the water solubility. The relative lability of palladium(II)-N linkages render thermodynamically favoured MOPs with relatively inert ethylenediamine chelating ligand. The excellent aqueous kinetic stability was ensured by the multitopicity of ligands—use of tri- and tetratopic linkers render MOPs water stable. Other variants of
cis-capped complex like
cis-[(tmeda)Pd(NO
3)
2] and
cis-[(bpy)Pd(NO
3)
2] gave water soluble architectures like MOP 66 and MOP 68 (
Figure 24) with enhanced stabilities suitable for carrying out applications like guest encapsulation and chemical transformation [
63,
189]. The generality of this approach was further demonstrated by other groups like Mukherjee [
190] and Klajn [
194] by using imidazole-containing ligands instead of pyridines/pyrimidines to prepare water soluble MOP 67 and MOP 69. Tri- and tetratopic imidazole-based organic linkers render hydrophobic cages that exhibit water solubility and good stability. Mukherjee and coworkers had reported that palladium(II)-based MOPs (
Figure 24) prepared from tetratopic imidazole linker can successfully be used to catalytically enhance the performance of cycloaddition reaction [
191].
Mixed-ligand strategy was employed to prepare N-donor coordinated MOP 65 with general formula M
II6L
2Pillar
3 (
Figure 24A) when triazine panels are mixed with cis-capped [(en)Pd(NO
3)
2] complex containing chelating ancillary ligands ethylenediamine (en) and ditopic pyridyl-based pillars [
195]. By choosing different sized pillars, the height of the MOPs can be adjusted with tunable functionalities for various applications like stabilizing dinucleotide base-pair duplex, stacking of heterometallic clusters containing soft
d10 metal ions, encapsulating electronically complementary guest molecules etc. [
192,
196].
Use of [(en)Pd(NO
3)
2] metal salt enables MOPs which are synthesized from hydrophobic ligands to become water soluble. Naked Pd metal ion originated from Pd(NO
3)
2 metal salt in combination with hydrophobic organic linkers render diverse class of MOPs which are soluble in organic solvents and often remain water-insoluble unless they are otherwise functionalized to make water-soluble. Additionally, labile Pd-N linkages render MOPs more susceptible to degradation, making extremely challenging for them to be useful for solid and solution state applications. Fujita and coworkers had reported the preparation of series of metal organic polyhedra prepared from Pd(NO
3)
2 metal salt and bent bipyridyl organic linkers with general formulas M
nL
2n and size upto ~8 nm. Four N-donating ligands contributed to form palladium(II) square-planar complexes are self-assembled into various MOP structures including octahedron, cuboctahedron, rhombicuboctahedron, Goldberg polyhedra etc. Nevertheless, water insolubility and instability raise the concern that whether such MOPs can become useful for real-world applications. General strategy to solubilize and stabilize such Pd-based MOPs include attaching water-soluble groups either pre-synthetically or post-synthetically onto the hydrophobic organic linkers. In an interesting study Fujita and coworkers had demonstrated that M
12L
24 cuboctahedron MOP exhibited remarkable stability under cold-spray ionization mass spectrometry condition [
72,
73]. The molecular ion species of cuboctahedron MOP can be detected from the mass spectrometry owing to the remarkable stabilization of cuboctahedron MOP through the cooperation of 48 Pd(II)-pyridine interactions. Two cuboctahedron MOPs separately prepared from two different bridging linkers didn’t undergo ligand exchange when they were mixed together in acetonitrile and allowed to stand overnight at room temperature. Comparative ligand exchange reaction in mononuclear metal complex, [Pd(py)
4](OTf)
2 is very fast (
kobs = 1.9 × 10
−2 s
−1) and the half-life is 105 times lower compared with the ligand exchange reaction in M
12L
