Abstract
(Photo-)electrocatalytic artificial photosynthesis driven by electrical and/or solar energy that converts water (H2O) and carbon dioxide (CO2) into hydrogen (H2), carbohydrates and oxygen (O2), has proven to be a promising and effective route for producing clean alternatives to fossil fuels, as well as for storing intermittent renewable energy, and thus to solve the energy crisis and climate change issues that we are facing today. Basic (photo-)electrocatalysis consists of three main processes: (1) light absorption, (2) the separation and transport of photogenerated charge carriers, and (3) the transfer of photogenerated charge carriers at the interfaces. With further research, scientists have found that these three steps are significantly affected by surface and interface properties (e.g., defect, dangling bonds, adsorption/desorption, surface recombination, electric double layer (EDL), surface dipole). Therefore, the catalytic performance, which to a great extent is determined by the physicochemical properties of surfaces and interfaces between catalyst and reactant, can be changed dramatically under working conditions. Common approaches for investigating these phenomena include X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), scanning probe microscopy (SPM), wide angle X-ray diffraction (WAXRD), auger electron spectroscopy (AES), transmission electron microscope (TEM), etc. Generally, these techniques can only be applied under ex situ conditions and cannot fully recover the changes of catalysts in real chemical reactions. How to identify and track alterations of the catalysts, and thus provide further insight into the complex mechanisms behind them, has become a major research topic in this field. The application of in situ/operando characterization techniques enables real-time monitoring and analysis of dynamic changes. Therefore, researchers can obtain physical and/or chemical information during the reaction (e.g., morphology, chemical bonding, valence state, photocurrent distribution, surface potential variation, surface reconstruction), or even by the combination of these techniques as a suite (e.g., atomic force microscopy-based infrared spectroscopy (AFM-IR), or near-ambient-pressure STM/XPS combined system (NAP STM-XPS)) to correlate the various properties simultaneously, so as to further reveal the reaction mechanisms. In this review, we briefly describe the working principles of in situ/operando surface/interface characterization technologies (i.e., SPM and X-ray spectroscopy) and discuss the recent progress in monitoring relevant surface/interface changes during water splitting and CO2 reduction reactions (CO2RR). We hope that this review will provide our readers with some ideas and guidance about how these in situ/operando characterization techniques can help us investigate the changes in catalyst surfaces/interfaces, and further promote the development of (photo-)electrocatalytic surface and interface engineering.
1. Introduction
Recognition of the climate crisis, environmental degradation and other negative impacts [1,2,3] caused by excessive usage of fossil fuels has led to the enacting of various policies targeting renewable energy development [4,5,6]. The entry into force of the Paris Agreement officially kicked off a new era of energy transition, and also formulated targets for all the signatories [7]. To fulfill the agreement, Net Zero (or called Carbon Neutrality elsewhere) [8,9] should be listed as the first priority to keep these targets attainable between 2050–2060. Both energy transitions and circular economy strategies [10] are key for accelerating progress towards “Net Zero” of greenhouse gas emissions and increasing resilience to climate change. Among the many energy transition technologies, artificial photosynthesis has emerged as a promising approach in pursuit of accomplishing “Net Zero” [9]. Artificial photosynthesis mimics natural photosynthesis, using either water splitting to generate hydrogen and reduce carbon emissions, or the CO2 reduction reaction (CO2RR) to directly produce other high-value-added chemicals [11,12,13,14] and convert atmospheric carbon. After Fujishima and Honda reported water splitting on TiO2 photoanode under UV light (Honda–Fujishima effect) [15] in 1972 for the first time, research involving (photo-)electrocatalytic artificial photosynthesis has attracted widespread attention [16,17,18,19,20,21].
Catalytic reactions are typically carried out on the surfaces of catalysts, and thus the microscopic reaction mechanism strongly depends on the physicochemical properties of surface/interface. The activity, selectivity and stability of catalysts are determined by several processes, including: (1) chemical adsorption of reactant on the surface or interface of catalyst; (2) activation of reactant to generate the intermediate, and (3) desorption of the final product from surfaces. In order to achieve a full understanding of the surface/interface engineering [22], enthusiastic efforts have been devoted to obtaining the physicochemical properties of the interface, and the structure of the double layer, including the study of substrate–adsorbate interactions that involve the sharing of electrons and orbital overlap (covalent bonds), as well as non-covalent electrostatic interactions that are weakly connecting the substrate and solvated ions and affect the potential drop (e.g., hydrogen bonding and van der Waals forces) [23].
Research on heterogeneous catalytic systems (such as gas/solid, gas/liquid and solid/liquid interfaces) [24] still faces a number of challenges. Conventional ex situ characterization techniques can only give a steady picture of the catalysts in a non-real-time state, instead of providing a real-time snapshot. For instance, as illustrated in Ref. [25], the authors loaded cobalt borate on a hematite surface and investigated the changes of cobalt borate through in situ XAS and ex situ XPS methods. While Co4+ was detected under a real-time reaction condition by in situ XAS, the ex situ XPS did not capture the presence of Co4+. Therefore, the ex situ characterization techniques are far from satisfying the need for measurement of the unsteady state of catalysts under working conditions, and do not provide a clear and accurate picture of the underlying reaction mechanisms (i.e., the reaction kinetics could not be studied).
A full understanding of reaction mechanisms can provide deep insights into the design of efficient and stable catalytic systems. In recent years, the burgeoning development of advanced in situ/operando characterization techniques has reshaped how the field of catalysis is studied. By employing in situ/operando techniques, people can facilitate the real-time analysis of interfacial carrier behavior [26], monitor the morphological and structural changes [27,28], probe the surface active sites [29,30,31,32,33], identify reaction transition states [34,35] and track the changes in adsorbed species [36,37]. This information can help us to understand the surface corrosion of materials, improve the catalytic efficiency of a system, establish the right reaction pathway, implement effective protection strategies, develop new catalysts, etc.
In this review, the applications of in situ/operando characterization techniques are summarized, with emphasis on the utilization of scanning tunneling microscopy (STM), scanning electrochemical microscopy (SECM), kelvin probe force microscopy (KPFM), X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) for studying (photo-)electrocatalysis, including surface potential, active sites, reaction intermediates, adsorption/desorption, phase and structural changes, etc. Furthermore, the aforementioned surface characterization techniques have also been extensively employed for the study of redox biomolecules coming from natural sources, which is another important area in the renewable energy field, demonstrating the wide applicability of in situ/operando in the investigations of various energy-related disciplines. The working principles of the listed techniques have been briefly described as well. We hope that this review will shed light on the necessity of in situ/operando characterization technologies for the development of the field of catalysis.
2. Scanning Probe Microscopy
Scanning probe microscopy (SPM) is a class of microscopic techniques that use probes to scan and image the surface topography of a specimen, including atomic force microscopy (AFM), scanning tunneling microscopy (STM) and scanning near-field optical microscopy (SNOM), etc. The surface imaged by SPM is attained by fully employing the physical interaction between the sharp tip and the topmost atoms from the sample, thus providing a nanoscale approach to investigate various systems. Furthermore, local chemical and physical properties other than morphology can also be interrogated by coupling SPM with other analytical methods and external fields, such as Kelvin probe, infrared spectroscopy (IR), X-ray photoelectron spectroscopy (XPS) and electrochemistry, to name a few. In this section, we will go through some applications of in situ/operando SPM techniques for the study of water splitting and CO2RR.
2.1. Scanning Tunneling Microscopy
In 1981, Binnig and Rohrer invented scanning tunneling microscopy (STM) with atomic resolution, which demonstrated a much improved spatial resolution compared to scanning electron microscopy (SEM) [38]. The emergence of STM offered an opportunity to observe the arrangement of the surface atoms, as well as their related physical and chemical properties in real time, which greatly promoted the development of surface/interface science [39]. The working principle of STM is based on the quantum tunneling effect. In brief, when an ultrasharp tip (e.g., a tungsten tip with 7 nm tip radius) approaches to a conductive sample within a sufficiently close distance (in nanometer range), the tunneling current will occur between the sample and the tip under an external applied bias [40]. This current exponentially increases with the decreasing distance between the tip and the sample surface. In general, the tip–sample separation is kept around 4–7 Å, which is barely above the sample surface, for the tip to sense the interactions (repulsive and attractive forces) between the two. For the sake of acquiring other physical/chemical information simultaneously with the surface scanning during a chemical reaction, multiple fields can be added and thereby the corresponding phase transformation and physical response can be obtained in situ. For instance, combining electrochemical techniques and STM together, termed EC-STM, not only allows atomic-level imaging of liquid/solid interfaces to be achieved, but also provides us with unique insight into real-time electrochemical processes. In 1988, Itaya and Tomita constructed the STM for operation in electrochemical conditions for the first time [41], pioneering the observation of solid/liquid surfaces under in situ conditions. Up to now, EC-STM has become a powerful means to provide structural information about the solid side of the electrode/electrolyte interface at the atomic-level [42].
One of the most common applications of in situ STM is to directly perceive the morphological change of materials during reaction processes. Fester and co-workers investigated the morphological changes of Co-O nanoislands deposited on the surface of Au (111) after hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) [43]. After 10 CV cycles at negative potential, well-defined hexagonal nanoislands with a size of 10–30 nm (Figure 1a) were found transforming into larger thin layers with uniform flat features (Figure 1b). The surface morphology after 10 OER cycles showed three distinctive regions (Figure 1c), of which region-(i) was consistent with O-Co-O/Au (111) after HER with respect to both height and morphology, and the typical smooth part of region-(ii) and Au single-atom deep pits (region-(iii)) were also observed.
Since Fujishima and Honda first reported water splitting on TiO2 under UV illumination in 1972 [15], TiO2 has received extensive attention and many water splitting models based on TiO2 have been proposed [44,45,46]. In the process of establishing numerous models, STM is indispensable because it provides a way to directly monitor the water splitting process and its products at the atomic level. Tan et al. [47] observed the photodissociation of H2O molecules adsorbed on the five-fold coordinated Ti (Ti5c) sites of the R-TiO2 (110)—1 × 1 surface, as shown in Figure 1d. The two H2O molecules adsorbed at the Ti5c sites (marked by white circles) under UV illumination can be dissociated into two types of OH species; one appeared at the adjacent oxygen bridging site (OHbr, marked by triangles), while the other occurred at the Ti5c sites away from where the initial H2O molecules stay, and even desorb from the surface.
The detection of active sites is of paramount importance in (photo-)electrocatalysis, which is not only a key step towards the improvement of the efficiency of catalysts, but also plays a pivotal role in determining the reaction mechanism of materials [48,49]. Mitterreiter et al. [50] investigated the active sites of MoS2 on the Au (111) layer under HER (the boundary between MoS2 and Au, as well as the steps on MoS2, are marked by open and filled triangles, respectively, in Figure 1e). From the EC-STM images near the boundary (Figure 1f,g) at different potentials and corresponding line profiles (Figure 1h,i), it was found that the sharp edge between Au (111) and MoS2 was clearly visible at −600 mV (corresponding to “HER off”). However, when the potential was gradually changed to −900 mV (corresponding to “HER on”), additional features appeared near the boundary, and the tunneling current around the edge became noisier, while the tunneling current of Au (111) and MoS2 surface was basically unaffected. According to Pfisterer et al. [51], the place where the additional “noise” characteristic of EC-STM occurred under reaction condition can be understood as actual active reaction sites, so the authors considered that the HER sites of the above sample were located near the edges and steps. Liang and co-workers [52] also revealed the hydrogen evolution catalytic sites in monoatomic-thick Pt and Pd on Au (111) substrate (Au/Pt and Au/Pd) by analyzing the tunneling current noise. The 3–4 Pd atoms (about 1 nm wide) near the boundary of the Au/Pd system showed the highest noise level (Figure 2a), demonstrating that the most active hydrogen evolution sites were located near the boundary of Pd atoms. However, the whole Pt overlayer of the Au/Pt system showed the highest noise level (Figure 2b), indicating that the catalytic centers were likely to be uniformly distributed over the Pt overlayer. The above research demonstrates that there is a significant difference in the location of active electrocatalytic centers for Pd/Au and Pt/Au systems. STM has also been extensively employed in the investigation of CO2RR. Gao et al. [53] prepared CeOx nanoislands on Au (111) to study the interaction between Au-CeOx interface and CO2. The in situ STM images (Figure 2c,d,g) clearly showed that the CO2 adsorption first occurred at the interfacial boundary near CeOx. In the following studies, CO2 tended to attach to the previous CO2 species and gradually diffused towards the center of the island (Figure 2e,f,h), but did not adsorb on the Au (111) surface. This result showed that the Au (111)-CeOx interface was not only the site of initial CO2 adsorption and activation, but also acted on the subsequent activity of CO2. This phenomenon also occurs in the field of water splitting; for instance, the reactants are first adsorbed and activated in the boundary region and gradually expand to the center of the island. In a recent work of Fester et al. [54], cobalt oxide nanoislands deposited on Au (111) surface (Figure 2i) were exposed to water vapor at room temperature. XPS analysis revealed that the lattice oxygen of the nanoisland decreased with exposure to water, while the number of hydroxyl groups continued to increase. The surface changes of single bilayers and double bilayers exposed to water vapor over time are shown in Figure 2j and Figure 2k, respectively. Point-like patterns appeared on the surface of both islands, which were the characteristic of the adsorption of H on lattice oxygen sites to form hydroxyl groups, but the hydroxyl groups on single bilayers appeared as weak protrusions, and the hydroxyl groups on double bilayers appeared as circular depressions. Combined with the atom-resolved STM movie, it was clear that the hydroxyl first originated from the edge of the island and then diffused to the basal plane, indicating that the edge of the island was the hydroxyl diffused from the edge of the island to the center. The applications of STM in the biological field are also not negligible. STM was successfully exploited to assess the electron transfer properties of cytochrome b protein molecules immobilized on gold surfaces [55]. This was the first study where the electron transfer was measured by controlling the protein orientation under pseudo-physiological conditions.