24 complex in organic solvents. The reasonable stability of Pd-based MOPs achieved through cooperative interaction encourages to bring them under aqueous environment to further expand their scopes. Accordingly, the bis-(pyridyl) ligands were successfully functionalized with water-solubilizing groups like sugar molecules, peptides, nucleotides to study their self-assembly in water. Later, Shionoya and coworkers had demonstrated that palladium metal ions in combination with banana-shaped bis-(pyridyl) ligand can be used to prepare M
2L
4-type MOP where two palladium metal ions are attached to four organic linkers in square planar geometry to occupy the top and bottom faces of the MOP, and four organic linkers are aligned across the four edges of the MOP [
197]. Clever group did extensive work to prepare palladium-based MOPs and studied their recognition properties in organic solvents [
198]. Yoshizawa and coworkers had first demonstrated the preparation of water-soluble version of Pd-based MOP from banana-shaped organic linkers tethered to water-solubilizing groups [
199,
200]. They had designed a new ligand bearing two anthracene panels and two methoxyethoxy groups on the central
m-phenylene ring. Mixture of Pd
II ions and organic linker in 1:2 ratio led to the exclusive formation of MOP 70 (
Figure 25A) which becomes soluble in mixture of water-methanol. Attaching additional methoxyethoxy group onto the ligand renders the water-soluble MOP. Pt-version of the MOP was also prepared and was used for guest encapsulation study in water. The corresponding water-soluble Pd-MOP was able to safely store radical initiators like AIBN which are generally unstable and are kept in dark at low temperature to avoid photochemical and thermal decomposition [
201]. Pd-based M
2L
4-type MOP will tend to be less stable in water compared with larger Pd
nL
2n-type cages due to the lack of significant number of cooperative interactions responsible for holding the MOP structure. In addition to using inert metal ions and attaching polar functional groups onto the outer surface of the MOP to shield the metal-ligand interactions from water attack, tuning the steric and electronic properties of coordinating ligand would render the cage more kinetically inert. Crowley and coworkers had reported the synthesis of kinetically inert M
2L
4 type MOPs by putting amino group onto the ortho and meta position of the pyridine unit [
202]. Three different MOPs, MOP 71: [Pd
2(tripy)
4](BF
4)
4, MOP 72: [Pd
2(2A-tripy)
4](BF
4)
4 and MOP 73: [Pd
2(3A-tripy)
4](BF
4)
4 were prepared from three different ligands only varying by substituents (H,
o-NH
2 and
m-NH
2) on the pyridine rings (
Figure 25B). However, they remain water-insoluble even after performing counter-anion exchange. Water soluble version of MOPs were prepared by functionalizing the organic linker with either hydroxymethyl or sugar molecules [
203]. Model palladium(II) N-heterocyclic carbene (NHC) probe complexes allow the determination of relative ranking of the donor abilities of pyridine ligands with the following order 2A-tripy > 3A-tripy > tripy. Enhanced electron-donating property of 3A-tripy ligand ensures that M
2L
4 MOP [Pd
2(3A-tripy)
4](BF
4)
4 is more stable compare with MOP [Pd
2(tripy)
4](BF
4)
4 in presence of common biological nucleophiles (Cl
−, histidine and cys). Comparatively, [Pd
2(2A-tripy)
4](BF
4)
4 MOP showed remarkably high stability (
Table 2) against all the nucleophiles with half-lives over 2 h whereas corresponding
t1/2 for the other MOPs were less than 30 min. Modestly stronger donor ability of 2A-tripy ligand can not fully justify the remarkable stability of [Pd
2(2A-tripy)
4](BF
4)
4 MOP, the steric shielding arising from 2-amino group on the
exo-faces of the [Pd
2(2A-tripy)
4](BF
4)
4 MOP protect the palladium (II) ions from the incoming nucleophiles. It appears from drug binding and cytotoxicity studies that the enhanced stability of MOPs through subtle structural modifications compromises their abilities to bind cisplatin and thus requires more inert metal ion-based MOPs.