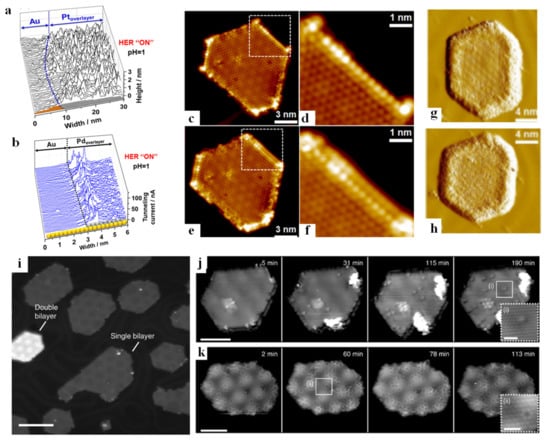
Figure 2.
Corresponding line scans within the rectangular area indicated in example of an n-ECSTM picture (constant current mode) recorded under the HER ON conditions for a boundary between a Pt overlayer and Au (111) (a). Boundary between a Pd overlayer on Au and Au surfaces under HER conditions in 0.1 M H2SO4. Pd atoms located close to the boundary have the largest comparative contribution to the overall activity (STM constant height mode) (b). Adapted with permission from ref. [52]. Copyright 2019 American Chemical Society. Interaction between CeOx/Au (111) and CO2. STM images of the CeOx-Au (111) interface before (c,d) and after (e,f) CO2 adsorption at 78 K. The square areas in (c) and (e) are magnified in (d) and (f), respectively. Sequential STM images of the CeOx island upon extended CO2 exposure at 78 K (g,h). Adapted with permission from ref. [53]. Copyright 2017 American Chemical Society. Representative STM image showing a 30% ML synthesis of CoO nanoislands. Single and double bilayers are labelled (i). Image sequences from atom-resolved STM movie (supplementary) recorded during water exposure showing the initial stage of hydroxylation on single- (j) and double-bilayer (k) CoO nanoislands. Adapted with permission from ref. [54]. Copyright 2017 Springer Nature.
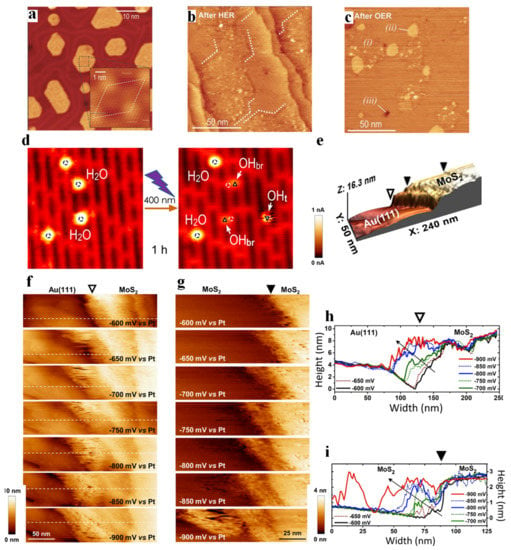
Figure 1.
STM images of Co−O bilayer nanoislands on Au (111) in STM (a), the cobalt oxide/Au (111) surface after HER (b). The Co−O/Au (111) surface after OER (c). Italic numbers indicate cobalt oxide (i), gold islands (ii) and single atom deep pits in the Au (111) surface (iii). Adapted with permission from ref. [43]. Copyright 2018 John Wiley & Sons, Inc. STM images (6.3 × 6.6 nm2, imaged at 1.0 V and 10 pA, 80 K) (d) showing the dissociation of water molecules under 400 nm UV irradiation for 1 h. Adapted with permission from ref. [47]. Copyright 2012 American Chemical Society. A boundary between the Au (111) substrate and a MoS2 flake (e). Constant current mode electrochemical STM images visualizing a MoS2/Au (111) boundary and a step edge at the surface of the MoS2 film under HER conditions with increasing overpotentials. A set of images characterizing the (f) MoS2/Au (111) boundary (open triangle) and (g) a step edge at the surface of the MoS2 film (filled triangle) at different electrode potentials as indicated in the figure. Line profiles across (h), (i) the same position for each potential from (f,g), respectively. Adapted with permission from ref. [50]. Copyright 2019 Springer Nature.
Due to the strict working conditions of STM, e.g., ultrasharp tip and extremely small separation between tip and sample that prefers a mirror-like flatness for the specimen, the quantum tunneling effect requires the specimen to be very conductive and uniform. These prerequisites limit the application of STM in a larger range of photoelectrode studies, where many of them are less conductive (studies of protection layers, metal–insulator–semiconductor structure), or flatness is not appreciated (nanostructures to increase surface aspect ratio, e.g., MOF, COF, inverse-opal). Moreover, the STM tips in EC-STM measurement are prepared using complex methods to deposit an electrophoretic paint coating to minimize the faradaic current at the tip/electrolyte interface. Thus, other techniques that can provide nanoscale information while having less stringent working requirements are needed to complement the study of surface/interface in photoelectrochemistry (PEC).
2.2. Atomic Force Microscopy
Ever since its invention by Binnig and Quate in 1986 [56], atomic force microscopy (AFM) has been extensively used in many different areas, such as biology, materials science, chemistry, physics, mechanical engineering, etc. In an AFM measurement, the sample’s morphological information (height fluctuation and shape, etc.) at the nanoscale or even atomic scale is obtained by the atomic force (attraction or repulsion) between the tip and the surface atoms. Such interaction exists in a range of 0.1–100 nm, regardless of the conductivity of the specimen. Therefore, AFM can not only overcome the material and flatness limitations of STM, but also can be operated under any environment (vacuum, air or liquid). The different operation modes of AFM such as contact and non-contact can be found in Reference [57]. Nowadays, there are many AFM-derived techniques, which make it not only possible to obtain information about the local topography of the sample, but they also provide surface potential [58], local conductance [59], active site distribution [60], electronic gaps [61] and other information simultaneously. Besides inorganic photocatalyst and electrocatalyst analysis, it has been successfully demonstrated that AFM also serves as a powerful tool for the study of biomolecule structure, redox mediators, surface charge density and EDL force [62]. For instance, AFM can be applied to elucidate the transition states of redox proteins like ferredoxin nicotin adenine dinucleaotide phosphate (NADP+) reductase towards its redox mediators, ferredoxin and flavodoxin [63] and also with the respective NADP+ cofactor molecule [64]. These were the first studies where redox flavoenzymes were studied at the single molecule level.
2.2.1. Kelvin Probe Force Microscopy
In 1898, the scanning Kelvin probe (SKP) technique, which was used to obtain the difference between the electronic work functions of two different metals, was first carried out by Kelvin [65]. For the first time in 1991, Nonnemacher et al. [66] introduced Kelvin probe technology into AFM, called Kelvin probe force microscopy (KPFM), which simultaneously allowed the imaging of surface topography and the measurement of the contact potential difference between the conductive tip and the sample. In KPFM analysis, when the conductive probe is close to the substrate, the contact potential difference is generated due to the different surface work functions between the two. The resulting contact potential difference can be eliminated with a known applied bias between the probe and the sample. Since the work function of the tip is known, the sample work function can be calculated [67]. A detailed explanation of the two different modes of KPFM (amplitude-modulated KPFM and frequency-modulated KPFM) can be found in references [67,68,69]. Thus far, KPFM has been developed as a powerful means to detect the surface charge distribution and/or work function on metal/semiconductor surfaces [70,71,72], and in situ/operando experiments can even image photogenerated charge carriers on the surface and interface of catalysts [73]. Although splendid progress has been made in KPFM, it is unfortunate that most KPFM operations thus far have been carried out under vacuum or ambient conditions, and few KPFM experiments in aqueous solution have been reported. This is due to the fact that the DC or AC bias will cause changes in the dynamics of the EDL [74], the electrochemical reactions and electrokinetic effects of both polar water molecules and electrolyte ions, resulting in difficulties in acquiring accurate and reliable measurements in aqueous solution [75,76,77,78,79,80,81]. Thus, in this section, we mainly focus on in situ observations under vacuum or ambient conditions rather than operando observations.
How to promote the separation and interfacial transfer of photogenerated charge carriers is a critical issue for improving the overall solar energy conversion efficiency in photocatalysis, and one of the effective methods is to load cocatalysts on photoelectrodes. Recently, various hydrogen evolution and oxygen evolution cocatalysts have been widely studied for promoting the separation and transfer of photogenerated electrons and holes at the interface, reducing the overpotential required for interfacial redox reactions, and inhibiting the corrosion of photoelectrodes. Yang et al. [17] investigated the surface potential of different oxygen evolution cocatalysts (NiO, CoO and NiCoO2) decorated on BVO, and found that the surface potential difference between the FTO substrate and NiCoO2/BVO was the smallest (Figure 3e,f). This result indicated that the upward bending of the band at the NiCoO2/BVO interface was the most severe among all the samples. This band structure could facilitate the separation of electron-hole pairs and guide the transport of charge carriers, resulting in improved charge separation efficiency, oxidation kinetics efficiency and photocurrent density of the NiCoO2/BVO photoanode. Hu et al. [82] electrodeposited AgOx, NiOx and AgOx/NiOx on the surface of BVO (AgOx/BVO, NiOx/BVO and AgOx/NiOx/BVO), and studied the surface potential changes of the above three photoanodes under illumination (Figure 3a–d). Among these three samples, AgOx/NiOx/BVO had the highest surface photovoltage (SPV, which is defined as the difference between contact potential difference (CPD) under light and CPD under dark [82,83]), which indicated that photogenerated holes in BVO were rapidly transferred towards the interface and accumulated in the composite AgOx/NiOx electrocatalyst. The concentration of accumulated photogenerated holes was the highest among the three. Meanwhile, the maximum SPV of AgOx/NiOx/BVO indicated that an efficient Schottky junction had been successfully established at the electrode–electrolyte interface, as demonstrated in the subsequent quantitative study of the absorbed photon to current efficiency on AgOx/NiOx/BVO. By artificially creating band bending by combining the cocatalysts with the semiconductor, the separation of electron-hole pairs can be effectively promoted, as is the improvement of the photocatalytic performance. In addition, spatial separation of photogenerated charge carriers can also be achieved through diffusion (sometimes called Dember effect, this has often been ignored because the built-in electric field is the main factor that facilitates the separation of charge carriers). In a recent work by Chen’s team [84], charge separation induced by diffusion (exploiting the difference in the mobility of electrons and holes) was found after cubic Cu2O was exposed to non-uniform illumination (asymmetric illumination), and the effect was stronger than that induced by the built-in electric field. By artificially creating asymmetric illumination, they found that the potential of the irradiated surface increased by 30 mV, while the potential of the unirradiated side decreased by 10 mV (Figure 3g,h). Subsequently, periodic illumination was used to continue the irradiation, and an opposite trend of the two facets was observed (Figure 3i). Combined with other auxiliary experiments, it was deduced that the SPV generated by charge separation caused by diffusion was 20 mV (holes move to the illuminated facet and electrons move to the shadow facet). This result quantitatively indicated that the efficiency of carrier separation caused by diffusion was even better than that of the built-in electric field.
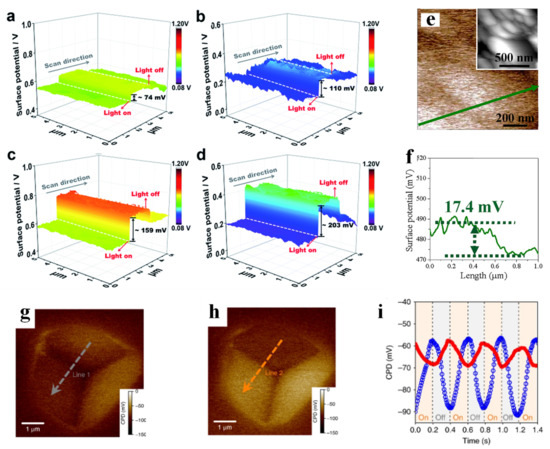
Figure 3.
Surface potential of pure BiVO4 (a), BiVO4/AgOx (b), BiVO4/NiOx (c), and BiVO4/AgOx/NiOx (d) photoanodes. Adapted with permission from ref. [82]. Copyright 2018 The Royal Society of Chemistry. The KPFM and surface potential results (e,f) for NiCoO2/BV; the insert figure is the morphology of sample. Adapted with permission from ref. [17]. Copyright 2019 Elsevier. Corresponding surface potential images in the dark state (g) and under illumination (h) (λ = 450 nm). Transient surface potential signals on the illuminated facet (blue) and shadow facet (red) collected with 5 Hz modulated light (i). Adapted with permission from ref. [84]. Copyright 2018 Springer Nature.
KPFM technology can also be used to probe the location of carriers and the distribution of active sites, which is due to the change of surface potential caused by the migration of charge carriers to a certain position on the surface. Wang et al. deposited Au NPs on the surface of rutile TiO2 (100) (Au/TiO2, as shown in Figure 4a) and detected the position distribution of the plasmonic holes for water oxidation on Au/TiO2 [85]. The surface potential of Au/TiO2 in the dark state (Figure 4b) clearly showed that the CPD of the interface between Au NP and TiO2 was significantly lower than that of TiO2, revealing that the Au/TiO2 interface has higher work function due to the formation of Schottky junction. In addition, a ring highlighted in purple can be found by differential spectroscopy (Figure 4d, which was obtained by Figure 4b,c), indicating that charge separation occurs at the Au/TiO2 interface, and holes were mainly concentrated at the interface. Moreover, DFT calculations also confirmed that this structure was favorable for water oxidation. Luna et al. [86] decorated Au nanoparticles (Au NPs) on TiO2 surface and investigated the effect of a single Au NP on photogenerated charge transfer and trapping under illumination. The CPD on top of a single Au NP in the dark was about 12 mV lower than that of the surrounding TiO2 (Figure 4e), from which it was inferred that the work function of Au NP was higher than that of TiO2. Further increase in the CPD difference between the single Au NP and TiO2 under illumination (up to 25 mV, Figure 4f) showed the transfer of photogenerated electrons from TiO2 to Au NPs. Following studies also quantitatively revealed that SPV difference increased logarithmically with the increase in light intensity in a certain range (Figure 4g). Wardhana et al. [87] deposited a Cr2O3 thin film on SrTiO3 (100) and found the accumulation of photogenerated holes on the surface of Cr2O3 thin film by recording photoinduced surface potential variations. Next, the effect of film thickness was also investigated, and it was found that the CPD in light and dark state was basically the same when the thickness was increased to 90 nm (Figure 5a), indicating that the inelastic mean free path of the photogenerated holes from the interface to the surface of the Cr2O3 film was within 90 nm. This work provides an approach for qualitatively calculating the mean free path of photogenerated carriers.
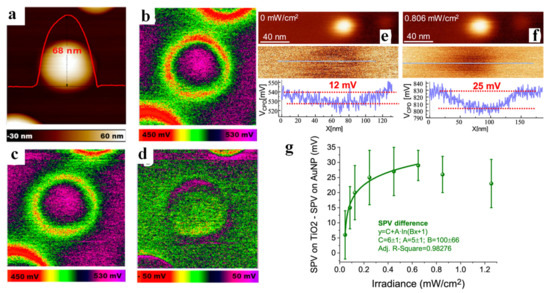
Figure 4.
AFM topography image of Au NP deposited on a TiO2 rutile single crystal. The height of a single Au NP is about 68 nm (a). KPFM images of Au/TiO2: in the dark (b) and upon 532 nm illumination (c). SPV image by subtracting the potential under dark conditions from that under 532 nm illumination (d) (SPV, ΔCPD = CPDlight − CPDdark). Adapted with permission from ref. [85]. Copyright 2017 American Chemical Society. Topography (top) and VCPD (bottom) images of a 3 nm diameter Au NP on bare TiO2 (110) (e) in the dark and (f) under UV illumination. VCPD profiles are shown at the bottom. Color scale in topography: (e) 3.6 nm and (f) 4 nm. Difference between SPV on bare TiO2 and SPV on the Au NP as a function of irradiance (g). Adapted with permission from ref. [86]. Copyright 2021 American Chemical Society.
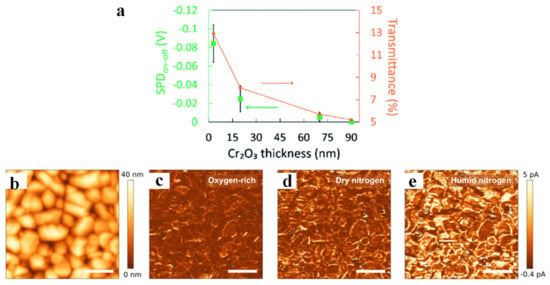
Figure 5.
The change in the values between dark and 470 nm irradiations from the 3, 20, and 70 nm, as well as 90 nm Cr2O3 film, including their transmittance (a). Adapted with permission from ref. [87]. Copyright 2022 The Royal Society of Chemistry. Topography (b) and current images under illumination (Vs = 1.75 V) in oxygen (c), dry (d), and humid (e) nitrogen atmosphere measured at identical areas. Adapted with permission from ref. [26]. Copyright 2018 American Chemical Society.
Combining Kelvin probe technique and photoconductive AFM, Eichhorn et al. studied water and oxygen adsorption on the surface of polycrystalline BVO film, and their effect on the transport and transfer of photogenerated charge carriers [26]. By observing the change of surface potential through KP technology, it was found that the adsorbed oxygen acted as an electron trap state on the surface of BVO, while the adsorbed water only formed a dipole layer on the surface to reduce the surface potential. The current distribution images of BVO under oxygen-rich, dry nitrogen and humid nitrogen are shown in Figure 5b–e, respectively. The minimum current in the oxygen-rich state was because the adsorbed oxygen acted as an electron trap site and captured the photogenerated electrons for desorption, leading to the decrease in the surface conductivity. The reason for the current increase under dry nitrogen was that the oxygen on the surface of BVO was replaced by nitrogen, while the apparent increase in conductivity under wet nitrogen might be related to the replacement of the residual oxygen on the surface of BVO by water molecules, and the water dipole arrangement affected the band alignment between the surface and the probe, thereby changing the injected current. Sometimes the presence of mobile ions can also affect KPFM experiments, and another new SPM technique that can be applied in liquid environments, electrochemical force microscopy, was developed and implemented by Collins et al. [79], which can also be used to study ion dynamics and electrochemical processes (such as adsorption/desorption, electron transfer, etc.).
2.2.2. Scanning Electrochemical Microscopy
Scanning electrochemical microscopy (SECM) can be categorized as an SPM technology that utilizes an ultramicroelectrode (UME) as electrochemical scanning probe to image and characterize the local morphology together with the corresponding electrochemical properties of various materials [88,89]. In the SECM analysis, the UME scans across the substrate surface to image the morphology, while the electrochemical responses occurring in the region around the probe and substrate have been simultaneously recorded [90]. A detailed explanation of the various operating modes, such as the feedback mode, the generation/collection mode and surface interrogation mode, can be found in References [91,92,93,94]. After decades of development, SECM has become a mature and attractive technology, which has received tremendous attention in the field of artificial photosynthesis, and it has been widely used in the detection of active sites, the study of interface reaction kinetics, etc. [95,96]. Moreover, the incorporation of SECM into the AFM system to form AFM-SECM (sometimes called potential-sensing electrochemical atomic force microscopy (PS-EC-AFM) elsewhere), further improves the spatial resolution and detection limits of this technique.
Bismuth vanadate (BVO) has become one of the most promising photoanode materials due to its narrow bandgap, non-toxicity, good photostability, appropriate band edge position to the redox potential of water, etc. [97,98]. Unfortunately, the poor electronic conductivity and rapid recombination of photogenerated carriers severely diminish its photocatalytic performance [33,99]. In response to this shortcoming, various optimization strategies have emerged, among which cobalt phosphate exhibits the outstanding advantage of easy incorporation onto the BiVO4 surface for surface modification [100]. Nellist et al. [101] prepared a thick BVO layer that completely covered the FTO substrate and supported a layer of cobalt (oxy)hydroxide phosphate (CoPi) on the surface. A bipotentiostat was in use to drive the PEC process, wherein the potential of the BVO (Vsub) was controlled by WE1, whilst the nanoelectrode probe (WE2) simultaneously scanned the electrode surface and measured the potential of CoPi (Vtip), as shown in Figure 6a. The potential of CoPi remained unchanged initially, then increased with an increase in the photocurrent when the potential of BVO reached to −0.75 V vs. εO2/OH−, indicating that CoPi collected photogenerated holes from the BVO. Another experiment by Nellist et al. corroborated these results [102]. They deposited CoPi on the surface of planar hematite semiconductor photoelectrodes, and as the potential of planar hematite (Vsub) varied from −0.8 V to −0.43 V vs. εO2/OH−, the tip potential (Vtip) was essentially unchanged (Figure 6c) because CoPi was not charged/reduced (and therefore not conductive). However, when Vsub = −0.33 V vs. εO2/OH−, the photogenerated holes were transferred to CoPi, which led to the oxidation of CoPi and thus turned it into a conductive state. They also investigated the influence of the thickness of CoPi coating and found that if the BVO layer was not dense (or defective), a thick CoPi would cause collected holes to be transferred to the FTO by forming a good electrical contact with the FTO (Figure 6b) [101]. This phenomenon reveals that the thickness of the co-catalyst is also an important factor affecting the PEC performance of the photoelectrode. Laskowski et al. deposited Ni nanoislands of different sizes on n-Si surface and found that both the Schottky barrier height in air and the photovoltage under open circuit potential were independent of the size of the Ni nanoislands. However, the photovoltage after photoelectric activation was actually size-dependent, due to the surface conversion of the deposited Ni to Ni(OH)2/NiOOH after the photoelectrochemical cycle. The authors explained this phenomenon by the pinch-off effect, and argued that the difference in photocurrent caused by the different thicknesses of the previous catalyst coatings should be explained by the pinch-off effect in addition to resistive losses and parasitic light absorption [103].
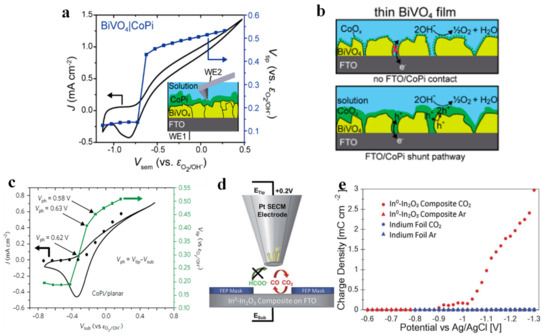
Figure 6.
Operando PS−EC−AFM potential stepping experiments. CV for CoPi−coated BiVO4 (black) and the measured tip potential (Vtip, purple) during potential stepping, while illuminated. The inset shows the operational setup (a). For thin films of BiVO4 with low CoPi loading, the catalyst does not significantly shunt to the underlying FTO (top), whereas at high loadings, the catalyst is in contact with both the BiVO4 and the FTO (bottom) (b). Adapted with permission from ref. [101]. Copyright 2018 American Chemical Society. In operando PS−EC−AFM potential-step photoelectrochemical experiments. The measured potential of CoPi on planar hematite (c). Adapted with permission from ref. [102]. Copyright 2017 Springer Nature. Schematic of the SG-TC SECM experiment showing the collection of CO but not COOH on a Pt tip electrode (d). Product collection charge density as a function of the potential applied to the catalytic electrode for both the In0−In2O3 composite and the In foil electrode in both CO2−saturated and Ar−saturated 0.1 M Na2SO4 (e). Adapted with permission from ref. [104]. Copyright 2017 The Royal Society of Chemistry.
SECM can also be used to identify electrochemical CO2RR products in a real-time PEC process. Shaughnessy et al. [104] prepared In0-In2O3 composites by electrochemically reducing the oxide layer on the surface of In2O3 to In, and they reported the application of substrate generation-tip collection scanning electrochemical microscopy (SG-TC SECM) to analyze the selectivity of the In0-In2O3 electrode. A schematic of SG-TC SECM is shown in Figure 6d. The amount of charge collected on the Pt tip under a series of potentials was measured to obtain the accurate reaction potential of CO production (Figure 6e). Mayer et al. [14] prepared three Sn/SnOx catalysts with different compositions (different relative contents of Sn, SnO and SnO2) and morphologies (from smooth surfaces to spherical nanoparticles) by pre-electroreduction. The specific SEM images and XPS spectra are shown in Figure 7a,b. These catalysts were then subjected to cyclic voltammetry tests under specific conditions, and the resulting peak current density features of each spatially resolved CV were attributed to formate, adsorbed CO (COads) and H2 oxidation, and the obtained Pt UME tip current densities were plotted as Figure 7c–e. The figures illustrated the highest formate oxidation current density and CO oxidation current density for pre-electroreduced Sn/SnOx at −1.25 V vs. Ag/AgCl, and lack of waves associated with H2 oxidation, which indicated that the CO2 to carbonaceous products (formate and CO) faradaic efficiency of electroreducted Sn/SnOx approached 100%. Sreekanth et al. [105] studied the pH and potential dependence on CO2RR behavior of Au substrate in 0.1 M KHCO3 solution, and showed different cyclic voltammetric responses under low alkaline (Figure 8a) and neutral conditions (Figure 8b). The characteristics of formic acid oxidation were shown under low alkaline conditions at −0.85 V vs. Ag/AgCl (13C NMR spectroscopy confirmed formic acid as the main product). However, when the bicarbonate solution was saturated with CO2, the pH changed from 8.3 to 6.8, resulting in the emergence of a sharp CO oxidation peak at 0.6 V vs. Ag/AgCl and a suppression of formic acid formation. The above results demonstrated that the products obtained by CO2RR can be controlled based on specific pH values and applied potentials. SECM based on specific modified UMEs can also be used to detect the local pH measurements. Monteiro et al. [106] first found a CO oxidation peak (peak I) at a substrate potential of −0.5 V, and a shoulder peak (peak II) emerged when the applied bias negatively shifted to −0.6 V. As the sample potential continued to move negatively, peak II gradually increased while peak I decreased. This phenomenon suggested that there were two different mechanisms of CO oxidation within a certain potential range. Since the applied potential caused a change in pH, the authors measured the pH of the local diffusion layer by a modified UME (Figure 8c), and they proposed three mechanisms of CO oxidation controlled by pH (Table 1) in combination with rotating disk electrode experiments. When pH ≤ 7, CO was oxidized by water, corresponding to peak I. When 11 > pH > 7, both peak I and peak II were presented, with peak II corresponding to CO oxidation by OH−. When pH ≥ 11, CO was only oxidized by OH−.
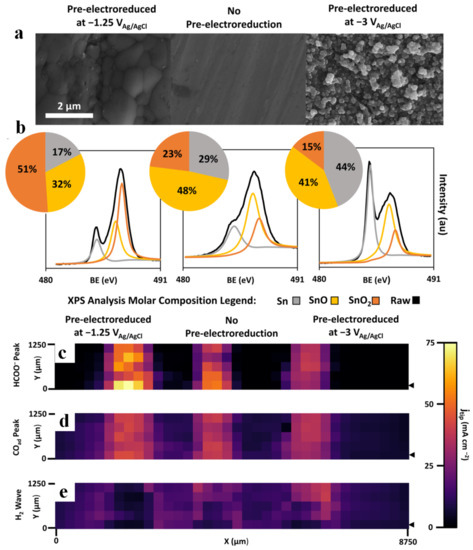
Figure 7.
Sn/SnOx catalyst array characterization. SEM imaging (a), XPS spectra and deconvolution at the Sn 3d 5/2 orbital with surface molar compositions calculated based on peak surface areas (b). Pt UME tip current densities obtained from the backward CV-SECM scan and attributed to the three products of CO2RR: HCOO− (c), COad (d), H2 (e). Pixel size: 250 × 250 μm. The black arrow indicates the starting position of the tip. Adapted with permission from ref. [14]. Copyright 2009 Springer Nature.
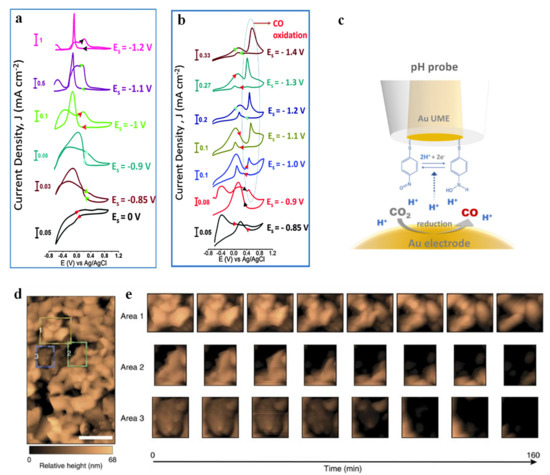
Figure 8.