Embedding charge on the backbones or on the outer surface of MOPs render water-soluble MOPs. Yoshizawa and coworkers had reported the preparation of polycationic capsular MOPs via self-assembly of Pd
II ions and bent bis-acridinium ligands [
204]. These architectures showed enhanced solubility in water due to their polycationic shells compared with MOPs containing pendant hydrophilic groups. Reaction of acridinium ligands with Pd(CH
3CN)
4(BF
4)
2 at 1:0.5 ratio in solvent mixture (D
2O/CD
3CN = 5:1) at room temperature afforded cationic M
2L
4 capsule MOP 74 (
Figure 25C). Interestingly, 1:1 reaction mixture in pure CD
3CN gave only M
2L
2 tubular structure MOP 75 indicating the stabilization of the highly charged structure in high dielectric solvent water with sufficient solubility (>5 mM) and stability under ambient conditions. Mukherjee and coworkers had reported the synthesis of water-soluble molecular cube MOP 76 from organic linkers bearing cationic methyl viologen units [
205]. Self-assembly of nitrate salt of triscationic organic linker with Pd(NO
3)
2 in water at 65 °C afforded cubic MOP 76 (
Figure 25D) featuring eight organic linker units located at each corner of the cube spanning across the adjacent faces to form six square planar complexes where each palladium(II) metal ion is coordinated by four linkers. Cooperative interplay between multiple weak interactions contributes to the MOP stability in highly dielectric solvent like water even at high temperature upto 90 °C. Interestingly, MOP 76 disintegrates in DMSO-d
6 at room temperature which was corroborated by the DFT calculation showing that the free energy gain during the formation of the complex in DMSO-d
6 is less than that in water explaining the inherent instability of MOP in DMSO-d
6. Large Fujita-type Pd-based cuboctahedron MOP was made and stabilized in water by Isaacs and coworkers [
206]. Attaching methyl viologen groups to the bis(pyridine) linkers render the Pd
12L
24 type MOP water soluble. Self-assembly of methyl viologen appended bis(pyridyl) ligand and Pd(NO
3)
2 in DMSO-d
6 followed by dialysis in water gave water-soluble version of Pd
12L
24 MOP in cuboctahedron geometry (
Figure 25E) with 12 square planar palladium(II) complexes linked together by 24 organic linkers. 24 methyl viologen units are attached to the outer surface of MOP (
Figure 24E). Post functionalization of MOP with macrocyclic host CB[7] and CB[8] led to the synthesis of CB[n]-capped MOPs (MOP 77 for CB[7]) which was then used to deliver prodrug of doxorubicin to the HeLa cells. CB[n]-loaded MOP was found to be stable in water upto pH slightly lower than physiological pH. Issacs and coworkers further synthesized the covalently linked CB[7]-functionalized Pd
12L
24-type MOP 78 (
Figure 25F) by self-assembling the CB[7]-appended bispyridyl ligand with Pd(NO
3)
2 in DMSO [
207]. Presence of cationic guests inside CB[7] cavity render the MOP water soluble and stable at physiological pH. Amphiphilic guest molecules bearing hydrophobic tail noncovalently attached to the appended CB[7] molecules backfold and agglomerate inside the MOP-cavity to create nanomicellar sphere which enables hydrophobic guest encapsulation within the cavity. Addition of competitive guest molecules destroys nanomicelles and triggers the stimuli-responsive cargoes delivery to the cancer cells.
Other strategy to stabilize Pd-based MOPs includes their encapsulation inside the pores of other supports like metal organic frameworks. Li and coworkers had demonstrated an efficient strategy called as hydrophilicity-directed strategy (
Figure 26A) to stabilize Pd-based MOP which is larger in size than the pore apertures of MOFs [
208]. As proof of principle chromium-based MOF MIL-101 was used to host M
6L
4-type Pd-based MOP 63. MIL-101 holds two types of mesoporous cavities with internal diameters of 29 and 34 Å, respectively with pore apertures in the range of ~12–16 Å. Palladium based M
6L
4 prepared from (en)Pd(NO
3)
2 and tris(pyridyl)triazine ligand is a hollow octahedron structure with diameter of 2.2 nm. As the pore windows are smaller than the diameter of M
6L
4-type MOP 63, the migration and leaching of M
6L
4 can be successfully prohibited to significantly enhance their stability. Hydrophilicity directed strategy (HDA) enables the subcomponents of M
6L
4 MOP to be encapsulated within the more hydrophilic inner surface of MOFs through capillary action. Activated MOF and the ligand L were first introduced into large volume of
n-hexane followed by the addition of small amount of aqueous solution of (en)Pd(NO
3)
2. Capillary force allows the subcomponents to go through the narrow pore apertures to enter within MOF cavities and forms M
6L
4 cage in situ to produce MOP 63ﬤ MIL-101-N (N = 3, 4, 6, or 10), where N denotes the average number of MOF cavities that accommodate one M
6L
4 inside, according to the amount of the precursors added in the reaction solution. Enhanced catalytic efficiency of M
6L
4 cage within the MOF demonstrated the advantages of using HDA strategy to prepare MOP ﬤ MOF compared with other approaches like soaking MOF particles in aqueous M
6L
4 solution or using one solvent instead by soaking MOF in aqueous mixture of the corresponding metal and ligand. Compare with HDA strategy, either approach led to poor loading of Pd-MOPs and they underwent obvious leaching to lose significant amount of palladium metal ions. Encapsulated MOPs quantitatively converted benzyl alcohol to benzaldehyde, which demonstrates the significant enhancement in stability of MOP and its performance compared with free MOP which gave only 60% converted product. Better reusability leading to higher turnover was demonstrated by encapsulated MOP materials as they remain unchanged upto five catalytic cycles compared with pristine MOPs which deactivated significantly. Enhanced catalytic efficacy of encapsulated MOP emphasizes better stability and dispersity of MOPs following their encapsulation within mesoporous MOF cavities.