Cyclic voltammetric responses of the Pt UME (10 mm) −tip probe to the products (HCOO) generated at the Au substrate in 0.1 M KHCO3 (a). Tip scan rate: 0.05 V s−1. Cyclic voltammetric responses of Pt UME (10 mm)—tip probe to the products (HCOO and CO) generated at Au substrate in 0.1 M KHCO3 solution saturated with CO2 (b). Adapted with permission from ref. [105]. Copyright 2014 The Royal Society of Chemistry. Schematic representation of the functionalized Au-UME used to measure pH (c). Adapted with permission from ref. [106]. Copyright 2020 American Chemical Society. Monitoring corrosion via in situ EC−AFM. EC−AFM scan (527,898 nm2; scale bar, 200 nm). The reported height is relative to a point on the underlying FTO substrate that is resolvable in all EC−AFM images. Common points have been used to align eight subsequent 11 mm2 scans of the same region, to correct for sample drift and rotation. Three coloured boxes indicate Regions 1 (yellow), 2 (green) and 3 (blue), whose temporal evolution were tracked in detail (d). Aligned EC-AFM scans in the three regions indicated in a were used to monitor corrosion-induced changes to BiVO4 mor-phology at 20 min increments in 1M KPi (pH 12.3) (e). Adapted with permission from ref. [107]. Copyright 2016 Springer Nature.

Table 1.
Relationship between pH, jlim species and bulk CO.
Electrochemical AFM (EC-AFM) is another type of AFM-based technique that focuses on the monitoring of morphology variation during the electrochemical reaction. Toma et al. [107] carried out an in situ EC-AFM measurement of BVO with an applied bias of 1.23 VRHE in dark, and they monitored the corrosion process of BVO films over time (Figure 8d,e). Area 1 exhibited uniform etching of the three solid–liquid interfaces. Area 2 had only one solid/liquid interface (diagonally across the upper left corner of the region, directly exposed to the electrolyte). Area 3 did not have a solid/liquid interface but there was a solid/solid grain boundary, exposing the top surface of the central grain, so this area was more vulnerable to chemical attack, with corrosion occurring almost entirely on the top surface. These results indicated that the corrosion of BVO was inevitable. Therefore, how to tackle the degradation of photoelectrodes has always been brought to the fore in PEC. Applying a protection layer onto an otherwise susceptible photocatalyst has become one of the most effective methods to solve this problem [108]. Recently, a kind of photoelectrode called “metal-insulator-semiconductor (MIS)” structure not only provides protection to the semiconductor, but also improves the solar-to-hydrogen efficiency with careful designs of band alignment and band bending [109,110,111]. Esposito et al. created a novel Si-based MIS photoelectrode [112]. They used a double-layer collector and replaced the native SiO2 layer with a rapid thermal oxidized SiO2. The former can increase the photovoltage while the latter can reduce the recombination of electron-hole pairs. It is noteworthy that the conventional MIS photocathode model failed to explain such high photocurrents found on this system and observed by scanning photocurrent microscopy and SECM. Therefore, the authors proposed an electrolyte-induced inversion channel layer and hydrogen spillover effect to elucidate this peculiar phenomenon that significant photocurrent and UME tip current can be observed at almost all locations on the surface of the photoelectrodes. AFM-SECM can also be used for bioelectrocatalytic research. Electron transfer of glucose oxidase (GOx) protein molecules can be quantified by AFM-SECM [113]. The resolution of this technique is in the order of femtoamperes (fA), which enables it to visualize the current of individual molecules through ferrocene chemical mediators to gold tips.
3. X-ray Characterization Techniques
X-ray is a kind of electromagnetic wave with short wavelength and high energy. First discovered by W. C. Röntgen in 1895 [114], X-ray now has been widely used in medical treatment, industrial flaw detection, communication engineering, material characterization, etc. Based on the interaction between X-ray and the irradiated sample, we can obtain a lot of useful information, including material thickness, elemental composition, chemical valence, material structure, etc. In addition, X-ray crystallography can be employed to decipher the tridimensional structure of redox biomolecules [115]. Thanks to the recent advancement of synchrotron radiation light sources, the generated X-ray possesses high luminous flux, high brightness, high purity, remarkable collimation, excellent monochromaticity and tunable energy, which allows us to obtain more accurate and specific information about the material. All these improvements of the synchrotron X-ray source pave the way for in situ/operando study of photoelectrocatalytic materials [116,117,118]. In this section, two X-ray characterization techniques (X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS)) will be introduced, and their application in the investigation of the underlying mechanisms of PEC water splitting and CO2RR is discussed.
3.1. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive X-ray technology with a probing depth less than 7–10 nm. The working principle of XPS is based on the renowned photoelectric effect. The incident X-ray can excite the core level electrons as well as the valence electrons from the topmost atoms. Once these electrons receive sufficient energy, they are ejected from the surface (photoejected free electrons) and then detected by the analyzer. These photoejected electrons carry bonding and valence state information, presented as the kinetic energy they possess [119,120]. However, the conventional XPS method cannot detect information alterations of the surface and interface under actual conditions (including light, humidity, applied potential, etc.). Because of the limited inelastic mean free path of photoelectrons generated by conventional X-ray sources, an ultra-high vacuum (UHV) environment is needed to minimize the electron scattering [120,121,122]. Ever since Siegbahn et al. developed the first ambient pressure (AP) XPS with a laboratory-based X-ray source in 1973, XPS has been extensively employed for liquid and/or gas phases surface/interface chemistry studies [123]. Recently, owing to the “dip and pull” method and optimized X-ray energy, we are able to probe solid/liquid and solid/gas interfaces under realistic (photo-)electrochemical reaction conditions [124,125,126].
We can utilize APXPS to determine whether water undergoes a simple adsorption process or a complex dissociation process on the catalyst surface. Through the different chemical behaviors of water vapor on pure Pt (111) and Pt/GaN (0001) systems, Zhang et al. [37] found that the selection of appropriate semiconductor supports can drive the dissociation of water. In the H2O/Pt (111) system, only the gas-phase water and the physically adsorbed water signals were observed; neither hydrolyzed dissociated products nor oxides were detected. However, when thin Pt films were deposited on GaN (0001), it was found that the dissociation of water was observed on Pt. Starr et al. [127] studied the chemical transformation on the surface of BVO (010) after the introduction of water vapor, and found that not only were water molecules adsorbed immediately at the surface, but also extensive hydroxylation occurred, and a large amount of reduced V (from V5+ to V4+) was also observed. Subsequently, the authors further studied the composition changes of BVO photoanode at 1.0 M KOH before and after illumination. The energy of incident photons was increased to probe the BVO thin film/0.1 M KPi interface, and the light-induced surface changes at the open circuit potential were also studied. O 1s spectra and Bi 4f spectra are shown in Figure 9a and Figure 9b, respectively. After illumination, it was found that (1) the BVO O 1s signal disappeared and the Bi 4f signal decreased, which was caused by an increase in the thickness of the aqueous electrolyte layer; (2) the ratio of the sum of adsorbed or dissolved phosphate ion peak and adsorbed hydroxyl group peak, to that of the overall liquid water peak, increased, which might originate from the migration of phosphate ions from the BVO thin film/0.1 M KPi interface to the 0.1 M KPi/vapor interface; and (3) Bi 4f spectra shifted to a higher binding energy due to the formation of BiPO4. Once back in the dark condition, the ratio of the peak intensity in (2) recovered to that of the original unilluminated state, and neither the peak associated with BiPO4 nor the BVO O 1s could be observed. Favaro et al. [128] conducted a similar experiment in polycrystalline BVO photoanode/0.1 M aqueous potassium phosphate (KPi) system. The intensity of HxPO4y− was also significantly increased relative to that of liquid-phase water (LPW) after illumination, and decreased upon subsequent reversion to the dark state (Figure 9c). Meanwhile, the ions in the electrolyte have also been studied, and are presented in Figure 9d,e for the K 2p and P 2p core levels, respectively. It can be seen that the intensity of K+ and P in HxPO4y− increases significantly under illumination, and the intensity variation caused by illumination was reversible after returning to dark, which coincided with the results of Starr et al.
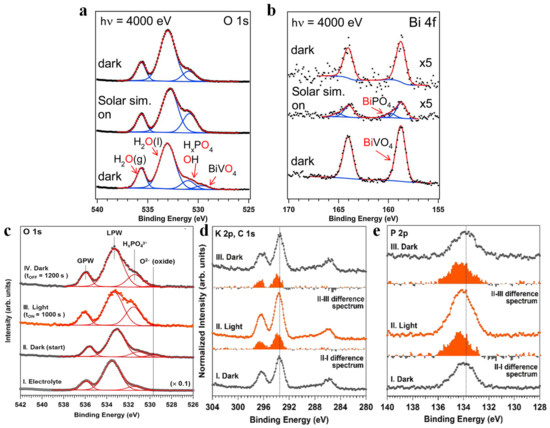
Figure 9.
O 1s (a) and Bi 4f (b) spectra of a BiVO4 thin film on FTO/glass under an approximately 21 nm thick potassium phosphate electrolyte at open circuit potential. Adapted with permission from ref. [127]. Copyright 2017 Elsevier B.V. In situ AP-HAXPES experiments performed on a polycrystalline BiVO4/liquid electrolyte interface. Selected O 1s spectra (c) and corresponding multipeak fitting procedure, I. KPi electrolyte only, II. The BiVO4/KPi electrolyte system initially in the dark, III. The BiVO4/KPi electrolyte system after 1000 s of illumination with a solar simulator, IV. The BiVO4/KPi electrolyte system 1200 s after returning to dark conditions. In situ AP-HAXPES experiments performed at room temperature and at p ∼ 17 Torr on a polycrystalline BiVO4/KPi aqueous electrolyte interface at the half-cell open circuit potential under illumination (∼0.92 sun) and dark conditions (hυ = 4.0 keV); K 2p and C 1s (d), and P 2p (e) photoelectron spectra. Adapted with permission from ref. [128]. Copyright 2017 American Chemical Society.
In recent years, the HER mechanism of many materials has been extensively explored [129,130,131,132]. However, OER has severely limited the overall development of water splitting due to its slow kinetic characteristics [133,134] Therefore, the study of efficient and stable OER catalysts has become a hot topic in the field of water splitting. Furaro et al. studied the surface chemistry and structure of polycrystalline Pt under 1.0 M KOH electrolyte as a function of applied potential [135]. The increase in the area of Pt-O and OHads was observed in the O 1s spectra (Figure 10a). Meanwhile, the area of H2Ochem increased only under OER, which was in line with the increase in electrode surface area. The relative positions and thicknesses of various substances as a function of the applied potential (Figure 10c) were further determined qualitatively in combination with Pt 4f spectra (Figure 10b). Interestingly, Pt(II) (including Pt(II)(OH)2 and Pt(II)O) and Ptδ-OHads appeared at relatively low potentials, and their quantities increased rapidly during OER, while Pt(IV)O2 was also detected. Stoerzinger conducted a similar study on polycrystalline Pt [136]. The O 1s and Pt 4f spectra are shown in Figure 10d and Figure 10e, respectively. The authors found that OER under alkaline conditions led to a partially irreversible oxidation of Pt, which was manifested in the increase in the amount of Ptδ-OHads and the formation of Pt(IV)O2. However, Pt(II)O and Pt(IV)O2 disappeared during HER, while the oxygen-free species Pt-H appeared. After driving HER for ~1 h, the chemical composition of the surface was found mostly unchanged under OCP.
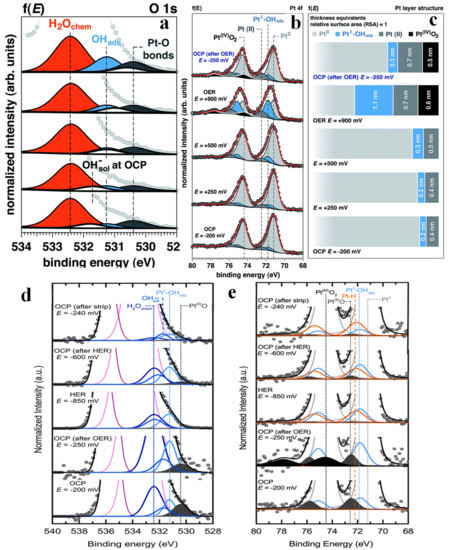
Figure 10.
Operando APXPS as a function of the applied potential. The magnification of the low binding energy spectrum tail of O 1s photoelectron peak acquired at 4 keV as a function of the applied potential (f(E)) to the working electrode (WE, from OCP to OER) (a). Evolution of the surface chemistry studied by operando APXPS, as a function of the applied potential. Pt 4f spectra acquired at 4 keV as a function of the applied potential (f(E)) to the working electrode (from OCP to OER) (b). Evolution of the surface structure as a function of the applied potential (f(E)), from OCP to OER (c). Adapted with permission from ref. [135]. Copyright 2017 The Royal Society of Chemistry. Magnification of the O 1s, background subtracted and normalized to the intensity of the Pt0, as a function of applied potential, illustrating the changing surface species (d). Operando APXPS Pt 4f photoelectron peak at 4 keV as a function of applied potential (e). Adapted with permission from ref. [136]. Copyright 2018 American Chemical Society.
Despite the superior performance of Pt catalysts, its scarcity and thus high and volatile price severely limits its large-scale application, so other non-noble metal catalysts are also being developed. Recently, transition metals and their derivatives have attracted increasing attention [133,134]. Han et al. [137] observed the surface species evolution of Co OER catalysts at different potentials in 0.1 M KOH electrolyte. Combined with Co 2p spectra (Figure 11a) and O 1s spectra (Figure 11b), it was found that when the potential increased to −0.4 V vs. Ag/AgCl, the formation of Co(OH)2 was detected, accompanied by the attenuation of the Co signal. When further increased to +0.4 V, the outmost surface of the Co(OH)2 layer was converted to CoOOH, as confirmed by APXPS with lower incident X-ray energy. The results indicated that, after the certain portion of Co that was in touch with the electrolyte was converted to Co(OH)2, it was consecutively oxidized to CoOOH under OER condition. Favaro et al. [138] studied the interfacial chemistry and structural changes of disordered Co(OH)2 biphase electrocatalysts covered on the Co3O4 surface at different potentials, as shown in Figure 11c. It was found that at the potential of converting Co3+ to Co4+ and above (including OER), all Co(OH)2 was converted to CoOOH, and a portion of Co3O4 also underwent a similar transformation (converted to CoOxOHy). It seems that in order to reduce the OER overpotential, the formation of CoOOH and CoOxOHy phase may need to be promoted kinetically. When the potential switches back from OER to OCP, part of the top layer CoO(OH) is restored to Co(OH)2, but there is still residual CoO(OH) remaining underneath the surface.
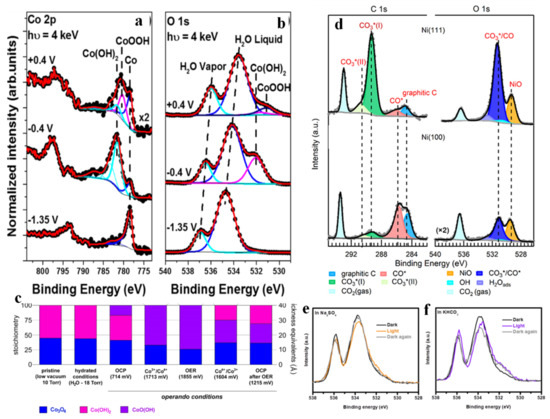
Figure 11.
Co 2p (a) and O 1s (b) spectra using the operando “dip and pull” method collected at −1.35, −0.4 and +0.4 V versus Ag/AgCl at hυ = 4 keV in 0.1 M KOH. Adapted with permission from ref. [137]. Copyright 2017 American Chemical Society. Evolution of the surface stoichiometry and structure of the biphasic catalyst as a function of the applied potential (c). Adapted with permission from ref. [138]. Copyright 2017 American Chemical Society. C 1s spectra on Ni (111) and Ni (100) and the corresponding O 1s spectra (d). Adapted with permission from ref. [139]. Copyright 2019 American Chemical Society. O 1s spectra of Cu2O in the dark, under illumination and in the dark again at OCP in 0.1 M Na2SO4 solution (e) and in 0.1 M KHCO3 solution (f). Adapted with permission from ref. [140]. Copyright 2021 Springer Nature.
Many transition-metal-based catalysts can also be applied to CO2RR, but their surface orientations may cause differences in CO2 activation and product. Cai et al. [139] investigated the interfacial reactions on Ni (111) and Ni (100) surfaces under pure CO2 and found that the amount of products with different surface orientations was significantly different. The C 1s spectra and O 1s spectra of Ni (111) and Ni (100) surfaces under 0.2 Torr CO2 are shown in Figure 11d. The Ni (100) surface was covered with adsorbed CO*, graphitic carbon and a small amount of carbonate, while the Ni (111) surface was topped with a plethora of carbonate species with a reduced amount of adsorbed CO* and graphitic carbon, which was also verified by DFT calculation. Sometimes the electrolyte also plays a key role in a catalytic system, as it may affect the performance of the photoelectrode under reaction condition. Liu et al. [140] used operando APXPS to track the variation of Cu2O in KHCO3 and Na2SO4 electrolytes. Although Cu2+-OH species were presented in both different electrolytes under illumination, the OH− feature remained at the interface of Cu2O-KHCO3 in dark (Figure 11f), while no OH− species could be found at the interface of Cu2O-Na2SO4 (Figure 11e).
3.2. X-ray Absorption Spectroscopy
When interacting with materials, the X-ray will be scattered and absorbed at first, then followed by an attenuation of the intensity of the X-ray during the transmission through the material. The X-ray absorption spectroscopy (XAS) is the technique that studies the relationship between the intensity of transmitted X-ray and the intensity of incident X-ray. According to the energy level of the emitted photoelectrons, X-ray absorption spectroscopy (XAS) is mainly composed of two regions, namely X-ray absorption near edge spectra (XANES) and extended X-ray absorption fine structure (EXAFS), which are formed by the interaction between photoelectrons and neighboring atoms. The former is located in a spectral region of about tens of eV around the absorption edge, which is a powerful technique to observe the alterations in the valence and electronic structure (including density of unoccupied states, and chemical bond hybridization) of absorbed atoms [141,142,143] The latter lies in the photon energy range of 50–1000 eV above the absorption edge, which can unravel the local geometric environment of the absorption atom, such as coordination number, coordination atoms, bond length and structural disorder [118,144,145]. Therefore, in situ/operando XAS can identify active components and track the structural evolution of catalysts and reaction intermediates under real-time electrochemical conditions [143,146,147].
Many reversible chemisorptions and electron transfer processes in interfacial reactions lead to the variations in the valence state and structure of catalysts. These processes are difficult (or even impossible) to detect by ex situ characterization techniques. A suitable approach to address these issues is in situ/operando XAS. Xi et al. reported that a cobalt borate (CoBi)-modified hematite photoanode achieves 1.12 mA cm−2 current density at 1.23 VRHE. An enormous enhancement of OER kinetics was attained when compared to the current density of pristine hematite (0.88 mA cm−2) [25]. Only Co2+ and Co3+ species were observed in the XPS spectra of the Co 2p3/2 scans of the fresh and used CoBi-modified hematite photoanodes, but the presence of Co4+ was revealed by in situ XAS results determining the average oxidation state. Specifically, the XAS results under different potentials and illumination showed the mean oxidation state of Co ions in the CoBi-modified hematite photoanode to be 3.38, which was in stark contrast to the XPS results the authors observed. The possible reason is that Co4+ species are unstable and exist only under in situ conditions. That work strengthens the importance of in situ spectroscopy when studying catalytic process. Merte et al. [148] described the surface adsorption and oxidation of Pt NPs loaded on glassy carbon at different potentials by XAS. Their work demonstrated the chemical states of Pt NPs at different potentials (Figure 12a), which can be classified into hydrogen chemisorption between 0 VRHE and 0.3 VRHE, oxygen chemisorption (including OH and/or O) from 0.3 VRHE to 0.96 VRHE, and platinum oxide formation from 0.96 VRHE to 1.26 VRHE. Particularly, when the potential was kept at 1.16 VRHE, the oxidation of Pt was found to proceed most fiercely. Möller et al. [149] tracked the chemical state and local structure changes of unsupported CuO nanocubes (U-NCs) during electrochemical CO2RR. XANES (Figure 12b) showed that, although the potential was kept constant, the absorption edge was shifted towards the lower energy side, and evidenced a decrease in the valence state of Cu. The local environment of Cu probed by EXAFS revealed an enhancement of the Cu-Cu peak accompanied by an attenuation of the Cu2O-related peak, demonstrating an increase in the metallic Cu-Cu coordination number and a decrease in the Cu-O coordination number. Linear fitting (Figure 12c) further found that the concentration of Cu0 species obtained from XANES was significantly higher than the coordination number of Cu0 species extracted from EXAFS, showcasing the existence of a large number of uncoordinated Cu sites. The above studies indicate that the initial cubic Cu2O was electrochemically reduced to the defect-rich Cu. A similar conclusion was reached by Velez et al. [150]. In their study, the bulk- and surface-sensitive versions had been achieved by total fluorescence yield mode (TFY), and by auger electron yield (AEY) and total electron yield (TEY), respectively, as shown in Figure 12d,e. Although the initial composition of ingredients was different, both surface and bulk were reduced to copper during CO2RR (in situ ECSEM also bore out this finding). Kornienko et al. [151] developed an amorphous cobalt sulfide (CoSx) HER catalyst and investigated the composition, local atomic structure and catalytic activity of CoSx under practical electrochemical conditions. Operando XANES (Figure 12f) showed the disappearance of the Co k-edge white line peak under HER conditions, and the spectrum resembled that of a transition metal sulfide. These results implied the disappearance of oxides and formation of cobalt sulfides. On the other hand, the increase in the intensity of the pre-edge peak can be explained by p-d hybridization of newly added cobalt sulfide. The Fourier transform EXAFS (Figure 12g) also confirmed an increase in the amount of cobalt sulfide, as a slight increase in average bond length was observed after HER.
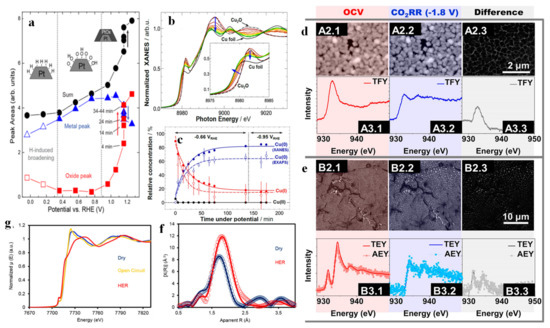
Figure 12.
Areas of the fitted components for the full set of spectra acquired for S1, as well as their sum, plotted as a function of the sample potential (a). Adapted with permission from ref. [148]. Copyright 2012 American Chemical Society. Cu K-edge XANES data of U-NC sample acquired under operando CO2 reduction conditions (b). Temporal evolution of the chemical composition of the Cu2O cubes during CO2 electroreduction obtained from the linear combination analysis of XANES data (filled circles) and coordination numbers from EXAFS data fitting (empty circles). Solid and dashed lines are guides for the eye. (c). Adapted with permission from ref. [149]. Copyright 2020 John Wiley & Sons, Inc. A2: In situ ECSEM measurements in 100 mM KHCO3 saturated in CO2 under OCV and CO2RR; A3: In situ XAS measurements in TFY in 100 mM KHCO3 saturated in CO2 under OCV and −1.8 V vs. Ag/AgCl (d); B2: In situ ECSEM measurements in 100 mM KHCO3 saturated in CO2 under OCV and CO2RR; B3: XAS measurements in TEY and AEY in 100 mM KHCO3 under OCV and −1.8 V vs. Ag/AgCl (e). Adapted with permission from ref. [150]. Copyright 2020 American Chemical Society. Operando Co K-edge XANES (f) reveals a line shape change that suggests transformation from cobalt oxide to cobalt sulfide. EXAFS (g) qualitatively indicates an increased average bond length and decreased shell contributions. Adapted with permission from ref. [151]. Copyright 2015 American Chemical Society.
Pt single-atom catalysts that consist only of isolated single atoms anchored onto FeOx nanocrystallites were first reported by Qiao and co-workers with high activity and stability for both CO oxidation and preferential oxidation of Co in H2 [152]. Since then, single-atom catalysts have aroused enthusiasm among researchers. Single-atom catalysts have many advantages, such as ultra-high atomic utilization (nearly 100%), large specific surface area, uniform distribution, etc. [153,154], and thus can tremendously improve the PEC performance of the photoelectrode. Fang’s group [155] synthesized atomically dispersed Pt on the N-C framework, and further tracked the valence state and atomic structure variations of the Pt sites under HER by operando XAS. Interestingly, the interaction and charge transfer between the Pt sites and the N-C carrier were weakened, tending to transition to a free metallic atom state. Wei et al. [156] synthesized a single-atom cobalt catalyst loaded on phosphorized carbon nitride, which exhibited outstanding HER performance. XANES and EXAFS showed that Co sites underwent a rise in oxidation state and a rearrangement in their local atomic structure from the ex situ state to the OCP and in situ states. Subsequently, the fitting and analysis spectra confirmed that HO-Co1-N2 acted as the real active site for HER, and the corresponding reaction mechanism was further proposed (Figure 13). The existence of the Co1-N4 moiety under ex situ condition was also speculated by Li et al. [157] through EXAFS.
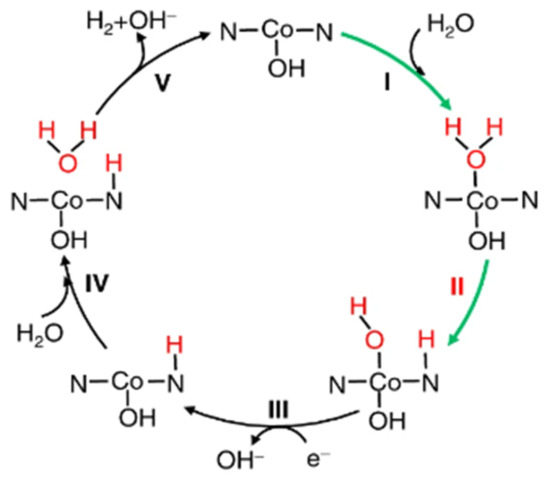
Figure 13.
Alkaline HER mechanism on HO-Co1/PCN. I: the catalytic cycle is initiated by adsorption of H2O onto Co; II: dissociation into adsorbed OH* and H* on the Co and nearby N, respectively (the asterisk denotes the adsorption site); III–V: another proton from an adjacent H2O molecule will react with the first H* to generate H2. It is well known that in alkaline HER, the first sluggish Volmer step is catalytic dissociation of a water molecule with the generation of H* (H2O + e− = H* + OH−). Adapted with permission from ref. [156]. Copyright 2018 Springer Nature.
4. Conclusions
In situ/operando analysis is crucial for the acquisition of a full understanding of catalytic reaction processes such as water splitting and CO2RR, in order to gain more insight into the dynamic changes of the catalyst under working conditions. Given that the property of the catalyst surface and interface is a vital factor affecting the catalytic performance, enthusiastic efforts have been devoted to researching surface and interface engineering in (photo-)electrocatalysis.
As shown in the aforementioned studies, in situ/operando characterization techniques have unique functions. The application of in situ/operando SPM can study active sites, surface potential, surface adsorption and dissociation, morphology and structure. In situ/operando X-ray spectroscopy can provide information on elemental composition, valence changes, atomic short-range structure and other information. Coupling advanced in situ/operando measurements and catalytic systems can definitively allow monitoring of interfacial carrier behavior, probing surface active sites, identifying reaction transition states and tracking changes in adsorbed species, providing valuable and accurate details of reaction dynamics in real time. The integration of fragmented information obtained with different in situ/operando characterization techniques will piece together the microscopic reaction mechanisms that occur at the surface/interface scale.