Fujita group had reported the synthesis of N-donor MOPs using kinetically inert metal ions like platinum (Pt). Like palladium, platinum metal ion also forms square-planar complex with N-donor ligands as they belong to the same group in the periodic table. Pitfalls to prepare ordered metal organic structures using kinetically inert metal ions include entrapment of chemicals in undesired architectures not allowing to converge them into ordered desired architectures. Thermal or chemical labilization often become helpful to overcome kinetic barrier to get to the thermodynamic minimum. Triazine tris(pyridyl) ligand with (en)Pt(NO
3)
2 in D
2O afforded kinetically distributed oligomer mixture which eventually converged into thermodynamically stable Pt-based MOP 80 (
Figure 26C) upon heating at 100 °C for 24 h in reasonable yield [
209]. Addition of suitable guest like sodium adamantane carboxylate to the aqueous mixture followed by stirring at 100 °C for 24 h induces the high-yield conversion of MOP. Both hydrophobic driving force and thermal labilization allow the guest-induced organization of the receptor. Use of inert Pt-N interactions render the cage exceptionally stable in the pH range ~1–11. Strong acid HNO
3, base K
2CO
3 or even strong nucleophile (NEt
3) were unable to destroy the framework. Comparatively, palladium(II) counterpart of the MOP immediately decomposed in presence of acid or nucleophilic base. This work paved the way to prepare stable MOPs using Pt-N interactions. In an interesting example, Fujita and coworkers had reported the preparation of platinum-based larger cuboctahedron MOPs [
210]. Reaction of bend bis-pyridine ligand with Pt(NO
3)
2 in DMSO was unable to guide the process toward thermodynamic minimum to render Pt
12L
24 type cuboctahedron MOP 81 (
Figure 26C). Temporary labilization of inert Pt(II)-pyridine bonds by the addition of strong hydrogen-bond donor 2,2,2-trifluoroethanol (TFE) weakens the pyridine-metal interaction allowing the formation of cuboctahedron cage. Cuboctahedron cage trapped inside thermodynamic minimum remains stable even after removal of solvents. Remarkable stability of cuboctahedron MOP 81 is noteworthy as the Pt-based MOP did not decompose upon addition of excess of nitric acid (480 equiv.). Elongated version of bent bispyridyl ligand unexpectedly gave cubic MOP Pt(II)
6L
12 when reacted with Pt(NO
3)
2, though the same ligand gave the Pd(II)
12L
24 sphere. Presumably Pt(II)
6L
12 cage is trapped as a metastable local-minimum structure on the potential surface in the self-assembly process, Pt(II)-pyridine interaction remain stronger even in presence of TFE. Mukherjee and coworkers had reported the synthesis of Pt-based MOP from tris-(imidazole) ligand and chiral
cis-[(1S,2S)-dch]Pt(NO
3)
2 [where (1S,2S)-dch = (1S,2S)-1,2-diaminocyclohexane] [
212]. 3:1 stoichiometric ratio in H
2O:MeOH yielded a [12 + 4] self-assembled chiral M
12L
4-type molecular tetrahedron. The Pt(II)
12L
4 tetrahedron MOP attains thermodynamic stability through positive cooperative effect of 24 Pt(II)-pym coordination bonds. The MOP features an interior cavity which successfully promotes the Michael addition reactions of a series of aromatic nitro-styrenes with indole through supramolecular catalysis.
N-donor based Pt MOP are generally robust by virtue of inert Pt-N interaction. In an interesting example Yang and coworkers [
211] had reported the further stabilization of N-donor based Pt MOPs following their inclusion within the pores of other supported materials (
Figure 26B). Corresponding starting materials allowed the subcomponent self-assembly to occur to prepare MOP 79 which was then further subjected to the verification of the hypothesis that mesoporous cavities can enhance the stability and activity of MOPs. Accordingly, mesoporous carbon FDU-16 was chosen. Porphyrin containing metallacycle MOP 79 was then successfully prepared within the pores of mesoporous carbon FDU-16 by adding the counterpart of metallacycle into the dipyridyl preloaded mesoporous matrix FDU-16. XRD, TEM and ICP measurements showed enhanced stability and maintenance of ordered structure without obvious aggregation of MOP. The greater stability and activity of MOP 79 within mesoporous cavity was further demonstrated by the enhanced catalytic performance of MOP following the encapsulation within the cavity. Porphyrins are known as photosensitizer to produce singlet oxygen
1O
2 upon photoirradiation. Embedding porphyrin macrocycles within metallacycle enables MOP 79 to produce singlet oxygen and improvement of singlet oxygen
1O
2 generation efficiency can be ascribed to the better stability and dispersity of MOP 79 in a confined space. Encapsulated MOP 79 was also used as heterogeneous catalyst for the oxidation of sulfide to sulfoxide. Photoirradiation exhibited better catalytic efficiency of MOP 79 to produce sulfoxide when MOP was encapsulated within the pores of mesoporous carbon FDU-16. Control experiment showed that encapsulation of MOP within the confined space has pronounced effect on the catalytic efficiency as the mixture of MOP 79 and mesoporous carbon as heterogenous catalyst is no better than MOP 79 only. Good reusability of mesoporous carbon confined MOP 79 catalyst was observed upto 5 cycles demonstrating the role of solid supports in enhancing the ability, dispersity and activity of MOPs.
Stang and coworkers have done extensive work on the preparation of platinum-based MOPs which showed enhanced kinetic stability due to the involvement of kinetically inert Pt-N interactions. Stang and coworkers have developed the methodology of preparing [
trans-Pt(PEt
3)
2NO
3] unit metathesized organic linkers to self-assemble with multitopic pyridine linkers for the preparation of Pt-based MOPs. Remarkable chemical and solution (including aqueous stability) stabilities render MOP to be useful for various solution-based applications like sensing and biomedical applications, particularly for the development of antitumor agents in the treatment of malignant tumors. Stang and coworkers had first envisaged that platinum-based self-assembled MOPs can be used to develop novel antitumor agents [
213]. Reaction of 2,6-bis(pyrid-4-ylethynyl)aniline and 2,9-bis[trans-Pt(PEt
3)
2(OTf)]phenanthrene afforded tetranuclear platinum-based rhomboidal polygon MOP 82 (
Figure 27A). MOP was found to be highly soluble and stable under physiological condition enabling them to be useful for biomedical applications. Remarkable fluorescence property of MOP 82 compared with free ligands allows them to be useful as contrast agent. No significant decrease in metabolism was observed from cytotoxicity studies suggesting low cytotoxicity of MOP 82 within the concentration range of 1 nM to 5 mM. Confocal microscopy showed that MOP 82 remained stable after cellular internalization and did not undergo any photobleaching. Significant tumor volume suppression (64%) was observed from in vivo efficacy study. This pioneering work demonstrated the promise of platinum-based self-assembled MOPs in developing novel antitumor agents. Following this pioneering work large body of research works have been carried out using Pt-based MOPs by Stang and other groups to demonstrate the promise of MOPs in general in biomedical and other potential applications [
40,
41]. Stang and other groups further extended the scope of using other inert metal ions to prepare stable MOPs containing N-donor ligands. Ruthenium is known to form inert metal complexes with pyridine-based organic ligands [
214]. Chi, Stang and coworkers had reported the synthesis of MOPs using multitopic pyridine-based ligands and diruthenium metal clusters [
215]. Dimeric ruthenium metal cluster was prepared by reacting bis-benzimidazole with [(
p-cymene)RuCl
2]
2 complex in presence of sodium acetate. Post-treatment with silver triflate salt in methanol afforded diruthenium molecular clip suitable for use in the self-assembly process in combination with di- and tritopic organic linkers in order to prepare Ru-based MOP 83 and MOP 84 (
Figure 27B). Both MOPs were found to be soluble and stable under physiological condition enabling them to demonstrate their antitumor activity toward various cancer cell lines. The antitumor action of these MOPs was evaluated, revealing that bis-benzimidazole-bridged MOPs have the potential to act as potent anticancer agents, particularly in cell lines which are resistant to other Pt-based molecules.