Numerous reviews have been published involving the application of in situ/operando characterization in the field of artificial photosynthesis, but most of them focus on a particular characterization method, or a particular type of catalytic reaction. In this review, we have summarized the recent advances in the in situ/operando surface/interface characterization techniques used in the study of (photo-)electrocatalytic reaction mechanisms on the basis of previous studies, covering STM, AFM (including KPFM, SECM, etc.), XPS and XAS, and we thus hope to provide readers with a more comprehensive reference and some inspiration when choosing appropriate surface/interface techniques for studying reaction mechanisms. The study of dynamic changes is important, not only as the basis of mechanism research, but also because it allows formulating design principles for catalytic systems.
Author Contributions
Conceptualization, H.L.; writing—original draft preparation, H.L.; writing—review and editing, H.L. and Z.Y.; supervision, G.Z.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the Southern University of Science and Technology (SUSTech) research startup grant. The authors would also like to acknowledge technical support from SUSTech Core Research Facilities, the Shenzhen Key Laboratory for Intelligence Robotics and Flexible Manufacturing Systems (RobFMS) and the SUSTech Energy Research Institute for Carbon Neutrality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, L.; Cheng, S.Y.; Li, J.B.; Huang, Y.F. Mitigating Environmental Pollution and Impacts from Fossil Fuels: The Role of Alternative Fuels. Energy Sources Part Recovery Util. Environ. Eff. 2007, 29, 1069–1080. [Google Scholar] [CrossRef]
- Rummukainen, M. Our commitment to climate change is dependent on past, present and future emissions and decisions. Clim. Res. 2015, 64, 7–14. [Google Scholar] [CrossRef]
- Uzair Ali, M.; Gong, Z.; Ali, M.U.; Asmi, F. and Muhammad, R.: CO2 emission, economic development, fossil fuel consumption and population density in India, Pakistan and Bangladesh: A panel investigation. Int. J. Finance Econ. 2022, 27, 18–31. [Google Scholar] [CrossRef]
- Chapman, A.J.; Itaoka, K. Energy transition to a future low-carbon energy society in Japan’s liberalizing electricity market: Precedents, policies and factors of successful transition. Renew. Sustain. Energy Rev. 2018, 81, 2019–2027. [Google Scholar] [CrossRef]
- Li, L. and Taeihagh, A.: An in-depth analysis of the evolution of the policy mix for the sustainable energy transition in China from 1981 to 2020. Appl. Energy 2020, 263, 114611. [Google Scholar] [CrossRef]
- Lindberg, M.B.; Markard, J.; Andersen, A.D. Policies, actors and sustainability transition pathways: A study of the EU’s energy policy mix. Res. Policy 2019, 48, 103668. [Google Scholar] [CrossRef]
- Akaev, A.A.; Davydova, O.I. The Paris Agreement on Climate Is Coming into Force: Will the Great Energy Transition Take Place? Her. Russ. Acad. Sci. 2020, 90, 588–599. [Google Scholar] [CrossRef]
- Huang, M.-T.; Zhai, P.-M. Achieving Paris Agreement temperature goals requires carbon neutrality by middle century with far-reaching transitions in the whole society. Adv. Clim. Chang. Res. 2021, 12, 281–286. [Google Scholar] [CrossRef]
- Li, Z.; Lu, L. Wastewater treatment meets artificial photosynthesis: Solar to green fuel production, water remediation and carbon emission reduction. Front. Environ. Sci. Eng. 2022, 16, 53. [Google Scholar] [CrossRef]
- Salviulo, G.; Lavagnolo, M.C.; Dabalà, M.; Bernardo, E.; Polimeno, A.; Sambi, M.; Bonollo, F.; Gross, S. Enabling Circular Economy: The Overlooked Role of Inorganic Materials Chemistry. Chem.—Eur. J. 2021, 27, 6676–6695. [Google Scholar] [CrossRef]
- Liu, M.; Pang, Y.; Zhang, B.; De Luna, P.; Voznyy, O.; Xu, J.; Zheng, X.; Dinh, C.T.; Fan, F.; Cao, C.; et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 2016, 537, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, Y.; Gao, C.; Wu, X. The enhanced photo-catalytic CO2 reduction performance of g-C3N4 with high selectivity by coupling CoNiSx. Mater. Res. Bull. 2021, 144, 111488. [Google Scholar] [CrossRef]
- Mayer, F.D.; Hosseini-Benhangi, P.; Sánchez-Sánchez, C.M.; Asselin, E.; Gyenge, E.L. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays. Commun. Chem. 2020, 3, 155. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, J.; An, H.; Yan, P.; Huang, B.; Chen, R.; Fan, F.; Li, C. Directly Probing Charge Separation at Interface of TiO2 Phase Junction. J. Phys. Chem. Lett. 2017, 8, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiong, Y.; Xiao, P.; Zhang, Y. Guiding charge transfer kinetics into cocatalyst for efficient solar water splitting. Electrochim. Acta 2019, 307, 43–50. [Google Scholar] [CrossRef]
- Nie, K.; Kashtanov, S.; Wei, Y.; Liu, Y.-S.; Zhang, H.; Kapilashrami, M.; Ye, Y.; Glans, P.-A.; Zhong, J.; Vayssieres, L.; et al. Atomic-scale understanding of the electronic structure-crystal facets synergy of nanopyramidal CoPi/BiVO4 hybrid photocatalyst for efficient solar water oxidation. Nano Energy 2018, 53, 483–491. [Google Scholar] [CrossRef]
- Ali-Löytty, H.; Louie, M.W.; Singh, M.R.; Li, L.; Sanchez Casalongue, H.G.; Ogasawara, H.; Crumlin, E.J.; Liu, Z.; Bell, A.T.; Nilsson, A.; et al. Ambient-Pressure XPS Study of a Ni–Fe Electrocatalyst for the Oxygen Evolution Reaction. J. Phys. Chem. C 2016, 120, 2247–2253. [Google Scholar]
- Lawrence, M.J.; Celorrio, V.; Sargeant, E.; Huang, H.; Rodríguez-López, J.; Zhu, Y.; Gu, M.; Russell, A.E.; Rodriguez, P. Insight into the Activity and Selectivity of Nanostructured Copper Titanates during Electrochemical Conversion of CO2 at Neutral pH via In Situ X-ray Absorption Spectroscopy. ACS Appl. Mater. Interfaces 2022, 14, 2742–2753. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Liu, Y.; Zhang, C.; Pan, Y. Structural regulation of single-atomic site catalysts for enhanced electrocatalytic CO2 reduction. Nano Res. 2022, 15, 4925–4941. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.-Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Frei, H.; Park, J.Y. Advancing the Frontiers in Nanocatalysis, Biointerfaces, and Renewable Energy Conversion by Innovations of Surface Techniques. J. Am. Chem. Soc. 2009, 131, 16589–16605. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Schwanke, C.; Zhou, D.; Drevon, D.; van de Krol, R.; Lange, K.M. In situ XAS study of CoBi modified hematite photoanodes. Dalton Trans. 2017, 46, 15719–15726. [Google Scholar] [CrossRef]
- Eichhorn, J.; Kastl, C.; Schwartzberg, A.M.; Sharp, I.D.; Toma, F.M. Disentangling the Role of Surface Chemical Interactions on Interfacial Charge Transport at BiVO4 Photoanodes. ACS Appl. Mater. Interfaces 2018, 10, 35129–35136. [Google Scholar] [CrossRef]
- Simon, G.H.; Kley, C.S.; Roldan Cuenya, B. Potential-Dependent Morphology of Copper Catalysts During CO2 Electroreduction Revealed by In Situ Atomic Force Microscopy. Angew. Chem. Int. Ed. 2021, 60, 2561–2568. [Google Scholar] [CrossRef]
- Timoshenko, J.; Jeon, H.S.; Sinev, I.; Haase, F.T.; Herzog, A.; Roldan Cuenya, B. Linking the evolution of catalytic properties and structural changes in copper–zinc nanocatalysts using operando EXAFS and neural-networks. Chem. Sci. 2020, 11, 3727–3736. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.-J.; Sokaras, D.; Weng, T.-C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef]
- Lu, F.; Yi, D.; Liu, S.; Zhan, F.; Zhou, B.; Gu, L.; Golberg, D.; Wang, X.; Yao, J. Engineering Platinum–Oxygen Dual Catalytic Sites via Charge Transfer towards Highly Efficient Hydrogen Evolution. Angew. Chem. 2020, 132, 17865–17871. [Google Scholar] [CrossRef]
- Casalongue, H.G.S.; Benck, J.D.; Tsai, C.; Karlsson, R.K.B.; Kaya, S.; Ng, M.L.; Pettersson, L.G.M.; Abild-Pedersen, F.; Nørskov, J.K.; Ogasawara, H.; et al. Operando Characterization of an Amorphous Molybdenum Sulfide Nanoparticle Catalyst during the Hydrogen Evolution Reaction. J. Phys. Chem. C 2014, 118, 29252–29259. [Google Scholar] [CrossRef]
- Hung, S.-F.; Chan, Y.-T.; Chang, C.-C.; Tsai, M.-K.; Liao, Y.-F.; Hiraoka, N.; Hsu, C.-S.; Chen, H.M. Identification of Stabilizing High-Valent Active Sites by Operando High-Energy Resolution Fluorescence-Detected X-ray Absorption Spectroscopy for High-Efficiency Water Oxidation. J. Am. Chem. Soc. 2018, 140, 17263–17270. [Google Scholar] [CrossRef] [PubMed]
- Ham, K.; Hong, S.; Kang, S.; Cho, K.; Lee, J. Extensive Active-Site Formation in Trirutile CoSb2O6 by Oxygen Vacancy for Oxygen Evolution Reaction in Anion Exchange Membrane Water Splitting. ACS Energy Lett. 2021, 6, 364–370. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.-S.; Kamat, P.V.; Ptasinska, S. Probing Interfacial Electrochemistry on a Co3O4 Water Oxidation Catalyst Using Lab-Based Ambient Pressure X-ray Photoelectron Spectroscopy. J. Phys. Chem. C 2018, 122, 13894–13901. [Google Scholar] [CrossRef]
- Tsai, F.-T.; Deng, Y.-T.; Pao, C.-W.; Chen, J.-L.; Lee, J.-F.; Lai, K.-T.; Liaw, W.-F. The HER/OER mechanistic study of an FeCoNi-based electrocatalyst for alkaline water splitting. J. Mater. Chem. A 2020, 8, 9939–9950. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Qian, J.; Su, H.; Lee, K.-J.; Cheng, T.; Xiao, H.; Yano, J.; Goddard, W.A.; Crumlin, E.J. Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper. Nat. Commun. 2019, 10, 1875. [Google Scholar] [CrossRef]
- Zhang, X.; Ptasinska, S. Dissociative adsorption of H2O onto a Pt thin film in direct contact with GaN (0001): Effect of electronic communications between catalyst and a semiconducting support. Appl. Surf. Sci. 2020, 516, 146127. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Surface Studies by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1982, 49, 57–61. [Google Scholar] [CrossRef]
- Song, Z.; Yi, J.; Qi, J.; Zheng, Q.; Zhu, Z.; Tao, L.; Cao, Y.; Li, Y.; Gao, Z.; Zhang, R.; et al. Line defects in monolayer TiSe2 with adsorption of Pt atoms potentially enable excellent catalytic activity. Nano Res. 2022, 15, 4687–4692. [Google Scholar] [CrossRef]
- Zheng, W.; Lee, L.Y.S. Observing Electrocatalytic Processes via In Situ Electrochemical Scanning Tunneling Microscopy: Latest Advances. Chemistry 2022, 17, e202200384. [Google Scholar] [CrossRef]
- Itaya, K.; Tomita, E. Scanning tunneling microscope for electrochemistry—A new concept for the in situ scanning tunneling microscope in electrolyte solutions. Surf. Sci. 1988, 201, L507–L512. [Google Scholar] [CrossRef]
- Liang, Y.; Pfisterer, J.H.K.; McLaughlin, D.; Csoklich, C.; Seidl, L.; Bandarenka, A.S.; Schneider, O. Electrochemical Scanning Probe Microscopies in Electrocatalysis. Small Methods 2019, 3, 1800387. [Google Scholar] [CrossRef]
- Fester, J.; Makoveev, A.; Grumelli, D.; Gutzler, R.; Sun, Z.; Rodríguez-Fernández, J.; Kern, K.; Lauritsen, J.V. The Structure of the Cobalt Oxide/Au Catalyst Interface in Electrochemical Water Splitting. Angew. Chem. Int. Ed. 2018, 57, 11893–11897. [Google Scholar] [CrossRef]
- Shirai, K.; Sugimoto, T.; Watanabe, K.; Haruta, M.; Kurata, H.; Matsumoto, Y. Effect of Water Adsorption on Carrier Trapping Dynamics at the Surface of Anatase TiO 2 Nanoparticles. Nano Lett. 2016, 16, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef]
- Agosta, L.; Brandt, E.G.; Lyubartsev, A.P. Diffusion and reaction pathways of water near fully hydrated TiO2 surfaces from ab initio molecular dynamics. J. Chem. Phys. 2017, 147, 024704. [Google Scholar] [CrossRef]
- Tan, S.; Feng, H.; Ji, Y.; Wang, Y.; Zhao, J.; Zhao, A.; Wang, B.; Luo, Y.; Yang, J.; Hou, J.G. Observation of Photocatalytic Dissociation of Water on Terminal Ti Sites of TiO 2 (110)-1 × 1 Surface. J. Am. Chem. Soc. 2012, 134, 9978–9985. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, S. An Investigation of Active Sites for electrochemical CO2 Reduction Reactions: From In Situ Characterization to Rational Design. Adv. Sci. 2021, 8, 2003579. [Google Scholar] [CrossRef]
- Hu, C.; Hu, Y.; Zhu, A.; Li, M.; Wei, J.; Zhang, Y.; Xie, W. Several Key Factors for Efficient Electrocatalytic Water Splitting: Active Site Coordination Environment, Morphology Changes and Intermediates Identification. Chem.—Eur. J. 2022, 28, e202200138. [Google Scholar] [CrossRef]
- Mitterreiter, E.; Liang, Y.; Golibrzuch, M.; McLaughlin, D.; Csoklich, C.; Bartl, J.D.; Holleitner, A.; Wurstbauer, U.; Bandarenka, A.S. In-situ visualization of hydrogen evolution sites on helium ion treated molybdenum dichalcogenides under reaction conditions. NPJ 2D Mater. Appl. 2019, 3, 25. [Google Scholar] [CrossRef]
- Pfisterer, J.H.K.; Liang, Y.; Schneider, O.; Bandarenka, A.S. Direct instrumental identification of catalytically active surface sites. Nature 2017, 549, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Csoklich, C.; McLaughlin, D.; Schneider, O.; Bandarenka, A.S. Revealing Active Sites for Hydrogen Evolution at Pt and Pd Atomic Layers on Au Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 12476–12480. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F.; et al. Enhancing CO2 Electroreduction with the Metal–Oxide Interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Fester, J.; García-Melchor, M.; Walton, A.S.; Bajdich, M.; Li, Z.; Lammich, L.; Vojvodic, A.; Lauritsen, J.V. Edge reactivity and water-assisted dissociation on cobalt oxide nanoislands. Nat. Commun. 2017, 8, 14169. [Google Scholar] [CrossRef]
- Della Pia, E.A.; Chi, Q.; Macdonald, J.E.; Ulstrup, J.; Jones, D.D.; Elliott, M. Fast electron transfer through a single molecule natively structured redox protein. Nanoscale 2012, 4, 7106. [Google Scholar] [CrossRef][Green Version]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Haugstad, G. Atomic Force Microscopy: Understanding Basic Modes and Advanced Applications; John Wiley and Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-63882-8. [Google Scholar]
- Yoo, H.; Bae, C.; Yang, Y.; Lee, S.; Kim, M.; Kim, H.; Kim, Y.; Shin, H. Spatial Charge Separation in Asymmetric Structure of Au Nanoparticle on TiO2 Nanotube by Light-Induced Surface Potential Imaging. Nano Lett. 2014, 14, 4413–4417. [Google Scholar] [CrossRef]
- Eichhorn, J.; Kastl, C.; Cooper, J.K.; Ziegler, D.; Schwartzberg, A.M.; Sharp, I.D.; Toma, F.M. Nanoscale imaging of charge carrier transport in water splitting photoanodes. Nat. Commun. 2018, 9, 2597. [Google Scholar] [CrossRef]
- Bentley, C.L.; Kang, M.; Unwin, P.R. Nanoscale Structure Dynamics within Electrocatalytic Materials. J. Am. Chem. Soc. 2017, 139, 16813–16821. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Mo, X.; Tse, E.C.M.; Cui, X. Electronic gap characterization at mesoscopic scale via scanning probe microscopy under ambient conditions. Nat. Commun. 2022, 13, 4648. [Google Scholar] [CrossRef]
- Sotres, J.; Baró, A.M. AFM Imaging and Analysis of Electrostatic Double Layer Forces on Single DNA Molecules. Biophys. J. 2010, 98, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; de Miguel, R.; Martínez-Júlvez, M.; Gómez-Moreno, C.; Lostao, A. Mechanostability of the Single-Electron-Transfer Complexes of Anabaena Ferredoxin-NADP+ Reductase. ChemPhysChem 2015, 16, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Domínguez, S.; Caballero-Mancebo, S.; Marcuello, C.; Martínez-Júlvez, M.; Medina, M.; Lostao, A. Nanomechanical Study of Enzyme: Coenzyme Complexes: Bipartite Sites in Plastidic Ferredoxin-NADP+ Reductase for the Interaction with NADP+. Antioxidants 2022, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.J. LVII. On the Charge of Electricity Carried by the Ions Produced by Röntgen Rays. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1898, 46, 528–545. [Google Scholar] [CrossRef][Green Version]
- Nonnenmacher, M.; O’Boyle, M.P.; Wickramasinghe, H.K. Kelvin probe force microscopy. Appl. Phys. Lett. 1991, 58, 2921. [Google Scholar] [CrossRef]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Glatzel, T.; Sadewasser, S.; Lux-Steiner, M.C. Amplitude or frequency modulation-detection in Kelvin probe force microscopy. Appl. Surf. Sci. 2003, 210, 84–89. [Google Scholar] [CrossRef]
- Collins, L.; Kilpatrick, J.I.; Kalinin, S.V.; Rodriguez, B.J. Towards nanoscale electrical measurements in liquid by advanced KPFM techniques: A review. Rep. Prog. Phys. 2018, 81, 086101. [Google Scholar] [CrossRef]
- Sadewasser, S.; Jelinek, P.; Fang, C.-K.; Custance, O.; Yamada, Y.; Sugimoto, Y.; Abe, M.; Morita, S. New insights on atomic-resolution frequency-modulation Kelvin-probe force-microscopy imaging of semiconductors. Phys. Rev. Lett. 2009, 103, 266103. [Google Scholar] [CrossRef]
- Tal, O.; Gao, W.; Chan, C.K.; Kahn, A.; Rosenwaks, Y. Measurement of interface potential change and space charge region across metal/organic/metal structures using Kelvin probe force microscopy. Appl. Phys. Lett. 2004, 85, 4148–4150. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, K.; Xiang, Y.; Wang, J.; Lin, W.; Guo, M.; Ma, G. Facet-Oriented Assembly of Mo:BiVO4 and Rh:SrTiO3 Particles: Integration of p–n Conjugated Photo-electrochemical System in a Particle Applied to Photocatalytic Overall Water Splitting. ACS Catal. 2022, 12, 2415–2425. [Google Scholar] [CrossRef]
- Chen, R.; Fan, F.; Dittrich, T.; Li, C. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem. Soc. Rev. 2018, 47, 8238–8262. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Kobayashi, K.; Oyabu, N.; Hirata, Y.; Matsushige, K.; Yamada, H. Practical aspects of Kelvin-probe force microscopy at solid/liquid interfaces in various liquid media. J. Appl. Phys. 2014, 116, 134307. [Google Scholar] [CrossRef]
- Kobayashi, N.; Asakawa, H.; Fukuma, T. Quantitative potential measurements of nanoparticles with different surface charges in liquid by open-loop electric potential microscopy. J. Appl. Phys. 2011, 110, 044315. [Google Scholar] [CrossRef]
- Kobayashi, N.; Asakawa, H.; Fukuma, T. Dual frequency open-loop electric potential microscopy for local potential measurements in electrolyte solution with high ionic strength. Rev. Sci. Instrum. 2012, 83, 033709. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Asakawa, H.; Fukuma, T. Nanoscale potential measurements in liquid by frequency modulation atomic force microscopy. Rev. Sci. Instrum. 2010, 81, 123705. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Kilpatrick, J.I.; Vlassiouk, I.V.; Tselev, A.; Weber, S.A.L.; Jesse, S.; Kalinin, S.V.; Rodriguez, B.J. Dual harmonic Kelvin probe force microscopy at the graphene–liquid interface. Appl. Phys. Lett. 2014, 104, 133103. [Google Scholar] [CrossRef]
- Collins, L.; Jesse, S.; Kilpatrick, J.I.; Tselev, A.; Varenyk, O.; Okatan, M.B.; Weber, S.A.L.; Kumar, A.; Balke, N.; Kalinin, S.V.; et al. Probing charge screening dynamics and electrochemical processes at the solid–liquid interface with electrochemical force microscopy. Nat. Commun. 2014, 5, 3871. [Google Scholar] [CrossRef]
- Hackl, T.; Schitter, G.; Mesquida, P. AC Kelvin Probe Force Microscopy Enables Charge Mapping in Water. ACS Nano 2022, 16, 17982–17990. [Google Scholar] [CrossRef]
- Hackl, T.; Poik, M.; Schitter, G. Influence of Imaging Parameters on AFM Surface Potential Measurements in Aqueous Solutions. In Proceedings of the 2022 IEEE 22nd International Conference on Nanotechnology (NANO), Palma de Mallorca, Spain, 4–8 July 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 39–42. [Google Scholar]
- Hu, Y.; Wu, Y.; Feng, J.; Huang, H.; Zhang, C.; Qian, Q.; Fang, T.; Xu, J.; Wang, P.; Li, Z.; et al. Rational design of electrocatalysts for simultaneously promoting bulk charge separation and surface charge transfer in solar water splitting photoelectrodes. J. Mater. Chem. A 2018, 6, 2568–2576. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, B.; Chen, H.; Li, Y.; Chang, S. Decreased Surface Photovoltage of ZnO Photoanode Films via Optimal Annealing Temperature for Enhanced Photoelectrochemical Performance. J. Nanomater. 2019, 2019, 9367573. [Google Scholar] [CrossRef]
- Chen, R.; Pang, S.; An, H.; Zhu, J.; Ye, S.; Gao, Y.; Fan, F.; Li, C. Charge separation via asymmetric illumination in photocatalytic Cu2O particles. Nat. Energy 2018, 3, 655–663. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Miao, S.; Liu, T.; Mu, L.; Li, R.; Fan, F.; Li, C. Positioning the Water Oxidation Reaction Sites in Plasmonic Photocatalysts. J. Am. Chem. Soc. 2017, 139, 11771–11778. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Barawi, M.; Gómez-Moñivas, S.; Colchero, J.; Rodríguez-Peña, M.; Yang, S.; Zhao, X.; Lu, Y.-H.; Chintala, R.; Reñones, P.; et al. Photoinduced Charge Transfer and Trapping on Single Gold Metal Nanoparticles on TiO 2. ACS Appl. Mater. Interfaces 2021, 13, 50531–50538. [Google Scholar] [CrossRef] [PubMed]
- Wardhana, A.C.; Yasuhara, S.; Yu, M.-W.; Yamaguchi, A.; Nagao, T.; Ishii, S.; Miyauchi, M. Direct imaging of visible-light-induced one-step charge separation at the chromium(iii) oxide–strontium titanate interface. J. Mater. Chem. A 2022, 10, 752–761. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kumatani, A.; Shiku, H.; Matsue, T. Scanning Probe Microscopy for Nanoscale Electrochemical Imaging. Anal. Chem. 2017, 89, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, M.V.; Nogala, W.; Velmurugan, J.; Wang, Y. Scanning electrochemical microscopy in the 21st century. Update 1: Five years after. Phys. Chem. Chem. Phys. 2011, 13, 21196. [Google Scholar] [CrossRef]
- Kranz, C.; Wächtler, M. Characterizing photocatalysts for water splitting: From atoms to bulk and from slow to ultrafast processes. Chem. Soc. Rev. 2021, 50, 1407–1437. [Google Scholar] [CrossRef]
- Zoski, C.G. Review—Advances in Scanning Electrochemical Microscopy (SECM). J. Electrochem. Soc. 2016, 163, H3088–H3100. [Google Scholar] [CrossRef]
- Ryu, C.H.; Nam, Y.; Ahn, H.S. Modern applications of scanning electrochemical microscopy in the analysis of electrocatalytic surface reactions. Chin. J. Catal. 2022, 43, 59–70. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Kong, W.; Fan, H. Recent advances in solar cells and photo-electrochemical water splitting by scanning electrochemical microscopy. Front. Optoelectron. 2018, 11, 333–347. [Google Scholar] [CrossRef]
- Pishgar, S.; Gulati, S.; Strain, J.M.; Liang, Y.; Mulvehill, M.C.; Spurgeon, J.M. In Situ Analytical Techniques for the Investigation of Material Stability and Interface Dynamics in Electrocatalytic and Photoelectrochemical Applications. Small Methods 2021, 5, 2100322. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ahn, H.S.; Bard, A.J. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, M.; Mleczko, M.J.; Koh, A.L.; Nishi, Y.; Pop, E.; Bard, A.J.; Zheng, X. Kinetic Study of Hydrogen Evolution Reaction over Strained MoS2 with Sulfur Vacancies Using Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 5123–5129. [Google Scholar] [CrossRef]
- Malathi, A.; J., M.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. Gen. 2018, 555, 47–74. [Google Scholar]
- Nguyen, T.D.; Nguyen, V.-H.; Nanda, S.; Vo, D.-V.N.; Nguyen, V.H.; Van Tran, T.; Nong, L.X.; Nguyen, T.T.; Bach, L.-G.; Abdullah, B.; et al. BiVO4 photocatalysis design and applications to oxygen production and degradation of organic compounds: A review. Environ. Chem. Lett. 2020, 18, 1779–1801. [Google Scholar] [CrossRef]
- Wang, S.; Cui, D.; Hao, W.; Du, Y. Roles of Cocatalysts on BiVO4 Photoanodes for Photoelectrochemical Water Oxidation: A Minireview. Energy Fuels 2022, 36, 11394–11403. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B.-K. Recent advances in BiVO4 semiconductor materials for hydrogen production using photoelectrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 111, 332–343. [Google Scholar] [CrossRef]
- Nellist, M.R.; Qiu, J.; Laskowski, F.A.L.; Toma, F.M.; Boettcher, S.W. Potential-Sensing Electrochemical AFM Shows CoPi as a Hole Collector and Oxygen Evolution Catalyst on BiVO4 Water-Splitting Photoanodes. ACS Energy Lett. 2018, 3, 2286–2291. [Google Scholar] [CrossRef]
- Nellist, M.R.; Laskowski, F.A.L.; Qiu, J.; Hajibabaei, H.; Sivula, K.; Hamann, T.W.; Boettcher, S.W. Potential-sensing electrochemical atomic force microscopy for in operando analysis of water-splitting catalysts and interfaces. Nat. Energy 2018, 3, 46–52. [Google Scholar] [CrossRef]
- Laskowski, F.A.L.; Oener, S.Z.; Nellist, M.R.; Gordon, A.M.; Bain, D.C.; Fehrs, J.L.; Boettcher, S.W. Nanoscale semiconductor/catalyst interfaces in photoelectrochemistry. Nat. Mater. 2020, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, C.I.; Jantz, D.T.; Leonard, K.C. Selective electrochemical CO2 reduction to CO using in situ reduced In2O3 nanocatalysts. J. Mater. Chem. A 2017, 5, 22743–22749. [Google Scholar] [CrossRef]
- Sreekanth, N.; Phani, K.L. Selective reduction of CO 2 to formate through bicarbonate reduction on metal electrodes: New insights gained from SG/TC mode of SECM. Chem. Commun. 2014, 50, 11143–11146. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Jacobse, L.; Koper, M.T.M. Understanding the Voltammetry of Bulk CO Electrooxidation in Neutral Media through Combined SECM Measurements. J. Phys. Chem. Lett. 2020, 11, 9708–9713. [Google Scholar] [CrossRef] [PubMed]
- Toma, F.M.; Cooper, J.K.; Kunzelmann, V.; McDowell, M.T.; Yu, J.; Larson, D.M.; Borys, N.J.; Abelyan, C.; Beeman, J.W.; Yu, K.M.; et al. Mechanistic insights into chemical and photochemical transformations of bismuth vanadate photoanodes. Nat. Commun. 2016, 7, 12012. [Google Scholar] [CrossRef]
- Zeng, G.; Pham, T.A.; Vanka, S.; Liu, G.; Song, C.; Cooper, J.K.; Mi, Z.; Ogitsu, T.; Toma, F.M. Development of a photoelectrochemically self-improving Si/GaN photocathode for efficient and durable H2 production. Nat. Mater. 2021, 20, 1130–1135. [Google Scholar] [CrossRef]
- Hemmerling, J.R.; Mathur, A.; Linic, S. Design Principles for Efficient and Stable Water Splitting Photoelectrocatalysts. Acc. Chem. Res. 2021, 54, 1992–2002. [Google Scholar] [CrossRef]
- Zhu, T.; Chong, M.N. Prospects of metal–insulator–semiconductor (MIS) nanojunction structures for enhanced hydrogen evolution in photoelectrochemical cells: A review. Nano Energy 2015, 12, 347–373. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, H.-J.; Jang, P.; Jung, C.; Seomoon, K. Properties of low-temperature passivation of silicon with ALD Al2O3 films and their PV applications. Electron. Mater. Lett. 2011, 7, 171–174. [Google Scholar] [CrossRef]
- Esposito, D.V.; Levin, I.; Moffat, T.P.; Talin, A.A. H2 evolution at Si-based metal–insulator–semiconductor photoelectrodes enhanced by inversion channel charge collection and H spillover. Nat. Mater. 2013, 12, 562–568. [Google Scholar] [CrossRef]
- Paiva, T.O.; Schneider, A.; Bataille, L.; Chovin, A.; Anne, A.; Michon, T.; Wege, C.; Demaille, C. Enzymatic activity of individual bioelectrocatalytic viral nanoparticles: Dependence of catalysis on the viral scaffold and its length. Nanoscale 2022, 14, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Röntgen, W.C. On a New Kind of Rays. Science 1896, 3, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Eo, Y.; Duong, M.T.H.; Ahn, H.-C. Structural Comparison of hMDH2 Complexed with Natural Substrates and Cofactors: The Importance of Phosphate Binding for Active Conformation and Catalysis. Biomolecules 2022, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wang, Y.; Guo, M.; Zhang, J.; Li, Z.; Deng, T.; Zhang, Z.; Yan, B. In-situ/operando techniques to identify active sites for thermochemical conversion of CO2 over heterogeneous catalysts. J. Energy Chem. 2021, 62, 153–171. [Google Scholar] [CrossRef]
- Dong, C.; Vayssieres, L. In Situ/Operando X-ray Spectroscopies for Advanced Investigation of Energy Materials. Chem.—Eur. J. 2018, 24, 18356–18373. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Q.; Lv, X.; Zhang, D.; Xu, H.; Ma, D.; Zhong, J. Understanding Photoelectrochemical Water Oxidation with X-ray Absorption Spectroscopy. ACS Energy Lett. 2020, 5, 975–993. [Google Scholar] [CrossRef]
- Ismail, N.; Qin, F.; Fang, C.; Liu, D.; Liu, B.; Liu, X.; Wu, Z.; Chen, Z.; Chen, W. Electrocatalytic acidic oxygen evolution reaction: From nanocrystals to single atoms. Aggregate 2021, 2, e106. [Google Scholar] [CrossRef]
- Axnanda, S.; Crumlin, E.J.; Mao, B.; Rani, S.; Chang, R.; Karlsson, P.G.; Edwards, M.O.M.; Lundqvist, M.; Moberg, R.; Ross, P.; et al. Using “Tender” X-ray Ambient Pressure X-Ray Photoelectron Spectroscopy as A Direct Probe of Solid-Liquid Interface. Sci. Rep. 2015, 5, 9788. [Google Scholar] [CrossRef]
- Cao, X.; Tan, D.; Wulan, B.; Hui, K.S.; Hui, K.N.; Zhang, J. In Situ Characterization for Boosting Electrocatalytic Carbon Dioxide Reduction. Small Methods 2021, 5, 2100700. [Google Scholar] [CrossRef]
- Roy, K.; Artiglia, L.; van Bokhoven, J.A. Ambient Pressure Photoelectron Spectroscopy: Opportunities in Catalysis from Solids to Liquids and Introducing Time Resolution. ChemCatChem 2018, 10, 666–682. [Google Scholar] [CrossRef]
- Siegbahn, H.; Siegbahn, K. ESCA applied to liquids. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 319–325. [Google Scholar] [CrossRef]
- Su, H.; Ye, Y.; Lee, K.-J.; Zeng, J.; Crumlin, E.J. Probing the nickel corrosion phenomena in alkaline electrolyte using tender x-ray ambient pressure x-ray photoelectron spectroscopy. J. Phys. Appl. Phys. 2021, 54, 374001. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Favaro, M.; Ross, P.N.; Hussain, Z.; Liu, Z.; Yano, J.; Crumlin, E.J. Stabilizing the Meniscus for Operando Characterization of Platinum During the Electrolyte-Consuming Alkaline Oxygen Evolution Reaction. Top. Catal. 2018, 61, 2152–2160. [Google Scholar] [CrossRef]
- Favaro, M.; Jeong, B.; Ross, P.N.; Yano, J.; Hussain, Z.; Liu, Z.; Crumlin, E.J. Unravelling the electrochemical double layer by direct probing of the solid/liquid interface. Nat. Commun. 2016, 7, 12695. [Google Scholar] [CrossRef]
- Starr, D.E.; Favaro, M.; Abdi, F.F.; Bluhm, H.; Crumlin, E.J.; van de Krol, R. Combined soft and hard X-ray ambient pressure photoelectron spectroscopy studies of semiconductor/electrolyte interfaces. J. Electron Spectrosc. Relat. Phenom. 2017, 221, 106–115. [Google Scholar] [CrossRef]
- Favaro, M.; Abdi, F.F.; Lamers, M.; Crumlin, E.J.; Liu, Z.; van de Krol, R.; Starr, D.E. Light-Induced Surface Reactions at the Bismuth Vanadate/Potassium Phosphate Interface. J. Phys. Chem. B 2018, 122, 801–809. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Huang, Z.; Guan, X.; Zong, S.; Cheng, C.; Zheng, B.; Guo, L. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: Enhanced photocatalytic H2-evolution activity and mechanism insight. Chem. Eng. J. 2020, 401, 126135. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, R.; Xu, Q. Metal–Organic Framework Based Catalysts for Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801193. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.-R.; Xiao, Y.-Y.; Yin, Y.-C.; Huang, W.-M.; Huang, Z.-C.; Zheng, Y.-Z.; Mu, F.-Y.; Huang, R.; Shi, G.-Y.; et al. Electronic metal–support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced Transition Metal-Based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Favaro, M.; Valero-Vidal, C.; Eichhorn, J.; Toma, F.M.; Ross, P.N.; Yano, J.; Liu, Z.; Crumlin, E.J. Elucidating the alkaline oxygen evolution reaction mechanism on platinum. J. Mater. Chem. A 2017, 5, 11634–11643. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Favaro, M.; Ross, P.N.; Yano, J.; Liu, Z.; Hussain, Z.; Crumlin, E.J. Probing the Surface of Platinum during the Hydrogen Evolution Reaction in Alkaline Electrolyte. J. Phys. Chem. B 2018, 122, 864–870. [Google Scholar] [CrossRef]
- Han, Y.; Axnanda, S.; Crumlin, E.J.; Chang, R.; Mao, B.; Hussain, Z.; Ross, P.N.; Li, Y.; Liu, Z. Observing the Electrochemical Oxidation of Co Metal at the Solid/Liquid Interface Using Ambient Pressure X-ray Photoelectron Spectroscopy. J. Phys. Chem. B 2018, 122, 666–671. [Google Scholar] [CrossRef]
- Favaro, M.; Yang, J.; Nappini, S.; Magnano, E.; Toma, F.M.; Crumlin, E.J.; Yano, J.; Sharp, I.D. Understanding the Oxygen Evolution Reaction Mechanism on CoOx using Operando Ambient-Pressure X-ray Photoelectron Spectroscopy. J. Am. Chem. Soc. 2017, 139, 8960–8970. [Google Scholar] [CrossRef]
- Cai, J.; Han, Y.; Chen, S.; Crumlin, E.J.; Yang, B.; Li, Y.; Liu, Z. CO2 Activation on Ni(111) and Ni(100) Surfaces in the Presence of H2 O: An Ambient-Pressure X-ray Photoelectron Spectroscopy Study. J. Phys. Chem. C 2019, 123, 12176–12182. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, F.; Li, J.; Zeng, G.; Ye, Y.; Larson, D.M.; Yano, J.; Crumlin, E.J.; Ager, J.W.; Wang, L.; et al. Investigation and mitigation of degradation mechanisms in Cu2O photoelectrodes for CO2 reduction to ethylene. Nat. Energy 2021, 6, 1124–1132. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Zou, L.; Wei, D.-Y.; Zheng, L.-L.; Wu, Y.-F.; Zhang, H.; Li, J.-F. In situ studies of energy-related electrochemical reactions using Raman and X-ray absorption spectroscopy. Chin. J. Catal. 2022, 43, 33–46. [Google Scholar] [CrossRef]
- Fabbri, E.; Abbott, D.F.; Nachtegaal, M.; Schmidt, T.J. Operando X-ray absorption spectroscopy: A powerful tool toward water splitting catalyst development. Curr. Opin. Electrochem. 2017, 5, 20–26. [Google Scholar] [CrossRef]
- Wang, B.; Chu, S.; Zheng, L.; Li, X.; Zhang, J.; Zhang, F. Application of X-Ray Absorption Spectroscopy in Electrocatalytic Water Splitting and CO2 Reduction. Small Sci. 2021, 1, 2100023. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Karuturi, S.; Catchpole, K.; Zhang, Q.; Zhao, C. Design and operando/in situ characterization of precious-metal-free electrocatalysts for alkaline water splitting. Carbon Energy 2020, 2, 582–613. [Google Scholar] [CrossRef]
- Timoshenko, J.; Roldan Cuenya, B. In Situ/Operando Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2021, 121, 882–961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Sun, F.; Zhou, S.; Hu, W.; Zhang, H.; Dong, J.; Jiang, Z.; Zhao, J.; Li, J.; Yan, W.; et al. Atomic-level insight into super-efficient electrocatalytic oxygen evolution on iron and vanadium co-doped nickel (oxy)hydroxide. Nat. Commun. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Deng, C.; Xie, S.; Li, Y.; Zhang, W.; Sheng, H.; Chen, C.; Zhao, J. Photocatalytic C–C Coupling from Carbon Dioxide Reduction on Copper Oxide with Mixed-Valence Copper(I)/Copper(II). J. Am. Chem. Soc. 2021, 143, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Merte, L.R.; Behafarid, F.; Miller, D.J.; Friebel, D.; Cho, S.; Mbuga, F.; Sokaras, D.; Alonso-Mori, R.; Weng, T.-C.; Nordlund, D.; et al. Electrochemical Oxidation of Size-Selected Pt Nanoparticles Studied Using in Situ High-Energy-Resolution X-ray Absorption Spectroscopy. ACS Catal. 2012, 2, 2371–2376. [Google Scholar] [CrossRef]
- Möller, T.; Scholten, F.; Thanh, T.N.; Sinev, I.; Timoshenko, J.; Wang, X.; Jovanov, Z.; Gliech, M.; Roldan Cuenya, B.; Varela, A.S.; et al. Electrocatalytic CO 2 Reduction on CuO x Nanocubes: Tracking the Evolution of Chemical State, Geometric Structure, and Catalytic Selectivity using Operando Spectroscopy. Angew. Chem. 2020, 132, 18130–18139. [Google Scholar] [CrossRef]
- Velasco-Velez, J.-J.; Mom, R.V.; Sandoval-Diaz, L.-E.; Falling, L.J.; Chuang, C.-H.; Gao, D.; Jones, T.E.; Zhu, Q.; Arrigo, R.; Roldan Cuenya, B.; et al. Revealing the Active Phase of Copper during the Electroreduction of CO2 in Aqueous Electrolyte by Correlating In Situ X-ray Spectroscopy and In Situ Electron Microscopy. ACS Energy Lett. 2020, 5, 2106–2111. [Google Scholar] [CrossRef]
- Kornienko, N.; Resasco, J.; Becknell, N.; Jiang, C.-M.; Liu, Y.-S.; Nie, K.; Sun, X.; Guo, J.; Leone, S.R.; Yang, P. Operando Spectroscopic Analysis of an Amorphous Cobalt Sulfide Hydrogen Evolution Electrocatalyst. J. Am. Chem. Soc. 2015, 137, 7448–7455. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Li, Y. Single-atom catalysis for carbon neutrality. Carbon Energy 2022, 4, 1021–1079. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.-Q.; Liang, S.; An, Q.; Fu, J.; Hu, J.-S. Coordination anchoring synthesis of high-density single-metal-atom sites for electrocatalysis. Coord. Chem. Rev. 2022, 466, 214603. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S.; et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Luo, Q.; Liu, W.; Lin, Y.; Liu, X.; Cao, Y.; Zhang, W.; Wu, Y.; Yang, J.; Yao, T.; et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2019, 2, 134–141. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Guo, Z.; Song, Y.; Tang, S.; Ma, Y.; Xing, X.; Wang, Q. Synthesis of Atomically Thin g-C3N4 Nanosheets via Supercritical CO2 Doping with Single-Atom Cobalt for Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 52560–52570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).